Abstract
Schizophrenia (SZ) is associated with deficits in both temporal and salience processing. The underlying neurological dysfunctions in both processes, which are interrelated and share neuroanatomical bases, remain poorly understood. The principal objective of this study was to elucidate whether there are any brain regions that show abnormal response during timing and oddball tasks in patients with SZ. To this end, we conducted a signed differential mapping (SDM) meta-analysis of functional magnetic resonance imaging (fMRI) studies assessing abnormal responses elicited by tasks based on the oddball paradigm in patients with SZ. We conducted a similar SDM meta-analysis of neuroimaging studies of timing tasks in SZ. Finally, we undertook a multimodal meta-analysis to detect the common findings of the two previous meta-analyses. We found that SZ patients showed hypoactivation in cortical and subcortical areas related to timing. The dysfunction observed during timing tasks partially coincided with deficiencies in change-detection functions (particularly in the case of preattentional processing in the mismatch negativity response). We hypothesize that a dysfunctional timing/change detection network underlies the cognitive impairment observed in SZ.
Keywords: Clinical psychology, Psychiatry, Neuroscience
1. Introduction
Schizophrenia (SZ) is characterized by various types of disturbance in perception, emotion, and cognition. Cognitive impairment, a core symptom in this disorder, is highly relevant for prognosis and functional outcome but poorly controlled by treatment (Green et al., 2000; Targum and Keefe, 2008) and has been proposed as a critical clinical and therapeutic target (Martinez-Aran and Vieta, 2015).
The neurocognitive profile of SZ comprises deficits across multiple domains, which include learning and memory, attention, working memory (WM), executive function, and social cognition, all within the context of general intellectual impairment (Hedman et al., 2013). Several studies using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) consensus cognitive battery (Keefe and Fenton, 2007; Kern et al., 2011; McCleery et al., 2014) have shown that individuals with SZ tend to suffer from disturbance in cognitive performance and that low scores indicate a worse prognosis.
Over recent years, timing dysfunction has become a focus of research interest in SZ. Extensive evidence (Alústiza et al., 2016; Lošák et al., 2016; Montalembert et al., 2015; Radua et al., 2014a,b) suggests that timing dysfunction is something underlying and at the core of the neurocognitive profile of SZ. Two recent meta-analyses of neuroimaging studies on timing identified a neural timing circuit that involved a group of cortical and subcortical regions: the supplementary motor area (SMA), the insula, the left inferior frontal region, the middle frontal gyrus, the left inferior parietal region, the left superior temporal gyrus, the right thalamus, the cerebellum, and the left putamen. This circuit was found in healthy subjects; in SZ, however, the participation of this neural timing circuit was significantly reduced, suggesting dysfunction, and there was broad hypoactivation in the right hemisphere (Ortuño et al., 2011; Wiener et al., 2010). It has been established that timing and other cognitive processes, such as attention, the automatic or controlled modification of behavior, the use of WM, and the adjustment of levels of concentration to task difficulty, employ the same brain networks (Gómez et al., 2014). Both of these broad categories of function have been reported to become interlinked when there is an increase in the level of cognitive effort demanded, both in healthy control subjects (Radua et al., 2014a,b) and in SZ patients (Alústiza et al., 2016).
As mentioned above, SZ patients experience deficits in diverse cognitive domains, including the basic ability, required in salience or novelty processing, that enables us to separate what is relevant from what is a distraction. A central abnormality in SZ is aberrant activity in the salience network (SN), which is thought to detect salient changes within a task and to respond to novel stimuli across sensory modalities (Menon, 2015). It involves activations in the temporal parietal junction (TPJ), the anterior insula, the anterior middle frontal gyrus, the bilateral anterior cingulate cortex (ACC), and the SMA. The SN operates on two levels: a primitive, automatic (bottom-up) level responsible for preattentional change detection; and a higher-order, controlled level, which involves attention and goal-oriented responses (Menon, 2015).
An experimental design widely used in the investigation of dysfunctional target processing is the oddball paradigm, in which infrequent target (oddball) stimuli are interspersed in a sequence of more numerous non-target (standard) stimuli. This paradigm can use either passive (“preattentional”) or active (“attentional”) tasks that assess neural responses to deviant/infrequent stimuli. SZ patients are impaired at such oddball detection tasks, as they are at other tasks of target detection (Kiehl et al., 2005; Kim et al., 2009). Oddball tasks involve two separate attentional processes: maintenance of attentional readiness, and detection of an infrequent change in the environment. This latter process of change detection might be regarded as novelty processing in the context of a framework of time, and the question arises of whether timing tasks might serve - in the same way that oddball tasks do - to assess a person's performance at change detection in perceptual processes.
Oddball detection studies have revealed two highly characteristic event-related potentials (ERP): the P300 wave, which is elicited by rare, cognitively relevant (target) stimuli; and mismatch negativity (MMN), which is the type of ERP elicited by deviants within a sequence of repetitive preattentional stimuli (Gaebler et al., 2015). Reduced MMN amplitude in the auditory modality is one of the best-replicated and documented neurophysiological deficits in SZ (Umbricht and Krljes, 2005). Some studies show evidence for the existence of MMN potential analogues in the visual and somatosensory modalities (Garrido et al., 2009). Because MMN underlies diverse neurobiological mechanisms connected with SZ cognitive deficits, including aberrant SN activity, reduced MMN amplitude has been proposed as a biomarker for different core impairments in SZ (Gaebler et al., 2015).
Deficits in target detection during oddball tasks in SZ have been well established by functional magnetic resonance imaging (fMRI) studies (Gur et al., 2007; Kiehl et al., 2005). Both the ventral (salience) and dorsal (sustained) attentional networks appear to contribute to the dysfunction in neural activation to salient events (Wynn et al., 2015). Neural generators of the P300 wave have been localized to several regions that partially overlap with the ventral attentional network; these regions include the TPJ, regions in the posterior superior parietal area, the ACC, the dorsolateral prefrontal cortex, the ventrolateral prefrontal cortex, and regions of the medial temporal zone (Bledowski et al., 2004; Jeon and Polich, 2003; Machado et al., 2014; Mulert et al., 2004).
Several studies suggest that there exists a connection between timing and saliency or novelty processing (Mikell et al., 2014; New and Scholl, 2009; Wittmann, 2015). Saliency processing requires a change detection mechanism capable of filtering and amplifying infrequent stimuli whether encountered in space or in time. The connection also involves shared neuroanatomical bases but can also be addressed from the perspective of allocation of cognitive resources to targets that imply a key function for survival (Uddin, 2017). The link between timing and saliency processing is not well understood as there is little solid research available. Therefore, dysfunctions involving both functions are little-studied.
The present study addresses the question of whether a dysfunctional timing network is associated with change detection deficits in SZ. We hypothesize that an aberrant timing network underlies the change detection network dysfunction in SZ subjects.
Our strategy is to identify, for SZ subjects, the neural regions underlying impaired timing in timing tasks and impaired change detection in oddball detection tasks and then to determine whether the dysfunctional timing activity pattern matches that observed for tasks based on the oddball paradigm. To this end, we carried out two signed differential mapping (SDM) meta-analyses - both of fMRI studies - in which SZ and HC subjects are compared in terms of brain response to timing tasks and to oddball detection tasks. After this, we undertook a third, multimodal, meta-analysis to identify brain regions common to patterns found in the two task-specific SDM meta-analyses.
2. Material and methods
We conducted three independent comprehensive literature searches for whole-brain fMRI studies that compared patients with SZ and HC using timing and oddball tasks. The searches were applied to the PubMed search engine up to November 2017. Keywords used in the first search were: ((Schizophrenia*) AND (functional magnetic resonance imaging*) AND (timing*)). In the second search, keywords used were: ((Schizophrenia*) AND (functional magnetic resonance imaging*) AND (oddball*)). Keywords used in the last search were: ((Schizophrenia*) AND (functional magnetic resonance imaging*) AND (mismatch negativity*)).
Inclusion criteria were that studies 1) used samples composed of both healthy volunteers and patients with SZ; 2) included use of a timing or oddball task whether standardized or designed specifically for the study concerned; 3) provided peak coordinates or statistical parametric maps in the published article; 4) made use of whole brain analyses; 5) employed a constant statistical threshold for each different region of the brain. A study was excluded if it 1) used a region-of-interest (ROI) approach; 2) did not report peak coordinates; 3) used different statistical thresholds for different brain regions; 4) was based on techniques other than fMRI (e.g., PET, SPECT); 5) was a case report, a qualitative study, a review or meta-analysis. Two reviewers independently assessed the studies in terms of the inclusion/exclusion criteria. The title and abstract were first screened for keywords, and after that, if the study was eligible, the full text was analyzed. When reviewers' independent decisions about inclusion or exclusion differed, the final decision was determined by discussion between the two reviewers.
From each dataset were obtained coordinates and t-values of the peaks of the clusters of activation differences between patients and controls. Spatial summarization of the data was carried out using anisotropic effect-size seed-based mapping software version 5.141 (AES-SDM; a quantitative voxel-based meta-analytic method formerly known as Signed Differential Mapping, http://www.sdmproject.com Radua and Mataix-Cols, 2009; Radua et al., 2011; Radua et al., 2014b). The SDM is a statistical technique for meta-analyzing studies that use neuroimage techniques (fMRI, PET, VBM or DTI) to differentiate brain activity or structure. In contrast to previous metanalytic methods (ALE or Kendel Density Analysis (KDA)), SDM addresses between-studies heterogeneity, incorporates meta-regression methods and allows multimodal meta-analysis (Radua and Mataix-Cols, 2012). First, we created a brain map of the effect size of the difference between the two groups for each study, based on the collected coordinates and t-values of cluster peaks. Second, we meta-analyzed these study maps fitting models for standard random-effects, which consider sample-size, intra-study variability, and between-study heterogeneity. Finally, we used a spatial permutation test to derive the statistical significance and we thresholded the results with the default parameters of the software.
2.1. Meta-analysis for timing studies
We conducted a SDM meta-analysis of fMRI studies that assessed HC and SZ subjects for brain response during timing tasks.
2.2. Meta-analysis for attentional oddball studies
For tasks based on the oddball paradigm, we did a similar SDM meta-analysis study to the one described above for timing. The analysis was of fMRI studies assessing HC and SZ subjects during oddball tasks that were almost exclusively attentional as opposed to pre-attentional; there was only one study of preattentional oddball detection that met our inclusion criteria.
2.3. Multimodal meta-analysis for timing and attentional oddball studies
We performed a multimodal meta-analysis to conflate the results of the timing meta-analysis with the results of the attentional oddball detection meta-analysis. The goal of this multimodal analysis was to find brain regions that show an abnormal response both in timing and in oddball tasks. To this end, the maps of the abnormal response to timing were overlapped with the map of the abnormal response to oddball detection. Rather than using a simple overlap of maps, the method used was a modification of the probability of the union of the maps (Radua et al., 2013) because this latter method is reported to better accommodate the presence of any P-value error in individual meta-analyses.
2.4. Multimodal meta-analysis for timing and preattentional oddball studies
We could not conduct a similar multimodal meta-analysis for timing and preattentional oddball studies because we had not been able to derive p-values for the latter. We conducted instead an exploratory overlap to detect brain regions that might show an abnormal response both in timing and in preattentional oddball tasks. Specifically, we selected those voxels that showed at least small effect sizes (0.2) in both the timing and preattentional oddball tasks. We only report clusters with an extent of at least 100 voxels.
3. Results
3.1. Studies included and their characteristics
Initial searching found several hundred papers, but only nine conformed to the inclusion criteria. Of these, three studied timing (a total of 53 SZ patients and 60 HC subjects), five examined attentional oddball detection (100 SZ; 122 HC), and one examined preattentional oddball detection (24 SZ; 24 HC) (see Supplementary Material, Tables 1, 2, and 3).
Table 1.
Studies of timing in SZ included in our SDM meta-analysis.
| Author | Sample | Task | Included contrast |
|---|---|---|---|
| 1. Davalos et al. (2011) | 16 SZ 18 HC |
An auditory time discrimination task | Difficult vs. easy level |
| 2. Lošák et al. (2016) | 28 SZ 27 HC |
A predictive motor timing paradigm: the Interception task | MISS contrast |
| 3. Volz et al. (2001) | 9 SZ 15 HC |
An auditory time estimation and a frequency discrimination task | Auditory time estimation vs. frequency discrimination |
Note: SZ, schizophrenia patients; HC, healthy controls.
Table 2.
Studies of attentional oddball in SZ included in our SDM meta-analysis.
| Author | Sample | Task | Included contrast |
|---|---|---|---|
| 1. Collier et al., 2014 | 20 SZ 22 HC |
An auditory and visual oddball task | Novel (auditory and visual) vs. baseline Target (visual) vs. baseline |
| 2. Laurens et al. (2005) | 28 SZ 28 HC |
An auditory oddball task | Novel vs. baseline (non target stimuli) |
| 3. Ngan et al., 2003 | 14 SZ 29 HC |
Auditory oddball task (two types) | Target (non speech stimuli) vs. baseline |
| 4. Wolf et al., 2008 | 17 SZ 21 HC |
An auditory 3-stimulus oddball task | Novel vs. baseline |
| 5. Wynn et al. (2015) | 21 SZ 22 HC |
A visual oddball task | Target vs. baseline (implicit) |
Note: SZ, schizophrenia patients; HC, healthy controls.
Table 3.
Studies of preattentional oddball in SZ included in our SDM meta-analysis.
| Author | Sample | Task | Included contrast |
|---|---|---|---|
| 1. Gaebler et al. (2015) | 24 SZ 24 HC |
An auditory mismatch task | Mismatch vs. baseline |
Note: SZ, schizophrenia patients; HC, healthy controls.
3.2. Meta-analysis results for timing studies
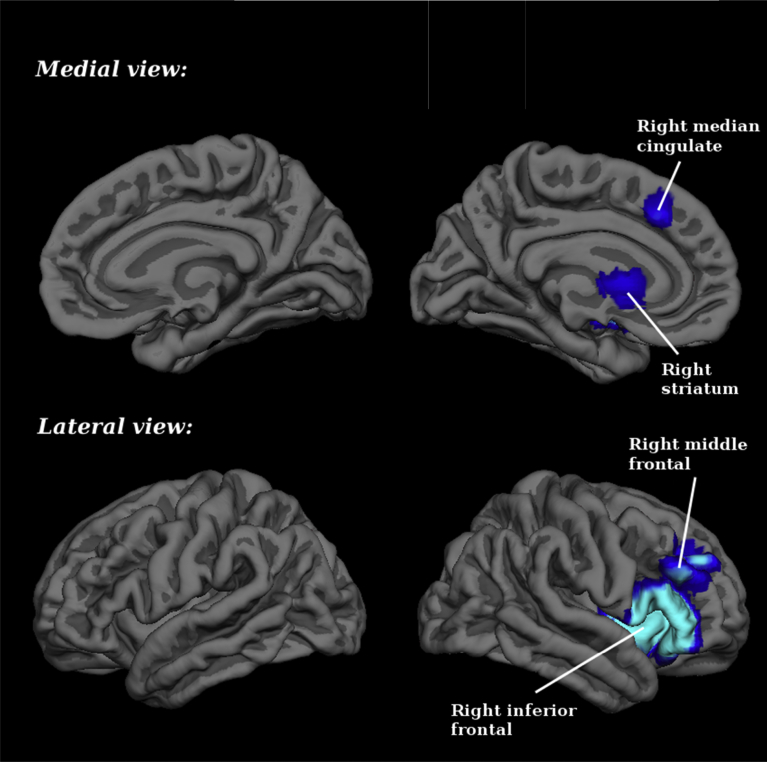
Relative to HC subjects, those with SZ demonstrated significant hypoactivation in the right striatum, the right middle frontal gyrus (BA 9 and 45), and the right median cingulate/paracingulate gyri (BA 32) (Table 4 and Fig. 1). Please see supplementary material for breakdown analysis.
Table 4.
Brain regions engaged during timing tasks: differences between schizophrenia patients and healthy controls. Hypoactivations. No hyperactivations or failures of deactivation found.
| Location | Peak |
|||
|---|---|---|---|---|
| MNI | Z | P | Voxels | |
| Right striatum | 26,14,0 | -3.015 | ∼0 | 2339 |
| Right middle frontal gyrus, BA 9 | 30,38,38 | -1.831 | 0.000851512 | 88 |
| Right middle frontal gyrus, BA 45 | 48,32,22 | -1.708 | 0.001460493 | 46 |
| Right median cingulate/paracingulate gyri, BA 32 | 4,26,40 | -1.637 | 0.001950800 | 36 |
Threshold: voxel P < 0.00500, peak SDM-Z > 1.000, cluster extent size ≥10 voxels. Breakdown regions with less than 10 voxels are not reported.
Fig. 1.
Brain regions engaged during timing tasks: differences between schizophrenia patients and healthy controls. Blue for hypoactivations (patients < controls in task > baseline).
3.3. Meta-analysis results for attentional oddball studies
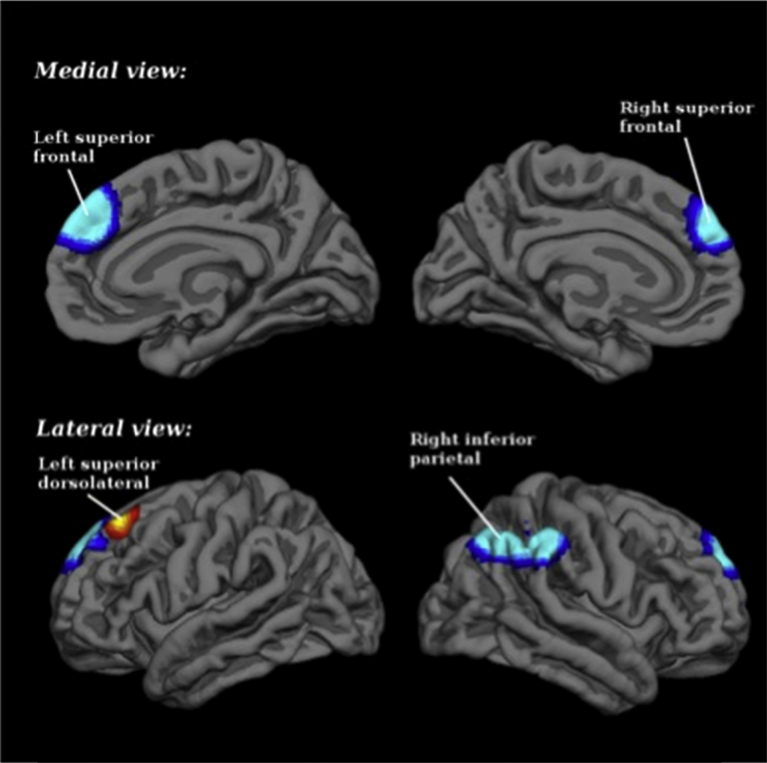
Relative to controls, SZ subjects had significantly decreased activation in the right inferior parietal gyri (BA 40) and the left superior medial gyrus. They also had significant hyperactivation or failure of deactivation in the left superior frontal gyrus, that is, in the dorsolateral prefrontal cortex (BA 9) (Table 5 and Fig. 2). Please see supplementary material for breakdown analysis.
Table 5.
Brain regions engaged during oddball detection tasks: differences between schizophrenia patients and healthy controls. Hyperactivations/failures of deactivation and hypoactivations.
| Location | Peak |
|||
|---|---|---|---|---|
| MNI | Z | P | Voxels | |
| Hyperactivations or failures of deactivation | ||||
| Left superior frontal gyrus, dorsolateral, BA 9 | -22,34,50 | 1.018 | 0.002910674 | 92 |
| (undefined) | -18,-32,14 | 1.106 | 0.002214015 | 16 |
| Hypoactivations | ||||
| Right inferior parietal (excluding supramarginal and angular) gyri, BA 40 | 52,-50,46 | -2.846 | 0.000216782 | 506 |
| Left superior medial gyrus | -8,44,38 | -2.702 | 0.000350952 | 323 |
Threshold: voxel P < 0.00500, peak SDM-Z > 1.000, cluster extent size ≥10 voxels. Breakdown regions with less than 10 voxels are not reported.
Fig. 2.
Brain regions engaged during attentional oddball tasks: differences between schizophrenia patients and healthy controls. Blue for hypoactivations (patients < controls in task > baseline), and yellow-red for hyperactivations or failures of deactivation (patients > controls in task > baseline; or patients < controls in task < baseline).
3.4. Multimodal meta-analysis results for timing and attentional oddball studies
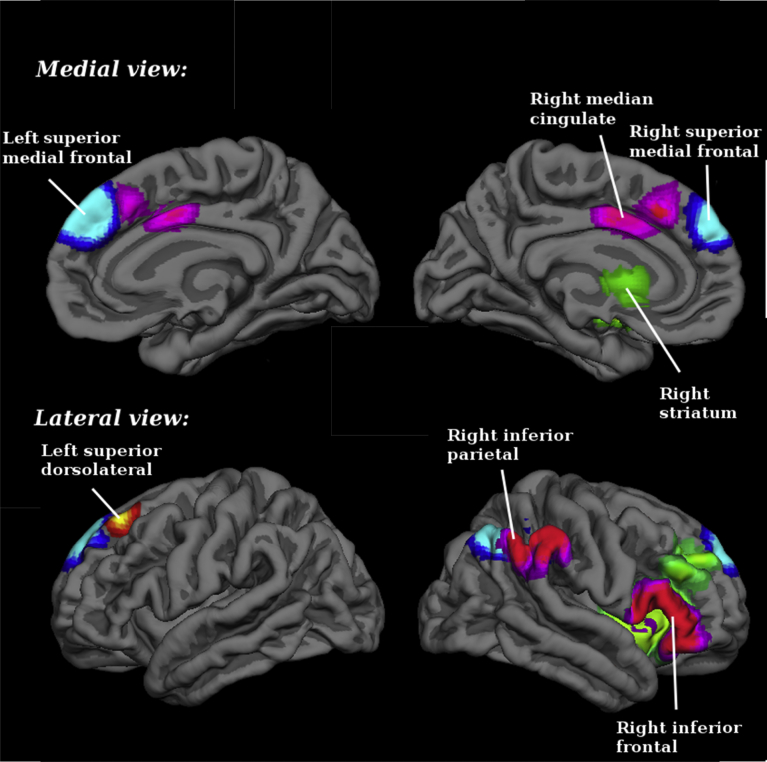
Regions that were hypoactivated by attentional oddball detection tasks in SZ patients relative to HC were found to overlap regions that were hypoactivated by timing tasks in these subjects. This overlapping occurred mainly in the right inferior frontal gyrus, triangular part (BA 45), but was also observed in the right median cingulate/paracingulate gyri (BA 24). In addition, the area corresponding to the right inferior parietal gyrus (BA 40), which was hypoactivated by attentional oddball tasks in SZ subjects relative to controls, was found to overlap with the map of the abnormal response observed with timing tasks (in SZ and HC subjects in general) (Table 6 and Fig. 3). Please see supplementary material for breakdown analysis.
Table 6.
Brain regions engaged during timing and attentional oddball detection tasks: differences between schizophrenia patients and healthy controls. Hypoactivations. No hyperactivations or failures of deactivation found.
| Location | Peak |
|
|---|---|---|
| MNI | Voxels | |
| Right inferior frontal gyrus, triangular part, BA 45 | 54,22,8 | 731 |
| Right inferior parietal (excluding supramarginal and angular) gyri, BA 40 | 48,-48,44 | 484 |
| Right median cingulate/paracingulate gyri, BA 24 | 2,-4,40 | 258 |
Threshold: voxel P < 0.00500, peak SDM-Z > 1.000, cluster extent size ≥10 voxels. Breakdown regions with less than 10 voxels are not reported.
Fig. 3.
Overlap between brain regions engaged during timing and attentional oddball tasks: differences between schizophrenia patients and healthy controls (magenta). Brain regions in neurological convention showing areas with statistically signification activation only during timing tasks (green) and during attentional oddball tasks (blue and red). Blue for hypoactivations (patients < controls in task > baseline), and red for hyperactivations or failures of deactivation (patients > controls in task > baseline; or patients < controls in task < baseline).
3.5. Multimodal meta-analysis results for timing and preattentional oddball studies
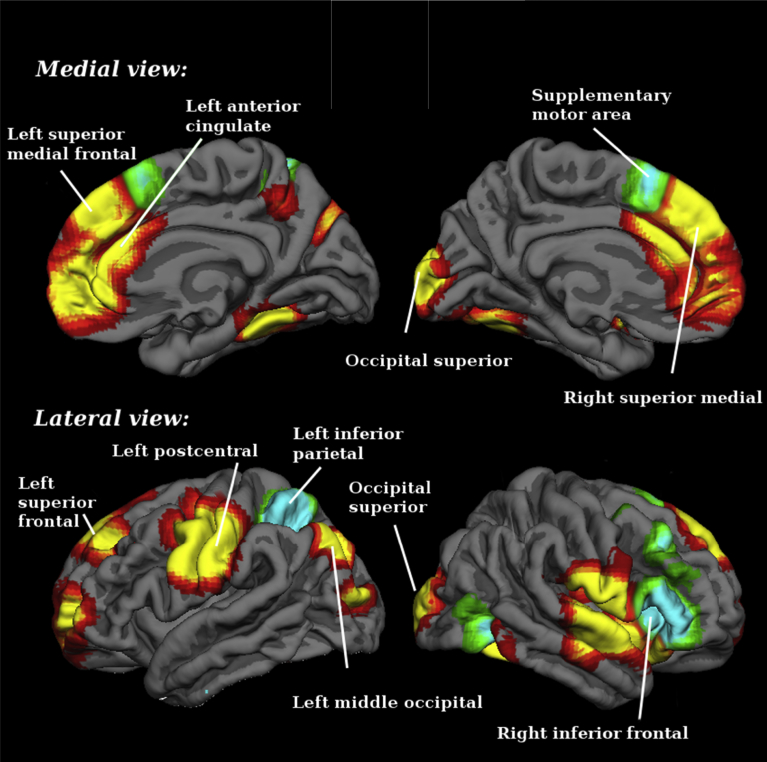
Overlapping in terms of hypoactivation in both timing and preattentional oddball occurred mainly in right insula and inferior frontal gyrus. In addition, the left parietal and the supplementary motor area overlapped with the maps of the abnormal responses observed when subjects performed both tasks. An overlap occurred also in different zones where an hypoactivation in preattentional oddball tasks and an hyperactivation in timing was found. This overlapping occurred mainly in bilateral superior frontal gyrus, mostly in BA 32. In addition, there was also overlapping in the right lateral temporal gyrus and in the gray matter around the central sulcus. Overlapping was also found in bilateral fusiform gyrus, in left middle occipital and in the left superior and middle frontal gyrus (Table 7 and Fig. 4). Please see supplementary material for breakdown analysis.
Table 7.
Brain regions engaged during timing and preattentional oddball detection tasks: differences between schizophrenia patients and healthy controls. Hypoactivations in preattentional oddball and hyperactivations or failures of deactivation in timing. Hypoactivations in preattentional oddball and timing.
| Location | Peak |
|
|---|---|---|
| MNI∗ | Voxels | |
| Hypoactivations in preattentional oddball and hyperactivations or failures of deactivation in timing | ||
| Bilateral superior frontal | 0,42,20 | 3363 |
| Right lateral temporal | 52,-4,0 | 1817 |
| Gray matter around central sulcus | 50,-10,38 | 1504 |
| Bilateral fusiform | 36,-66,-18 -28,-46,-16 |
613 350 |
| Left middle occipital | -30,-74,38 -34,-90,10 |
462 143 |
| Left superior and middle frontal gyrus | -28,56,2 -20,38,38 |
317 113 |
| Hypoactivations | ||
| Right insula and inferior frontal | 40,22,2 | 1266 |
| Left parietal | -30,-54,52 | 604 |
| Supplementary motor area | 2,16,54 | 228 |
MNI equals centers of gravity (COG) as center calculated using fsl cluster.
Fig. 4.
Overlap between brain regions engaged during timing and preattentional oddball tasks: differences between schizophrenia patients and healthy controls. Yellow-red (red for edges, yellow for center) for overlapping between preattentional oddball hypoactivations (patients < controls in task > baseline) and timing hyperactivations or failures of deactivation (patients > controls in task > baseline; or patients < controls in task < baseline), and blue-green (green edges, blue center) for overlapping between preattentional oddball and timing hypoactivations (patients < controls in task > baseline).
4. Discussion
The current study provides evidence that, in SZ subjects, tasks that involve timing and those that involve oddball detection engage or fail to engage certain neural regions in common. The study, therefore, supports the idea of an interrelationship between temporal processing and salience processing (of both attentional and preattentional stimuli) in SZ.
Timing is a primary cognitive domain that underlies or broadly influences other cognitive processes (Alústiza et al., 2017). In previous studies, we established that timing structures can be activated by cognitive demands such as cognitive control tasks in which there is variation in the degree of difficulty (Alústiza et al., 2016). To explain this finding, we suggested the existence of a temporal-cognitive control network (Alústiza et al., 2016), proposing that this network responds to changes in how demanding a task is. The idea of a temporal-cognitive control network leads on to the notion that, because temporality is change related, neural circuits involved in timing might be expected to overlap with other networks involved in cognitive functions whenever a change in the degree of task difficulty occurs (Alústiza et al., 2017). This is the reasoning by which we came to look at the change detection mechanism as a target for study. As the change detection mechanism is a core feature of novelty processing, we hypothesized that regions implicated in timing may overlap with the cognitive saliency network. Having carried out our research, we conclude that timing dysfunction can be related to any kind of cognitive task, whether it is salience detection or cognitive control, as long as it involves change detection.
Our results show dysfunctional participation of frontal, cingulate, and parietal regions during both timing and attentional oddball tasks in SZ. In line with previous fMRI studies (Duncan, 2010), we observed a pattern of frontal and parietal activity associated with heterogeneous cognitive demands (i.e., attention, WM, executive control, response selection). This is in agreement with a “multiple-demand system” thought to extend over a group of regions in the prefrontal and parietal cortex. These regions - to be more precise - comprise the cortex in and surrounding the posterior part of the inferior frontal sulcus, the cortex in the anterior insula and the adjacent frontal operculum, the cortex in the pre-SMA and the dorsal anterior cingulate, and the cortex in the area of the intraparietal sulcus (Duncan, 2010). For this reason, and in conjunction with the fact that the timing and oddball tasks involved in the various studies all engage diverse cognitive demands, our observation of general fronto-parietal activation in connection with attentional activity is not surprising.
Regarding the fMRI overlapping observed with timing and preattentional oddball paradigm-based tasks, SZ subjects showed significant hypoactivation, relative to HC subjects, in timing-related cortical-subcortical areas (particularly in the right frontal region, parietal region, insula, SMA, anterior cingulate, striatum, and thalamus). This finding is in agreement with previous meta-analyses (Ortuño et al., 2011; Wiener et al., 2010) carried out to explore the neuroanatomical basis of timing: SZ subjects showed, in comparison to controls, significantly lower activation of most right hemisphere regions of the time circuit.
Neuroimaging studies comparing brain activation in SZ and HC subjects during cognitive functions have revealed aberrant activations patterns in SZ patients. The published results are inconclusive with regard to the nature of brain activations in SZ and whether they are hypo- or hyper-activations (Nygard et al., 2012). Although several studies have found lower activation in networks related to cognitive processes and interpreted this as evidence of deficient functioning in SZ subjects (Ortuño et al., 2011; Alústiza et al., 2016, 2017), other authors (Hugdahl et al., 2009; Guerrero-Pedraza et al., 2012) have suggested that both hypo- and hyper-activation might be necessary for efficient cognitive functioning because the up-regulation of one network will require the down-regulation of another (Nygard et al., 2012). In the light of this latter theory and in explanation of the rather equivocal research results, it has been hypothesized that SZ subjects present difficulties in shifting from a baseline network activity to networks implicated in task performance. This hypothesis is explored by Kim et al. (2009) in their study of an auditory oddball task. Nygard et al. (2012) conducted research to compare the decrease and increase of signal during fMRI epochs that alternate in terms of whether tasks are present or absent. They found sustained hyper-activation of the default mode of a network and a reduced signal in some networks implicated during cognitive tasks. Their results suggest that SZ subjects have difficulty in reallocating cognitive resources when transitioning between a state of relative cognitive rest to a situation that requires active processing. Since most cognitive functions are related to timing, we hypothesize that the deactivation or low activation in SZ subjects is related to timing deficits. According to our own previous meta-analysis, lower recruitment in timing regions, especially in the right hemisphere is associated with worse performance in temporality tasks (Ortuño et al., 2011; Alústiza et al., 2017).
Several studies report the involvement of a dysfunctional, neurophysiological cortical-subcortical circuit connected with temporal and salience processing in SZ (Mikell et al., 2014; New and Scholl, 2009; Wittmann, 2015). The current study provides further support to these observations. We found that the dysfunctional regions associated with timing were engaged by oddball style tasks in SZ (particularly with preattentional stimuli during MMN). This overlap went beyond regions that are expected for a “multiple-demand system” operative for attentional activity (frontal dorsolateral and parietal) (Duncan, 2010), suggesting a wider common denominator. Furthermore, our study found that SZ patients had deficits in areas (i.e., the frontal region, parietal, insula, cingulate, SMA) that are known to mediate SN in HC subject. Thus, our results are in agreement with other neuroimaging studies that have found SZ subjects to have significantly lower activation, relative to controls, of most regions of the SN (Menon, 2015).
In line with previous combined ERP and fMRI studies (Wynn et al., 2015), our findings reinforce the idea that deficits during an oddball task in SZ are linked with a specific location of dysfunction: the ventral (salience) attentional network. Like Wynn et al., we also found that SZ subjects exhibited reduced fMRI neural activation, compared with controls, not just in the ventral but also in the dorsal attentional network. In contrast with the dorsal network, which is believed to focus attention to a task in a general sense relative to other tasks, the ventral network is involved in the detection of salient changes within a task that is already underway (Corbetta and Shulman, 2002). Whereas the dorsal network comprises activation in the inferior frontal junction, medial intraparietal sulcus, superior parietal lobule, and middle temporal area, the ventral network comprises activation in the temporal parietal junction, anterior insula, anterior middle frontal gyrus, bilateral anterior cingulate cortex, and SMA (Wynn et al., 2015).
Laurens et al. (2005) suggested that SZ patients are less efficient when it comes to the reorientation of processing resources used by an ongoing task that involves the detection of and response to task-relevant target stimuli. The results of the current study support this hypothesis. During novel stimulus processing, SZ subjects were characterized, relative to HC subjects, by hypoactivity in the right amygdala–hippocampus, within the paralimbic cortex in the rostral anterior and posterior cingulate cortices and the right frontal operculum, and in the association cortex at the right temporo-parietal-occipital junction, bilateral intraparietal sulcus, and the bilateral dorsal frontal cortex. With regard to the subcortex, relative hypoactivation during the processing of novel stimuli occurred in the cerebellum, thalamus, and basal ganglia. Our results show no significant group differences between HC and SZ subjects in cerebellum activation. In this respect, an absence of cerebellar participation when dealing with timing tasks was also reported by Lewis and Miall (2006).
In addition to playing an important role in the detection of salient events and reacting to them, the SN also facilitates access to attention and WM resources when a salient event is detected (Menon, 2015). The overlap between regions involved in both temporal and salience processing could be interpreted to indicate that both functions require similar cognitive abilities, such as attention, WM, or executive functions. Parallel to the observation that certain brain regions traditionally thought to be involved with timing (such as frontal, thalamus, and striatum) are engaged during oddball tasks in response to target detection, we hypothesize that specific brain regions usually associated with cognitive domains (such as the prefrontal cortex and fronto-parietal regions) are engaged during timing tasks. We also suggest the possibility that the timing dysfunction (variability and inaccuracy) and the change detection impairments observed in SZ subjects are interrelated in SZ. Our current findings agree with previous studies - Livesey et al. (2007) reported the engagement of timing network by an increase in the difficulty of non-temporal tasks - that support the notion that timing is change-related. Thus, the temporal-salience network might underlie all mental processes involved in or activated by change detection.
Sabri et al. (2006), using simultaneous fMRI and ERP techniques, examined attentional modulation as it occurred during a task whose difficulty was varied, and which was based on an oddball-like paradigm requiring detection of irrelevant deviance. They found that processing of stimuli that were not the main focus of attention depended on the nature and scale of the attentional demands required to process features of the stimulus. Additionally, and in line with our results, they found that the degree of difficulty of a task affected several critical ERP components, such as MMN and N1 and P3a. MMN, which measures bottom-up passive deviance detection, was relatively higher for an easy version of the task and was associated with more dorsal activation of the supratemporal plane. N1 and P3a components, which are related to top-down involuntary shifts of attention to deviant stimuli, were relatively higher for a difficult version of the task. Processing of sensory deviance was associated with brain activations in the ventral areas of the superior temporal cortex. Therefore, these results show that dorsal regions are primarily activated in response to passive detection of MMN between the standard stimuli and the incoming deviant stimuli. In a broader sense, Sabri et al.'s results suggest that both dorsal and ventral auditory regions in the superior temporal gyrus are modulated by ongoing attentional demands. Thus, it could be argued that MMN is preattentive, and cognitive control is a factor that participates in the regulation of and transition between preattentional and attentional tasks.
We, therefore, conclude with a working hypothesis that whereas preattentional oddball detection is related to an automatic timing system, which gauges time without recourse to cognitive modulation (and is mainly concerned with time of less than a second in duration), attentional oddball detection relates to a cognitively-controlled timing system, which is based on higher-level cognitive circuits (that are primarily needed for gauging time intervals that last for several seconds or longer).
Walsh (2003) suggested that there exists a general system that underlies perception of different magnitudes, such as space, quantity, and time. Apparently, these perceptual functions are interrelated and share neuroanatomical bases (Magnani et al., 2014). Our proposal of a cognitive common denominator in timing and salience processing goes beyond the Walsh “General Magnitude System.” We further suggest that the timing network involves both magnitude and cognitive (preattentional and attentional) information processing.
The philosophical notion that time is related to how we perceive changes in what is salient to us has been around since the days of Aristotle. Change and hence time is ubiquitous in nature and in the human environment, and consequently, the human mind needs to be able to perceive what is changing and respond appropriately (Ortuño and Alústiza, 2014). In this context, the existence of neurological networks in common to deal with time and salience would hardly be surprising.
Meta-analyses, in general, suffer from limitations, and this study is no exception. In particular, the fact that peak-based meta-analyses are based on coordinates from published studies rather than raw statistical brain maps makes data less accurate. It should be born in mind; however, that voxel-wise meta-analytical methods, such as those used here, tend towards false negatives rather than the false positives (Radua et al., 2013). A principal drawback of our meta-analysis is the small number of studies included; this was due to the scarcity of suitable publications. Further studies are required to verify the accuracy and reliability of our results.
5. Conclusions
In conclusion, SZ subjects show consistent brain activity deficits in brain regions that are conventionally associated not only with timing but also with change detection. The implication is that timing dysfunction might underlie aberrant SN and MMN, and therefore be a primary cognitive deficit in SZ. From our findings, we suggest that the temporal/change-detection impairment displayed in SZ subjects is connected specifically with a fronto-thalamo-striatal dysfunction. Our findings indicate that disruption in time processing is the central cognitive deficit in this disorder. The results reported here are, however, preliminary, and further studies are needed to address the specific role of timing on cognition in SZ.
Declarations
Author contribution statement
Irene Alústiza, María Sol Garcés, Javier Goena: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Aleix Solanes, Joaquim Radua: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Marta Ortuño, Patricio Molero, Felipe Ortuño: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank David Burdon for English proofreading.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Alústiza I., Radua J., Albajes-Eizagirre A., Domínguez M., Aubá E., Ortuño F. Meta-analysis of functional neuroimaging and cognitive control studies in schizophrenia: preliminary elucidation of a core dysfunctional timing network. Front. Psychol. 2016;7:1–12. doi: 10.3389/fpsyg.2016.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alústiza I., Radua J., Pla M., Martin R., Ortuño F. Meta-analysis of functional magnetic resonance imaging studies of timing and cognitive control in schizophrenia and bipolar disorder: evidence of a primary time deficit. Schizophr. Res. 2017;188:21–32. doi: 10.1016/j.schres.2017.01.039. [DOI] [PubMed] [Google Scholar]
- Bledowski C., Prvulovic D., Hoechstetter K., Scherg M., Wibral M., Goebel R. Localizing P300 generators in visual target and distractor processing: a combined event-related potential and functional magnetic resonance imaging study. J. Neurosci. 2004;24:9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A.K., Wolf D.H., Valdez J.N., Turetsky B.I., Elliott M.A., Gur R.E., Gur R.C. Comparison of auditory and visual oddball fMRI in schizophrenia. Schizophr. Res. 2014;158(1–3):183–188. doi: 10.1016/j.schres.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Davalos D., Rojas D., Tregellas J. Temporal processing in schizophrenia: effects of task-difficulty on behavioral discrimination and neuronal responses. Schizophr. Res. 2011;127:123–130. doi: 10.1016/j.schres.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cognit. Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Gaebler A.J., Mathiak K., Koten J.W., Jr., Konig A.A., Koush Y., Weyer D. Auditory mismatch impairments are characterized by core neural dysfunctions in schizophrenia. Brain. 2015;138:1410–1423. doi: 10.1093/brain/awv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido M.I., Kilner J.M., Stephan K.E., Friston K.J. The mismatch negativity: a review of underlying mechanisms. Clin. Neurophysiol. 2009;120(3):453–463. doi: 10.1016/j.clinph.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez J., Marín-Méndez J., Molero P., Atakan Z., Ortuño F. Time perception net-works and cognition in schizophrenia: a review and a proposal. Psychiatr. Res. 2014;220:737–744. doi: 10.1016/j.psychres.2014.07.048. [DOI] [PubMed] [Google Scholar]
- Green M.F., Kern R.S., Braff D.L., Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff?”. Schizophr. Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Guerrero-Pedraza A., McKenna P.J., Gomar J.J., Sarró S., Salvador R., Amann B., Carrión M.I., Landin-Romero R., Blanch J., Pomarol-Clotet E. First-episode psychosis is characterized by failure of deactivation but not by hypo- or hyperfrontality. Psychol. Med. 2012;42(01):73–84. doi: 10.1017/S0033291711001073. [DOI] [PubMed] [Google Scholar]
- Gur R.E., Turetsky B.I., Loughead J., Snyder W., Kohler C., Elliott M. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonances imaging. Am. J. Psychol. 2007;164 doi: 10.1176/ajp.2007.164.3.442. 442–229. [DOI] [PubMed] [Google Scholar]
- Hedman A.M., van Haren N.E., van Baal C.G., Kahn R.S., Hulshoff Pol H.E. IQ change over time in schizophrenia and healthy individuals: a meta-analysis. Schizophr. Res. 2013;146:201–208. doi: 10.1016/j.schres.2013.01.027. [DOI] [PubMed] [Google Scholar]
- Hugdahl K., Løberg E.-M., Nygård M. Left temporal lobe structural and functional abnormality underlying auditory hallucinations in schizophrenia. Front. Neurosci. 2009;3(1):34–45. doi: 10.3389/neuro.01.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y.W., Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Fenton W.S. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr. Bull. 2007;33:912–920. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern R.S., Gold J.M., Dickinson D., Green M.F., Nuechterlein K.H., Baade L.E. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr. Res. 2011;126:124–131. doi: 10.1016/j.schres.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K., Stevens M., Celone K., Kurtz M., Krystal J. Abnormal hemodynamics in schizophrenia during an auditory oddball task. Biol. Psychiatry. 2005;57:1029–1040. doi: 10.1016/j.biopsych.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.I., Mathalon D.H., Ford J.M., Mannell M., Turner J.A., Brown G.G. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophr. Bull. 2009;35:67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens K.R., Kiehl K.A., Ngan E.T., Liddle P.F. Attention orientation dysfunction during salient novel stimulus processing in schizophrenia. Schizophr. Res. 2005;75:159–171. doi: 10.1016/j.schres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Lewis P.A., Miall R.C. Remembering the time: a continuous clock. Trends Cognit. Sci. 2006;10:401–406. doi: 10.1016/j.tics.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Livesey A.C., Wall M.B., Smith A.T. Time perception: manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia. 2007;45:321–331. doi: 10.1016/j.neuropsychologia.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Lošák J., Huttlova J., Lipova P., Marecek R., Bares M., Filip P. Predictive motor timing and the cerebellar vermis in schizophrenia: an fMRI study. Schizophr. Bull. 2016;42:1517–1527. doi: 10.1093/schbul/sbw065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado S., Arias-Carrión O., Sampaio I., Bittencourt J., Velasques B., Teixeira S. Source imaging of P300 visual evoked potentials and cognitive functions in healthy subjects. Clin. EEG Neurosci. 2014;45:262–268. doi: 10.1177/1550059413514389. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A., Vieta E. Cognition as a target in schizophrenia, bipolar disorder and depression. Eur. Neuropsychopharmacol. 2015;25:151–157. doi: 10.1016/j.euroneuro.2015.01.007. [DOI] [PubMed] [Google Scholar]
- McCleery A., Ventura J., Kern R.S., Subotnik K.L., Gretchen-Doorly D., Green M.F. Cognitive functioning in first-episode schizophrenia: MATRICS consensus cognitive battery (MCCB) profile of impairment. Schizophr. Res. 2014;157:33–39. doi: 10.1016/j.schres.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Salience network. In: Toga Arthur W., editor. Brain Mapping: An Encyclopedic Reference. Vol. 2. Academic Press: Elsevier; 2015. pp. 597–611. [Google Scholar]
- Mikell C.B., Sheehy J.P., Youngerman B.E., McGovern R.A., Wojtasiewicz T.J., Chan A. Features and timing of the response of single neurons to novelty in the substantia nigra. Brain Res. 2014;1542:79–84. doi: 10.1016/j.brainres.2013.10.033. [DOI] [PubMed] [Google Scholar]
- Montalembert M., Tordjman S., Bonnot O., Coulon N. Perception temporelle et schizophrénie: approche phénoménologique et neuropsychologique. Encéphale. 2015;41:S56–S61. doi: 10.1016/S0013-7006(15)30008-7. [DOI] [PubMed] [Google Scholar]
- Mulert C., Jäger L., Schmitt R., Bussfeld P., Pogarell O., Möller Integration of fMRI and simultaneous EEG: towards a comprehensive under- standing of localization and time-course of brain activity in target detection. Neuroimage. 2004;22:83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- New J.J., Scholl B.J. Subjective time dilation: spatially local, object-based, or a global visual experience? J. Vis. 2009;4:1–11. doi: 10.1167/9.2.4. [DOI] [PubMed] [Google Scholar]
- Ngan E.T., Vouloumanos A., Cairo T.A., Laurens K.R., Bates A.T., Anderson C.M., Werker J.F., Liddle P.F. Abnormal processing of speech during oddball target detection in schizophrenia. NeuroImage. 2003;20(2):889–897. doi: 10.1016/S1053-8119(03)00385-9. [DOI] [PubMed] [Google Scholar]
- Nygard M., Eichele T., Loberg E.M., Jorgensen H.A., Johnsen E., Kroken R.A., Berle J.O., Hugdahi K. Patients with schizophrenia fail to up-regulate task-positive and down-regulate task-negative brain networks: an fMRI study using ICA analysis approach. Front. Hum. Neurosci. 2012;6:149. doi: 10.3389/fnhum.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortuño F., Alústiza I. Aristotle got it right again! Med. Hypotheses. 2014;83:509–510. doi: 10.1016/j.mehy.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Ortuño F., Guillén-Grima F., López-García P., Gómez J., Pla J. Functional neural networks of time perception: challenge and opportunity for schizophrenia research. Schizophr. Res. 2011;125(2–3):129–135. doi: 10.1016/j.schres.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D. Meta-analytic methods for neuroimaging data explained. Biol. Mood Anxiety Disord. 2012;2:2–6. doi: 10.1186/2045-5380-2-6. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D., Phillips M.L., El-Hage W., Kronhaus D.M., Cardoner N., Surguladze S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatr. 2011;27:605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Radua J., Romeo M., Mataix-Cols D., Fusar-Poli P. A general approach for combining voxel-based meta-analysis conducted in different neuroimaging modalities. Curr. Med. Chem. 2013;20:462–466. [PubMed] [Google Scholar]
- Radua J., Ojeda Del Pozo N., Gómez J., Guillén-Grima F., Ortuño F. Meta-analysis of functional neuroimaging studies indicates that an increase of cognitive difficulty during executive tasks engages brain regions associated with time perception. Neuropsychologia. 2014;58:14–22. doi: 10.1016/j.neuropsychologia.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Radua J., Rubia K., Canales-Rodríguez E.J., Pomarol-Clotet E., FusarPoli P., Mataix-Cols D. Anisotropic kernels for coordinate based meta-analyses of neuroimaging studies. Front. Psychiatr. 2014;5:13. doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri M., Liebenthal E., Waldron E.J., Medler D.A., Binder J.R. Attentional modulation in the detection of irrelevant deviance: a simultaneous ERP/fMRI study. J. Cognit. Neurosci. 2006;18:689–700. doi: 10.1162/jocn.2006.18.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targum S.D., Keefe R.S. Cognition and schizophrenia: is there a role for cognitive assessments in diagnosis and treatment? Psychiatry (Edgmont) 2008;5:55–59. [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q. Salience Network and the Human Brain. Elsevier; London: 2017. What is salience? pp. 1–4. [Google Scholar]
- Umbricht D., Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr. Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Volz H.P., Nenadic I., Gaser C., Rammsayer T., Häger F., Sauer H. Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. Neuroreport. 2001;12:313–316. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- Walsh V. A theory of magnitude: common cortical metrics of time, space and quantity. Trends Cogn. Sci. 2003;7:483–488. doi: 10.1016/j.tics.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Wiener M., Turkeltaub P., Coslett H.B. The image of time: a voxel-wise meta-analysis. Neuroimage. 2010;49:1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
- Wittmann M. Modulations of the experience of self and time. Conscious. Cogn. 2015;38:172–181. doi: 10.1016/j.concog.2015.06.008. Academic Press. [DOI] [PubMed] [Google Scholar]
- Wolf D.H., Turetsky B.I., Loughead J., Elliott M.A., Pratiwadi R., Gur R.E., Gur R.C. Auditory oddball fMRI in schizophrenia: association of negative symptoms with regional hypoactivation to novel distractors. Brain Imaging Behav. 2008;2(2):132–145. doi: 10.1007/s11682-008-9022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn J.K., Jiménez A.M., Roach B.J., Korb A., Lee J., Horan W.P., Ford J.M. Impaired target detection in schizophrenia and the ventral attentional network: findings from a joint event-related potential-functional MRI analysis. Neuroimage Clin. 2015;9:95–102. doi: 10.1016/j.nicl.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.