Abstract
Objectives
To identify the association between night shift work and the risk of various cancers with a comprehensive perspective and to explore sex differences in this association.
Methods
We searched PubMed, Embase, and Web of Science for studies on the effect of night shift work on cancer, including case-control, cohort, and nested case-control studies. We computed risk estimates with 95% confidence intervals (CIs) in a random or fixed effects model and quantified heterogeneity using the I 2 statistic. Subgroup, metaregression, and sensitivity analyses were performed to explore potential sources of heterogeneity. Contour-enhanced funnel plots and the trim and fill method were used together to analyze bias. Linear dose–response analysis was used to quantitatively estimate the accumulative effect of night shift work on the risk of cancer.
Results
Fifty-eight studies were eligible for our meta-analysis, including 5,143,838 participants. In the random effects model, the pooled odds ratio (OR) of cancers was 1.15 (95% CI = 1.08–1.22, P < 0.001; I 2 = 76.2%). Night shift work increased the cancer risk in both men (OR = 1.14, 95% CI = 1.05–1.25, P = 0.003) and women (OR = 1.12, 95% CI = 1.04–1.20, P = 0.002). Subgroup analyses showed that night shift work positively increased the risk of breast (OR = 1.22, 95% CI = 1.08–1.38), prostate (OR = 1.26, 95% CI = 1.05–1.52), and digestive system (OR = 1.15, 95% CI = 1.01–1.32) cancers. For every 5 years of night shift work, the cancer risk increased by 3.2% (OR = 1.032, 95% CI = 1.013–1.051).
Conclusion
This is the first meta-analysis identifying the positive association between night shift work and the risk of cancer and verifying that there is no sex difference in the effect of night shift work on cancer risk. Cancer risk increases with cumulative years of night shift work.
1. Introduction
Recent years have witnessed a rise in the number of people working late or night shifts in different employment sectors, such as healthcare, construction, transportation, and food preparation [1, 2]. The rate of shift work can exceed 15% of the workforce in many countries of North America, continental Europe, and Australia [2], and the trend is increasing. Night shift workers not only have higher short-term safety risks because of decreased alertness [3] but also have greater long-term health risks, including for diabetes [4], obesity [5], cardiovascular disease [6], depression [7], and cancer [8]. In 2007, a report by the International Agency for Research on Cancer (IARC) classified night shift work involving circadian disruption as “probably carcinogenic to humans” based on sufficient evidence in animal experiment and limited evidence in humans [9]. Therefore, investigating the influence of night shift work has captured attention. Most previous original studies verified the effect of night shift work on cancer risk, but the results are controversial for different cancers. Some findings have indicated that night shift work is significantly associated with higher cancer risk [10–39] whereas other studies have provided insignificant evidence for this relationship [40–66], motivating further study.
There were several postulated causal mechanisms that explain how night shift work multiplies cancer risk. First, melatonin, a marker of circadian rhythms, has a fundamental impact on inhibiting carcinogenesis through antioxidation, regulation of immunity, free radical scavenging, and antiangiogenesis [67]. Generally, night shift workers have a substantially decreased melatonin level during the nighttime [68, 69]. Melatonin suppression has been reported in breast [69], prostate [70], lung [71], ovarian [67], and gastrointestinal [72] cancers. Second, the 24-hour circadian rhythm is generated via interacting feedback loops of the circadian genes in all cells of both the hypothalamic suprachiasmatic nucleus (SCN) and all peripheral tissues [73, 74]. Night shift work can induce a conflict between the endogenous circadian clock and the external shifted sleep period and feeding behavior, leading to a dampening of the gene expression rhythm (25% of circadian genes) and subsequent disordered expression of transcription and translation in these cells [73, 74]. These disturbances can interfere with cell proliferation, apoptosis, hormonal balancing, metabolism, DNA damage repair, and immune and neuroendocrine functions. Recent studies have uncovered that the disruptive expression of circadian genes especially increases the risk of cancers in the immune, skeletal, digestive, and reproductive systems in which cell proliferation, metabolism, and DNA damage repair are required to maintain daily function [74]. Overall, the mechanisms based on hormonal and molecular levels manifest that the influence of night shift work on cancer is systemic and is not limited to a specific organ. However, many previous meta-analyses have identified the association of night shift work with only one type of cancer, including breast [75–78], prostate [79–81], and colorectal [82] cancers, among others. Only one study [8] analyzed the relationship between night shift work and the risk of cancers in women. Accordingly, we aimed to classify the association between night shift work and the risk of multiple cancers from a comprehensive perspective.
Previous studies have revealed that the circadian timing system differs in the sexes, which is mediated by different neuroendocrine contexts, such as sex hormones and their receptors in SCN [16, 83, 84]. Compared with male sex, female sex has been associated with earlier timing and larger amplitude of melatonin and earlier timing and longer duration of sleep [85, 86]. After night shift work, women showed greater impaired performance in health and cognition compared with men. For example, accuracy, alertness, the amplitude of melatonin, and working memory deteriorate more in women [3, 86, 87], enabling us to understand why female was more susceptible to sleep and wake disturbances after shift work [3]. More intense response to shift work in women reminds us whether the effect of night shift work on cancers varies with different genders.
Consequently, we conducted a meta-analysis to investigate this sex difference. We also expanded upon previous meta-analyses by not only evaluating the association between night shift work and a specific cancer but also estimating whether there was a dose–response relationship between night shift work and the risk of multiple cancers.
2. Methods
2.1. Search Strategy
We conducted a comprehensive updated search through May 2018 using PubMed, Embase, and Web of Science databases. Two investigators searched for eligible English articles independently. The search terms were “night shift work” or “rotating shift work” or “night work” or “shift work” and “carcinoma” or “neoplasm” or “tumor” or “cancer”. In addition, we manually reviewed the reference lists of articles for additional relevant studies.
2.2. Inclusion and Exclusion Criteria
Studies were included if they satisfied the following criteria: (i) the research was a case-control study, cohort study, or nested case-control study; (ii) the exposure of interest was night shift work, and the outcome of interest was the risk of any type of cancer; (iii) the study reported adjusted risk estimates (odds ratio, OR; relative risk, RR; hazard ratio, HR) with 95% confidence intervals (CIs) or provided sufficient data to allow calculation. Studies were excluded if they satisfied the following criteria: (i) the study did not provide sufficient data; (ii) the study mentioned recurrent cancer; (iii) when more than one article was based on the same study population, we only included the study with the largest number of cases.
2.3. Data Extraction
Data extraction was conducted independently by two authors for the following items: first author, publication year, study location, study design, number of cases, occupation, quality score, definition of exposure, participant sex, type of cancer, adjusted OR with 95% CI, adjusted covariates, and exposure assessment. As the prevalence of tumor is very low, we considered that ORs equaled RRs or HRs, providing similar risk estimates [88]. According to the definition of work schedule, we divided work schedules into rotating shift (working a regular shift schedule), fixed shift (permanent night work), and mixed (with no clear work schedule). ORs of the longest versus shortest exposure time were extracted from articles as the exposure indicator for statistical analysis. We also extracted dose information from ordinal categorical data (≥3 levels of the exposure category) for dose–response meta-analysis.
2.4. Quality Assessment
Two authors performed quality assessment using the Newcastle-Ottawa Quality Assessment Scale (NOS) [89]. The scale comprises a total of 9 points on the three parts of the NOS, including participant selection (0–4 points), comparability (0–2 points), and exposure or outcome assessment (0–3 points). Scores of ≥7 indicate a high quality.
2.5. Statistical Analysis
All statistical analyses were performed using Stata version 12.0 (StataCorp, College Station, TX, USA). We preferentially extracted adjusted ORs from original articles to evaluate the association between night shift work and cancer risk. If there were no adjusted ORs for specific subgroup analyses, a number of cases and participants would be extracted to calculate OR. The inverse variance method was used to combine ORs. If I 2 for the heterogeneity test was ≤50%, a fixed effects model was adopted to pool ORs; otherwise, a random effects model was selected. To explore potential heterogeneity, we performed subgroup analyses, metaregression analyses, and sensitivity analyses. One subgroup analysis was the classification of work schedules; we used a random effects model to evaluate the effect size for cancer on different work schedules [80]. To confirm the stability of results, a sensitivity analysis was conducted by omitting one study and then recalculating the rest of studies. The leave-one-out analysis was used to examine the weight of influence of each study on pooled OR [90].
A generalized least-squares trend (GLST) model was used to estimate the overall dose–response relationship of night shift work and the risk of cancer by computing risk estimates for different ordinal levels of night shift work. There were at least three ordinal levels of the exposure category in each study. The midpoint of the upper and lower boundaries of each level was considered the average exposure. The upper boundary of the highest level was considered the same as the adjacent category if it was not provided [76]. We used a two-stage random effect model to evaluate the linearity between night shift work and the risk of cancer.
Potential publication bias was estimated with the Begg funnel plot. Furthermore, the contour-enhanced funnel plot and the trim and fill method were used together to analyze the cause of bias. All reported P values were two-sided, and statistical significance was set at P ≤ 0.05.
3. Results
3.1. Study Selection
Figure 1 illustrates the results of the literature search and the process of selection. A total of 753 articles were initially identified from PubMed, Embase, and Web of Science databases. After screening based on the title and abstract, 143 articles were selected for full-text assessment; 53 studies were eligible for the final analysis. We also retrieved four relevant articles from the reference lists. Finally, 57 studies [10–66] were included in the analysis of the association of night shift work with risk of cancer.
Figure 1.
Flow chart of identification of relevant studies.
3.2. Study Characteristics
The characteristics of the abovementioned studies are summarized in Table 1. Fifty-seven articles were included in this meta-analysis, including 21 case-control studies, 6 nested case-control studies, and 30 cohort studies. One article [10] included two cohorts, the Nurses' Health Study (NHS) and Nurses' Health Study II (NHS II). Therefore, a total of 58 studies were finally enrolled, involving 225,976 cases and 5,143,838 participants. We extracted information about sex in each article, except these articles that did not include classification by sex [12, 15, 48], to analyze the effect of night shift work on cancers in men and women separately. Several studies [20, 28, 43, 44, 53] analyzed the association between night shift work and different kinds of cancers. We also classified all kinds of cancer analyzed in the included studies into seven categories, including digestive system, hematological system, prostate, breast, reproductive system, lung, and skin cancers. A total 27 articles were from Europe, 11 from Asia, 17 from North America, and 3 from Australia. Most studies were based on a population with no specific occupation whereas other studies involved participants with a specific occupation, such as nurses, textile workers, women in the military, and pilots. According to the definition of night shift work, work schedules were classified as rotating shift (29 studies), fixed shift (9 studies), or mixed shift (27 studies). In fact, a cross section of studies described different work schedules, which we extracted simultaneously. Exposure assessment was performed using a questionnaire, interview, or databases. A total 43 studies were adjusted for more than four confounders and 15 studies for fewer than four confounders. The average NOS score was 7.2, and scores ranged from 4 to 8.
Table 1.
Characteristics of studies included in meta-analysis.
| Study (year) | Region | Study design | No. of cases | Occupation | Exposure | Adjusted OR (95% CI) | Type of cancer | Adjusted item | Exposure assessment method |
|---|---|---|---|---|---|---|---|---|---|
| Walasa et al. (2018) [40] | Australia | Case-control study | 350 | NA | Night, never vs. 7.5+ years | 0.95 (0.57–1.58) | Colorectal cancer | Age group, education level, socioeconomic status, lifetime cigarette smoking, and alcohol intake 10 years ago | Questionnaire |
| Talibov et al. (2018) [41] | Europe | Case-control study | 131,594 | NA | Rotating, never vs. 20+ years | 1.033 (0.984–1.084) | Hematological system cancer | Cumulative benzene, formaldehyde, and ionizing radiation | Questionnaire |
| Tse et al. (2017) [11] | Asia | Case-control study | 431 | NA | Night, never vs. ever | 1.76 (1.07–2.89) | Prostate cancer | Age at interview, marital status, unemployment status, family prostate cancer history, consumption of deep fried food, consumption of pickled vegetable, green tea drinking habits, and cbpai | Interview |
| Heckman et al. (2017) [44] | North America | Cohort study | 4854 | Nurses | Rotating, never vs. 10+ years | 0.794 (0.711–0.888) | Skin cancer | Years of shift work, hours of sleep, sleep adequacy, sleepy days per week, snoring, restless legs syndrome, family history of melanoma, hours spent in sun, number of severe sunburns, sunburn severity, artificial tanning frequency, annual uv at residence, moles on lower legs, natural hair color in adolescence, marital status, financial status, BMI, physical activity, smoking status, menopausal status, postmenopausal hormones, oral contraceptive use, and healthy eating index | Questionnaire |
| Papantoniou (2017) [12] | Europe | Case-control study | 1626 | NA | Night and rotating, never vs. 15+ years | 1.28 (1.06–1.56) | Colorectal cancer | Age, centre, educational level, sex, history of colorectal cancer, BMI, smoking, leisure time physical activity, alcohol consumption, total energy intake in grams/day, all red meat consumption, sleep duration, bisphosphonates, and NSAIDs | Questionnaire |
| Devore et al. (2017) [45] | North America | Cohort study | 3014 | Nurses | Rotating, never vs. 10+ years | 0.96 (0.83, 1.11) | Colorectal adenoma | Age, time period of first lower endoscopy, reason for endoscopy, family history of cancer, height, BMI, physical activity, smoking, alcohol intake, menopausal status, menopausal hormone use, oral contraceptive use, multivitamin use, total calcium intake, vitamin d intake, red meat intake, NSAIDs use, and predicted vitamin D score | Questionnaire |
| Vistisen et al. (2017) [42] | Europe | Cohort study | 1245 | NA | Night, never vs. ever | 0.90 (0.80–1.01) | Breast cancer | Calendar year, age, age at birth of first child, number of births, family history of breast cancer or ovarian cancer, oral contraception, hormone replacement therapy, other sex hormones, medication related to alcoholism, mammography screening attendance, and highest family educational level | Database |
| Wegrzyn et al. (2017)a [10] | North America | Cohort study | 5971 | Nurses | Rotating, never vs. 30+ years | 0.95 (0.77–1.17) | Breast cancer | Age, height, BMI, BMI at age 18, adolescent body size, age at menarche, age at first birth and parity combined, breast feeding, type of menopause and age at menopause, combined menopausal hormone therapy, duration of estrogen alone menopausal hormone therapy, duration of estrogen and progesterone menopausal hormone therapy, first-degree family history of breast cancer, history of benign breast disease, alcohol consumption, physical activity, and current mammography use. | Questionnaire |
| Wegrzyn et al. (2017)a [10] | North America | Cohort study | 3570 | Nurses | Rotating, never vs. 20+ years | 2.15 (1.23–3.73) | Breast cancer | Age, height, BMI, BMI at age 18, adolescent body size, age at menarche, age at first birth and parity combined, breast feeding, type of menopause and age at menopause, combined menopausal hormone therapy, duration of estrogen alone menopausal hormone therapy, duration of estrogen and progesterone menopausal hormone therapy, first-degree family history of breast cancer, history of benign breast disease, alcohol consumption, physical activity, and mammography use. | Questionnaire |
| Jørgensen et al. (2017) [43] | Europe | Cohort study | 945 | Nurses | Rotating, night, and rotating, never vs. ever | 0.91 (0.77–1.08) | Unclassified cancer | Age, smoking, pack-years, physical activity, BMI, alcohol consumption, diet (vegetables, fruit, and fatty meat consumption), preexisting diseases (hypertension, diabetes, and myocardial infarction), self-reported health, stressful work environment, marital status, female reproductive factors (birth, use of hormone therapy, and oral contraceptives) | Questionnaire |
| Behrens et al. (2017) [13] | Europe | Cohort study | 76 | NA | Rotating, never vs. 20+ years | 3.08 (1.67–5.69) | Prostate cancer | Age, smoking, family history of prostate cancer, level of school education, and equivalent income | Interview |
| Akerstedt et al. (2017) [46] | Europe | Cohort study | 454 | NA | Night, never vs. ever | 0.91 (0.74–1.12) | Prostate cancer | Age, education level, tobacco consumption, BMI, having children, coffee consumption, and previous cancer | Interview |
| Dickerman et al. (2016) [49] | Europe | Cohort study | 602 | NA | Rotating, never vs. ever | 1.0 (0.7–1.2) | Prostate cancer | Age, education, BMI, physical activity, social class, smoking status, alcohol use, snoring, and zygosity | Questionnaire |
| Papantoniou et al. (2016) [47] | Europe | Case-control study | 1708 | NA | Night and rotating, never vs. 15+ years | 1.21 (0.89–1.65) | Breast cancer | Age, centre, educational level, parity, menopausal status, family history of breast cancer, BMI, smoking status, oral contraceptive use, leisure time physical activity, alcohol consumption, and sleep duration | Interview |
| Gyarmati et al. (2016) [48] | Europe | Case-control study | 374 | NA | Night and rotating, never vs. 20+ years | 1.1 (0.8–1.6) | Stomach cancer | Age, sex, educational level, centre, BMI, cigarette smoking status, family history, physical activity level, total energy intake, grams of red meat, grams of vegetables, and grams of fruit and alcohol consumption | Interview |
| Costas et al. (2016) [15] | Europe | Case-control study | 321 | NA | Night, never vs. 20+ years | 1.77 (1.14–2.74) | Chronic lymphocytic leukemia | Region, age, sex, worked on a farm, family history of hematologic malignancies, BMI, tobacco consumption (never, past, and current), sleep problems, and education | Interview |
| Bai et al. (2016) [16] | Asia | Cohort study | 1251 | NA | Night, never vs. 20+ years | 1.08 (0.90–1.29) | Unclassified cancer | Age, BMI, family history of cancer, alcohol drinking and smoking status, number of children, menopausal status, hormone replacement therapy, and contraception status | Questionnaire |
| Travis et al. (2016) [14] | Europe | Cohort study | 4809 | NA | Night, never vs. ever | 1.00 (0.92–1.08) | Breast cancer | Socioeconomic status, parity and age at first birth, BMI, alcohol intake, strenuous physical activity, family history of breast cancer, age at menarche, oral contraceptive use, smoking, living with a partner, and hormone therapy | Questionnaire |
| Gu et al. (2015) [20] | North America | Cohort study | 5413 | Nurses | Rotating, never vs. 15+ years | 1.08 (0.98–1.19) | Unclassified cancer | Age, alcohol consumption, physical exercise, multivitamin use, menopausal status and postmenopausal hormone use, physical exam in the past 2 years, healthy eating score, smoking status, pack-years, BMI, and husband's education | Questionnaire |
| Wang P. et al. (2015) [17] | Asia | Case-control study | 712 | NA | Night, never vs. ever | 1.34 (1.05–1.72) | Breast cancer | Age, education, BMI, age at menarche, menopausal status, parity, physical activity, breast feeding, and family history of cancer | Interview |
| Papantoniou et al. (2015) [18] | Europe | Case-control study | 1115 | NA | Night and rotating, never vs. 28+ years | 1.38 (1.05–1.81) | Prostate cancer | Age, centre, educational level, family history of prostate cancer, physical activity over the past decade, smoking status, past sun exposure, and daily meat consumption | Interview |
| Li et al. (2015) [51] | Asia | Nested case-control study | 1709 | Industry | Night, never vs. ever | 0.73 (0.66–0.82) | Breast cancer | Age at the beginning of follow-up | Questionnaire |
| Lin et al. (2015) [50] | Asia | Cohort study | 94 | NA | Rotating, never vs. ever | 1.43 (0.78–2.63) | Biliary tract cancer | Age, BMI, history of cholelithiasis, history of diabetes, cigarette smoking, alcohol drinking, perceived stress, and sleep time | Questionnaire |
| Kwon et al. (2015) [52] | Asia | Nested case-control study | 1451 | Industry | Rotating, never vs. 30.6+ years | 0.88 (0.69–1.12) | Lung cancer | Adjusted for age, smoking, parity, and endotoxin | Database |
| Akerstedt et al. (2015) [21] | Europe | Cohort study | 463 | NA | Night, never vs. 21+ years | 1.77 (1.03–3.04) | Breast cancer | Age, education level, tobacco consumption, BMI, having children, coffee consumption, previous cancer, and use of hormones including oral contraceptives | Interview |
| Hammer et al. (2015) [19] | Europe | Cohort study | 337 | Industry | Rotating, never vs. ever | 0.93 (0.73–1.18) | Prostate cancer | Age and professional status | Database |
| Gapstur et al. (2014) [55] | North America | Cohort study | 4974 | NA | Rotating, night, and evening, never vs. ever | 1.08 (0.95–1.22) | Prostate cancer | Age, race, education, BMI, smoking status, family history of prostate cancer, and painful/frequent urination | Questionnaire |
| Koppes et al. (2014) [54] | Australia | Cohort study | 2531 | NA | Night, never vs. ever | 0.87 (0.72–1.05) | Breast cancer | Night work, age, origin, children in household, education, occupation, job tenure (years), and contractual working hours | Interview |
| Carter et al. (2014) [23] | North America | Cohort study | 1289 | NA | Rotating, night, and evening, never vs. ever | 1.27 (1.03–1.56) | Ovarian cancer | Oral contraceptive use, age at menarche and menopause, tubal ligation, parity, postmenopausal estrogen use, race, family history of cancers, exercise, BMI, and height | Questionnaire |
| Yong et al. (2014) [53] | Europe | Cohort study | 10,873 | Industry | Rotating, never vs. ever | 1.04 (0.89–1.21) | Unclassified cancer | Age, job level, cigarette smoking, and employment duration in categories | Questionnaire |
| Datta et al. (2014) [56] | Asia | Case-control study | 50 | Industry | Night, never vs. ever | 1.51 (0.27–8.52) | Breast cancer | None | Interview |
| Truong et al. (2014) [22] | Europe | Case-control study | 1126 | NA | Night, never vs. ever | 1.32 (1.02–1.72) | Breast cancer | Age, study area, parity, age at first full-term pregnancy, age at menarche, family history of breast cancer, current use of hormonal replacement therapy, BMI, tobacco, and alcohol | Interview |
| Knutsson et al. (2013) [26] | Europe | Cohort study | 94 | NA | Night, never vs. ever | 2.02 (1.03–3.95) | Breast cancer | Height, weight, waist, hip circumference, educational level, number of children, smoking, menopausal status, oral contraceptive use, hormones other than contraceptives, alcohol intake, educational level, BMI, and waist–hip ratio | Questionnaire |
| Rabstein et al. (2013) [57] | Europe | Case-control study | 857 | NA | Night, never vs. ever | 1.01 (0.68–1.5) | Breast cancer | Age, adjusted for family history of breast cancer, hormone replacement use, and number of mammograms | Interview |
| Fritschi et al. (2013) [59] | Australia | Case-control study | 1205 | NA | Night, never vs. 20+ years | 1.02 (0.71–1.45) | Breast cancer | Light at night, phase shift and sleep disruption, poor diet, lack of physical activity and little time outdoors, and age | Questionnaire |
| Schernhammer et al. (2013) [24] | North America | Cohort study | 1455 | Nurses | Rotating, never vs. 15+ years | 1.28 (1.07–1.53) | Lung cancer | Age, smoking status, age at start of smoking, cigarettes smoked per day among current smoker, time since quitting among past smokers, fruit intake, vegetable intake, BMI, and environmental smoking exposures | Questionnaire |
| Menegaux et al. (2013) [25] | Europe | Case-control study | 1232 | NA | Night, never vs. 4.5+ years | 1.40 (1.01–1.92) | Breast cancer | Age, study area, parity, age at first full-term pregnancy, age at menarche, family history of breast cancer, current hormonal replacement therapy, BMI, tobacco, and alcohol | Interview |
| Grundy et al. (2013) [27] | North America | Case-control study | 1134 | NA | Mixed, never vs. 30+ years | 2.21 (1.14–4.31) | Breast cancer | Age and centre | Questionnaire |
| Bhatti et al. (2013) [60] | North America | Case-control study | 1490 | NA | Night, never vs. 7+ years | 1.02 (0.74–1.42) | Ovarian cancer | Age at reference, county, reference year, duration of oral contraceptive use, number of full-term pregnancies, and BMI | Interview |
| Lin et al. (2013) [58] | Asia | Cohort study | 127 | Industry | Rotating, never vs. ever | 0.83 (0.43–1.60) | Pancreatic cancer | Age, BMI, history of diabetes, alcohol drinking, cigarette smoking, perceived stress, and sleep time | Questionnaire |
| Hansen and Stevens (2012) [30] | Europe | Nested case-control study | 267 | Nurses | Night and evening, never vs. 20+ years | 2.1 (1.3–3.2) | Breast cancer | Adjusted for age, weight regularity, use of hormone replacement therapy, age at menarche, menstrual regularity, menopausal status, age at birth of first child, breast cancer in mother or sister, and total duration of lactation | Interview |
| Hansen and Lassen (2012) [31] | Europe | Nested case-control study | 132 | Industry | Evening, never vs. 15+ years | 2.1 (1.0–4.5) | Breast cancer | Adjusted for age, hormone replacement therapy, number of childbirths, age at menarche, years of education, occasional sunbathing frequency, and tobacco smoking status | Questionnaire |
| Parent et al. (2012) [28] | North America | Case-control study | 3137 | NA | Night, never vs. 10+ years | 2.016 (1.246–3.261) | Unclassified cancer | None | Interview |
| Natti et al. (2012) [29] | Europe | Cohort study | 99 | NA | Night, never vs. ever | 2.148 (1.178–3.917) | Unclassified cancer | Age, longstanding illness (among men), and smoking status | Interview |
| Kubo et al. (2011) [32] | Asia | Cohort study | 17 | Industry | Rotating, never vs. ever | 1.79 (0.57–5.68) | Prostate cancer | Age, BMI, alcohol intake, smoking, exercise, and marital status | Database |
| Lie et al. (2011) [62] | Europe | Nested case-control study | 699 | Nurses | Night, never vs. 12+ years | 1.3 (0.9–1.8) | Breast cancer | Age, period of diagnosis, parity, family history of breast cancer in mother or sister, and frequency of alcohol consumption at time of diagnosis | Interview |
| Poole et al. (2011) [61] | North America | Cohort study | 718 | Nurses | Rotating, never vs. 20+ years | 0.8 (0.51–1.23) | Ovarian cancer | Age, duration of oral contraceptive use, parity, BMI, smoking status, tubal ligation history, menopausal status, family history of ovarian cancer (yes/no), and duration of breastfeeding | Questionnaire |
| Pesch et al. (2010) [64] | Europe | Case-control study | 753 | NA | Night, never vs. 20+ years | 2.48 (0.62–9.99) | Breast cancer | Age in 5-year groups, adjusted for family history of breast cancer, hormone replacement use, and number of mammograms | Interview |
| Pronk et al. (2010) [63] | Asia | Cohort study | 349 | NA | Mixed, never vs. 17+ years | 0.8 (0.5–1.2) | Breast cancer | Age, education, family history of breast cancer, number of pregnancies, age at first birth, and physical activity | Interview |
| Lahti et al. (2008) [33] | Europe | Cohort study | 6307 | NA | Rotating, never vs. ever | 1.07 (1.01–1.13) | Non-Hodgkin lymphoma | Age, social class, and cohort period | Database |
| Viswanathan et al. (2007) [34] | North America | Cohort study | 515 | Nurses | Rotating, never vs. 20+ years | 1.47 (1.03–2.10) | Endometrial cancer | Age, age at menarche, age at menopause, parity, BMI, oral contraceptive use, use and duration of postmenopausal hormones, hypertension, diabetes, and smoking | Questionnaire |
| Lie et al. (2006) [36] | Europe | Nested case-control study | 537 | Nurses | Rotating, never vs. 30+ years | 2.21 (1.10–4.45) | Breast cancer | Total employment time as a nurse and parity | Database |
| O'Leary et al. (2006) [65] | North America | Case-control study | 487 | NA | Mixed, never vs. ever | 1.04 (0.79–1.38) | Breast cancer | Age at reference date, parity, family history, education, and history of benign breast disease | Interview |
| Schernhammer et al. (2006) [35] | North America | Cohort study | 1352 | Nurses | Rotating, never vs. 20+ years | 1.79 (1.06–3.01) | Breast cancer | Age, age at menarche, menopausal status, age at menopause, age at first birth, BMI, current alcohol consumption, oral contraceptive use, postmenopausal hormone use, smoking status, benign breast disease, family history of breast cancer, and physical activity | Questionnaire |
| Kubo et al. (2006) [37] | Asia | Cohort study | 31 | Industry | Rotating, never vs. ever | 3.0 (1.2–7.7) | Prostate cancer | Age, study area, family history of prostate cancer, BMI, smoking, alcohol drinking, job type, physical activity at work, workplace, perceived stress, educational level, and marriage status | Questionnaire |
| Schernhammer et al. (2003) [38] | North America | Cohort study | 602 | Nurses | Rotating, never vs. 15+ years | 1.35 (1.03–1.77) | Colorectal cancer | Age in years, smoking, BMI, physical activity in quintiles, regular aspirin use, colorectal cancer in parent or sibling, screening endoscopy during the study period, consumption of beef, pork, or lamb as a main dish, alcohol consumption status, total caloric intake in quintiles, use of postmenopausal hormones, menopausal status, and height in seven categories | Questionnaire |
| Davis et al. (2001) [66] | North America | Case-control study | 767 | NA | Mixed, never vs. 3+ years | 1.6 (0.8–3.2) | Breast cancer | Parity, family history of breast cancer (mother or sister), oral contraceptive use (ever), and recent (<5 years) discontinued use of hormone replacement therapy | Interview |
| Hansen (2001) [39] | Europe | Case-control study | 6281 | NA | Night, never vs. 0.6+ years | 1.5 (1.3–1.7) | Breast cancer | Age, social class, age at birth of first child, age at birth of last child, and number of children | Interview |
Abbreviations: BMI: body mass index; NSAIDs: nonsteroidal anti-inflammatory drugs; NA: not available. aThis study included two prospective cohorts (NHS and NHS2).
3.3. Association between Night Shift Work and the Risk of Various Cancers
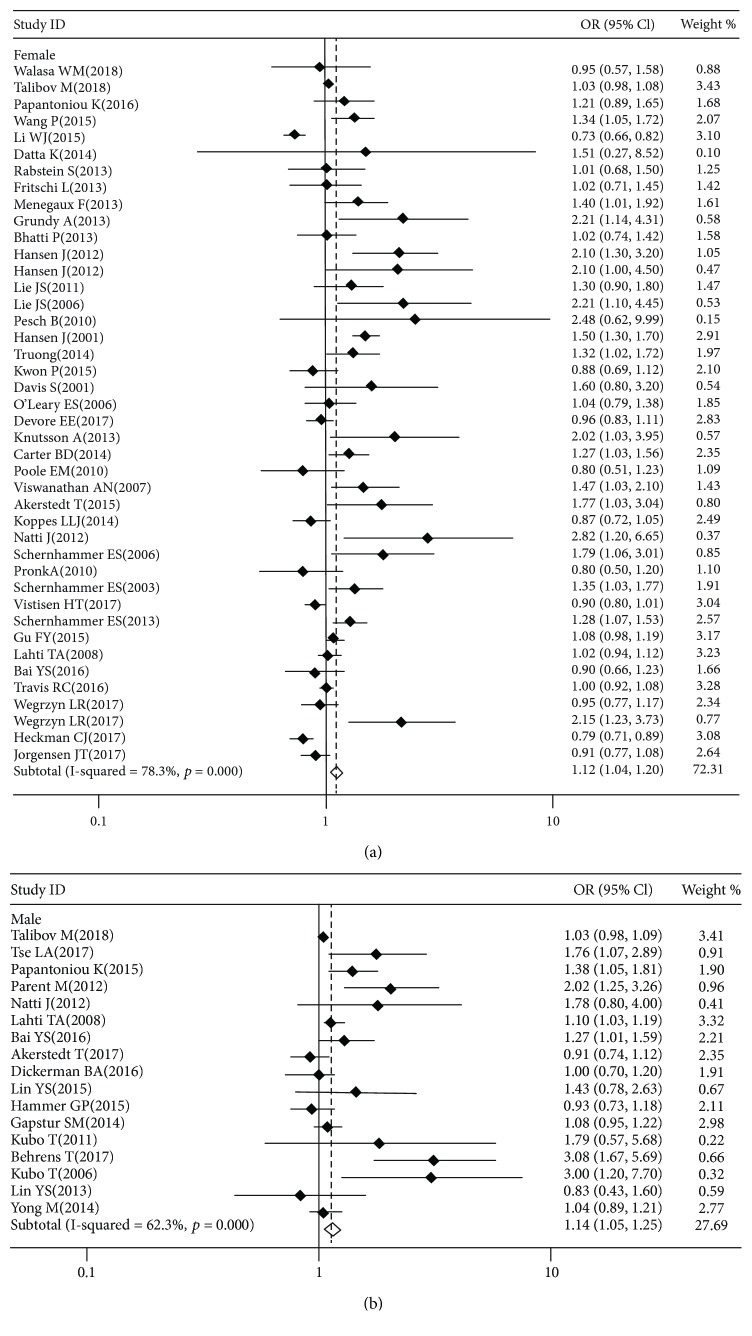
The random effects model was used to pool the ORs, indicating the relationship between night shift work and risk of multiple cancers. The pooled OR was 1.15 (95% CI = 1.08–1.22, P < 0.001), with high heterogeneity (I 2 = 76.2%, P ≤ 0.001) (shown in online Figure S1). We observed that night shift work could increase the risk of cancers both in men (OR = 1.14, 95% CI = 1.05–1.25, P = 0.003) and women (OR = 1.12, 95% CI = 1.04–1.20, P = 0.002), with high heterogeneity in men (I 2 = 78.3%, P ≤ 0.001) and women (I 2 = 62.3%, P ≤ 0.001) (Figure 2). In cancers that can occur in both men and women (i.e., excluding breast, prostate, and reproductive system cancers, such as ovarian, endometrial, and testis cancer), night shift work demonstrated a positive association with the risk of cancer in men (OR = 1.09, 95% CI = 1.01–1.17, P = 0.031) but not in women (OR = 1.02, 95% CI = 0.94–1.12, P = 0.637).
Figure 2.
Forest plots of studies describing the association between night shift work and the risk of multiple cancers in women (a) and men (b) separately. I 2: the indicator for judging the degree of heterogeneity; OR: odds ratio; CI: confidence interval. The squares and horizontal lines represent the study-specific OR and 95% CI. The diamond represents the pooled OR and 95% CI.
3.4. Subgroup Analysis and Metaregression Analysis
To explore the source of potential heterogeneity and assess the influence of specific characteristics of night shift work and cancer risk, we conducted subgroup analyses, including for shift schedule, type of cancer, study region, participant occupation, study design, exposure assessment, number of adjusted variables, and NOS score (Table 2). Among the different work schedules, rotating shift work (OR = 1.14, 95% CI = 1.04–1.24) increased cancer risk whereas fixed shift work (OR = 1.09, 95% CI = 0.90–1.31) did not. A significant relationship was observed for breast cancer (OR = 1.22, 95% CI = 1.08–1.38), prostate cancer (OR = 1.26, 95% CI = 1.05–1.52), and digestive system cancer (OR = 1.15, 95% CI = 1.01–1.32). With respect to region, studies in Europe (OR = 1.18, 95% CI = 1.10–1.28) and North America (OR = 1.16, 95% CI = 1.04–1.31) showed higher ORs than those in Asia and Australia. When stratified by study design, a positive association was revealed for case-control studies (OR = 1.28, 95% CI = 1.15–1.42) and cohort studies (OR = 1.07, 95% CI = 1.00-1.15) but not nested case-control studies. For different occupations, studies based on populations in which no specific occupation was classified showed higher risk estimates (OR = 1.17, 95% CI = 1.10–1.25). Nurses (OR = 1.17, 95% CI = 1.02–1.35) had elevated cancer risk, but participants with industrial occupations did not. The interview group, which had more comprehensive information collection, presented a higher risk estimate (OR = 1.32, 95% CI = 1.17–1.49) than studies using questionnaires and databases to collect information. Regarding NOS score, studies with high-quality scores were associated with increased risk (OR = 1.14, 95% CI = 1.08–1.21) and decreased heterogeneity (I 2 = 61.6%, P ≤ 0.001) whereas those with low-quality scores did not show this positive relationship and had high heterogeneity (I 2 = 86.9%, P ≤ 0.001). Additionally, increased risk was present in studies with more than four adjusted variables. Studies with fewer than four adjusted variables showed no elevated risk of cancer, with high heterogeneity (I 2 = 82.7%, P ≤ 0.001). We performed metaregression analyses to assess the potential heterogeneity sources (Table 2); however, the results showed that none of the subgroups generated the potential heterogeneity.
Table 2.
The results of subgroup analyses and metaregression analyses on the association between night shift work and the risk of cancers.
| Subgroup | No. of studies | Weight (%) | OR (95% CI) | I 2 | P for heterogeneity | P ∗ for interaction |
|---|---|---|---|---|---|---|
| Shift schedulea | 0.570 | |||||
| Rotating shift | 29 | 46.97 | 1.14 (1.04–1.24) | 68.7% | <0.001 | |
| Fixed shift | 9 | 11.19 | 1.09 (0.90–1.31) | 51.1% | 0.037 | |
| Mixed shift | 27 | 41.84 | 1.20 (0.82–1.77) | 80.7% | <0.001 | |
| Type of cancerb | 0.298 | |||||
| Digestive system cancer | 11 | 15.72 | 1.15 (1.01–1.32) | 40.2% | 0.081 | |
| Hematological system cancer | 5 | 9.12 | 1.08 (0.99–1.17) | 54.7% | 0.066 | |
| Prostate cancer | 11 | 16.10 | 1.26 (1.05–1.52) | 73.2% | <0.001 | |
| Breast cancer | 37 | 39.62 | 1.22 (1.08–1.38) | 81.2% | <0.001 | |
| Reproductive system cancer | 6 | 7.99 | 1.06 (0.85–1.32) | 49.5% | 0.078 | |
| Lung cancer | 5 | 7.53 | 1.08 (0.87–1.35) | 53.4% | 0.073 | |
| Skin cancer | 3 | 3.92 | 0.93 (0.50–1.74) | 74.9% | 0.019 | |
| Region | 0.298 | |||||
| Australia | 3 | 5.03 | 0.91 (0.77–1.06) | 0.0% | 0.728 | |
| Europe | 27 | 48.92 | 1.18 (1.10–1.28) | 75.1% | <0.001 | |
| Asia | 11 | 14.29 | 1.11 (0.88–1.39) | 78.3% | <0.001 | |
| North America | 17 | 31.75 | 1.16 (1.04–1.31) | 76.1% | <0.001 | |
| Occupation | 0.795 | |||||
| Unclassified occupation | 35 | 61.45 | 1.17 (1.10–1.25) | 69.7% | <0.001 | |
| Industry | 9 | 12.09 | 1.00 (0.81–1.24) | 72.8% | <0.001 | |
| Nurses | 14 | 26.46 | 1.17 (1.02–1.35) | 80.6% | <0.001 | |
| Study design | 0.845 | |||||
| Case-control study | 21 | 32.23 | 1.28 (1.15–1.42) | 66.5% | <0.001 | |
| Nested case-control study | 6 | 9.08 | 1.30 (0.89–1.90) | 88.0% | <0.001 | |
| Cohort study | 31 | 58.69 | 1.07 (1.00–1.15) | 70.9% | <0.001 | |
| Exposure assessment | 0.075 | |||||
| Questionnaire | 28 | 53.99 | 1.08 (1.00–1.17) | 77.1% | <0.001 | |
| Interview | 24 | 34.52 | 1.32 (1.17–1.49) | 66.3% | <0.001 | |
| Database | 6 | 9.06 | 1.00 (0.85–1.18) | 77.1% | 0.004 | |
| Number of adjusted variables | 0.926 | |||||
| ≤4 | 15 | 22.84 | 1.13 (0.99–1.28) | 82.7% | <0.001 | |
| >4 | 43 | 77.16 | 1.16 (1.08–1.24) | 72.8% | <0.001 | |
| Study score | 0.585 | |||||
| Low quality | 17 | 25.20 | 1.16 (0.98–1.37) | 86.9% | <0.001 | |
| High quality | 41 | 74.80 | 1.14 (1.08–1.21) | 61.6% | <0.001 |
a,bFive studies report their studies including different kinds of cancer; nine articles report their studies including different types of shift schedules. ∗ P values for metaregression.
3.5. Sensitivity Analysis
Sensitivity analysis showed that the pooled ORs were stable and did not identify the origins of heterogeneity. After omitting 19 studies by the leave-one-out analyses, we found a stable positive relationship (OR = 1.06, 95% CI = 1.02–1.11) between night shift work and the risk of cancer, with low heterogeneity (I 2 = 29.8%). It was found that none of the individual studies could powerfully change the positive result.
3.6. Dose–Response Analysis of Night Shift Work and the Risk of Cancers
Twenty-nine studies, which involved at least three levels of night shift exposure, were included in the dose–response analysis of night shift work and cancer risk. We used the two-stage random effects model to evaluate the linearity relationship (P < 0.001). For every 5 years of night shift work, the risk of cancer increased by 3.2% (OR = 1.032, 95% CI = 1.013–1.051) (Figure 3).
Figure 3.
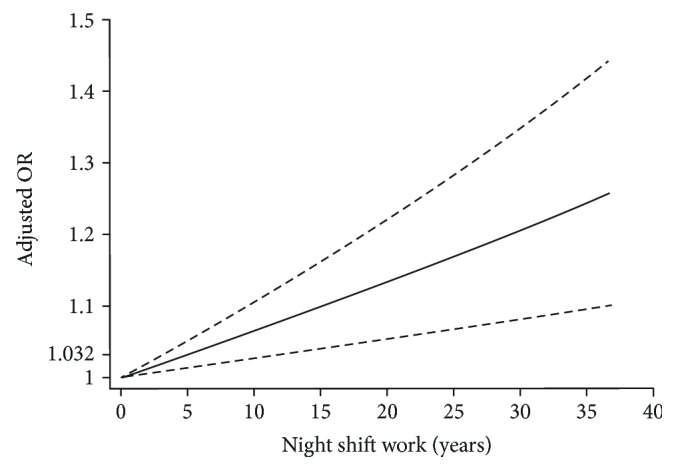
Dose–response relation plots between night shift work and the risk of multiple cancers. OR: odds ratio. Solid line represents the estimated OR by years, and the dotted lines represent the low and upper limits of 95% CIs separately.
3.7. Publication Bias
The Begg test showed a potential publication bias among all enrolled studies (P = 0.001). After combining the trim and fill method and contour-enhanced funnel plot, the result showed that most of the filled studies were outside the 10% line, which indicated that the previously verified bias was mostly caused by the high heterogeneity, not the publication bias (Figure 4). The filled risk estimate was still positive, as before (OR = 1.06, 95% CI = 1.01–1.11), such that the pooled OR was stable in our study.
Figure 4.
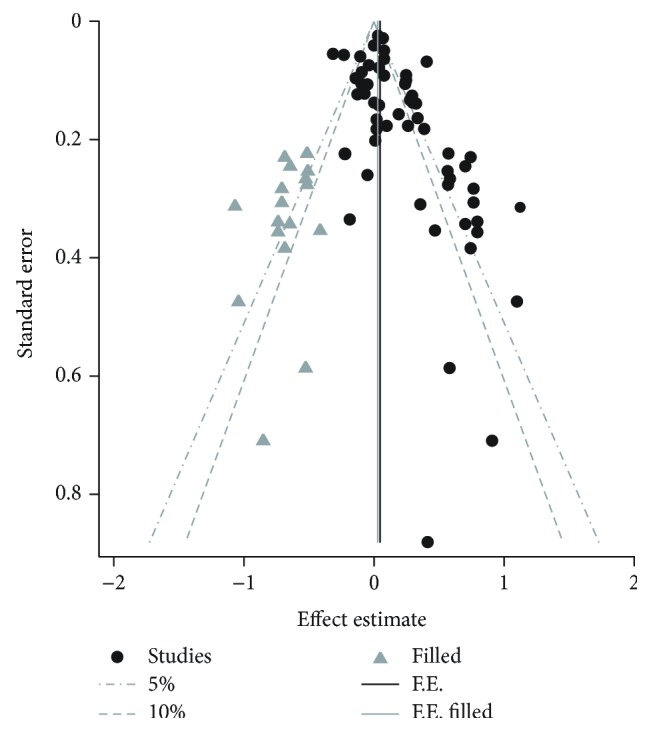
The contour-enhanced funnel plot of studies assessing the association between night shift work and the risk of cancers after using the trim and fill method.
4. Discussion
This meta-analysis, consisting of 58 studies with 225,976 cases and 5,143,838 participants, revealed a positive relationship between night shift work and the risk of cancer. Compared with people who never experience working late, the risk of cancer was found to be increased by 15% in all shift workers, by 12% in female workers and 14% in male workers. A linear dose–response relationship showed a positive gradient of cancer risk with cumulative years of night shift work; for every 5 years of night shift work, cancer risk increased by 3.2%. Yuan et al. [8] confirmed that night shift work elevates the risk of multiple cancers in women, especially breast cancer. Several meta-analyses [79–81] have verified the positive relationship between night shift work and risk of prostate cancer. We obtained the same result, i.e., that long-time night shift work was associated with a higher risk of breast cancer (OR = 1.22, 95% CI = 1.08–1.38), prostate cancer (OR = 1.26, 95% CI = 1.05–1.52), and cancers in women (OR = 1.12, 95% CI = 1.04–1.20). As far as we know, this is the first meta-analysis to comprehensively explore the effect of night shift work on multiple cancers in the whole population and separately in men and women.
Tissue-specific functions and output circadian rhythms are related to the different cell-based clock genes in periphery [83]. To exclude the tissue-specific influence, we only analyzed cancers that can occur in both men and women and found that night shift work increased cancer risk in men (OR = 1.09, 95% CI = 1.02–1.17) but not in women (OR = 1.02, 95% CI = 0.94–1.12). One meta-analysis involving colorectal cancer [82] demonstrated that night shift work could increase the risk of this type of cancer in women. However, we did not find a risk relationship for either men or women based on more studies of colorectal cancer (data not shown). Although there were considerably fewer articles on other cancers than on breast and prostate cancers, the low heterogeneity for digestive system cancer (P = 0.081, I 2 = 40.2%), hematological system cancer (P = 0.066, I 2 = 54.7%), and lung cancer (P = 0.078, I 2 = 49.5%) presented a more reliable conclusion. Previous studies have suggested that a common mechanism might be shared among hormone-dependent cancers including prostate cancer in men and breast and ovarian cancers in women [91, 92]. Melatonin has been implicated in antiproliferation effects in vivo and in vitro, and an elevated PSA level has been strongly connected with night shift work [91, 93], which could illustrate why breast and prostate cancers are more sensitive to night shift work than other common cancers.
One meta-analysis [8] analyzing the influence of night shift work on the risk of multiple cancers in women included up to 61 articles. Although light at night (LAN) [94] has been considered one of the risk factors for cancer, studies describing LAN were not included in our meta-analysis if the analysis of LAN was not connected to night shift work. We also excluded cross-sectional studies or studies only describing sleep duration. Therefore, the exposure of all 58 studies in our article was night shift work, which could decrease the clinical heterogeneity, making a more reliable result possible. Whereas the definition of night shift work differs largely among studies, we further divided work schedules into fixed shift, rotating shift, and mixed schedule, to reduce heterogeneity. Consistent with Mancio et al. [79], rotating shift workers had evidence of a higher risk of cancer whereas no association was observed in fixed shift workers. One speculation was that constant and rapid changing work times among rotating shift workers may necessitate a severe circadian disruption, causing failed adaptation, whereas fixed night shift workers had sufficient time to adapt almost completely to the shift cycle [95]. Consequently, rotating shift work resulted in a more profound effect on carcinogenesis through severe circadian disruption.
Our subgroup analyses also uncovered other meaningful results. One finding demonstrated that prostate, breast, and digestive system cancers were connected with night shift work whereas night shift work did not raise the risk of cancers of the hematological system, reproductive system, lung, and skin. In addition, Yuan et al. [8] found that female night shift workers in Europe and North America have greater risk of cancer than women in Asia and Australia. Based on the whole population, our results were consistent with those findings and indicate that the association of cancer risk with night shift work is not largely different between men and women. The different associations might be attributed to the limitations of the study populations. Many studies from Asia were limited to industrial workers whereas most studies from Europe and North America were based on the general population. However, the contrasting results might essentially be owing to differences in ethnicity or sensitivity. More specific exploration based on ethnicity is indispensable in future research. Moreover, studies based on the general population showed a higher cancer risk than those among nurses and industrial workers, and the pooled ORs in population could be better generalized to the overall population. Cohort studies, meaning higher-quality study designs, also indicated the same positive association between night shift work and the risk of cancer. Accordingly, the higher pooled ORs in these subgroups could confirm this association more powerfully.
Through analyzing Q and I 2 values, we found a significant heterogeneity among the studies included in this article (P < 0.001, I 2 = 76.2%); therefore, we used a random effects model to decrease the heterogeneity. After subgroup analyses, we found that fixed shift work, digestive system cancer, reproductive system cancer, unclassified occupation, interview data collection, and high-quality studies were related to less heterogeneity, representing more reliable results. However, all P values in the metaregression analyses did not reflect a statistical difference, such that heterogeneity could not be explained by metaregression analysis. One-by-one-omitted sensitivity and leave-one-out analyses showed that the pooled risk estimates were stable and positive, even when 19 studies were omitted, until heterogeneity was reduced to 29.8%. Although we did not find an obvious source of heterogeneity, the specific subgroup analyses, such as a more uniform definition of work schedules, unclassified occupation based on population, more detailed interviews, and high-quality studies, could decrease the potential heterogeneity.
Theoretical biological mechanisms for the positive relationship between night shift work and cancer risk are complex. First, night shift work and LAN could disturb the normal synchrony with the day–night rhythm and sleeping and diet patterns and bring about circadian disruption, which could suppress the secretion of melatonin [80]. Melatonin plays a pivotal role in inhibiting carcinogenesis through antioxidation, regulation of the immune system, free radical scavenging, and antiangiogenesis [67]. Decreased melatonin levels might disturb its antiproliferation effects on prostate cancer cells both in vivo and in vitro [93] and induce continuous secretion of estrogen, to increase the risk of breast cancer [77]. Second, night shift work can reduce the exposure time to sunlight and subsequently decrease vitamin D levels [80]. Studies have supported the inverse association between circulating vitamin D levels and risk of breast [96], colorectal [97], and prostate cancer [98]. Third, from a molecular perspective, night shift work could constitute a disruption of the feedback loops of circadian genes and lead to subsequent disordered expression of transcription and translation in all cells, which could pose a threat to cell proliferation, metabolism, regulation of the immune system, and DNA damage repair, causing carcinogenesis [73, 74].
To the best of our knowledge, this meta-analysis is the first and most comprehensive of its kind to identify the association between night shift work and risk of cancer from the perspective of diverse cancers and by sex. There were several strengths in our meta-analysis. First, we enrolled a large number of articles, even using strict inclusion criteria. The massive study population could enhance statistical power and ensure more accurate risk estimation. Second, a linear dose–response analysis was used to quantify the association between accumulative years of night shift work and cancer risk. Third, we classified work schedules and found that rotating shift work could increase cancer risk whereas fixed shift work could not. The classification of work schedules could decrease clinical heterogeneity to make the results more reliable. Fourth, 34 of 57 studies were carried out among the general population, such that the pooled OR could be better extended to the entire population. Our meta-analysis also had several limitations. First, a significantly high heterogeneity was discovered. We observed significant variability in the study design, risk estimates, study population, definition of night shift work, and exposure assessment. Each of these aspects may generate heterogeneity. Even though many statistical methods were used, we still had trouble finding an obvious source of potential heterogeneity; therefore, the conclusions reached in our meta-analysis should be interpreted with caution. Second, the lack of a consistent definition of night shift work may lead to a certain degree of misclassification and result in a dilution of the pooled OR [8]. Third, given that most night shift workers tend to have lower socioeconomic status, a lower uptake of screening and response rates may result in underestimation of the pooled risk estimates. Finally, studies using interviews could actively collect more detailed information, presenting a stronger risk compared with studies using questionnaire- and database-based data collection. In addition, there is inherent recall bias when conducting interviews or questionnaires. Hence, different exposure assessment methods and studies with lower quality or a less number of adjusted variables can cause information bias.
In conclusion, our meta-analysis identified a positive relationship between night shift work and cancer risk, using a comprehensive perspective of common cancers. We revealed that the risk of cancer increases cumulatively by 3.2% for every 5 years of night shift work. Moreover, we found no difference between men and women in the association between night shift work and the risk of cancer. Overall, on the grounds that public health is adversely affected by night shift work and its prevalence is on the rise, it is indispensable to develop shift work schedules with the aim of reducing cancer risk. Our meta-analysis does not merely increase public awareness, it also supports the recommendation for regular cancer screening among night shift workers.
Acknowledgments
This study was partly funded by the National Natural Science Foundation of China (Grant Nos. NSFC 81502195 and NSFC 81672512) and Medicine and Health Science Technology Development Project of Shandong Province (No. 2016WS0258). We also thank Analisa Avila, ELS, of Liwen Bianji, Edanz Group China (http://www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Contributor Information
Lijiang Sun, Email: slijiang999@126.com.
Guiming Zhang, Email: zhangguiming9@126.com.
Conflicts of Interest
The authors declare no competing financial interests.
Supplementary Materials
Figure S1: forest plots of studies describing the association between night shift work and the risk of multiple cancers. I 2: the indicator for judging the degree of heterogeneity; OR: odds ratio; CI: confidence interval. The squares and horizontal lines represent the study-specific OR and 95% CI. The diamond represents the pooled OR and 95% CI.
References
- 1.Aisbett B., Condo D., Zacharewicz E., Lamon S. The impact of shift work on skeletal muscle health. Nutrients. 2017;9(3) doi: 10.3390/nu9030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright K. P., Jr., Bogan R. K., Wyatt J. K. Shift work and the assessment and management of shift work disorder (SWD) Sleep Medicine Reviews. 2013;17(1):41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Boivin D. B., Shechter A., Boudreau P., Begum E. A., Ng Ying-Kin N. M. K. Diurnal and circadian variation of sleep and alertness in men vs. naturally cycling women. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(39):10980–10985. doi: 10.1073/pnas.1524484113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vimalananda V. G., Palmer J. R., Gerlovin H., et al. Night-shift work and incident diabetes among African-American women. Diabetologia. 2015;58(4):699–706. doi: 10.1007/s00125-014-3480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peplonska B., Bukowska A., Sobala W. Association of rotating night shift work with BMI and abdominal obesity among nurses and midwives. PLoS One. 2015;10(7, article e0133761) doi: 10.1371/journal.pone.0133761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pimenta A. M., Kac G., Campos e Souza R. R., Maria de Barros Almeida Ferreira L., Maria de Fátima Silqueira S. Night-shift work and cardiovascular risk among employees of a public university. Revista Da Associacao Medica Brasileira. 2012;58(2):168–177. doi: 10.1016/s0104-4230(12)70177-x. [DOI] [PubMed] [Google Scholar]
- 7.Kalmbach D. A., Pillai V., Cheng P., Arnedt J. T., Drake C. L. Shift work disorder, depression, and anxiety in the transition to rotating shifts: the role of sleep reactivity. Sleep Medicine. 2015;16(12):1532–1538. doi: 10.1016/j.sleep.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan X., Zhu C., Wang M., Mo F., Du W., Ma X. Night shift work increases the risks of multiple primary cancers in women: a systematic review and meta-analysis of 61 articles. Cancer Epidemiology, Biomarkers & Prevention. 2018;27(1):25–40. doi: 10.1158/1055-9965.EPI-17-0221. [DOI] [PubMed] [Google Scholar]
- 9.Straif K., Baan R., Grosse Y., et al. Carcinogenicity of shift-work, painting, and fire-fighting. The Lancet Oncology. 2007;8(12):1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 10.Wegrzyn L. R., Tamimi R. M., Rosner B. A., et al. Rotating night-shift work and the risk of breast cancer in the Nurses’ Health Studies. American Journal of Epidemiology. 2017;186(5):532–540. doi: 10.1093/aje/kwx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tse L. A., Lee P. M. Y., Ho W. M., et al. Bisphenol A and other environmental risk factors for prostate cancer in Hong Kong. Environment International. 2017;107:1–7. doi: 10.1016/j.envint.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Papantoniou K., Castaño Vinyals G., Espinosa A., et al. Shift work and colorectal cancer risk in the MCC-Spain case-control study. Scandinavian Journal of Work, Environment & Health. 2017;43(3):250–259. doi: 10.5271/sjweh.3626. [DOI] [PubMed] [Google Scholar]
- 13.Behrens T., Rabstein S., Wichert K., et al. Shift work and the incidence of prostate cancer: a 10-year follow-up of a German population-based cohort study. Scandinavian Journal of Work, Environment & Health. 2017;43(6):560–568. doi: 10.5271/sjweh.3666. [DOI] [PubMed] [Google Scholar]
- 14.Travis R. C., Balkwill A., Fensom G. K., et al. Night shift work and breast cancer incidence: three prospective studies and meta-analysis of published studies. Journal of the National Cancer Institute. 2016;108(12, article djw169) doi: 10.1093/jnci/djw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costas L., Benavente Y., Olmedo-Requena R., et al. Night shift work and chronic lymphocytic leukemia in the MCC-Spain case-control study. International Journal of Cancer. 2016;139(9):1994–2000. doi: 10.1002/ijc.30272. [DOI] [PubMed] [Google Scholar]
- 16.Bai Y., Li X., Wang K., et al. Association of shift-work, daytime napping, and nighttime sleep with cancer incidence and cancer-caused mortality in Dongfeng-tongji cohort study. Annals of Medicine. 2016;48(8):641–651. doi: 10.1080/07853890.2016.1217037. [DOI] [PubMed] [Google Scholar]
- 17.Wang P., Ren F. M., Lin Y., et al. Night-shift work, sleep duration, daytime napping, and breast cancer risk. Sleep Medicine. 2015;16(4):462–468. doi: 10.1016/j.sleep.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Papantoniou K., Castaño-Vinyals G., Espinosa A., et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. International Journal of Cancer. 2015;137(5):1147–1157. doi: 10.1002/ijc.29400. [DOI] [PubMed] [Google Scholar]
- 19.Hammer G. P., Emrich K., Nasterlack M., Blettner M., Yong M. Shift work and prostate cancer incidence in industrial workers: a historical cohort study in a German chemical company. Deutsches Aerzteblatt Online. 2015;112(27-28):463–470. doi: 10.3238/arztebl.2015.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu F., Han J., Laden F., et al. Total and cause-specific mortality of U.S. nurses working rotating night shifts. American Journal of Preventive Medicine. 2015;48(3):241–252. doi: 10.1016/j.amepre.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akerstedt T., Knutsson A., Narusyte J., Svedberg P., Kecklund G., Alexanderson K. Night work and breast cancer in women: a Swedish cohort study. BMJ Open. 2015;5(4, article e008127) doi: 10.1136/bmjopen-2015-008127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong T., Liquet B., Menegaux F., et al. Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocrine-Related Cancer. 2014;21(4):629–638. doi: 10.1530/ERC-14-0121. [DOI] [PubMed] [Google Scholar]
- 23.Carter B. D., Ryan Diver W., Hildebrand J. S., Patel A. V., Gapstur S. M. Circadian disruption and fatal ovarian cancer. American Journal of Preventive Medicine. 2014;46(3):S34–S41. doi: 10.1016/j.amepre.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Schernhammer E. S., Feskanich D., Liang G., Han J. Rotating night-shift work and lung cancer risk among female nurses in the United States. American Journal of Epidemiology. 2013;178(9):1434–1441. doi: 10.1093/aje/kwt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menegaux F., Truong T., Anger A., et al. Night work and breast cancer: a population-based case-control study in France (the CECILE study) International Journal of Cancer. 2013;132(4):924–931. doi: 10.1002/ijc.27669. [DOI] [PubMed] [Google Scholar]
- 26.Knutsson A., Alfredsson L., Karlsson B., et al. Breast cancer among shift workers: results of the WOLF longitudinal cohort study. Scandinavian Journal of Work, Environment & Health. 2013;39(2):170–177. doi: 10.5271/sjweh.3323. [DOI] [PubMed] [Google Scholar]
- 27.Grundy A., Richardson H., Burstyn I., et al. Increased risk of breast cancer associated with long-term shift work in Canada. Occupational and Environmental Medicine. 2013;70(12):831–838. doi: 10.1136/oemed-2013-101482. [DOI] [PubMed] [Google Scholar]
- 28.Parent M. E., El-Zein M., Rousseau M. C., Pintos J., Siemiatycki J. Night work and the risk of cancer among men. American Journal of Epidemiology. 2012;176(9):751–759. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 29.Natti J., Anttila T., Oinas T., Mustosmaki A. Night work and mortality: prospective study among Finnish employees over the time span 1984 to 2008. Chronobiology International. 2012;29(5):601–609. doi: 10.3109/07420528.2012.675262. [DOI] [PubMed] [Google Scholar]
- 30.Hansen J., Stevens R. G. Case-control study of shift-work and breast cancer risk in Danish nurses: impact of shift systems. European Journal of Cancer. 2012;48(11):1722–1729. doi: 10.1016/j.ejca.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Hansen J., Lassen C. F. Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occupational and Environmental Medicine. 2012;69(8):551–556. doi: 10.1136/oemed-2011-100240. [DOI] [PubMed] [Google Scholar]
- 32.Kubo T., Oyama I., Nakamura T., et al. Industry-based retrospective cohort study of the risk of prostate cancer among rotating-shift workers. International Journal of Urology. 2011;18(3):206–211. doi: 10.1111/j.1442-2042.2010.02714.x. [DOI] [PubMed] [Google Scholar]
- 33.Lahti T. A., Partonen T., Kyyrönen P., Kauppinen T., Pukkala E. Night-time work predisposes to non-Hodgkin lymphoma. International Journal of Cancer. 2008;123(9):2148–2151. doi: 10.1002/ijc.23566. [DOI] [PubMed] [Google Scholar]
- 34.Viswanathan A. N., Hankinson S. E., Schernhammer E. S. Night shift work and the risk of endometrial cancer. Cancer Research. 2007;67(21):10618–10622. doi: 10.1158/0008-5472.CAN-07-2485. [DOI] [PubMed] [Google Scholar]
- 35.Schernhammer E. S., Kroenke C. H., Laden F., Hankinson S. E. Night work and risk of breast cancer. Epidemiology. 2006;17(1):108–111. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- 36.Lie J. A. S., Roessink J., Kjærheim K. Breast cancer and night work among Norwegian nurses. Cancer Causes & Control. 2006;17(1):39–44. doi: 10.1007/s10552-005-3639-2. [DOI] [PubMed] [Google Scholar]
- 37.Kubo T., Ozasa K., Mikami K., et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. American Journal of Epidemiology. 2006;164(6):549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 38.Schernhammer E. S., Laden F., Speizer F. E., et al. Night-shift work and risk of colorectal cancer in the Nurses’ Health Study. Journal of the National Cancer Institute. 2003;95(11):825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 39.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12(1):74–77. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Walasa W. M., Carey R. N., Si S., et al. Association between shiftwork and the risk of colorectal cancer in females: a population-based case-control study. Occupational and Environmental Medicine. 2018;75(5):344–350. doi: 10.1136/oemed-2017-104657. [DOI] [PubMed] [Google Scholar]
- 41.Talibov M., Pukkala E., Martinsen J. I., Tryggvadottir L., Weiderpass E., Hansen J. Night-shift work and hematological cancers: a population based case-control study in three Nordic countries. Scandinavian Journal of Work, Environment & Health. 2018;44(3):258–264. doi: 10.5271/sjweh.3705. [DOI] [PubMed] [Google Scholar]
- 42.Vistisen H. T., Garde A. H., Frydenberg M., et al. Short-term effects of night shift work on breast cancer risk: a cohort study of payroll data. Scandinavian Journal of Work, Environment & Health. 2017;43(1):59–67. doi: 10.5271/sjweh.3603. [DOI] [PubMed] [Google Scholar]
- 43.Jørgensen J. T., Karlsen S., Stayner L., Hansen J., Andersen Z. J. Shift work and overall and cause-specific mortality in the Danish nurse cohort. Scandinavian Journal of Work, Environment & Health. 2017;43(2):117–126. doi: 10.5271/sjweh.3612. [DOI] [PubMed] [Google Scholar]
- 44.Heckman C. J., Kloss J. D., Feskanich D., Culnan E., Schernhammer E. S. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occupational and Environmental Medicine. 2017;74(3):169–175. doi: 10.1136/oemed-2016-103783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devore E. E., Massa J., Papantoniou K., et al. Rotating night shift work, sleep, and colorectal adenoma in women. International Journal of Colorectal Disease. 2017;32(7):1013–1018. doi: 10.1007/s00384-017-2758-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akerstedt T., Narusyte J., Svedberg P., Kecklund G., Alexanderson K. Night work and prostate cancer in men: a Swedish prospective cohort study. BMJ Open. 2017;7(6, article e015751) doi: 10.1136/bmjopen-2016-015751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papantoniou K., Castaño-Vinyals G., Espinosa A., et al. Breast cancer risk and night shift work in a case-control study in a Spanish population. European Journal of Epidemiology. 2016;31(9):867–878. doi: 10.1007/s10654-015-0073-y. [DOI] [PubMed] [Google Scholar]
- 48.Gyarmati G., Turner M. C., Castaño-Vinyals G., et al. Night shift work and stomach cancer risk in the MCC-Spain study. Occupational and Environmental Medicine. 2016;73(8):520–527. doi: 10.1136/oemed-2016-103597. [DOI] [PubMed] [Google Scholar]
- 49.Dickerman B. A., Markt S. C., Koskenvuo M., et al. Sleep disruption, chronotype, shift work, and prostate cancer risk and mortality: a 30-year prospective cohort study of Finnish twins. Cancer Causes & Control. 2016;27(11):1361–1370. doi: 10.1007/s10552-016-0815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Y., Nishiyama T., Kurosawa M., et al. Association between shift work and the risk of death from biliary tract cancer in Japanese men. BMC Cancer. 2015;15(1):p. 757. doi: 10.1186/s12885-015-1722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W., Ray R. M., Thomas D. B., et al. Shift work and breast cancer among women textile workers in Shanghai, China. Cancer Causes & Control. 2015;26(1):143–150. doi: 10.1007/s10552-014-0493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwon P., Lundin J., Li W., et al. Night shift work and lung cancer risk among female textile workers in Shanghai, China. Journal of Occupational and Environmental Hygiene. 2015;12(5):334–341. doi: 10.1080/15459624.2014.993472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yong M., Blettner M., Emrich K., Nasterlack M., Oberlinner C., Hammer G. P. A retrospective cohort study of shift work and risk of incident cancer among German male chemical workers. Scandinavian Journal of Work, Environment & Health. 2014;40(5):502–510. doi: 10.5271/sjweh.3438. [DOI] [PubMed] [Google Scholar]
- 54.Koppes L. L. J., Geuskens G. A., Pronk A., Vermeulen R. C. H., de Vroome E. M. M. Night work and breast cancer risk in a general population prospective cohort study in the Netherlands. European Journal of Epidemiology. 2014;29(8):577–584. doi: 10.1007/s10654-014-9938-8. [DOI] [PubMed] [Google Scholar]
- 55.Gapstur S. M., Diver W. R., Stevens V. L., Carter B. D., Teras L. R., Jacobs E. J. Work schedule, sleep duration, insomnia, and risk of fatal prostate cancer. American Journal of Preventive Medicine. 2014;46(3):S26–S33. doi: 10.1016/j.amepre.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 56.Datta K., Roy A., Nanda D., et al. Association of breast cancer with sleep pattern—a pilot case control study in a regional cancer centre in South Asia. Asian Pacific Journal of Cancer Prevention. 2014;15(20):8641–8645. doi: 10.7314/APJCP.2014.15.20.8641. [DOI] [PubMed] [Google Scholar]
- 57.Rabstein S., Harth V., Pesch B., et al. Night work and breast cancer estrogen receptor status—results from the German GENICA study. Scandinavian Journal of Work, Environment & Health. 2013;39(5):448–455. doi: 10.5271/sjweh.3360. [DOI] [PubMed] [Google Scholar]
- 58.Lin Y., Ueda J., Yagyu K., Kurosawa M., Tamakoshi A., Kikuchi S. A prospective cohort study of shift work and the risk of death from pancreatic cancer in Japanese men. Cancer Causes & Control. 2013;24(7):1357–1361. doi: 10.1007/s10552-013-0214-0. [DOI] [PubMed] [Google Scholar]
- 59.Fritschi L., Erren T. C., Glass D. C., et al. The association between different night shiftwork factors and breast cancer: a case-control study. British Journal of Cancer. 2013;109(9):2472–2480. doi: 10.1038/bjc.2013.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhatti P., Cushing-Haugen K. L., Wicklund K. G., Doherty J. A., Rossing M. A. Nightshift work and risk of ovarian cancer. Occupational and Environmental Medicine. 2013;70(4):231–237. doi: 10.1136/oemed-2012-101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poole E. M., Schernhammer E. S., Tworoger S. S. Rotating night shift work and risk of ovarian cancer. Cancer Epidemiology, Biomarkers & Prevention. 2011;20(5):934–938. doi: 10.1158/1055-9965.EPI-11-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lie J. A. S., Kjuus H., Zienolddiny S., Haugen A., Stevens R. G., Kjaerheim K. Night work and breast cancer risk among Norwegian nurses: assessment by different exposure metrics. American Journal of Epidemiology. 2011;173(11):1272–1279. doi: 10.1093/aje/kwr014. [DOI] [PubMed] [Google Scholar]
- 63.Pronk A., Ji B. T., Shu X. O., et al. Night-shift work and breast cancer risk in a cohort of Chinese women. American Journal of Epidemiology. 2010;171(9):953–959. doi: 10.1093/aje/kwq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pesch B., Harth V., Rabstein S., et al. Night work and breast cancer—results from the German GENICA study. Scandinavian Journal of Work, Environment & Health. 2010;36(2):134–141. doi: 10.5271/sjweh.2890. [DOI] [PubMed] [Google Scholar]
- 65.O'Leary E. S., Schoenfeld E. R., Stevens R. G., et al. Shift work, light at night, and breast cancer on Long Island, New York. American Journal of Epidemiology. 2006;164(4):358–366. doi: 10.1093/aje/kwj211. [DOI] [PubMed] [Google Scholar]
- 66.Davis S., Mirick D. K., Stevens R. G. Night shift work, light at night, and risk of breast cancer. Journal of the National Cancer Institute. 2001;93(20):1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 67.Zhao M., Wan J., Zeng K., et al. The reduction in circulating melatonin level may contribute to the pathogenesis of ovarian cancer: a retrospective study. Journal of Cancer. 2016;7(7):831–836. doi: 10.7150/jca.14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirick D. K., Bhatti P., Chen C., Nordt F., Stanczyk F. Z., Davis S. Night shift work and levels of 6-sulfatoxymelatonin and cortisol in men. Cancer Epidemiology, Biomarkers & Prevention. 2013;22(6):1079–1087. doi: 10.1158/1055-9965.EPI-12-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davis S., Mirick D. K., Chen C., Stanczyk F. Z. Night shift work and hormone levels in women. Cancer Epidemiology, Biomarkers & Prevention. 2012;21(4):609–618. doi: 10.1158/1055-9965.EPI-11-1128. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez-Garcia A., Mayo J. C., Hevia D., Quiros-Gonzalez I., Navarro M., Sainz R. M. Phenotypic changes caused by melatonin increased sensitivity of prostate cancer cells to cytokine-induced apoptosis. Journal of Pineal Research. 2013;54(1):33–45. doi: 10.1111/j.1600-079X.2012.01017.x. [DOI] [PubMed] [Google Scholar]
- 71.Lissoni P., Chilelli M., Villa S., Cerizza L., Tancini G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. Journal of Pineal Research. 2003;35(1):12–15. doi: 10.1034/j.1600-079X.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 72.Persson C., Glimelius B., Ronnelid J., Nygren P. Impact of fish oil and melatonin on cachexia in patients with advanced gastrointestinal cancer: a randomized pilot study. Nutrition. 2005;21(2):170–178. doi: 10.1016/j.nut.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 73.Kervezee L., Cuesta M., Cermakian N., Boivin D. B. Simulated night shift work induces circadian misalignment of the human peripheral blood mononuclear cell transcriptome. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(21):5540–5545. doi: 10.1073/pnas.1720719115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kettner N. M., Katchy C. A., Fu L. Circadian gene variants in cancer. Annals of Medicine. 2014;46(4):208–220. doi: 10.3109/07853890.2014.914808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee H. E., Lee J., Jang T. W., Kim I. A., Park J., Song J. The relationship between night work and breast cancer. Annals of Occupational and Environmental Medicine. 2018;30(1):p. 11. doi: 10.1186/s40557-018-0221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He C., Anand S. T., Ebell M. H., Vena J. E., Robb S. W. Circadian disrupting exposures and breast cancer risk: a meta-analysis. International Archives of Occupational and Environmental Health. 2015;88(5):533–547. doi: 10.1007/s00420-014-0986-x. [DOI] [PubMed] [Google Scholar]
- 77.Wang F., Yeung K. L., Chan W. C., et al. A meta-analysis on dose–response relationship between night shift work and the risk of breast cancer. Annals of Oncology. 2013;24(11):2724–2732. doi: 10.1093/annonc/mdt283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamdar B. B., Tergas A. I., Mateen F. J., Bhayani N. H., Oh J. Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Research and Treatment. 2013;138(1):291–301. doi: 10.1007/s10549-013-2433-1. [DOI] [PubMed] [Google Scholar]
- 79.Mancio J., Leal C., Ferreira M., Norton P., Lunet N. Does the association of prostate cancer with night-shift work differ according to rotating vs. fixed schedule? A systematic review and meta-analysis. Prostate Cancer and Prostatic Diseases. 2018;21(3):337–344. doi: 10.1038/s41391-018-0040-2. [DOI] [PubMed] [Google Scholar]
- 80.Gan Y., Li L., Zhang L., et al. Association between shift work and risk of prostate cancer: a systematic review and meta-analysis of observational studies. Carcinogenesis. 2018;39(2):87–97. doi: 10.1093/carcin/bgx129. [DOI] [PubMed] [Google Scholar]
- 81.Du H. B., Bin K. Y., Liu W. H., Yang F. S. Shift work, night work, and the risk of prostate cancer: a meta-analysis based on 9 cohort studies. Medicine. 2017;96(46, article e8537) doi: 10.1097/MD.0000000000008537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X., Ji A., Zhu Y., et al. A meta-analysis including dose–response relationship between night shift work and the risk of colorectal cancer. Oncotarget. 2015;6(28):25046–25060. doi: 10.18632/oncotarget.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yan L., Silver R. Neuroendocrine underpinnings of sex differences in circadian timing systems. The Journal of Steroid Biochemistry and Molecular Biology. 2016;160:118–126. doi: 10.1016/j.jsbmb.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kruijver F. P. M., Swaab D. F. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology. 2002;75(5):296–305. doi: 10.1159/000057339. [DOI] [PubMed] [Google Scholar]
- 85.Cain S. W., Dennison C. F., Zeitzer J. M., et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. Journal of Biological Rhythms. 2010;25(4):288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Santhi N., Lazar A. S., McCabe P. J., Lo J. C., Groeger J. A., Dijk D. J. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(19):E2730–E2739. doi: 10.1073/pnas.1521637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rangtell F. H., Karamchedu S., Andersson P., et al. A single night of sleep loss impairs objective but not subjective working memory performance in a sex-dependent manner. Journal of Sleep Research. 2018 doi: 10.1111/jsr.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiologic Reviews. 1987;9(1):1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 89.Wells G. A., Shea B., O’Connell D., et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa Hospital Research Institute; 2014. [Google Scholar]
- 90.Wallace B. C., Schmid C. H., Lau J., Trikalinos T. A. Meta-analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Medical Research Methodology. 2009;9(1) doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flynn-Evans E. E., Mucci L., Stevens R. G., Lockley S. W. Shiftwork and prostate-specific antigen in the National Health and Nutrition Examination Survey. Journal of the National Cancer Institute. 2013;105(17):1292–1297. doi: 10.1093/jnci/djt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu Y., Zheng T., Stevens R. G., Zhang Y., Boyle P. Does “clock” matter in prostate cancer? Cancer Epidemiology, Biomarkers & Prevention. 2006;15(1):3–5. doi: 10.1158/1055-9965.EPI-05-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jung-Hynes B., Schmit T. L., Reagan-Shaw S. R., Siddiqui I. A., Mukhtar H., Ahmad N. Melatonin, a novel Sirt 1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. Journal of Pineal Research. 2011;50(2):140–149. doi: 10.1111/j.1600-079X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hunter C. M., Figueiro M. G. Measuring light at night and melatonin levels in shift workers: a review of the literature. Biological Research for Nursing. 2017;19(4):365–374. doi: 10.1177/1099800417714069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams C. Work-life balance of shift workers. Perspectives on Labour & Income. 2008;9:5–16. [Google Scholar]
- 96.Obaidi J., Musallam E., Al-Ghzawi H. M., Azzeghaiby S. N., Alzoghaibi I. N. Vitamin D and its relationship with breast cancer: an evidence based practice paper. Global Journal of Health Science. 2014;7(1):261–266. doi: 10.5539/gjhs.v7n1p261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giovannucci E. Epidemiology of vitamin D and colorectal cancer. Anti-Cancer Agents in Medicinal Chemistry. 2013;13(1):11–19. doi: 10.2174/187152013804487254. [DOI] [PubMed] [Google Scholar]
- 98.Schwartz G. G. Vitamin D, sunlight, and the epidemiology of prostate cancer. Anti-Cancer Agents in Medicinal Chemistry. 2013;13(1):45–57. doi: 10.2174/187152013804487344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: forest plots of studies describing the association between night shift work and the risk of multiple cancers. I 2: the indicator for judging the degree of heterogeneity; OR: odds ratio; CI: confidence interval. The squares and horizontal lines represent the study-specific OR and 95% CI. The diamond represents the pooled OR and 95% CI.