Abstract
With rapidly increased construction of nuclear power plants worldwide to reduce energy shortage and subsequent environment contamination, routine use of radiotherapy and radiodiagnosis equipment in the clinical medicine, the research on the health effect of radiation exposure has become a very important area to explore. Traditional Chinese Medicine (TCM) may be an ideal candidate therapy as it usually produces fewer side effects even with long-term administration. In this paper, we reviewed current therapeutic approaches to prevent radiation-induced brain neuropathological and functional changes. Neuroprotective effects of TCM in different brain injury models have been briefly summarized. We then reviewed the neuroprotective and radioprotective effect of TCM in different radiation exposure models and discussed the potential molecular mechanism(s) of the neuroprotective and radioprotective effect of TCM. The conclusions and future research directions were made in the last part of the paper.
1. Introduction
Radiation-Induced Brain Dysfunction. Nuclear accidents such as radiation leakage from the Fukushima nuclear power plant in Japan in 2011, extensive use of X-ray, Computed Tomography (CT scan), Positron Emission Tomography (PET) in medical diagnosis, radiotherapy (RT) for treatment of human cancers, space travel, and atomic weapons testing have significantly increased the chance of radiation exposures [1]. Exposure to high doses/dose rates of radiation leads to an increased risk for cancer and noncancerous diseases including atherosclerotic, cardiovascular, cerebrovascular, and neurodegenerative effects [2]. Irradiation of eukaryotic cells induces damage to proteins, lipids, and DNA directly or indirectly due to free radical formation. Cell signaling events in response to radiation depend on environmental conditions besides genetic and physiological features of the biological systems [3]. In the mammalian brain, severe structural and functional injury occurs after acute or fractionated high dose radiation exposure [4]. Low doses/dose rates of radiation exposure may produce cognitive impairment even without any significant morphological alterations [5]. Ionizing radiation (IR) provokes cognitive deficits, especially during childhood and adolescence.
Different psychiatric disorders, including depression, bipolar disorder, and schizophrenia, may be related to hippocampal neurogenesis disturbances. There is evidence of an increased incidence of schizophrenia spectrum disorders following exposure to atomic bombing radiation, radiotherapy, or environment with high natural IR level [6]. Alzheimer's disease (AD) is a human neurodegenerative disease, and its global prevalence has been predicted to increase dramatically in the following decades. Mounting evidence suggests that exposure to IR may result in the development of AD [7]. On the other hand, retrospective studies involving the general population and those with brain radiotherapy did not show any association between RT and Alzheimer's disease [8, 9]. From a therapeutic point of view, so far, only Amifostine has been used as an important adjunct to radiotherapy to reduce radiation-induced damage to normal tissues or cells, particularly in skin, intestine, marrow, mucosa, and salivary glands with lesser activity in kidney and lung and none in brain [10]. However, toxic side effects of Amifostine have restricted its use in clinical treatment of radiation-induced diseases [11]. Therefore, it is important to develop compounds which can protect against radiation-induced brain damage with less side-effect.
In the behavioral tests to evaluate the effect of Traditional Chinese Medicine (TCM) on learning and memory, Morris water maze test has been commonly used. The maze consists of a pool, with a hidden platform submerged just below the water surface. During the Morris water maze test, the rat or mouse learns to escape from the water by locating a hidden platform with the help of visual cues. The learning ability is quantified as escape time. The shorter escape time a mouse or a rat needed to find the central platform, the better its spatial memory [12].
In this review paper, a comprehensive literature research was carried out using key words “Traditional Chinese Medicine (TCM), radiation or irradiation, neuronal damage, brain, neuroprotection, or radioprotection” by means of the scientific engine Google Scholar (http://scholar.google.com/), and via the databases, PubMed (http://www.ncbi.nlm.nih.gov/pubmed). The following Chinese websites, http://acad.cnki.net/Kns55/brief/result.aspx?dbPrefix=CJFQ from China National Knowledge Infrastructure (CNKI), http://g.wanfangdata.com.cn/ from WanFang, and http://qikan.cqvip.com/ from WEIPU, were also searched.
2. Current Treatment For Radiation-Induced Brain Dysfunction
Radiation-induced neuronal apoptosis results from oxidative stress, and antioxidant treatments prevent radiation-induced brain damage [13]. Amifostine has been found to decrease the reactive oxygen species (ROS) levels [14] and suppress radiation-induced cell death in developing cerebellar granular cells [15]. Amifostine significantly attenuated recognition memory defects in adult mice exposed to low dose radiation [16] and has been widely used as a radioprotective agent.
Irradiation induces the activation of inflammatory cells and the release of inflammatory cytokines [17]. Anti-inflammation therapy has been proved to be radioprotective. Eicosapentaenoic acid is an anti-inflammation agent and could effectively protect hippocampal neurons from damage by whole body irradiation [18, 19]. Pretreatment with anti-inflammatory drugs such as indomethacin or a peroxisome proliferator-activated receptor-α agonist combined with fenofibrate prevented microglial activation and impairment of neurogenesis [20]. Treatment with the angiotensin converting enzyme inhibitors AT1RA L-158,809 and ramipril ameliorated radiation-induced cognitive deficits and reduced apoptosis among subgranular zone (SGZ) progenitors and inflammatory disruption within the SGZ microenvironment in the rat model [21, 22]. The administration of atorvastatin combined with ramipril appeared to synergistically ameliorate radiation-induced inhibition of neurogenesis [23]. Therefore, anti-inflammatory therapy may be a potential therapeutic approach for radiation-induced brain injury.
3. Traditional Chinese Medicine (TCM) with Neuroprotective Effect
Several TCM have been tested for their neuroprotective activity after brain insults (Table 1).
Table 1.
Neuroprotective effect of Traditional Chinese Medicine (TCM) after brain insults.
| No. | Herbs and plant extract | Main acting constituents | Test system | Brain insults | Effect | Mechanism | References |
|---|---|---|---|---|---|---|---|
| 1 | Morinda officinalis | Rubiadin, Rubiadin-1-methylether, etc. | Rat, mouse, human | Alzheimer's disease (AD), memory disorder | Improve learning and memory, Anti-AD, protect brain function, anti-depression, anti-aging, improve language function | Anti-oxidative stress, anti-apoptosis, enhanced LTP function in hippocampal synapse, improving glucose metabolism | [24–30] |
|
| |||||||
| 2 | Lycium barbarum | Lycium barbarum polysaccharide, Betane, etc. | Rat, mouse | Memory disorder resulted by lead expose or manganese poisoning | Improve learning and memory, protect brain function | Anti-oxidative stress, anti-apoptosis, promoting hippocampal neurogenesis | [31–34] |
|
| |||||||
| 3 | Safflor | Carthamin, Safflow yellow, etc. | Rat, mouse | Memory disorder | Improve learning and memory | Anti-oxidative stress, anti-apoptosis, | [35–38] |
|
| |||||||
| 4 | Epimedium | Icariin, Icariside, etc. | Rat, mouse | Dementia | Improve learning and memory, Anti-AD, protect brain function, anti-aging | Anti-oxidative stress, anti-apoptosis, reducing the entanglement of nerve fibers (Tau), promoting hippocampal neurogenesis, inhibiting inflammatory mediators, increase estrogen levels, increase the activity of sodium pump and calcium pump |
[39, 40] |
|
| |||||||
| 5 | Radix polygalae | Onjisaponin, Ketone, etc. | Rat, mouse | AD | Improve learning and memory, Anti-AD, protect brain function | Anti-oxidative stress, anti-apoptosis, enhancing LTP function in hippocampal synapse, increasing the expression of CREB in the hippocampus, inhibiting inflammatory mediators, improve the entanglement of nerve fibers(Tau) promoting hippocampal neurogenesis | [41–48] |
|
| |||||||
| 6 | Acrous tatrinouill shotts | β-Asarone, α-Asarone, etc. | Rat, mouse | Memory impairment by scopolamine; cortical neuron damage; depression | Improve learning and memory, Anti-AD, protect brain function, anti-depression | Anti-oxidative stress, anti-apoptosis, increasing the expression of CREB in the hippocampus |
[49–51] |
|
| |||||||
| 7 | Polygona sibiricum | Polygonatum Polysaccharide, Street soap shake, etc. | Rat, mouse | Memory impairment by scopolamine | Improve learning and memory, Anti- AD, protect brain function, anti-depression | Anti-oxidative stress, anti-apoptosis, improving cerebral ischemia | [52, 53] |
|
| |||||||
| 8 | Cynomorium songaricum Rupr | Anthocyanin, Triterpenoid saponins, etc. | Rat, mouse | AD | Improve learning and memory, Anti-AD, protect brain function, anti-aging | Anti-oxidative stress | [54–56] |
|
| |||||||
| 9 | Alpinia oxyphylla miq. Fruit | Sesquiterpene, Monoterpene, etc. | Rat, mouse | Scopolamine-induced dementia | Improve learning and memory, protect brain function, anti-aging | Anti-oxidative stress, anti-apoptosis | [57, 58] |
|
| |||||||
| 10 | Broomrape | Ergosterin, Cistanche glycoside, etc. |
Mouse, human | AD; Memory impairment; cerebral ischemia | Improve learning and memory, protect brain function | Anti-oxidative stress, anti-apoptosis | [59–67] |
3.1. Morinda officinalis
Bajisin is a glycoside monomer extracted from TCM Morinda officinalis. It protects brain cells and has antiaging and antidepression effect. In the rat model of acute cerebral ischemic injury, Bajisin increased the activity of superoxide dismutase (SOD), glutathione peroxidase, and glucose production and reduced lipid peroxide in the brain tissue of senile mice. It had no obvious influence on the nitrogen monoxide (NO) [24]. In D-galactose and sodium nitrite-induced Alzheimer disease model, Morinda officinalis significantly increased the learning and memory ability [25]. It also had antiaging effect. Morinda officinalis significantly decreased malondialdehyde (MDA) and the apoptotic index of Purkinje fibers [26]. So far, five compounds from Morinda officinalis were isolated and their structures were identified as asrubiadin-1-methylether (I), 2-hydroxy-1-methoxyanthraquinone (II), scopoletin (III), isofraxidin (IV), and anthraquinone-2-aldehyde (V) [27]. When the forced swimming tests in mice and rats and differential-reinforcement-of-low-rate 72 second schedule (DRL72 s) in rats were used, the extracts induced significant reduction in the immobility periods in the forced swimming tests and elicited significant increases in reinforcers in DRL72 s [28]. Clinical trials suggested that Bajitian oligosaccharide capsule improved the symptoms of patients with mild or moderate depression. The efficacy was similar to fluoxetine, but it produced fewer side effects [29, 30].
3.2. Lycium barbarum
Regulates immunity, has antiaging effect, and is able to scavenge free radicals. Administration of Lycium barbarum juice significantly improved learning and memory ability and increased the activities of acetylcholinesterase (AchE) and SOD, while the contents of MDA in brains decreased obviously when compared to aging mice [31]. It increased the learning and memory ability in manganese [32] or lead [33] poisoning mice model. Lycium barbarum could also induce differentiation of bone marrow stromal cells (BMSCs) into neurons [34].
3.3. Safflor
Improved the learning and memory ability of AD rats induced by AB1-42[35]. This effect may be related to the decrease of oxidative stress and the increase of cholinergic nerve function in brain tissue [36–38].
3.4. Epimedium
Is a genus of flowering plants in the family Berberidaceae. The active component of Epimedium extracts Icariin inhibits tumor, enhances immunity, improves heart and cerebral vessels, and regulates endocrine function. Recent studies suggest that Icariin also has neuroprotective effects in the central nervous system. Icariin increased the SOD activity of brain tissue, reduced MDA and AchE activity, and therefore protected the hippocampus and improved the learning and memory ability in the rat model [39]. Icariin also promoted neurogenesis in the dentate gyrus (DG) of the hippocampus [40].
3.5. Radix polygalae
Is usually used for the treatment of human insomnia or coughing. In the rat model of Alzheimer's disease (AD), Radix polygalae could effectively improve learning and memory ability by inhibiting the activity of brain AchE, reducing MDA, free radical levels, and oxidative stress injury, and increasing SOD [41]. It significantly decreased the escape latency in hidden platform and increased the time spent in target quadrant and the number of crossing times in the spatial probe test [42]. Radix polygalae also protected neurons from the toxic effect of AB1-40 and reduced the hyperphosphorylation of tau (Ser 396) in the neurons of AD rats by activating the expression of protein phosphatase 2A (PP2A) and inhibiting the expression of protein kinase A (PKA) [43]. The main active component of Radix polygalae, Tenuigenin, promoted the differentiation of neural stem cell into nerve cells [44, 45]. Radix polygalae improved hippocampal-dependent learning and memory and had potential antidepressant properties [46–48].
3.6. Acrous tatrinouill shotts
Is widely used in clinical practice in epilepsy, fever, phlegm syncope, faint, forgetful, stroke aphasia, tinnitus, and Alzheimer's disease. B-asarone is its main ingredient. Acrous tatrinouill shotts can improve the learning and memory ability of rats induced by scopolamine, which may be linked to the reduction of the expression of glial fibrillary acidic protein (GFAP) and MDA in hippocampal astrocytes [49]. Glutamate exposure to cultured rat cortical neurons induced morphological changes and lactate dehydrogenase (LDH) leakage, increased intracellular calcium concentration, and decreased cell survival rate. B-asarone could reduce intracellular calcium concentration, LDH leakage, and apoptosis ratio and therefore increase cell survival [50]. The depression was relieved effectively by B-asarone in the rat model which may be related to the improvement of the expression of Bcl-2, brain-derived neurotrophic factor (BNDF), tyrosine kinase receptor B (TrkB), and mitogen-activated protein kinases (MAPK) [51].
3.7. Polygona sibiricum
Has antiaging, lowering increasing coronary blood flow, and anticancer effect. Polygona sibiricum improved the learning and memory ability in mice. Alcohol extract from the rhizome of Polygonatum sibiricum improved acquisition of impairment of memory induced by scopolamine (SCO) in mice. It also extended the survival time of mice subjected to cerebral ischemia by the occlusion of the bilateral carotid arteries and that PS ethanolic extract 2.0 mg/mL and 10.0 mg/mL inhibited MDA formation in the rat brain tissue [52]. Polygona sibiricum also reduced the deposition of Aβ in the hippocampus of AD rats [53].
3.8. Cynomorium songaricum Rupr
Has the role of tonifying kidney yang, enhancing aphrodisiac action, and laxative effect. It markedly improved the learning and memory ability in the rat AD model by reducing oxidative stress in the brain tissue and promoting the formation of synapses [54–56].
3.9. Alpinia oxyphylla miq
Is a dried ripe fruit of the perennial plant of the ginger family. It significantly improved the learning and memory ability in mice induced by scopolamine [57]. The Alpinia oxyphylla Miq could prevent the injury of hippocampal CA3 neurons in the restrained stress rat model [58].
3.10. Broomrape
Has different biological activities, such as immune regulation, memory enhancement, antioxidative, antiaging, and radiation protection. A significant improvement of memory was observed in mice with learning disabilities induced by hydrocortisone [59, 60]. Broomrape protected the brain cells by scavenging oxygen free radicals and reducing lipid peroxidation damage to brain tissue [61, 62]. It improved the learning and memory of AD mice by decreasing the content of MDA, increasing the activity of SOD, GSH-Px, and decreasing the activity of AChE, the apoptosis rate of brain cells, and the accumulation of calcium in brain tissue [63–65]. In AD patients, Broomrape improved the cognitive and self-care ability and delayed the progress of dementia [66]. The total glycosides of Cistanche deserticola extract could significantly improve the behavioral characteristics in the mouse Parkinson's disease model induced by 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and increase the content of dopamine in striatum and the expression of tyrosine hydroxylase in the substantia nigra [67].
4. Traditional Chinese Medicine with Radioprotective Effects
4.1. Astragalus membranaceus
Hydroponically grown root extracts from Astragalus membranaceus significantly reduced UVA-induced DNA damage in cultured human lung and skin fibroblasts [68]. In the brain of rats with acute encephalopathy caused by 60Co irradiation, the intraperitoneal injection of Astragalus parenteral solution decreased the nitric oxide and ameliorated the cognitive ability, suggesting that astragale may protect radiation-induced brain injury [69, 70]. The acute and chronic electromagnetic field (EMF) irradiation could initiate neurologic damage in hippocampus. Chinese medicine diet (CMD) which comprised ferulic acid, gimenoside, astragalus polysaccharide, an ingredient of Astragalus, and rhodiola sachaliens has protective effect on the impaired learning and memory, the neuronal apoptosis, and the peroxidation damage induced by electromagnetic field irradiation. CMD intervention played a significant protective role in antagonizing neurological damage in the later stage of acute irradiation and chronic irradiation [71, 72]. Astragalus also significantly protected neuronal apoptosis induced by radiation injury at a single-dose X-ray exposure of 30 Gy [73]. While radiotherapy prolongs the survival time of patients with head and neck tumor, the side effects such as radiation optic neuropathy may lead to irreversible visual loss which seriously affects the quality of life of patients. Recent studies suggested that Astragalus membranaceus significantly improved the visual acuity of irradiated rats or patients with nasopharyngeal carcinoma after radiotherapy [74, 75].
4.2. Salvia miltiorrhiza
The bioactive constituents of Salvia miltiorrhiza, i.e., tanshinones and depsides, protect against β-amyloid-induced toxicity by the anti-inflammatory mechanisms. The two constituents enhance the antiapoptotic B-cell leukemia protein-2 family members, decrease the translocation of cytochrome c, and have an activity on vascular endothelial growth factor. In addition, depsides decrease caspase-3, intracellular Ca(2+), and reactive oxygen species while tanshinones enhance the activities of superoxide dismutase and glutathione peroxidase [76]. In the mouse whole brain irradiation model, Salvia miltiorrhiza prevented the high dose radiation-induced brain structural and functional changes and improved the quality of life by ameliorating the primary events [77]. Microwave irradiation induced a significant decrease of ATPase activity and a remarkable increase of Na+, Ca2+ contents in the hippocampus. However, Salvia miltiorrhiza could significantly lower the inhibition of ATPase activity and the increase of Na+, Ca2+ in the hippocampus. The neuronal damage was also ameliorated substantially [78]. The behavioral test indicated that Salvia miltiorrhiza could improve the learning and memory ability of rats [79]. This was confirmed by another study showing that Salvia miltiorrhiza improved the ionizing radiation-induced cognition impairment by reducing lipid peroxide (LPO) and intercellular cell adhesion molecule-1 (ICAM-1) expression in the mouse model [80]. The brain radioprotective effect of Salvia miltiorrhiza was also confirmed in clinical studies showing that radiotherapy combined with administration of Salvia miltiorrhiza significantly reduced radiation-induced brain injury [81–83].
4.3. Ligusticum chuanxiong Hort
Ligusticum chuanxiong Hort and its bioactive ingredient, tetramethylpyrazine (TMP), have been used to treat cardiovascular diseases and to relieve various neurological symptoms. TMP effectively protected neuronal apoptosis, which was associated with the inhibition of oxidative stress and a change in the levels of apoptosis-related proteins, Bcl-2 and Bax. Furthermore, TMP reduced the expression of proinflammatory cytokines such as TNF-α and IL-8, which likely contributes to its cytoprotective effects [84]. Clinical study has shown that TMP significantly improves the symptoms of patients with radiotherapy-induced encephalopathy [85] and optic neuropathy [86].
4.4. Broomrape
Citanche glycoside is an active component of Broomrape. It facilitated the repairing process of radiation-induced damage to the biological membranes and cells of sensitive organs in the mice [87].
4.5. Horse Chestnut P.E.
β-aescine sodium is an ingredient from Horse Chestnut P.E. Cerebral edema is a radiation injury at acute stages after the exposure. Early aggressive treatment of cerebral edema could relieve the symptoms of intracranial hypertension, delay or block the development of the disease, and prevent the occurrence of cerebral hernia. Clinical data indicated that patients with radiation-induced brain edema could be effectively controlled by β-aescine sodium or mannitol and dexamethasone [88].
4.6. Radix Hedysari
In γ-ray irradiated rat model, administration of Radix hedysari significantly increased SOD, but decreased MDA activity in the brain tissue. It suggests that Radix hedysari may serve as an antioxidant drug and be helpful for the recovery of radiation-induced brain damage [89, 90]. Radix hedysari capsule increased the wet weight of liver, spleen, brain, and testicular tissue of irradiated mice, suggesting that it has radioprotective effect [91].
4.7. Safflower
12C6+ irradiation induces cognitive dysfunction and impairment of the blood brain barrier, significantly decreased SOD, and increased MDA activity in the brain tissue. Hydroxysafflor yellow A, an ingredient of safflower, dose dependently improved cognitive dysfunction, protected the blood-brain barrier, increased SOD, and decreased MDA activity. It suggests that hydroxysafflor yellow A may have a radioprotective effect on radiation-induced brain injury [92].
4.8. Arnebiae Radix
Shikonin, a bioactive ingredient of Arnebiae Radix, improved 12C6+ ion beam-induced brain injury by its modulating effects on the oxidative stress [93].
4.9. Ginkgo
Ginkgo flavonoid, an extract from Ginkgo, prevented age-related spatial memory deficits in both animal study [94] and clinical trial [95]. After high dose irradiation, Ginkgo flavonoid could prevent the radiation-induced hippocampal injury [96].
4.10. Ginseng
Panaxoside Rgl, a bioactive ingredient of Ginseng, reduced neuron apoptosis by controlling Cdk5 and played a protective role in radiation-induced hippocampal damage [97].
4.11. Kang-fu-ling
Kang-fu-ling (KFL) is a polybotanical dietary supplement with antioxidant properties. KFL reversed high power microwave-induced memory loss and the histopathological changes in hippocampus of rats. In addition, KFL displayed a protective effect against HPM-induced oxidative stress and activated the nuclear factor-E2-related factor 2 (Nrf2) and its target genes in the hippocampus of rats. The Nrf2-antioxidant response element (ARE) signaling pathway may be involved in the neuroprotective effects of KFL against HPM-induced oxidative stress. The dietary supplement KFL may therefore be a promising natural radioprotector [98].
4.12. Shenqi
Shenqi Fuzheng Injection (SFI) was extracted from a number of medicinal herbs, such as Radix Codonopsis (root of Codonopsis pilosula) and Radix Astragali (root of Astragalus), and was approved by the State Food and Drug Administration of China in 1999. Administration of SFI effectively attenuates irradiation-induced brain injury via inhibition of the NF-κB signaling pathway and microglial activation [99].
4.13. 978-1
A TCM of destagnation and renal invigoration (978-1) was effective to prevent or treat the damage of learning and memory ability caused by irradiation in mice. It was able to prevent or treat radiation-induced subventricular cell damage by downregulation of p53 and C-jun expression and inhibition of apoptosis [100, 101].
5. The Molecular Mechanisms of Neuroprotective and Radioprotective Effect of TCM
It has been well documented that radiation induces brain oxidative stress, microglial activation, acute and chronic inflammatory responses, apoptosis, autophagy, abnormal angiogenesis and neurogenesis, redistribution or imbalanced neurotransmitter and receptor systems, and downregulation of neural growth factors [102]. TCM may play roles in antioxidative stress and antiapoptosis. It could promote hippocampal neurogenesis, improve microcirculation, inhibit microglial activation and inflammation, and reduce TAU production (Table 2).
Table 2.
Neuroprotection effect of TCM after radiation exposure.
| No. | Herbs and plant extract | Main acting constituents | Test system | Brain insults (radiation source, dose, dose rate) | Effect | Mechanism | References |
|---|---|---|---|---|---|---|---|
| 1 | Astragalus membranaceus | Astragalus polysaccharides, Total saponins of Astragalus, etc. |
Rat, mouse, Human, cell |
60Co, 4.5Gy/min, 160-170s, one time, single-dose X-rays exposure of 30 Gy |
Improve learning and memory, increase SOD activity, decrease MDA | Scavenge oxygen free radicals, reduce nitric oxide production | [68–75] |
|
| |||||||
| 2 | Salvia miltiorrhiza | Tanshinone, Cryptotanshinone, etc. |
Mouse, Rat, Human |
Varian-600, X-rays, 22Gy | Improve learning and memory | Reduce lipid peroxide in brain tissue and inhibit the adhesion of endothelial cells factor 1 expression | [76–83] |
|
| |||||||
| 3 | Ligusticum chuanxiong Hort | Chuanxiongzine, Ligustilide, etc. | Human | Patients had a history of radiotherapy for head and neck cancer | Reduce radiation encephalopathy | Improve microcirculation, expand blood vessels, inhibit the generation of oxygen free radicals | [84–86] |
|
| |||||||
| 4 | Broomrape | Ergosterin, Cistanche glycoside, etc. |
Mouse | 5 Gy 60Co-γ | Improve viability | Scavenge oxygen free radicals, Strengthen immunity | [87] |
|
| |||||||
| 5 | Horse Chestnut P.E | Aescine, etc. | Human | Patients had a history of radiotherapy for head cancer | Prevent brain edema | Stabilize endothelial cells, | [88] |
|
| |||||||
| 6 | Radix Hedysari | Hedysarum polysaccharide, etc. | Rat, Mouse | 2 Gy 60Co-γ | Increase SOD activity, decrease MDA | Reduce oxidative stress | [89–91] |
|
| |||||||
| 7 | safflower | Carthamin, Safflow yellow, etc. | Mouse | 4 Gy 12C6+ | Increase SOD activity, decrease MDA | Reduce oxidative stress | [92] |
|
| |||||||
| 8 | Arnebiae Radix | Shikonin, Acetylshikonin, etc. | Mouse | 12C6+ ion beam, dose rate of approximately 0.5 Gy/min | Improve the spatial memory deficits | Reduce oxidative stress | [93] |
|
| |||||||
| 9 | Ginkgo | Flavonoids, ginkgolides, etc. |
Rat | 12MEV, 20 Gy | Inhibition of brain cell edema | Scavenge oxygen free radicals, apoptosis inhibition | [94–96] |
|
| |||||||
| 10 | Ginseng | Ginsenoside, etc. | Rat | 30 Gy | Protect the hippocampal neurons | Inhibit apoptosis | [97] |
|
| |||||||
| 11 | Kang-fu-ling | Total glucoside of Astragalus, total glucoside of Radix Paeoniae Rubra and Tanshinone | Rat | HPM at 30 mW cm 2 for 15 min |
Improve spatial memory | Modulate ROS formation and antioxidant enzymes. | [98] |
|
| |||||||
| 12 | Shenqi | Codonopsis polysaccharides, Astragalus polysaccharides, etc. |
Mouse | A single dose of cranial radiotherapy (CRT) with 20Gy | Improve the physical status, survival, and spatial learning, attenuate all the CRT-induced changes in the brain tissues. | Inhibit NF-κB signaling pathway and microglial activation | [99] |
|
| |||||||
| 13 | Renal invigoration (978-1) | Icariin, Lignin, etc. | Mouse | A single dose of 20 Gy | Prevent impairment of learning and memory | Inhibit apoptosis | [100, 101] |
The roles of antioxidative stress and antiapoptosis of TCM have been well accepted. Morinda officinalis increases the activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in the brain tissue of rats with acute cerebral ischemia and aging and reduce the content of lipid peroxide (LPO) [24, 26]. In the Lycium barbarum-treated aging mice induced by D-galactose, the animal learning, memory, and brain AchE and SOD increased significantly, while brain MDA decreased obviously. Similar changes were also found in Lead poisoning mice [31, 33]. Safflor increased SOD and GSH-Px, but decreased MDA in the cortical tissue of AD rats [35–38, 92]. Epimedium improved animal learning and memory ability by increasing brain SOD, decreasing MDA and Ach E, and reducing the damage of hippocampal neurons by D-gal AlCl3 treatment [39]. In the AD model induced by the hippocampal injection of amyloid-β25~35, the brain SOD were decreased, but AchE and MDA were increased obviously leading to learning and memory impairment. Radix polygalae treatment increased SOD, reduced AchE and MDA, and improved learning and memory ability [41]. Similarly, Acrous tatrinouill shots [49, 51, 103], Polygona sibiricum [52], and Cynomorium songaricum Rupr [54–56, 104, 105] also increased the brain SOD, reduced MDA, and improved the impairment of learning and memory, whereas Alpinia oxyphylla miq. fruit, Broomrape, and Astragalus membranaceus are antioxidative and antiapoptotic [57–75, 87], and Salvia miltiorrhiza decreased the brain lipid peroxidase in AD rats [76, 80, 81]. Ligusticum chuanxiong Hort inhibited free radicals in hypoxia and reduced neuronal apoptosis [85]. In 60Co-γ irradiated animals, Radix Hedysari treatment increased the brain superoxide dismutase activity but reduced maleic dialdehyde [89, 90]. Other antioxidative stress and antiapoptosis TCM include Arnebiae Radix, Ginkgo, Ginseng, Kang-fu-ling, Shenqi, and Renal invigoration (978-1) [93–101].
TCM may also promote neurogenesis to prevent radiation-induced cognitive impairment. Morinda officinalis and Lycium barbarum promoted neurogenesis in the subgranular zone of the dentate gyrus [29, 32, 34]. Morinda officinalis also increased the number of dendrites and their branches of the hippocampal neurons [29], whereas Lycium barbarum had inductive effect on differentiation of bone marrow stromal cells (BMSCs) into neurons [32, 34]. Epimedium significantly reduced senile plaques in the hippocampus and increased the number of BrdU+ cells in the dentate gyrus. Our previous study showed that epimedium extract prevented the loss of proliferation cells, newly generated neurons, and interneurons in the hilus, in particular, the subgranular zone of the dentate gyrus [40, 106]. In vitro study showed that adding tenuigenin to the neural stem cell medium increased the number of newly formed neurospheres and promoted the differentiation of the hippocampal neural stem cells (NSCs) into neurons [44–46, 48].
TCM improved radiation-induced inflammation and microcirculation changes. TMP reduced the expression of proinflammatory cytokines such as TNF-α and IL-8, which may also contribute to its cytoprotective effects [84]. Horse Chestnut P.E. improved microcirculation and stabilizing vascular endothelial cells and has been used to treat radiation-induced brain edema [88]. In rats with AB25-35 induced Alzheimer's disease, Epimedium was used to improve spatial learning and memory by inhibiting TNF-a, IL-6, and caspase-3 expression [107]. Radix polygalae reduced the brain tau level, the phosphorylation of tau protein, and the expression of PKA, but increased the expression of PP2A in amyloid β peptide 1-40 (Aβ1-40)-injected mice [43]. Salvia miltiorrhiza inhibited the radiation-induced senile plaques and neurofibrillary tangles in the mouse brain [77], whereas Shengqifuzheng effectively attenuated irradiation-induced brain injury by inhibiting NF-κB signaling pathway and microglial activation [99].
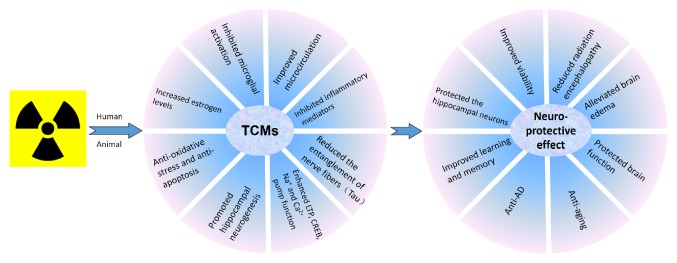
TCM may increase estrogen level and change the Ca2+ Na+ function to protect brain injury. Acrous tatrinouill shots could reduce intracellular calcium concentration [50]. The oral administration of icariin increased serum estradiol(E_2) level and improved the learning and memory ability in AD rats [108]. Salvia miltiorrhiza increased ATPase activity and reduced Na+, Ca2+ in the hippocampus (CA1 area) and improved microwave radiation-induced brain damage [78] (refer to Figure 1).
Figure 1.
Traditional Chinese Medicine (TCM) reduces radiation-induced microglial activation and production of inflammatory cytokines and chemokines to inhibit brain inflammation. TCMs increase the superoxide dismutase activity and decrease malondialdehyde and nitric oxide production to reduce oxidative stress. Upregulation of Bcl-2, downregulation of Bax-2, downregulation of p53 and c-jun expression, and increased ratio of Bcl-2/Bax by TCMs prevent radiation-induced apoptosis. TCMs may also increase brain-derived neurotrophic factor (BNDF), tyrosine kinase receptor B (TrkB), and mitogen-activated protein kinases (MAPK) to promote neurogenesis and improve radiation-induced impairment of cognition. These drugs also enhance LTP, CREB, Na+ and Ca2+ pump function, increase estrogen levels, and reduce the entanglement of nerve fibers (Tau) to improve radiation-induced neurocognitive impairment.
6. Limitation of TCM as Radio-Neuro-Protectants
While it seems promising to use TCM as potential radio-neuro-protectants, it should be emphasized that the ingredients of TCM are very complex and have not been fully identified and purified. Improper processing, dispensing, compatibility, excessive dosage, and individual differences may significantly affect clinical use of TCM [109]. As individual reponse to a same TCM may be different, it may compromise the usage of TCM for treatment of victims with massive radiation exposure. TCM Formulae with two or more herbs often produce better curative efficacies and fewer side effects than a single herb, but improper use may produce more harm than benefit [110]. Disbelief of TCM may also limit its use worldwidely.
7. Conclusions and Future Research Directions
Extensive publications on neuroprotective and radioprotective effect of TCM suggest that TCM may be effective in prevention of radiation-induced glial cell activation and proliferation, neuroinflammation, oxidative stress, apoptosis, and neurodegeneration. TCM may also promote brain neurogenesis and improve radiation-induced impairment of cognition. However, different doses and combination of TCM, animal species, strains, ages, and sexes were used in different research laboratories in previous studies. The variations in radiation sources, doses/dose rates, and irradiation patterns (acute or fractionated) made it difficult to evaluate if the positive effect of TCM in one animal model or laboratory could be applied to other models or laboratories. The neuroprotective or radioprotective effect of TCM administered before irradiation may not be observed when TCM are injected after irradiation. The routes of TCM administration, i.e., oral or intraperitoneal injection, may also compromise the data translation. Furthermore, the purity of the components and composition of the compounds of TCM were not clearly mentioned in most of the previous studies which may limit its clinical use.
TCM with radioprotective effect on the brain are far less investigated than on other organs. Further extensive studies in the following areas may still be needed: (1) Radiosensitivity varies significantly among different strains of animals. The effect of TCM may have to be tested in the same strain of animals for comparison in order to make solid conclusions. Animal age and sex should be chosen carefully as TCM effect may be age- and sex-dependent. (2) When animals are exposed to the same dose of radiation, radiation dose rate may affect radiosensitivity. Therefore, the same dose rate of radiation exposure should be used to compare TCM effect. The radiation source and component of radiation may also affect TCM effect. (3) The mechanism of high dose/dose rate radiation-induced brain damage may be different from low dose/dose rate radiation-induced injury. Comparative study of radioprotective effect of TCM in animals exposed to high and low doses of radiation exposures, in particular, the latter, may shed light on further understanding the mechanisms of the two patterns of radiation-induced brain damage.
Acknowledgments
This work was supported by Sciences Foundation of the Hubei Provincial Department of Education (Q20161305), Hubei Province Health and Family Planning Scientific Research Project (WJ2016-Y-10) to HJR, and National Research Foundation (NRF) of Singapore to TFR.
Contributor Information
Bo Xu Ren, Email: boxuren188@163.com.
Feng Ru Tang, Email: tangfr@gmail.com.
Conflicts of Interest
The authors have no actual or potential conflicts of interest.
Authors' Contributions
Xiao Chun Peng and Jiang Rong Huang contributed equally to this work.
References
- 1.Kim J. S., Yang M., Kim S. H., Shin T., Moon C. Neurobiological toxicity of radiation in hippocampal cells. Histology and Histopathology. 2013;28(1):301–310. doi: 10.14670/HH-28.301. [DOI] [PubMed] [Google Scholar]
- 2.Marazziti D., Baroni S., Catena-Dell'Osso M., et al. Cognitive, psychological and psychiatric effects of ionizing radiation exposure. Current Medicinal Chemistry. 2012;19(12):1864–1869. doi: 10.2174/092986712800099776. [DOI] [PubMed] [Google Scholar]
- 3.United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation. United Nations Scientific Committee on the Effects of Atomic Radiation; 2008. [Google Scholar]
- 4.Tofilon P. J., Fike J. R. The radioresponse of the central nervous system: A dynamic process. Journal of Radiation Research. 2000;153(4):357–370. doi: 10.1667/0033-7587(2000)153[0357:TROTCN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Kim J. S., Lee H. J., Kim J. C., et al. Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiationsyndrome is associated with inhibition of hippocampal neurogenesis. Journal of Radiation Research. 2008;49(5):517–526. doi: 10.1269/jrr.08020. [DOI] [PubMed] [Google Scholar]
- 6.Loganovsky K. N., Volovik S. V., Manton K. G., Bazyka D. A., Flor-Henry P. Whether ionizing radiation is a risk factor for schizophrenia spectrum disorders? The World Journal of Biological Psychiatry. 2005;6(4):212–230. doi: 10.1080/15622970510029876. [DOI] [PubMed] [Google Scholar]
- 7.Begum N., Wang B., Mori M., Vares G. Does ionizing radiation influence Alzheimer's disease risk? Journal of Radiation Research. 2012;53(6):815–822. doi: 10.1093/jrr/rrs036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeAngelis L. M., Delattre J.-Y., Posner J. B. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–796. doi: 10.1212/WNL.39.6.789. [DOI] [PubMed] [Google Scholar]
- 9.Peper M., Steinvorth S., Schraube P., et al. Neurobehavioral toxicity of total body irradiation: A follow-up in long- term survivors. International Journal of Radiation Oncology Biology Physics. 2000;46(2):303–311. doi: 10.1016/S0360-3016(99)00442-3. [DOI] [PubMed] [Google Scholar]
- 10.Phillips T. L. Rationale for initial clinical trials and future development of radioprotectors. American Journal of Clinical Oncology. 1980;3(2):165–173. [PubMed] [Google Scholar]
- 11.Jagetia G. C. Radioprotective potential of plants and herbs against the effects of ionizing radiation. Journal of Clinical Biochemistry and Nutrition. 2007;40(2):74–81. doi: 10.3164/jcbn.40.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Peng X., Zeng X., et al. Estrogen secreted by mesenchymal stem cells necessarily determines their feasibility of therapeutical application. Scientific Reports. 2015;5(1) doi: 10.1038/srep15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sleeper E., Tamm C., Frisén J., Zhivotovsky B., Orrenius S., Ceccatelli S. Cell death in adult neural stem cells. Cell Death & Differentiation. 2002;9(12):1377–1378. doi: 10.1038/sj.cdd.4401127. [DOI] [PubMed] [Google Scholar]
- 14.Yang M., Song M. S., Kim S. H., et al. Cytotoxicity of gamma-ray in rat immature hippocampal neurons. Journal of Veterinary Science. 2011;12(3):203–207. doi: 10.4142/jvs.2011.12.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guelman L. R., Cabana J. I., Pagotto R. M., Zieher L. M. Ionizing radiation-induced damage on developing cerebellar granule cells cultures can be prevented by an early amifostine post-treatment. International Journal of Developmental Neuroscience. 2005;23(1):1–7. doi: 10.1016/j.ijdevneu.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Lee H., Kim J., Song M., et al. Amifostine ameliorates recognition memory defect in acute radiation syndrome caused by relatively low-dose of gamma radiation. Journal of Veterinary Science. 2010;11(1):81–83. doi: 10.4142/jvs.2010.11.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feiock C., Yagi M., Maidman A., Rendahl A., Hui S., Seelig D. Central nervous system injury - A newly observed bystander effect of radiation. PLoS ONE. 2016;11(9) doi: 10.1371/journal.pone.0163233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babcock T., Helton W. S., Espat N. J. Eicosapentaenoic acid (EPA): An antiinflammatory ω-3 fat with potential clinical applications. Nutrition Journal . 2000;16(11-12):1116–1118. doi: 10.1016/S0899-9007(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 19.Lonergan P. E., Martin D. S. D., Horrobin D. F., Lynch M. A. Neuroprotective effect of eicosapentaenoic acid in hippocampus of rats exposed to γ-irradiation. The Journal of Biological Chemistry. 2002;277(23):20804–20811. doi: 10.1074/jbc.M202387200. [DOI] [PubMed] [Google Scholar]
- 20.Ramanan S., Kooshki M., Zhao W., Hsu F., Riddle D. R., Robbins M. E. The PPARα agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. International Journal of Radiation Oncology Biology Physics. 2009;75(3):870–877. doi: 10.1016/j.ijrobp.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenrow K. A., Brown S. L., Liu J., Kolozsvary A., Lapanowski K., Kim J. H. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Journal of Radiation Oncology. 2010;5(1, article no. 6) doi: 10.1186/1748-717X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee T. C., Greene-Schloesser D., Payne V., et al. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Journal of Radiation Research. 2012;178(1):45–56. doi: 10.1667/rr2731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenrow K. A., Liu J., Brown S. L., Kolozsvary A., Lapanowski K., Kim J. H. Combined atorvastatin and ramipril mitigate radiation-induced impairment of dentate gyrus neurogenesis. Journal of Neuro-Oncology. 2011;101(3):449–456. doi: 10.1007/s11060-010-0282-x. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z. F., Tan B. X., Chen J. W., Li X. Y., Lin R. S., Lin L. Mechanism of Bajisins protective effect on acute cerebral ischemic injury. Journal of Guangzhou University Of TCM. 2000;17(3):215–217. [Google Scholar]
- 25.Wang X., Li J., Liao Y. L., Deng C. L. Study on protective eeffct of water extract from morindae officinalis on alzheimer disease model rats. China Pharmacy. 2013;24(31):2908–2910. [Google Scholar]
- 26.Chen Z., Fu R. F., Cheng L. X. Effect of BEM on cerebellum of D-galactose induced aged rat. Journal of Chinese Medicine. 2010;25(150):903–907. [Google Scholar]
- 27.Li Y. B., Wang L. L., Lai X. P., Feng F., Zhou Y. J. Active anti-aging constituents in Morinda officinalis. Central South Pharmacy. 2011;9(2):101–103. [Google Scholar]
- 28.Zhang Z. Q., Yuan L., Zhao N., Xu Y. K., Yang M., Luo Z. P. Antidepressant effect of the ehanolic extracts of the roots of Morinda officinalis in rats and mice. Chinese Pharmaceutical Journal. 2000;35(11):739–741. [Google Scholar]
- 29.Zou L.-Y., Zhang H.-Y. Research advance of morinda officinalis oligosaccharides in treatment of depression. Chinese Journal of New Drugs. 2012;21(16):1889–1945. [Google Scholar]
- 30.Zhang T. L., Liu J., Bahati Halia., Li B. Y., Liu W. Y. The value of CT perfusion and functional magnetic resonance imaging in the treatment of vascular dementia Morinda officinalis oligosaccharides Bajijiasu treatment of vascular dementia with short-term and long-term effect. Chinese Journal of Gerontology. 2016;36(19):4765–4767. [Google Scholar]
- 31.Ji J. J., Han L. S., Xu J., Yang Y., Xu C. Y., Yan Y. Effects of fructus lycii on learning and memory of D-galactose induced aging mice. Chinese Journal of Public Health. 2007;23(3):359–360. [Google Scholar]
- 32.Wen J., Yang B. N., Ren D. Effects of Lycium barbarum polysaccharides on neurogenesis and learning and memory in manganese poisoning mice. Chinese Journal of Integrated Traditional and Western Medicine. 2010;30(3):295–298. [PubMed] [Google Scholar]
- 33.Cai T. G., Cai Y., Yu S. L. Influence of Lycium barbarum polysaccharides on neurogenesis and learning and memory and protein kinase C in hippocamous in lead exposed mice. Chinese Journal of Public Health. 2010;26(1):38–39. [Google Scholar]
- 34.Liu X., Shan W., Zeng R.-X., Fang Y., Li D.-H., Qin S.-J. Differentiation of rat bone marrow mesenchymal stem cells into neuron-like cells induced by lycium barbarum polysaccharide. Journal of Clinical Rehabilitative Tissue Engineering Research. 2009;13(14):2667–2672. [Google Scholar]
- 35.Ma Q., Xu H., Ruang Y. Y., Shi X. M., Wang Z. X., Hu Y. L. Effects of safflower yellower on learning and memory disorders of dementia rats induced by AB1-42. Pharmacology and Clinics of Chinese Materia Medica. 2014;30(5):64–66. [Google Scholar]
- 36.Hu Y. L., Wang P. L., Zhang R., Liu M. Effects of safflower yellow pigment on learning and memory in mice induced by scopolamine. Chinese Journal of Gerontology. 2012;10(32):4197–4198. [Google Scholar]
- 37.Xu H., Ma Q., Wang Z. X., Hu Y. L. Effects of safflower yellow on D-galactose/sodium nitrite induced learning and memory disorders in mice. Pharmacology and Clinics of Materia Medica. 2013;29(2):59–61. [Google Scholar]
- 38.Xu H., Ma Q., Wang Z. X., Hu Y. L. Effects of safflower yellow on learning and memory impairments of vascular dementia in rats in mice. Chinese Pharmacentical Journal. 2014;49(12):1032–1035. [Google Scholar]
- 39.Deng W., Zheng M. G., Zhang J., Huang C. Effects of total flavonoids from two species of the central Guizhou on the memory of rats with dementia. Lishizhen Medicine and Materia Medica Research. 2012;23:627–629. [Google Scholar]
- 40.Cai R., Li F., Dong H. X., Shi J. S. Effects of icariin on the learning and memory function and hippocampus neurogenesis of Tg2576 mice. Journal of Zunyi Medical University. 2015;38(4):350–354. [Google Scholar]
- 41.Zhan H. T., Li H. Y., Zeng X. X., Wu J. F. Effects of mechanism of polygala hongkongensis hemsl extracts on learning and memory in Alzheimer's disease model rats. Journal of Apoplexy and Nervous Diseases. 2012;29(3):243–245. [Google Scholar]
- 42.Guo C. R. Effects of Radix polygalae by different processing methods on learning memory in model mice induce by scopolamine. Shandong Chemical Industry. 2015;44(4):10–12. [Google Scholar]
- 43.Xu K. L., Chen Q., Liu W., et al. Effects of tenuigenin on tau protein phosphory lation at Ser 396 site in neurons of AD rats induced by AB1-40. Chinese Journal of Pathophysiology. 2012;28(9):1605–1609. [Google Scholar]
- 44.Huang L., Kuang S. Y. Effects of volatile oil extracted from Alipinia oxyphylla on the apoptosis of substantial nigra neuron in PD mice model. China Pharmacy. 2011;22(47):4430–4433. [Google Scholar]
- 45.Chen Y., Huang X., Chen W., Wang N., Li L. Tenuigenin promotes proliferation and differentiation of hippocampal neural stem cells. Neurochemical Research. 2012;37(4):771–777. doi: 10.1007/s11064-011-0671-3. [DOI] [PubMed] [Google Scholar]
- 46.Xue W., Hu J.-F., Yuan Y.-H., et al. Polygalasaponin XXXII from polygala tenuifolia root improves hippocampal-dependent learning and memory. Acta Pharmacologica Sinica. 2009;30(9):1211–1219. doi: 10.1038/aps.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu Y., Liu P., Guo D.-H., Rahman K., Wang D.-X., Xie T.-T. Antidepressant effects of the extract YZ-50 from Polygala tenuifolia in chronic mild stress treated rats and its possible mechanisms. Pharmaceutical Biology. 2010;48(7):794–800. doi: 10.3109/13880200903280034. [DOI] [PubMed] [Google Scholar]
- 48.Liu P., Hu Y., Guo D.-H., et al. Potential antidepressant properties of Radix Polygalae (Yuan Zhi) Phytomedicine. 2010;17(10):794–799. doi: 10.1016/j.phymed.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Zhou X. J., Dai S. J., Chen H. S., Xie F. R., Li C. Y., Yang Y. X. Effects of acorus tatarinowii schott volatile oil on learning and memory impairment rats induced by scopolamine and its possible mechanism. Journal of Gansu College of TCM. 2015;32(1):1–6. [Google Scholar]
- 50.Chen Y.-Z., Wang Q.-W., Liang Y., Fang Y.-Q. Protective effects of beta-asarone on cultured rat cortical neurons damage induced by glutamate. Journal of Chinese Medicinal Materials. 2007;30(4):436–439. [PubMed] [Google Scholar]
- 51.Sun Y. R., Dong H. Y., Wang X. Y., et al. Influences of B -asarone on Rat behaviors and expression of hippo campal neurons in rats with depression. Journal of Beijing University of Traditional Chinese Medicine. 2013;36(8):546–549. [Google Scholar]
- 52.Sun L. R., Li X., Guo Y. Y., Wang D., Li Z. A study on the effects of the rhizome of pokygonatum sibiricum red on acquisition of memory impairment induced by scopolamine in mice and the others. Journal of Shenyang Pharmaceutical University. 2001;18:286–289. [Google Scholar]
- 53.Yi Y. L., Wu S. X., Ye M. S., Zeng Y., Zhang P., Xie Y. Q. Effect of Aβ 1-42 injection on hippocampus cells in rats and protective role of polygona-polysaccharose for Alzheimer’s disease. Journal of Central South University (Medical Sciences) 2014;39:344–348. doi: 10.3969/j.issn.1672-7347.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Zhao Y.-Q., Wang Z.-W., Jing Y.-H. The study on the effect of Suoyang on ultrastructural in the relevant brain areas of learning and memory in the rat's model of Alzheimer' disease. Chinese Journal of Clinical Rehabilitation. 2002;6(15):2220–2221. [Google Scholar]
- 55.Tian F. Z., Chang H. Y., Zhou J. Y., Lu Y. Effects of ethyl acetate extract from cynomorim songaricum on learning and memory function and hippocampa neurons in ovariectomized rat model with Alzheimer's disease. Journal of Beijing University of Traditional Chinese Medicine. 2014;37(11):763–766. [Google Scholar]
- 56.Wu M., Liu L., Hu Y. L., Liu J. L. Effect of water extracts of Cynomorium songaricum on scopolamine induced learning and memory impairment of mice. Xinjiang Journal of Traditional Chinese Medicine. 2015;33(2):29–31. [Google Scholar]
- 57.Li Y. Q., Wang Y. F., Shi X. L., Li T. Y., Zhang Y. Effect of Ethanol extract of fruit of Alpinia oxyphylla Miq on learning and memory ability in scopolamine-induced dementia mice. Journal of Capital Normal University. 2015;36(1):54–56. [Google Scholar]
- 58.Sun L., Chen Y. J., Liu C., Zhou Y. The FAO may have an effect on the injury of hippocampal CA3 neurons in restrained stress rat. Journal of Dalian University of Technology. 2009;6:87–90. [Google Scholar]
- 59.Hu Y. M., Hu Y. X., Liu X. Y., Ma Z., Yi C. Z. Effects of total glycosides of Cistanche deserticola on learning and memory function of normal mice. Chinese Journal of Preventive Medicine. 2007;8(4):370–373. [Google Scholar]
- 60.Gao C., Wang C. S., Wu G. Z., Tu P. F. Effects of total glycosides of Cistanche deserticola on learning and memory function of mice with kidney yang deficiency induced by hydrocortisone. Chinese Journal of Traditional Chinese Medicine. 2005;11(5):330–332. [Google Scholar]
- 61.Pu X. P., Li X. R., Li H. N., Tu Z. F., Song Z. H., Li C. L. Protective effect of Cistanche deserticola campneoside on apoptosis induced by neurotoxin MPP+ Journal of Peking University (Health Sciences) 2001;33(3):p. 217. [Google Scholar]
- 62.Meng X. Z., Wang X. W., Jiang X. Y., Liu F. X., Paer H. T. Protective effects of total glycosides of Cistanche deserticola on cerebral ischemia reperfusion injury in conscious mice. Chinese Journal of Clinical Neuroscience. 2003;11(3):p. 239. [Google Scholar]
- 63.Luo L., Wang X. W., Liu F. X., Yang S., Wang T. The protective effect of meat Chengdu glycosides on aluminium trichloride induced learning and memory impairment of mice. Chinese Journal of New Drugs and Clinical Remedies. 2007;26(1):33–36. [Google Scholar]
- 64.Liu F. X., Wang X. W., Wang X. F., Luo L. Effect of glycosides of cistanche on learning and memory in Alzheimer's disease mice and its mechanism. Xinjiang Medical University. 2005;28(12):1131–1135. [Google Scholar]
- 65.Liu F. X., Wang X. W., Luo L., Xin H., Wang X. F. Effects and mechanisms of total glycosides of Cistanche deserticola on learning and memory in mice with Alzheimer's disease caused by B - amyloid peptide. Chinese Pharmacological Bulletin. 2006;22(5):595–599. [Google Scholar]
- 66.Wang Q. The aclinicl research of Roucongrong Zonggan Jiaonang in treatment of Alzheimer's disease. Strait Pharmaceutical Journal. 2009;21(3):103–104. [Google Scholar]
- 67.Geng X. C., Song L. W., Pu X. P., Tu P. Neuroprotective effects of phenylethanoid glycosides from Cistanehes salsa agalnstl-methyl-4-pheny1-1, 2, 3, 6-tetrahydropyridine(MPIP)-induced dopaminergic toxicity in C57 mice. Biological & Pharmaceutical Bulletin. 2004;27(6):797–801. doi: 10.1248/bpb.27.797. [DOI] [PubMed] [Google Scholar]
- 68.Curnow A., Owen S. J. An evaluation of root phytochemicals derived from althea officinalis (Marshmallow) and astragalus membranaceus as potential natural components of UV protecting dermatological formulations. Oxidative Medicine and Cellular Longevity. 2016;2016:9. doi: 10.1155/2016/7053897.7053897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao Y.-C., Chen Y. Interventional effect of astragale parenteral solution on the cognitive ability and behavioral changes of rats with acute radioactive injury. Chinese Journal of Clinical Rehabilitation. 2005;9(36):71–73. [Google Scholar]
- 70.Bai C. Z., Zhong Q. M., Wu Y. P., Jia L. L., Niu Y. Y., Feng M. L. Experimental study on the protective effects of 5 kinds of Chinese herbs such as Astragalus membranaceus on radiation injury in mice. Chinese Journal of Cellular and Molecular Immunology. 2013;29(10):1052–1054. [Google Scholar]
- 71.Gong Q. F., Yang X. S., Deng C. H., Zhang G. B., Yu P. Protective effect of nutritional intervention on microwave induced oxidative damage in hippocampus of rats. Chinese Journal of Radiology Health. 2008;17(4):401–403. [Google Scholar]
- 72.Gong Q. F., Yang X. S., Tu L., Zhang G. B., Yu Z. P. The chinese medicine nutrient diet intervention prevent against the neurologic damage induce by EMF irradiation in rat hippocampus. Chinese Journal of Applied Physiology. 2013;29(4):346–350. [PubMed] [Google Scholar]
- 73.Tang H. H., Chen Y., Luo J. Protective effect of astragalus injection on radiation injury inprimary cultured rat hippocampal neuron. Chinese Journal of Cancer Prevention and Treatment. 2010;17(14):1049–1051. [Google Scholar]
- 74.Zhu D., Zeng P., Liu J., Sun L., Huang Y. T. Traditional Chinese medicine for the treatment of radiation-induced optic neuropathy. Hubei Journal of Traditional Chinese Medicine. 2008;30(4):39–40. [Google Scholar]
- 75.Ji J. P., Cheng X. H., Lian L. H., Liu H., Chen L., Li Z. Y. Tongqiao stasis method on retinal vascular endothelial and optic nerve injury in rats and the preventive effect of radiation. Journal of Guangzhou University of Traditional Chinese Medicine. 2015;32(4):715–719. [Google Scholar]
- 76.Bonaccini L., Karioti A., Bergonzi M. C., Bilia A. R. Effects of salvia miltiorrhiza on CNS neuronal injury and degeneration: a plausible complementary role of tanshinones and depsides. Planta Medica. 2015;81(12-13):1003–1016. doi: 10.1055/s-0035-1546196. [DOI] [PubMed] [Google Scholar]
- 77.Yi J. L., Liu Y. J., Miao Y. J., Yang W. Z., M Cai W. Radiation-induced late brain injury and the protective effect of traditional chinese medicine. Chinese Journal of Radiation Oncology. 2004;13(2):107–110. [Google Scholar]
- 78.Zhang J. N., Zhang X., Yi S. Y., Li A. M. Neuronal damage in hippocampus( CA-1) region after microwave radiation with and without salvia miltiorrhiza treatment. Chinese Neurosurgical Journal. 1999;15(1):18–20. [Google Scholar]
- 79.Jiang S. J., Wu W. P., Zhang X. S., et al. The improvement of Salvia miltiorrhiza on temporal lobe ischemia spatial cognitive impairment rats and on the expression of platelet-derived growth factor influence. Chinese Neurosurgical Journal. 1999;32(1):290–292. [Google Scholar]
- 80.Yi J. L., Liu Y. J., Yang W. Z., Huang X. D., Miao Y. J., Cai W. M. Radiation-induced acute brain injury and the protective effect of traditional chinese medicine-Salvia Miltiorrhiza. Chinese Journal of Radiation Oncology. 2003;12(2):112–115. [Google Scholar]
- 81.Mou J. G., Zheng H. T., Zhang C. J. Effect of compound salvia miltiorrhiza injection on prevention of acute radiation-induced brain injury. Sichuan Tumor Prevention. 2004;17(3):181–182. [Google Scholar]
- 82.Duan Y. L., Fan X. H., Liu Q. G., Zhao C., Guan J. Clinical observation of tanshinone IIA combined with radiotherapy in the treatment of elderly patients with brain metastases. Chinese Journal of Practical Medicine. 2011;38(2):101–102. [Google Scholar]
- 83.Duan Y. L., Fan X. H., Liu Q. G., Zhao C., Guan J. Clinical observation on Tanshinone IIA in radiation induced senile brain injury. Modern Oncology. 2011;19(4):674–675. [Google Scholar]
- 84.Zhang C., Teng F., Tu J., Zhang D., Annunziato L. Ultrasound-enhanced protective effect of tetramethylpyrazine against cerebral ischemia/reperfusion injury. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0113673.e113673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang F. L., Luo D. P., Tong Y. H. Salviae miltiorrhizae liguspyragine hydrochloride and glucose injection treatment of radiation encephalopathy randomized controlled study. Chinese Journal of Clinical Research. 2014;27(3):285–287. [Google Scholar]
- 86.Chen X. H., Li Z. Y., Ji J. P. Changes of visual vield and VEP in patients of radiation-induced optic neuropathy treated with ligustrazine combined with xingnaojing injection. Chinese Journal of Experimental Traditional Medical Formulae. 2013;19(5):296–299. [Google Scholar]
- 87.Jiang X. Y., Wang X. W., Shang X. Y., Wulia Y. M., Wang X. F. Protective effects of Cistanche glycosides on ultrastructure of sensitive organs of 60Co irradiated mice. Northwest Pharmaceutical Journal. 2001;16(2):66–67. [Google Scholar]
- 88.Wu G. H., Yu J., Zhou R. H., Lin Q., Wang J., Piao S. D. Effect of β-aescine sodium with mannito on radiation-induced brain edema. Journal of Practical Diagnosis and Therapy. 2008;22(2):110–111. [Google Scholar]
- 89.Li Y. R., Tan J., Wang J., Xu S. L., Guo J. Effect of radix hedysari capsule on 60Co 7 after radiation in lung and brain of rats, the serum SOD activity and MDA content. Shanxi University of Traditional Chinese Medicine. 2010;31(3):363–364. [Google Scholar]
- 90.Li Y. R., Tan J., Wang J., et al. The radiation-protective effect of radix hedysari capsules on mice. Pharmaceutical Journal of Chinese People’s Libration Army. 2010;26(5):400–402. [Google Scholar]
- 91.Xie Y. H., Guo J., Yang Q., Wang S. W., Li Y. R. The protective effect of hongqi capsule on low-dose radiation injured mice. Progress in Modern Biomedicine. 2015;15(18):3452–3457. [Google Scholar]
- 92.Gan L., Wang Z. H., Zhang H., Ma C. J., Li G. Protective effects of hydroxy safflower yellow A on radiation-induced brain injury induced by carbon ion beam irradiation. Nuclear Techniques. 2012;35(8):624–629. [Google Scholar]
- 93.Gan L., Wang Z. H., Zhang H., et al. Protective effects of shikonin on brain injury induced by carbon ion beam irradiation in mice. Biomedical and Environmental Sciences. 2015;28(2):148–151. doi: 10.3967/bes2015.019. [DOI] [PubMed] [Google Scholar]
- 94.Stackman R. W., Eckenstein F., Frei B., Kulhanek D., Nowlin J., Quinn J. F. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer's disease by chronic Ginkgo biloba treatment. Experimental Neurology. 2003;184(1):510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- 95.van Dongen M. C. J. M., van Rossum E., Kessels A. G. H., Sielhorst H. J. G., Knipschild P. G. The efficacy of ginkgo for elderly people with dementia and age-associated memory impairment: New results of a randomized clinical trial. Journal of the American Geriatrics Society. 2000;48(10):1183–1194. doi: 10.1111/j.1532-5415.2000.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 96.Ding Y. Q., Wang Y., Chen X. J. Effect of Ginkgo flavonoid on acute radiation-induced hippocampal injury in rats. Jiangsu Medical Journal. 2013;39(20):2379–2382. [Google Scholar]
- 97.Sun A. M., Li C. G., Lin S. M., Zhang Y. Q., Xia Q. The importance of regulatory role of panaxoside Rg1 in Cdk5 on hippocampal neuron radioactive damage protection. Journal of Chinese Physician. 2014;16(5):584–587. [Google Scholar]
- 98.Hu S., Peng R., Wang C., et al. Neuroprotective effects of dietary supplement Kang-fu-ling against high-power microwave through antioxidant action. Food & Function Journal. 2014;5(9):2243–2251. doi: 10.1039/C4FO00257A. [DOI] [PubMed] [Google Scholar]
- 99.Zhang J., Tong F., Cai Q., et al. Shenqi Fuzheng Injection attenuates irradiation-induced brain injury in mice via inhibition of the NF-κB signaling pathway and microglial activation. Acta Pharmacologica Sinica. 2015;36(11):1288–1299. doi: 10.1038/aps.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu G. Y., Cai W. M., Liang K. Preliminary study on prevention and treatment of radiation brain injury in acute stage by 978-1. Chinese Journal of Radiological Medicine and Protection. 2000;20(3):p. 163. [Google Scholar]
- 101.Zhu G. Y., Liang K., Cai W. M. Mechanism and effect of Chinese medicine destagnation and renal invigoration on radiation brain injury in mice. Chinese Journal of Radiation Oncology. 2001;10(1):38–41. [Google Scholar]
- 102.Yang B., Ren B. X., Tang F. R. Prenatal irradiation–induced brain neuropathology and cognitive impairment. Brain & Development. 2017;39(1):10–22. doi: 10.1016/j.braindev.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 103.Wang J. P., Zhang X. J., Yu X. W., Xing G. H., Sun Y. R., Rong W. Effects of Beta-asarone on hippocampal neurons of depression rat model. Journal of Guangzhou University of TCM. 2014;31(6):924–927. [Google Scholar]
- 104.Hu G. Z., Nie R. Q., Xiao Y. S., Zhang J., Wen Z., Wu D. F. Influence of polygonatum polysaccharide on apotosis of primary cultured neonate rat cerebral cortical neurons caused by hypoxia. Pharmacology and Clinics of Chinese Materia Medica. 2005;21(4):37–39. [Google Scholar]
- 105.Huang X. Y., Wang Y., Zhang X. M., Zhao J. C., Zhu X. F. Effects of polygalaceae ssponins on neural stem cell differentiation into neurons. Neural Injury and Functional Reconstruction. 2011;6(2):90–93. [Google Scholar]
- 106.Wang S. W., Ren B. X., Qian F., et al. Radioprotective effect of epimedium on neurogenesis and cognitionafter acute radiation exposure. Neuroscience Research. 2018;S0168-0102(18):30380–30388. doi: 10.1016/j.neures.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 107.Jin F., Chen C., Jin H., et al. Protective effects of icariin on spatial learning and memory in rats with AB25-35 induced Alzheimer's disease via inhibiting TNF-a, IL-6 and caspase-3 expression. Chinese Journal of New Drugs and Clinical Remedies. 2013;32(10):833–837. [Google Scholar]
- 108.Zhou W. Q., Deng J. Y., Bi M. G., Du G. H. Correlation between effect of icariin on learning and memory and level of estradiol in serum in senescence accelerated mouse prone 8. Chinese Journal of Experimental Traditional Medical Formulae. 2009;15(9):p. 341. [Google Scholar]
- 109.Zhang X. F. Toxic and side effects of Chinese medicine and preventive measures. Chinese Medicine Modern Distance Education of China. 2012;10(11):65–66. [Google Scholar]
- 110.Zhou M., Hong Y., Lin X., Shen L., Feng Y. Recent pharmaceutical evidence on the compatibility rationality of traditional Chinese medicine. Journal of Ethnopharmacology. 2017;206:363–375. doi: 10.1016/j.jep.2017.06.007. [DOI] [PubMed] [Google Scholar]