Abstract
In order to study the relationship between the structure and function of AmpG, structure, site-specific mutation, and gene complementary experiments have been performed against the clinical isolates of Pseudomonas aeruginosa. We found that there are 51 nucleotide variations at 34 loci over the ampG genes from 24 of 35 P. aeruginosa strains detected, of which 7 nucleotide variations resulted in amino acid change. The ampG variants with the changed nucleotides (amino acids) could complement the function of ampG deleted PA01 (PA01ΔG). The ampicillin minimum inhibitory concentration (MIC) of PA01ΔG complemented with 32 ampG variants was up to 512 μg/ml, similar to the original PA01 (P. aeruginosa PA01). Furthermore, site-directed mutation of two conservative amino acids (I53 and W90) showed that when I53 was mutated to 53S or 53T (I53S or I53T), the ampicillin MIC level dropped drastically, and the activity of AmpC β-lactamase decreased as well. By contrast, the ampicillin MIC and the activity of AmpC β-lactamase remained unchanged for W90R and W90S mutants. Our studies demonstrated that although nucleotide variations occurred in most of the ampG genes, the structure of AmpG protein in clinical isolates is stable, and conservative amino acid is necessary to maintain normal function of AmpG.
1. Introduction
Pseudomonas aeruginosa is a common opportunistic pathogen and often infects people with low immunity. P. aeruginosa infection leads to a high fatality rate in burn patients or those needing mechanically assisted ventilation. P. aeruginosa also plays an important role in chronic respiratory infection, especially for those with cystic fibrosis (CF) and some other chronic respiratory system infections [1]. With the abused use of antibiotics in clinics and agriculture, some strains of P. aeruginosa become resistant to most if not all antibiotics, causing serious consequences especially for patients in the intensive care unit (ICU) or with chronic respiratory diseases. The main mechanism of resistance is selective mutations of the chromosome leading to a high yield of the cephalosporin lytic enzyme AmpC. ampC is located on the chromosome, its expression inducible by β-lactams, and is found in most Enterobacteria, P. aeruginosa, and other nonfermentative Gram-negative bacilli [2]. β-lactamases are normally expressed in low levels, but can be induced by β-lactam antibiotics especially cefoxitin and imipenem. The only exception is Escherichia coli and Shigella due to the lack of ampR [3]. Therefore, the prolonged and wide use of β-lactam antibiotics can result in multiple β-lactam-resistant bacteria that produce high levels of β-lactamases, causing therapeutic failures [4, 5].
The induction of AmpC production is intimately linked to peptidoglycan recycling [6]. A number of genes including ampG, ampR, ampD, and ampE are involved in the process [7]. ampG encodes a transmembrane protein functioning as a specific permease to transport 1,6-GldNAc-anhydro-MurNAc and 1,6-GldNAc-anhydro-MurNAc peptide, which are the signal molecules involved in ampC expression [8]. ampR encodes a DNA-binding protein belonging to the LysR superfamily [9]. There are two regulatory characteristics of ampR: in the absence of a β-lactam inducer, it binds to the UDP-MurNAc pentapeptide to promote the formation of an AmpR-DNA complex that represses ampC transcription, while in the presence of a β-lactam antibiotic, peptidoglycan fragments accumulate in the cytoplasm [10], and the 1,6-anhydro-MurNAc tripeptide (or pentapeptide) competitively displaces the UDP-MurNAc pentapeptide and converts AmpR into an activator, triggering the ampC expression or production of β-lactamase [9]. ampD encodes a cytosolic N-acetyl-anhydromuramyl-L-alanine amidase and specifically hydrolyzes the 1,6-anhydro-MurNAc peptide, thus inhibiting the ampC expression [11, 12]. ampE encodes a cytoplasmic membrane protein acting as a sensory transducer molecule required for ampC induction [13], but the exact role of AmpE is not fully understood. It has been shown that there are two ampG homologues in P. aeruginosa, ampG (PA4393) and ampGh1 (PA4218), and only the product of ampG is a functional protein; the inactivation of ampG by mutation or deletion confers noninducible and low-level β-lactamase expression [14, 15]. Herein, we studied the relationship between the structure and function of ampG in wild type P. aeruginosa with different resistance levels against ampicillin. Our findings may provide more theoretical basis for identifying molecular features of AmpG and help design methods to screen candidate agents to inhibit the function of AmpG, thus prolonging the use of commonly used β-lactams in clinics.
2. Materials and Methods
2.1. Bacterial Strain and Plasmid
Two hundred and eleven (211) nonduplicate wild strains of P. aeruginosa were isolated from the clinical samples at the First Affiliated Hospital of Wenzhou Medial University, China, between 2009 and 2011. The strains were identified by the Vitek-60 microorganism autoanalysis system (BioMerieux Corporate, Lyon, France). P. aeruginosa PA01 and plasmids pUCP24 [14]were obtained from the Laboratory of Microbial Genetics, University of Florida, Gainesville, USA. The strains and plasmids used or constructed in this work are listed in Table 1.
Table 1.
Bacterial strains and plasmids.
| Strains or plasmids | Relevant characteristic (s) |
|---|---|
| Strains | |
| E. coli JM109 | endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 (argF-lacZYA) U169 80dlacZ |
| PA01 | Reference strain; genome completely sequenced |
| PA01ΔampG | ampG (PA4393) deleted PA0114 |
|
| |
| Plasmids | |
| pMD18-ampG1-35 | pMD18 vectors carrying ampGs of 35 wild type P. aeruginosa strains, respectively (this work) |
| pMD18-ampGPA | pMD18 vector carrying ampG of PA01 (this work) |
| pUCP24 | pUC18-derived broad-host-range vector; Gmr |
| pUCP24-ampG1-35 | ampG genes from pMD18-ampG1-35 cloned into pUCP24, respectively; Gmr (this work) |
| pUCP24-ampGPA | ampG gene of pMD18-ampGPA cloned into pUCP24; Gmr (this work) |
| pUCP24-ampGmut | pUCP24 vector carrying site-directedly mutated ampG of PA01 (this work) |
| pUCP24-ampGPA-I53S | |
| pUCP24-ampGPA-I53T | |
| pUCP24-ampGPA-W90R | |
| pUCP24-ampGPA-W90S | |
2.2. Antibiotic Susceptibility Test
Minimal inhibitory concentrations (MICs) to the antimicrobial agents were determined by the agar dilution method for the control and recombinant strains in accordance with the guidelines of the Clinical and Laboratory Standards Institute (CLSI). Antimicrobial agents were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (NICPBP) and the pharmaceutical companies in China. E. coli ATCC 25922 was used as a quality control for the MIC test.
2.3. Cloning and Comparative Analysis of the ampG Genes
Genomic DNA was extracted from the strains using an AxyPrep Bacterial Genomic DNA Miniprep kit (Axygen Scientific, Union City, CA, USA) and PCR- (polymerase chain reaction-) amplified to clone the ampG genes. The primers were designed by using Primer 5.0, and a pair of flanking restriction endonuclease adapters were added at the 5′ end of the primers (BamHI for the forward primer PAampG-F and HindIII for the reverse primer PAampG-R, respectively, Table 2) and synthesized by Shanghai Sunny Biotechnology Co., Ltd (Shanghai, China). PCR amplification was carried out under the following conditions, i.e., an initial 5 min denaturation at 95°C, followed by 35 cycles of denaturation (94°C for 45 s), annealing (65°C for 45 s), and extension (72°C for 90 s), and a final extension step at 72°C for 10 min. The PCR products of ampGs were first cloned into a pMD18-T vector. The recombinant plasmid (pMD18-ampG) was identified initially by PCR and then verified by sequencing. Blast programs from NCBI (http://www.ncbi.nlm.nih.gov/BLAST) and MEGA5.05 (molecular evolutionary genetics analysis software, http://www.megasoftware.net) were used to analyze the similarities of the ampG nucleotide sequences and the AmpG amino acid sequences.
Table 2.
Primers used in this work.
| Primer | Sequence∗ | Purpose |
|---|---|---|
| PAampG-F | 5′GGGATCCCAACGCGCACGCTTGCGCGAGGA 3′(BamHI) | Cloning of ampG of PA01 |
| PAampG-R | 5′GAAGCTTTCAGTGCTGCTCGGCGTTCTGGT3′(HindIII) | |
| PAampG-I53S | 5′CCCAGCCAACTGGCGAAACCgctGGTATCCCGCGCCACGC3′ | Forward primer for I53S |
| PAampG-I53T | 5′CCCAGCCAACTGGCGAAACCggtGGTATCCCGCGCCACGC3′ | Forward primer for I53T |
| PAampG-53R | 5′GGTTTCGCCAGTTGGCTGGGGCTGGTGTACGCCTTCAAGT3′ | |
| PAampG-W90R | 5′AGCACCTGCGAGAACACCAGccgGGAACGGCGCCGGCCGA3′ | Forward primer for W90R |
| PAampG-W90S | 5′AGCACCTGCGAGAACACCAGcgaGGAACGGCGCCGGCCGA3′ | Forward primer for W90S |
| PAampG-90R | 5′CTGGTGTTCTCGCAGGTGCTGATCGCCCTGGGACTGCTCG3′ |
∗Underlined are restriction endonuclease sites; nucleotide sequence corresponding to the mutated amino acids is shown in lowercase.
2.3. Genetic Complementation Assays to Determine the Function of the Cloned ampG Genes
The verified recombinant pMD18-ampG plasmid was digested with BamHI and HindIII restriction enzymes. The ampG fragment was recovered and then ligated into a pUCP24 vector digested with the same restriction enzymes (BamHI and HindIII). The recombinant plasmid pUCP24-ampG was transformed into E. coli JM109, and the recombinant E. coli JM109-pUCP24-ampG was further identified by PCR. pUCP24-ampG was extracted and introduced into PA01∆ampG as described previously [16]. MIC to ampicillin was performed for the recombinant PA01∆ampG-pUCP24-ampG to detect the function of the cloned ampGs. PA01∆ampG carrying the vector pUCP24 was used as a negative control.
2.4. Site-Directed Mutation of the Conservative Amino Acids in the ampG Gene
We used the ampG gene sequence of PA01 as the template to design primers for amplification of the 5′ and 3′ end fragments of the ampG gene, respectively. The forward primer for amplification of the 5′ end fragment (PAampG-F with a BamHI recognition site at the 5′ end) and the reverse primer for amplification of the 3′ end fragment (PAampG-R with a HindIII recognition site at the 5′ end) were the same as described above (Table 2). The corresponding nucleotide mutations were inserted into the downstream primers of 5′ end and the upstream primer of 3′ end fragments of the ampG gene, and then the mixture of the PCR products of the 5′ end and the 3′ end fragments (mole ratio of 1:1) was used as the template, and the upstream primer of the 5′ end and the downstream primer of the 3′ end were used as the primers for amplification of ampG variants with the point mutations. Different mutations in the primers are shown in Table 2. PCRs were performed under the following conditions: an initial 5 min denaturation at 95°C followed by 35 amplification cycles, each consisting of a 40 sec denaturation step at 95°C, a 30 sec annealing step at 55°C, and a 40 sec extension step at 72°C followed by a 10 min final extension at 72°C (ExTaq from TaKaRa, Dalian, China). The PCR products (ampGmut) were purified and inserted into pMD18-T vectors. The recombinant plasmid pMD18-ampGmut was initially identified by PCR and then verified by sequencing. The verified pMD18-ampGmut recombinant plasmids were digested with BamHI and HindIII. The ampGmut fragments were recovered and ligated into the pUCP24 vectors digested with the same restriction enzymes (BamHI and HindIII). The recombinant pUCP24-ampGmut was then transformed into PA01∆ampG to detect the complementary effect of ampicillin resistance in the host.
2.5. Detection of β-Lactamase Activity
The detection procedures were performed as described in [14]. P. aeruginosa cells were induced for 1 h with 4 μg/ml cefoxitin (Calbiochem, San Diego, USA) and for 2 h with 50 μg/ml cefoxitin, respectively. Crude cell extracts were prepared by sonication, and the protein content of crude extracts was determined using BCA protein assay reagent (Pierce, USA) [17]. The β-lactamase activity was quantified with an UV spectrophotometer using 100 µM of nitrocefin as the substrate. The activity of the β-lactamase was defined as nanomole of nitrocefin hydrolyzed at 30°C per min by 1 g protein. All the induction experiments were performed in triplicate, and the result represents an average of the three replicates.
3. Results
3.1. Ampicillin MIC Detection of the P. aeruginosa Strains
The MIC to ampicillin for 211 strains of P. aeruginosa showed that most of the strains had a high resistance level. Only 2.84 % (6/211) strain had a low MIC level (≤128 μg/ml), and more than a half of them (61.61 %, 130/211) showed a very high MIC level of ≥2048 μg/ml (Figure 1).
Figure 1.
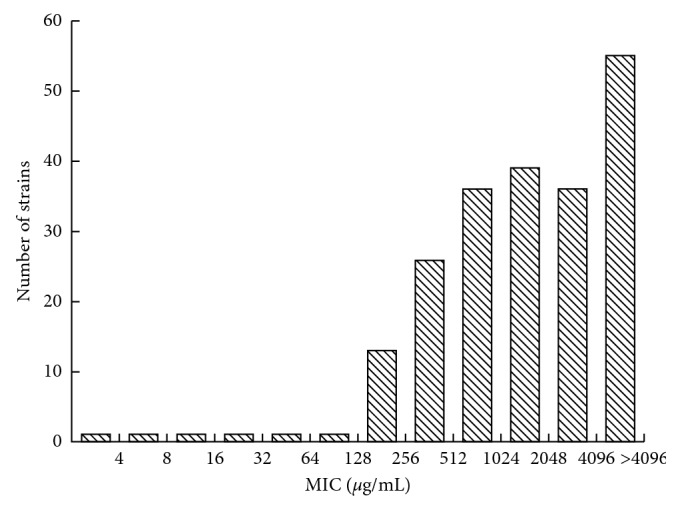
The MIC to ampicillin for 211 strains of P. aeruginosa had a high resistance level.
3.2. Sequence Variations of the ampG Genes among the Strains with Different MIC Levels
To compare the ampG gene structure of P. aeruginosa strains with different ampicillin MIC levels, we cloned and sequenced the ampG gene of 35 strains with high MIC levels (512 to −≥8192 μg/ml). Compared with the ampG gene of PA01, a total of 51 nucleotide variations over 34 loci in 24 ampG genes were identified. Among these nucleotide variations, seven led to amino acid changes (Table 3). Three amino acids were located in the transmembrane regions, but none of them corresponded to the 51 conserved amino acids described in a recent report [18].
Table 3.
Sequence variation and function of ampGs in clinical P. aeruginosa strains.
| Nucleotide | Amino acid | MIC∗∗ | |||
|---|---|---|---|---|---|
| Position | Variation | Position | Variation | (μg/ml) | |
| PA01ΔampG-pUCP24-ampG1 | 618 | C-A | 206 | / | 512/512 |
| PA01ΔampG-pUCP24-ampG2 | 715 | C-T | 239 | / | 512/1024 |
| 1080 | G-C | 360 | / | ||
| 1317 | C-T | 439 | / | ||
| PA01ΔampG-pUCP24-ampG3 | 715 | C-T | 239 | / | 512/1024 |
| 1080 | G-C | 360 | / | ||
| 1317 | C-T | 439 | / | ||
| PA01ΔampG-pUCP24-ampG4 | 715 | C-T | 239 | / | 512/1024 |
| 1080 | G-C | 360 | / | ||
| 1317 | C-T | 439 | / | ||
| PA01ΔampG-pUCP24-ampG7 | 618 | C-A | 206 | / | 512/512 |
| 916 | G-C | 306 | D-H | ||
| PA01ΔampG-pUCP24-ampG8 | 27 | C-A | 9 | / | 512/1024 |
| PA01ΔampG-pUCP24-ampG9 | 27 | C-A | 9 | / | 512/512 |
| PA01ΔampG-pUCP24-ampG10 | 1329 | G-A | 443 | / | 512/1024 |
| PA01ΔampG-pUCP24-ampG11 | 666 | C-T | 222 | / | 256/512 |
| 819 | G-A | 273 | / | ||
| 942 | G-A | 314 | / | ||
| 1062 | A-G | 354 | / | ||
| 1077 | C-A | 539 | / | ||
| 1113 | C-T | 371 | / | ||
| PA01ΔampG-pUCP24-ampG12 | 618 | C-A | 206 | / | 512/1024 |
| PA01ΔampG-pUCP24-ampG13 | 1423 | G-A | 475 | D-N | 512/1024 |
| PA01ΔampG-pUCP24-ampG14 | 1423 | G-A | 475 | D-N | 512/1024 |
| PA01ΔampG-pUCP24-ampG15 | 336 | C-T | 112 | / | 512/4096 |
| PA01ΔampG-pUCP24-ampG16 | 1747 | G-A | 583 | / | 512/1024 |
| PA01ΔampG-pUCP24-ampG17 | 153 | T-C | 51 | / | 512/≥8192 |
| PA01ΔampG-pUCP24-ampG20 | 213 | G-A | 71 | / | 256/4096 |
| PA01ΔampG-pUCP24-ampG24 | 450 | C-T | 150 | / | 1024/4096 |
| PA01ΔampG-pUCP24-ampG25 | 336 | C-T | 112 | / | 1024/≥8192 |
| 1027 | C-T | 343 | / | ||
| PA01ΔampG-pUCP24-ampG26 | 1182 | G-A | 394 | / | 512/4096 |
| PA01ΔampG-pUCP24-ampG28 | 657 | C-T | 219 | / | 512/≥8192 |
| 729 | C-T | 243 | / | ||
| 837 | C-T | 279 | / | ||
| 990 | C-T | 330 | / | ||
| 1062 | A-G | 354 | / | ||
| 1239 | T-C | 443 | / | ||
| 1316 | T-C | 439 | I-T(11)∗ | ||
| PA01ΔampG-pUCP24-ampG29 | 1143 | C-T | 381 | / | 512/≥8192 |
| PA01ΔampG-pUCP24-ampG30 | 27 | C-A | 9 | / | 512/≥8192 |
| 1329 | G-A | 443 | / | ||
| PA01ΔampG-pUCP24-ampG31 | 27 | C-T | 9 | / | 256/4096 |
| 558 | G-A | 186 | / | ||
| 1002 | C-T | 334 | / | ||
| 1062 | A-G | 354 | / | ||
| 1113 | C-T | 371 | / | ||
| PA01ΔampG-pUCP24-ampG35 | 617 | C-T | 206 | T-I (6)∗ | 512/≥8192 |
| 708 | T-A | 236 | / | ||
| 1193 | G-A | 398 | G-E (9)∗ | ||
| 1364 | T-C | 455 | L-P | ||
| PA01ΔampG-pUCP24-ampGPA | / | / | / | / | 512 |
| PA01ΔampG | / | / | / | / | 32 |
| PA01 | / | / | / | / | 512 |
| ATCC 25922 | / | / | / | / | 4 |
D: aspartic acid; H: histidine; N: asparagine; I: isoleucine; T: threonine; G: glycine; E: glutamate; L: leucine; P: proline; ∗transmembrane region; ∗∗MIC values of the cloned ORF/wild strain.
3.3. Function Analysis of ampG Variants from Clinically Isolated P. aeruginosa Strains
In order to detect the function of ampGs, the ampG genes from 24 strains with sequence variation were further cloned into pUCP24 and transformed into the PA01ΔampG to perform genetic complementation analysis. All the cloned ampG genes complemented the function of the deleted ampG gene in PA01ΔampG. The MIC levels for ampicillin were between 256 and 1024 μg/ml, close to the level of PA01 or PA01ΔampG-pUCP24-ampGPA (512 μg/ml). The AmpC β-lactamase activity of the genetically complemented recombinants also showed similar results (Table 3). These data indicate that the cloned ampGs have similar function despite their structural differences.
3.4. Effect of the Mutations of the Conservative Amino Acids on AmpG Function
In order to analyze the correlation of the conservative amino acids with the function of AmpG, 2 conservative amino acids I53 and W90 located in the transmembrane regions 2 and 3, respectively, were randomly chosen for site-directed mutagenesis. According to the chemical properties of the amino acids, 4 to 6 different amino acid substitutions were designed for each conserved amino acid. Finally, a total of 4 mutated genes were successfully cloned; these mutants were transformed into the recipient cell PA01ΔampG; their MIC levels to ampicillin and activities of AmpC type β-lactamase were tested (Table 4). PA01 Δ ampG-pUCP24-ampGPA-W90R and PA01 Δ ampG-pUCP24-ampGPA-W90S had high resistance level to ampicillin, similar to that of the original P. aeruginosa PA01, but significantly different from that of the I53S and I53T mutants that had much lower resistance level to ampicillin and showed significantly lower AmpC type β-lactamase activities (Table 4).
Table 4.
MIC levels to ampicillin and AmpC β-lactamase activity of the site-directed mutants.
| Strain | Amino acid variation | β-Lactamase activity | MIC (μg/ml) |
|---|---|---|---|
| PA01ΔampG-pUCP24-ampGPA-I53S | Ile (atc)-Ser (agc) | 69.8 | 32 |
| PA01ΔampG-pUCP24-ampGPA-I53T | Ile (atc)-Thr (acc) | 56.7 | 16 |
| PA01ΔampG-pUCP24-ampGPA-W90R | Try (tgg)-Arg (cgg) | 25296.4 | 512 |
| PA01ΔampG-pUCP24-ampGPA-WA90S | Try (tgg)-Ser (tcg) | 12628.9 | 512 |
| PA01 | / | 1981.6 | 512 |
| PA01ΔampG | / | 44.8 | 32 |
| PA01ΔampG-pUCP24-ampGPA | / | 5019.3 | 1024 |
| ATCC 25922 | / | 0 | 4 |
4. Discussion
The clinical isolates of P. aeruginosa often show resistance to most antibiotics [19, 20]. The mechanism of multiple resistance is mainly related to inactivated enzyme production, overexpression of efflux systems, antibiotic target alteration, biofilm formation, and acquisition of foreign drug-resistant genes by means of horizontal gene transfer [21–23]. The inactivating enzymes to β-lactam antibiotics produced by P. aeruginosa were AmpC, the extended spectrum β-lactamase (ESBLs), and the metalloenzyme (MBL), of which AmpC has attracted more attention because its production can be induced [24]. Besides being encoded in P. aeruginosa, ampC extensively exists in the chromosomes or plasmids of Gram-negative bacillus such as Enterobacter, Citrobacter, and Acinetobacter [25, 26]. The muropeptide derivatives are essential for the induction of AmpC type β-lactamase expression [27]. Schmidt et al. [28] used nitrosoguanidine (NTG) to induce mutations in E. coli SN0301 (carrying ampC and ampR of E. cloacae) and obtained three mutants with mutated ampG genes including ampG1 (G151D), ampG3 (G268D), and ampG5 (G373D), respectively. They all belong to the conserved amino acids located on the transmembrane regions 5 (158G), 10 (419G), and 13 (544G), respectively, in AmpG of P. aeruginosa PA01 [18]. In this work, we also performed site-directed mutation for two conserved amino acids (I53 and W59). As a result, the AmpC activity of the mutated AmpGs (I53S or I53T) located in the transmembrane regions 2 was drastically lowered. Our findings suggest that the conserved amino acids play an important role in keeping the normal function of AmpG. A single amino acid might be enough to determine the substrate specificity and the transportation activity of AmpG. The length of the AmpG proteins from different species differs from each other and certainly the transmembrane regions of the AmpGs also vary [29, 30]. A previous work from our team showed that the longest AmpG protein was from P. aeruginosa (CDR92618), which consists of 598 amino acids and is predicted to have 14 transmembrane regions. The shortest was from Microcoleus with only 401 AA and 11 transmembrane regions. Most of the AmpG proteins contain 12 or 14 transmembrane regions. Analysis of the conservative amino acid against 134 AmpGs showed that there were 51 conserved amino acids identified in 12 transmembrane regions excluding the corresponding transmembrane regions 7 and 8 of PA01. In P. aeruginosa PA01, transmembrane regions 1, 2, and 4 contained more conserved amino acids (with 8, 7, and 9 conserved amino acids, respectively), and transmembrane regions 6, 11, 12, and 14 contained only 1 or 2 conserved amino acids. In this work, we sequenced 35 ampG genes from clinically isolated P. aeruginosa with different MIC levels to ampicillin (512 to ≥8192 μg/ml) and found 24 genes harboring 51 nucleotide variations over 34 loci. Among them, only 6 loci in 5 bacterial strains showed amino acid variations. Four strains had one amino acid difference, and one strain had 3 mutated amino acids. Amino acid change in PA01ΔampG-pUCP24-ampG28 (I439T) was located in transmembrane 11, and two of the three amino acid changes in PA01ΔampG-pUCP24-ampG35 (T206I and G398E) were located in transmembrane regions 6 and 9, respectively. They did not belong to the 51 conserved amino acids predicted in our previous work [18]. The MIC levels for ampicillin indicated that all of these 24 genes had normal functions, suggesting that these variations both in nucleotide and amino acid sequences did not affect the function of AmpGs.
In the recent years, the clinically isolated pathogens became more and more resistant to most antibiotics. Studies on the polymorphism and relationship between the structure and function of AmpGs will help to establish a theoretical basis for the development of inhibitors against AmpG. Effective inhibitors for AmpG will shed light on prolonging the clinical use of β-lactam antibiotics.
Acknowledgments
This study was supported by the Natural Science Foundation of Zhejiang Province, China (LY14C060005 and LQ17H190001), the Science and Technology Foundation of National Health and Family Planning Commission of China (WKJ2012-2-032), and the National Natural Science Foundation of China (81401702, 81501808, 80215049, and 31500109).
Abbreviations
- ATCC:
American Type Culture Collection
- BLAST:
Basic local alignment search tool
- CF:
Cystic fibrosis
- CLSI:
Clinical and Laboratory Standards Institute
- ESBL:
Extended spectrum β-lactamase
- ICU:
Intensive care unit
- MIC:
Minimum inhibitory concentration
- NICPBP:
National Institute for the Control of Pharmaceutical and Biological Products
- ORF:
Open reading frame
- P. aeruginosa:
Pseudomonas aeruginosa
- PCR:
Polymerase chain reaction.
Contributor Information
Shunfei Lu, Email: lslsf@163.com.
Jinsong Li, Email: strongli72@sina.com.
Data Availability
The sequence variation data used to support the findings of this study are included within Table 3 in this article. The MIC levels data used to support the findings of this study are included within Table 4 in this article. Previously reported research data were used to support this study and are available at doi:10.1371/journal.pone.0168060, and these prior studies (and datasets) are cited at relevant places within the text as references [16].
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Qingli Chang and Chongyang Wu contributed equally to this work.
References
- 1.Bodnar R., Meszaros A., Olah M., Agh T., et al. Inhaled antibiotics for the treatment of chronic Pseudomonas aeruginosa infection in cystic fibrosis patients: challenges to treatment adherence and strategies to improve outcomes. Patient Preference and Adherence. 2016;10:183–193. doi: 10.2147/ppa.s53653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xia J., Gao J., Tang W. Nosocomial infection and its molecular mechanisms of antibiotic resistance. BioScience Trends. 2016;10(1):14–21. doi: 10.5582/bst.2016.01020. [DOI] [PubMed] [Google Scholar]
- 3.Normark S. beta-Lactamase induction in gram-negative bacteria is intimately linked to peptidoglycan recycling. Microbial Drug Resistance. 1995;1(2):111–114. doi: 10.1089/mdr.1995.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Meletis G., Chatzidimitriou D., Malisiovas N. Double- and multi-carbapenemase-producers: the excessively armored bacilli of the current decade. European Journal of Clinical Microbiology and Infectious Diseases. 2015;34(8):1487–1493. doi: 10.1007/s10096-015-2379-9. [DOI] [PubMed] [Google Scholar]
- 5.Tang S. S., Apisarnthanarak A., Hsu L. Y. Mechanisms of beta-lactam antimicrobial resistance and epidemiology of major community- and healthcare-associated multidrug-resistant bacteria. Advanced Drug Delivery Reviews. 2014;78(3):3–13. doi: 10.1016/j.addr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Kong K. F., Aguila A., Schneper L., Mathee K., et al. Pseudomonas aeruginosa beta-lactamase induction requires two permeases, AmpG and AmpP. BMC Microbiology. 2010;10(1):p. 328. doi: 10.1186/1471-2180-10-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luan Y., Li G.-L., Duo L.-B., et al. DHA-1 plasmid-mediated AmpC beta-lactamase expression and regulation of Klebsiella pnuemoniae isolates. Molecular Medicine Reports. 2015;11(4):3069–3077. doi: 10.3892/mmr.2014.3054. [DOI] [PubMed] [Google Scholar]
- 8.Fisher J. F., Mobashery S. The sentinel role of peptidoglycan recycling in the beta-lactam resistance of the Gram-negative Enterobacteriaceae and Pseudomonas aeruginosa. Bioorganic Chemistry. 2014;56:41–48. doi: 10.1016/j.bioorg.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caille O., Zincke D., Merighi M., et al. Structural and functional characterization of Pseudomonas aeruginosa global regulator AmpR. Journal of Bacteriology. 2014;196(22):3890–3902. doi: 10.1128/jb.01997-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vadlamani G., Thomas M. D., Patel T. R., et al. The beta-lactamase gene regulator AmpR is a tetramer that recognizes and binds the D-Ala-D-Ala motif of its repressor UDP-N-acetylmuramic acid (MurNAc)-pentapeptide. Journal of Biological Chemistry. 2015;290(5):2630–2643. doi: 10.1074/jbc.m114.618199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang J., Kim H. S. Cell wall recycling-linked coregulation of AmpC and PenB beta-lactamases through ampD mutations in Burkholderia cenocepacia. Antimicrobial Agents and Chemotherapy. 2015;59(12):7602–7610. doi: 10.1128/aac.01068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Q., Park J. T. Substrate specificity of the AmpG permease required for recycling of cell wall anhydro-muropeptides. Journal of Bacteriology. 2002;184(23):6434–6436. doi: 10.1128/jb.184.23.6434-6436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindquist S., Galleni M., Lindberg F., Normark S. Signalling proteins in enterobacterial AmpC beta-lactamase regulation. Molecular Microbiology. 1989;3(8):1091–1102. doi: 10.1111/j.1365-2958.1989.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Bao Q., Gagnon L. A., et al. ampG gene of Pseudomonas aeruginosa and its role in beta-lactamase expression. Antimicrobial Agents and Chemotherapy. 2010;54(11):4772–4779. doi: 10.1128/aac.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamorano L., Reeve T. M., Juan C., et al. AmpG inactivation restores susceptibility of pan-beta-lactam-resistant Pseudomonas aeruginosa clinical strains. Antimicrobial Agents and Chemotherapy. 2011;55(5):1990–1996. doi: 10.1128/aac.01688-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nigro G., Fazio L. L., Martino M. C., et al. Muramylpeptide shedding modulates cell sensing of Shigella flexneri. Cellular Microbiology. 2008;10(3):682–695. doi: 10.1111/j.1462-5822.2007.01075.x. [DOI] [PubMed] [Google Scholar]
- 17.Trepanier S., Prince A., Huletsky A. Characterization of the penA and penR genes of Burkholderia cepacia 249 which encode the chromosomal class A penicillinase and its LysR-type transcriptional regulator. Antimicrobial Agents and Chemotherapy. 1997;41(11):2399–2405. doi: 10.1128/aac.41.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biedenbach D. J., Giao P. T., Van P. H., et al. Antimicrobial-resistant Pseudomonas aeruginosa and Acinetobacter baumannii from patients with hospital-acquired or ventilator-associated pneumonia in Vietnam. Clinical Therapeutics. 2016;38(9):2098–2105. doi: 10.1016/j.clinthera.2016.07.172. [DOI] [PubMed] [Google Scholar]
- 19.Ding C., Yang Z., Wang J., et al. Prevalence of Pseudomonas aeruginosa and antimicrobial-resistant Pseudomonas aeruginosa in patients with pneumonia in mainland China: a systematic review and meta-analysis. International Journal of Infectious Diseases. 2016;49:119–128. doi: 10.1016/j.ijid.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Holmes A. H., Moore L. S. P., Sundsfjord A., et al. Understanding the mechanisms and drivers of antimicrobial resistance. The Lancet. 2016;387(10014):176–187. doi: 10.1016/s0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 21.Sharma V. K., Johnson N., Cizmas L., McDonald T. J., Kim H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere. 2016;150:702–714. doi: 10.1016/j.chemosphere.2015.12.084. [DOI] [PubMed] [Google Scholar]
- 22.Sun J., Deng Z., Yan A. Bacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitations. Biochemical and Biophysical Research Communications. 2014;453(2):254–267. doi: 10.1016/j.bbrc.2014.05.090. [DOI] [PubMed] [Google Scholar]
- 23.Zeng X., Lin J. Beta-lactamase induction and cell wall metabolism in Gram-negative bacteria. Frontiers in Microbiology. 2013;4:p. 128. doi: 10.3389/fmicb.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luk S., Wong W.-K., Ho A. Y.-M., Yu K. C.-H., To W.-K., Ng T.-K. Clinical features and molecular epidemiology of plasmid-mediated DHA-type AmpC beta-lactamase-producing Klebsiella pneumoniae blood culture isolates, Hong Kong. Journal of Global Antimicrobial Resistance. 2016;7:37–42. doi: 10.1016/j.jgar.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Patel M. H., Trivedi G. R., Patel S. M., Vegad M. M. Antibiotic susceptibility pattern in urinary isolates of gram negative bacilli with special reference to AmpC beta-lactamase in a tertiary care hospital. Urology Annals. 2010;2(1):7–11. doi: 10.4103/0974-7796.62915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanson N. D., Sanders C. C. Regulation of inducible AmpC beta-lactamase expression among Enterobacteriaceae. Current Pharmaceutical Design. 1999;5(11):881–894. [PubMed] [Google Scholar]
- 27.Schmidt H., Korfmann G., Barth H., Martin H. H. The signal transducer encoded by ampG is essential for induction of chromosomal AmpC beta-lactamase in Escherichia coli by beta-lactam antibiotics and ’unspecific’ inducers. Microbiology. 1995;141(5):1085–1092. doi: 10.1099/13500872-141-5-1085. [DOI] [PubMed] [Google Scholar]
- 28.Li P., Ying J., Yang G., et al. Structure-function analysis of the transmembrane protein AmpG from Pseudomonas aeruginosa. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0168060.e0168060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chahboune A., Decaffmeyer M., Brasseur R., Joris B. Membrane topology of the Escherichia coli AmpG permease required for recycling of cell wall anhydromuropeptides and AmpC beta-lactamase induction. Antimicrobial Agents and Chemotherapy. 2005;49(3):1145–1149. doi: 10.1128/aac.49.3.1145-1149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenthal R. S., Nogami W., Cookson B. T., Goldman W. E., Folkening W. J. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infection and Immunity. 1987;55(9):2117–2120. doi: 10.1128/iai.55.9.2117-2120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequence variation data used to support the findings of this study are included within Table 3 in this article. The MIC levels data used to support the findings of this study are included within Table 4 in this article. Previously reported research data were used to support this study and are available at doi:10.1371/journal.pone.0168060, and these prior studies (and datasets) are cited at relevant places within the text as references [16].


