Summary
One and key of the priorities in laboratory medicine is improvement of quality management system for patient safety. Quality in the health care is tightly connected to the level of excellence of the health care provided in relation to the current level of knowledge and technical development. Accreditation is an effective way to demonstrate competence of the laboratory, a tool to recognize laboratories world-wide, is linked to periodical audits, to stimulate the maintenance and improvement of the quality, which leads to high standard of services for clients (patients, health care providers, etc.). The strategic plans of IFCC and EFLM include focusing on accreditation of labs based on ISO standards and cooperation with European Accreditation and national accreditation bodies. IFCC and EFLM recognised that ISO 15189:2012 Medical laboratories – Requirements for quality and competence, encompasses all the assessment criteria specified in the policy of quality. The last version is oriented to process approach with detailed division and clearly defined requirements. The accreditation of labs improves facilitation of accurate and rapid diagnostics, efficiency of treatment and reduction of errors in the laboratory process. Accreditation is not about who the best is, but who has a system of standard procedures with aim to improve the quality and patient safety. Quality system is about people, with people and for people.
Keywords: Accreditation, ISO 15189, Quality system
Kratak sadržaj
Prvi i ključni prioritet u laboratorijskoj medicinu je poboljšanje kvaliteta sistema menadžmenta radi sigurnosti pacijenata. Kvalitet u zdravstvenom sistemu je čvrsto povezan sa nivoom uspešnosti zdravstvene brige obezbeđene sa trenutnim razvojem znanja i tehničkih mogućnosti. Akreditacija je efikasan način da ukaže na kompetentnost laboratorije i prepoznavanje laboratorija u širim razmerama, i periodičnim preispitivanjima koja utiču na stalno poboljšanje kvaliteta, omogućavajući visok standard usluga svojim klijentima (pacijentima i pružaocima zdravstvenih usluga, itd.) Strateški planovi IFCC i EFLM su fokusirani na akreditaciju laboratorija na osnovu ISO standarda i saradnju sa evropskim i nacionalnim akreditacionim telima. IFCC i EFLM su prepoznali da ISO 15189:2012 Medical laboratories-Requirement for quality and competence obuhvata sve kriterijume specifične za politiku kvaliteta u medicinskim laboratorijama. Poslednja verzija je usmerena na procese i jasno definiše sve neophodne zahteve. Akreditacija laboratorija poboljšava tačnu i brzu dijagnostiku, efiksanost tretmana i umanjenje grešaka u laboratorijskom procesu. Akreditacija nije ko je najbolji, već ko ima sistem standardnih procedura čiji je cilj da poboljša kvalitet i sigurnost pacijenta. Sistem kvaliteta je namenjen ljudima, sa ljudima i za ljude.
Ključne reči: akreditacija, ISO 15189, sistem kvaliteta
Introduction
Today s patient focused medicine is oriented on the prevention, early diagnosis and tailored therapy. There are some defined priorities in medicine and bioscience as evidence-based medicine, target therapy, using the stem cells, nanotechnology, biotechnology, and improvement of quality. The laboratory medicine has defined priorities such a s laboratory automation, consolidation of laboratories, integrated diagnostics – organisation and IT, molecular diagnostics (NGS, DNA microarrays, chips, etc.), imaging analysis, POCT and accreditation of laboratories aiming to improve the quality of patient care. The three pillars for laboratories presented by Beastall (1) are: 1) Quality – analytical quality, quality assurance and accreditation, 2) Clinical effectiveness – clinical outcomes, patient focus, timeliness, and 3) Cost effectiveness – total cost, value for money, appropriate use (1).
The health care sector is regulated by different factors – prices of health care services, market structure, capacity, public opinion, advertisement and of course, the quality. Quality in the health care is the level of excellence of the health care provided in relation to the current level of knowledge and technical development with customer orientation and patient centred (2). One and key of the priorities in laboratory medicine is improvement of quality management system for patient safety. Laboratory attempts to improve quality aim to reduce diagnostic errors and decrease turnaround time with traceability of all laboratory procedures. The accreditation body must be legally identifiable, impartial, and independent of external influences (3).
What are the differences between accreditation and certification?
Certification is procedure by which a third party gives written assurance that a product, process or service conforms to specific requirements.
Each country has multiple certification bodies
Example of certification bodies: AENOR, AFNOR, BVQI, CERMET, IQNet, TüV, …
Requirements for a quality management system (only)
ISO 9001
Accreditation is procedure by which an authoritative body gives formal recognition that a body or person is competent to carry out specific tasks.
There is only one recognized national accreditation body in each country
Examples of accreditation body: COFRAC, UKAS, CIA….
Requirements for a quality management system + requirements regarding technical & analytical competence
ISO 17025 and ISO 15189
The history of quality systems in labs started many decades ago. The first steps are implementation of internal (IQA) and external quality control (EQA) systems and their basic principles in daily laboratory practice.
An important set of criteria was done in EN 45 001 (European Standard), specifying general criteria for the operation of a testing laboratory. Next step for accreditation was documented in ISO 17025 (General Requirements for the Competence of Testing and Calibration Laboratories) (4). This standard is widely used for testing laboratories in whole world in industry and also in medicine. The ISO 17025:2005 is the basis for the accreditation. This standard requires a management system and how the laboratory be found competent to perform specific tests/calibrations or types of tests/calibration.
Other national or international standards as Joint Commission International (JCI), SLIPTA –WHO, Clinical Pathology Accreditation (CPA) in UK, College of American Pathologists (CAP) in USA, CCKL Code of Practice in the Netherlands or standards, based on the International Society for Quality in Healthcare (ISQUa) are also used in some countries for accreditation.
The strategic plans of EFLM and IFCC are focusing on lab accreditation based on ISO standards with cooperation of European Accreditation and national accreditation bodies. EFLM and IFCC recognises that ISO 15189 is precisely describing standard for labs. The first issue of standard was in 2003, next 2007 and now was implemented the third issue – ISO 15189:2012 – Medical laboratories – Requirements for quality and competence (5), which is oriented on processes approach with detailed division with clearly defined requirements. The quality management system provides integration of all processes required quality policy with needs and requirements of the users.
The European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) established a Quality and Regulation Committee with Working Group - Accreditation and ISO/CEN standards (WG-A/ISO) which cooperate with EA, International Organization for Standardization’s Technical Committee 212 (ISO/TC 212) and European Committee for Standardization’s Technical Committee 140 (CEN/TC140) with aim to harmonize accreditation of medical laboratories.
We cannot forget that the most critical parameter for improving the quality of labs is educational activities inside and outside the labs, which are the key points in accreditation and quality management systems.
Accreditation in the world
People are asking why we need accredited labs. Accreditation is a good way to demonstrate competence of the laboratory, is a tool to recognize laboratories world-wide, is linked to periodical audits stimulating to keep and improve the quality, leads to high standard of services for clients (e.g. patients, health care providers) (Table I).
Table I.
Why we accredited medical laboratories?
| Accreditation is a good way to demonstrate competence of the laboratory |
| Accreditation is a tool to recognize laboratories worldwide |
| In some countries accreditation is mandatory or will be mandatory in the future |
| Accreditation and the linked periodical audits are a stimulant for keeping the quality system alive |
| Improve quality of our services |
| High standard of services for clients – patients, physicians |
| Interest of management – institute and hospital |
| Better documentation of processes and responsibilities |
Accreditation is mandatory in some countries or will be mandatory in the future (e.g. France) or some specific analyses should be accredited (e.g. Germany) or accredited labs have better reimbursement or contract with health insurance companies (e.g. Sweden, Belgium, Czech Republic). According the French Law No. 2013-442 from May 30, 2013, all tests in medical laboratories will be accredited to November 1, 2020.
The majority countries have no mandatory accreditation only in some areas as molecular biology tests (Belgium, Germany), new-born screening (Germany), immunohematology and blood transfusion (Ireland) or biochemistry and haematology (Lithuania). France and Hungary have declared mandatory accreditation for all the fields of laboratory medicine (6).
The data from survey shows that 50% European countries use only ISO 15189, 31% both ISO 15189 (preferably) and ISO 17025, 15% both ISO 15189 and ISO 17025 and 4% only ISO 17025, for medical laboratory accreditation. The main field (more than 80 %) for accreditation are clinical chemistry, haematology, microbiology, immunology and molecular genetics.
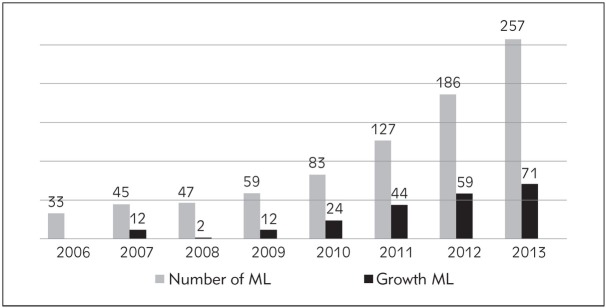
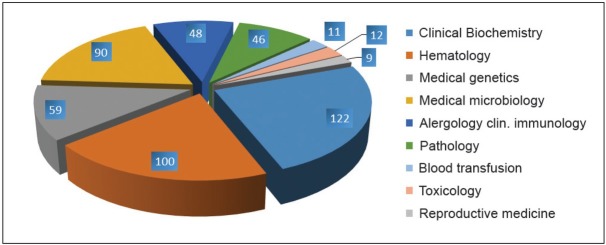
In Czech Republic we started process of medical laboratories accreditation in the beginning of the 21st century. The first labs were accredited according ISO 17025 and continuously were reaccredited to ISO 15189. Number of accredited labs in Czech Republic is continuously growing and now has 260 (May 2017) accredited labs in all areas of laboratory medicine and reassessment according to new ISO which started in winter 2013 was successfully done (Figure 1 and 2).
Figure 1.
Number of accredited medical labs in Czech Republic.
Figure 2.
Scope of accreditation in medical labs Czech Republic 2016.
The standard for accreditation ISO 15189:2012 – Medical laboratories – Requirements for quality and competence (5)
Accreditation is a procedure by which an authoritative body gives formal recognition that a body or person is competent to carry out specific tasks, it is an independent process (7, 8). The gold standard and the most recognized standard is ISO 15189 for clinical laboratories accreditation in Europe. The last version from 2012 has more and better definitions, which are connected to patient safety, is structured via processes and have many notes and examples. There are some new parts, which will be mentioned later and laboratory information management (5.10.) and ethical conduct (4.1.1.3.) are normative and showing the impact of these topics for patients.
There are some examples of areas with better definitions connected to patient safety
automated selection and reporting of results
biological reference interval
critical – alert interval
primary sample specimen
quality indicator
Next part is focused only some parts of standard where some changes, adoptions or new parts have importance to patient safety. During all processes lab has a memory that welfare of patient is a priority.
Parts of ISO 15189:2012
4. Management requirement (5)
The processes for selecting, evaluating and transfer of samples and results between the labs and referral laboratories are precisely described with responsibilities for both sites. Evaluation and audits (4.14.) contain new chapters leading to better interactions with clients (user feedback) and staff of laboratory (4.14.4.). Risk management (4.14.6.) is important part to identify the potential risk for patient safety and laboratory processes with aim to eliminate them. The labs shall describe the quality quantitative indicators with regular reviewing at all laboratory processes.
4.1. Organization and management responsibility
4.1.1.3. Ethics in laboratory medicine
Principles, collection of information/samples, Potential conflicts of interests – openly and appropriately declared
Confidentiality of information is maintained
Ethical codex for patients – confidence!
Ethical codex for personnel
4.5. Examination by referral laboratory
4.5.1. Selecting and evaluating referral laboratories and consultants
The report shall indicate which examinations were performed by a referral laboratory or consultant.
4.8. Resolution of complaints
4.9. Identification and control of nonconformities
4.10. Corrective action
Procedure, relevance, types of nonconformities
4.11. Preventive action
Identification of possible nonconformities – procedure for determining the root causes of potential nonconformities
4.12. Continual improvement
Systematic processes
Education and training
The laboratory shall continually improve the quality management system, including all processes in labs, including corrective actions and preventive actions. Improvement activities shall be directed at areas of highest priority based on risk assessments.
4.13. Control of records
4.14. Evaluation and audit
Periodic review of requests
Assessment of user feedback
Staff suggestions
Management shall more activate staff to make suggestions for the improvement in labs with and feedback provided to the staff.
Internal audits (plan-programme–protocol– recommendation–conformity)
Risk management
The laboratory shall evaluate the processes and potential failures on examination results as they affect patient safety, and shall update processes to reduce or eliminate the identified risks.
Quality indicators
14.15. Management review
5. Technical requirements (5)
For personnel is important to have precise job description with competencies and responsibilities with evaluation of effectiveness of continual professional development. Laboratory equipment newly adopts the reporting of adverse incident. We need opened mind description of excessively detailed requirements for consumables. Precise determination of biological reference intervals and critical values are important for correct communications connected to patient safety and laboratory information management (5.10.) are normative.
5.1. Personnel
Qualification, education and training, job description, management,
Responsibilities, competences, continual education incl. QMS
Confidentiality of information
Periodical reassessment
Plan and system of education
5.1.8. Continuing education and professional development
A continuing education programme shall be available to the personnel who participates in managerial and technical processes. Personnel shall take part in continuing education. The effectiveness of the continuing education programme shall be periodically reviewed.
5.2. Accommodation and environmental conditions
Safety place for staff and patients – adequate space
Minimize the risk of injury
Place for correct performance of examination or main lab processes
5.3. Laboratory equipment, reagents, and consumables
Capable to comply, programme for proper calibration and metrological traceability, function of instruments, reagents and analytical system and consumables
5.4. Pre-examination process
Laboratory is responsible for management of pre-analytical phase
5.4.3. Request form information
The request form – patient identification, including gender, date of birth, and the location/contact details of the patient, and a unique identifier, clinically relevant information about the patient and the request, for examination performance and result interpretation purposes
Primary sample collection manual
Instruction for proper collection and handling of primary samples
Sample transport (e.g. temperature monitoring)
Criteria for acceptance or rejection of samples
5.5. Examination processes
Appropriate examination procedures
The identity of persons performing activities in examination processes
Validation process – documentation
Calibration, reference materials
Analytical characteristics of methods
Biological reference intervals
5.5.2. Biological reference intervals or clinical decision values
The biological reference intervals or clinical decision values are communicated to users, with information of appropriate changes.
5.6. Assuring quality of examination procedures
Internal quality control system IQA – control materials
Programme/plan of calibration of measuring systems
Analytical parameters e.g. reproducibility, trueness, uncertainty.
Measurement uncertainty of measured quantity values
Traceable results (SI units)
Participation in EQA
5.7. Post-examination processes
Review the results, conformity with clinical information and reporting results
Authorized personal for releasing the result (possibility of automatic releasing results)
Critical values – action!
Reference values – annual reviewing
5.8. Reporting results
5.9. Release of results
5.9.2. Automated selection and reporting of results
System for automated selection and reporting of results should ensure:
the criteria for automated selection and reporting are defined, approved, readily available and understood by the staff
the criteria are validated for proper functioning before use and verified after changes to the system that might affect their functioning
there is a process for indicating the presence of sample interferences (e.g. haemolysis, icterus, lipemia) that may alter the results of the examination
5.10. Laboratory information management
The laboratory shall have access to the data and information needed to provide a service, which meets the needs and requirements of the user.
5.10.2. Authorities and responsibilities
The authorities and responsibilities for the management of the information system are defined, including the maintenance and modification to the information systems that may affect patient care. The authorities and responsibilities of all personnel who use the system and who: access patient data and information, enter or change patient data and examination results, authorize the release of examination results and reports are defined.
Accreditation process
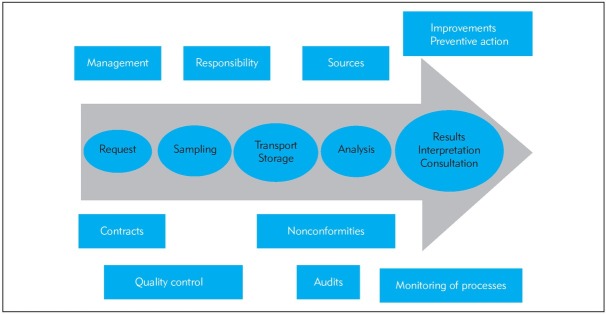
To accredit or not to accredit, that is the question! Starting the process of accreditation is particularly an interest in improving the quality of lab services with better documentation of processes and responsibilities or interest of management (institute, hospital, owner, government, etc.). The first step before accreditation is building enthusiastic team with education on quality management system, selection of methods for accreditation, describing the processes in the lab, developing or improving the metrology system, definition and structure of documents, preparation of a quality manual, SOPs and…(Figure 3) (2, 9, 10).
Figure 3.
Scheme of accreditation process.
SWOT analysis looks for four criteria, there should be mentioned only the positives – benefits and negatives from accreditation process. The benefits include standardization of all processes, responsibility of each member of team, personal policy, demonstrability of results, systematic evaluation of suppliers, risk management prestige, better communication with partners (11). Each process has not only positive features, but also the negatives, which in accreditation could be more investments to equipment, facilities, QMS or education, expenses for accreditation and consultation bodies and spent time of each member of staff.
The accreditation of labs improves laboratory medicine and all processes in laboratories, which include – reduction of errors in the pre-analytical, analytical and post analytical processes, facilitation of accurate and rapid diagnostics, participation in acceleration and efficiency of treatment, facilitation of personalised medicine development, and stimulates continuous improvement.
Laboratory medicine will be the centre of attention regarding quality due to their wide impact on patient care for correct medical decision on diagnosis and treatment and in preventive action. Accreditation is more an instrument than the aim that increases the quality of services for clients – patients, physicians. Accreditation is not about who the best is, but who has a system of standard procedures. Improvement of quality system in labs is ambitious and never ending story. Don’t forget that quality system is about people, with people and for people.
Acknowledgment
Supported by RVO MZ CR VFN64165.
Footnotes
Conflict of interest
Conflict of interest statement: The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Beastall GH. Shaping the Future of Laboratory Medicine: The Great Debate’ IFCC.
- 2.Zima T. Accreditation in clinical laboratories. Biochemia Medica. 2010;20/2:215. –. [Google Scholar]
- 3.Dybkaer R. Quality Assurance, Accreditation, and Certification: Needs and Possibilities. Clinical Chemistry. 1994;40/7:1416. –. [PubMed] [Google Scholar]
- 4.EN ISO. – General Requirements for the Competence of Testing and Calibration Laboratories 17025:2005. [Google Scholar]
- 5.EN ISO 15189:2012 –. Medical laboratories – Requirements for quality and competence [Google Scholar]
- 6.Boursier G, Vukasovic I, Mesko Brguljan P, Lohmander M, Ghita I, Bernabeu FA, Barrett E, Brugnoni D, Kroupis Ch, Sprongl L, Thelen MHM, Vanstapel F, Vodnik T, Huisman W, Vaubourdolle M. Accreditation process in European countries – an EFLM survey. Clin Chem Lab Med. 2016;54(4):545. doi: 10.1515/cclm-2015-0780. –. [DOI] [PubMed] [Google Scholar]
- 7.Guzel O, Guner EI. ISI 15189 Accreditation: Requirements for quality and competence of medical laboratories, experience of a laboratory I. Clinical Biochemistry. 2009;42:274. doi: 10.1016/j.clinbiochem.2008.09.011. –. [DOI] [PubMed] [Google Scholar]
- 8.Yanikkaya-Demirel G.. ISO 15189 accreditation: Requirements for quality and competence of medical laboratories, experience of a laboratory II. Clinical Biochemistry. 2009;42:279. doi: 10.1016/j.clinbiochem.2008.09.099. –. [DOI] [PubMed] [Google Scholar]
- 9.Braga F, Infusino I, Panteghini M. Role and responsibilities of laboratory medicine specialists in the verification of metrological traceability of in vitro medical diagnostics. J Med Biochem. 2015;34:282. doi: 10.1515/jomb-2015-0004. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theodorsson E. Quality assurance in clinical chemistry: a touch of statistics and a lot of common sense. J Med Biochem. 2016;35:103. doi: 10.1515/jomb-2016-0012. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huisman W, Horvath AR, Burnett D, Blaton V. Accreditation of medical laboratories in the European Union. Clin Chem Lab Med. 2007;45(2):268. doi: 10.1515/CCLM.2007.037. et al. –. [DOI] [PubMed] [Google Scholar]