Summary
Background
Polycystic ovary syndrome (PCOS) is associated with reproductive and metabolic abnormalities. The aim of this study was to analyse risk of cardiovascular disease (CVD) in PCOS, to define individual risk factors and assess their ability to predict risk.
Methods
Fifty-four young women with PCOS (22 obese and 32 normal weight) were compared to 46 respective controls (17 obese and 29 normal weight). Anthropometric parameters, lipid status parameters, inflammation markers, concentrations of glucose, transaminases, sex and anterior pituitary hormones, sex hormone binding globulin (SHBG) and androgens were measured. Cardiovascular Risk Score (CVRS), indices for identifying Non-Alcoholic Fatty Liver Disease (NAFLD) and the Index of Central Obesity (ICO) were calculated.
Results
Significantly higher CVRS values (p<0.05) were found in obese PCOS women compared to normal weight control and normal weight PCOS groups. Anthropometric parameters, lipid status parameters and fibrinogen (p<0.001, p<0.01) were higher in women with higher CVRS. The most significant CVRS predictors in all PCOS women were SHBG, androstenedione, follicle-stimulating hormone (FSH) and dehydroepiandrosterone sulphate (DHEAS). ICO and all NAFLD indices exhibited significant positive correlation with CVRS and a model consisting of these indices provided good diagnostic accuracy (AUC>0.8) in identifying patients with increased cardiovascular risk (CVR).
Conclusions
Obesity is a higher risk for developing CVD than PCOS alone. Anthropometric parameters, lipid parameters, fibrinogen, NAFLD indices and ICO increase CVR in PCOS women. For the prediction of CVR in PCOS, we suggest a combination of NAFLD indices and ICO.
Keywords: polycystic ovary syndrome, cardiovascular disease, cardiovascular risk score, non-alcoholic fatty liver disease, central obesity index
Kratak sadržaj
Uvod
Sindrom policističnih jajnika (PCOS) povezan je sa reproduktivnim i metaboličkim abnormalnostima. Cilj ove studije je bio da se ispita rizik za razvoj kardiovaskularnih bolesti u sindromu policističnih jajnika, da se definišu pojedinačni faktori rizika i da se proceni njihova sposobnost da predvide rizik.
Metode
Pedeset četiri mlade žene sa PCOS (22 gojazne i 32 normalno uhranjene) poređene su sa 46 ispitanica koje su činile odgovarajuću kontrolnu grupu (17 gojaznih i 29 normalno uhra njenih). Određivani su antropometrijski parametri, parametri lipidnog statusa, markeri inflamacije, koncentracije glukoze, transaminaza, polnih, adenohipofiznih hormona, globulina koji vezuje polne hormone (SHBG) i androgena. Računati su skor za procenu rizika za nastanak kardiovaskularnih bolesti (CVRS), indeksi koji se koriste za dijagnozu nealkoholne masne bolesti jetre (NAFLD) i indeks centralne gojaznosti (ICO).
Rezultati
Uočene su značajno više vrednosti CVRS (p<0,05) kod gojaznih PCOS žena u odnosu na normalno uhranjenu kontrolnu i PCOS grupu. Kod žena sa višim CVRS utvrđene su više vrednosti antropometrijskih parametara, parametara lipidnog statusa i fibrinogena (p<0,001, p<0,01). Najvažniji hormonski prediktori rizika kod svih žena sa PCOS su bili: SHBG, androstenedion, folikulostimulirajući hormon (FSH) i dehidroepiandrosteron-sulfat (DHEAS). ICO i svi NAFLD indeksi su pokazali značajnu pozitivnu korelaciju sa CVRS, a model sastavljen od kombinacije ovih indeksa je pokazao dobru dijagnostičku tačnost (AUC>0,8) u izdvajanju pacijenata sa povišenim kardiovaskularnim rizikom.
Zaključak
Gojaznost je značajniji rizik za razvoj kardiovaskularnih bolesti od samog PCOS. Antropometrijski parametri, parametri lipidnog statusa, fibrinogen, NAFLD indeksi i ICO povećavaju kardiovaskularni rizik kod žena sa PCOS. Za procenu kardiovaskularnog rizika u PCOS predlažemo kombinaciju NAFLD indeksa i ICO.
Ključne reči: sindrom policističnih jajnika, kardiovaskularne bolesti, procena kardiovaskularnog rizika, nealkoholna masna bolest jetre, indeks centralne gojaznosti
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age with a prevalence of 6 to 20% (depending on the criteria used for the definition of PCOS), while cardiovascular disease (CVD) is the most common cause of death in the world both in women and men (1, 2). Many studies have demonstrated relationships between CVD risk factors such as abdominal obesity, dyslipidemia, glucose intolerance, diabetes and hypertension and signs of PCOS (3, 4). However, the existence of risk factors does not mean the same thing as increased risk. Therefore, the connection between PCOS and increased risk of developing CVD is debatable (5).
Many factors in PCOS, including both metabolic and hormonal, affect cardiovascular risk (CVR) (6). Non-alcoholic fatty liver disease (NAFLD) and central obesity are both risk factors for developing CVD (7). In addition, NAFLD occurs with an increased incidence in women with PCOS, while central obesity increases the probability of developing PCOS (8). It is known that NAFLD is associated with insulin resistance and CVR, but it is not clear whether PCOS and NAFLD act additively on the CVR. In PCOS, the connection between the CVR and NAFLD and central obesity can be established using indices for the diagnosis of NAFLD (Aspartate aminotransferase to Platelet Ratio Index, APRI; Hepatic Steatosis Index, HIS and Lipid Accumulation Product, LAP), as well as the Index of Central Obesity–ICO (9, 10, 11, 12).
Due to the existence of different criteria to define PCOS, there are conflicting data on the risk of CVD in women with PCOS. The aim of this study was to analyse the risk for CVD in our population. A secondary aim was to define individual risk factors and grouped risk factors for developing CVD for their ability to predict risk.
Materials and Methods
The study included 100 women of similar age (between 18 and 35 years) who were examined between January 2007 and December 2007 in the outpatient department of the Clinic of Internal Medicine at the »Dr Dragisa Misović–Dedinje« Clinical Hospital Centre in Belgrade, after obtaining informed written consent. The study was approved by the Institute’s Ethics Committee and conducted respecting the principles set out in the latest amendment to the Declaration of Helsinki (Edinburgh, 2000). The women were divided into 2 main groups, a control group (n=46) and a group with diagnosed PCOS (n=54). Each group, depending on the nutritional status, was divided into two subgroups according to body mass index (BMI): a subgroup of normal weight women (BMI ≤ 25 kg/m2) and a subgroup of obese women (BMI > 25 kg/m2).
The control group consisted of 29 healthy normal weight and 17 obese women. The subjects of the control group were not taking contraceptives, had normal glucose metabolism, did not smoke or consume alcohol.
Patients with proven PCOS included 32 normal weight women and 22 obese women. Diagnosis of PCOS was determined based on the Rotterdam criteria with exclusion of other causes of hyperandrogenism, menstrual disorders and infertility (13). None of the women were taking oral contraceptive therapy, anti-androgens or drugs that could have affected regulation of blood pressure, blood lipid and carbohydrate metabolism in the previous six months.
Measurements of body weight and height were carried out in the morning in a fasting state. Body mass index (BMI) was calculated based on the obtained values. The waist circumference (WC) was measured by a flexible tape measure, simply at the smallest circumference of the natural waist, usually just above the belly button, and the hip circumference was measured at the widest part of the buttocks or hip. Waist-to-hip ratio (WHR) was calculated using these values. Upper arm circumference (UAC) is the circumference of the left upper arm measured at the mid-point between the tip of the shoulder and the tip of the elbow.
Blood samples were collected in the morning in a fasting state (at least 12 hours after the last meal) on the second or third day of the menstrual cycle (except for those women for whom the period between the two cycles was longer than six weeks, in this case blood samples were collected after this period).
The erythrocyte sedimentation rate (ESR) was determined using a Westergren tube (BD, Plymouth, UK). The following serum analyses were performed: transaminases and lipid profile – total cholesterol (TC) and triglycerides (TG) by standard enzymatic methods on an Abbott Spectrum chemistry analyser (Abbott Diagnostics, Illinois, USA). The concentration of high-density lipoprotein cholesterol (HDL-C) was determined by the phosphotungstate/magnesium method, while the concentration of low-density lipoprotein cholesterol (LDL-C) was determined by the Friedewald formula (14). Blood glucose was measured by the glucose oxidase method on a Beckman glucose analyser (Beckman Coulter Inc., Brea, USA). Fibrinogen was determined by the Clauss method on a BFT II analyser (Siemens Healthcare GmbH, Germany). Testosterone, sex hormone binding globulin (SHBG), follicle-stimulating hormone (FSH), luteinising hormone (LH), estradiol (E2), progesterone, androstenedione and dehydroepiandrosterone-sulphate (DHEAS) were measured by chemiluminescence immunoassays (CLIA) on an Immulite 1000 (Siemens Healthcare GmbH, Germany). LH/FSH index was determined by calculation. Free Androgen Index (FAI) was calculated by the formula [(100 × total testosterone)/SHBG].
The risk score for the development of cardiovascular disease (CVRS) in young people later in life was calculated (15) by adding the points for each risk factor (eg. BMI, low HDL-cholesterol, high non-HDL-cholesterol, smoking, high blood pressure and fasting glucose, as well as the age of the patient) (Table I).
Table I.
Scoring in calculating the risk score for cardiovascular disease.
| Factors | Points |
|---|---|
| Gender (W) | -1 |
| non-HDL (mmol/L) | |
| <3.37 | 0 |
| 3.37–4.12 | 2 |
| 4.14–4.90 | 4 |
| 4.92–5.67 | 6 |
| >5.70 | 8 |
| HDL (mmol/L) | |
| <1.036 | 1 |
| 1.036–1.530 | 0 |
| >1.550 | -1 |
| Smoking | +1 |
| Blood pressure | |
| Normal | 0 |
| High | 4 |
| Hyperglycemia | 5 |
| Age (years) | |
| 15–19 | 0 |
| 20–24 | 5 |
| 25–29 | 10 |
| 30–34 | 15 |
-
Dyslipidemia existed if there were increased levels of cholesterol (≥ 5.20 mmol/L), triglycerides (≥ 1.70 mmol/L), LDL-cholesterol (≥ 3.40 mmol/L), or if the HDL-cholesterol was decreased (< 1.30 mmol/L). The index of central obesity (ICO) and NAFLD indices (APRI, HIS and LAP) were calculated using formulas (Table II).
Table II.
Calculation of ICO and NAFLD indices.
| Indices | Calculating formulas | Cut-off |
|---|---|---|
| ICO | WC [cm]/ body height [cm] | >0.53 |
| APRI | (AST [IU/L]/upper referent range level for AST[IU/L])/ (PLT count [109/L]) × 100 | >1.50 |
| LAP | (WC [cm] – 58) × (TG [mmol/L]) | >54.2 |
| HIS | 8 x ALT [IU/L]/AST [IU/L] + BMI (+2 points for women,+2 for DM status) | >36.0 |
ICO – index of central obesity, APRI – AST to Platelet Ratio Index, HIS – Hepatic steatosis Index, LAP – Lipid Accumulation Product, ALT – alanine aminotransferase, AST – aspartate aminotransferase, BMI – body mass index
Statistical analysis was performed using the Statistical Package for the Social Sciences (version 18.0 for Windows, SPSS Inc., Chicago, IL, USA). Results are presented as mean ± standard deviation (⎯x ± SD). Differences between groups were evaluated by the ANOVA test followed by post hoc Tukey-Snedecor test. Spearman correlation analyses and multiple linear regression analyses were also used to determine the relationships between the determined parameters. Receiver Operating Characteristic (ROC) curves were constructed to assess the ability of the individual parameters and the selected models to predict cardiovascular risk where CVRS was a dependent variable. A risk score value greater than 13 (the threshold of the third tertile for this group of women) was taken as the limit for an increased risk of developing CVD. P values less than 0.05 were statistically significant.
Results
The subjects involved in this current study were divided in four groups according to their obesity status and PCOS presence. Anthropometric, biochemical parameters and hormones that showed significant difference between these groups of women are presented in Table III.
Table III.
Anthropometric, biochemical parameters and hormones in obese and non-obese women in PCOS and control groups.
| Parameter | PCOS obese (n=22) | PCOS normal weight (n=32) | Control group obese (n=29) | Control group normal weight (n=17) | P |
|---|---|---|---|---|---|
| BMI, kg/m2 | 34.5±5.48 | 19.9±1.83††† | 30.9±4.68†,‡‡‡ | 20.9±1.93†††,§§§ | <0.001 |
| WC, cm | 105.0±15.40 | 71.0±5.17††† | 97.5±10.49‡‡‡ | 74.8±7.66†††,§§§ | <0.001 |
| UAC, cm | 33.7±4.07 | 23.6±2.08††† | 32.9±3.37‡‡‡ | 25.8±2.53†††,‡,§§§ | <0.001 |
| WHR | 0.861±0.079 | 0.831±0.038 | 0.828±0.074 | 0.763±0.059†††,‡‡‡,§§§ | <0.001 |
| ESR, mm/h | 16.60±9.160 | 8.91±3.541†† | 15.90±10.690‡‡ | 9.24±4.830††,§ | <0.001 |
| Glucose, mmol/L | 4.60±0.530 | 4.48±0.453 | 5.13±0.396††,‡‡‡ | 4.76±0.368§ | <0.001 |
| ALT, IU/L | 25.8±12.78 | 18.1±8.81† | 19.9±5.92 | 16.3±8.53†† | <0.01 |
| HDL-C, mmol/L | 1.19±0.245 | 1.53±0.333††† | 1.18±0.198‡‡‡ | 1.42±0.235††,§ | <0.001 |
| non-HDL-C, mmol/L | 4.34±1.172 | 3.33±0.772†† | 3.82±1.087 | 3.52±0.684† | <0.01 |
| TG, mmol/L | 1.810±0.896 | 0.931±0.402†† | 1.270±0.684† | 0.797±0.322†††,§ | <0.001 |
| TG/HDL-C | 1.640±0.993 | 0.646±0.348†† | 1.120±0.640†,‡ | 0.580±0.266†††,§ | <0.001 |
| Fibrinogen, g/L | 3.69±0.645 | 2.89±0.561††† | 3.45±0.701‡‡ | 2.96±0.556††† | <0.001 |
| Testosterone, nmol/ L | 2.35±0.797 | 2.09±0.714 | 1.66±0.919† | 1.53±0.578††,‡ | <0.001 |
| SHBG, nmol/L | 38.1±29.58 | 35.2±10.86 | 73.1±9.10†††,‡‡‡ | 67.3±25.47†††,‡‡‡,§§ | <0.001 |
| LH, IU/L | 9.51±3.827 | 9.21±2.867 | 2.85±1.161†††,‡‡‡ | 5.97±3.386††,‡‡‡,§§ | <0.001 |
| Estradiol, pmol/L | 90.1±44.19 | 93.5±65.17 | 124.8±66.17 | 169.7±95.26††,‡‡‡ | <0.001 |
| Androstenedion, ng/mL | 3.54±1.380 | 3.36±0.985 | 2.09±0.793†††,‡‡‡ | 2.01±0.798†††,‡‡‡ | <0.001 |
| LH/FSH | 1.40±0.493 | 1.39±0.373 | 0.47±0.229† | 0.79±0.331††,‡ | <0.001 |
| FAI | 0.083±0.0518 | 0.065±0.0282 | 0.023±0.0118†††,‡‡ | 0.273±0.0193 †††,‡‡‡ | <0.001 |
| Dyslipidemia no/yes (%) | 68.2/31.8 | 90.6/9.4 | 64.7/35.3 | 93.1/6.9 | χ2<=0.05 10.3 |
BMI – Body mass index; WC – waist circumference; UAC – upper arm circumference; WHR – waist to hip ratio; ESR – erythrocyte sedimentation rate; ALT – alanine aminotransferase; HDL-C – high-density lipoprotein cholesterol; TG – triglycerides; SHBG – sex hormone binding globulin; LH – luteinising hormone; E2 – estradiol; LH/FSH ratio; FAI – free androgen index; P from ANOVA test, followed by post hoc Tukey-Snedecor test
†, ††, ††† – the difference of any group and first group (PCOS obese group)
‡, ‡‡, ‡‡‡ – the difference of the third and/or the fourth group with second group (PCOS normal weight group)
§, §§, §§§ – the difference of the fourth group with the third group (control obese group)
P<0.05, 0.01, 0.001
After post hoc analyses, the following results were observed. Normal weight PCOS patients had significantly lower BMI and WC than obese PCOS patients. However, their WHR was similar and significantly higher than in the normal weight control subjects, although BMI and WC between both normal weight groups (PCOS patients and control subjects) were not significantly different. Obese subjects from both groups had higher values of inflammatory parameters (ESR and fibrinogen) compared to normal weight subjects, lower values of HDL-C and elevated triglyceride concentrations compared to normal weight subjects, while the concentration of non HDL-C was highest in obese PCOS patients. Levels of testosterone, androstenedione, LH and the LH/FSH ratio were significantly higher in PCOS patients compared to the control group, while the levels of SHBG and estradiol were significantly lower.
In order to assess risk for cardiovascular disease development in later life, we used the CVRS calculation. The results are presented in Figure 1.
Figure 1.
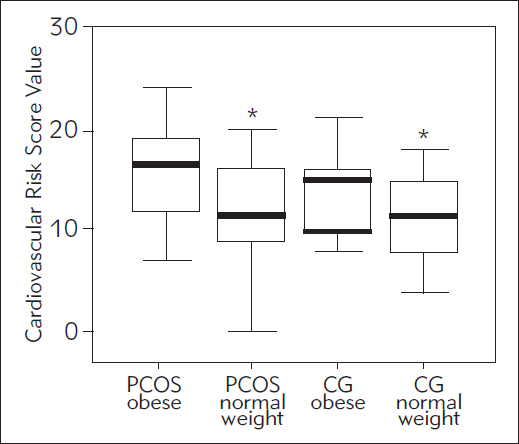
CVRS in obese and non-obese PCOS and control women.
* P<0.05 compared to PCOS overweight or obese women, according to ANOVA
Using ANOVA analysis with Tukey-Snedecor post hoc test, we found significantly higher CVRS values in obese PCOS compared to both normal weight groups (PCOS and CG, p<0.05 respectively). Significantly higher CVRS values were found in obese PCOS patients compared with normal weight PCOS patients and normal weight control subjects, p<0.05 respectively (using ANOVA/Tukey-Snedecor post hoc test). According to the results of CVRS calculations, we then divided our study subjects into CVRS tertiles (low risk, medium risk and higher risk). We compared
other measured parameters that were not included in CVRS calculations to conclude if CVRS level was related to other parameters (for example, hormones measured in PCOS patients). The results are presented in Table IV. We found significantly higher values of fibrinogen in women with medium CVRS (second tertile) compared with women with low CVRS (first tertile). Anthropometric parameters (except UAC and WHR), lipid parameters and fibrinogen, which were not included in CVRS calculation, exhibited significantly higher concentrations in women with higher CVRS compared with women with low or medium CVRS. In addition, women with higher CVRS did not differ from those with medium CVRS with respect to values of UAC, WHR and fibrinogen.
Table IV.
Anthropometric, biochemical parameters and hormones according to CVRS tertiles.
| Parameter | CVRS Tertiles | |||
|---|---|---|---|---|
| CVRS range, points | First (n=37) 0–11 | Second (n=39) 11–17 | 11–17 Third (n=24) 18–24 | P |
| WC, cm | 78.0±13.35 | 83.6±17.82 | 94.3±17.92††,‡ | <0.01 |
| UAC, cm | 25.9±4.18 | 28.3±5.61 | 30.8±4.89†† | <0.01 |
| WHR | 0.80±0.065 | 0.81±0.067 | 0.85±0.076†† | <0.01 |
| AST, U/L | 24.6±4.96 | 25.4±5.54 | 31.5±11.61††,‡‡ | <0.01 |
| TG, mmol/L | 0.831±0.308 | 1.160±0.733 | 1.600±0.804†††,‡ | <0.001 |
| TG/HDL-C | 0.592±0.270 | 0.924±0.663 | 1.450±0.954†††,‡‡ | <0.001 |
| Fibrinogen, g/L | 2.88±0.628 | 3.29±0.706† | 3.46±0.553†† | <0.01 |
| Dyslipidemia no/yes, % | 100/0 | 87.2/12.8 | 45.8/54.2 | χ2 =30.1, P<0.001 |
CVRS – cardiovascular risk score; WC – waist circumference; UAC – upper arm circumference; WHR – waist to hip ratio; TG – triglycerides
P from ANOVA test, followed by post hoc Tukey test
†, ††, ††† – degree of statistical significance found between second or third tertile with first tertile
‡, ‡‡, ‡‡‡ – degree of statistical significance found between the third tertile with the second tertile
P<0.05, 0.01, 0.001
Multiple linear regression analysis was implemented to determine possible influence of hormonal status of both PCOS groups on CVRS. The initial model consisted of all measured hormones combined with backward selection. The analysis revealed the best models for PCOS patients (Table V).
Table V.
Multiple linear regression analysis with CVRS (dependent variable) and measured hormones (independent predictors).
| PCOS all | PCOS obese | PCOS normal weight | |||
|---|---|---|---|---|---|
| SHBG | 0.025 ( 0.0008–0.0492)* | FSH | 0.391 (0.022–0.761)* | E2 | -0.00933 (-0.01721– 0.00145)* |
| FSH | 0.419 (0.168–0.671)** | DHEAS | 0.000887 (0.0000144–0.001759)* | ||
| Androstenedione | 0.800 (0.238–1.364)** | ||||
| DHEAS | -0.001 (-0.002– -0.0002)* | ||||
| Adjusted R2 | 0.398 | 0.319 | 0.460 | ||
SHBG – sex hormone binding globulin; FSH – follicle-stimulating hormone, E2 – estradiol, DHEAS – dehydroepiandrosterone-sulfate Data show B (CI); *P<0.05, **P<0.01, ***P<0.001 according to MLR analysis
The key CVRS predictors for the whole PCOS group were SHBG, FSH and androstenedione (positive correlations) and DHEAS (negative correlation). The adjusted R2 was 0.398 which indicated that approximately 40% variability in CVRS values was related to the selected parameters model. In obese PCOS women, the most important predictor was only FSH (positive correlation) (with approximately 30% of variation). In normal weight PCOS women, the key predictors were estradiol (negative correlation) and DHEAS (positive correlation) with approximately 46% influence on CVRS variability.
In women with PCOS, non-alcoholic fatty liver disease (NAFLD) is common, which itself is an additional risk for CVD development. In order to estimate additional cardiovascular risk, we calculated several NAFLD indices: APRI, LAP and HIS, as well as the anthropometric parameter – index of central obesity (ICO). Figure 2 shows NAFLD scores and ICO in the study subgroups.
Figure 2.
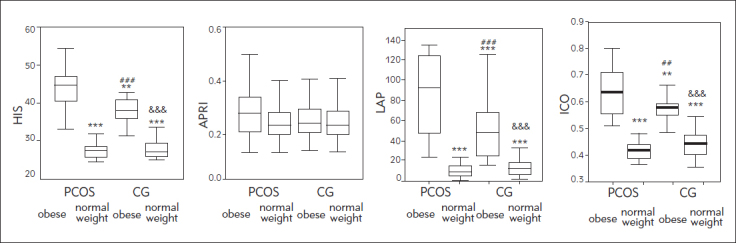
HIS, APRI, LAP and ICO in the study subgroups.
*P<0.05 compared to PCOS overweight or obese women, according to ANOVA
***P<0.001,**P<0.01 vs. PCOS obese, ##P<0.01, ###P<0.001 &&& P<0.001 vs. PCOS normal weight.
Clearly, HIS, LAP and ICO indices were significantly higher in obese PCOS women compared to other subgroups. In addition, normal weight PCOS and normal weight control subjects did not differ regarding these two NAFLD indicies and ICO. No differences in the APRI index were found between any of the subgroups. Spearman's non-parametric correlation between CVRS and NAFLD indices and ICO are presented in Figure 3. All NAFLD-related parameters as well as the ICO index showed significant positive correlation with CVRS. LAP showed the strongest correlation with CVRS.
Figure 3.
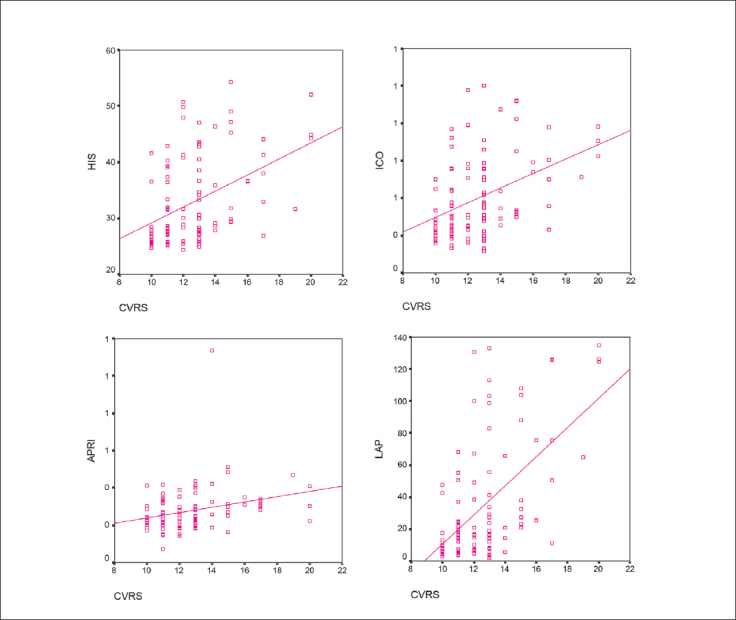
Correlation between CVRS and NAFLD indices.
Spearman’s non-parametric correlation coefficient ρ: CVRS with HIS 0.427 (P<0.001), APRI 0.278 (P<0.01), LAP 0.566 (P<0.001), ICO 0.417 (<0.001).
Receiver operating characteristic (ROC) curves were constructed in order to compare the ability of LAP, HIS, APRI and ICO indices to predict CVRS (Figure 4). We compared these parameters using the area under the curve (AUC) values (Table VI). According to ROC analysis, LAP was the best predictor for CVRS (Figure 4A, Table VI). Additionally, we constructed a model consisting of 3 NAFLD indices (LAP, HIS, APRI) and the anthropometric parameter ICO using logistic regression analysis-generated predictive probabilities (Figure 4B). The model showed a satisfactory diagostic accuracy in discriminating patients with high CVRS (AUC > 0.8 which was excellent diagnostic accuracy).
Figure 4.
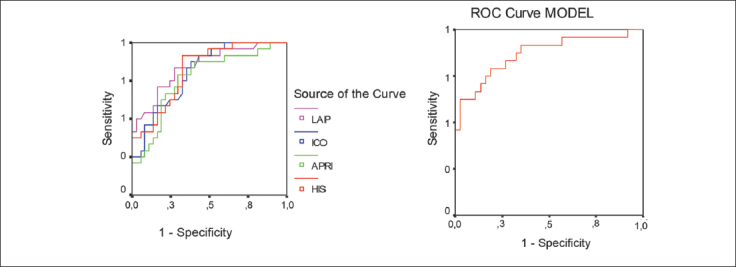
A. ROC curve is showing the ability of NAFLD indices and ICO to predict cardiovascular risk, B. ROC curve of model (integrated ICO, LAP, APRI and HIS indices) to predict cardiovascular risk.
Table VI.
Area under the curves for NAFLD indices and ICO calculated after constructing ROC curves (Figure 4A) and area under the curve for model (integrated ICO, LAP, APRI and HIS indices) (Figure 4B).
| 95% Confidence | |||
|---|---|---|---|
| Test Result Variable (s) | AUC (SE) | Interval | P |
| ICO | 0.751 (0.052) | 0.648–0.853 | <0.001 |
| LAP | 0.776 (0.053) | 0.673–0.879 | < 0 .001 |
| HIS | 0.757 (0.051) | 0.657–0.858 | <0.001 |
| APRI | 0.757 (0.057) | 0.644–0.869 | <0.001 |
| Predicted probability – Model | 0.815 (0.056) | 0.705–0.925 | <0.001 |
AUC – Area Under the Curve; a Under the non-parametric assumption; b Null hypothesis: True area = 0.5
Discussion
In order to assess CVD risk in women with PCOS, we compared the values of measured parameters to determine those that were significantly different between the examined subgroups. Our results revealed that obese women had higher anthropometric measurements and that there was a significant difference in the levels of hormones in PCOS and healthy subjects. As expected, women with PCOS in our study had significantly higher concentrations of androgens (testosterone, androstenedione) and FAI and significantly lower concentrations of SHBG compared to control groups (16). Also, LH and the LH/FSH ratio were significantly higher in both PCOS groups compared to the control groups. In PCOS, the secretion of LH is favoured in relation to FSH and this disorder of gonadotropin secretion, namely an inverse ratio of FSH to LH, can be seen in 75% of women with this syndrome (17).
We used a modified risk score (CVRS) (15) because the classical risk assessment for coronary heart disease is common practice among the adult population and evidence for successful implementation in young adults is not available, except for those with familial hypercholesterolemia (18). The maximum number of points in CVRS for women is 24 points. CVRS values greater than 17 indicate that there is a 20% probability of developing clinically significant lesions in the left anterior descending artery and right coronary artery. Clinically significant lesions in the left coronary artery are lesions in the fourth and fifth degree according to the criteria of the American Heart Association (AHA) (15, 19).
After calculating CVRS and finding that there was no significant difference in the CVRS between the two groups of obese patients (PCOS and controls), while the difference was present between the obese PCOS group and normal weight control group, the real question was how PCOS could influence cardiovascular risk and how much influence was from obesity. Target lesions in the right coronary artery are those that cover 9% or more of the intimal surface of the blood vessel (15). Orio et al. (20) noted that young PCOS women had increased left ventricular mass and diastolic dysfunction which was not associated with weight, and suggested that PCOS women were candidates for development of early CVD. Bickerton et al. (21) found no significant difference when criteria for assessing cardiovascular risk using any of the biochemical parameters that assess the risk of atherothrombosis or vascular inflammation in PCOS women were compared to controls matched according to weight and age. Elting et al. (22) showed that a higher prevalence of hypertension was related to obesity, while Chen et al. (23) demonstrated that hyperandrogenemia in even young women with PCOS was associated with hypertension independently of insulin resistance, BMI and dyslipidemia.
Taking into account these published reports, in our study we examined the impact of each factor on the CVRS in both PCOS groups of women as well as the influence of each factor on increased CVRS.
We found that women with high CVRS had the highest WC, UAC and WHR as the greatest risk are related to obesity. According to the latest guidelines, increased CVD risk exists in women with PCOS having any of the following risk factors: obesity, dyslipidemia, smoking, hypertension, impaired glucose tolerance and family history of premature CVD (24). In addition, we found the highest percentage of dyslipidemia in women with high CVRS. Dyslipidemia is one of the most common cardiometabolic complications of PCOS. It occurs in about 70% of patients (25, 17) and it usually manifests itself in the form of atherogenic dyslipidemia characterised by hypertriglyceridemia with low concentrations of HDL-C (26). It is believed that hyperandrogenemia impacts on the occurrence of dyslipidemia. Namely, it is possible that androgen excess modifies LDL-C early in life and leads to a more atherogenic lipid profile (27). In recent years, a number of studies observed an increased level of LDL in women with PCOS, but there was considerable variability in the level of LDL, which was likely due to several factors such as ethnicity, phenotype, diet quality and body weight (28). Due to the influence of hormonal status in PCOS on CVD, multiple linear regression analysis was performed to assess impact on the cardiovascular risks of all PCOS women, normal weight or obese PCOS subjects, respectively (Table V). Negative correlation between estradiol and CVRS in the normal weight PCOS group confirmed that in this group of women estrogen probably exerted a protective effect on the cardiovascular system (29, 30).
FSH positively correlated with CVRS in all PCOS patients, including obese PCOS patients (Table V). In pre-menopausal women, the increase in FSH above 7 IU/L was associated with a significant increase in both total and LDL-cholesterol levels and therefore with increased cardiovascular risk (31).
Hyperandrogenism is present in women with PCOS. The relationship between DHEAS and cardiovascular risk in PCOS is uncertain. Goodarzi et al. (1) proposed a DHEAS-mediated protective impact on the cardiovascular system through an uknown mechanism. In addition, Carmina and Lobo found that PCOS patients with increased DHEAS generally had more favourable metabolic and cardiovascular parameters (32). In our study, we confirmed the existence of negative correlation between CVRS and DHEAS in all PCOS subjects, while the normal weight PCOS subjects still showed positive correlation between DHEAS and CVRS. In contrast, Schaffrath et al. (33) did not establish a link between the concentration of sex hormones and cardiovascular diseases using multivariable analysis. The same study also stated that, in studies that dealt with PCOS as the primary problem, SHBG and DHEAS stood out as inversely associated with subclinical CVD, but these studies were limited by their cross-sectional nature or only a small number of women were involved. In contrast, our study revealed positive correlation between the concentration of SHBG and CVRS, which cannot be explained by searching current published literature.
By including NAFLD indices into the study and relating them to CVRS, we noticed the positioning of the LAP index as a significant predictor of CVD. This conclusion is not surprising considering that the LAP index has proved to be better than BMI for identifying risk of developing CVD (10). Obese PCOS patients had an elevated LAP index compared with obese patients in the control group (Figure 2). The significant increase in LAP index in PCOS patients indicated metabolic disorders in this syndrome (34). The index of central obesity (ICO) was significantly higher in obese patients with PCOS compared with all other study groups (Figure 2). Also, the WHR in normal weight PCOS women was significantly higher than the WHR in normal weight control group (although their BMI is not significantly different) (Table III). Carmina et al. noted that PCOS women had more abdominal fat, but similar amounts of total fat, compared with controls after adjusting for body weight (35). Fat tissue in the abdominal wall and visceral adipose tissue are metabolically active tissues more sensitive to catecholamines than to insulin. Central obesity is associated with insulin resistance, glucose intolerance, diabetes, an increased production of androgens and reduced SHBG synthesis. It has been shown that increased content of abdominal/visceral fat correlates with cardiometabolic risk factors such as insulin resistance, dyslipidemia, hypertension and metabolic syndrome in general (35). This relation with CVD risk was confirmed through the positive correlation of ICO index and CVRS (Figure 3). ICO together with NAFLD indices showed good diagnostic accuracy to segregate patients with increased cardiovascular risk (Figure 4B, Table VI). Central obesity is a more specific risk factor for PCOS compared to general obesity. As the relationship between ICO and CVRS is clear, ICO links PCOS to cardiovascular risk.
APRI and HIS indices proved to be good predictors of general cardiovascular risk, particularly in combination with LAP and ICO indices (Figure 4, Table VI). To calculate these indices, aminotransferase levels are used, with the highest level of ALT in obese PCOS patients (Table III) and the highest level of AST in the third cardiovascular risk score tertile (Table IV). APRI is directly proportional to the concentration of AST which indicates that AST has the ability to predict cardiovascular risk. Several studies have evaluated the ability of transaminases to predict cardiovascular risk. Monami et al. (36) found a correlation between increased concentrations of AST and in creased risk for CVD. This indicates that these parameters should be monitored, as PCOS is a complex disorder with significant changes in metabolism which can lead to further damage of the cardiovascular system not primarily caused by the syndrome. Our study indicated that PCOS did not contribute to a higher risk of developing CVD compared with obesity but rather demonstrated the effect of other factors (anthropometric parameters – WC, lipid parameters – TG, TG/HDL-C, fibrinogen, NAFLD indices, ICO) on the increase in cardiovascular risk in women with PCOS. For the prediction of cardiovascular risk in PCOS, our study suggests the combination of NAFLD indices and ICO.
In order to broaden our scientific knowledge about PCOS, comprehensive syndrome phenotyping and understanding reproductive and metabolic abnormalities within phenotypes will require a larger number of patients and longer follow-up patient monitoring periods.
Acknowledgements
This study was conducted as a part of project No. 175035 – J. Kotur-Stevuljevic and No. 175036 – S. Ignjatovic, financially supported by the Ministry of Science and Technology of the Republic of Serbia. The study funder had no role in study design, collection, analysis and interpretation of data; nor in the writing of the report and decision to submit the manuscript for publication.
List of abbreviations
- APRI,
aspartate aminotransferase to platelet ratio index;
- CVD,
cardiovascular disease;
- CVR,
cardiovascular risk;
- CVRS,
cardiovascular risk score;
- HIS,
hepatic steatosis index;
- ICO,
index of central obesity;
- LAP,
lipid accumulation product;
- UAC,
upper arm circumference;
- WC,
waist circumference.
Footnotes
Conflict of interest
Conflict of interest statement: The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Goodarzi M, Carmina E, Azziz R.. DHEA, DHEAS and PCOS. J Ster Bioch. 2015;145:213. doi: 10.1016/j.jsbmb.2014.06.003. –. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation, Global Health Observatory data, Mortality and morbidity, Cardiovascular diseases. http://www.who.int/gho/ncd/mortality_morbidity/cvd/en/ [04.09.2016.]
- 3.Birdsall MA, Farquhar CM, White HD. Association between polycystic ovaries and extent of coronary artery disease in women having cardiac catheterization. Ann Intern Med. 1997;126:32. doi: 10.7326/0003-4819-126-1-199701010-00005. –. [DOI] [PubMed] [Google Scholar]
- 4.Legro RS. Polycystic Ovary Syndrome and Cardiovascular Disease: A Premature Association? End Rev. 2003;24:302. doi: 10.1210/er.2003-0004. –. [DOI] [PubMed] [Google Scholar]
- 5.Temple R. Are surrogate markers adequate to assess cardiovascular disease drugs? JAMA. 1999;282:790. doi: 10.1001/jama.282.8.790. –. [DOI] [PubMed] [Google Scholar]
- 6.Dokras A. Cardiovascular disease risk in women with PCOS. Steroids. 2013;78:773. doi: 10.1016/j.steroids.2013.04.009. –. [DOI] [PubMed] [Google Scholar]
- 7.Lonardo A, Sookoian S, Pirola CJ, Targher G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism. 2015;65:1136. doi: 10.1016/j.metabol.2015.09.017. –. [DOI] [PubMed] [Google Scholar]
- 8.Cerda C, Pérez-Ayuso RM, Riquelme A, Soza A, Villaseca P, Sir-Petermann T. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome. J Hepatology. 2007;47:412. doi: 10.1016/j.jhep.2007.04.012. et al. –. [DOI] [PubMed] [Google Scholar]
- 9.Xiang S, Hua F, Chen L, Tang Y, Jiang X, Liu Z. Lipid Accumulation Product is Related to Metabolic Syndrome in Women with Polycystic Ovary Syndrome. Exp Clin End Diab. 2013;121:115. doi: 10.1055/s-0032-1333261. –. [DOI] [PubMed] [Google Scholar]
- 10.Sert A, Pirgon O, Aypar E, Yilmaz H, Dündar B. Relationship between aspartate aminotransferase-to-platelet ratio index and carotid intima-media thickness in obese adolescents with non-alcoholic fatty liver disease. J Clin Res Ped End. 2013;5:182. doi: 10.4274/Jcrpe.891. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W. Hepatic steatosis index: A simple screening tool reflecting non-alcoholic fatty liver disease. Dig Liver Dis. 2010;42:503. doi: 10.1016/j.dld.2009.08.002. et al. –. [DOI] [PubMed] [Google Scholar]
- 12.Parikh RM, Joshi SR, Menon PS, Shah NS. Index of central obesity – A novel parameter. Med Hypotheses. 2007;68:1272. doi: 10.1016/j.mehy.2006.10.038. –. [DOI] [PubMed] [Google Scholar]
- 13.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod 2004; 2004;19:41. doi: 10.1093/humrep/deh098. –. [DOI] [PubMed] [Google Scholar]
- 14.Warnick GR, Knopp RH, Fitzpatrick V, Branson L. Estimating low-density lipoprotein cholesterol by the Friede-wald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin Chem. 1990;36:15. –. [PubMed] [Google Scholar]
- 15.McMahan CA, Gidding SS, Fayad ZA, Zieske AW, Malcom GT, Tracy RE. Risk scores predict atherosclerotic lesions in young people. Arch Intern Med. 2005;165:883. doi: 10.1001/archinte.165.8.883. et al. –. [DOI] [PubMed] [Google Scholar]
- 16.Pasquali R, Zanotti L, Fanelli F, Mezzullo M, Fazzini A, Morselli Labate AM. Defining Hyperandrogenism in Women With Polycystic Ovary Syndrome: A Challenging Perspective. J Clin End Met. 2016;101:2013. doi: 10.1210/jc.2015-4009. et al. –. [DOI] [PubMed] [Google Scholar]
- 17.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456. doi: 10.1016/j.fertnstert.2008.06.035. et al. –. [DOI] [PubMed] [Google Scholar]
- 18.Wiegman A, Hutten BA, de Groot E, Rodenburg J, Bakker HD, Büller HR. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA. 2004;292:331. doi: 10.1001/jama.292.3.331. et al. –. [DOI] [PubMed] [Google Scholar]
- 19.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512. doi: 10.1161/01.atv.15.9.1512. et al. –. [DOI] [PubMed] [Google Scholar]
- 20.Orio F Jr, Palomba S, Spinelli L, Cascella T, Tauchmanovà L, Zullo F. The Cardiovascular Risk of Young Women with Polycystic Ovary Syndrome: An Observational, Analytical, Prospective Case-Control Study. J Clin End Met. 2003;89:3696. doi: 10.1210/jc.2003-032049. et al. –. [DOI] [PubMed] [Google Scholar]
- 21.Bickerton AS, Clark N, Meeking D, Shaw KM, Crook M, Lumb P. Cardiovascular risk in women with polycystic ovarian syndrome (PCOS) J Clin Pathol. 2005;58:151. doi: 10.1136/jcp.2003.015271. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elting MW, Korsen TJ, Bezemer PD, Schoemaker J. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum Reprod. 2001;16:556. doi: 10.1093/humrep/16.3.556. –. [DOI] [PubMed] [Google Scholar]
- 23.Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49:1442. doi: 10.1161/HYPERTENSIONAHA.106.083972. –. [DOI] [PubMed] [Google Scholar]
- 24.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin End Met. 2010;95:2038. doi: 10.1210/jc.2009-2724. et al. –. [DOI] [PubMed] [Google Scholar]
- 25.Conway GS, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A. The Polycystic Ovary Syndrome: an Endocrinological Perspective from the European Society of Endocrinology. Eur J End. 2014;171:489. doi: 10.1530/EJE-14-0253. et al. –. [DOI] [PubMed] [Google Scholar]
- 26.Macut D, Bjekic-Macut J, Savic-Radojevic A. Dyslipidemia and oxidative stress in PCOS. Front Horm Res. 2013;40:51. doi: 10.1159/000341683. –. [DOI] [PubMed] [Google Scholar]
- 27.Macut D, Damjanovic S, Panidis D, Spanos N, Glisic B, Petakov M. Oxidised low-density lipoprotein concentration – early marker of an altered lipid metabolism in young women with PCOS. Eur J End. 2006;155:131. doi: 10.1530/eje.1.02187. et al. –. [DOI] [PubMed] [Google Scholar]
- 28.Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta analysis. Fertil Steril. 2011;95:1073. doi: 10.1016/j.fertnstert.2010.12.027. –. [DOI] [PubMed] [Google Scholar]
- 29.Skafar DF, Xu R, Morales J, Ram J, Sowers JR. Female sex hormones and cardiovascular disease in women. J Clin End Met. 1997;82:3913. doi: 10.1210/jcem.82.12.4443. –. [DOI] [PubMed] [Google Scholar]
- 30.Mendelsohn ME, Karas RH. The Progective Effects of Estrogen on the Cardiovascular System. The New Engl J Med. 1999;340:1801. doi: 10.1056/NEJM199906103402306. –. [DOI] [PubMed] [Google Scholar]
- 31.Chu MC, Rath KM, Huie J, Taylor HS. Elevated basal FSH in normal cycling women is associated with unfavourable lipid levels and increased cardiovascular risk. Hum Repr. 2003;18:1570. doi: 10.1093/humrep/deg330. –. [DOI] [PubMed] [Google Scholar]
- 32.Carmina E, Lobo RA. Prevalence and metabolic characteristics of adrenal androgen excess in hyperandrogenic women with different phenotype. J End Invest. 2007;30:111. doi: 10.1007/BF03347408. –. [DOI] [PubMed] [Google Scholar]
- 33.Schaffrath G, Kische H, Gross S, Wallaschofski H, Völzke H, Dörr M. Association of sex hormones with incident 10-year cardiovascular disease and mortality in women. Maturitas. 2015;82:424. doi: 10.1016/j.maturitas.2015.08.009. et al. –. [DOI] [PubMed] [Google Scholar]
- 34.Wiltgen D, Benedetto IG, Mastella LS, Spritzer PM. Lipid accumulation product index: a reliable marker of cardiovascular risk in polycystic ovary syndrome. Hum Repr. 2009;24:1726. doi: 10.1093/humrep/dep072. –. [DOI] [PubMed] [Google Scholar]
- 35.Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. JCEM. 2007;92:2500. doi: 10.1210/jc.2006-2725. et al. –. [DOI] [PubMed] [Google Scholar]
- 36.Monami M, Bardini G, Lammana C, Pala L, Cresci B, Francesconi P. Liver enzymes and risk of diabetes and cardiovascular disease: Results of the Firenze Bagno a Ripoli (FIBAR) study. Metabolism. 2008;57:387. doi: 10.1016/j.metabol.2007.10.015. et al. –. [DOI] [PubMed] [Google Scholar]


