Summary
Background
Coronary artery disease (CAD) is the most common cause of mortality and disability from incommunicable disease in the world. Although the association between the single nucleotide polymorphisms (SNPs) in protein-coding genes and the risk of CAD has been investigated extensively, very few heart-disease associated studies concerning the SNPs in miRNA genes have been reported. The present study was performed to elucidate the association between the pre-microRNA-149 (miR-149) SNP rs2292832 and the risk of CAD in an Iranian population.
Methods
Polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) were performed to identify the genotypes of the miR-149 SNP rs2292832 in 421 unrelated subjects (272 with CAD and 149 controls).
Results
Our analysis revealed that the TT genotype was more frequent in CAD patients than control subjects (P=0.02) implying that TT genotype should be considered as a risk factor in CAD development (TT vs. TC+CC p=0.02, OR=1.88).
Conclusions
The present study suggests that rs2292832-TT in pre-miR-149 is associated with CAD in an Iranian population.
Keywords: miR-149, single nucleotide polymorphisms, rs2292832, coronary artery disease, cardiovascular disorders
Kratak sadržaj
Uvod
Koronarna arterijska bolest najčešči je uzrok smrti i invaliditeta usled hronične bolesti na svetu. Iako postoje broj - ne studije o povezanosti između pojedinačnih polimorfizama nukleotida (SNPs) u genima koji kodiraju proteine i rizika za koronarnu arterijsku bolest (KAB), prijavljen je vrlo mali broj studija vezanih za srčane bolesti koje se tiču SNPs u genima za miRNK. Cilj ove studije bio je da se ispita povezanost između pre-mikroRNK-149 (miR-149) SNP rs2292832 i rizika za KAB u populaciji Iranaca.
Metode
Polimerazna lančana reakcija (PCR) i polimorfizam dužine restrikcionih fragmenata (RFLP) upotrebljeni su za identifikovanje genotipova miR-149 SNP rs2292832 kod 421 ispitanika koji nisu u srodstvu (272 sa KAB i 149 kontrola).
Rezultati
Naša analiza je pokazala da je TT genotip bio češči kod obolelih od KAB nego kod kontrolnih subjekata (P=0,02) {to govori da bi TT genotip trebalo razmotriti kao faktor rizika za razvoj KAB (TT vs. TC+CC p=0,02, OR=1,88).
Zaključak
Ova studija ukazuje na povezanost između rs2292832-TT u pre-miR-149 i KAB u iranskoj populaciji.
Ključne reči: miR-149, pojedinačni polimorfizmi nukleotida, rs2292832, koronarna arterijska bolest, kardiovaskularna oboljenja
Introduction
Coronary artery disease (CAD) is the most common cause of mortality and disability from incommunicable disease across the globe (1, 2, 3). The prevalence of CAD in the United States was one of every six deaths in 2010 (4) and it is estimated to be a factor in nearly 50% of all deaths each year in Iran (5). Moreover, it is estimated that the prevalence of CAD will increase to approximately 18% by 2030 (1, 4). CAD is a multifactorial disorder caused by the interplay of environmental and genetic risk factors. It is well known that atherosclerosis is the initial process in CAD pathology (6).
Atherosclerosis is a chronic and inflammatory disease caused by the accumulation of lipids, inflammatory cells and fibrous molecules in vessel endothelium, by which it leads to the narrowing and thickening of the artery walls. This in turn leads to reduced oxygen supply to the heart (7) and, ultimately, sudden cardiac death (2). The exact mechanisms that lead to the development of CAD remain to be understood, but it is believed that CAD has a strong genetic component. There is supporting evidence to suggest that up to 60% of the disease risk is contributed by genetic factors. Until recently, the investigation of genetic factors that predispose humans to the disease has focused mainly on single nucleotide polymorphisms (SNPs) in coding regions. However, many genetic signals discovered through genome-wide association studies (GWAS) can be mapped to non-protein coding sequences.
MicroRNAs (miRNAs) comprise a class of single-strand, non-coding and small (22-24nt) ribonucleic acid molecules. These endogenous conserved molecules are not translated into proteins but instead regulate gene expression at the post-transcriptional level (8).
Recently, miRNAs have attracted a great deal of attention in cardiovascular research. Several reports have indicated that miRNAs have a pivotal regulatory role in the cellular processes that are implicated in CAD pathogenesis. By controlling the behavior of key players in atherosclerosis (i.e., macrophages, endothelial cells and smooth-muscle cells) miRNAs exert a regulatory role on the process (9). There is evidence to suggest that miRNA dysregulation is relevant to CAD development, progression and prognosis.
SNPs within the miRNA regulome (regulatory elements which either regulate miRNA expression or are regulated by miRNA functions) have potential to modulate miRNA-mediated regulation and thereby predispose individuals to different disorders (10, 11). Recent studies have shown that the polymorphism rs2292832 in pre-miR-149 is associated with different cancers (12, 13, 14, 15, 16), stroke (17, 18) and asthma (19). However, it has not been studied in CAD patients. Therefore, in this study we aimed to investigate rs2292832 association with CAD development risk in an Iranian cohort.
Materials and Methods
Study subjects
This case-control study included a total of 421 Iranian subjects (272 with CAD and 149 controls with normal angiogram), referred to Shahid Rajaei Cardiovascular Center (Tehran, Iran). The CAD and control groups were matched for age and gender (Table I). Other demographic and anthropometric data was obtained from medical records. The diagnosis of CAD was based on coronary angiogram. The patient had at least one stenosed epicardial coronary artery (≥50% stenosis) and controls had normal coronary angiograms (<5% stenosis). Written informed consent was obtained from each participant. The study was performed in compliance with the principles of the Helsinki Declaration and was approved by the organization’s ethical committee. We excluded all patients with diabetes, malignancy, autoimmune diseases, infectious diseases, renal failure, hematological disorders, thyroid and liver diseases, history of recent myocardial infarction, familial hypercholesterolemia and history of coronary artery bypass grafting (CABG).
Table I.
Anthropometric and biochemical characteristics of the study subjects.
| Parameter | CAD (n = 272) | Controls (n = 149) | P value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Sex (male/female) | 173/99 | 96/53 | 0.916 |
| Smoker (no/yes) | 70/202 | 27/122 | 0.090 |
| Age (years) | 53.80±10.89 | 52.54±11.02 | 0.253 |
| BMI (kg/m2) | 27.93±3.82 | 25.31±3.39 | <0.001 |
| Systolic blood pressure (mm Hg) | 130.31±17.30 | 126.73±16.32 | 0.037 |
| Diastolic blood pressure (mm Hg) | 75.22±10.97 | 77.47±11.50 | <0.001 |
| LDL-Cholesterol (mmol/L) | 2.76±0.78 | 2.54±1.01 | 0.011 |
| HDL-Cholesterol (mmol/L) | 1.01±0.26 | 1.07±0.25 | 0.033 |
| Triglyceride (mmol/L) | 2.21±1.12 | 1.81±0.67 | <0.001 |
| Total Cholesterol (mmol/L) | 4.79±1.07 | 4.28±1.03 | <0.001 |
Biochemical measurements
Blood samples were collected after a 12-hour fasting period. Serum levels of triglyceride (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-c) were measured in an automated system by routine laboratory methods. Friedwald’s equation was used to calculate low-density lipoprotein cholesterol (LDL-c) levels.
Genotyping analysis
We followed the standard salting out procedure to extract genomic DNA, which was stored at –20 °C until use. The rs2292832 T/C polymorphism was genotyped by Polymerase Chain Reaction - Restriction Fragment Length Polymorphism (PCR-RFLP) using the forward primer 5’-TGT CTT CAC TCC CGT GCT TGT CC-3’ and the reverse primer 5’-TGA GGC CCG AAA CAC CCG TA-3’(12). PCR amplification reaction (25 μL) contained DNA template (50–100 ng), each dNTP (0.5 mmol/L), MgCl2 (1.5 mmol/L) and 1U Taq polymerase (CinnaGen, Tehran, Iran), 5% DMSO and 0.5 μmol/L of each forward and reverse primer. The PCR thermal conditions were: 95 °C for 5 minutes for initial denaturation followed by 30 cycles of 95° for 30 seconds, 61 °C for 30 seconds, 72 °C for 1 minute and a final step at 72° for 10 minutes. The PCR product of 254 base pairs (bp) was digested by the PvuII restriction enzyme (Feremetas, Germany) at 37 °C for 1 hour. The digested fragments were separated by electrophoresis on a 2% agarose gel premixed with SYBR Green (CinnaGen, Tehran, Iran) and then were visualized under UV light. These included a 254 bp fragment for CC homozygotes; fragments of 254 bp, 194 bp and 60 bp for CT heterozygotes; and 194 bp and 60 bp fragments for TT homozygotes.
Bioinformatics analysis
The evolutionary sequence conservation was assessed through multiple sequence alignment (MSA) using the Clustal Omega program (20). Since sequence variations may affect transcript structures, we modeled the secondary structure of the pri- and pre-miR-149 using the RNAsnp software (21) and RNAfold WebServer (22) to estimate the effects of rs2292832 on the corresponding structures.
Statistical analysis
We used SPSS version 18 (Chicago, IL, USA) and R platform programs to perform statistical analysis. The Shapiro-Wilk test was used for testing the normality of data. Depending on whether the data was normally distributed, quantitative variables were expressed as mean ±SD or median. The compatibility of genotype frequencies with Hardy–Weinberg equilibrium expectations was checked through the chi-square goodness-of-fit test with one degree of freedom. All genetic association study statistics were calculated by the SNPassoc package (23) in R platform. The odds ratio (OR) and 95% confidence intervals (CIs) were calculated as a measure of the correlation between rs2292832 and CAD. P value ≤0.05 was considered statistically significant.
Results
Main characteristics of the study population
The anthropometric and biochemical characteristics of the included subjects in both groups (272 patients and 149 controls) are shown in Table I. The study groups were matched for age and sex (P=0.253 and 0.916, respectively). We found significant differences between CAD patients and controls for body mass index (BMI) (mean value of 27.93± 3.82 vs. 25.31±3.39 kg/m2, P<0.001), systolic blood pressure (SBP) (mean value of 130.31±17.30 vs. 126.73±16.32 mmHg, P=0.037), diastolic blood pressure (DBP) (mean value of 75.22±10.97 vs. 77.47±11.50 mmHg, P<0.001), LDL-c (mean level of 2.76±0.78 vs. 2.54±1.01 mmol/L, P=0.011), HDL-c (mean level of 1.01±0.26 vs. 1.07±0.25 mmol/L, P=0.033), TG (triglyceride) (mean level of 2.21±1.12 vs. 1.81±0.67 mmol/L, P<0.001) and TC (total cholesterol) (mean level of 4.79±1.07 vs. 4.28±1.03 mmol/L, P<0.001).
Genotypes and allele frequencies of rs2292832
The genotype frequency was in agreement with the Hardy-Weinberg equilibrium (χ2=0.00076 P>0.05). The distribution of rs2292832 genotypes and the frequency of alleles between CAD patients and control subjects are presented in Table II. We analyzed the rs2292832 genotype association with
Table II.
Genotypes and allele frequencies of rs2292832 in CAD patients and controls.
| Models | Control | CAD | OR (95% CI) | P value | AIC |
|---|---|---|---|---|---|
| Allele | |||||
| C | 185 (62.0) | 319 (58.6) | |||
| T | 113 (38.0) | 225 (41.4) | 1.15 (0.86–1.54) | 0.34 | |
| Co-dominant | 548.1 | ||||
| CC | 53 (35.6) | 95 (34.9) | 0.07 | ||
| CT | 79 (53.0) | 124 (45.6) | |||
| TT | 17 (11.4) | 53 (19.5) | |||
| Dominant | 551.1 | ||||
| CC | 53 (35.6) | 95 (34.9) | |||
| CT+TT | 96 (64.4) | 177 (65.1) | 1.03 (0.68–1.56) | 0.89 | |
| Recessive | 546.4 | ||||
| CC+CT | 132 (88.6) | 219 (80.5) | |||
| TT | 17 (11.4) | 53 (19.5) | 1.88 (1.04–3.38) | 0.02 | |
| Over-dominant | 549.0 | ||||
| CC+TT | 70 (47.0) | 148 (54.4) | |||
| CT | 79 (53.0) | 124 (45.6) | 0.74 (0.50–1.11) | 0.14 | |
| log-Additive | 549.6 | ||||
| 0,1,2 | 149 (35.4) | 272 (64.6) | 1.20 (0.90–1.60) | 0.21 |
CAD under four genetic models (co-dominant, dominant, recessive and over-dominant). The AIC (Akaike’s information criterion) was calculated to choose the most appropriate model of inheritance. Accordingly, the recessive model was the best fit for the inheritance model with the lowest AIC for rs2292832. Our analysis revealed that the TT genotype was more frequent in CAD patients than controls (P=0.02; OR=1.88; 95% CI: 1.04–3.38) under the recessive model.
In-silico analysis
Multiple-sequence alignment
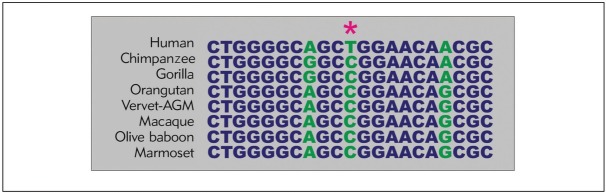
MSA (Multiple-Sequence Alignment) analysis revealed that rs2292832 and the flanking sequence are well conserved, at least in primate species. However, as Figure 1 shows, most primate species have the C allele at the rs2292832 position, whereas this allele was substituted by the T allele in Homo sapiens.
Figure 1.
Multiple sequence alignment for rs2292832 and flanking sequence.
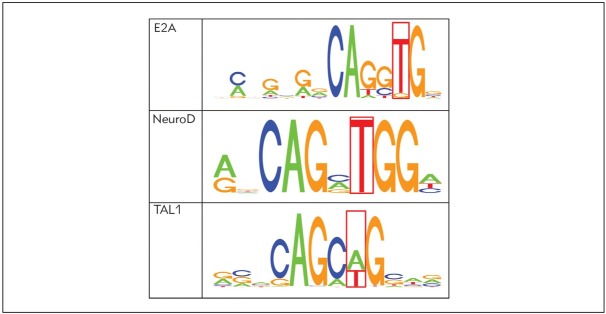
Evidence exists to support the potential functional relevance of rs2292832. The ENCODE DNase footprinting assay experiments indicated that rs22928320 is part of a canonical binding motif for the E2A, NeuroD and TAL1 transcriptional factors (Figure 2).
Figure 2.
Canonical motif for E2A, NeuroD and TAL1 transcriptional factors.
The effect of rs2292832 on the secondary structure of pre-miR-149
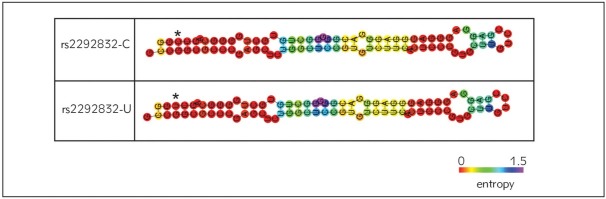
We employed the RNAsnp algorithm to test whether the rs2292832 SNP affects the pre-miR-149 secondary structure. Our results showed that this SNP is not structure-disruptive (Pdmax: 0.95). Furthermore, we did so in order to estimate the SNP impact on the stability of the miRNA hairpin structure, using the RNAWebServer. We modeled the minimum free energy (MFE) and centroid structures (weighted average of possible structural conformations) for the precursor hsa-miR-149 with and without the rs2292832 variant. There was no significant structure modification due to the different alleles of rs2292832 (Figure 3).
Figure 3.
Schematic view of the RNAfold predicted secondary structures of the pre-miR-149 containing either the wild type or the mutant allele of rs2292832. The SNP position is shown by asterisk. There is no difference in ΔG between hsa-miR-149 with mutant and wild-type allele.
Discussion
In the present study, we investigated the possible association between rs2292832 T/C SNP in miR-149 and CAD in an Iranian population. Although we did not observe a significant difference in the alleles’ frequencies between patients and controls (P=0.34), the rs2292832-TT genotype was significantly more prevalent in patients (P=0.02; OR=1.88; 95% CI: 1.04–3.38). Of note, this is the first report that describes the effects of this SNP in an Iranian cohort. Jing Xu et al. (24) investigated the possible association between rs2292832 and congenital heart disease (CHD) in a Chinese population. Nevertheless, they failed to show significant association between the SNP genotypes and CHD development. These contradictory results could be explained mainly by disease heterogeneity. However, differences in sample size and subject ethnicity may also contribute to different results.
Recent studies have shown that genetic variations in microRNA could increase and/or decrease miRNA expression levels and thereby contribute to many pathologic states (25). Despite the fact that many studies have addressed the association between the SNPs in protein-coding regions and the risk of CAD, very few association studies have investigated the association of SNPs in non-coding regions (i.e., miRNA genes) and CAD (26). Several other studies have demonstrated that variations in miR-149 sequence are linked with a range of human diseases including cancers, ischemic stroke (IS) and asthma (12, 13, 14, 15, 17, 18, 19). Young Joo Jeon et al. (17) reported that miR-149 rs2292832 was associated with IS risk and silent brain infarction in a Korean population. Furthermore, they showed that miR-149 was involved in the regulation of the tumor necrosis factor-α (TNF-α) signaling pathway. It is well-established that TNF-α plays a crucial role in the initiation and progression of endothelial dysfunction (27).
It is largely accepted that elevated levels of blood lipids and systolic blood pressure are the risk factors for CAD susceptibility and development (28). We also found that the serum levels of BMI, SBP, DBP, LDL-c, HDL-c, TG and TC in CAD patients were higher than controls. This may influence our conclusion regarding the association of miR-149 rs2292832 with CAD development.
To further investigate the role of miR-149 in CAD pathogenesis, we performed a gene-set enrichment analysis on miR-149 target genes using the Enrichr web server (29) and target genes from the miRTarBase database (30). We found that the targets were enriched in inflammatory and apoptotic pathways. It is well documented that inflammation is implicated in the initial processes of CAD development (31, 32). It has been shown that miR-149 negatively regulates the TLR triggered inflammatory response of cytokine production through a mechanism of targeting the adaptor molecule MyD88 (33). Additionally, by targeting the puma protein, miR-149 negatively regulates the apoptosis process. Thus, this miRNA inhibits the mitochondrial fission and increases cardiac muscle cell viability (34). This evidence further supports the notion that miR-149 down-regulation is implicated in CAD development (1, 4, 35).
The ENCODE DNase footprinting assay experiments revealed that rs22928320 is part of a canonical binding motif for the E2A, NeuroD and TAL1 transcriptional factors (36). These transcription factors have different roles in cellular physiology, including apoptosis, cell growth and cell-to-cell communication. The disturbance of these processes is implicated in different diseases, including CAD.
The MSA analysis revealed that, at the rs2292832 position, the ancestral allele C is substituted with T in humans. A part of the human difference with respect to primate ancestors may be confirmed by such variation. In fact, it has been proposed that this kind of SNP is involved in the development of human-specific traits/phenotypes (32, 37). Further bioinformatics analysis has revealed that rs2292832 is not structure-disruptive in the secondary structure of pri-miR-149 nor pre-miR-149. Further functional studies are warranted to elucidate the effect of rs2292832 on the miR-149 transcript structure. Since rs2292832 is found to reside outside the sequence of mature miR-149, it is suggested that SNP may influence the maturation process and consequently down-regulate the miR-149 expression level (15). Therefore, our current study showed that rs2292832 in miR-149 is associated with CAD development in an Iranian population.
Acknowledgments
The authors appreciate the financial support from the Shahid Beheshti University of Medical Sciences (No. 194).
List of abbreviations
- PCR
polymerase chain reaction
- RFLP
restriction fragment length polymorphism
- CAD
coronary artery disease
- SNPs
single nucleotide polymorphisms
- GWAS
genome-wide association studies
- CABG
coronary artery bypass grafting
- MSA
multiple sequence alignment
- BMI
body mass index
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- AIC
Akaike’s information criterion
- MFE
minimum free energy
- CHD
congenital heart disease
- TNF-α
tumor necrosis factor-α
Footnotes
Conflict of interest
Conflict of interest statement: The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.Ali Sheikh MS, Xia K, Li F, Deng X, Salma U, Deng H. Circulating miR-765 and miR-149: Potential noninvasive diagnostic biomarkers for geriatric coronary artery disease patients. BioMed research international. 2015;9:63. doi: 10.1155/2015/740301. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai X, Wiernek S, Evans JP, Runge MS. Genetics of coronary artery disease and myocardial infarction. World Journal of Cardiology. 2016;8:1. doi: 10.4330/wjc.v8.i1.1. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin F, Mehdi Jahani M, Ghaedi H, Alipoor B, Fatemi A, Tajik M, Sharifi Z, Golmohammadi T, Askari M, Azarnejad A, Alipoor S, Valipour A, Mousavizadeh K. Genetic variants of cytochrome b-245, alpha polypeptide gene and premature acute myocardial infarction risk in an Iranian population. J Med Biochem. 2015;34:402. doi: 10.2478/jomb-2014-0066. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sayed ASM, Xia K, Li F, Deng X, Salma U, Li T. The diagnostic value of circulating microRNAs for middleaged (40–60-year-old) coronary artery disease patients. Clinics. 2015;70:257. doi: 10.6061/clinics/2015(04)07. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatmi Z, Tahvildari S, Motlag AG, Kashani AS. Prevalence of coronary artery disease risk factors in Iran: a population based survey. BMC Cardiovascular Disorders. 2007;7:1. doi: 10.1186/1471-2261-7-32. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badimon L, Padró T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. European Heart Journal: Acute Cardiovascular Care. 2012;1:60. doi: 10.1177/2048872612441582. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosin AA, Prasad A, Viiri LE, Davies AH, Shalhoub J. MicroRNAs in atherosclerosis. Journal of Vascular Research. 2014;51:338. doi: 10.1159/000368193. –. [DOI] [PubMed] [Google Scholar]
- 8.Saadatian Z, Masotti A, Fam ZNS, Alipoor B, Bastami M, Ghaedi H. Single-nucleotide polymorphisms within micrornas sequences and their 3’utr target sites may regulate gene expression in gastrointestinal tract cancers. Iranian Red Crescent Medical Journal. 2014;16:2261. doi: 10.5812/ircmj.16659. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreou I, Sun X, Stone PH, Edelman ER, Feinberg MW. miRNAs in atherosclerotic plaque initiation, progression, and rupture. Trends in molecular medicine. 2015;21:307. doi: 10.1016/j.molmed.2015.02.003. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulik Sullivan B, Selitsky S, Sethupathy P. Prioritization of genetic variants in the microRNA regulome as functional candidates in genome wide association studies. Human mutation. 2013;34(8):1049. doi: 10.1002/humu.22337. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghaedi H, Bastami M, Zare-Abdollahi D, Alipoor B, Movafagh A, Mirfakhraie R. Bioinformatics prioritization of SNPs perturbing microRNA regulation of hematological malignancy-implicated genes. Genomics. 2015;106(6):360. doi: 10.1016/j.ygeno.2015.10.004. et al. –. [DOI] [PubMed] [Google Scholar]
- 12.Huang GL, Lu Y, Pu XX, He YX, Chen ML, Li YZ. Association study between miR 149 gene polymorphism and nasopharyngeal carcinoma. Biomedical Reports. 2013;1:599. doi: 10.3892/br.2013.97. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Z, Chen J, Tian T, Zhou X, Gu H, Xu L. Genetic variants of miRNA sequences and non–small cell lung cancer survival. The Journal of Clinical Investigation. 2008;118:2600. doi: 10.1172/JCI34934. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Ma YL, Zhang P, Shen TY, Shi CZ, Yang YZ. SP1 mediates the link between methylation of the tumour suppressor miR 149 and outcome in colorectal cancer. The Journal of Pathology. 2013;229:12. doi: 10.1002/path.4078. et al. –. [DOI] [PubMed] [Google Scholar]
- 15.Wei W-J, Lu Z-W, Li D-S, Wang Y, Zhu Y-X, Wang Z-Y. Association of the miR-149 Rs2292832 polymorphism with papillary thyroid cancer risk and clinico-pathologic characteristics in a Chinese population. International Journal of Molecular Sciences. 2014;15:20968. doi: 10.3390/ijms151120968. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M, Chen W, He Y, Gu Y. Role of miR-149C> T polymorphisms on the risk of hepatocellular carcinoma in a Chinese population. Genet Mol Res. 2014;13:7184. doi: 10.4238/2014.September.5.4. –. [DOI] [PubMed] [Google Scholar]
- 17.Jeon YJ, Kim OJ, Kim SY, Oh SH, Oh D, Kim OJ. Association of the miR-146a, miR-149, miR-196a2, and miR-499 polymorphisms with ischemic stroke and silent brain infarction risk. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33:420. doi: 10.1161/ATVBAHA.112.300251. et al. –. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Liu N, Ma J, Fang Y, Cao Y, Li H. Effect of a pre-microRNA-149 (miR-149) genetic variation on the risk of ischemic stroke in a Chinese Han population. Genet Mol Res. 2015;14:2582. doi: 10.4238/2015.March.30.17. et al. –. [DOI] [PubMed] [Google Scholar]
- 19.Kishore A, Borucka J, Petrkova J, Petrek M. Novel in-sights into miRNA in lung and heart inflammatory diseases. Mediators of Inflammation. 2014;213:60. doi: 10.1155/2014/259131. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sievers F, Higgins DG. Clustal Omega, accurate alignment of very large numbers of sequences. Multiple sequence alignment methods. 2014;1079:105. doi: 10.1007/978-1-62703-646-7_6. –. [DOI] [PubMed] [Google Scholar]
- 21.Sabarinathan R, Tafer H, Seemann SE, Hofacker IL, Stadler PF, Gorodkin J. The RNAsnp web server: predicting SNP effects on local RNA secondary structure. Nucleic Acids Research. 2013;41(W1):W475. doi: 10.1093/nar/gkt291. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofacker IL. Vienna RNA secondary structure server. Nucleic Acids Research. 2003;31(13):3429. doi: 10.1093/nar/gkg599. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19(1):149. doi: 10.1093/bioinformatics/19.1.149. –. [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Hu Z, Xu Z, Gu H, Yi L, Cao H. Functional variant in microRNA 196a2 contributes to the susceptibility of congenital heart disease in a Chinese population. Human Mutation. 2009;30:1231. doi: 10.1002/humu.21044. et al. –. [DOI] [PubMed] [Google Scholar]
- 25.Ghaedi H, Tabasinezhad M, Alipoor B, Shokri F, Movafagh A, Mirfakhraie R. The pre-mir-27a variant rs895819 may contribute to type 2 diabetes mellitus susceptibility in an Iranian cohort. Journal of Endocrinological Investigation. 2016;10:1187. doi: 10.1007/s40618-016-0499-4. et al. –. [DOI] [PubMed] [Google Scholar]
- 26.Xiong X-d, Cho M, Cai X-p, Cheng J, Jing X, Cen J-m. A common variant in pre-miR-146 is associated with coronary artery disease risk and its mature miRNA expression. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2014;761:15. doi: 10.1016/j.mrfmmm.2014.01.001. et al. –. [DOI] [PubMed] [Google Scholar]
- 27.Palmieri D, Capponi S, Geroldi A, Mura M, Mandich P, Palombo D. TNFα induces the expression of genes associated with endothelial dysfunction through p38MAPK-mediated down-regulation of miR-149. Biochemical and Biophysical Research Communications. 2014;443:246. doi: 10.1016/j.bbrc.2013.11.092. –. [DOI] [PubMed] [Google Scholar]
- 28.Wilson PW. Established risk factors and coronary artery disease: the Framingham Study. American Journal of Hypertension. 1994;7:7S. doi: 10.1093/ajh/7.7.7s. –. S.29. [DOI] [PubMed] [Google Scholar]
- 29.Enricher. Enricher. 2016. http://amp.pharm.mssm.edu/Enrichr/ [27/6/2016]. Available from. [Google Scholar]
- 30.miRTarBase. miRTarBase 2016 [27/06/2016]. Available from. www.mirtarbase.mbc.nctu.edu.tw
- 31.Spagnoli LG, Bonanno E, Sangiorgi G, Mauriello A. Role of inflammation in atherosclerosis. Journal of Nuclear Medicine. 2007;48(11):1800. doi: 10.2967/jnumed.107.038661. –. [DOI] [PubMed] [Google Scholar]
- 32.Tousoulis D, Kampoli A-M, Papageorgiou N, Androulakis E, Antoniades C, Toutouzas K. Pathophysiology of atherosclerosis: the role of inflammation. Current Pharmaceutical Design. 2011;17(37):4089. doi: 10.2174/138161211798764843. et al. –. [DOI] [PubMed] [Google Scholar]
- 33.Xu G, Zhang Z, Xing Y, Wei J, Ge Z, Liu X. MicroRNA 149 Negatively Regulates TLR Triggered Inflammatory Response in Macrophages by Targeting MyD88. Journal of Cellular Biochemistry. 2014;115:919. doi: 10.1002/jcb.24734. et al. –. [DOI] [PubMed] [Google Scholar]
- 34.Ding S-L, Wang J-X, Jiao J-Q, Tu X, Wang Q, Liu F. A pre-microRNA-149 (miR-149) genetic variation affects miR-149 maturation and its ability to regulate the Puma protein in apoptosis. Journal of Biological Chemistry. 2013;288:26865. doi: 10.1074/jbc.M112.440453. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong D-l, Yang B-f. Role of microRNAs in cardiac hypertrophy, myocardial fibrosis and heart failure. Acta Pharmaceutica Sinica B. 2011;1:1. –. [Google Scholar]
- 36.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M. Annotation of functional variation in personal genomes using RegulomeDB. Genome Research. 2012;22:1790. doi: 10.1101/gr.137323.112. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed M, Liang P. Study of modern human evolution via comparative analysis with the Neanderthal genome. Genomics & informatics. 2013;11(4):230. doi: 10.5808/GI.2013.11.4.230. –. [DOI] [PMC free article] [PubMed] [Google Scholar]