Abstract
Systemic cobaltism is a debilitating complication of metal-on-metal (MoM) arthroplasty. In this report, we review a case of a 54-year-old female with metallosis from a MoM hip resurfacing and varying degrees of black discoloration of her tongue and metallic gustation as a result of systemic cobaltism. After explanting the metal components, thorough debridement, and conversion to ceramic-on-polyethylene arthroplasty, the patient’s oral mucosal discoloration and metallic gustation resolved. This represents the first documentation of systemic cobaltism from MoM hip resurfacing manifesting as oral mucosal discoloration and metallic gustation with resolution after explant, debridement, and conversion to ceramic-on-polyethylene total hip arthroplasty.
Keywords: Metallosis, Metal on metal, Hip resurfacing, Systemic cobaltism, Tongue discoloration
Introduction
Metallosis is a well-described complication of metal-on-metal (MoM) hip resurfacing and can precipitate systemic toxicity. Metallosis results from wear and corrosion of metal-containing implants causing deposition of metal particles into the local tissue; this can be seen at any bearing joint including the head-acetabulum junction and trunnion [1], [2]. Prior reports have articulated a distinction between local and systemic deposition of metal debris. Wear and corrosion may result in large particulate, nanoparticulate, and ionic metal debris that can metastasize locally through lymphatics or undergo phagocytosis and systemic transport via serum proteins or intracellularly in endosomes depending on its valence [3], [4], [5]. Particulate debris may manifest as local discoloration and adjacent epidermal tattooing, pseudotumor, and adverse local tissue reaction (ALTR) (metallosis) [2], [3], [6], [7], [8], [9], [10]. Systemic metal toxicity, notably systemic cobaltism, may potentiate organ failure and distant discoloration, seen once before as green tongue discoloration with vanadium toxicity from ceramic-on-ceramic components [5], [11], [12], [13].
However, to our knowledge, there are no prior reports of black discoloration of the oral mucosa or metallic gustation from systemic cobaltism in the presence of a MoM hip resurfacing. To illustrate this, we present a case of a 54-year-old female with black tongue discoloration, metallic gustation, and elevated serum cobalt and chromium that resolved after explant of a Birmingham Hip Resurfacing (BHR) system (Smith & Nephew, Inc., Memphis, TN) MoM hip resurfacing prosthesis and conversion to a ceramic-on-polyethylene (CoP) total hip arthroplasty.
Case history
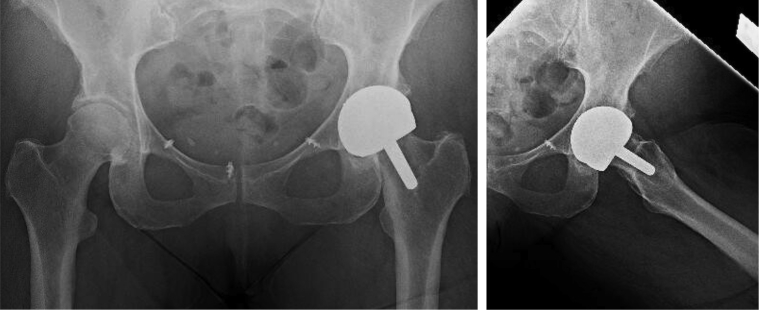
Informed consent was obtained from the patient before publication of this case history. A 54-year-old female with stage IV ovarian cancer in remission after debulking surgery and chemotherapy and stage IB melanoma locally resected underwent uncomplicated MoM hip resurfacing with the BHR system in 2006. She presented to our clinic in October 2016 with complaints of knee pain refractory to corticosteroid injections and intermittent buttock pain. Plain radiographs of the resurfaced hip and symptomatic knee were obtained at that time, revealing no evidence of calcar resorption, component loosening, or other radiographic marker of implant failure (Fig. 1). Knee radiographs were unremarkable. Laboratory analysis of erythrocyte sedimentation rate, C-reactive protein, and white blood cell count resulted as normal. With concern for occult infection vs early manifestation of metallosis, an ultrasound-guided hip aspiration was performed, revealing cell count of 1293 nucleated cells, 50.8% neutrophils and negative for alpha-defensins. Serum laboratory reports were also obtained and notable for cobalt levels of 34 ppb and chromium of 28.1 ppb. Magnetic resonance imaging was considered to evaluate for ALTR, but not pursued, as her serum metal ions alone were indicative of ALTR [14], [15], and there was no evidence of abductor deficiency on examination. Conversion arthroplasty was offered but declined due to the patient’s high level of function. Her Harris Hip Score [16] at the time of initial presentation was 82.0.
Figure 1.
Preoperative radiographs demonstrating MoM hip resurfacing, dated July 31, 2017. There is no evidence of calcar resorption or component loosening to suggest metallosis. There are prior suture anchors in the anterior pelvis from an unrelated surgery.
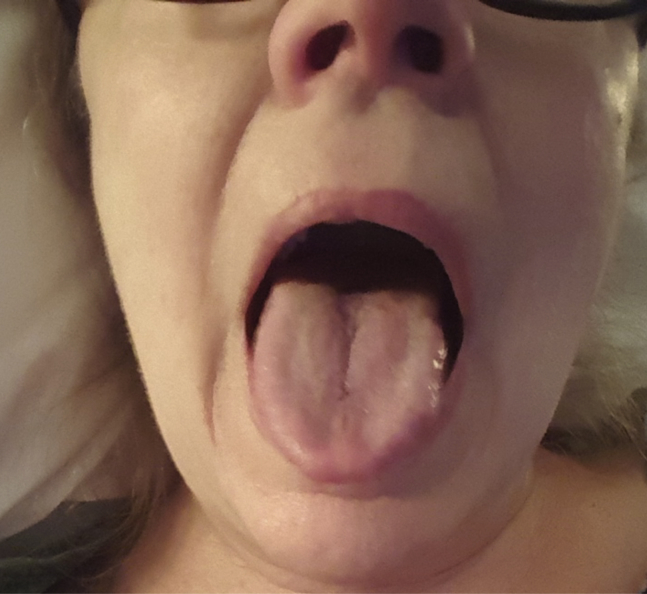
The patient returned to clinic 6 months later (March 2017) with resolved knee pain but worsening buttock pain and complaints of exercise fatigue. Her Harris Hip Score was 61.1. Serum ion levels were repeated revealing an uptrend in serum cobalt level of 39 ppb and chromium of 47.2 ppb. She had significant pain with hip internal rotation and flexion, with no evidence of contracture or abductor deficiency. The patient was additionally found to have black tongue discoloration on complete preoperative examination which had been present for 6 months and refractory to instrumented debridement by oral health-care providers and prescription oral hygienics including toothpastes and mouthwash (Fig. 2). The patient reported that this discoloration was constant but varied inconsistently in intensity of coloration. She also reported mild metallic gustation and aversion to some foods. No biopsy of the discoloration was performed. No evidence of discoloration was apparent on her dentition which appeared to be in good health.
Figure 2.
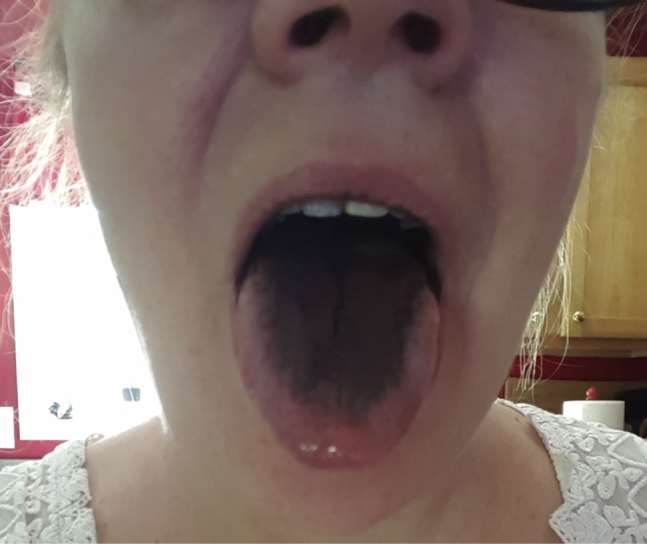
Clinical photograph of black discoloration of the patient’s tongue on June 15, 2017, coinciding with serum cobalt level of 39 ppb and chromium of 47.2 ppb.
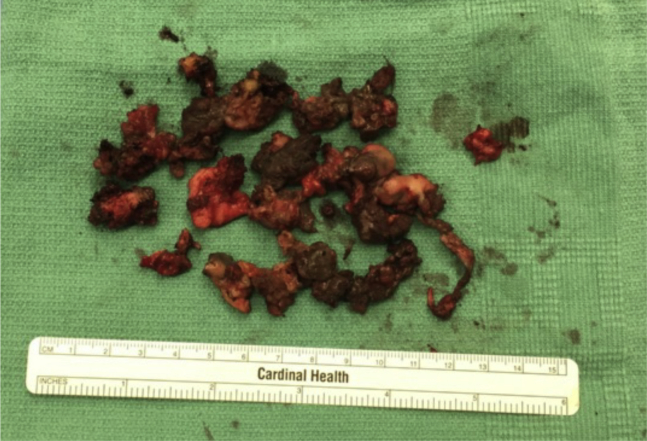
In August 2017, the patient underwent conversion arthroplasty to CoP total hip arthroplasty. A standard posterior approach to the hip was used. Upon exposing the implant, there was a very large black fluid collection with extensive black-gray staining of the pericapsular tissues, muscle, and bone (Fig. 3). There was significant fat necrosis with friable fat and capsule. The abductors were superficially friable and debrided; the majority were spared. Four specimens sent to the microbiology laboratory were finalized as negative for infection after 14 days. The remaining stained and friable pericapsular tissue was debrided, and the wound was thoroughly irrigated before implantation of a Stryker Accolade II Femoral Stem and Stryker Trident Hemispherical Acetabular liner with modular dual mobility liner (Kalamazoo, MI) with a CeramTec BIOLOX femoral head (Plochingen, Germany). Although there was possibility for mechanically assisted crevice corrosion with this implant, the average serum metal ion level at 2-year follow-up has been shown to be less than 1.0 ppb, which is the accepted cutoff for a well-functioning hip arthroplasty [17]. In similar fashion, use of a ceramic head mitigated the risk of trunnion corrosion [18]. In addition, the modular dual mobility liner afforded enhanced stability when considering abductor deficiency associated with metallosis [17]. Thus, the benefit of implant stability outweighed the risk of further metallosis, and this implant was chosen. On postoperative day 1, the patient noted improvement in her tongue coloration, and by postoperative day thirteen, her tongue had returned to normal coloration, and gustation returned to baseline (Fig. 4).
Figure 3.
Intraoperative photograph taken on August 8, 2017, of black-gray discoloration of periarticular tissue including fat, muscle, and capsule.
Figure 4.
Clinical photograph of the patient’s resolved tongue coloration on August 21, 2017, after debridement and conversion arthroplasty, coinciding with serum cobalt 8.7 ppb and chromium 18.5 ppb.
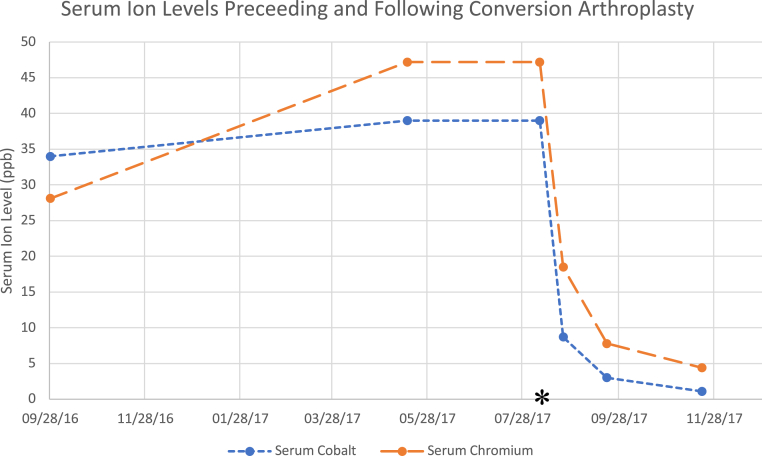
The patient’s serum ion levels continued to downtrend postoperatively. At 2 weeks postoperatively, her serum cobalt level was 8.7 ppb and serum chromium was 18.5 ppb (Fig. 5). At 6 weeks postoperatively, her serum cobalt level was 3.0 ppb and serum Chromium was 7.8. ppb. At 12-weeks postoperatively, her serum cobalt was 1.1 ppb and serum chromium was 4.4 ppb (Fig. 6). By this time, the patient had returned to a high level of function. Her Harris Hip Score was 95.7. Although not available at time of this report, laboratory assessment of serum metal ion levels is planned at 1 year postoperatively to ensure levels are less than 1 ppb.
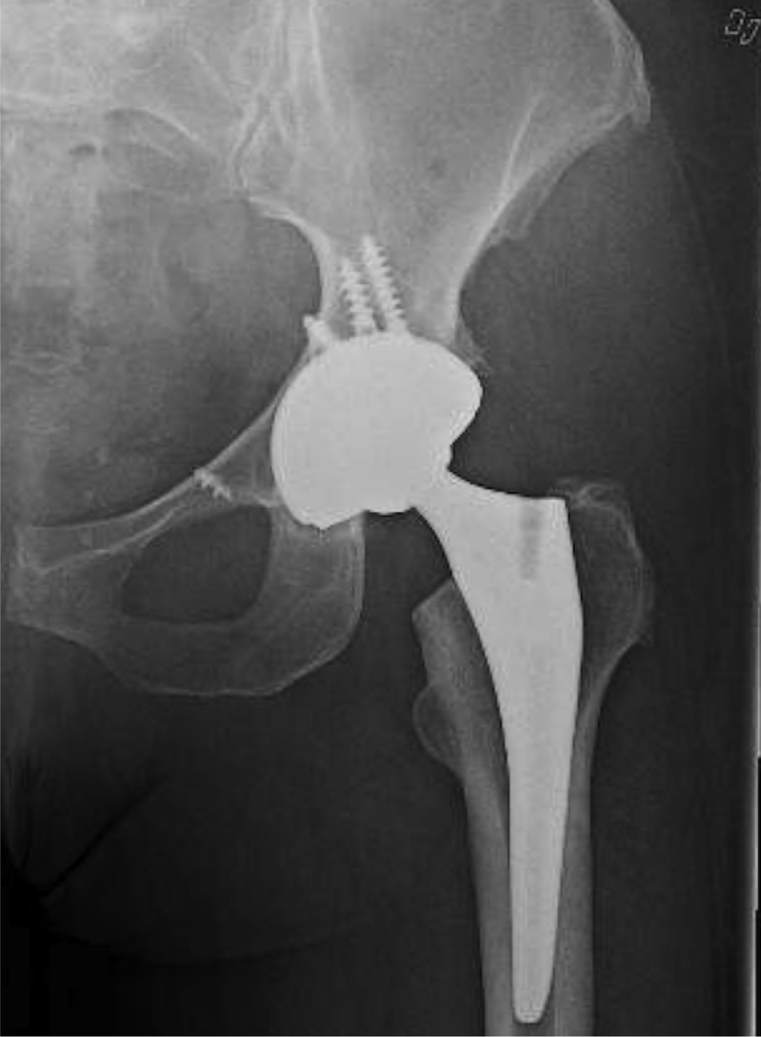
Figure 5.
Postoperative radiograph demonstrating CoP arthroplasty without adverse features, dated August 23, 2017.
Figure 6.
Line chart depicting uptrend, preoperatively, and downtrend, postoperatively, of serum metal ion levels. The asterisk represents the date of conversion arthroplasty.
Discussion
MoM hip resurfacing was approved for use by the FDA in 2006 and popularized for use in the younger and more active individual with femoroacetabular arthritis because of reports of less volumetric wear, preserved bone stock, increased stability, and enhanced ability to return to high-impact activity compared with conventional total hip arthroplasty [19], [20], [21]. Nevertheless, nearly 13% of MoM hip resurfacings were revised within 10 years compared with 20% of MoM total hip arthroplasties and less than 4% of metal-on-polyethylene (MoP) total hip arthroplasties. This uptrend in revision rate has previously been attributed to metal particulate fretting and corrosion debris in MoM arthroplasties [2].
Metallic debris can be released as a function of wear and corrosion that can occur at any bearing joint of a MoM implant, including the head-acetabular junction, trunnion, and other modular components. Wear occurs during interaction of surfaces under relative motion and includes abrasion where a harder surface wears against a softer surface, adhesion from shear stresses, and fatigue from exceeding the material’s fatigue strength. Corrosion occurs when metal comes into contact with air and undergoes oxidation [1]. Wimmer et al. [22] demonstrated that corrosion can be beneficial, as oxidation of the metal components before implantation forms a protective tribochemical film that reduces wear through lubrication. However, the same oxide film's relative softness results in fretting wear and repeat oxidation, causing generation and delamination of the film, and potentiating a vicious cycle of protection and wear, causing deposition of local metal debris of varying microscopic and macroscopic sizes [1], [22].
Locally, metallosis may cause local metallic discoloration and tissue necrosis, pseudotumor, and aseptic lymphocyte-dominated vasculitis-associated lesion [2], [10], [12]. Patients with local metal toxicity can present with pain, antalgic gait, weakness, reduced range of motion, and instability. It is not uncommon for patients to present with complaints of clicking, clunking, or grinding in the hip, signifying early instability before a dislocation event [2], [23]. Owing to the low specificity of these complaints, it is imperative to investigate other complications including infection, aseptic loosening, malposition of components, fracture, and dislocation when metallosis is suspected.
Systemic metal ions are significantly higher in patients with MoM prostheses than in those with MoP prostheses and even higher in those with worse outcomes in terms of pain and level of function [24], [25], [26]. Systemically, metal toxicity, including cobaltism, can present with fatigue, weakness, hypothyroidism, polycythemia, cognitive dysfunction, neuropathy, encephalopathy, and organ failure [10], [12], [13], [27], [28]. Fatal cardiomyopathy has also been demonstrated with extremely high cobalt levels from orthopedic implants [27], [29]. Molecularly, chromium and cobalt ions induce DNA damage both directly at the level of the nucleus and indirectly by inducing reactive oxygen species [30]. Carcinogenic forms of chromium have been identified in cardiac, hepatic, and splenic tissue in postmortem analysis of patients who underwent MoP hip arthroplasty [5].
Heavy-metal discoloration in the oral mucosa is well described in the dental literature through exogenous deposition from increased blood levels. Deposition can be seen with metal-containing prescription drugs, for example, in arsenicals, which were used to treat syphilis as well as unintentional exposure to heavy metals, for example lead, which presents as a blue-black line along the gingiva known as Burtonian line. Metal depositions in the oropharynx are typically diffuse and bilateral [31], [32]. Oral mucosal discoloration has been seen previously after arthroplasty in 1 report as green tongue discoloration from systemic vanadium toxicity after ceramic-on-ceramic total hip arthroplasty [11]. Dysgeusia and a lingual film were reported by Oldenburg et al. in a patient with severe systemic cobaltism after a failed and revised CoP total hip arthroplasty, but there was no further elucidation of the changes in gustation or coloration of the film [33].
Mechanistically, the surface of cobalt-chromium alloy used in MoM resurfacings undergoes oxidation before implantation, resulting in a protective chromium oxide–rich passivation film that can fail after implantation because of wear, likely fatigue and fretting in this case, and unintended corrosion from micromotion [1], [5], [22]. This releases cobalt-chrome alloy particles that can be phagocytosed, degraded into ionic or nanoparticulate form by lysosomal enzymes, and transported hematogenously in endosomes [4], [34]. Changes in endosomal pH cause release of cobalt-chrome into tissues [5], and the cycle continues. It is possible that our patient's tongue coloration and gustation changed alongside the varying acidity of ingested foods. Further investigation of this problem could be simply evaluated by safely changing the patient's oral pH and monitoring clinically. Biopsy would be needed to confirm this theory. This ionic [4] or nanoparticulate [34] mechanism differs from that of local metallic staining, local tissue reactions, and spread of larger particulate debris to the overlying epidermis through lymphatic metastasis, which results from abrasion and causes tattooing rather than transient discoloration [2], [3], [6], [7], [8], [9].
To date, there is no consensus laboratory value for the diagnosis of metallosis. The diagnosis of metallosis is made on a combination of clinical concern, elevated serum metal ion levels, and abnormalities on cross-sectional imaging, including pseudotumor, muscle atrophy, and soft-tissue inflammation [2], [14]. Recently, an external multicenter study from Europe validated fewer missed incidences of adverse reactions to metal debris with implant-specific thresholds than the fixed thresholds recommended in Europe and the United States. Specifically, thresholds of 2.15 ppb of cobalt for unilateral BHR, 5.5 ppb of either cobalt or chromium for bilateral BHR, and 3.57 ppb of cobalt for unilateral DePuy Corail-Pinnacle (Leeds, UK) implants were validated. Using implant-specific thresholds, an absolute reduction of 0.2%, 2.1%, and 3.7% in missed incidences of adverse reactions to metal debris compared to fixed thresholds of 3.0 ppb, 7.0 ppb, and 10.0 ppb, respectively, was demonstrated [35]. In addition, Fillingham et al. [15] found that a threshold of 1.0 ppb for serum cobalt demonstrated high sensitivity (100%) and specificity (90%) in identifying the presence of ALTR in MoP arthroplasty from trunnion corrosion (mechanically assisted crevice corrosion). Although serum chromium levels greater than 0.15 ppb and a ratio of serum cobalt to chromium greater than 1:4 were also implicated in the study, lower specificity (50% and 70%, respectively) was demonstrated in correlating ALTR. These data suggest that a lower and perhaps implant-specific threshold should be entertained by surgeons when diagnosing metallosis and systemic toxicity. As the use of obtaining serum metal ion levels continues to be elucidated in the diagnosis of systemic metal ion toxicity, findings such as black tongue discoloration and metallic gustation may provide further diagnostic insight when found in conjunction with elevated serum metal ion levels.
We do appreciate that this patient had significant medical comorbidities including stage IV ovarian carcinoma and stage IB melanoma and that chemotherapy may cause tongue hyperpigmentation or discoloration [36], [37]. The patient received 6 cycles of paclitaxel and carboplatin, followed by 12 additional cycles of paclitaxel in 2008 after optimal debulking of serous ovarian adenocarcinoma, 2 years after MoM hip resurfacing and nearly a decade before the conversion arthroplasty. She remains on tamoxifen consolidation therapy. She received local resection for the melanoma and no chemotherapy. Although it is possible that the combination of chemotherapy and systemic cobaltism manifested as tongue discoloration, the timing, nature, and resolution of tongue discoloration and altered gustation correlates with both symptomatic metallosis and objective laboratory evidence. Thus, it is most prudent to conclude that her tongue discoloration and altered gustation were a direct result of serum metal ion burden.
Neither the sensitivity nor specificity of tongue discoloration in predicting systemic metal ion toxicity can be elucidated from this report. However, it poses an intriguing physical examination finding that can be readily recognized by orthopedic surgeons, primary care physicians, and oral health-care providers. Early recognition through oropharyngeal and integument examination may assist in the diagnosis of metal ion toxicity and hasten the explant of components before the development of systemic toxicity or even organ failure and should be researched further. Once a diagnosis of systemic cobaltism is made, nonmusculoskeletal manifestations should be entertained by the orthopedic surgeon and elucidated with a thorough history, complete physical examination, and relevant laboratory and imaging studies to facilitate expeditious treatment of concurrent systemic pathology.
Summary
MoM hip resurfacing is associated with metallosis and systemic metal ion toxicity including systemic cobaltism. Tongue discoloration may demonstrate the profound impact of serum metal ion burden and may be an early indicator of impending or ongoing systemic toxicity. Inspection of the oral mucosa and integument is an inexpensive and facile tool that can potentially aid in the diagnosis and expeditious treatment of systemic metal ion toxicity when serum metal ion levels are elevated. Further clinical investigation is warranted to ascertain the specificity and sensitivity of this finding.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2018.06.005.
Appendix A. Supplementary data
References
- 1.Stewart T.D. Tribology of artificial joints. Orthop Trauma. 2010;24:435. [Google Scholar]
- 2.Drummond J., Tran P., Fary C. Metal-on-metal hip arthroplasty: a review of adverse reactions and patient management. J Funct Biomater. 2015;6:486. doi: 10.3390/jfb6030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creighton-Smith M., McGrory B.J., Tolocica I. Long-term tattooing as a manifestation of metallosis in acetabular liner wear-through: a report of two cases. Curr Orthop Pract. 2015;26:64. [Google Scholar]
- 4.Walter L.R., Marel E., Harbury R., Wearne J. Distribution of chromium and cobalt ions in various blood fractions after resurfacing hip arthroplasty. J Arthroplasty. 2008;23:814. doi: 10.1016/j.arth.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Swiatkowska I., Mosselmans J.F.W., Geraki T. Synchrotron analysis of human organ tissue exposed to implant material. J Trace Elem Med Biol. 2018;46:128. doi: 10.1016/j.jtemb.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Park J.Y., Shin D.H., Choi J.S., Kim K.H. Metallic discoloration on the right shin caused by titanium alloy prostheses in a patient with right total knee replacement. Ann Dermatol. 2013;25:356. doi: 10.5021/ad.2013.25.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sporer S.M., Chalmers P.N. Cutaneous manifestation of metallosis in a metal-on-metal total hip arthroplasty after acetabular liner dissociation. J Arthroplasty. 2012;27:1580.e13. doi: 10.1016/j.arth.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Callender V.D., Cardwell L.A., Munhutu M.N., Bigby U., Rodney I.J. Cutaneous metallosis in patient with knee prosthesis composed of cobalt-chromium molybdenum alloy and titanium-aluminum-vanadium alloy. JAAD Case Rep. 2014;1:36. doi: 10.1016/j.jdcr.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willis-Owen C.A., Keene G.C., Oakeshott R.D. Early metallosis-related failure after total knee replacement: a report of 15 cases. J Bone Joint Surg Br. 2011;93:205. doi: 10.1302/0301-620X.93B2.25150. [DOI] [PubMed] [Google Scholar]
- 10.Willert H.-G., Buchhorn G.H., Fayyazi A. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 11.Pesce V., Maccagnano G., Vicenti G. First case report of vanadium metallosis after ceramic-on-ceramic total hip arthroplasty. J Biol Regul Homeost Agents. 2013;27:1063. [PubMed] [Google Scholar]
- 12.Pelclova D., Sklensky M., Janicek P., Lach K. Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clin Toxicol (Phila) 2012;50:262. doi: 10.3109/15563650.2012.670244. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda T., Takahashi K., Kabata T., Sakagoshi D., Tomita K., Yamada M. Polyneuropathy caused by cobalt-chromium metallosis after total hip replacement. Muscle Nerve. 2010;42:140. doi: 10.1002/mus.21638. [DOI] [PubMed] [Google Scholar]
- 14.Berber R., Skinner J.A., Hart A.J. Management of metal-on-metal hip implant patients: who, when and how to revise? World J Orthop. 2016;7:272. doi: 10.5312/wjo.v7.i5.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fillingham Y.A., Della Valle C.J., Bohl D.D. Serum metal levels for diagnosis of adverse local tissue reactions secondary to corrosion in metal-on-polyethylene total hip arthroplasty. J Arthroplasty. 2017;32:S272. doi: 10.1016/j.arth.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Harris W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737. [PubMed] [Google Scholar]
- 17.Matsen Ko L.J., Pollag K.E., Yoo J.Y., Sharkey P.F. Serum metal ion levels following total hip arthroplasty with modular dual mobility components. J Arthroplasty. 2016;31:186. doi: 10.1016/j.arth.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 18.Kocagoz S.B., Underwood R.J., MacDonald D.W., Gilbert J.L., Kurtz S.M. Ceramic heads decrease metal release caused by head-taper fretting and corrosion. Clin Orthop Relat Res. 2016;474:985. doi: 10.1007/s11999-015-4683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman E.J., Ashley B., Sheth N.P. Metal-on-metal total hip arthroplasty: is there still a role in 2016? Curr Rev Musculoskelet Med. 2016;9:93. doi: 10.1007/s12178-016-9323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams D., Royle M., Norton M. Metal-on-metal hip resurfacing: the effect of cup position and component size on range of motion to impingement. J Arthroplasty. 2009;24:144. doi: 10.1016/j.arth.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Bedigrew K.M., Ruh E.L., Zhang Q., Clohisy J.C., Barrack R.L., Nunley R.M. 2011 Marshall Urist Young Investigator Award: when to release patients to high-impact activities after hip resurfacing. Clin Orthop. 2012;470:299. doi: 10.1007/s11999-011-2131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wimmer M.A., Sprecher C., Hauert R., Täger G., Fischer A. Tribochemical reaction on metal-on-metal hip joint bearings: a comparison between in-vitro and in-vivo results. Wear. 2003;255:1007. [Google Scholar]
- 23.Langton D.J., Joyce T.J., Jameson S.S. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93:164. doi: 10.1302/0301-620X.93B2.25099. [DOI] [PubMed] [Google Scholar]
- 24.Engh C.A., MacDonald S.J., Sritulanondha S., Korczak A., Naudie D., Engh C. Metal ion levels after metal-on-metal total hip arthroplasty: a five-year, prospective randomized trial. J Bone Joint Surg Am. 2014;96:448. doi: 10.2106/JBJS.M.00164. [DOI] [PubMed] [Google Scholar]
- 25.Hailer N.P., Bengtsson M., Lundberg C., Milbrink J. High metal ion levels after use of the ASRTM device correlate with development of pseudotumors and T cell activation. Clin Orthop. 2014;472:953. doi: 10.1007/s11999-013-3307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Günther K.-P., Lützner J., Hannemann F. Update on metal-on-metal hip joints. Orthopade. 2013;42:373. doi: 10.1007/s00132-013-2100-6. quiz 388. [DOI] [PubMed] [Google Scholar]
- 27.Zywiel M.G., Brandt J.-M., Overgaard C.B., Cheung A.C., Turgeon T.R., Syed K.A. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95-B:31. doi: 10.1302/0301-620X.95B1.30060. [DOI] [PubMed] [Google Scholar]
- 28.Gessner B.D., Steck T., Woelber E., Tower S.S. A systematic review of systemic cobaltism after wear or corrosion of chrome-cobalt hip implants. J Patient Saf. 2015:1. doi: 10.1097/PTS.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin J.R., Spencer-Gardner L., Camp C.L., Stulak J.M., Sierra R.J. Cardiac cobaltism: a rare complication after bilateral metal-on-metal total hip arthroplasty. Arthroplast Today. 2015;1:99. doi: 10.1016/j.artd.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raghunathan V.K., Devey M., Hawkins S. Influence of particle size and reactive oxygen species on cobalt chrome nanoparticle-mediated genotoxicity. Biomaterials. 2013;34:3559. doi: 10.1016/j.biomaterials.2013.01.085. [DOI] [PubMed] [Google Scholar]
- 31.Sreeja C., Ramakrishnan K., Vijayalakshmi D., Devi M., Aesha I., Vijayabanu B. Oral pigmentation: a review. J Pharm Bioallied Sci. 2015;7:403. doi: 10.4103/0975-7406.163471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarakji B., Umair A., Prasad D., Alsakran Altamimi M. Diagnosis of oral pigmentations and malignant transformations. Singapore Dent J. 2014;35:39. doi: 10.1016/j.sdj.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Oldenburg M., Wegner R., Baur X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J Arthroplasty. 2009;24:825.e15. doi: 10.1016/j.arth.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Wan R., Mo Y., Zhang Z., Jiang M., Tang S., Zhang Q. Cobalt nanoparticles induce lung injury, DNA damage and mutations in mice. Part Fibre Toxicol. 2017;14:38. doi: 10.1186/s12989-017-0219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matharu G.S., Berryman F., Judge A. Blood metal ion thresholds to identify patients with metal-on-metal hip implants at risk of adverse reactions to metal debris: an external multicenter validation study of Birmingham hip resurfacing and corail-pinnacle implants. J Bone Joint Surg Am. 2017;99:1532. doi: 10.2106/JBJS.16.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfreijat M. Tongue hyperpigmentation associated with chemotherapy. J Community Hosp Intern Med Perspect. 2013;3 doi: 10.3402/jchimp.v3i3-4.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casamiquela K.M., Cohen P.R. Chemotherapy-associated tongue hyperpigmentation and blue lunula. J Drugs Dermatol. 2013;12:223. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.