Abstract
In the circulation, testosterone and other sex hormones are bound to binding proteins, which play an important role in regulating their transport, distribution, metabolism, and biological activity. According to the free hormone hypothesis, which has been debated extensively, only the unbound or free fraction is biologically active in target tissues. Consequently, accurate determination of the partitioning of testosterone between bound and free fractions is central to our understanding of how its delivery to the target tissues and biological activity are regulated and consequently to the diagnosis and treatment of androgen disorders in men and women. Here, we present a historical perspective on the evolution of our understanding of the binding of testosterone to circulating binding proteins. On the basis of an appraisal of the literature as well as experimental data, we show that the assumptions of stoichiometry, binding dynamics, and the affinity of the prevailing models of testosterone binding to sex hormone-binding globulin and human serum albumin are not supported by published experimental data and are most likely inaccurate. This review offers some guiding principles for the application of free testosterone measurements in the diagnosis and treatment of patients with androgen disorders. The growing number of testosterone prescriptions and widely recognized problems with the direct measurement as well as the computation of free testosterone concentrations render this critical review timely and clinically relevant.
This appraisal of the dynamics of testosterone binding to proteins and of the free hormone hypothesis offers guidance for the application of free testosterone in the evaluation of androgen disorders.
Essential Points
Most circulating testosterone is bound to its cognate binding proteins—sex hormone−binding globulin (SHBG), human serum albumin (HSA), cortisol-binding globulin, and orosomucoid; these binding proteins play an important role in regulating the transport, tissue delivery, bioactivity, and metabolism of testosterone
The physiochemical characteristics and dynamics of the binding of testosterone to its binding proteins are poorly understood; oversimplified assumptions of stoichiometry, binding dynamics, and binding affinity have contributed to the development of inaccurate linear binding models of testosterone to SHBG and HSA
The ensemble allosteric model of the binding of testosterone to SHBG developed from recent studies using modern biophysical techniques suggests that testosterone binding to SHBG is a complex, multistep process that involves interbinding site allostery
The dynamics of the binding of testosterone to HSA, orosomucoid, and corticosteroid-binding globulin also require careful reexamination because the roles of these binding proteins in regulating circulating testosterone concentrations remain incompletely understood
If the free hormone hypothesis is correct (i.e., only free testosterone is biologically active), accurate determination and harmonized reference ranges for free testosterone are necessary to diagnose androgen disorders in men and women
Methods for the measurement of free testosterone levels are fraught with potential problems, including poor precision, inaccuracy, and low specificity, and reliable assays are not readily available to practicing clinicians; therefore, algorithms based on valid binding models that can be used to estimate circulating free testosterone levels are needed to facilitate sound clinical decision making
Binding proteins in the peripheral circulation are important in regulating the transport, bioavailability, and metabolism of their cognate ligands, such as steroid hormones, fatty acids, vitamins, and drugs. The major sex steroid hormones—testosterone, 5α-dihydrotestosterone, and 17β-estradiol—bind predominantly to sex hormone−binding globulin (SHBG) and to human serum albumin (HSA) and to a lesser extent to corticosteroid-binding globulin (CBG) and orosomucoid. SHBG, which is secreted by the liver, binds to testosterone with high affinity and is an important determinant of the distribution of circulating testosterone into its bound and free fractions (1). HSA is one of the most abundant and versatile proteins in circulation; although it binds testosterone with lower affinity than SHBG does, its high binding capacity and high concentration allow it to buffer fluctuations in testosterone levels (1). The characteristics of testosterone binding to CBG and orosomucoid and the biological roles of these binding proteins in regulating testosterone bioavailability remain incompletely understood.
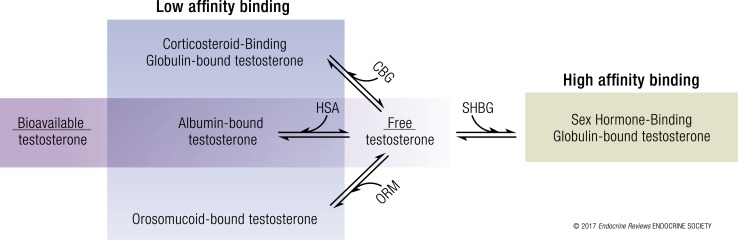
Total testosterone refers to the sum of the concentrations of protein-bound and unbound testosterone in circulation. The fraction of circulating testosterone that is unbound to any plasma protein is referred to as the free testosterone fraction. The term bioavailable testosterone refers to the fraction of circulating testosterone that is not bound to SHBG and largely represents the sum of free testosterone plus HSA-bound testosterone (Fig. 1) (2); the term reflects the view that HSA-bound testosterone, which is bound with low affinity, can dissociate from HSA in the tissue capillaries and effectively be available for biological activity. The free testosterone fraction can be measured directly by the equilibrium dialysis or ultrafiltration method or calculated from total testosterone, SHBG, and HSA concentrations using published mass action binding algorithms (3–6). The bioavailable fraction can be measured using the ammonium sulfate precipitation method or the concanavalin A method, or it can be calculated from total testosterone, SHBG, and HSA concentrations (7). Although the pioneers who originated the concept of bioavailable testosterone envisioned it as the sum of HSA-bound and unbound fractions of circulating testosterone (2), the methods used to measure bioavailable testosterone concentrations, namely, the ammonium sulfate precipitation and concanavalin A methods, quantitate it as the non−SHBG-bound fraction of circulating testosterone, which approximates but is not equivalent to its original conceptualization as the sum of HSA-bound plus unbound testosterone levels (8).
Figure 1.
Partitioning of testosterone in the systemic circulation. Circulating testosterone is bound tightly to SHBG (green = high affinity binding) and weakly to albumin, orosomucoid (ORM), and CBG (blue = low affinity binding) (11). Only 1% to 4% of circulating testosterone is unbound or free. The combination of free and albumin-bound testosterone is also referred to as the “bioavailable testosterone” fraction.
The validity of calculated bioavailable and free testosterone is predicated on the accuracy of binding protein and testosterone concentrations and on the veracity of the assumptions of the association stoichiometry, binding affinities, and binding dynamics underlying the molecular binding model. The foundational assumptions about the relationship between testosterone and its binding proteins and estimates of the biophysical parameters of testosterone binding to its cognate binding proteins, upon which many extant algorithms for computing free testosterone are based, have undergone recent reappraisal and are discussed later. Data from the experimental studies performed in the 1960s and 1970s have been extrapolated without acknowledgment of the lack of experimental support for the underlying assumptions about linearity (3, 9–11) or of the methodological limitations described by the original authors. Collectively, these have led to an oversimplification of binding models based on somewhat erroneous assumptions of stoichiometry, binding affinity, and binding dynamics.
The rapid growth of testosterone prescriptions during the last decade (12) has refocused attention on the critical need for accurate determination of free testosterone in the diagnostic evaluation of men with a suspected androgen deficiency and for rational dosing and monitoring of testosterone replacement therapy. Accordingly, an expository review of the published data and prevailing models of testosterone binding is timely. Here, we present a historical perspective of the evolution of our understanding of the binding and bioavailability of testosterone. This review attempts to provide a comprehensive and critical appraisal of the prevailing models of testosterone binding to SHBG and HSA, the associated biophysical parameters, and their underlying assumptions and limitations. We discuss how recent advances in the computational and biophysical techniques have begun to unravel the multistep dynamics of testosterone binding to its cognate binding proteins, including the allosteric interactions between the testosterone binding sites on the SHBG dimer. This review also provides a contemporary perspective on the validity of the free hormone hypothesis and the clinical implications of these findings in the diagnosis, treatment, and monitoring of men with hypogonadism.
Biology of Binding Proteins and Their Role in the Transport, Distribution, Metabolism, and Bioavailability of Testosterone
At least four structurally distinct binding proteins are known to bind testosterone in human circulation: SHBG, HSA, CBG, and orosomucoid. Among these, SHBG has received the most attention because of its high binding affinity for testosterone. These binding proteins influence the tissue bioavailability and metabolic clearance rate of testosterone by regulating the amount of free testosterone available for biological action in the tissue. The roles of HSA, CBG, and orosomucoid in regulating testosterone’s bioavailability are less well understood, and we do not know how disease states or conditions that may differentially alter the circulating concentrations of HSA, CBG, and orosomucoid impact the binding of testosterone to SHBG. Current computations of free and bioavailable testosterone account only for the potential impact of alterations in HSA and SHBG, ignoring CBG and orosomucoid and other potentially interacting proteins and steroid hormones.
SHBG
SHBG, a homodimeric glycoprotein with a molecular mass of approximately 90 kDa (13), was first identified by Mercier et al. (14), who separated a testosterone-binding β-globulin by electrophoresis. An estradiol-binding protein was independently isolated the same year (15), and competitive steroid binding studies showed that the two proteins were identical (16). Consequently, it became known as the testosterone-estradiol binding globulin. This binding protein has since been shown to bind to and act as a transport protein for other sex steroid hormones as well and is therefore more commonly known as the SHBG (4, 11).
The SHBG protein is encoded by a single gene on the short arm of chromosome 17, which includes eight exons (17). Three distinct promoters—PL, PT, and PN—can initiate transcription from three separate sites in exon 1, resulting in three variants: 1L, 1T, and 1N. The typical wild-type SHBG protein is the product of translation of a transcript produced under the influence of promoter PL and the other seven exons. A variant, SHBG-T, is missing exon 7 but includes the product of exon 1T produced under the influence of promoter PT (18).
SHBG circulates as a homodimer. Calcium and zinc ions are required for holding the dimer together (19); thus, chelating agents, such as EDTA, can dissociate the SHBG dimer. Each SHBG monomer contains two laminin G−like (LG) domains at the N-terminal end of the protein, encoded by exons 2 to 5 (20). These LG domains form pockets that enable the binding of sex hormones. The serine residue within this binding pocket is important in androgen and estrogen binding and forms hydrogen bonds with functional groups at the C3 position of the A ring of testosterone (21) and with the C17 hydroxyl group in the D ring of estradiol (22). Thus, the binding of androgens and estrogens imparts different conformations to the SHBG molecule. The SHBG protein contains three oligosaccharides; two oligosaccharides are attached at two N-glycosylation sites on asparagine and one at an O-glycosylation site on threonine (23). SHBG levels, which typically range from 10 to 56 nmol/L, can be measured using immunofluorometric and chemiluminescent assays or by dihydrotestosterone binding assays (24).
Although reports indicate that SHBG has been produced locally in the testes, uterus, and brain, most circulating SHBG in humans is produced in the liver. The product of the SHBG gene in the testes is called the androgen-binding protein, which has different oligosaccharides and is not secreted into the circulation. SHBG production in the liver is inhibited by hepatic lipids and by tumor necrosis factor-α and interleukin-1, rather than by insulin directly, which was reported previously (25). Thus, the low SHBG levels seen in obesity and diabetes are most likely the result of low-grade inflammation and increased amounts of hepatic lipids rather than high insulin levels (26). Selva and Hammond have shown that thyroid hormones increase SHBG production indirectly by increasing hepatocyte nuclear 4 alpha gene expression, which is a major regulator of SHBG transcription (27).
The distribution of SHBG-bound testosterone differs in men and women: In the presence of estradiol, about 20% of binding sites are occupied by testosterone (11). The reported association constant for binding of testosterone to SHBG has varied among published studies depending on the experimental conditions, but it is consistently reported to be around 1 × 109 L/mol with two binding sites on each SHBG homodimer (4, 5, 28–31). Known variants, including the rs6258, rs143521188, rs143269613, rs146779355, and rs373769356 polymorphisms, decrease affinity for testosterone and higher equilibrium dissociation constant (Kd) values (32, 33). Notably, previous binding studies have assumed that the two binding sites on the SHBG homodimer are equivalent. A recent reappraisal of testosterone binding to SHBG using modern biophysical techniques indicated that the two binding sites on the SHBG dimer are not equivalent and that there is an allosteric interaction between the binding sites on the SHBG dimer such that the second testosterone molecule binds SHBG with a substantially different affinity than the first binding site (34). The allosteric model of the multistep binding of testosterone to SHBG is discussed later in this review.
HSA
HSA is the most abundant protein in the human circulation, accounting for 60% of the total serum protein content and having a concentration of 30 to 50 g/L (450 to 750 µM) (35, 36). From 33% to 54% of testosterone binds with low affinity to HSA, with an association constant of 2.0 to 4.1 × 104 L/mol at 37°C (4, 5, 37–39). Albumin Catania (580 Lys-Leu-Pro-COOH) (40) and albumin Roma (321 Glu-Lys) (41) are known variants that impact the affinity of HSA for testosterone; albumin Roma has a decreased affinity for testosterone, and it is unknown if albumin Catania has an increased or decreased affinity.
The high capacity of HSA for binding steroids is particularly highlighted during pregnancy, when the circulating sex steroid concentrations increase very substantially; however, even during pregnancy, more than 99% of available binding sites on HSA remain unoccupied (11). It has long been hypothesized that HSA-bound testosterone may dissociate in the capillary bed of organs with long transit times, such as the liver and the brain, and may become biologically active (bioavailable) in these organs in addition to the unbound testosterone (2, 42).
The HSA protein is encoded by a gene on chromosome 4 (43), which contains 15 exons placed symmetrically in three domains that likely arose by triplication of a single ancestral gene. The HSA gene is translated into a 609−amino acid product from which a signal peptide and a propeptide are cleaved, yielding a 585−amino acid mature protein that is secreted into the circulation. HSA in circulation can undergo nonenzymatic glycation by formation of a Schiff base between ε-amino groups of lysine and arginine residues and glucose (44). HSA is generally measured with dye-binding assays such as bromocresol green or bromocresol purple or with immunoassays (45). The bromocresol green methods may overestimate HSA because of interference by acute-phase reactant proteins (46–48), whereas the bromocresol purple method reportedly has high concordance with immunoassays (49, 50).
Major gaps remain in our understanding of the dynamics of free testosterone regulation by HSA. Pardridge (42) hypothesized that within the tissue capillaries, conformational changes in the HSA molecule caused by interactions between HSA and the endothelial wall could lead to an opening of the binding site coil and enhanced dissociation of testosterone from HSA. Indeed, the dissociation of testosterone from bovine serum albumin in the brain capillary is ∼50 times faster than dissociation from albumin in vitro (42). This increase in transportability of HSA-bound testosterone may result from interactions of HSA with specific receptors in the microcirculation; however, in vivo studies of HSA transport into the brain (51) or liver (52) microcirculation showed that the volume of distribution of HSA was no greater than in the vascular space. Others have postulated that the enhanced dissociation of testosterone from HSA in the capillaries results from secretion of binding inhibitors from the endothelium (18). Current models of the binding of testosterone to HSA are discussed further in a subsequent section.
CBG
CBG, or transcortin, is the primary transporter for glucocorticoids (cortisol and corticosterone) and progestins (progesterone and 17-hydroxyprogesterone), and it regulates the partitioning of circulating cortisol into bound and unbound fractions (53). CBG is an α-globulin produced in the liver and encoded by the SERPIN A6 gene on chromosome 14q32.13 in a chromosomal region that contains a number of other related serine protease inhibitor genes (53). It circulates at a concentration of 1.7 to 3.1 mg/dL.
Some data indicate that CBG binds up to ∼4% of circulating testosterone (Fig. 1) (11) with a low association constant (5.3 × 106 L/mol) (11, 54), such that the binding of testosterone to CBG has been largely discounted in estimating free testosterone (11, 24). Cooke et al. (55) showed that the fraction of non−SHBG-bound testosterone is lower between the hours of 0300 to 0700 in men, coinciding with the early morning increase in cortisol and suggesting that the high circulating cortisol concentrations during this period could potentially displace testosterone from CBG, resulting in a higher fraction of testosterone being bound to SHBG. In follow-up in vitro studies that investigated the influence of other steroids on protein-bound testosterone, the experimentally observed decrease in non−SHBG-bound testosterone in response to the addition of cortisol was 15.4% (54). However, simulation studies that used the published estimates of testosterone’s binding affinity to CBG predicted a substantially smaller decrease in the percentage of non−SHBG-bound testosterone than the 15.4% decrease observed experimentally. This discordance between the predicted and experimentally obtained values raises the possibility that published estimates of testosterone binding to CBG are incorrect and that a higher amount of circulating testosterone is bound to CBG than was assumed. It is also possible that the interaction of cortisol and testosterone for shared or interacting binding sites on HSA plays a role. It is also possible that conformational changes at the estradiol and cortisol binding sites on CBG occur in the rat liver microcirculation, leading to variations in the bioavailability of cortisol (56). Further studies are needed to clarify the role of CBG in regulating the bioavailability of testosterone in human circulation.
α1-Acid glycoprotein, or orosomucoid
α1-Acid glycoprotein, or orosomucoid, is an acute phase α-globulin that is synthesized in hepatocytes, is regulated by interleukin-1 and tumor necrosis factor, and circulates at plasma concentrations between 0.6 and 1.2 mg/mL (57). Whereas HSA acts as a carrier of acidic (negative) and neutral drugs, orosomucoid acts as a carrier of basic (positive) and neutrally charged lipophilic compounds.
Kerkay and Westphal (58) found that testosterone associated linearly with orosomucoid at a single primary binding site and with a binding affinity that varied with the temperature. Thus, at 4°C and 37°C, the binding constants were 7.3 × 105 L/mol and 3.0 × 105 L/mol, respectively. The low solubility of the steroids studied did not permit the use of sufficiently high steroid concentrations in equilibrium dialysis experiments to determine whether there were any secondary binding sites. Other studies of the binding of testosterone to orosomucoid were conducted by monitoring the ultraviolet absorption spectra of testosterone, which is not a very sensitive technique. The application of modern biophysical methods, including derivative spectroscopy and environmentally sensitive fluorescent probes such as 4,4′-Dianilino-1,1′-binaphthyl-5,5′-disulfonate (bis-ANS) (59), which offer a substantially higher level of sensitivity than ultraviolet absorption, could enable a more refined estimation of the affinity and dynamics of the binding interactions of testosterone with orosomucoid.
Appraisal of the Prevailing Models of Testosterone Binding to Plasma Proteins
Most of the experimental data characterizing the association of testosterone with HSA and SHBG, which led to the conception of linear binding models of testosterone’s association with SHBG and HSA, including those by Vermeulen et al. (3), Södergard et al. (4), and Mazur (5), were generated in the 1950s through the 1980s. The resolution of the crystal structure of the liganded SHBG domains in the early 2000s was a major advance in our understanding of testosterone binding to SHBG. However, as we discuss subsequently, a paucity of experimental data supports the widely used assumptions of stoichiometry and the affinity of testosterone’s binding to SHBG (Table 1).
Table 1.
Factors Contributing to Erroneous Assumptions of Binding Affinity and Stoichiometry in Linear Models of Testosterone Binding to Its Cognate Binding Proteins
| Contributing Factor |
|---|
| 1:1 Binding stoichiometry assumed without supporting experimental data |
| Use of Scatchard plots to force a straight line through nonlinear experimental binding data |
| Failure to account for alteration of binding equilibria during separation of free and bound testosterone |
| Variations in the estimates of binding affinity because of differences in the temperature at which binding isotherms and dialysis experiments were performed |
| Variations in the estimates of binding affinity due to differences in dialysis conditions, including differences in the assay buffer composition and relative volumes of serum and assay buffers |
| Limited ability to detect additional binding sites on SHBG and HSA because of the narrow range of testosterone concentrations used in the binding experiments |
Critical evaluation of the current model of testosterone binding to HSA
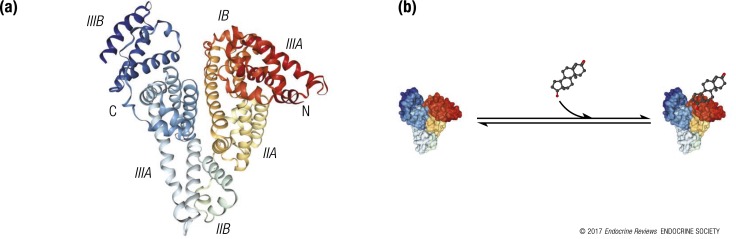
HSA consists of three domains [Fig. 2(a)] (60); both domains II and III have a binding pocket formed mostly of hydrophobic and positively charged residues in which a variety of compounds bind (61). It is widely believed that testosterone binds to HSA at a single site on domain IIA (62–64) with low-to-moderate affinity (i.e., an association constant of 2.0 to 4.1 × 104 L/mol at 37°C) and a fast dissociation half-time (∼1 second) (4, 5, 38, 39, 65). As a result, all the equations for calculating free testosterone have used 1:1 stoichiometry of testosterone binding to HSA [Fig. 2(b)] (3–5, 34).
Figure 2.
Multiple domains of HSA are involved in the binding and transport of biomolecules. (a) Crystal structure of HSA (PDB:1AO6) depicting multiple domains (I to III) of HSA that participate in the binding and transport of hormones, nutrients, other biomolecules, and drugs. Testosterone is thought to bind to a binding site in domain II of HSA (41, 62, 63). (b) A linear model of testosterone binding to HSA based on the assumption of 1:1 stoichiometry. Although structural and functional studies of HSA have shown several hydrophobic pockets for ligands, the prevailing models assume only 1:1 stoichiometry of testosterone binding to HSA.
In the years since these studies were published, limited experimental data in the literature have supported the commonly assumed 1:1 stoichiometry for the binding of testosterone to HSA (Table 1) (66). For instance, experimental data published as early as 1954 by Eik-Nes et al. (67) suggested multiple, noninteracting testosterone binding sites on HSA. In 1978, Moll et al. (68) performed a detailed evaluation of the association of testosterone with HSA, and these authors also suspected multiple, identical, noninteracting testosterone binding sites on HSA. In 1982, Södergard et al. (4) conducted thermodynamic studies of the association of dihydrotestosterone with HSA and reported that the data pointed toward multiple binding sites on HSA. Ryan (69) suggested the possibility of multiple binding sites for testosterone on HSA and a nonlinear binding relationship. The calculations of binding parameters based on the assumption of 1:1 stoichiometry may also be invalid (Table 1) (70). Thus, although these trailblazers were suspicious of 1:1 stoichiometry, the methods and computational tools available to them were inherently limited in providing definitive evidence of stoichiometry, multiple binding sites with different binding affinities, or allostery in the binding of testosterone to HSA. Regardless, this same set of papers has been cited repeatedly over the years as the basis of the 1:1 stoichiometry for the binding of testosterone to HSA, although, in fact, these pioneering studies did not provide experimental data to support this assumption (Table 1).
As recently as the 1990s, Fischer et al. (71) concluded on the basis of studies that used equilibrium dialysis and circular dichroism that the second domain of the HSA molecule contained the primary binding site(s) for testosterone and acknowledged that “the data indicated the existence of cooperativity between secondary fatty acid binding sites and the primary testosterone binding site.” Others also showed that for many ligands, the multiple binding sites on the HSA domains are allosterically coupled (72). It is conceivable that testosterone, like other ligands, may also have multiple binding sites with distinct affinities on HSA. Oversimplification of binding models and potentially erroneous assumptions can have major implications not only on estimates of testosterone’s bioavailability but also on putative competitive interactions with fatty acids and other hormones and drugs.
The dynamics of testosterone binding to HSA requires careful reexamination using modern experimental tools. Previous methods of comparing the solubility of testosterone in aqueous buffer solution with its solubility in similarly buffered bovine serum albumin and using Scatchard analysis for equilibrium dialysis of testosterone with HSA were incapable of confirming multiple binding sites or identifying allosteric interactions between binding sites. For instance, novel conformational probes that exhibit perturbations in their ground or excited-state optical properties in response to changes in their electronic environment can facilitate characterization of the binding of hormones and drugs to HSA and evaluation of the competitive displacement by ligands. In addition, magnetic resonance spectroscopy using 13C-enriched probes can help map the spatial pockets of testosterone binding to HSA.
Critical evaluation of the current model of testosterone binding to SHBG
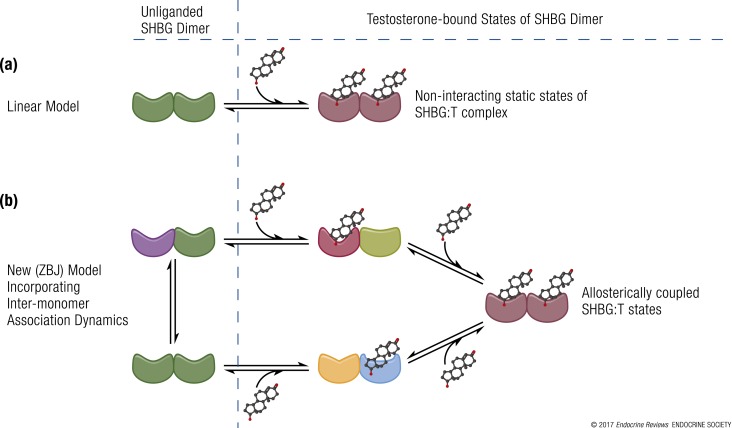
From the early days of its discovery, SHBG has been recognized as the high-affinity binding protein for testosterone. Initial estimates of its binding association constant for testosterone in the 1960s were in the nanomolar range (38). Subsequently, the resolution of the crystal structure of the N-terminal LG domain of SHBG (19, 21) in the early 2000s revealed that the dimerization and steroid-binding domains of the homodimeric SHBG molecule are distinct and that both monomers within the complex are capable of binding sex steroids (30). Together, these observations led to the assumption that the monomers must have identical affinities in solution [Fig. 3(a)] (73–75).
Figure 3.
Schematic representation of experimental models of testosterone binding to SHBG. (a) Linear model of testosterone (T) binding to SHBG as conceptualized by Vermeulen et al. (3), Södergard et al. (4), and Mazer (5). (b) New model (ZBJ, schematic adaptation) proposed by Zakharov et al. (34) incorporating the dynamics of allosteric regulation in testosterone binding to SHBG. The different shapes represent conformationally distinct states of SHBG in the dynamic repartitioning of free testosterone into bound forms. Recent evidence derived from new biophysical techniques indicates that the binding of testosterone to SHBG is a dynamic, multistep process. The binding of one molecule of testosterone to the first binding site on an SHBG dimer leads to conformational rearrangement and allostery between the two binding sites, such that the second testosterone molecule binds to the second binding site with a different binding affinity; there is readjustment of equilibria between these interconverting microstates. This multistep, allosteric model provides validated estimates of free testosterone, which have close correspondence with values measured using equilibrium dialysis.
Recently, we demonstrated that the linear model of testosterone binding to SHBG, which has formed the basis of many law-of-mass-action equations, was not consistent with the experimental binding data [Fig. 3(a)] (34) and that the binding affinities of the two binding sites on the SHBG dimer were not identical. Consistent with our data, Heinrich-Balard et al. (76), who used surface plasmon resonance to study molecular interactions between the immobilized testosterone ligand and SHBG over a large range of SHBG concentrations, found that the binding affinity of SHBG for testosterone varied with the total plasma SHBG concentration. Within the narrow physiological range of SHBG concentrations, the measured association constants approximated those described in the literature (3, 8), but when the SHBG concentrations were either low or high (<15 nmol/L or >100 nmol/L), the observed association constants differed substantially from those reported in the literature (8).
In complementary studies using binding isotherms, testosterone depletion curves, and isothermal calorimetry, we showed that the interaction of testosterone with SHBG is a dynamic, nonlinear, multistep process that involves allosteric interaction between the two binding sites on the SHBG homodimer [Fig. 3(b)] (34). Several possible models of ligand binding to SHBG were examined using experimental data in a three-step approach: First, the fits of the binding isotherms for each model were examined using analysis of residuals and a reduced χ2 statistic; second, we evaluated whether the models captured the key characteristic features of the binding isotherms; and third, the predicted free testosterone values derived using the models were compared with those directly measured using equilibrium dialysis. Free testosterone concentrations derived from the model that accounted for allosteric regulation between the SHBG monomers eliminated the systematic deviations observed in the calculated free testosterone values derived from the linear models using the law of mass action. Additional studies should examine the influence of SHBG mutations and polymorphisms (32, 33) observed in the wider population on the performance of the allosteric models.
Additional Potential Roles of SHBG and Orosomucoid
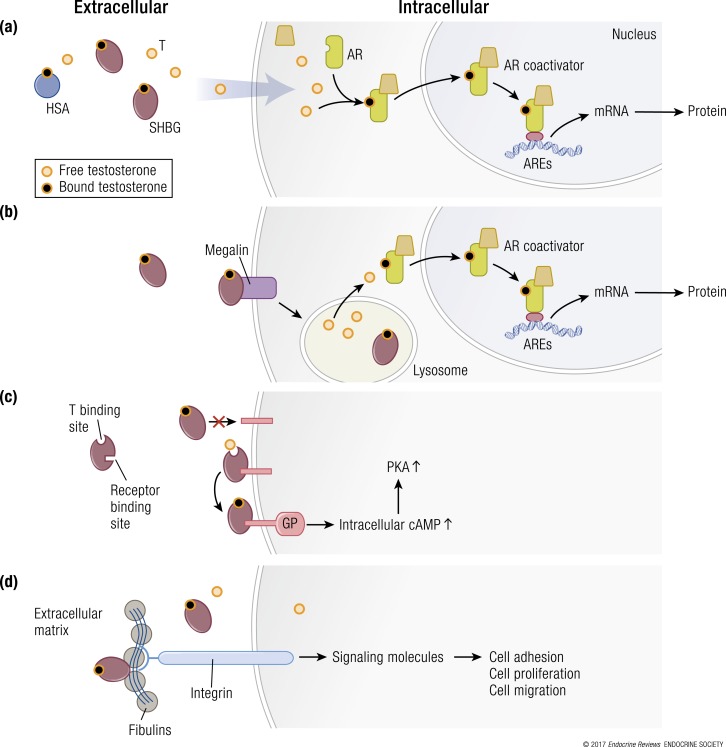
The classic genomic signaling that mediates the biologic actions of testosterone involves its passive diffusion into the cellular cytoplasm [Fig. 4(a)], association with the androgen receptor, translocation into the nucleus, and binding to the DNA response element to modulate transcription of specific androgen-responsive genes. Although passive diffusion is widely observed in multiple cell types, the globulin family proteins are postulated to facilitate cellular steroid uptake [Fig. 4(b)–4(d)]. Binding proteins, such as SHBG, have been described as multifunctional proteins, capable of regulating the response to steroid hormones as well as their entry into cells (13, 77–79). These binding proteins are also postulated to serve other functions, as described later (80–88).
Figure 4.
Multiple hypothetical mechanisms for the cellular uptake of testosterone and downstream signaling. (a) The model depicting the “free” hormone hypothesis. In this model, testosterone (T) that is not bound to SHBG or HSA or other binding proteins diffuses across the plasma membrane and binds to the androgen receptor (AR). The liganded AR recruits coregulators and chaperone proteins, translocates to the nucleus, and binds to androgen response elements (AREs) on androgen-responsive target genes, which activates the transcription of target genes. (b) The megalin-dependent mode of testosterone entry. According to this model, SHBG-bound testosterone is internalized into the cell through an endocytic process mediated by the membrane protein megalin. Once internalized, SHBG-bound testosterone is released at the low pH within the lysosome. (c) The SHBG receptor-testosterone system. The SHBG dimer has multiple binding sites—two sites (simplified as one in this model) bind testosterone, and one site binds to a membrane receptor. It may be that only unbound SHBG is able to bind to the receptor, then the SHBG-receptor-testosterone complex is coupled to the activation of a G protein (GP), the accumulation of intracellular cyclic adenosine monophosphate (cAMP), and activation of protein kinase A (PKA). PKA may modulate AR function by activating AR through phosphorylation (not depicted) (92). (d) Steroid ligand−dependent interactions between SHBG and at least two matrix-associated proteins in the fibulin family (fibulin-1D and fibulin-2) contribute to the extravascular sequestration of SHBG in some tissues, such as the breast, prostate, and endometrial stroma. According to this model, ligand-dependent interactions between SHBG and fibulins modulate their binding to various signaling molecules, such as integrins, to modify signaling pathways that regulate cell adhesion, proliferation, and migration. mRNA, messenger RNA.
Potential role of SHBG in the prostate
In the 1990s, several investigators reported that SHBG might bind to cell surface binding sites on prostate cells and activate intracellular signaling on its own [Fig. 4(c)] (89–91). However, the cell surface receptors for SHBG have not been isolated or fully characterized. Therefore, we do not know whether SHBG has an independent role in regulating prostate growth or function. The postulated SHBG receptor−testosterone system as well as the megalin-dependent transport of testosterone into the cell are discussed in later sections [Fig. 4(b)].
Potential role of SHBG and fibulins in the endometrium
Fibulins are secreted glycoproteins in the blood and extracellular matrix that act as bridging peptides between elastin fibers and cell surface integrins and become incorporated into the fibrillar extracellular matrix. There are seven members of the fibulin family, each with a different biological role. Steroid hormone−dependent interactions between SHBG and at least two fibulin family members (fibulin-1D and fibulin-2) may contribute to the extravascular accumulation and distribution of SHBG within the endometrial stroma, where it has been reported to control sex steroid access to target cells (93). This interaction may provide a molecular scaffold for signaling molecules such as integrins and represents a new mechanism of steroid hormone action [Fig. 4(d)] (93–96). These protein−protein interactions suggest additional regulation of the bioavailability of testosterone at the tissue level through tissue-binding proteins such as the fibulins.
Circulating SHBG level as a biomarker of metabolic risk
In epidemiologic studies, low total testosterone levels have been associated with increased risks of diabetes and metabolic syndrome, a cluster of conditions including hypertension, insulin resistance, central obesity, and dyslipidemia, which predispose individuals to an increased risk of cardiovascular disease. In longitudinal analyses, SHBG levels rather than total or free testosterone levels have been independently and prospectively associated with incident diabetes and metabolic syndrome after adjustments for age, adiposity, and comorbid conditions (97, 98). Among children and adolescents, SHBG may also be a biomarker for metabolic syndrome risk (99), and lower levels were more robustly associated with the risk of metabolic syndrome in boys than in girls (100). We do not know whether SHBG is merely a marker of metabolic risk or whether SHBG plays a causal role in the pathophysiology of metabolic disorders such as diabetes and metabolic syndrome.
Role of orosomucoid in acute and chronic infections
Orosomucoid, an acute phase reactant, evolved from the immunoglobulin protein superfamily (101). Inflammatory modulators, such as cytokines and chemokines, influence the expression of the AGP gene and orosomucoid synthesis (102). Circulating orosomucoid concentrations are increased in the setting of infection (103, 104), and orosomucoid was recently established as an effective prognostic marker of the severity of sepsis (105). Orosomucoid plays an important role in the inflammatory response by inhibiting neutrophil migration in sepsis through a nitric oxide−dependent mechanism (103). It may also have a protective function by binding to lipopolysaccharide and enhancing its clearance from the body (106) and by inhibiting platelet aggregation to prevent hypercoagulability in sepsis (107, 108). Orosomucoid has also been reported to regulate the bioavailability of protease inhibitors in persons with chronic HIV infection (109), which may have important implications for therapeutic drug monitoring (110). Orosomucoid may play a similar role in the distribution and bioavailability of testosterone in persons infected with HIV or hepatitis C virus (HCV), who often display marked alterations in binding protein (Table 2) concentrations.
Table 2.
Conditions That Alter SHBG Concentrations
| Conditions Associated With High SHBG Concentrations | Conditions Associated With Low SHBG Concentrations |
|---|---|
| Aging (112) | Obesity (113, 114) |
| Chronic infections such as HIV (115, 116) and hepatitis C (117, 118) | Diabetes, insulin resistance states (97, 98) |
| Hyperthyroidism (119) | Hypothyroidism (119) |
| Elevated estrogen [advanced liver disease (117), pregnancy (120), alcohol consumption (121)] | Polycystic ovarian syndrome (122) |
| Medications [thiazolidinediones (123−126), anticonvulsants (127), oral contraceptives (128, 129), selective estrogen receptor modulators (130)] | Medications [glucocorticoids (131), tyrosine kinase inhibitors (132), androgens (133)] |
| SHBG polymorphisms (rs6258, rs12150660) (32) | SHBG polymorphisms (rs6257, rs6259, rs727428, rs1799941) (33, 134) |
| Extreme weight loss (anorexia nervosa) (135) | Nephrotic syndrome (136) |
| Acromegaly (137) |
What is the Biologically Active Fraction of Circulating Testosterone? The Historical Evolution of the Free Hormone Hypothesis
According to the free hormone hypothesis, only the free testosterone fraction is able to diffuse into the cell and exert a biologic effect either by binding to the androgen receptor or after its conversion by a family of steroid 5α-reductase enzymes to 5α-dihydrotestosterone (2, 42, 111). The circulating free testosterone concentration is regulated by gonadotropins through complex interlinked feedback and feed-forward loops. If the free hormone hypothesis is correct, an increase in SHBG concentrations would transiently decrease free testosterone concentrations, which would trigger the feedback and feed-forward mechanisms within the hypothalamic-pituitary-testicular axis to stimulate luteinizing hormone (LH) and testosterone production until free testosterone levels were restored.
The free hormone hypothesis has been at the center of active academic debate for nearly 60 years. Experimental support for the free hormone hypothesis came from early studies in which the entry of testosterone into the cell was blocked by the addition of SHBG, suggesting that only the free hormone can enter the cells (138, 139). In rodent studies, the addition of increasing amounts of human or animal serum that contained the binding proteins or human pregnancy serum with a high SHBG concentration inhibited uptake by the cell of testosterone and its biological action (140). Similarly, the addition of high concentrations of HSA attenuated the aromatization of testosterone by human placental microsomes in vitro (141).
In the 1980s and 1990s, the free hormone hypothesis was challenged by various studies (Fig. 4). Pardridge (42), Manni et al. (2), and others postulated that HSA-bound testosterone, being loosely bound, could dissociate in the tissue capillaries, enter the cell, and thus become bioavailable [Fig. 4(a)]. This led to the concept that unbound testosterone plus HSA-bound testosterone fractions in circulation were biologically relevant to tissue action, and these two fractions together came to be known as bioavailable testosterone.
A second set of studies reported that SHBG-bound testosterone may be internalized into the cell through an endocytic process mediated by the membrane protein megalin [Fig. 4(b)] (142). According to this proposal, once internalized, SHBG-bound testosterone is released at the low pH within the lysosome. Genetic disruption of megalin in mice was associated with anomalies of the reproductive tract, including impaired descent of the testes into the scrotum, abnormal persistence of the cranial suspensory ligament in males, and blockade of the vaginal opening in females (142). There was no difference in androgen levels in megalin−/− mice compared with wild-type controls. In an editorial that accompanied this paper, Adams et al. (118) speculated that similar to the internalization of low-density lipoprotein cholesterol (143), trafficking chaperones may exist to deliver androgen to specific intracellular proteins, including receptors and metabolizing enzymes (144). However, because of the lack of follow-up studies to clarify the nature of megalin-mediated endocytic transport, the putative role of the protein in the transport of sex hormones has remained elusive.
Tissue-level regulation of testosterone action remains incompletely understood. It has been postulated that in certain tissues, such as the prostate, extracellular SHBG may bind to an SHBG receptor to form a complex that is coupled to an adenylate cyclase−mediated signaling cascade and may exert an independent biological effect [Fig. 4(c)] (145–147). Additional reports of the identification of androgen-binding protein (ABP)/SHBG membrane receptors in many potential target tissues, including the epididymis, testis, prostate, skeletal muscle, and the liver of rats, led to speculation that these proteins may have a much broader function in modulating sex steroid action (148).
Evaluation of the free hormone hypothesis using data from epidemiologic and other clinical studies
In multiple epidemiologic studies, bioavailable and free testosterone levels were more robustly associated with certain androgen-dependent outcomes than was total testosterone (149–151). For instance, total testosterone levels have been associated with an increased risk of diabetes and metabolic syndrome (84, 152, 153); however, free testosterone is either weakly associated or not significantly associated with the risk of incident diabetes or metabolic syndrome. When analyses of the association of total testosterone with metabolic syndrome were adjusted for SHBG, total testosterone was no longer significantly associated (97). Also, in middle-aged and older men, circulating non−SHBG-bound testosterone levels were more strongly associated with muscle strength, bone mineral density and bone quality, and fat mass than total testosterone levels were (154). Similarly, free testosterone levels were more robustly associated with depression and hypogonadal symptoms (155, 156) than total testosterone levels were (157).
The European Male Aging Study is a large epidemiologic investigation of the physical, psychological, and sexual health of a diverse population of aging European men in relation to their sex hormone levels (158). Three sexual symptoms—decreased libido, weak morning erections, and erectile dysfunction—had a significant syndromic association with total testosterone level <11 nmol/L and free testosterone level <220 pmol/L (158). Further analyses of the European Male Aging Study data revealed that men with normal total testosterone but low free testosterone concentrations had higher LH levels, reported more sexual and physical symptoms, and had lower hemoglobin values and bone mineral density than men with normal total and free testosterone concentrations as well as men with low total testosterone and normal free testosterone concentrations. Thus, low free testosterone concentration, even in the presence of normal total testosterone concentration, was associated with evidence of androgen deficiency, whereas low total testosterone concentration in the presence of normal free testosterone concentration was not, providing support for the free hormone hypothesis. The authors noted that the addition of free testosterone did not have a substantial effect on the association between symptoms and total testosterone when the total testosterone level was <8 nmol/L. Thus, measurement of free testosterone concentrations may be particularly valuable in patients with borderline total testosterone concentrations.
The levels of binding proteins may be altered by aging and disease states, including liver disease, nephrotic syndrome, thyroid dysfunction, malnutrition, obesity, inflammatory and infectious conditions, and acute illness (Table 2) (159, 160). In a study of men with HCV infection, Rao et al. (161) found twofold-higher SHBG levels in patients with HCV, which were associated with high-normal total testosterone levels but low-normal free testosterone levels. This reduction in free testosterone levels in this population of men with HCV was associated with a high prevalence of sexual dysfunction even in those with normal total testosterone levels. Some men with HIV may have markedly elevated SHBG levels (162), leading to high-normal or high total testosterone levels, even though free testosterone levels in a significant fraction of men with HIV were low normal or even low in the face of elevated total testosterone levels (163–165), leading many HIV experts to recommend measurement of free testosterone levels in the evaluation of hypogonadism in this population (166).
Experiments of nature: studies of men with genetic variations in the SHBG gene
Several genetic polymorphisms in the human SHBG gene have been associated with altered SHBG levels (32, 167–174) and some with the production of SHBG protein with altered testosterone-binding properties (32, 33). Patients with undetectable SHBG concentrations serve as unique experiments of nature for examining the free hormone hypothesis. Vos et al. (172) recently identified a brother and sister pair who were homozygous for a missense mutation within the SHBG gene (p.G224R SHBG), resulting in retention of the mutant protein in the endoplasmic reticulum, failure to secrete SHBG, and undetectable serum SHBG levels. As expected with an undetectable SHBG level, the male patient with SHBG deficiency had a very low total testosterone level of 4.8 nmol/L (reported normal range, 10 to 30 nmol/L) but a normal free testosterone level (174 pmol/L; normal range of the assay, 120 to 750 pmol/L). Both siblings had normal gonadal development and function. Furthermore, the LH level in the male was not elevated, and he had normal sexual development and normal secondary sex characteristics with normal spermatogenesis, suggesting that free testosterone concentrations, rather than total testosterone concentrations, regulate sexual development and feedback inhibition of gonadotropins.
Evaluation of the free hormone hypothesis in preclinical models
ABP/SHBG has been described in many mammals including humans, rats, and mice (175). ABP and SHBG share considerable homology in their amino acid sequence, but differences in glycosylation may be responsible for their different physiological roles (175–178). SHBG regulates the bioavailability of circulating sex steroids, whereas ABP is thought to regulate spermatogenesis and sperm maturation by maintaining high androgen levels in the testes and the epididymis (179, 180).
The extrapolation of rodent studies to human physiology is difficult because of the substantial differences in synthesis and physiology of ABP/SHBG in rodents and humans. SHBG is secreted from the liver into the bloodstream in most mammalian species, with the notable exception of rodents. The rodent liver does not produce SHBG postnatally, and rat SHBG is expressed in the liver for only a few days during late fetal maturation (181). To understand the functional role of the human SHBG gene in regulating reproductive function, several groups have expressed the human SHBG gene or portions of the human SHBG gene in transgenic mice. These studies revealed that the transgenes encoding the human SHBG sequences were expressed most abundantly in the mouse liver and kidney, in contrast to the transgene encoding the rat ABP/SHBG gene, in which expression was confined mainly to the mouse testis (182, 183). Transgenic male mice that hyperexpress human SHBG had markedly elevated serum total testosterone levels that were 10 to 100 times higher than those in wild-type littermates (184). Despite markedly elevated total testosterone levels, free testosterone concentrations and reproductive function in these mice were normal (185), providing further support for the free hormone hypothesis.
Because circulating levels of ABP/SHBG in the adult wild-type rat are very low, most of the circulating testosterone in rats is bound to albumin (186, 187). To study the impact of albumin on testosterone, Masakazu et al. (188) used Nagase analbuminemic rats (NARs), a mutant strain of Sprague-Dawley rats characterized by a total lack of serum albumin (188, 189). Total testosterone concentration in the NAR serum was significantly lower than that in normal rats, whereas serum free testosterone and gonadotropin concentrations were similar in the two groups. The NARs had small testes (190) but normal reproductive capacity and apparently normal sexual function, consistent with the free hormone hypothesis. Mendel et al. (191) used the same NARs to further evaluate the roles of free and bound testosterone. They reasoned that if albumin-bound testosterone was the biologically active fraction of circulating testosterone, the NARs would have to compensate for their lack of albumin by increasing the plasma concentration of free testosterone. In contrast, if intracellular testosterone concentrations were dependent on the concentration of free testosterone in the plasma, the NARs would have normal plasma concentrations of free testosterone. They found that circulating free testosterone concentrations in NARs were not different from those in rats with normal albumin concentrations, supporting the free hormone hypothesis.
“Although a diligently conducted equilibrium dialysis assay accurately measures free testosterone levels, the method is fraught with operator-dependent errors.”
Methods for Determination of Free Testosterone
Considering the high affinity of SHBG for testosterone binding, the SHBG-bound fraction is generally considered unavailable for biological action, and only the free and bioavailable testosterone fractions have been viewed as biologically active. The need for accurate assessment of free testosterone levels in the diagnosis and treatment of hypogonadism has stimulated the development of a variety of methods (Table 3), which are discussed in detail in the following sections.
Table 3.
The Relative Merits and Demerits of Various Methods of Measuring Free and Bioavailable Testosterone Levels
|
Method
|
Merits
|
Problems
|
|---|---|---|
| Bioavailable Testosterone | ||
| Ammonium sulfate precipitation of SHBG-bound testosterone | • Correlates well with free testosterone obtained by equilibrium dialysis | • Technically difficult • Not easily automated • Few clinical laboratories measure it routinely • Conceptually measures non−SHBG-bound testosterone, which approximates but does not equal HSA-bound plus unbound testosterone |
| Concanavalin A method | • More selective and less variable than ammonium sulfate precipitation to precipitate SHBG | • Technically difficult • Not easily automated • Not used currently by clinical laboratories • Measures non−SHBG-bound testosterone, which approximates but does not equal HSA-bound plus unbound testosterone |
| Calculated bioavailable testosterone | • Based on law-of-mass-action theory or empirical equations • Simple to obtain | • Correlation between different algorithms is poor unless revalidated in a local laboratory • Dependent on correct estimation of the association constants for the binding of testosterone to SHBG (KT) and HSA (KHSA) • Results affected by the quality of total testosterone and SHBG and HSA measurements |
| Free Testosterone | ||
|---|---|---|
| Equilibrium dialysis | • The reference method against which other methods are compared | • Technically difficult; operations in which the dialysis is performed vary across laboratories, contributing to high interlaboratory variability • Not easily automated • Few hospital clinical laboratories perform this assay • Expensive • Relies on accuracy and precision of total testosterone |
| Ultracentrifugation | • Comparable to equilibrium dialysis | • Technically difficult • Not easily automated • Few clinical laboratories measure it routinely • Expensive • Relies on accuracy and precision of total testosterone |
| Free androgen index | • Represents the ratio of total testosterone/SHBG • Has been shown to correlate with free testosterone measurements • Simple to obtain | • Overly simplistic and inaccurate measure of free testosterone concentrations • Poor indicator of gonadal status • Dependent on accurate measurements of total testosterone and SHBG • Most experts do not favor its use |
| Analogue immunoassays | • Commercially available kits • High throughput and precision • Has been shown to correlate with free testosterone measurements | • Provides inaccurate estimates of free testosterone • Experts recommend against the use of direct analogue assays for measurement of free testosterone. |
| Salivary testosterone | • Simple to obtain | • May not be an accurate marker of circulating free testosterone concentrations • Affected by sample desiccation, contamination by food and blood |
| Calculated free testosterone | • Easy to use algorithms based on various models of testosterone binding to SHBG or empirical equations • Simple to obtain | • Dependent upon correct estimates of the association constants and stoichiometry for binding of testosterone to SHBG and HSA • Accuracy and precision affected by the accuracy and precision of the total testosterone and SHBG assays |
Equilibrium dialysis and its various embodiments
Equilibrium dialysis is widely considered the reference method against which other methods are compared. It is technically demanding, and its performance is affected by assay conditions, which can result in high assay variability (192). Typically, the equilibrium dialysis procedure involves the dialysis of serum or plasma samples across a semipermeable cellulose membrane with a low-molecular-weight cutoff; protein-bound testosterone is retained, whereas free testosterone equilibrates across the dialysis membrane and can be measured in the dialysate either directly using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay or immunoassay or indirectly using a tracer. Indirect methods require adding a trace amount of radioactively labeled testosterone to the sample, and after equilibrium has been achieved, the proportion of tracer in the dialysate provides a measure of the percentage of free testosterone. Because free testosterone concentration can then be calculated by multiplying the percentage of free fraction with the total testosterone concentration obtained from the same sample in a separate assay, accurate determination of total testosterone levels is necessary for accurate determination of free testosterone levels by this method.
Although a diligently conducted equilibrium dialysis assay accurately measures free testosterone level, the method is fraught with operator-dependent errors. The protocol itself is labor-intensive, requiring repeated purification of the radioactive tracer, and is not readily amenable to high throughput. Even some large commercial diagnostic laboratories have stopped offering this assay. Although equilibrium dialysis is widely considered to be the gold standard for measuring free testosterone, this method is subject to various sources of error that may contribute to inaccuracy and imprecision. For instance, the dilution of serum or plasma may disturb the equilibrium between SHBG and its ligands (193). Results may also be altered when solutes become attached to the dialysis apparatus or membrane or when there is unequal distribution of free ligands between the two compartments as a result of (1) inadequate time to reach equilibrium; (2) release of materials from the plate or membrane that interferes with the determination of concentration; and (3) the Donnan effect at low ionic strengths, which alters the distribution of charged particles near a semipermeable membrane so that they may not distribute evenly across the two sides of the membrane (194, 195). The ionic strength and pH of the dialysis buffer and the temperature at which dialysis is performed affect the equilibrium and the estimates of binding parameters. The batch-to-batch variability in adsorption characteristics of dialysis plates from different manufacturers may be an additional source of interassay variation. The Centers for Disease Control and Prevention’s (CDC’s) hormone standardization program is invested in improving clinical assays and minimizing factors that affect measurement variability (196).
Effects of temperature variations
Steroid binding is affected by the temperature and may be 2.5 times higher at 4°C than at 37°C (9, 197, 198). The seminal testosterone-binding experiments were performed at varying temperatures—some studies were performed with ice-cold ammonium sulfate (4°C) (2) or at 25°C (9), which may affect binding equilibrium (Table 1). For example, in a separate study characterizing temperature effects on cortisol protein binding by the equilibrium dialysis method, raising the temperature from 37°C to 41°C led to an increase of ∼80% in serum free cortisol level (199).
Effects of assay buffer composition and buffer volumes
The composition and ionic strength of the dialysis buffer affect the results of equilibrium dialysis experiments. Experiments should ideally be performed using a dialysis buffer with an ionic composition that resembles that of human plasma, but this has not been the case in all studies. The assumption that the concentration of free ligands is equal on both sides of the membrane at equilibrium is not always valid (Table 1). Most proteins have a charge and accumulate a set of neutralizing counterions. The Donnan effect, discussed previously, is a consequence of maintaining the overall electrical neutrality of the solution and may give spurious evidence of an association between a ligand and a protein of opposite charge when charged counterions are present in the buffer. Differences in the ratios of volumes of dialysis buffer to sample may also affect estimates of free testosterone; when the binding is nonlinear, the decrease in total analyte concentration can alter the free fraction (Table 1).
Alteration of equilibria during physical separation of free and bound testosterone fractions
Traditional assays for determining stoichiometry and association constants usually involve separation of bound and free forms of testosterone using equilibrium dialysis, ultracentrifugation, ammonium sulfate precipitation, or other chromatographic separation methods with a subsequent Scatchard plot of the ratio of bound testosterone to unbound testosterone [(bound/free testosterone); ordinate] plotted against the bound testosterone concentration [(bound); abscissa] (200). The Scatchard analysis is a method of “linearizing” data from a saturation binding experiment to determine binding constants and estimates of stoichiometry of the noninteracting sites. However, under several experimental conditions, the underlying assumptions in the Scatchard analysis are not met, and the use of the Scatchard analysis may yield inaccurate parameter estimation (Table 1).
Achieving standardization of dialysis conditions across laboratories has been difficult, resulting in substantial interlaboratory variations in reported results. Authors who measure free testosterone by equilibrium dialysis should provide details about their methodology to ensure reproducibility and interlaboratory comparability.
Direct analogue immunoassays
Direct analogue immunoassays for the measurement of free testosterone levels provide high throughput and precision, but they lack accuracy. Numerous studies have shown that direct immunoassays for free testosterone significantly underestimated these concentrations compared with values obtained by equilibrium dialysis (201, 202). In addition, free testosterone values determined using direct analogue immunoassays varied with SHBG concentrations (203). Moreno et al. (204) showed that in a population of ambulatory men, the numerical values for free testosterone derived using analogue immunoassays were approximately one-eighth of the values calculated using the law-of-mass-action equation. The Endocrine Society does not recommend the use of analogue immunoassays for free testosterone (205, 206). Despite the Endocrine Society’s recommendation against the use of direct immunoassays, in a recent survey more than 70% of clinical laboratories were using these immunoassays to measure free testosterone levels (204).
Salivary testosterone
Some laboratories offer salivary testosterone (207), which is thought to be unaffected by variations in circulating binding proteins (208), as a marker of free testosterone levels. Clifton et al. (209) reported that morning salivary testosterone in 3722 community-dwelling people was associated with a range of self-reported health markers in men but not in women. Keevil et al. (210) used a more advanced mass spectrometer and found that salivary testosterone correlated significantly with serum calculated free testosterone more than serum total testosterone in both men and women. The measurements of salivary testosterone levels can be affected adversely by uneven desiccation of salivary samples and by impurities introduced from the oral cavity, including bleeding from the gums, which can interfere with or contribute to assay imprecision and inaccuracy. Furthermore, a growing body of data suggests that testosterone may undergo local metabolism in the salivary glands (211) so that salivary testosterone may not accurately reflect the unbound fraction in the plasma.
Methods for Determination of Bioavailable Testosterone
Bioavailable testosterone is the sum of unbound and HSA-bound testosterone (Fig. 1). Methods of direct determination of bioavailable testosterone include the ammonium sulfate precipitation method and the concanavalin A method. Most extant bioavailable assays use precipitation of SHBG-bound testosterone with saturated ammonium sulfate solution, followed by the direct measurement of testosterone in the supernatant. In a variation of this method, serum is preincubated with tritiated testosterone (2), and the fraction of tritiated testosterone not precipitated by ammonium sulfate is multiplied by the total testosterone concentration to derive the bioavailable testosterone concentration (212). Concanavalin A, which binds SHBG, has been used as an alternative approach to remove SHBG-bound testosterone; testosterone in the fraction, which does not bind to concanavalin A, can then be measured using LC-MS/MS or an immunoassay (213).
Methods for the determination of bioavailable testosterone assume complete depletion of SHBG by the addition of saturated ammonium sulfate. However, the separation of SHBG-bound testosterone from non-SHBG testosterone is affected greatly by the experimental conditions, including temperature and ammonium sulfate saturation. Consequently, the separation of SHBG-bound testosterone and the fraction not bound to SHBG may not be complete, resulting in imprecision and inaccuracy in the estimates of bioavailable testosterone concentrations. Bioavailable testosterone assays are operationally difficult to perform and are not easily automated, and they suffer from high levels of imprecision and inaccuracy. Because of the high levels of imprecision and inaccuracy in bioavailable testosterone assays, we do not favor the direct measurement of bioavailable testosterone measurement in clinical practice.
Computational Algorithms for Estimating Free and Bioavailable Testosterone Concentrations: Pitfalls and the Compelling Need for Accuracy in Calculated Free Testosterone
Most hospital and commercial laboratories do not offer an equilibrium dialysis assay for free testosterone, most likely because of operational complexities in performing the assay and difficulties in automating the procedure; only a few academic and commercial laboratories offer this assay. Furthermore, efforts to standardize experimental conditions for the performance of equilibrium dialysis across the few commercial and academic laboratories that offer it have proven challenging. Fortunately, LC-MS/MS methods for precise total testosterone measurements and high-sensitivity SHBG enzyme-linked immunosorbent assay are widely available. Accordingly, an accurate algorithm, validated against the equilibrium dialysis measurement, can provide calculated free testosterone values with significantly higher precision and lower cost than can be achieved with equilibrium dialysis in many hospital laboratories.
Recognizing the practical difficulties that practicing clinicians face in obtaining precise and accurate measurements of free testosterone concentrations by the equilibrium dialysis method, an expert panel of the Endocrine Society concluded that “calculated free testosterone, using high quality testosterone and SHBG assays with well-defined reference intervals, is the most useful clinical marker…” (205). Accordingly, several groups have developed frameworks for computing free testosterone from SHBG, total testosterone, and HSA concentrations that can be broadly classified into three categories: (1) algorithms based on linear models of testosterone binding to SHBG, (2) algorithms derived from empiric bootstrapping of data fits to mathematical forms, and (3) algorithms based on nonlinear models incorporating allostery in the SHBG dimer.
Calculated free testosterone based on linear models
The algorithms published by Vermeulen et al. (3), Södergard et al. (4), and Mazer (5) are all based on the linear model of testosterone binding to SHBG [Fig. 3(a)]. They all used Scatchard analysis to linearize data from a saturation-binding experiment to determine binding constants and estimates of stoichiometry of noninteracting sites. However, under several experimental conditions, the underlying assumptions in the Scatchard analysis are not met, and the Scatchard analysis may yield inaccurate parameter estimation (Table 1). For instance, the assumptions of linear regression, that the scatter of points about a line follows a normal (or Gaussian) distribution and the standard deviation is the same at every concentration of the analyte, are violated in a Scatchard plot, which alters the relationship between bound and free fractions. Use of the calculated values of the bound/free steroids further violates the assumption of linear regression that all uncertainty is in the Y variable, whereas the X variable is known with complete certainty. Because of the nonlinear nature of binding and allosteric interactions between binding sites, the linear transformation of the binding data to force a straight line through nonlinear data renders these historical estimates of binding affinity and capacity prone to error. Nonlinear computational tools may be more suitable for binding events, which involve allosteric interactions and are nonlinear; however, these methods have not been used in the literature for the analyses of data related to testosterone binding to SHBG or HSA.
These algorithms use different association constants of testosterone binding to HSA and SHBG and therefore yield slightly different estimates of free testosterone. For example, the Vermeulen et al. (3) equation used association constants of 3.6 × 104 and 1 × 109 L/mol for HSA and SHBG, respectively, whereas the Södergard et al. (4) algorithm used 4.06 × 104 and 5.97 × 108 L/ mol, respectively. All three equations, especially the Vermeulen et al. (3) equation, have been widely used in the literature and in commercial laboratories. The calculated free testosterone values derived using these equations correlated with free testosterone concentrations measured by equilibrium dialysis in some studies (3, 4, 214) but displayed substantial systematic differences from values derived using the equilibrium dialysis method and from each other (6, 34, 215–218).
Hackbarth et al. (218) evaluated five separate equations in two patient groups with different sex distributions. They defined percentage differences above 20% to be unacceptable; depending on the equation, 32% to 72% of males and 29% to 57% of females displayed an unacceptable agreement between levels of calculated free testosterone and measured free testosterone by equilibrium dialysis. In addition, 14.9% of males and 11.1% of females showed poor fit by all five equations.
Calculated free testosterone from empiric and bootstrap fitting approaches
Ly and Handelsman (6) analyzed a data set comprising >4000 blood samples in which free and total testosterone and SHBG concentrations were measured; dividing the data set into samples with serum total testosterone above and below 5 nM, they used a bootstrap regression modeling approach free of assumptions about theoretical binding equilibria to develop an empirical equation for free testosterone in terms of total testosterone and SHBG. Later, Sartorius et al. (217) created a variety of formulas for evaluation by bootstrap resampling to identify the best-fit model according to entropy reduction and improve upon the previous empirical calculated free testosterone equation. This algorithm, like the others, is highly dependent on the accuracy and precision of the total testosterone and SHBG assays, which affects the accuracy and precision of calculated free testosterone (219). Furthermore, regression equations derived empirically in one patient population may not necessarily apply to another population, especially to a population with substantially different SHBG concentrations. In a different patient population, there is no reason to believe that best-fit parameters will be the same as in the test population. In addition, these methods do not have a testable binding model that can be subjected to experimental validation or improved upon to incorporate other variables or new knowledge of the dynamics of testosterone binding to its cognate binding proteins or for personalization to specific conditions or disease states.
Calculated free testosterone using an algorithm that incorporates experimentally observed nonlinear binding dynamics and allosteric interaction between binding sites
We recently investigated the source of systematic discrepancies between free testosterone values computed using the simple linear model, which formed the basis of the Vermeulen et al. (3), Södergard et al. (4), and Mazer (5) equations, and free testosterone measured using equilibrium dialysis. These discrepancies between free testosterone calculated using these linear binding models and free testosterone measured using equilibrium dialysis are most likely the result of the erroneous assumptions of the dynamics of testosterone binding to SHBG. Recent studies of testosterone binding to SHBG using modern biophysical techniques suggest that SHBG circulates as a homodimer and that there is complex allosteric interaction between the two binding sites on the SHBG dimer, such that the binding affinities of the two sites are not identical (34). The computational algorithm based on this novel multistep ensemble allosteric model (EAM) (34) of testosterone binding to SHBG provided estimates of free testosterone levels that closely matched free testosterone levels measured using the equilibrium dialysis method in samples derived from men and women in two randomized clinical trials (220, 221). The calculated free testosterone level obtained using the prevailing linear model was systematically lower than those measured by equilibrium dialysis. In Table 4, we show that calculated free testosterone values across age deciles in the Framingham Heart Study computed by the linear model are lower than those computed by the allosteric model. The EAM model is based on experimentally derived binding affinity and dynamics, which can be verified experimentally and improved upon with additional information about other variables that determine free testosterone concentrations.
“Discrepancies between free testosterone calculated using these linear binding models and equilibrium dialysis are most likely the result of the erroneous assumptions of the dynamics of testosterone binding to SHBG.”
Table 4.
Comparison of Free Testosterone Determined Using the Linear and Allosteric Models for the Framingham Heart Study Data
| Age Decile (y) | Number of Participants (%) | SHBG (nmol/L) | Total Testosterone (ng/dL) | Calculated Free Testosterone, Linear Model (pg/mL) | Calculated Free Testosterone, Allosteric Model (pg/mL) |
|---|---|---|---|---|---|
| <30 | 217 (7%) | 36.49 | 715.02 | 145.35 (45.8) | 238.39 (71.77) |
| 30–39 | 621 (20%) | 37.82 | 658.23 | 129.39 (47.63) | 215.52 (77.82) |
| 40–49 | 811 (26%) | 38.80 | 42.05 | 113.48 (43.09) | 196.42 (71.74) |
| 50–59 | 736 (23.4%) | 51.67 | 616.82 | 97.64 (34.98) | 185.93 (70.87) |
| 60–69 | 465 (15%) | 58.93 | 568.57 | 80.72 (28.89) | 161.98 (63.12) |
| 70–79 | 270 (9%) | 67.13 | 571.94 | 71.98 (30.25) | 154.53 (71.89) |
| 80+ | 22 (0.7%) | 85.28 | 590.31 | 56.63 (21.75) | 136.73 (65.63) |
Data for models are presented as mean ± standard deviation. Of note, albumin concentrations of 4.3 g/dL were used for computations.
Lack of Standardization of Free Testosterone Measurement Methods and Unavailability of Harmonized Reference Ranges for Free Testosterone
Le et al. (222) surveyed 120 academic and community laboratories in the United States to characterize the distribution of assays and the associated reference values for free testosterone. In all, 84% of the surveyed laboratories sent their samples for free testosterone measurement to larger centralized reference laboratories (222). These large commercial laboratories offered a variety of methods, including ultracentrifugation, radioimmunoassay, and calculation-based algorithms, as well as equilibrium dialysis (222). Many clinical laboratories used calculated free testosterone based on published linear equations (3). The laboratories reported wide variations in the reference ranges. Only 30 of the laboratories surveyed would confirm that validation studies had been performed, and the authors advised that reference ranges provided by manufacturers and laboratories should be interpreted with caution.
In a survey of 12 academic laboratories, 12 community medical laboratories, and one national laboratory, Lazarou et al. (223) found 17 and 13 different sets of reference values for total and free testosterone, respectively, which were established largely without clinical considerations. Recently, Bhasin et al. (224) reported reference ranges for calculated free testosterone concentrations in a large, rigorously collected sample of community-dwelling men. In healthy young men of the Framingham Heart Study who were 19 to 40 years of age, the lower limit of the normal range, defined as the 2.5th percentile of calculated free testosterone, was 70 pg/mL (242.7 pmol/L) (198).
Clinical Implications and Recommendations
Male hypogonadism is a clinical condition characterized by the presence of typical signs and symptoms in the setting of consistently low serum testosterone concentrations. The Endocrine Society guidelines currently suggest measuring free testosterone levels in men in whom total testosterone concentrations are near the lower limit of the normal range and in men with conditions that affect SHBG concentrations and render total testosterone a less reliable index of gonadal function (206). If the free hormone hypothesis is correct, free testosterone should serve as the benchmark for biochemical confirmation of hypogonadism. Accurate determination of free testosterone values is therefore central to an accurate diagnosis of hypogonadism.
The direct analogue assays for free testosterone determination are inaccurate and should not be used. However, a confluence of factors related to the regulatory process, economic considerations, and difficulties in performing equilibrium dialysis methods in many hospital laboratories has led to their surprising endurance despite their known inaccuracy. Historically, laboratory-certifying bodies, such as the Clinical Laboratory Improvement Amendments, have certified laboratories and assays mostly on the basis of process measures; unlike the CDC and its Hormone Assay Standardization program for testosterone, these bodies have generally not required accuracy-based benchmarks. Similarly, the requirement in the assay approval process for demonstration of comparability to a previously approved assay enables new tracer analogue assays to be approved because they can demonstrate comparability to previously approved analogue methods.
Equilibrium dialysis is the reference method for free testosterone determination, but this assay is not always available to clinicians in all hospital laboratories; in addition, there are substantial interlaboratory variations because of the lack of standardization of assay conditions, making it difficult for practicing endocrinologists to interpret free testosterone levels. Mechanisms to harmonize the equilibrium dialysis procedure across laboratories are needed. Until equilibrium dialysis methods can be standardized across laboratories, a computational framework that accurately captures the dynamics of testosterone to SHBG and HSA interactions in calculating free testosterone values is an unmet need for precise clinical diagnosis. The EAM appears to be an accurate and testable model for calculating free testosterone levels, but this model needs further validation in large populations.
Total testosterone, which can be measured with high accuracy using LC-MS/MS assays in CDC-certified laboratories, and free testosterone are highly correlated, and it is only in individuals with altered binding-protein concentrations that the associations begin to diverge. For the time being, we therefore suggest continuing to follow the Endocrine Society’s guidelines to measure total testosterone level and, in circumstances of suspected alterations in SHBG and albumin concentrations and/or binding, checking free testosterone level by equilibrium dialysis. Efforts are underway to standardize the procedures for free testosterone measurement and to generate harmonized reference ranges. Until that time, clinicians should be aware that inaccuracies in free testosterone measurements and calculations and poorly defined reference ranges can increase the risk of misclassification in the diagnosis of androgen disorders.
Synthesis
Sex steroid bioactivity and the respective roles of SHBG and HSA are more complex than originally believed. The oversimplified assumptions of stoichiometry, binding dynamics, and binding affinity have contributed to the development of inaccurate linear binding models, which have been propagated without much critical reappraisal until now. These historical linear models and the resulting equations for calculating free testosterone based on these legacy models are widely used and may potentially increase the risk of misclassifying men seeking testosterone therapy. A novel multistep EAM of the binding of testosterone to SHBG provides close approximation of free testosterone levels using equilibrium dialysis, but clinical experience with this new model is currently limited. Harmonized reference ranges for free testosterone are needed to demarcate individuals who are eugonadal from those who are hypogonadal, acknowledging that different symptoms may have different thresholds. These steps would reduce the risk of disease misclassification and optimize clinical decision making in the management of androgen disorders in men and women.
Acknowledgments
This work was partially supported by SBIR grant R43AG045011 (to R.J.) and grant 5R01AG037193 (to S.B.) and by the Boston Claude D. Pepper Older Americans Independence Center grant P30AG031679 (to S.B.).
Disclosure Summary: S.B. received research support from AbbVie, Transition Therapeutics, and Regeneron; consulting fees from Novartis, AbbVie, and Lilly; and equity from Function Promoting Therapies, LLC. F.C.W.W. received research support from Besins, Eli Lily, Bayer, and Mereo; consulting fees from Besins and Repros Therapeutics; and royalties from UpToDate. A.M.M. received research support from AbbVie and GSK; consulting fees from AbbVie, Endo, Lilly, and Lipocine; and royalties from UpToDate. R.J. received equity from Function Promoting Therapies, LLC, and LeadInvent Pharma. The remaining authors have nothing to disclose.
Abbreviations:
- ABP
androgen-binding protein
- CBG
corticosteroid-binding globulin
- CDC
Centers for Disease Control and Prevention
- EAM
ensemble allosteric model
- HCV
hepatitis C virus
- HSA
human serum albumin
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LG
laminin G−like
- LH
luteinizing hormone
- NAR
Nagase analbuminemic rat
- SHBG
sex hormone-binding globulin.
References
- 1. Hammond GL. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol. 2016;230(1):R13–R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manni A, Pardridge WM, Cefalu W, Nisula BC, Bardin CW, Santner SJ, Santen RJ. Bioavailability of albumin-bound testosterone. J Clin Endocrinol Metab. 1985;61(4):705–710. [DOI] [PubMed] [Google Scholar]
- 3. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 4. Södergard R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. [DOI] [PubMed] [Google Scholar]
- 5. Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74(6):512–519. [DOI] [PubMed] [Google Scholar]
- 6. Ly LP, Handelsman DJ. Empirical estimation of free testosterone from testosterone and sex hormone-binding globulin immunoassays. Eur J Endocrinol. 2005;152(3):471–478. [DOI] [PubMed] [Google Scholar]
- 7. Giton F, Guéchot J, Fiet J. Comparative determinations of non SHBG-bound serum testosterone, using ammonium sulfate precipitation, concanavalin A binding or calculation in men. Steroids. 2012;77(12):1306–1311. [DOI] [PubMed] [Google Scholar]
- 8. Giton F, Fiet J, Guéchot J, Ibrahim F, Bronsard F, Chopin D, Raynaud JP. Serum bioavailable testosterone: assayed or calculated? Clin Chem. 2006;52(3):474–481. [DOI] [PubMed] [Google Scholar]
- 9. Pearlman WH, Crépy O. Steroid-protein interaction with particular reference to testosterone binding by human serum. J Biol Chem. 1967;242(2):182–189. [PubMed] [Google Scholar]
- 10. Nisula BC, Dunn JF. Measurement of the testosterone binding parameters for both testosterone-estradiol binding globulin and albumin in individual serum samples. Steroids. 1979;34(7):771–791. [DOI] [PubMed] [Google Scholar]
- 11. Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981;53(1):58–68. [DOI] [PubMed] [Google Scholar]
- 12. Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548–551. [DOI] [PubMed] [Google Scholar]
- 13. Khosla S. Sex hormone binding globulin: inhibitor or facilitator (or both) of sex steroid action [editorial]? J Clin Endocrinol Metab. 2006;91(12):4764–4766. [DOI] [PubMed] [Google Scholar]
- 14. Mercier C, Alfsen A, Baulieu E-E. A testosterone binding globulin. In: Proceedings of the Second Symposium on Steroid Hormones; 1965; Ghent, Belgium. Excerpta Medica International Congress Series. 1966;101:212.
- 15. Rosenbaum W, Christy NP, Kelly WG. Electrophoretic evidence for the presence of an estrogen-binding beta-globulin in human plasma. J Clin Endocrinol Metab. 1966;26(12):1399–1403. [DOI] [PubMed] [Google Scholar]
- 16. Murphy BE. Binding of testosterone and estradiol in plasma. Can J Biochem. 1968;46(4):299–302. [DOI] [PubMed] [Google Scholar]
- 17. Bérubé D, Séralini GE, Gagné R, Hammond GL. Localization of the human sex hormone-binding globulin gene (SHBG) to the short arm of chromosome 17 (17p12----p13). Cytogenet Cell Genet. 1990;54(1-2):65–67. [DOI] [PubMed] [Google Scholar]
- 18. Nakhla AM, Hryb DJ, Rosner W, Romas NA, Xiang Z, Kahn SM. Human sex hormone-binding globulin gene expression: multiple promoters and complex alternative splicing. BMC Mol Biol. 2009;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grishkovskaya I, Avvakumov GV, Sklenar G, Dales D, Hammond GL, Muller YA. Crystal structure of human sex hormone-binding globulin: steroid transport by a laminin G-like domain. EMBO J. 2000;19(4):504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod. 2011;85(3):431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grishkovskaya I, Avvakumov GV, Hammond GL, Catalano MG, Muller YA. Steroid ligands bind human sex hormone-binding globulin in specific orientations and produce distinct changes in protein conformation. J Biol Chem. 2002;277(35):32086–32093. [DOI] [PubMed] [Google Scholar]
- 22. Avvakumov GV, Grishkovskaya I, Muller YA, Hammond GL. Crystal structure of human sex hormone-binding globulin in complex with 2-methoxyestradiol reveals the molecular basis for high affinity interactions with C-2 derivatives of estradiol. J Biol Chem. 2002;277(47):45219–45225. [DOI] [PubMed] [Google Scholar]
- 23. Hammond GL, Underhill DA, Smith CL, Goping IS, Harley MJ, Musto NA, Cheng CY, Bardin CW. The cDNA-deduced primary structure of human sex hormone-binding globulin and location of its steroid-binding domain. FEBS Lett. 1987;215(1):100–104. [DOI] [PubMed] [Google Scholar]
- 24. Thaler M, Metzger J, Schreiegg A, Denk B, Gleixner A, Hauptmann H, Luppa PB. Immunoassay for sex hormone-binding globulin in undiluted serum is influenced by high-molecular-mass aggregates. Clin Chem. 2005;51(2):401–407. [DOI] [PubMed] [Google Scholar]
- 25. Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67(3):460–464. [DOI] [PubMed] [Google Scholar]
- 26. Wang Q, Kangas AJ, Soininen P, Tiainen M, Tynkkynen T, Puukka K, Ruokonen A, Viikari J, Kähönen M, Lehtimäki T, Salomaa V, Perola M, Davey Smith G, Raitakari OT, Järvelin MR, Würtz P, Kettunen J, Ala-Korpela M. Sex hormone-binding globulin associations with circulating lipids and metabolites and the risk for type 2 diabetes: observational and causal effect estimates. Int J Epidemiol. 2015;44(2):623–637. [DOI] [PubMed] [Google Scholar]
- 27. Selva DM, Hammond GL. Thyroid hormones act indirectly to increase sex hormone-binding globulin production by liver via hepatocyte nuclear factor-4alpha. J Mol Endocrinol. 2009;43(1):19–27. [DOI] [PubMed] [Google Scholar]
- 28. Petra PH. The plasma sex steroid binding protein (SBP or SHBG): a critical review of recent developments on the structure, molecular biology and function. J Steroid Biochem Mol Biol. 1991;40(4-6):735–753. [DOI] [PubMed] [Google Scholar]
- 29. Hammond GL, Bocchinfuso WP. Sex hormone-binding globulin/androgen-binding protein: steroid-binding and dimerization domains. J Steroid Biochem Mol Biol. 1995;53(1-6):543–552. [DOI] [PubMed] [Google Scholar]
- 30. Avvakumov GV, Grishkovskaya I, Muller YA, Hammond GL. Resolution of the human sex hormone-binding globulin dimer interface and evidence for two steroid-binding sites per homodimer. J Biol Chem. 2001;276(37):34453–34457. [DOI] [PubMed] [Google Scholar]
- 31. Metzger J, Schnitzbauer A, Meyer M, Söder M, Cuilleron CY, Hauptmann H, Huber E, Luppa PB. Binding analysis of 1alpha- and 17alpha-dihydrotestosterone derivatives to homodimeric sex hormone-binding globulin. Biochemistry. 2003;42(46):13735–13745. [DOI] [PubMed] [Google Scholar]
- 32. Ohlsson C, Wallaschofski H, Lunetta KL, Stolk L, Perry JR, Koster A, Petersen AK, Eriksson J, Lehtimäki T, Huhtaniemi IT, Hammond GL, Maggio M, Coviello AD, Ferrucci L, Heier M, Hofman A, Holliday KL, Jansson JO, Kähönen M, Karasik D, Karlsson MK, Kiel DP, Liu Y, Ljunggren O, Lorentzon M, Lyytikäinen LP, Meitinger T, Mellström D, Melzer D, Miljkovic I, Nauck M, Nilsson M, Penninx B, Pye SR, Vasan RS, Reincke M, Rivadeneira F, Tajar A, Teumer A, Uitterlinden AG, Ulloor J, Viikari J, Völker U, Völzke H, Wichmann HE, Wu TS, Zhuang WV, Ziv E, Wu FC, Raitakari O, Eriksson A, Bidlingmaier M, Harris TB, Murray A, de Jong FH, Murabito JM, Bhasin S, Vandenput L, Haring R; EMAS Study Group . Genetic determinants of serum testosterone concentrations in men. PLoS Genet. 2011;7(10):e1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu TS, Hammond GL. Naturally occurring mutants inform SHBG structure and function. Mol Endocrinol. 2014;28(7):1026–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zakharov MN, Bhasin S, Travison TG, Xue R, Ulloor J, Vasan RS, Carter E, Wu F, Jasuja R. A multi-step, dynamic allosteric model of testosterone’s binding to sex hormone binding globulin. Mol Cell Endocrinol. 2015;399:190–200. [DOI] [PubMed] [Google Scholar]
- 35. Tietz NW, ed. Textbook of Clinical Chemistry. Philadelphia, PA: Saunders; 1986. [Google Scholar]
- 36. Peters T Jr. All About Albumin: Biochemistry, Genetics, and Medical Applications. San Diego, CA: Academic Press; 1996.
- 37. Griffin JE, Wilson JD. Disorders of the testis and the male reproduction tract. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, eds. Williams Textbook of Endocrinology. 9th ed. Philadelphia, PA: WB Saunders; 1998. [Google Scholar]
- 38. Vermeulen A, Verdonck L. Studies on the binding of testosterone to human plasma. Steroids. 1968;11(5):609–635. [PubMed] [Google Scholar]
- 39. Burke CW, Anderson DC. Sex-hormone-binding globulin is an oestrogen amplifier. Nature. 1972;240(5375):38–40. [DOI] [PubMed] [Google Scholar]
- 40. Banfi G, Daverio R, Bonini P. Reduced levels of free testosterone in four Catania-type alloalbuminemic males. J Clin Lab Anal. 1992;6(3):123–124. [DOI] [PubMed] [Google Scholar]
- 41. Kragh-Hansen U, Minchiotti L, Brennan SO, Sugita O. Hormone binding to natural mutants of human serum albumin. Eur J Biochem. 1990;193(1):169–174. [DOI] [PubMed] [Google Scholar]
- 42. Pardridge WM. Serum bioavailability of sex steroid hormones. Clin Endocrinol Metab. 1986;15(2):259–278. [DOI] [PubMed] [Google Scholar]
- 43. Kurnit DM, Philipp BW, Bruns GA. Confirmation of the mapping assignment of human serum albumin to chromosome 4 using a cloned human albumin gene. Cytogenet Cell Genet. 1982;34(4):282–288. [DOI] [PubMed] [Google Scholar]
- 44. Arasteh A, Farahi S, Habibi-Rezaei M, Moosavi-Movahedi AA. Glycated albumin: an overview of the in vitro models of an in vivo potential disease marker. J Diabetes Metab Disord. 2014;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Helmersson-Karlqvist J, Flodin M, Havelka AM, Xu XY, Larsson A. The Roche immunoturbidimetric albumin method on Cobas c 501 gives higher values than the Abbott and Roche BCP methods when analyzing patient plasma samples. J Clin Lab Anal. 2016;30(5):677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brackeen GL, Dover JS, Long CL. Serum albumin: differences in assay specificity. Nutr Clin Pract. 1989;4(6):203–205. [DOI] [PubMed]
- 47. Speicher CE, Widish JR, Gaudot FJ, Hepler BR. An evaluation of the overestimation of serum albumin by bromcresol green. Am J Clin Pathol. 1978;69(3):347–350. [DOI] [PubMed] [Google Scholar]
- 48. Gustafsson JE. Improved specificity of serum albumin determination and estimation of “acute phase reactants” by use of the bromcresol green reaction. Clin Chem. 1976;22(5):616–622. [PubMed] [Google Scholar]
- 49. Pinnell AE, Northam BE. New automated dye-binding method for serum albumin determination with bromcresol purple. Clin Chem. 1978;24(1):80–86. [PubMed] [Google Scholar]
- 50. Wells FE, Addison GM, Postlethwaite RJ. Albumin analysis in serum of haemodialysis patients: discrepancies between bromocresol purple, bromocresol green and electroimmunoassay. Ann Clin Biochem. 1985;22(Pt 3):304–309. [DOI] [PubMed] [Google Scholar]
- 51. Pardridge WM, Eisenberg J, Cefalu WT. Absence of albumin receptor on brain capillaries in vivo or in vitro. Am J Physiol. 1985;249(3):E264–E267. [DOI] [PubMed]
- 52. Stollman YR, Gärtner U, Theilmann L, Ohmi N, Wolkoff AW. Hepatic bilirubin uptake in the isolated perfused rat liver is not facilitated by albumin binding. J Clin Invest. 1983;72(2):718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bolton JL, Hayward C, Direk N, Lewis JG, Hammond GL, Hill LA, Anderson A, Huffman J, Wilson JF, Campbell H, Rudan I, Wright A, Hastie N, Wild SH, Velders FP, Hofman A, Uitterlinden AG, Lahti J, Räikkönen K, Kajantie E, Widen E, Palotie A, Eriksson JG, Kaakinen M, Järvelin MR, Timpson NJ, Davey Smith G, Ring SM, Evans DM, St Pourcain B, Tanaka T, Milaneschi Y, Bandinelli S, Ferrucci L, van der Harst P, Rosmalen JG, Bakker SJ, Verweij N, Dullaart RP, Mahajan A, Lindgren CM, Morris A, Lind L, Ingelsson E, Anderson LN, Pennell CE, Lye SJ, Matthews SG, Eriksson J, Mellstrom D, Ohlsson C, Price JF, Strachan MW, Reynolds RM, Tiemeier H, Walker BR; CORtisol NETwork (CORNET) Consortium . Genome wide association identifies common variants at the SERPINA6/SERPINA1 locus influencing plasma cortisol and corticosteroid binding globulin. PLoS Genet. 2014;10(7):e1004474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cooke RR, McIntosh JE, Murray-McIntosh RP. Effect of cortisol on percentage of non-sex-hormone-bound steroid: implications for distribution of steroids on binding proteins in serum. Clin Chem. 1996;42(2):249–254. [PubMed] [Google Scholar]
- 55. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39(2):163–171. [DOI] [PubMed] [Google Scholar]
- 56. Pardridge WM. Transport of protein-bound hormones into tissues in vivo. Endocr Rev. 1981;2(1):103–123. [DOI] [PubMed] [Google Scholar]
- 57. Colombo S, Buclin T, Décosterd LA, Telenti A, Furrer H, Lee BL, Biollaz J, Eap CB; Swiss HIV Cohort Study . Orosomucoid (alpha1-acid glycoprotein) plasma concentration and genetic variants: effects on human immunodeficiency virus protease inhibitor clearance and cellular accumulation. Clin Pharmacol Ther. 2006;80(4):307–318. [DOI] [PubMed] [Google Scholar]
- 58. Kerkay J, Westphal U. Steroid-protein interactions, XIX: complex formation between alpha 1-acid glycoprotein and steroid hormones. Biochim Biophys Acta. 1968;170(2):324–333. [DOI] [PubMed]
- 59. Hawe A, Sutter M, Jiskoot W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm Res. 2008;25(7):1487–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brown JR. Structural origins of mammalian albumin. Fed Proc. 1976;35(10):2141–2144. [PubMed] [Google Scholar]
- 61. Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K. Crystal structure of human serum albumin at 2.5 A resolution. Protein Eng. 1999;12(6):439–446. [DOI] [PubMed] [Google Scholar]
- 62. Kratochwil NA, Huber W, Müller F, Kansy M, Gerber PR. Predicting plasma protein binding of drugs: a new approach. Biochem Pharmacol. 2002;64(9):1355–1374. [DOI] [PubMed] [Google Scholar]
- 63. Matsushita S, Isima Y, Chuang VT, Watanabe H, Tanase S, Maruyama T, Otagiri M. Functional analysis of recombinant human serum albumin domains for pharmaceutical applications. Pharm Res. 2004;21(10):1924–1932. [DOI] [PubMed] [Google Scholar]
- 64. Ryan MT, Gibbs G. Analysis of ultraviolet spectral perturbations arising in the interaction of steroids and human serum albumin. Arch Biochem Biophys. 1970;136(1):65–72. [DOI] [PubMed] [Google Scholar]
- 65. Emadi-Konjin P, Bain J, Bromberg IL. Evaluation of an algorithm for calculation of serum “bioavailable” testosterone (BAT). Clin Biochem. 2003;36(8):591–596. [DOI] [PubMed] [Google Scholar]
- 66. Chanphai P, Vesper AR, Bekale L, Bérubé G, Tajmir-Riahi HA. Transporting testosterone and its dimers by serum proteins. J Photochem Photobiol B. 2015;153:173–183. [DOI] [PubMed] [Google Scholar]
- 67. Eik-Nes K, Schellman JA, Lumry R, Samuels LT. The binding of steroids to protein, I: solubility determinations. J Biol Chem. 1954;206(1):411–419. [PubMed]
- 68. Moll GW Jr, Rosenfield RL. Some limitations of using equilibrium dialysis to study human serum albumin-testosterone interaction. J Clin Endocrinol Metab. 1978;46(3):501–503. [DOI] [PubMed]
- 69. Ryan MT. Influence of steroid binding on the tryptic hydrolysis of serum albumin. Biochemistry. 1973;12(12):2221–2230. [DOI] [PubMed] [Google Scholar]
- 70. Rosner W, Christy NP, Kelly WG. Partial purification and preliminary characterization of estrogen-binding globulins from human plasma. Biochemistry. 1969;8(7):3100–3108. [DOI] [PubMed] [Google Scholar]
- 71. Fischer MJ, Bos OJ, van der Linden RF, Wilting J, Janssen LH. Steroid binding to human serum albumin and fragments thereof: role of protein conformation and fatty acid content. Biochem Pharmacol. 1993;45(12):2411–2416. [DOI] [PubMed]
- 72. Ascenzi P, Bocedi A, Notari S, Fanali G, Fesce R, Fasano M. Allosteric modulation of drug binding to human serum albumin. Mini Rev Med Chem. 2006;6(4):483–489. [DOI] [PubMed] [Google Scholar]
- 73. Iqbal MJ, Johnson MW. Purification and characterization of human sex hormone binding globulin. J Steroid Biochem. 1979;10(5):535–540. [DOI] [PubMed] [Google Scholar]
- 74. Hammond GL, Robinson PA, Sugino H, Ward DN, Finne J. Physicochemical characteristics of human sex hormone binding globulin: evidence for two identical subunits. J Steroid Biochem. 1986 Apr;24(4):815–24. [DOI] [PubMed]
- 75. Petra PH, Stanczyk FZ, Senear DF, Namkung PC, Novy MJ, Ross JBA, Turner E and Brown JA. Current status of the molecular structure and function of the plasma sex steroid-binding protein (SBP). J Steroid Biochem. (1983) 699–706. [DOI] [PubMed]
- 76. Heinrich-Balard L, Zeinyeh W, Déchaud H, Rivory P, Roux A, Pugeat M, Cohen R. Inverse relationship between hSHBG affinity for testosterone and hSHBG concentration revealed by surface plasmon resonance. Mol Cell Endocrinol. 2015;399:201–207. [DOI] [PubMed] [Google Scholar]
- 77. Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membrane. J Steroid Biochem Mol Biol. 1999;69(1-6):481–485. [DOI] [PubMed] [Google Scholar]
- 78. Kahn SM, Hryb DJ, Nakhla AM, Romas NA, Rosner W. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002;175(1):113–120. [DOI] [PubMed] [Google Scholar]
- 79. Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Sex hormone-binding globulin: anatomy and physiology of a new regulatory system. J Steroid Biochem Mol Biol. 1991;40(4-6):813–820. [DOI] [PubMed] [Google Scholar]
- 80. Kalme T, Seppälä M, Qiao Q, Koistinen R, Nissinen A, Harrela M, Loukovaara M, Leinonen P, Tuomilehto J. Sex hormone-binding globulin and insulin-like growth factor-binding protein-1 as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. J Clin Endocrinol Metab. 2005;90(3):1550–1556. [DOI] [PubMed] [Google Scholar]
- 81. Pascual-Figal DA, Tornel PL, Nicolás F, Sánchez-Más J, Martínez MD, Gracia MR, Garrido IP, Ruipérez JA, Valdés M. Sex hormone-binding globulin: a new marker of disease severity and prognosis in men with chronic heart failure. Rev Esp Cardiol. 2009;62(12):1381–1387. [DOI] [PubMed] [Google Scholar]
- 82. Osuna JA, Gómez-Pérez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl. 2006;52(5):355–361. [DOI] [PubMed] [Google Scholar]
- 83. De Simone M, Verrotti A, Iughetti L, Palumbo M, Farello G, Di Cesare E, Bernabei R, Rosato T, Lozzi S, Criscione S. Increased visceral adipose tissue is associated with increased circulating insulin and decreased sex hormone binding globulin levels in massively obese adolescent girls. J Endocrinol Invest. 2001;24(6):438–444. [DOI] [PubMed] [Google Scholar]
- 84. Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91(3):843–850. [DOI] [PubMed] [Google Scholar]
- 85. Pascal N, Amouzou EK, Sanni A, Namour F, Abdelmouttaleb I, Vidailhet M, Guéant JL. Serum concentrations of sex hormone binding globulin are elevated in kwashiorkor and anorexia nervosa but not in marasmus. Am J Clin Nutr. 2002;76(1):239–244. [DOI] [PubMed] [Google Scholar]
- 86. Estour B, Pugeat M, Lang F, Dechaud H, Pellet J, Rousset H. Sex hormone binding globulin in women with anorexia nervosa. Clin Endocrinol (Oxf). 1986;24(5):571–576. [DOI] [PubMed] [Google Scholar]
- 87. Thaler MA, Seifert-Klauss V, Luppa PB. The biomarker sex hormone-binding globulin: from established applications to emerging trends in clinical medicine. Best Pract Res Clin Endocrinol Metab. 2015;29(5):749–760. [DOI] [PubMed]
- 88. Hammond GL, Wu TS, Simard M. Evolving utility of sex hormone-binding globulin measurements in clinical medicine. Curr Opin Endocrinol Diabetes Obes. 2012;19(3):183–189. [DOI] [PubMed] [Google Scholar]
- 89. Ding VD, Moller DE, Feeney WP, Didolkar V, Nakhla AM, Rhodes L, Rosner W, Smith RG. Sex hormone-binding globulin mediates prostate androgen receptor action via a novel signaling pathway. Endocrinology. 1998;139(1):213–218. [DOI] [PubMed] [Google Scholar]
- 90. Hryb DJ, Khan MS, Romas NA, Rosner W. Solubilization and partial characterization of the sex hormone-binding globulin receptor from human prostate. J Biol Chem. 1989;264(10):5378–5383. [PubMed] [Google Scholar]
- 91. Nakhla AM, Ding VD, Khan MS, Romas NA, Rhodes L, Smith RG, Rosner W. 5 alpha-Androstan-3 alpha,17 beta-diol is a hormone: stimulation of cAMP accumulation in human and dog prostate. J Clin Endocrinol Metab. 1995;80(7):2259–2262. [DOI] [PubMed] [Google Scholar]
- 92. Sadar MD, Hussain M, Bruchovsky N. Prostate cancer: molecular biology of early progression to androgen independence. Endocr Relat Cancer. 1999;6(4):487–502. [DOI] [PubMed] [Google Scholar]
- 93. Ng KM, Catalano MG, Pinós T, Selva DM, Avvakumov GV, Munell F, Hammond GL. Evidence that fibulin family members contribute to the steroid-dependent extravascular sequestration of sex hormone-binding globulin. J Biol Chem. 2006;281(23):15853–15861. [DOI] [PubMed] [Google Scholar]
- 94. Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120(3):577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hemler ME. Integrin associated proteins. Curr Opin Cell Biol. 1998;10(5):578–585. [DOI] [PubMed] [Google Scholar]
- 96. Horwitz GD, Newsome WT. Neurophysiology: sensing and categorizing. Curr Biol. 1998;8(11):R376–R378. [DOI] [PubMed] [Google Scholar]
- 97. Bhasin S, Jasjua GK, Pencina M, D’Agostino R Sr, Coviello AD, Vasan RS, Travison TG. Sex hormone-binding globulin, but not testosterone, is associated prospectively and independently with incident metabolic syndrome in men: the Framingham Heart Study. Diabetes Care. 2011;34(11):2464–2470. [DOI] [PMC free article] [PubMed]
- 98. Lakshman KM, Bhasin S, Araujo AB. Sex hormone-binding globulin as an independent predictor of incident type 2 diabetes mellitus in men. J Gerontol A Biol Sci Med Sci. 2010;65A(5):503–509. [DOI] [PMC free article] [PubMed]
- 99. De Oya I, Schoppen S, Lasunción MA, Lopez-Simon L, Riestra P, De Oya M, Garcés C. Sex hormone-binding globulin levels and metabolic syndrome and its features in adolescents. Pediatr Diabetes. 2010;11(3):188–194. [DOI] [PubMed]
- 100. Al-Daghri NM, Khan N, Sabico S, Al-Attas OS, Alokail MS, Kumar S. Gender-specific associations of serum sex hormone-binding globulin with features of metabolic syndrome in children. Diabetol Metab Syndr. 2016;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schmid K. Human plasma alpha 1-acid glycoprotein: biochemical properties, the amino acid sequence and the structure of the carbohydrate moiety, variants and polymorphism. Prog Clin Biol Res. 1989;300:7–22. [PubMed]
- 102. Fournier T, Medjoubi-N N, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482(1-2):157–171. [DOI] [PubMed] [Google Scholar]
- 103. Mestriner FL, Spiller F, Laure HJ, Souto FO, Tavares-Murta BM, Rosa JC, Basile-Filho A, Ferreira SH, Greene LJ, Cunha FQ. Acute-phase protein alpha-1-acid glycoprotein mediates neutrophil migration failure in sepsis by a nitric oxide-dependent mechanism. Proc Natl Acad Sci USA. 2007;104(49):19595–19600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Brinkman-van Der Linden EC, van Ommen EC, van Dijk W. Glycosylation of alpha 1-acid glycoprotein in septic shock: changes in degree of branching and in expression of sialyl Lewis(x) groups. Glycoconj J. 1996;13(1):27–31. [DOI] [PubMed]
- 105. Xiao K, Su L, Yan P, Han B, Li J, Wang H, Jia Y, Li X, Xie L. α-1-Acid glycoprotein as a biomarker for the early diagnosis and monitoring the prognosis of sepsis. J Crit Care. 2015;30(4):744–751. [DOI] [PubMed] [Google Scholar]
- 106. Moore DF, Rosenfeld MR, Gribbon PM, Winlove CP, Tsai CM. Alpha-1-acid (AAG, orosomucoid) glycoprotein: interaction with bacterial lipopolysaccharide and protection from sepsis. Inflammation. 1997;21(1):69–82. [DOI] [PubMed] [Google Scholar]
- 107. Osikov MV, Makarov EV, Krivokhizhina LV. Effects of alpha1-acid glycoprotein on hemostasis in experimental septic peritonitis. Bull Exp Biol Med. 2007;144(2):178–180. [DOI] [PubMed] [Google Scholar]
- 108. Snyder S, Coodley EL. Inhibition of platelet aggregation by alpha1-acid glycoprotein. Arch Intern Med. 1976;136(7):778–781. [PubMed] [Google Scholar]
- 109. Schön A, del Mar Ingaramo M, Freire E. The binding of HIV-1 protease inhibitors to human serum proteins. Biophys Chem. 2003;105(2-3):221–230. [DOI] [PubMed] [Google Scholar]
- 110. Ofotokun I, Lennox JL, Eaton ME, Ritchie JC, Easley KA, Masalovich SE, Long MC, Acosta EP. Immune activation mediated change in alpha-1-acid glycoprotein: impact on total and free lopinavir plasma exposure. J Clin Pharmacol. 2011;51(11):1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev. 1989;10(3):232–274. [DOI] [PubMed] [Google Scholar]
- 112. Yeap BB. Testosterone and ill-health in aging men. Nat Clin Pract Endocrinol Metab. 2009;5(2):113–121. [DOI] [PubMed] [Google Scholar]
- 113. de Moor P, Joossens JV. An inverse relation between body weight and the activity of the steroid binding -globulin in human plasma. Steroidologia. 1970;1(3):129–136. [PubMed] [Google Scholar]
- 114. Kopelman PG, Pilkington TR, White N, Jeffcoate SL. Abnormal sex steroid secretion and binding in massively obese women. Clin Endocrinol (Oxf). 1980;12(4):363–369. [DOI] [PubMed] [Google Scholar]
- 115. Martin ME, Benassayag C, Amiel C, Canton P, Nunez EA. Alterations in the concentrations and binding properties of sex steroid binding protein and corticosteroid-binding globulin in HIV+patients. J Endocrinol Invest. 1992;15(8):597–603. [DOI] [PubMed] [Google Scholar]
- 116. Monroe AK, Dobs AS, Xu X, Palella FJ, Kingsley LA, Witt MD, Brown TT. Sex hormones, insulin resistance, and diabetes mellitus among men with or at risk for HIV infection. J Acquir Immune Defic Syndr. 2011;58(2):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Nguyen HV, Mollison LC, Taylor TW, Chubb SA, Yeap BB. Chronic hepatitis C infection and sex hormone levels: effect of disease severity and recombinant interferon-alpha therapy. Intern Med J. 2006;36(6):362–366. [DOI] [PubMed] [Google Scholar]
- 118. Barreca T, Picciotto A, Franceschini R, Varagona G, Garibaldi A, Valle F, Cataldi A, D'Agostino S, Rolandi E. Sex hormones and sex hormone-binding globulin in males with chronic viral hepatitis during recombinant interferon-alpha 2b therapy. J Interferon Res. 1993;13(3):209–211. [DOI] [PubMed] [Google Scholar]
- 119. Földes J, Banos C, Lakatos P, Tarjan G. [Serum sex hormone-binding globulin levels in thyroid diseases]. Orv Hetil. 1990;131(29):1579–1582. [PubMed] [Google Scholar]
- 120. Kerlan V, Nahoul K, Le Martelot MT, Bercovici JP. Longitudinal study of maternal plasma bioavailable testosterone and androstanediol glucuronide levels during pregnancy. Clin Endocrinol (Oxf). 1994;40(2):263–267. [DOI] [PubMed] [Google Scholar]
- 121. Hirko KA, Spiegelman D, Willett WC, Hankinson SE, Eliassen AH. Alcohol consumption in relation to plasma sex hormones, prolactin, and sex hormone-binding globulin in premenopausal women. Cancer Epidemiol Biomarkers Prev. Dec;23(12):2943–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nehir Aytan A, Bastu E, Demiral I, Bulut H, Dogan M, Buyru F. Relationship between hyperandrogenism, obesity, inflammation and polycystic ovary syndrome. Gynecol Endocrinol. 2016;32(9):709–713. [DOI] [PubMed] [Google Scholar]
- 123. Rautio K, Tapanainen JS, Ruokonen A, Morin-Papunen LC. Endocrine and metabolic effects of rosiglitazone in overweight women with PCOS: a randomized placebo-controlled study. Hum Reprod. 2006;21(6):1400–1407. [DOI] [PubMed] [Google Scholar]
- 124. Tarkun I, Cetinarslan B, Turemen E, Sahin T, Canturk Z, Komsuoglu B. Effect of rosiglitazone on insulin resistance, C-reactive protein and endothelial function in non-obese young women with polycystic ovary syndrome. Eur J Endocrinol. 2005;153(1):115–121. [DOI] [PubMed] [Google Scholar]
- 125. Garmes HM, Tambascia MA, Zantut-Wittmann DE. Endocrine-metabolic effects of the treatment with pioglitazone in obese patients with polycystic ovary syndrome. Gynecol Endocrinol. 2005;21(6):317–323. [DOI] [PubMed] [Google Scholar]
- 126. Sepilian V,, Nagamani M. Effects of rosiglitazone in obese women with polycystic ovary syndrome and severe insulin resistance . J Clin. [DOI] [PubMed] [Google Scholar]
- 127. Svalheim S, Sveberg L, Mochol M, Tauboll E. Interactions between antiepileptic drugs and hormones. Seizure. 2015;28:12–17. [DOI] [PubMed] [Google Scholar]
- 128. van Rooijen M, Silveira A, Hamsten A, Bremme K. Sex hormone–binding globulin–a surrogate marker for the prothrombotic effects of combined oral contraceptives. Am J Obstet Gynecol. 2004;190(2):332–337. [DOI] [PubMed] [Google Scholar]
- 129. De Leo V, Di Sabatino A, Musacchio MC, Morgante G, Scolaro V, Cianci A, Petraglia F. Effect of oral contraceptives on markers of hyperandrogenism and SHBG in women with polycystic ovary syndrome. Contraception. 2010;82(3):276–280. [DOI] [PubMed] [Google Scholar]
- 130. Lin XF, Wu RR, Du J, Liao YC, Du Y, Ye Y, Wang Y, Zhang XB, Wu C, Chen A. Exploring the significance of sex hormone-binding globulin examination in the treament of women with polycystic ovarian syndrome (PCOS). Clin Exp Obstet Gynecol. 2015;42(3):315–320. [PubMed] [Google Scholar]
- 131. Cunningham SK, Loughlin T, Culliton M, McKenna TJ. The relationship between sex steroids and sex-hormone-binding globulin in plasma in physiological and pathological conditions. Ann Clin Biochem. 1985;22(Pt 5):489–497. [DOI] [PubMed] [Google Scholar]
- 132. Weickhardt AJ, Doebele RC, Purcell WT, Bunn PA, Oton AB, Rothman MS, Wierman ME, Mok T, Popat S, Bauman J, Nieva J, Novello S, Ou SH, Camidge DR. Symptomatic reduction in free testosterone levels secondary to crizotinib use in male cancer patients. Cancer. 2013;119(13):2383–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bonetti A, Tirelli F, Catapano A, Dazzi D, Dei Cas A, Solito F, Ceda G, Reverberi C, Monica C, Pipitone S, Elia G, Spattini M, Magnati G. Side effects of anabolic androgenic steroids abuse. Int J Sports Med. 2008;29(8):679–687. [DOI] [PubMed] [Google Scholar]
- 134. Abu-Hijleh TM, Gammoh E, Al-Busaidi AS, Malalla ZH, Madan S, Mahmood N, Almawi WY. Common Variants in the Sex Hormone-Binding Globulin (SHBG) Gene Influence SHBG Levels in Women with Polycystic Ovary Syndrome. Ann Nutr Metab. 2016;68(1):66–74. [DOI] [PubMed] [Google Scholar]
- 135. Baranowska B, Zgliczynski S. The role of sex hormones in the mechanism of inhibited LH release in female patients with anorexia nervosa. Acta Endocrinol (Copenh). 1982;99(3):334–338. [DOI] [PubMed] [Google Scholar]
- 136. Elias AN, Carreon G, Vaziri ND, Pandian MR, Oveisi F. The pituitary-gonadal axis in experimental nephrotic syndrome in male rats. J Lab Clin Med. 1992;120(6):949–954. [PubMed] [Google Scholar]
- 137. Kaltsas GA, Mukherjee JJ, Jenkins PJ, Satta MA, Islam N, Monson JP, Besser GM, Grossman AB. Menstrual irregularity in women with acromegaly. J Clin Endocrinol Metab. 1999;84(8):2731–2735. [DOI] [PubMed] [Google Scholar]
- 138. Damassa DA, Lin TM, Sonnenschein C, Soto AM. Biological effects of sex hormone-binding globulin on androgen-induced proliferation and androgen metabolism in LNCaP prostate cells. Endocrinology. 1991;129(1):75–84. [DOI] [PubMed] [Google Scholar]
- 139. Giorgi EP, Stein WD. The transport of steroids into animal cells in culture. Endocrinology. 1981;108(2):688–697. [DOI] [PubMed] [Google Scholar]
- 140. Lasnitzki I, Franklin HR. The influence of serum on uptake, conversion and action of testosterone in rat prostate glands in organ culture. J Endocrinol. 1972;54(2):333–342. [DOI] [PubMed] [Google Scholar]
- 141. Mowszowicz I, Kahn D, Dray F. Influence of testosterone binding to serum proteins on aromatization by enzymes of human placental microsomes. J Clin Endocrinol Metab. 1970;31(5):584–586. [DOI] [PubMed] [Google Scholar]
- 142. Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, Willnow TE. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122(5):751–762. [DOI] [PubMed] [Google Scholar]
- 143. Soccio RE, Breslow JL. Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol. 2004;24(7):1150–1160. [DOI] [PubMed] [Google Scholar]
- 144. Adams JS, Chen H, Chun R, Gacad MA, Encinas C, Ren S, Nguyen L, Wu S, Hewison M, Barsony J. Response element binding proteins and intracellular vitamin D binding proteins: novel regulators of vitamin D trafficking, action and metabolism. J Steroid Biochem Mol Biol. 2004;89-90(1-5):461–465. [DOI] [PubMed] [Google Scholar]
- 145. Porto CS, Lazari MF, Abreu LC, Bardin CW, Gunsalus GL. Receptors for androgen-binding proteins: internalization and intracellular signalling. J Steroid Biochem Mol Biol. 1995;53(1-6):561–565. [DOI] [PubMed] [Google Scholar]
- 146. Rosnera W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Androgen and estrogen signaling at the cell membrane via G-proteins and cyclic adenosine monophosphate. Steroids. 1999;64(1-2):100–106. [DOI] [PubMed]
- 147. Nakhla AM, Leonard J, Hryb DJ, Rosner W. Sex hormone-binding globulin receptor signal transduction proceeds via a G protein. Steroids. 1999;64(3):213–216. [DOI] [PubMed] [Google Scholar]
- 148. Krupenko SA, Krupenko NI, Danzo BJ. Interaction of sex hormone-binding globulin with plasma membranes from the rat epididymis and other tissues. J Steroid Biochem Mol Biol. 1994;51(1-2):115–124. [DOI] [PubMed] [Google Scholar]
- 149. Rove KO, Crawford ED, Perachino M, Morote J, Klotz L, Lange PH, Andriole GL, Matsumoto AM, Taneja SS, Eisenberger MA, Reis LO. Maximal testosterone suppression in prostate cancer: free vs total testosterone. Urology. 2014;83(6):1217–1222. [DOI] [PMC free article] [PubMed]
- 150. Matsumoto AM, Getzenberg RH, Coss C, Hancock ML, Si X, Dalton JT, Steiner MS. The free hormone hypothesis: Correlation of decreases in PSA with free testosterone rather than total testosterone in men with advanced prostate cancer treated with GTx-758. Chicago, IL: ASCO; 2013. [Google Scholar]
- 151. Rizvi SJ, Kennedy SH, Ravindran LN, Giacobbe P, Eisfeld BS, Mancini D, McIntyre RS. The relationship between testosterone and sexual function in depressed and healthy men. J Sex Med. 2010;7(2 Pt 1):816–825. [DOI] [PubMed] [Google Scholar]
- 152. Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90(5):2618–2623. [DOI] [PubMed] [Google Scholar]
- 153. Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27(5):1036–1041. [DOI] [PubMed] [Google Scholar]
- 154. van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85(9):3276–3282. [DOI] [PubMed] [Google Scholar]
- 155. Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicker L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry. 2008;65(3):283–289. [DOI] [PubMed] [Google Scholar]
- 156. Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241–4247. [DOI] [PubMed] [Google Scholar]
- 157. Yeap BB, Almeida OP, Hyde Z, Chubb SA, Hankey GJ, Jamrozik K, Flicker L. Higher serum free testosterone is associated with better cognitive function in older men, while total testosterone is not: The Health In Men Study. Clin Endocrinol (Oxf). 2008;68(3):404–412. [DOI] [PubMed]
- 158. Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Boonen S, Vanderschueren D, Labrie F, Huhtaniemi IT; EMAS Group . Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135. [DOI] [PubMed] [Google Scholar]
- 159. Campion EW, deLabry LO, Glynn RJ. The effect of age on serum albumin in healthy males: report from the Normative Aging Study. J Gerontol. 1988;43(1):M18–M20. [DOI] [PubMed] [Google Scholar]
- 160. Cooper JK, Gardner C. Effect of aging on serum albumin. J Am Geriatr Soc. 1989;37(11):1039–1042. [DOI] [PubMed] [Google Scholar]
- 161. Rao J, Danoff A, Bini EJ. Elevated sex hormone binding globulin levels may contribute to sexual dysfunction in men with chronic hepatitis C virus infection. J Clin Gastroenterol. 2009;43(1):94–95. [DOI] [PubMed] [Google Scholar]
- 162. Rochira V, Guaraldi G. Hypogonadism in the HIV-infected man. Endocrinol Metab Clin North Am. 2014;43(3):709–730. [DOI] [PubMed] [Google Scholar]
- 163. Moreno-Pérez O, Escoín C, Serna-Candel C, Portilla J, Boix V, Alfayate R, González-Sánchez V, Mauri M, Sánchez-Payá J, Picó A. The determination of total testosterone and free testosterone (RIA) are not applicable to the evaluation of gonadal function in HIV-infected males. J Sex Med. 2010;7(8):2873–2883. [DOI] [PubMed] [Google Scholar]
- 164. Monroe AK, Dobs AS, Palella FJ, Kingsley LA, Witt MD, Brown TT. Morning free and total testosterone in HIV-infected men: implications for the assessment of hypogonadism. AIDS Res Ther. 2014;11(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Monroe AK, Brown TT. Free testosterone for hypogonadism assessment in HIV-infected men. Clin Infect Dis. 2014;58(11):1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Blick G. Optimal diagnostic measures and thresholds for hypogonadism in men with HIV/AIDS: comparison between 2 transdermal testosterone replacement therapy gels. Postgrad Med. 2013;125(2):30–39. [DOI] [PubMed] [Google Scholar]
- 167. Haiman CA, Riley SE, Freedman ML, Setiawan VW, Conti DV, Le Marchand L. Common genetic variation in the sex steroid hormone-binding globulin (SHBG) gene and circulating shbg levels among postmenopausal women: the Multiethnic Cohort. J Clin Endocrinol Metab. 2005;90(4):2198–2204. [DOI] [PubMed] [Google Scholar]
- 168. Cousin P, Calemard-Michel L, Lejeune H, Raverot G, Yessaad N, Emptoz-Bonneton A, Morel Y, Pugeat M. Influence of SHBG gene pentanucleotide TAAAA repeat and D327N polymorphism on serum sex hormone-binding globulin concentration in hirsute women. J Clin Endocrinol Metab. 2004;89(2):917–924. [DOI] [PubMed] [Google Scholar]
- 169. Xita N, Tsatsoulis A, Chatzikyriakidou A, Georgiou I. Association of the (TAAAA)n repeat polymorphism in the sex hormone-binding globulin (SHBG) gene with polycystic ovary syndrome and relation to SHBG serum levels. J Clin Endocrinol Metab. 2003;88(12):5976–5980. [DOI] [PubMed] [Google Scholar]
- 170. Dunning AM, Dowsett M, Healey CS, Tee L, Luben RN, Folkerd E, Novik KL, Kelemen L, Ogata S, Pharoah PD, Easton DF, Day NE, Ponder BA. Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J Natl Cancer Inst. 2004;96(12):936–945. [DOI] [PubMed] [Google Scholar]
- 171. Low YL, Taylor JI, Grace PB, Dowsett M, Folkerd E, Doody D, Dunning AM, Scollen S, Mulligan AA, Welch AA, Luben RN, Khaw KT, Day NE, Wareham NJ, Bingham SA. Polymorphisms in the CYP19 gene may affect the positive correlations between serum and urine phytoestrogen metabolites and plasma androgen concentrations in men. J Nutr. 2005;135(11):2680–2686. [DOI] [PubMed] [Google Scholar]
- 172. Vos MJ, Mijnhout GS, Rondeel JM, Baron W, Groeneveld PH. Sex hormone binding globulin deficiency due to a homozygous missense mutation. J Clin Endocrinol Metab. 2014;99(9):E1798–E1802. [DOI] [PubMed] [Google Scholar]
- 173. Dupont Ahrentsen O, Jensen HK, Johnsen SG. Sex-hormone-binding globulin deficiency. Lancet. 1982;320(8294)):377. [DOI] [PubMed] [Google Scholar]
- 174. Hogeveen KN, Cousin P, Pugeat M, Dewailly D, Soudan B, Hammond GL. Human sex hormone-binding globulin variants associated with hyperandrogenism and ovarian dysfunction. J Clin Invest. 2002;109(7):973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Joseph DR. Structure, function, and regulation of androgen-binding protein/sex hormone-binding globulin. Vitam Horm. 1994;49:197–280. [DOI] [PubMed] [Google Scholar]
- 176. Joseph DR, Hall SH, Conti M, French FS. The gene structure of rat androgen-binding protein: identification of potential regulatory deoxyribonucleic acid elements of a follicle-stimulating hormone-regulated protein. Mol Endocrinol. 1988;2(1):3–13. [DOI] [PubMed] [Google Scholar]
- 177. Hammond GL, Underhill DA, Rykse HM, Smith CL. The human sex hormone-binding globulin gene contains exons for androgen-binding protein and two other testicular messenger RNAs. Mol Endocrinol. 1989;3(11):1869–1876. [DOI] [PubMed] [Google Scholar]
- 178. Danzo BJ, Black JH, Bell BW. Analysis of the oligosaccharides on androgen-binding proteins: implications concerning their role in structure/function relationships. J Steroid Biochem Mol Biol. 1991;40(4-6):821–831. [DOI] [PubMed] [Google Scholar]
- 179. Anthony CT, Danzo BJ, Orgebin-Crist MC. Investigations on the relationship between sperm fertilizing ability and androgen-binding protein in the hypophysectomized, pregnenoloneenolone-injected rat. Endocrinology. 1984;114(4):1419–1425. [DOI] [PubMed] [Google Scholar]
- 180. Anthony CT, Danzo BJ, Orgebin-Crist MC. Investigations on the relationship between sperm fertilizing ability and androgen-binding protein in the restricted rat. Endocrinology. 1984;114(4):1413–1418. [DOI] [PubMed] [Google Scholar]
- 181. Sullivan PM, Petrusz P, Szpirer C, Joseph DR. Alternative processing of androgen-binding protein RNA transcripts in fetal rat liver: identification of a transcript formed by trans splicing. J Biol Chem. 1991;266(1):143–154. [PubMed]
- 182. Reventos J, Sullivan PM, Joseph DR, Gordon JW. Tissue-specific expression of the rat androgen-binding protein/sex hormone-binding globulin gene in transgenic mice. Mol Cell Endocrinol. 1993;96(1-2):69–73. [DOI] [PubMed] [Google Scholar]
- 183. Joseph DR, O’Brien DA, Sullivan PM, Becchis M, Tsuruta JK, Petrusz P. Overexpression of androgen-binding protein/sex hormone-binding globulin in male transgenic mice: tissue distribution and phenotypic disorders. Biol Reprod. 1997;56(1):21–32. [DOI] [PubMed] [Google Scholar]
- 184. Jänne M, Deol HK, Power SG, Yee SP, Hammond GL. Human sex hormone-binding globulin gene expression in transgenic mice. Mol Endocrinol. 1998;12(1):123–136. [DOI] [PubMed] [Google Scholar]
- 185. Laurent MR, Hammond GL, Blokland M, Jardí F, Antonio L, Dubois V, Khalil R, Sterk SS, Gielen E, Decallonne B, Carmeliet G, Kaufman JM, Fiers T, Huhtaniemi IT, Vanderschueren D, Claessens F. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: validation of the free hormone hypothesis. Sci Rep. 2016;6:35539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Suzuki Y, Ito M, Sinohara H. Isolation and characterization of sex-steroid-binding protein from rat and rabbit plasma. J Biochem. 1981;89(1):231–236. [DOI] [PubMed] [Google Scholar]
- 187. Westphal U. Steroid-protein interactions II. Monogr Endocrinol. 1986;27:1–7. [PubMed]
- 188. Masakazu T, Hiroshi I, Bun-ichi T, Sumi N. Reduced activity of androgen biosynthesis in the testes of rats with analbuminemia. J Steroid Biochem. 1986;24(4):871–876. [DOI] [PubMed]
- 189. Nagase S, Shimamune K, Shumiya S. Albumin-deficient rat mutant: an animal model for analbuminemia. Jikken Dobutsu. 1980;29(1):33–38. [DOI] [PubMed] [Google Scholar]
- 190. Takahashi M, Wakabayashi K, Nagase S. Hormone levels of anterior pituitary gland and serum in Nagase analbuminemia rats. Endocrinol Jpn. 1984;31(2):185–193. [DOI] [PubMed] [Google Scholar]
- 191. Mendel CM, Murai JT, Siiteri PK, Monroe SE, Inoue M. Conservation of free but not total or non-sex-hormone-binding-globulin-bound testosterone in serum from Nagase analbuminemic rats. Endocrinology. 1989;124(6):3128–3130. [DOI] [PubMed] [Google Scholar]
- 192. Miller KK, Rosner W, Lee H, Hier J, Sesmilo G, Schoenfeld D, Neubauer G, Klibanski A. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab. 2004;89(2):525–533. [DOI] [PubMed] [Google Scholar]
- 193. Van Uytfanghe K, Stöckl D, Kaufman JM, Fiers T, Ross HA, De Leenheer AP, Thienpont LM. Evaluation of a candidate reference measurement procedure for serum free testosterone based on ultrafiltration and isotope dilution-gas chromatography-mass spectrometry. Clin Chem. 2004;50(11):2101–2110. [DOI] [PubMed] [Google Scholar]
- 194. Thode J, Fogh-Andersen N, Siggaard-Andersen M, Siggaard-Andersen O. Donnan effect or protein interference in ionised calcium measurements? Ann Clin Biochem. 1983;20(5):271–273. [DOI] [PubMed]
- 195. Fogh-Andersen N, Bjerrum PJ, Siggaard-Andersen O. Ionic binding, net charge, and Donnan effect of human serum albumin as a function of pH. Clin Chem. 1993;39(1):48–52. [PubMed] [Google Scholar]
- 196. Vesper HW, Botelho JC, Wang Y. Challenges and improvements in testosterone and estradiol testing. Asian J Androl. 2014;16(2):178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Selby C. Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem. 1990;27(6):532–541. [DOI] [PubMed]
- 198. Steeno O, Heyns W, Van Baelen H, de Moor P. Testosterone binding in human plasma. Ann Endocrinol (Paris). 1968;29(Suppl 29):141–148. [PubMed]
- 199. Vogeser M, Briegel J. Effect of temperature on protein binding of cortisol. Clin Biochem. 2007;40(9-10):724–727. [DOI] [PubMed] [Google Scholar]
- 200. Scatchard G. The attractions of proteins for small molecules and ions. Ann N Y Acad Sci. 1949;51:660–672.
- 201. Bhasin S, Zhang A, Coviello A, Jasuja R, Ulloor J, Singh R, Vesper H, Vasan RS. The impact of assay quality and reference ranges on clinical decision making in the diagnosis of androgen disorders. Steroids. 2008;73(13):1311–1317. [DOI] [PubMed] [Google Scholar]
- 202. Morales A, Collier CP, Clark AF. A critical appraisal of accuracy and cost of laboratory methodologies for the diagnosis of hypogonadism: the role of free testosterone assays. Can J Urol. 2012;19(3):6314–6318. [PubMed] [Google Scholar]
- 203. Winters SJ, Kelley DE, Goodpaster B. The analog free testosterone assay: are the results in men clinically useful? Clin Chem. 1998;44(10):2178–2182. [PubMed] [Google Scholar]
- 204. Moreno SA, Shyam A, Morgentaler A. Comparison of free testosterone results by analog radioimmunoassay and calculated free testosterone in an ambulatory clinical population. J Sex Med. 2010;7(5):1948–1953. [DOI] [PubMed] [Google Scholar]
- 205. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 2007;92(2):405–413. [DOI] [PubMed]
- 206. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society . Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. [DOI] [PubMed] [Google Scholar]
- 207. Pearce S, Dowsett M, Jeffcoate SL. Three methods compared for estimating the fraction of testosterone and estradiol not bound to sex-hormone-binding globulin. Clin Chem. 1989;35(4):632–635. [PubMed] [Google Scholar]
- 208. Vining RF, McGinley RA, Symons RG. Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clin Chem. 1983;29(10):1752–1756. [PubMed] [Google Scholar]
- 209. Clifton S, Macdowall W, Copas AJ, Tanton C, Keevil BG, Lee DM, Mitchell KR, Field N, Sonnenberg P, Bancroft J, Mercer CH, Wallace AM, Johnson AM, Wellings K, Wu FC. Salivary testosterone levels and health status in men and women in the British general population: findings from the Third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). J Clin Endocrinol Metab. 2016;101(11):3939–3951. [DOI] [PMC free article] [PubMed]
- 210. Keevil BG, MacDonald P, Macdowall W, Lee DM, Wu FC; NATSAL Team. Salivary testosterone measurement by liquid chromatography tandem mass spectrometry in adult males and females. Ann Clin Biochem. 2014;51(3):368–378. [DOI] [PMC free article] [PubMed]
- 211. Blom T, Ojanotko-Harri A, Laine M, Huhtaniemi I. Metabolism of progesterone and testosterone in human parotid and submandibular salivary glands in vitro. J Steroid Biochem Mol Biol. 1993;44(1):69–76. [DOI] [PubMed] [Google Scholar]
- 212. Loric S, Guéchot J, Duron F, Aubert P, Giboudeau J. Determination of testosterone in serum not bound by sex-hormone-binding globulin: diagnostic value in hirsute women. Clin Chem. 1988;34(9):1826–1829. [PubMed] [Google Scholar]
- 213. Yamamoto K, Koh E, Matsui F, Sugimoto K, Sin HS, Maeda Y, Namiki M. Measurement-specific bioavailable testosterone using concanavalin A precipitation: comparison of calculated and assayed bioavailable testosterone. Int J Urol. 2009;16(11):894–901. [DOI] [PubMed] [Google Scholar]
- 214. Rinaldi S, Geay A, Déchaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1065–1071. [PubMed] [Google Scholar]
- 215. de Ronde W, van der Schouw YT, Pols HA, Gooren LJ, Muller M, Grobbee DE, de Jong FH. Calculation of bioavailable and free testosterone in men: a comparison of 5 published algorithms. Clin Chem. 2006;52(9):1777–1784. [DOI] [PubMed] [Google Scholar]
- 216. Ho CK, Stoddart M, Walton M, Anderson RA, Beckett GJ. Calculated free testosterone in men: comparison of four equations and with free androgen index. Ann Clin Biochem. 2006;43(5):389–397. [DOI] [PubMed]
- 217. Sartorius G, Ly LP, Sikaris K, McLachlan R, Handelsman DJ. Predictive accuracy and sources of variability in calculated free testosterone estimates. Ann Clin Biochem. 2009;46(2):137–143. [DOI] [PubMed]
- 218. Hackbarth JS, Hoyne JB, Grebe SK, Singh RJ. Accuracy of calculated free testosterone differs between equations and depends on gender and SHBG concentration. Steroids. 2011;76(1-2):48–55. [DOI] [PubMed] [Google Scholar]
- 219. Ho CK, Beckett GJ. Late-onset male hypogonadism: clinical and laboratory evaluation. J Clin Pathol. 2011;64(6):459–465. [DOI] [PubMed]
- 220. Spitzer M, Basaria S, Travison TG, Davda MN, Paley A, Cohen B, Mazer NA, Knapp PE, Hanka S, Lakshman KM, Ulloor J, Zhang A, Orwoll K, Eder R, Collins L, Mohammed N, Rosen RC, DeRogatis L, Bhasin S. Effect of testosterone replacement on response to sildenafil citrate in men with erectile dysfunction: a parallel, randomized trial. Ann Intern Med. 2012;157(10):681–691. [DOI] [PubMed] [Google Scholar]
- 221. Huang G, Basaria S, Travison TG, Ho MH, Davda M, Mazer NA, Miciek R, Knapp PE, Zhang A, Collins L, Ursino M, Appleman E, Dzekov C, Stroh H, Ouellette M, Rundell T, Baby M, Bhatia NN, Khorram O, Friedman T, Storer TW, Bhasin S. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21(6):612–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Le M, Flores D, May D, Gourley E, Nangia AK. Current practices of measuring and reference range reporting of free and total testosterone in the United States. J Urol. 2016;195(5):1556–1561. [DOI] [PubMed]
- 223. Lazarou S, Reyes-Vallejo L, Morgentaler A. Wide variability in laboratory reference values for serum testosterone. J Sex Med. 2011;3(6):1085–1089. [DOI] [PubMed]
- 224. Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, Wang PY, Nielson C, Wu F, Tajar A, Labrie F, Vesper H, Zhang A, Ulloor J, Singh R, D’Agostino R, Vasan RS. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]