Abstract
Introduction:
Increasing evidence has expanded the role of green tea from a traditional beverage to a source of pharmacologically active molecules with diverse health benefits. However, conclusive clinical results are needed to better elucidate the cancer-preventive and therapeutic effects of green tea polyphenols (GTPs).
Areas covered:
The authors describe GTPs’ chemical compositions and metabolic biotransformations, and their recent developments in drug discovery, focusing on their cancer chemopreventive and therapeutic effects. They then review the recent development of GTP-loaded nanoparticles and GTP prodrugs.
Expert opinion:
GTPs possess potent anticarcinogenic activities through interfering with the initiation, development and progression phases of cancer. There are several challenges (e.g., poor bioavailability) in developing GTPs as therapeutic agents. Use of nanoparticle-based delivery systems has provided unique advantages over purified GTPs. However, there is still a need to determine the actual magnitude and pharmacological mechanisms of GTPs encapsulated in nanoparticles, in order to address newly emerging safety issues associated with the potential “local overdose” effect. The use of Pro- epigallocatechin gallate (Pro-EGCG) as a prodrug appears to offer improved in vitro stability as well as better in vivo bioavailability and efficacies in a number of animal studies, suggesting its potential as a therapeutic agent for further study and development.
Keywords: Bioavailability, biotransformation, cancer, catechin, epigenetic target, EGCG, nanoparticles, pharmacology, pro-EGCG, target delivery
1. Introduction
Tea, made from the leaves of the Camellia senenisis plant in the family Theaceae, is now the second most consumed beverage worldwide after water, with more than two-thirds of the world population being consuming this beverage. Consumption of tea is believed to date back to ancient China and India, where it was mostly used as a medicinal plant to help rid the body of excess fluid, control bleeding, heal wounds, and improve heart health. In past decades, interest in tea consumption is growing due to accumulating evidence from cellular, animal, and epidemiological studies that have linked tea consumption to various health benefits, such as chemoprevention of various cancers (prostate, breast, lung, skin, pancreatic, colon and head and neck cancers), chronic inflammation, heart disease, diabetes, and neurodegenerative diseases [1–7]. Although all these health benefits have not been consistently achieved in reported intervention trials, positive results from clinical trials have provided direct evidence supporting the protective effect of tea against human cancer [8,9]. In addition, multiple mechanisms of action have been discovered that could explain how the green tea polyphenols (GTPs) exert their chemopreventive effects [10, 11]. Recently, we did a literature search in PubMed database with the keywords “tea polyphenol and cancer”, and obtained 1272 publications (1976–2018), suggesting that tea polyphenol has been a promising candidate for chemoprevention and therapeutic application of human cancers.
In this review article, after a brief description of GTPs, we will focus on their recent developments in drug discovery, with a special emphasis on potential molecular mechanisms involved in therapeutic implication of GTPs in various cancers and other diseases.
2. Chemical compositions and structures of GTPs
To date, more than three hundred varieties of tea are produced by different manufacturing processes, of which green, black, oolong, white, yellow and post fermented teas are six major commercial forms depending on the extent of fermentation. Green tea (unfermented, 20% of total tea consumption) is produced by steaming and drying the fresh tea leaves to prevent fermentation via inactivating the polyphenol oxidase activity. Black tea (fermented) is the most consumed type in Europe and United States, accounting for 78% of global tea production and consumption according to a recent estimation of the United Nations [12]. It is produced by allowing the oxidation and polymerization of tea catechins by polyphenol oxidases during fermentation to form oligomeric polyphenols (theaflavins) and polymeric derivatives (thearubigins), which contribute to the characteristic aroma and color of black tea. Oolong tea (semi-fermented, 2% of total tea consumption) is especially popular in south China and Southeast Asia, and it is produced through a process including withering the plant under strong sun and oxidation before curling and twisting. White tea and yellow tea are considered as semi-fermented tea. White tea is produced similarly to oolong tea, but minor differences between them are that white tea does not require bruising, oxidation, and curing steps, while yellow tea’s production is very close to that of green tea but needs an extra yellowing process.
In general, tea polyphenols are the major component of these teas, accounting for up to 30% of the dry weight of tea leaves. These polyphenols are composed of flavanols, flavandiols and phenolic acids. The major monomeric flavanols are referred to as catechins, including: (+)-catechin (C), (−)-catechin gallate (CG), (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC), (−)-gallocatechin (GC), (−)-gallocatechin gallate (GCG) and (−)-epigallocatechin gallate (EGCG). Their chemical structures are illustrated in Figure 1. These catechins contain the same basic structural features, which are two aromatic rings (rings A and B) joined by three carbons and an oxygen forming a cyclic pyran ring (ring C). The catechins differ in the number and distribution of hydroxyl groups on the B-ring, the type of configuration (cis- or trans-isomers), and the presence or absence of a galloyl moiety (ring D). EGCG is the most abundant in these catechins, representing 50~80% of total catechins in green tea and about 16.5% wt in the water extraction fraction of green tea. It is also considered to be the major contributor to the various health benefits of green tea in cell culture and animal studies, as well as in clinical studies [13–16]. Commercial products derived from green tea are mainly in a powdered form (pill or capsule). In general, these green tea extract supplements contain about 90% total polyphenols with EGCG as primary active ingredient, which provide more catechins than the daily intake from a typical green tea beverage in order to achieve the proclaimed health benefits. Besides these, green tea contains some other constituents including gallic acid, chlorogenic acid, caffeic acid and flavonols such as kaempferol, myricetin and quercetin.
Figure 1.
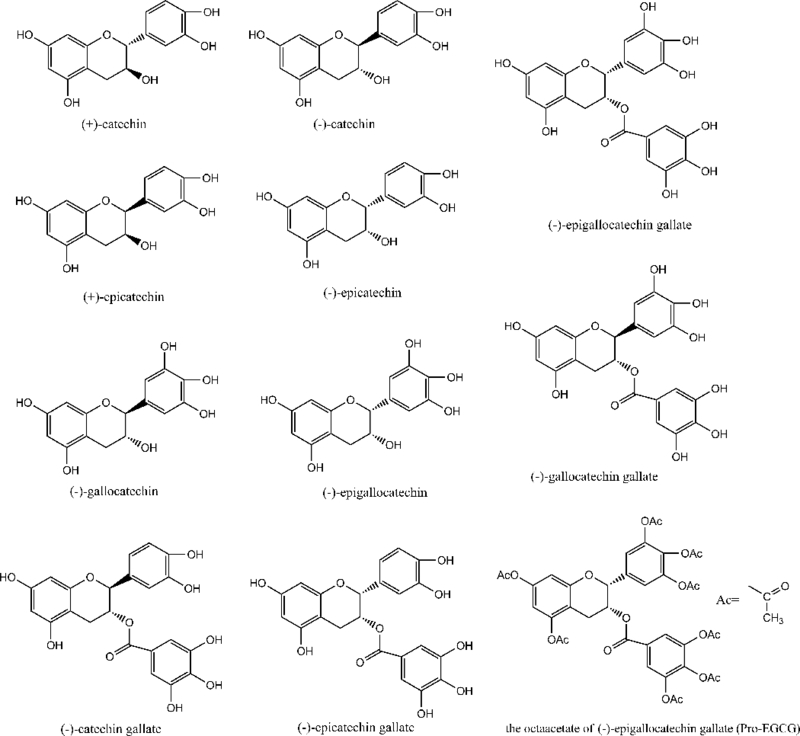
Chemical structures of major tea catechins and the octaacetate of (−)-epigallocatechin gallate (Pro-EGCG).
3. Metabolism, biotransformation, bioavailability and toxicology of GTPs
Encouraging data from epidemiological, cellular, animal and clinical studies support numerous health benefits of GTPs and green tea major active ingredient EGCG. However, intervention trials have generally not yet achieved a consistent conclusion in terms of the efficiency and the dose for all the illnesses and diseases of interest [17]. For example, reports from U.S. Food and Drug Administration (FDA) revealed that “there is no credible evidence to support qualified health claims for green tea or green tea extract reducing the risk of heart disease,” and “it is highly unlikely that green tea reduces the risk of breast cancer or prostate cancer.” [18, 19]. This discrepancy might be attributed to a variety of factors, such as the composition of tea extract, treatment dose and times, individual and local variables, environmental conditions, and even lifestyles of the patients. Especially, metabolic biotransformation of GTPs under physiological conditions is a critical factor influencing their bioavailability and therapeutic application in vivo [20].
The metabolic biotransformation of green tea catechins affects their absorption, distribution, metabolism, excretion, and toxicity, as well as overall efficiency in disease prevention and therapy. Metabolic fate of green tea catechins has been extensively investigated in in vitro systems (including human liver microsomes, human placental cytosol, human jejunal cytosol, human Caco-2 cytosol and human saliva), animals (mice, rats and rabbits), and some human models [21–23], and their metabolic pathways including methylation, glucuronidation, sulfation and ring-fission in the liver, kidney and gastrointestinal tract [24, 25] have been reported (Figure 2). Moreover, it is evident that these biotransformation reactions are species- or tissue-dependent, and the type of finished metabolic products vary depending on the types of parental catechins and the tissues where metabolic transformation takes place. For instance, the glucuronidation of EGCG was much greater than that of EGC. The greatest catalytic efficiency for glucuronidation is observed in mouse small intestinal microsomes, followed by mouse liver, human liver, rat liver, and rat small intestine for EGCG-4”-O-glucuronide. In the case of EGC-3’-O-glucuronide, the catalytic efficiency in microsomes was decreased in the order of: mouse liver > human liver > rat liver > rat or mouse small intestine [26]. EGCG is also sulfated depending on time and concentration in human, mouse, and rat liver cytosol, with the rat having the strongest catalytic activity. The concentration of the parental catechins also affects the type of the metabolic products. A good example is the methylation of EGCG by rat liver cytosol: the demethylated form is predominate at low EGCG concentrations (< 1.0 μM) while the monomethylated species could prevail at high EGCG concentrations (> 3.0 μM) [27].
Figure 2.
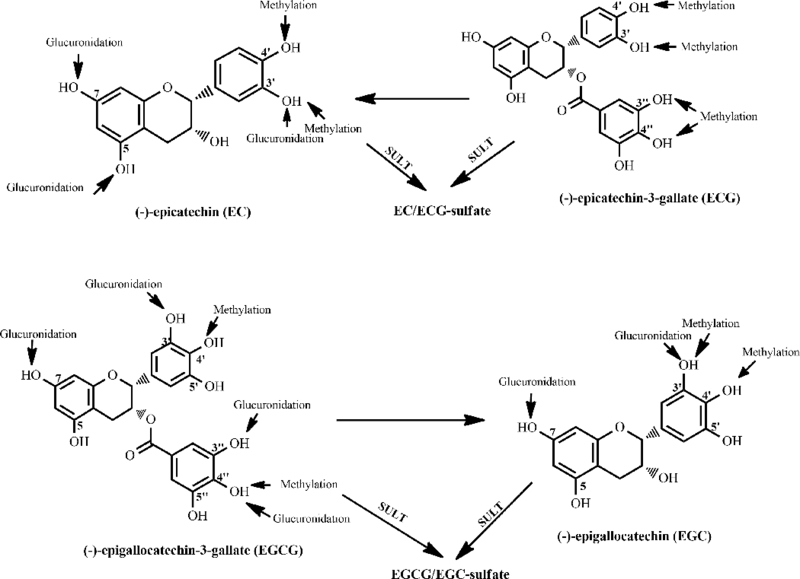
Structure sites for methylation, glucuronidation and sulfation of major tea catechins by human and rodent enzymes.
To better understand the mechanism of actions behind various pharmacological activities of GTPs, bioactivities of some tea polyphenol metabolites have been examined. In general, these metabolites formed by the glucuronidation, methylation, and sulfation of green tea polyphenols exhibited decreased bioactivities (i.e. antioxidant, anti-inflammatory and cancer-preventive activities) [28]. Studies on structure-activity relationship of some analogs of green tea catechins suggest that the addition of a methyl group on the B- or D-ring of (−)-EGCG or (−)-ECG led to decreased proteasome-inhibitory activity and, as the number of methyl groups increased, the inhibitory potencies further decreased [29]. Taken together, these results imply that the metabolic transformation of GTPs is an important issue leading to a poor bioavailability and restricting their clinical applications as therapeutic agents.
The oxidation of EGCG is another important aspect associated to pharmacological activities of GTPs. The chemical structures of EGCG endows it highly reactive property, making them very susceptible to oxidation in air under neutral or alkaline conditions [30]. EGCG oxidation involves many pathways including auto-oxidation, transition metal ion or oxidant-promoted oxidation and enzyme-catalyzed oxidation [31]. The auto-oxidation of EGCG occurs not only in cell culture conditions, but also in different tissues and circulating bloods. However, compared with in vitro conditions, the auto-oxidation degree of EGCG in vivo is believed lower due to more complicated environmental conditions [32], such as the lower oxygen partial pressure (< normal oxygen partial pressure of 160 mmHg), and the presence of antioxidant enzymes in tissues. However, studies showed that some in vivo factors promoted the EGCG oxidation degree, including cytochrome P450 peroxidase, myeloperoxidase and tyrosinase, oxidants released from neutrophils and endogenous copper [33, 34]. It is evident that the pro-oxidant effects of EGCG or its oxidation was affected by the dose levels and the environment. Numerous EGCG oxidation products have been characterized and their biological activities have been evaluated using in vitro and in vivo models. Compared to the parental catechin, some enzyme-catalyzed catechin polymers exhibited higher antioxidant and xanthine oxidase-inhibitory activity and anti-hyperglycemic effect [35, 36]. Interestingly, some EGCG auto-oxidation products have equivalent cytotoxic activities to EGCG, but enhanced capacity for cysteine depletion, suggesting that anti-cancer activity of EGCG involves a joint contribution of EGCG and vast numbers of bioactive EGCG oxidation products formed extracellularly [31]. The generation of ROS as a result of the auto-oxidation of EGCG is shown to induce ERK1/2 activation in Jurkat cells [37]. Although the in vitro mechanisms report cannot explain the effect of EGCG in vivo, the bio-activity of EGCG is likely independent of its oxidation.
It is noteworthy that studies from experimental animals and epidemiological surveys have shown that GTPs have a dose-dependent toxicology [38]. When the mice were administrated with diets containing 0.01% and 0.1% GTPs, a hepato-protective activity was observed. By contrast, the treatment with a high dose of GTPs (1%) exhibited symptoms of nephrotoxicity, such as the increased serum creatinine level. Moreover, this treatment deteriorated oxidative damage and decreased the expression of antioxidant enzymes and heat shock proteins (HSPs) in colitis and normal mice. Some case reports also indicated that excessive intake of tea extracts induce liver toxicity [39], which is probably due to the pro-oxidant property of tea polyphenols [40]. It is proposed that low and moderate doses of tea polyphenols produced lower levels of ROS which activate Nrf2 to attenuate oxidative stress whilst high dose of tea polyphenols produced high levels of ROS to induce toxicity [1]. These findings suggest that dose-dependent functionality and toxicology of GTPs should be considered when GTPs or related supplements are used to obtain beneficial effects.
4. GTPs and cancer prevention and therapy
4.1. GTPs and prostate cancer
Prostate cancer (PC) appears to be a suitable model for chemoprevention by means of diet intervention, considering that there is a relatively longer intervention period before the symptoms accompanied with prostate cancer arise and a diagnosis is finally established [41]. It is evident that the redox homeostasis imbalance resulting from reactive oxygen species (ROS) and cellular antioxidant defense is one of the important mechanisms in initiation and progression of PC, and interactions between multiple genetic and ambient factors are significant determinants in PC development [42].
GTPs and EGCG have shown chemopreventive and anti-carcinogenic effects on prostate cells, as supported by the data from cellular, animal experiments, and human clinical trials. For example, the relationship between the prostate cancer risk and habitual green tea intake among Chinese men was investigated in Hong Kong, and results showed an inverse correlation between PC risk and green tea consumption and EGCG intake [43]. The typical chemical characteristics of tea polyphenols allow them to affect cellular redox homeostasis by exerting antioxidant or pro-oxidant activity. The antioxidant/pro-oxidant behavior of green tea polyphenols varies with different experimental conditions, such as concentration, temperature, pH, presence of metal ions, and differences in culture medium composition. For example, the influences of EGCG concentrations and pH on the net anti-oxidant and pro-oxidant activity of EGCG in flaxseed oil-in-water (o/w) emulsions are examined [44]. In low pH (pH 2–4) emulsions, EGCG (1–100 μM) was observed to yield pro-oxidant effects. However, with the increase of EGCG concentration (500 μM), a competition between antioxidant and pro-oxidant activities of EGCG was observed, leading to lower TBARS concentrations than those observed at 100 μM EGCG. In high pH (pH 5–7) emulsions, high EGCG concentrations showed the best antioxidant effects with 500 μM EGCG resulting in the lowest TBARS concentrations in emulsions. The presence of oxygen and temperature significantly affected the stability [32]. In addition, the presence of some transition metals Cu(II) plays important roles in affecting antioxidant or pro-oxidant activity of EGCG. Co-treatment of HL-60 human leukemia cells with tea catechins and Cu(II) led to the formation of 8-oxo-guanosine [45]. But co-treated with tea catechins and the catalase or the copper chelator, bathocuproine, catechin-mediated oxidative damage was decreased.
GTPs have shown the effects on intracellular ROS production and antioxidant enzymes activity [46]. Besides the anti-oxidant and pro-oxidant activity, some other specific mechanisms are involved in the chemopreventive actions of GTPs, including regulation of the activities of cell surface receptors and their signaling pathways [i.e. Receptor tyrosine kinases (RTKs), insulin-like growth factor receptor 1 (IGF-R1)], modulation of gene expression through either direct effect on transcription factors (such as Sp1, NF-kB, AP-1, STAT1, STAT3 and Nrf2) [47, 48], or indirect epigenetic mechanisms [DNA methylation, histone modifications, and expression of noncoding regulatory micro RNA (miRNAs)] [49]. In addition, GTPs are found to interfere with intracellular proteostasis at various levels, such as to target HSPs and proteasome functions, and to interfere with the autophagic flux [50, 51]. Moreover, some EGCG analogues have been identified as novel heat shock protein 90 (Hsp90) inhibitors [52]. Table 1 summarizes the reported effects of GTPs against prostate cancer in experimental systems.
Table 1.
Summary on the anticancer effects of green tea polyphenols against prostate cancer in experimental systems.
| Models | Compounds used | Concentration | Methods | Results | Conclusion | References | |
|---|---|---|---|---|---|---|---|
| Cell lines | LNCaP CV1 22Rν1 C4–2 |
EGCG | 0–60 μM |
In silico modeling, MTT assay, transient transfection and reporter assay, fluorescence resonance energy transfer assay, immunohistochemical and RT-PCR analysis |
Inhibited cell growth, androgen (AR)-mediated transcriptional activation, AR nuclear translocation and protein expression. ↓ miRNA-21, ↑ miRNA-330. |
Antiandrogenic action | [53] |
| PC-3 LNCaP |
EGCG + quercetin | EGCG 40 μM quercetin 20 μM | Cellular absorption analysis, MTT assay, flow cytometry analysis | Inhibited cell proliferation, induced cell cycle arrest and apoptosis in PC-3 cells, stronger antiproliferative activity in LNCaP cells. | Quercetin enhanced the anticancer effect of EGCG via increasing the cellular uptake and decreasing methylation of EGCG. | [54] | |
| LNCaP | Tea polyphenols | 20–80 mg/mL | Proliferation assay, methylene blue staining and quantification, DNA fragmentation assay, cell death assay, western blot, ChIP assay, cell cycle analysis and apoptosis detection | Promoted apoptosis regardless of p53 status, ↑ FAS through activation of c-jun-N-terminal kinase, ↑ FADD phosphorylation, caspase-8 activation. |
Induced prostate cancer cell death by two distinct mechanisms regardless of p53 status. | [55] | |
| LNCaP PCa DU145 PrEC |
Tea polyphenols EGCG | 1–10 μg/mL | DNA methyltransferase activity assay, HDAC activity assay, bisulfite treatment and methyl-specific PCR, genomic bisulfite sequencing, western blot, fluorescence immunocytochemistry, ChIP assay. | Caused re-expression of GSTP1, demethylation in the proximal GSTP1 promoter and regions distal to the transcription factor binding sites. ↓ the mRNA and protein levels of MBD1, MBD4 and MeCP2, ↑ acetylated histone H3 and H4, ↓ MBD2 association with Sp1 binding sites. | GTP has dual potential to alter DNA methylation and chromatin modeling | [56] | |
| LNCaP PrECs | EGCG | 100 μM | Western blot | Recapitulated ATM expression and activity, ↓ the fusion transcript appearance. | Therapeutic effect of EGCG in mitigating the exacerbation of the disease with reference to the fusion transcripts. | [57] | |
| DU145 LNCaP | EGCG | 10–80 mg/mL | DNA ladder assay, confocal microscopy analysis, flow cytometry analysis, cell cycle analysis, Western blot. | Caused cell cycle arrest, apoptosis, a dose-dependent increase of p53 in LNCaP cells of induction of cyclin kinase inhibitor WAF1/p21. | Antiproliferative effects against both androgen- sensitive and androgen- insensitive human PCA cells, and this effect is mediated by deregulation in cell cycle and induction of apoptosis. | [58] | |
| KYSE HT-29 PC3 | EGCG | 5–50 μM | DNA methyltransferase assay, In Silico molecular modeling studies, Bisulfite modification and methylation-specific PCR, RT-PCR, Western blot | Caused a reversal of hypermethylation of p16INK4a, retinoic acid receptor β, O6-methylguanine methyltransferase, and human mutL homologue 1. Reactivated some methylation-silenced genes. |
Inhibited DNMT activity and reactivated methylation-silenced genes in cancer cells. |
[59] | |
| PC-3 LNCaP |
Tea polyphenols | 10–80 mg/mL | HDAC enzyme activity, RT-PCR, Western blot, ChIP assay, apoptosis analysis | Inhibited class I HDAC enzyme activity, ↑ acetylated histone H3 in total cellular chromatin, ↑ expression of p21/waf1 and Bax at the protein and message levels. | Anticancer effects of GTP may be due, in part, to HDAC inhibition. | [60] | |
| LNCaP DU145 |
EGCG | 5–40 μg/mL | Cell cycle analysis, apoptosis analysis, morphological analysis, Western blotting, immunoprecipitation |
↑ protein expression of WAF1/p21, KIP1/p27, INK4a/p16, and INK4c/p18, ↓ protein expression of cyclin D1, cyclin E, cdk2, cdk4, and cdk6. ↑ the binding of cyclin D1 toward WAF1/p21 and KIP1/p27, ↓the binding of cyclin E toward cdk2. | EGCG caused cell cycle arrest, which may be an irreversible process ultimately leading to apoptotic cell death. | [4] | |
| LNCaP | EGCG | 20–80 μm | TUNEL assay, ELISA, DEVDase assay, reporter assays | Induced stabilization of p53, ↑ its transcriptional activity, its downstream targets p21/WAF1 and Bax. This altered expression of Bcl-2 family members triggered the activation of initiator caspases 9 and 8. | EGCG induces apoptosis by shifting the balance between pro- and antiapoptotic proteins in favor of apoptosis. | [61] | |
| LNCaP | EGCG | 20–40 μM | MTT assay, fluorescence microscopy analysis, Western blotting, gelatin zymography, invasion assay, Wound healing assay. | Enhanced apoptotic activity in LNCaP cells. ↑ poly(ADP-ribose) polymerase cleavage and modulation of pro- and antiapoptotic Bcl2 family of proteins, regulated death-inducing signaling cascade complex. |
EGCG sensitizes TRAIL-resistant LNCaP cells to TRAIL-mediated apoptosis through modulation of intrinsic and extrinsic apoptotic pathways. | [62] | |
| DU145 LNCaP | EGCG | 10–40 mg/mL | Western blotting | ↓levels of PI3K and phospho‐Akt, ↑ Erk1/2 in both DU145 and LNCaP cells. | Modulation of the constitutive activation of PI3K/Akt and Erk1/2 pathways by EGCG | [63] | |
| RWPE-1, PC-3 | EGCG | 0–50 μM | MTS assay, Immunoblot detection | inhibited PC-3 cell proliferation, activation of the extracellular signal-regulated kinase (ERK1/2) pathway | ERK1/2 activation via a MEK-independent, PI3K-dependent signaling pathway is partially responsible for the antiproliferative effects of EGCG. | [64] | |
| Animal experiments | PC-3 C57BL/SV129 | EGCG | 20 or 100 mM, 100 mg/kg by oral gavage |
Reporter gene assays, cell viability assays, RT-PCR assays, promoter analysis, microarray data analysis | Attenuated AP-1 activation, ↓Nrf2-dependent genes, Conserved TFBS signatures were identified in the promoter regions of Nrf2, AP-1, ATF2, and ELK-1. | The anticancer effects was mediated via concerted modulation of Nrf2 and AP-1 pathways in the prostate. |
[65] |
| LNCaP PC-3 CWR22Rν1 mice |
EGCG Tea polyphenols |
EGCG (10–40 μM, 0.1% GTP |
Cell growth and viability, fluorescence microscopy analysis, Western blotting, ELISA | Inhibited cell growth and induced apoptosis, ↓ peroxisome proliferator activated receptor γ, ↑ tumor growth inhibition, ↓prostate-specific antigen levels, insulin-like growth factor-I levels. |
Green tea polyphenols and selective cyclooxygenase-2 inhibitors inhibited the growth of human prostate cancer cells both in vitro and in vivo. | [66] | |
| C57BL TRAMP mice |
Tea polyphenols | 0.1% | Immunoblot analysis, densitometric analysis | ↓NF-κB, IKKα, IKKβ, RANK, NIK and STAT-3. ↓levels of transcription factor osteopontin. |
Inhibition of osteopontin and NF-κB signaling may contribute to induction of apoptosis. | [47] | |
| C57BL/6 TRAMP mice |
Tea polyphenols | 0.1% | Magnetic resonance imaging, Insulin-like growth factor-I and insulin-like growth factor binding protein-3 assay, immunoblotting and apoptosis detection. | Inhibited CaP development. Delayed primary tumor incidence and tumor burden. Inhibitied serum insulin-like growth factor-I and restorated insulin-like growth factor binding protein-3 levels, ↓protein expression of proliferating cell nuclear antigen in the prostate. |
GTP inhibited the growth and progression of CaP in TRAMP mice. | [67] | |
| TRAMP mice |
EGCG | 0.06% | Apoptosis analysis, immunohistochemistry, immunoblot analyses, ELISA | Inhibited early but not late stage PCa, reduced cell proliferation, induced apoptosis, ↓androgen receptor, insulin-like growth factor-1 (IGF-1), IGF-1 receptor, phospho- extracellular signal-regulated kinases 1 and 2, cyclooxygenase-2 and inducible NO synthase. | EGCG suppressed prostate cancer without toxicity. | [68] | |
| C57BL/6 TRAMP mice |
Tea polyphenols | 0.1% | Ultrasound imaging, ELISA kits, immunoblot analysis, histology and immunohistochemical analysis. | Inhibited IGF-I and its downstream targets including phosphatidylinositol 3-kinase, pAkt, and phosphorylated extracellular signal-regulated kinase |
Chemopreventive potential of GTP decreases with advancing stage of the disease. |
[69] |
4.2. GTPs and lung cancer
Lung cancer is the leading cause of cancer-related deaths worldwide in both men and women, which could be categorized into two histological groups: small cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC). NSCLCs include adenocarcinoma, squamous cell carcinoma (SqCC), and large cell carcinoma [70]. SCLC accounts for approximately 15% of all lung cancers and has a poor prognosis, with the 5-year survival at diagnosis rarely exceeding 15%. EGCG shows similar inhibitory effects on drug-sensitive (H69) and drug-resistant (H69VP) SCLC cells, which is attributed to their abilities in reducing telomerase, inducing caspases 3/9 activities and blocking the cell-cycle in S phase [71]. NSCLC represents 85% of lung cancers, and patients of stage IIIB & IV disease have the five-year survival less than 10%. Treatment with 10–50 μM of EGCG for 3 days led to promoter demethylation and restoration of WIF-1, an antagonist of Wnt proto-oncogene in NSCLC cells (H460 and A549) [72]. EGCG is a potent inhibitor of cell proliferation, independent of EGRF inhibition, in several NSCLC cell lines including those resistant to EGFR kinase inhibitors and those overexpressing c-Met (H2122, H358, H460, H1975 and H1993 cells). EGCG is able to completely inhibit ligand-induced c-Met phosphorylation and partially inhibit EGFR phosphorylation. When EGCG is used in combination with erlotinib, a synergistic inhibition of cell proliferation and colony formation was observed [73]. Co-treatment of NSCLC cells (i.e. PC-9, A549 and ChaGo K-1 cells) with EGCG and celecoxib also shows synergistic induction of apoptosis by upregulation of GADD153 genes, which might be attributed to the activation of the mitogen-activated protein kinases (MAPKs), such as ERK1/2 and p38 MAPK pathways [74]. EGCG is also effective in attenuating cardiopulmonary bypass-related lung injury by reducing lung edema, pulmonary neutrophil infiltration, and presumably initiating poly (ADP-ribose) polymerase (PARP) dependent cell death signaling [75].
Studies on lung cancer-related micro RNA (miRNA) is a research focus recently due to the potential roles of miRNAs in lung tumorgenesis. The identified miRNAs include miR34-c, miR145, miR142–5p and miR210. EGCG upregulates the expression of microRNA (i.e. miR-210) by binding HIF-1α in mouse (CL13) and human NSCLC cells (H1299, H460 and A549), resulting in reduced cell proliferation and anchorage-independent growth [76]. The overall miRNA levels in the oncogenic Ƙ-ras-induced mouse lung cancer is decreased [77], which is consistent with the findings that that lung-specific knockout of Dicer results in the abnormality of lung development and function [78]. Furthermore, the genomic and bioinformatics approaches were employed to analyze the role of microRNA in the EGCG inhibition of tobacco carcinogen-induced lung tumors in A/J mice [79]. Results showed that the expression levels of 21 miRNAs had modest changes and 26 potential targeted genes of the 21 microRNAs were identified by the computation method. Further pathway analysis revealed that the most impacted pathways of EGCG treatment are the regulatory networks associated to AKT, NF-κB, MAPKs, and cell cycle, and the identified miRNA targets are involved in the networks of AKT, MAPKs and cell cycle regulation.
4.3. GTPs and breast cancer
Breast cancer is the leading cause of cancer death among females worldwide, with an estimated 14 million new cases and roughly 8 million deaths each year [70]. Breast cancer is categorized into three main subtypes according to their molecular profiles: hormone receptor-positive (Estrogen receptor-positive/ ER+ve), HER2-positive (ErbB2-positive, a member of EGFR family) and triple-negative tumors. The heterogeneity of breast cancer, with the distinct morphological and phenotypic profiles through gene expression, proliferation, and metastatic potential, make it difficult to treat with chemotherapy.
It is found that GTPs, particularly EGCG, inhibit proliferation, migration, angiogenesis, and tumor growth in breast cancer, as well as inducing apoptosis and cell cycle arrest in the cancerous cells [80]. EGCG has been shown to interfere with estrogen receptor function, inhibit estrogen-induced breast cancer cell proliferation, and sensitize hormone responsive tumors to drugs that target steroid receptors (e.g. tamoxifen) [81]. For example, treatment with 10 μM EGCG for 72 h led to reactivation of estrogen receptor (ER)-α expression in low basal ER-α MDA-MB-231 breast cancer cells. Moreover, this effect is enhanced when combined with a histone deacetylase (HDAC) inhibitor [82]. The combination of EGCG and curcumin was efficacious in both in vitro and in vivo models of ER-α-positive breast cancer. EGCG possesses potent anti-invasive activity by activating the FOXO3a/ERα/MTA3/E-cadherin pathway and overcomes trastuzumab drug resistance of HER2 positive breast cancer cells [83]. In addition, EGCG treatment reduced cell proliferation, ATP production, and Akt activity, and a concomitant elevated level of nuclear FOXO3a and p27Kip1 levels in trastuzumab-resistant breast cancer cells, suggesting that the combination of EGCG with trastuzumab may be an effective approach to treat HER2-overexpressing breast cancers [84]. Moreover, GTPs are able to target breast cancer stem cells (CSCs) and their signaling pathways, by blocking Wnt signaling through a transcriptional repressor. Considering that CSCs are responsible for the initiation of tumor growth, cancer recurrence, anticancer drug resistance, and metastasis, they are believed to be promising therapeutic targets for breast cancer in the future [85].
GTPs and EGCG are able to modulate epigenetic events to breast cancer prevention and therapy, and are thus used as epi-drugs for prevention, prognosis, and perhaps treatment, which might aid in overcoming the drawback of standard therapy for estrogen-dependent breast cancer by using the selective ER modulators [86]. EGCG reduced DNA methyltransferase 1 (DNMT1) activity by either reducing S-adenosyl-L-methionine (SAM) and increasing S-adenosyl homocysteine (SAH), or blocking the entry of the key nucleotide cytosine into its active site by hydrogen bonding [87, 88]. Also, EGCG specifically inhibited histone acetyl transferase (HAT) but not histone deacetylases (HDAC) in B lymphocytes [89]. When used in combination with trichostatin A, EGCG reactivated ERα expression via remodeling of the chromatin structure of the ERα promoter by altering the status of histone acetylation and methylation [82]. In addition, EGCG affected miRNAs to cause subtle changes in multiple molecular targets and pathways. For example, treatment with EGCG led to upregulation of MiR-16 in tumor cells, which down-regulates IκB kinase α and subsequently induces IκB accumulation in tumor-associated macrophages and inhibits M2 polarization [90].
Taken together, there are several proposed mechanisms of action of green tea polyphenols against breast cancer, including anti-angiogenesis, interaction with target proteins [PI3K, 67-kDa laminin receptor, Ras-GTPase activating protein (GAP) SH3 domain-binding protein 1 (G3BP1), Bcl-xL and Bcl-2, vimentin, Fyn, GRP78, and insulin like growth factor 1 receptor (IGF-1R)], inhibition of cell signaling pathways [EGFR, Wnt, hepatocyte growth factor signaling pathway, Met phosphorylation and ERK-1/2, as well as Akt/protein kinase B (PKB)], inhibition of enzyme activities (CDK 2/CDK4, proteasomes), induction of cell cycle arrest and apoptosis, and effects on microRNAs [80].
4.4. GTPs and neurodegenerative diseases
Excessive ROS production causes oxidative damages to normal neuronal biomolecules and leads to accumulation of iron in specific brain area, which is believed to contribute to the major pathological aspects of Parkinson’s disease (PD) and Alzheimer’s disease (AD) [91]. Green tea extract and EGCG are found to exert neuroprotective/neurorescue activities in a wide array of cellular and animal models of neurological disorders.
Accumulation of phosphorylated tau protein (P-tau) aggregates and the 42 amino acid form of β-amyloid (Aβ42) is a pathological hallmark of AD. EGCG has been implicated as a drug candidate that is able to modulate aggregation of proteins, such as Huntingtin, β-amyloid and α-synuclein in neurodegenerative diseases [92]. For instance, treatment with 10 μM EGCG provides a protective effect on cultured rat primary prefrontal cortical neurons against iAβ-induced cytotoxicity via the activation of the AKT pathway [93] or by increasing the levels of acetylcholine [94]. Moreover, EGCG is effective in suppressing Al-induced Aβ42 fibrillation by preventing further conversion of Aβ42 monomers into a folded conformation [95]. EGCG also inhibits the aggregation of tau protein into toxic oligomers at ten- to hundred-fold substoichiometric concentrations to rescue neuronal cells from tau-induced neurotoxicity [96]. Results from Chesser et al. (2016) revealed that EGCG treatment enhances the clearance of three AD-relevant phosphorylated tau epitopes in primary neurons [97]. These clearance are likely through increasing adaptor protein expression in a highly specific manner, which differs from the previously reported mechanisms such as those independent of both Nrf2 activation and enhanced autophagy [98].
PD is neuropathologically characterized by the misfolding and aggregation of α-synuclein protein, damage and loss of dopamine (DA) neurons in the substantia nigra pars compacta (SN), and mitochondrial dysfunction caused by oxidative stress [99]. EGCG treatment inhibits the α-synuclein protein aggregation in a time- and concentration-dependent manner with the ED50 of 250 nM [100]. Similar results were obtained in in vivo human brain tissues. It is suggested that the inhibitory effect of EGCG on α-synuclein aggregation is attributed to intermolecular hydrophobic interactions. Studies on animal experiments show that oral administration of EGCG is able to significantly reduce dopamine neuron loss in the substantia nigra pars compacta and can also prevent striatal dopamine and tyrosine hydroxylase protein level depletion [101]. Some proposed molecular mechanisms behind therapeutic actions of catechins includes scavenging of ROS and iron chelating, inhibition of lipid peroxidation, induction of endogenous detoxifying enzymes, modulation of cell signaling pathways (activation of PKC and PI3K/Akt signaling pathways and inhibition of MAPK signaling pathway), and regulation of cell survival and apoptotic gene expression [102, 103]. By interaction with these signaling cascade, EGCG further strengthens the response of the cell to environment or the stressor, ultimately leading to responses such as cell proliferation, apoptosis, synthesis of inflammatory mediators, and neurite growth.
GTPs and diabetes
According to the WHO report, about 347 million people worldwide have diabetes mellitus (DM), from which total deaths are projected to rise by more than 50% in the next 10 years. Type 2 DM (T2DM) accounts for approximately 90% of all diabetes in almost all countries [104]. There are some discrepancies regarding the effect of tea consumption on diabetes based on human epidemiological studies. Results from some cohort studies in Taiwan and Japan show that the consumption of green tea is effective against type 2 diabetes [105, 106]. However, some randomized trials showed contrary results that daily consumption of green tea by patients with diabetes for several months failed to ameliorate diabetes-related parameters [107].
Some mechanisms of actions of GTPs against diabetes have been proposed. First, oxidative stress has been implicated in the progression of diabetic complications. The health beneficial effects of tea polyphenols on type 2 diabetes are partly attributed to their antioxidant activity, as well as their modulatory action towards some oxidative stress-related signals [108]. Secondly, EGCG is capable of inhibiting cellular glucose uptake either by blockade of insulin signaling or by direct competition with glucose for GLUTs. Thirdly, tea catechins also affect the insulin pathway either by inhibiting the carbohydrate digestive enzymes α-amylase, α-glucosidase, and intestinal sucrase in the intestine of the rats, or by enhancing the insulin sensitivity and insulinotropic activity [109]. In addition, GTPs are also effective in improving endothelial dysfunction and modulation of inflammation (cytokine expression).
4.6. GTPs and endometriosis
Endometriosis is a disorder due to the implantation of endometrial glands and stroma outside the uterine cavity. It is generally accepted that endometriosis is an angiogenesis-dependent disorder [110]. New blood vessel formation to deliver the oxygen and nutrient supply is essential to the development and progression of endometriosis [111]. Animal models have confirmed that angiogenesis occurs in endometriosis [112,113]. Anti-angiogenesis therapy therefore offers an opportunity for the treatment of endometriosis [114]. The anti-angiogenic activity of EGCG has been previously demonstrated in vitro and in vivo [115]. Furthermore, EGCG suppressed the angiogenesis signaling pathway and inhibited the growth of experimental endometriosis in mice [113,116]. According to one study, EGCG inhibited the E2-stimulated activation, proliferation and VEGF expression of endometrial cells in vitro [117]. EGCG also selectively suppressed angiogenesis and blood perfusion of endometriotic lesions in vivo.
4.7. GTPs and angiogenesis
Accumulating data based on in vitro and in vivo tumor models has confirmed the antiangiogenic properties of GTPs. Various molecular mechanisms have been proposed, such as the suppression of cell proliferation, induction of apoptosis, inhibition the expression of angiogenic factors, suppression the phosphorylation of receptors and inhibition of binding of VEGF to its receptor. For example, green tea extract significantly reduced the expression of Flt-1 and KDR/Flk-1 in HUVEC, suggesting that they may have preventive effects on tumor angiogenesis and metastasis through reduction of expression of VEGF receptors [118]. EGCG is recognized as the only catechin that inhibits VEGF binding to its receptor. It was able to reduce the expression of VEGF and therefore regulates VEGF expression through blocking ERK and Akt phosphorylation [109]. In addition, EGCG significantly decreased the expression of HIF-1α, a strong activator of VEGF expression [120]. It also was able to block the signal transduction events of VEGF, such as phosphorylation of ERK1/2, autophosphorylation of VEGF-R1 and VEGF-R2 and the expression of early growth response-1 (Egr-1) transcription factor in human umbilical arterial endothelial cells [121]. Recently, GTPs, especially EGCG, exhibited influence on some miRNAs and activation/inhibition of various cellular/molecular pathways which are involved in angiogenesis in various cancer types [122]. For example, the incubation of MCF-7 cells with low concentration of Polyphenon-60 (green tea extract) for 48 h lead to significant changes of 23 miRNAs [123]. With more and more studies being carried forward in the future, some new miRNAs and novel roles of miRNAs involved in anti-angiogenesis effect of GTPs may be discovered. Figure 3 summarizes the biological activities and physiological mechanisms of green tea catechins as reviewed above. Please also see Section 7 for anti-angiogenesis effects of pro-EGCG.
Figure 3.
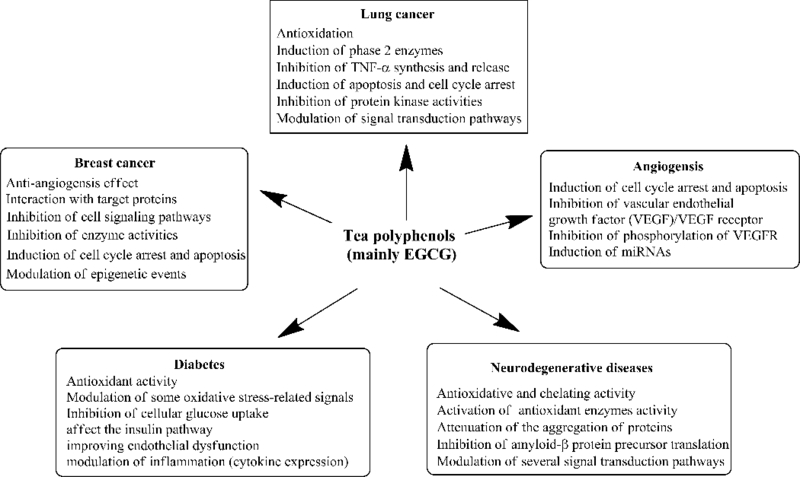
Biological activities and physiological mechanisms of green tea catechins.
5. Challenges for use of GTPs as therapeutic agents
Diverse health-promoting activities of green tea and its major constituent EGCG have been supported by the evidence from cellular, animal and clinical studies. Case-control and cohort studies have reported beneficial effects of green tea on patients with various cancers, such as breast, colon and rectum, pancreas, stomach, ovary, and lung, as well as prevention of recurrence in Stage I-II breast cancer patients. However, some studies did not show an association between the intake of tea and/or polyphenols and the decreased risk of human cancers. For instance, a phase II clinical trial was conducted in Italy to investigate the effect of green tea catechins in patients with high-grade prostatic intraepithelial neoplasia (HG-PIN). After sixty volunteers with HG-PIN took daily 600 mg of green tea catechins (Categ Plus®) for 6 and 12 months, no any statistical difference in PCa incidence was observed between the experimental groups through prostatic biopsy and measurements of prostate-specific antigen (PSA) [124]. Results from a meta-analysis of prospective cohort studies in Asian populations revealed that cumulative tea consumption in the highest consumption range (> 5 cups per day) led to a 22% reduction of the relative risk of liver cancer, but significantly only for women, not in men [125]. This limited effect in a subpopulation of Asian women does not endow a general recommendation for green tea as a preventive agent of hepatocellular carcinoma. Furthermore, meta-analysis including two studies and breast cancer recurrence and seven studies of breast cancer incidence did not find a significant effect of green tea on breast cancer prevention [80]. Many published reviews have discussed the possible reasons between the in vitro and in vivo studies as wells as between the epidemiological and clinical trials [1,10,126,127]. Taken together, the offered explanations include: a) higher quantities of GTPs used in in vitro and in animal studies as compared to the doses used for human clinical trials. The concentrations of GTPs used for in vitro studies usually range from 20 μM to 100 μM or even higher. By contrast, these levels cannot be achieved in vivo, especially inside or surrounding cancer cells [10]. Even after 7 to 9 cups of tea, the physiological level of tea in blood is less than 1 μM. Furthermore, the concentration of catechins in the tissues is affected by the duration of tea intake; b) Experimental systems are generally simple and homogenous in terms of genetic, host and lifestyle factors, and experimental conditions are easily controlled. However, clinical trials faced more complicated experimental conditions, such as the additional variables of host and lifestyle factors, exposure complexity and metabolic competence; c) Time of exposure to green tea might be different. For example, for the chemopreventive effect of GTPs, exposure is normally before, during and after carcinogen treatment in experimental models when cells/tissue/processes are norm while the administration of green tea in clinical trials is after damage/disease, high-risk individuals/cancer patients. These inconsistent results suggest that there is still a long way to go before the clinical use of GTPs as therapeutic agents.
The low bioavailability of GTPs is still a major hurdle that hinders their clinical application as therapeutic agents. The rates of absorption and bioavailability are extremely low in orally administered EGCG (perhaps < 3.5% of the dose) [128]. Many EGCG bioactivities observed in vitro are not reproduced in vivo, suggesting that the irrelevance of in vitro results associated with tea polyphenols. Molecular and cellular mechanisms associated with low bioavailability of GTPs have been proposed [20], including loss or inactivation owing to metabolic transformation or degradation, low cellular uptake due to poor hydrophobicity to cross the cell membrane, and active efflux of many polyphenolic compounds by the multidrug resistance-associated protein 2.
Many strategies aiming improving bioavailability and therapeutic efficiency of GTPs have been put into practice. First, it might be applicable to synthesize more GTPs-based analogs or prodrugs to find more potent, stable and specific active agents. Second, design and development of effective delivery systems, such as nanoparticles and liposome, might assist in improving the bioavailability of GTPs. Various nanoparticles are believed to be promising candidates for the encapsulation of chemically labile bioactive substances since they manifested some unique advantages over enhancing performance of the loaded drugs, including higher stability and bioavailability, improved water solubility, sustainable and controlled release, and higher absorption property [129]. Encapsulation of catechins in carbohydrate nanoparticles and liposomes significantly improved their therapeutic efficacy [130]. Third, the combined use of GTPs with other drugs and inhibitors as therapeutic agents might be a promising approach. This combination generates more effective therapeutic activity, such as a synergistic effect, than that of individual drug alone. For example, combination of EGCG and NS-398 significantly resulted in enhanced cell growth inhibition, apoptosis induction, proapoptotic proteins expression, as well as inhibition of nuclear factor-κB compared to the additive effects of the two agents alone [66]. The combination of EGCG and erlotinib induces apoptosis of SCCHN cells by regulating Bim and Bcl-2 at the posttranscriptional level [131]. Some combinations of EGCG and anticancer compounds induced similar synergistic anticancer effects for both in vitro and in vivo experiments, with an average reduction in tumor volume reaching by 70.3% in xenograft mouse models [132].
6. Development of nanoparticle-based delivery system of GTPs
Various attempts have been made in the past decades to overcome the above-mentioned limitations of GTPs, among which, nanoparticle-based delivery system appears to be a promising alternative for improving the bioavailability of GTPs due to their unique advantages over traditional delivery methods, including improved stability, enhanced anticancer performance, higher bioavailability, and sustainable and controlled release and targeted delivery to the site of action. A summary of the listed references on the development of nanoparticle-based delivery systems of GTPs is shown in Table 2.
Table 2.
Summary of the listed references on the development of nanoparticle-based delivery systems of green tea polyphenols.
| Delivery systems | Drugs | Coating materials | Methods | Advantages | References |
|---|---|---|---|---|---|
| Nanomicell | Tea polyphenols | Gelatin, dextran, genipin | Sustained release of tea polyphenol in vitro. Comparable or stronger cytotoxicity against MCF-7 cells than free tea polyphenol. |
[133] | |
| Nanoliposome | EGCG | Chitosan, soy lecithin, cholesterol | Enhanced stability, sustained release, increased intracellular content in MCF7 cells, induced apoptosis, inhibited proliferation. | [134] | |
| EGCG | Phospholipid S75, cholesterol, Tween 80 | Sustained release, improved stability in simulated intestinal fluid, slowed the degenerations of in vitro antioxidant activities of EGCG. | [135] | ||
| EGCG | Carboxymethyl chitosan, hexadecyl dimethylammonium chloride, cholesterol | Good in vitro digestion stability, Sustained release, slowed the degradation of EGCG in simulated intestinal fluid. |
[136] | ||
| Tea polyphenol | Phospholipid, cholesterol, Tween 80 |
Ethanol injection method with dynamic high- pressure microfluidization |
Retained antioxidant activities, sustained release property, improved the stability of tea polyphenol in alkaline solution. | [137] | |
| Solid lipid nanoparticles | Green tea extract | Tween 20/80, synperonic PE/F68, phosphatidylcholine |
high shear homogenization technique | High antioxidant activity, highly efficient against E. coli bacteria. |
[138] |
| EGCG | Phosphatidylcholine, kolliphor HS15, α-tocopherol acetate | Increased EGCG stability, sustained release, high binding affinity to and uptake by macrophages, increased macrophage EGCG content, and decreased macrophage MCP-1 mRNA levels and protein secretion. | [139] | ||
| Nanoemulsion | EGCG | Carrageenan, β-lactoglobulin | High-pressure homogenization | Enhanced in vitro anticancer activity. | [140] |
| Tea extract | Cholesterol, phytosterol, cetech-3, cetyl phosphate, glycerine | Sprayed through a high-pressure emulsifier | Increased bioavailability and hypocholesterolemic effects in vivo. | [141] | |
| Catechins | Soy protein, oil | Improved the stability, bioaccessibility and permeability of green tea catechins. | [142] | ||
| Lipid nanoparticle | EGCG | Glycerol, cationic lipid, Softisan®100, soybean phosphatidylcholine | Multiple emulsion technique | Confirmed the possibility of lipid-based systems for EGCG ocular drug delivery. | [143] |
| EGCG | Glycerol, cationic lipid, Softisan®100, soybean phosphatidylcholine | Double-emulsion technique | Prolonged EGCG release, ability to reach both anterior and posterior segment of the eye, safety and non-irritant nature. | [144] | |
| Multiple emulsion | Catechin, curcumin | Olive oil, polyglycerol polyricinoleate, gelatin | Two-step emulsification method | Co-loading of curcumin and catechin did not have adverse effects on either compound’s stability or bioaccessibility. | [145] |
| Polyelectrolyte complex nanoparticles | EGCG | Polylactic acid, polyethylene glycol | Biological effectiveness with over 10-fold dose advantage for exerting its proapoptotic and angiogenesis inhibitory effects. | [130] | |
| EGCG | Gelatin, glutaraldehyde | two-step desolvation method | Blocked hepatocyte growth factor (HGF)-induced intracellular signaling in MBA-MD-231 cells. | [146] | |
| Tea catechins | Chitosan, poly(γ-glutamic acid) |
Polyelectrolyte self-assembly method | pH-responsive, retained the activity of tea catechins, increased the paracellular transport. | [147] | |
| EGCG | Chitosan, polyaspartic acid |
Polyelectrolyte self-assembly method | pH-responsive, improved effectiveness of EGCG against rabbit atherosclerosis. | [148] | |
| Hydrogel nanoparticles | Tea catechins | Chitosan, sodium tripolyphosphate |
Ionic gelation | Controlled release of tea catechins. | [149] |
| Tea polyphenols | Carboxymethyl chitosan, chitosan hydrochloride | Ionic gelation | Sustained release, antitumor activities towards HepG2 cancer cells. | [150] | |
| EGCG | Chitosan, pentasodium tripolyphosphate hexahydrate | Ionic gelation | a slow release in acidic pH and faster release in simulated intestinal fluid, improved therapeutic benefit against PCa tumors. | [151] | |
| EGCG | Chitosan, pentasodium tripolyphosphate hexahydrate | Ionic gelation | Induction apoptosis, inhibited cell cycle. | [152] | |
| Green tea extract | Chitosan, sodium tripolyphosphate | Ionic gelation | Enhanced uptake in HepG2 cells, removed the extracellular collagen caused by CCl4 in the hepatic fibrosis rat liver. | [153] | |
| Polymeric nanoparticles | EGCG, paclitaxel | Poly-L-lactide-co- glycolic acid, casein |
Emulsion–precipitation method | Sustained and sequential release of both the drugs in plasma, prolonged circulation, enhanced availability, sensitized Ptx resistant MDA-MB-231 cells to Ptx, induced apoptosis, inhibited NF-κB activation, downregulated the key genes related to angiogenesis, tumor metastasis and survival. | [154,155] |
| EGCG | Poly-L-lactide-co- glycolic acid, PEG polymer, pseudomimetic dipeptide |
Selective in vitro efficacy against PSMA-expressing PCa cells. | [156] |
6.1. Protecting GTPs against harsh conditions
GTPs are very unstable and reactive owing to their chemical structure, and thus highly susceptible to chemical, physical and biological stress factors such as light, oxygen, thermal processing, pH changes, metal ions, and other harsh conditions. Nanoparticle-based delivery systems are considered as plausible options to overcome these limitations. For example, encapsulation in chitosan-tripolyphosphate nanoparticles is effective in stabilizing tea catechins in alkaline solution [157]. It took 8 and 24 hours for the non-encapsulated and encapsulated (+)-catechin, respectively, to be degraded to 50% of their initial levels, and the corresponding values for the nonencapsulated and encapsulated EGCG were 10 and 40 minutes, respectively. Shpigelman et al. (2010) used thermally modified β-lactoglobulin to form co-assembled nanovehicles for delivery of EGCG, and found that these complexes conferred considerable protection to EGCG against oxidative degradation. A 33-fold lower initial degradation rate and a 3.2-fold slower degradation over 8 days were observed for nano-entrapped compared to unprotected EGCG [158].
6.2. Sustainable and control release of GTPs
The half-life for EGCG is about 5 hours, and for EGC and EC the half-lives vary between 2.4 and 3.4 hours [159]. Such a short half-life greatly hinders its clinical development. Extensive studies have been conducted on the sustainable release of GTPs by various nanoparticle delivery systems. Colloidal complexes from association of methylcellulose and EGCG presented a sustained EGCG release from the complex with around 60% release in intestinal pH at the end of 2 hours [160]. Electrospray-aided nanoencapsulation process improved the release and permeability properties of catechins, especially at lower core-to-wall ratios (1:50 or 1:10) compared to higher core-to-wall ratio (1:05) or as catechins in free form [161]. In addition, some pH-responsive and magnetic-responsive nanoparticle systems have been developed by incorporating pH/magnetic-responsive polymers in nanoparticles [162]. Self-assembled nanoparticles composed of chitosan (CS) and an edible polypeptide, poly(γ-glutamic acid) (γ-PGA) for the delivery of tea catechins, were pH-responsive and demonstrated different tea catechin release profiles in simulated gastrointestinal tract (GI tract) media [147].
In vitro release studies show that the release of GTPs from nanoparticles is affected by pH values, temperature of the release medium, and structure of the nanoparticles, whilst the loading concentrations of drugs seem to be independent to the cumulative percentage of the drug release [163]. In GTPs-loaded liposomal nanoparticles, when the pH value of release medium changed from 3.0 to 9.0, the cumulative release percentages of GTPs in 48 h were about 50% and 74%, respectively. The cumulative release ratio of the drug increased with the temperature. Under the same conditions, about 53%, 60% and 79% of GTPs were released within 48 h at room temperature, 37°C and 45°C, respectively. These differences might be explained that the pH and temperature were able to affect the structure of the liposomal nanoparticles or the binding between liposomes and GTPs. Release kinetics analysis shows that the drug release for nanoparticulate dosage forms or sustained release formulations fitted with Zero order kinetics with anomalous mode of transport. In chitosan-based nanoparticles, GTPs release in vitro was divided into two phases: a very rapid initial burst (about 6 h) and a slow release (up to 48 h). The composition and structure of chitosan nanoparticles play important roles in the release behavior of GTPs from chitosan-based nanoparticles delivery system [150].
6.3. Improved bioavailability of GTPs
Results from the study on EGCG-loaded solid lipid nanoparticles (EGCG-SLN) showed that the lipid core of nanoparticles not only provides an additional structural reinforcement to the nanoparticle assembly, but also makes it biologically compatible. The cytotoxicity of EGCG-SLN was found to be 8.1 times higher against MDA-MB 231 human breast cancer cells and 3.8 times higher against DU-145 human prostate cancer cells than that of the pure EGCG [164]. Compared with free EGCG, EGCG encapsulated in polylactic acid-polyethylene glycol nanoparticles retained its biological effectiveness with over 10-fold dose advantage for exerting its proapoptotic and angiogenesis inhibitory effects in cell assays, and also showed anti-tumor effect in a tumor xenograft mouse model [165]. Similarly improved bioavailability of EGCG was also observed by Hu et al., who synthesized a novel type of nanochemoprevention by encapsulation of EGCG with caseinophosphopeptides and chitosan nanoparticles, and found an enhanced penetration of EGCG through Caco-2 cell monolayers [166].
6.4. Targeted delivery of GTPs
Targeted treatments are aimed at blocking specific biologic transduction pathways or cancer proteins that are involved in tumor growth and progression, i.e. molecular targets (receptors, growth factors, kinase cascades or molecules related with apoptosis and angiogenesis) that are present in normal tissues, but are found overexpressed or mutated in cancer. Modification of the surface of the coating material not only provides active targeting characteristics to the particles, but also improves therapeutic efficacy of encapsulated drugs and overcomes the multidrug resistance (MDR) [167]. Various ligands are extensively used to functionalize the surface of nanoparticles for encapsulation of EGCG, such as albumin, hyaluronic acid, biotin, folate, transferrin, and monoclonal antibodies [168]. In order to selectively deliver EGCG to cancer cells, EGCG-loaded nanoparticles consisting of PLGA-PEG functionalized with small molecules like the prostate specific membrane antigen (PSMA) were found to exhibit a selective in vitro efficacy against PSMA-expressing prostate cancer cells [156].
7. Pro-drug of GTPs
Our laboratory successfully introduced chemical modifications to GTPs to form prodrugs and synthesized a series of analogs based on structure-activity relationship [169, 170]. Pro-EGCG (Figure 1), the octaacetate of EGCG, was found to be 6 times more stable than (−)-EGCG in a culture medium (RMPI) which mimics the body fluid with a pH value around 8. Therefore, peracetate protection of the phenol groups of (−)-EGCG aids in stabilizing in culture (presumably physiological) conditions [169]. When pro-EGCG was incubated with an extract of leukemia Jurkat T cells, it was degraded with the formation of EGCG, suggesting that pro-EGCG can be hydrolyzed presumably by cellular esterases to give EGCG. (−)-EGCG has been found to potently inhibit the chymotrypsin-like activity of proteasomes in vitro and in vivo [171]. In contrast, Pro-EGCG, of itself, was completely inactive in inhibiting the chymotrypsin-like activity of the purified 20S of proteasome. On the other hand, Pro-EGCG is equally potent to, if not more potent than, (−)-EGCG in inhibiting the proteasomal activity in intact Jurket cells, suggesting again that Pro-EGCG can be converted to EGCG inside the cells [169].
The in vivo activity of Pro-EGCG has also been examined in animal models. Intragastric administration of Pro-EGCG to CF-1 mice resulted in higher bioavailability of EGCG in plasma compared with administration of equimolar doses of EGCG [172]. To investigate the potential efficacy of Pro-EGCG in vivo, breast cancer MDA-MB-231 tumors were induced in nude mice, followed by treatment with Pro-EGCG or (−)-EGCG for 31 days. Results showed a significant inhibition of breast tumor growth by Pro-EGCG, compared with (−)-EGCG [173]. Superior efficacy of Pro-EGCG as compared to EGCG was also observed on their inhibitory effect on androgen-independent prostate cancer, CWR22R, xenograft model in nude mice [174]. In mouse models of xenograft of human endometrial cancer, Pro-EGCG inhibited tumor angiogenesis through down-regulation of vascular endothelial growth factor A (VEGFA) and hypoxia inducible factor 1 alpha (HIF1α) in tumor cells and chemokine (C-X-C motif) ligand 12 (CXCL12) in host stroma [175]. To evaluate the potential of cancer preventive effects, dietary administration of Pro-EGCG and EGCG in dextran sulfate sodium (DSS)-induced colitis in mice was studied. The results indicated that Pro-EGCG administration was more effective than EGCG in preventing the shortening of colon length, the formation of aberrant crypt foci (ACF) and lymphoid nodules (LN) in mouse colon stimulated by DSS [176]. In a mouse model of endometriosis, both EGCG and pro-EGCG significantly decreased the growth of endometrial implants and reduced the lesion size and weight, as well as inhibited functional and structural microvessels in the lesions. However, the inhibition by pro-EGCG in all the angiogenesis parameters was significantly better than that by EGCG [177].
8. Conclusion
Accumulating evidence has supported the potential roles of GTPs and its major constituent EGCG in chemopreventive and therapy of various cancers and other diseases. Unfortunately, intervention studies have not yet confirmed these health benefits of GTPs. Nanoparticle-based delivery techniques hold great promise for addressing issues related to poor stability, solubility and bioavailability encountered in medical applications of GTPs. However, there is still much more to study in terms of optimizing the performance of nanoparticle-based delivery systems for GTPs in the future. Using the pro-drug approach, Pro-EGCG, the octaacetate of EGCG, has been found to have superior stability in vitro and better activities and efficacy than EGCG in a number of animal models in vivo. It offers the potential to be further developed into a therapeutic agent.
9. Expert opinion
The key findings in the green tea field so far include that GTPs possess potent anticarcinogenic activities by interfering with the initiation, development, and progression of cancer; EGCG suppresses the angiogenesis signaling pathway and inhibits neovascularization, which can contribute to not only its anticancer activities, but also the growth inhibition of experimental endometriosis in mice [113, 116]. Another key finding in this field is that GTPs have numerous conformations due to the presence of the galloyl group, enabling them to interact with various types of molecules, such as nucleic acids and proteins. For instance, EGCG is capable of entering into nucleus and binding to both DNA and RNA in CpG-rich regions [178]. Although the exact mechanism by which the EGCG accumulates in nucleus remains unclear, these interactions might play an important role in regulating gene functions. EGCG is also found to bind with a variety of proteins, such as histidine-rich glycoproteins, kinases, anti- and pro-apoptotic proteins, insulin-like growth factor receptor, proteasomes, and so on. The interactions between EGCG and these targets may play regulatory roles in multiple signaling events. Moreover, EGCG has cell type-and environment-specific responses. Therefore, it is important in the future to identify more molecular targets or biomarkers that respond to physiologically effective concentrations of EGCG. Such future studies will contribute an in-depth understanding of the chemopreventive and therapeutic mechanism of GTPs towards various cancers, as well as will provide a better guidance for the targeted therapy of cancers with GTPs as clinical therapeutic agents in the future.
There are some weaknesses or challenges related to GTPs that have to be overcome before their clinical use for human health benefits. The poor bioavailability is still a major challenge for the development of GTPs as a therapeutic agent for clinical application. In order to maximize chemopreventive and therapeutic efficiency of GTPs (mainly EGCG) as those observed in various in vitro cellular assays, continuous efforts aiming at improving their bioavailability should be made in the future. The use of pro-drug such as Pro-EGCG may help in resolving the poor bioavailability issue even though further studies are clearly required. In addition, by using various polymeric carrier systems, i.e. nanoparticles, liposomes, solid liposome nanoparticle and micelles, some advancement has been achieved in enhancing bioavailability and stability of GTPs. Nanoparticles-based molecular targeted cancer therapy is a promising strategy to overcome the lack of specificity of conventional chemotherapeutic agents. It should provide more specific targeting to tumor tissues via improved pharmacokinetics and pharmacodynamics and active intracellular uptake [179]. Ultimately, active targeting via the inclusion of a specific ligand on the nanoparticles is envisioned to provide the most effective therapy, and some ligand-targeted nanotherapeutics are either approved or under clinical evaluation [180]. As far as the biological targets are concerned, tumor-associated antigens appeared to be suitable biological targets for therapeutic intervention. The translation of these bioconjugates into clinical practice is expected soon, even though there is continuing interest in developing a localized therapeutic option for treatment of various cancers [156].
Although these drug carrier systems provide us with more options for targeted anticancer therapies, a big challenge associated with it is to understand the intrinsic characteristics of these carriers that determine their interactive actions with target organs/cells or various biological systems. The knowledge is needed in the field is determination of pharmacokinetic and therapeutic effectives of the loaded drugs as well as their disease-targeting selectivity. Therefore, in the coming years researchers should put more effort on developing novel GTPs (e.g., EGCG)-loaded carriers that are able to target disease tissues selectively and specifically. In addition, one would expect to see the progress in determination of the actual magnitude and pharmacological mechanisms of GTPs encapsulated in nanoparticles, as compared to those free, in order to address newly emerging safely issues associated with the potential “local overdose” effect for nanotechnology-enabled encapsulation system.
Another big challenge in cancer chemotherapy field is time-dependent multidrug resistance to chemotherapy and radiotherapy, along with treatment discontinuation caused by various side effects. One future strategy to overcome this shortcoming is to develop novel combination treatments of chemotherapy and bioactive dietary compounds. Multidrug chemotherapy has become a research focus in the recent years because of better therapeutic efficiency and lower adverse effects compared to the conventional monotherapy. More future attention should be paid to the combinations of GTPs or EGCG as adjuvant with chemotherapy or radiotherapy. It should be noted that antagonistic effects could be generated when GTPs are combined with other therapeutic drugs in some cases. However, in most of cases additive or synergistic interaction between EGCG and conventional anticancer agents could be generated that should enhance the therapeutic efficiency and reduce adverse side effects of conventional anticancer drugs. For example, combinations of green tea extract or EGCG with some conventional anticancer drugs, such as cisplatin, tamoxifen, etc., have exhibited some encouraging preclinical data, suggesting that the combinations are more effective than monotherapy [181]. However, there are only few clinical studies on such combinations. More efforts are needed in the future to search for promising combinations of EGCG with other anticancer agents preclinically and in clinical studies to validate such combinations for cancer therapy. In addition, the inconsistent results of the cellular assays, animal experiments and human intervention trials imply that it may be unwise to extrapolate the results of in vitro studies to in vivo situation. Taken into accounting the confounding and modulating factors that affect the in vivo anti-cancer performance of GTPs (e.g. quantities of tea polyphenol, duration time of tea intake, varied aetiology in different populations and population heterogeneity, and so on), well-designed cohort studies and human intervention trials to minimize the undesired influence of these factors are meaningful and needed. Studies on the selection of approximate dosages and biomarkers for clinical trials treated with GTPs are needed in the future.
Article highlights.
Accumulating evidence from cellular, animal and epidemiological studies has supported the diverse health benefits of green tea polyphenols and its major constituent EGCG.
The beneficial effects of green tea polyphenols are limited by their poor stability, absorption and bioavailability.
The metabolic biotransformation of green tea catechins affects their absorption, distribution, metabolism, excretion and toxicity, as well as overall efficiency in disease prevention and therapy.
Effects of EGCG are dependent on cell types and environments.
Nanoparticle-based delivery techniques could improve stability, solubility and bioavailability of green tea polyphenols.
The use of Pro-EGCG as a prodrug of EGCG offers improved stability, bioavailability and efficacy in animal models.
Acknowledgments
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 31201417, 31571836, 81772492/H1615), the Shandong Provincial Natural Science Foundation (Grant No. ZR2018BC063) as well as by a National Cancer Institute grant R21CA184788 (to QP Dou) and a National Institutes of Health grant P30 CA022453 (to the Karmanos Cancer Institute at Wayne State University).
Footnotes
Declaration of interest
QP Dou and TH Chan are inventors of the issued patent claiming the use of pro-EGCG for proteasome inhibition and the treatment of cancer. TH Chan is also the co-inventor of the patent for use of Pro-EGCG in the treatment of endometriosis (US9,713,603). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Bibliography
- 1.Yang CS, Wang X, Lu G, et al. Cancer prevention by tea: animal studies, molecular mechanisms and human revelance. Nat Rev Cancer 2009;9(6):429–39.*A comprehansive review on tea and cancer prevention.
- 2.Yang CS, Wang H. Cancer preventive activities of tea catechins. Molecules 2016;21(12):1679–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang CS, Wang H, Sheridan ZP. Studies on prevention of obesity, metabolic syndrome, diabetes, cardiovascular diseases and cancer by tea. J Food Drug Anal 2018;26(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Hussain T, Mukhtar H. Molecular pathway for (−)-epigallocatechin- 3-gallate-induced cell cycle arrest and apoptosis of human prostate carcinoma cells. Arch Biochem Biophys 2003;410(1):177–85.** The first systematic study showing EGCG-mediated effects on each component of cdk inhibitor-cyclin-cdk machinery during cell cycle arrest and apoptosis of human prostate carcinoma cells.
- 5.Stuart EC, Scandlyn MJ, Rosengren RJ. Role of epigallocatechin gallate (EGCG) in the treatment of breast and prostate cancer. Life Sci 2006;79(25):2329–36. [DOI] [PubMed] [Google Scholar]
- 6.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res 2010;302(2):71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji BT, Chow WH, Hsing AW, et al. Green tea consumption and the risk of pancreatic and colorectal cancers. Intl J Cancer 1997;70(3):255–58.* One of large population-based case-control studies showing that green tea drinking may lower the risk of colorectal and pancreatic cancers.
- 8.Saito E, Inoue M, Sawada N, et al. Association of green tea consumption with mortality due to all causes and major causes of death in a Japanese population: the Japan Public Health Center-based Prospective Study. Ann Epidemiol 2015; 25(7):512–18. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Liu S, Zhou H, et al. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a Chinese cohort of 165,000 adult men. Eur J Epidemiol 2016;31(9):853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peter B, Bosze S, Horvath R. Biophysical characteristics of proteins and living cells exposed to the green tea polyphenol epigallocatechin-3-gallate (EGCg): review of recent advances from molecular mechanisms to nanomedicine and clinical trials. Eur Biophys J 2017;46(1):1–24. [DOI] [PubMed] [Google Scholar]
- 11.Thakur VS, Gupta K, Gupta S. The chemopreventive and chemotherapeutic potentials of tea polyphenols. Curr Pharm Biotechnol 2012;13(1):191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang K World tea production and trade: current and future development. Roma: Italy; 2015. [Google Scholar]
- 13.Chu C, Deng J, Man Y, et al. Green tea extracts epigallocatechin-3-gallate for different treatments. BioMed Res Intl 2017;2017:5615647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eng QY, Thanikachalam PV, Ramamurthy S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J Ethnopharmacol 2018;210:296–310. [DOI] [PubMed] [Google Scholar]
- 15.Xu Q, Langley M, Kanthasamy AG, et al. Epigallocatechin gallate has a neurorescue effect in a mouse model of Parkinson disease. J Nutr 2017; 147(10):1926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang L, Li L, Yang J, et al. Potential benefit of (−)-epigallocatechin-3 -gallate for macrovascular complications in diabetes. Braz J Med Biol Res 2017;50(10):e6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohn T Dietary factors affecting polyphenol bioavailability. Nutr Rev 2014;72(7):429–52. [DOI] [PubMed] [Google Scholar]
- 18.Administration USFaD. Letter responding to health claim petition dated January 27, 2004: Green tea and reduced risk of cancer health claim. In: 2004Q-0083 Dn, ed. 2005.
- 19.Administration USFaD. Qualified health claims: letter of denial – green tea and reduced risk of cardiovascular disease. Docket number 2005Q-0297 2006 May 9 2006.
- 20.Huo C, Wan SB, Lam WH, et al. The challenge of developing green tea polyphenols as therapeutic agents. Inflammopharmacology 2008;16(5):248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aura AM, Mattila I, Seppänen-Laakso T, et al. Microbial metabolism of catechin stereoisomers by human faecal microbiota: Comparison of targeted analysis and a non-targeted metabolomics method. Phytochem Lett 2008;1(1):18–22. [Google Scholar]
- 22.Lee MJ, Wang ZY, Li H, et al. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol Biomarkers Prev 1995;4(4):393–99. [PubMed] [Google Scholar]
- 23.Das NP. Studies on flavonoid metabolism: Absorption and metabolism of (+)-catechin in man. Biochem Pharmacol 1971;20(12):3435–45. [DOI] [PubMed] [Google Scholar]
- 24.Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res Fundam Mol Mech Mutagen 2003;523–524(1):201–8. [DOI] [PubMed] [Google Scholar]
- 25.Hu M, Chen J, Lin H. Metabolism of flavonoids via enteric eecycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther 2003;307(1):314–21. [DOI] [PubMed] [Google Scholar]
- 26.Lambert JD, Sang S, Yang CS. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol Pharm 2007;4(6):819–25. [DOI] [PubMed] [Google Scholar]
- 27.Lu H, Meng X, Yang CS. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab Dispos 2003;31(5):572–9. [DOI] [PubMed] [Google Scholar]
- 28.Wu AH, Tseng CC, Van Den Berg D, et al. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res 2003;63(21):7526–29. [PubMed] [Google Scholar]
- 29.Dou QP, Landis-Piwowar KR, Chen D, et al. Green tea polyphenols as a natural tumour cell proteasome inhibitor. Inflammopharmacology 2008;16(5):208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sang S, Lambert JD, Ho CT, et al. The chemistry and biotransformation of tea constituents. Pharmacol Res 2011;64(2):87–99. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y, Chen P, Ling T, et al. Certain (−)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem 2016;204:218–26. [DOI] [PubMed] [Google Scholar]
- 32.Sang S, Lee MJ, Hou Z, et al. Stability of tea polyphenol (−)-epigallocatechin-3 -gallate and formation of dimers and epimers under common experimental conditions. J Agric Food Chem 2005;53(24):9478–84. [DOI] [PubMed] [Google Scholar]
- 33.Dickerhof N, Magon NJ, Tyndall JD, et al. Potent inhibition of macrophage migration inhibitory factor (MIF) by myeloperoxidase-dependent oxidation of epicatechins. Biochem J 2014;462(2):303–14. [DOI] [PubMed] [Google Scholar]
- 34.Moridani MY, Scobie H, Salehi P, et al. Catechin metabolism: Glutathione conjugate formation catalyzed by tyrosinase, peroxidase, and cytochrome P450. Chem Res Toxicol 2001;14(7):841–8. [DOI] [PubMed] [Google Scholar]
- 35.Kurisawa M, Chung JE, Kim YJ, et al. Amplification of antioxidant activity and xanthine oxidase inhibition of catechin by enzymatic polymerization. Biomacromolecules 2003;4(3):469–71. [DOI] [PubMed] [Google Scholar]
- 36.Jeon SY, Oh S, Kim E, et al. Glucosidase inhibiton and antiglycation activity of laccase-catalyzed catechin polymers. J Agric Food Chem 2013;61(19):4577–84. [DOI] [PubMed] [Google Scholar]
- 37.Song S, Huang YW, Tian Y, et al. Mechanism of action of (−)-epigallocatechin-3 -gallate: autooxidation-dependent activation of extracellular signal-regulated kinase 1/2 in Jurkat cells. Chin J Nat Med 2014;12(9):654–62. [DOI] [PubMed] [Google Scholar]
- 38.Murakami A Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch Biochem Biophys 2014;557:3–10. [DOI] [PubMed] [Google Scholar]
- 39.Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis). Ann Intern Med 2006;144(1):68–71. [DOI] [PubMed] [Google Scholar]
- 40.Lambert JD, Sang S, Yang CS. Possible controversy over dietary polyphenols: benefits vs risks. Chem Res Toxicol 2007;20(4):583–5. [DOI] [PubMed] [Google Scholar]
- 41.Khan N, Mukhtar H. Modulation of signaling pathways in prostate cancer by green tea polyphenols. Biochem Pharmacol 2013;85(5):10.1016/j.bcp.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson WG, De Marzo AM, DeWeese TL, et al. The role of inflammation in the pathogenesis of prostate cancer. J Urol 2004;172(5):S6–S12. [DOI] [PubMed] [Google Scholar]
- 43.Lee PMY, Ng CF, Liu ZM, et al. Reduced prostate cancer risk with green tea and epigallocatechin 3-gallate intake among Hong Kong Chinese men. Prostate Cancer Prostatic Dis 2017;20(3):318–22. [DOI] [PubMed] [Google Scholar]
- 44.Zhou L, Elias RJ. Antioxidant and pro-oxidant activity of (−)-epigallocatechin-3 -gallate in food emulsions: Influence of pH and phenolic concentration. Food Chem 2013;138(2):1503–9. [DOI] [PubMed] [Google Scholar]
- 45.Oikawa S, Furukawaa A, Asada H, et al. Catechins induce oxidative damage to cellular and isolated DNA through the generation of reactive oxygen species. Free Radic Res 2003;37(8):881–90. [DOI] [PubMed] [Google Scholar]
- 46.Oh B, Figtree G, Costa D, et al. Oxidative stress in prostate cancer patients: A systematic review of case control studies. Prostate Intl 2016;4(3):71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddiqui IA, Shukla Y, Adhami VM, et al. Suppression of NFκB and its regulated gene products by oral administration of green tea polyphenols in an autochthonous mouse prostate cancer model. Pharm Res 2008;25(9):2135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol 2011;82(12):1807–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henning SM, Wang P, Carpenter CL, et al. Epigenetic effects of green tea polyphenols in cancer. Epigenomics 2013;5(6):729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moses MA, Henry EC, Ricke WA, et al. The heat shock protein 90 inhibitor, epigallocatechin gallate, has anti-cancer activity in a novel human prostate cancer progression model. Cancer Prev Res 2015;8(3):249–57.* A study showing that EGCG preferentially targets cancer cells and inhibits a molecular chaperone supportive of the malignant phenotype.
- 51.Naponelli V, Modernelli A, Bettuzzi S, et al. Roles of autophagy induced by natural compounds in prostate cancer. BioMed Res Intl 2015;2015(5):121826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khandelwal A, Hall JA, Blagg BSJ. Synthesis and structure-activity relationships of EGCG analogues, a recently identified Hsp90 inhibitor. J Org Chem 2013;78(16):7859–84.** First study to evaluate the structure−activity relationships between epigallocatechin-3-gallate (EGCG) and heat shock protein 90 (Hsp90).
- 53.Siddiqui IA, Asim M, Hafeez BB, et al. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J 2011;25(4):1198–207.**The first study that clearly correlated miRNA-regulation with catechins’ treatment in prostate cancer.
- 54.Wang P, Heber D, Henning SM. Quercetin increased the antiproliferative activity of green tea polyphenol (−)-epigallocatechin gallate in prostate cancer cells. Nutr Cancer 2012;64(4):580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta K, Thakur VS, Bhaskaran N, et al. Green tea polyphenols induce p53-dependent and p53-independent apoptosis in prostate cancer cells through two distinct mechanisms. PLoS One 2012;7(12):e52572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pandey M, Shukla S, Gupta S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int J Cancer 2010;126(11):2520–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farooqi AA, Mansoor Q, Ismail M, et al. Therapeutic effect of epigallocatechin-3-gallate (EGCG) and silibinin on ATM dynamics in prostate cancer cell line LNCaP. World J Oncol 2010;1(6):242–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta S, Ahmad N, Nieminen AL, et al. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol Appl Pharmacol 2000;164(1):82–90. [DOI] [PubMed] [Google Scholar]
- 59.Fang MZ, Wang YW, Ai N, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res 2003;63(2):7563–70. * The first study showing that the DNA methylation was inhibited by a commonly consumed dietary constituent and suggest the potential use of EGCG for the prevention or reversal of related gene silencing in the prevention of carcinogenesis.
- 60.Thakur VS, Gupta K, Gupta S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis 2012;33(2):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hastak K, Gupta S, Ahmad N, et al. Role of p53 and NF-κB in epigallocatechin-3 -gallate-induced apoptosis of LNCaP cells. Oncogene 2015;22(31):4851–9. [DOI] [PubMed] [Google Scholar]
- 62.Siddiqui IA, Malik A, Adhami VM, et al. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene 2008;27(14):2055–63. * The first study showing that EGCG sensitizes TRAIL-resistant LNCaP cells to TRAIL-mediated apoptosis through modulation of intrinsic and extrinsic apoptoticpathways.
- 63.Siddiqui IA, Adhami VM, Afaq F, et al. Modulation of phosphatidylinositol-3- kinase/protein kinase B- and mitogen-activated protein kinase-pathways by tea polyphenols in human prostate cancer cells. J Cell Biochem 2004;91(2):232–42. * A study shows that the EGCG modulated the constitutive activation of PI3K/Akt and Erk1/2 pathways.
- 64.Albrecht DS, Clubbs EA, Ferruzzi M, et al. Epigallocatechin-3- gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK- independent ERK1/2 activation. Chem Biol Interact 2008;171(1):89–95. [DOI] [PubMed] [Google Scholar]
- 65.Nair S, Barve A, Khor TO, et al. Regulation of Nrf2- and AP-1-mediated gene expression by epigallocatechin-3-gallate and sulforaphane in prostate of Nrf2-knockout or C57BL/6J mice and PC-3 AP-1 human prostate cancer cells. Acta Pharmacol Sin 2010;31(9):1223–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adhami VM, Malik A, Zaman N, et al. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin Cancer Res 2007; 13(5):1611–9. [DOI] [PubMed] [Google Scholar]
- 67.Gupta S, Hastak K, Ahmad N, et al. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci USA 2001;98(18):10350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harper CE, Patel BB, Wang J, et al. Epigallocatechin‐3‐gallate suppresses early stage, but not late stage prostate cancer in TRAMP mice: Mechanisms of action. Prostate 2007;67(14):1576–89. [DOI] [PubMed] [Google Scholar]
- 69.Adhami VM, Siddiqui IA, Sarfaraz S, et al. Effective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on the stage of the disease. Clin Cancer Res 2009;15(6):1947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 71.Sadava D, Whitlock E, Kane SE. The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem Biophys Res Commun 2007;360(1):233–37. [DOI] [PubMed] [Google Scholar]
- 72.Gao Z, Xu Z, Hung MS, et al. Promoter demethylation of WIF-1 by epigallocatechin-3-gallate in lung cancer cells. Anticancer Res 2009;29(6):2025–30. [PubMed] [Google Scholar]
- 73.Milligan SA, Burke P, Coleman DT, et al. The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clin Cancer Res 2009;15(15):4885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suganuma M, Kurusu M, Suzuki K, et al. Green tea polyphenol stimulates cancer preventive effects of celecoxib in human lung cancer cells by upregulation of GADD153 gene. Int J Cancer 2006;119(1):33–40. [DOI] [PubMed] [Google Scholar]
- 75.Kasper B, Salameh A, Krausch M, et al. Epigallocatechin gallate attenuates cardiopulmonary bypass–associated lung injury. J Surg Res 2016;201(2):313–25. [DOI] [PubMed] [Google Scholar]
- 76.Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1alpha. Carcinogenesis 2011;32(12):1881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435(7043):834–38. [DOI] [PubMed] [Google Scholar]
- 78.Harris KS, Zhang Z, McManus MT, et al. Dicer function is essential for lung epithelium morphogenesis. PNAS 2006;103(7):2208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou H, Chen JX, Yang CS, et al. Gene regulation mediated by microRNAs in response to green tea polyphenol EGCG in mouse lung cancer. BMC genomics 2014;15(S11):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li MJ, Yin YC, Wang J, et al. Green tea compounds in breast cancer prevention and treatment. World J Clin Oncol 2014;5(3):520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tu SH, Ku CY, Ho CT, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits nicotine- and estrogen-induced α9-nicotinic acetylcholine receptor upregulation in human breast cancer cells. Mol Nutr Food Res 2011;55(3):455–66. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Yuan YY, Meeran SM, et al. Synergistic epigenetic reactivation of estrogen receptor-α (ERα) by combined green tea polyphenol and histone deacetylase inhibitor in ERα-negative breast cancer cells. Mol Cancer 2010;9(1):274.* A study showing that EGCG can restore ERα expression by regulating epigenetic mechanisms, and this effect is enhanced when combined with an HDAC inhibitor, providing a basis for use of combination approaches to treat breast cancer.
- 83.Belguise K, Guo S, Sonenshein GE. Activation of FOXO3a by the green tea polyphenol epigallocatechin-3-gallate induces estrogen receptor α expression reversing invasive phenotype of breast cancer cells. Cancer Res 2007;67(12):5763–70. [DOI] [PubMed] [Google Scholar]
- 84.Eddy SF, Kane SE, Sonenshein GE. Trastuzumab-resistant HER2-driven breast cancer cells are sensitive to epigallocatechin-3 gallate. Cancer Res 2007;67(19):9018–23. [DOI] [PubMed] [Google Scholar]
- 85.Dandawate PR, Subramaniam D, Jensen RA, et al. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin Cancer Biol 2016;40-41:192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khan SI, Aumsuwan P, Khan IA, et al. Epigenetic events associated with breast cancer and their prevention by dietary components targeting the epigenome. Chem Res Toxicol 2012;25(1):61–73. [DOI] [PubMed] [Google Scholar]
- 87.Fang MZ, Wang Y, Ai N, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res 2003;63(22):7563–70. [PubMed] [Google Scholar]
- 88.Lee WJ, Shim J-Y, Zhu BT. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharm 2005;68(4):1018–30. [DOI] [PubMed] [Google Scholar]
- 89.Choi KC, Jung MG, Lee YH, et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res 2009;69(2):583–92. [DOI] [PubMed] [Google Scholar]
- 90.Jang J-Y, Lee J-K, Jeon Y- K, et al. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer 2013;13:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riederer P, Sofic E, Rausch W, et al. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 1989;52(2):515–20. [DOI] [PubMed] [Google Scholar]
- 92.Bieschke J, Russ J, Friedrich RP, et al. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. PNAS 2010;107(17):7710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qin XY, Cheng Y, Yu LC. Potential protection of green tea polyphenols against intracellular amyloid beta-induced toxicity on primary cultured prefrontal cortical neurons of rats. Neurosci Lett 2012;513(2):170–3. [DOI] [PubMed] [Google Scholar]
- 94.Okello EJ, Leylabi R, McDougall GJ. Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. Food Func 2012;3(6):651–61. [DOI] [PubMed] [Google Scholar]
- 95.Singh NA, Mandal AKA, Khan ZA. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr J 2016;15(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wobst HJ, Sharma A, Diamond MI, et al. The green tea polyphenol (−)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett 2015;589(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chesser AS, Ganeshan V, Yang J, et al. Epigallocatechin-3-gallate enhances clearance of phosphorylated tau in primary neurons. Nutr Neurosci 2016;19(1):21–31. [DOI] [PubMed] [Google Scholar]
- 98.Irimie AI, Braicu C, Zanoaga O, et al. Epigallocatechin-3-gallate suppresses cell proliferation and promotes apoptosis and autophagy in oral cancer SSC-4 cells. Onco Targets Ther 2015;8(8):461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agrawal M, Biswas A. Molecular diagnostics of neurodegenerative disorders. Front Mol Biosci 2015;2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu Y, Zhang Y, Quan Z, et al. Epigallocatechin gallate (EGCG) inhibits alpha-synuclein aggregation: a potential agent for Parkinson’s disease. Neurochem Res 2016;41(10):2788–96. [DOI] [PubMed] [Google Scholar]
- 101.Levites Y, Weinreb O, Maor G, et al. Green tea polyphenol (−)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem 2001;78(5):1073–82. [DOI] [PubMed] [Google Scholar]
- 102.Erba D, Riso P, Bordoni A, et al. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J Nutr Biochem 2005;16(3):144–9. [DOI] [PubMed] [Google Scholar]
- 103.Mandel S, Amit T, Kalfon L, et al. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: special reference to epigallocatechin gallate (EGCG). J Alzheimers Dis 2008;15(2):211–22. [DOI] [PubMed] [Google Scholar]
- 104.Yang J, Mao QX, Xu HX, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open 2014;4(7):e005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iso H, Date C, Wakai K, et al. The relationship between green tea intake and type 2 diabetes in japanese adults. Ann Intern Med 2006;144(8):I–28. [DOI] [PubMed] [Google Scholar]
- 106.Wu C-H, Lu F-H, Chang C- S, et al. Relationship among habitual tea consumption, percent body fat, and body fat distribution. Obes Res 2003;11(9):1088–95. [DOI] [PubMed] [Google Scholar]
- 107.Hsu C-H, Liao Y-L, Lin S- C, et al. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern Med Rev 2011;16(2):157–63. [PubMed] [Google Scholar]
- 108.Martin MA, Goya L, Ramos S. Protective effects of tea, red wine and cocoa in diabetes. Evidences from human studies. Food Chem Toxicol 2017;109(Part 1):302–14. [DOI] [PubMed] [Google Scholar]
- 109.Pastoriza S, Mesías M, Cabrera C, et al. Healthy properties of green and white teas: an update. Food Func 2017;8(8):2650–62. [DOI] [PubMed] [Google Scholar]
- 110.Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann N Y Acad Sci 2002;955(1):89–100. [DOI] [PubMed] [Google Scholar]
- 111.Groothuis PG, Nap AW, Winterhager E, et al. Vascular development in endometriosis. Angiogenesis 2005;8(2):147–56. [DOI] [PubMed] [Google Scholar]
- 112.Becker CM, Rohwer N, Funakoshi T, et al. 2-Methoxyestradiol inhibits hypoxia-inducible factor-1a and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol 2008;172(2):534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu H, Lui WT, Chu CY, et al. Antti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum Reprod 2009;24(3):608–18. [DOI] [PubMed] [Google Scholar]
- 114.Hull ML, Charnock-Jones DS, Chan CL, et al. Antiangiogenic agents are effective inhibitors of endometriosis. J Clin Endocrinol Metab 2003;88(6):2889–99. [DOI] [PubMed] [Google Scholar]
- 115.Cao YH, Cao RH. Angiogenesis inhibited by drinking tea. Nature 1999;398:381. [DOI] [PubMed] [Google Scholar]
- 116.Xu H, Becker CM, Lui WT, et al. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil Steril 2011;96(4):1021–8. [DOI] [PubMed] [Google Scholar]
- 117.Laschke MW, Schwender C, Scheuer C, et al. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum Reprod 2008;23(10):2308–18. [DOI] [PubMed] [Google Scholar]
- 118.Kojima-Yuasa A, Hua JJ, Kennedy DO, et al. Green tea extracts inhibits angiogenesis of human umbilical vein endothelial cells through reduction of expression of VEGF receptors. Life Sci 2003;73(10):1299–313. [DOI] [PubMed] [Google Scholar]
- 119.Shirakami Y, Shimizu M, Adachi S, et al. Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor–vascular endothelial growth factor receptor axis. Cancer Sci 2009;100(10):1957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kurbitz C, Heise D, Redmer T, et al. , Epicatechin gallate and catechin gallate are superior to epigallocatechin gallate in growth suppression and anti-inflammatory activities in pancreatic tumor cells. Cancer Sci 2011;102(4):728–34. [DOI] [PubMed] [Google Scholar]
- 121.Ahn HY, Hadizadeh KR, Seul C, et al. Epigallocathechin-3 gallate selectively inhibits the PDGF-BB-induced intracellular signaling transduction pathway in vascular smooth muscle cells and inhibits transformation of sis-transfected NIH 3T3 fibroblasts and human glioblastoma cells (A172). Mol Biol Cell 1999; 10(4):1093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rashidi B, Malekzadeh M, Goodarzi M, et al. Green tea and its anti-angiogenesis effects. Biomed Pharm 2017;89:949–56. [DOI] [PubMed] [Google Scholar]
- 123.Fix LN, Shah M, Efferth T, et al. MicroRNA expression profile of MCF-7 human breast cancer cells and the effect of green tea polyphenon-60. Cancer Genomics-Proteomics 2010;7(5):261–77. [PubMed] [Google Scholar]
- 124.Micali S, Territo A, Pirola GM, et al. Effect of green tea catechins in patients with high-grade prostatic intraepithelial neoplasia: Results of a short-term double-blind placebo controlled phase II clinical trial. Arch Ital Urol Androl 2017;89(3):197–202. [DOI] [PubMed] [Google Scholar]
- 125.Huang YQ, Lu X, Min H, et al. Green tea and liver cancer risk: A meta-analysis of prospective cohort studies in Asian populations. Nutrition 2016;32(1):3–8. [DOI] [PubMed] [Google Scholar]
- 126.Teschke R, Schulze J. Green tea and the question of reduced liver cancer risk: the dawn of potential clinical relevance? Hepatobiliary Surg Nutr 2017;6(2):122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang CS, Li G, Yang Z, et al. Cancer prevention by tocopherols and tea polyphenols. Cancer lett 2013;334(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kida K, Suzuki M, Matsumoto N, et al. Identification of biliary metabolites of (−)-epigallocatechin gallate in rats. J Agric Food Chem 2000;48(9):4151–55. [DOI] [PubMed] [Google Scholar]
- 129.Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nano 2007;2(12):751–60. [DOI] [PubMed] [Google Scholar]
- 130.Siddiqui IA, Adhami VM, Bharali DJ, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res 2009;69(5):1712–6.* Provides a basis for the use of nanoparticle-mediated delivery to enhance bioavailability and limit any unwanted toxicity of EGCG.
- 131.Haque A, Rahman MA, Chen ZG, et al. Combination of erlotinib and EGCG induces apoptosis of head and neck cancers through posttranscriptional regulation of Bim and Bcl-2. Apoptosis 2015;20(7):986–95. [DOI] [PubMed] [Google Scholar]
- 132.Fujiki H, Sueoka E, Watanabe T, et al. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J Cancer Res Clin Oncol 2015;141(9):1511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou H, Sun X, Zhang L, et al. Fabrication of biopolymeric complex coacervation core micelles for efficient tea polyphenol delivery via a green process. Langmuir 2012;28(41):14553–61. [DOI] [PubMed] [Google Scholar]
- 134.de Pace RC, Liu X, Sun M, et al. Anticancer activities of (−)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J Liposome Res 2013;23(3):187–96. [DOI] [PubMed] [Google Scholar]
- 135.Zou L, Peng S, Liu W, et al. Improved in vitro digestion stability of (−)-epigallocatechin gallate through nanoliposome encapsulation. Food Res Int 2014;64:492–9. [DOI] [PubMed] [Google Scholar]
- 136.Zou L, Peng S, Liu W, et al. A novel delivery system dextran sulfate coated amphiphilic chitosan derivatives-based nanoliposome: Capacity to improve in vitro digestion stability of (−)-epigallocatechin gallate. Food Res Int 2015;69(1):114–20. [Google Scholar]
- 137.Zou L, Liu W, Liu W, et al. Characterization and bioavailability of tea polyphenol nanoliposome prepared by combining an ethanol injection method with dynamic high-pressure microfluidization. J Agric Food Chem 2014;62(4):934–41. [DOI] [PubMed] [Google Scholar]
- 138.Manea AM., Andronescu C, Meghea A Green tea extract loaded into solid lipid nanoparticles. UPB Sci Bull 2014;76(2):125–36. [Google Scholar]
- 139.Zhang J, Nie S, Martinez-Zaguilan R, et al. Formulation, characteristics and antiatherogenic bioactivities of CD36-targeted epigallocatechin gallate (EGCG)-loaded nanoparticles. J Nutr Biochem 2016;30:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ru Q, Yu H, Huang Q. Encapsulation of epigallocatechin-3-gallate (EGCG) using oil-in-water (O/W) submicrometer emulsions stabilized by icarrageenan and b-lactoglobulin. J Agric Food Chem 2010;58(19):10373–81. [DOI] [PubMed] [Google Scholar]
- 141.Kim YJ, Houng SJ, Kim JH, et al. Nanoemulsified green tea extract shows improved hypocholesterolemic effects in C57BL/6 mice. J Nutr Biochem 2012;23(2):186–91. [DOI] [PubMed] [Google Scholar]
- 142.Anu-Bhushani J, Karthik P, Anandharamakrishnan C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2 cell monolayer permeability of green tea catechins. Food Hydrocoll 2016;56:372–82. [Google Scholar]
- 143.Fangueiro JF, Andreani T, Fernandes L, et al. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf B Biointerfaces 2014;123:452–60. [DOI] [PubMed] [Google Scholar]
- 144.Fangueiro JF, Calpena AC, Clares B, et al. Biopharmaceutical evaluation of epigallocatechin gallate-loaded cationic lipid nanoparticles (EGCG-LNs): In vivo, in vitro and ex vivo studies. Int J Pharm 2016;502(1–2):161–9. [DOI] [PubMed] [Google Scholar]
- 145.Aditya NP, Aditya S, Yang H, et al. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem 2015;173(173):7–13. [DOI] [PubMed] [Google Scholar]
- 146.Shutava TG, Balkundi SS, Vangala P, et al. Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano 2009;3(7):1877–85. [DOI] [PubMed] [Google Scholar]
- 147.Tang DW, Yu SH, Ho YC, et al. Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocoll 2013;30(1):33–41. [Google Scholar]
- 148.Hong Z, Xu Y, Yin JF, et al. Improving the effectiveness of (−)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCGloaded nanoparticles prepared from chitosan and polyaspartic acid. J Agric Food Chem 2014;62(52):12603–9. [DOI] [PubMed] [Google Scholar]
- 149.Hu B, Pan C, Sun Y, et al. Optimization of fabrication parameters to produce chitosantripolyphosphate nanoparticles for delivery of tea catechins. J Agric Food Chem 2008;56(16):7451–8. [DOI] [PubMed] [Google Scholar]
- 150.Liang J, Li F, Fang Y, et al. Synthesis, characterization and cytotoxicity studies of chitosan-coated tea polyphenols nanoparticles. Colloids Surf B Biointerfaces 2011;82(2):297–301. [DOI] [PubMed] [Google Scholar]
- 151.Khan N, Bharali DJ, Adhami VM, et al. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014;35(2):415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Siddiqui IA, Bharali DJ, Nihal M, et al. Excellent anti-proliferative and pro-apoptotic effects of (−)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomedicine 2014;10(8):1619–26. [DOI] [PubMed] [Google Scholar]
- 153.Safer AM, Hanafy NA, Bharali DJ, et al. Effect of green tea extract encapsulated into chitosan nanoparticles on hepatic fibrosis collagen fibers assessed by atomic force microscopy in rat hepatic fibrosis model. J Nanosci Nanotechnol 2015;15(9):6452–9. [DOI] [PubMed] [Google Scholar]
- 154.Narayanan S, Mony U, Vijaykumar DK, et al. Sequential release of Epigallocatechin gallate and paclitaxel from PLGA-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cells. Nanomedicine 2015;11(6):1399–406. [DOI] [PubMed] [Google Scholar]
- 155.Narayanan S, Pavithran M, Viswanath A, et al. Sequentially releasing dual-drug-loaded PLGA-casein core/shell nanomedicine: Design, synthesis, biocompatibility and pharmacokinetics. Acta Biomater 2014;10(5):2112–24. [DOI] [PubMed] [Google Scholar]
- 156.Sanna V, Pintus G, Roggio AM, et al. Targeted biocompatible nanoparticles for the delivery of (−)-Epigallocatechin 3-gallate to prostate cancer cells. J Med Chem 2011;54(5):1321–32. [DOI] [PubMed] [Google Scholar]
- 157.Dube A, Ng K, Nicolazzo JA, et al. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem 2010;122(3):662–7. [Google Scholar]
- 158.Shpigelman A, Israeli G, Livney YD. Thermally-induced protein–polyphenol co-assemblies: beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocoll 2010;24(8):735–43. [Google Scholar]
- 159.Yang CS, Kim S, Yang GY, et al. Inhibition of carcinogenesis by tea: bioavailability of tea polyphenols and mechanisms of actions. Proc Soc Exp Biol Med 1999;220(4):213–17. [DOI] [PubMed] [Google Scholar]
- 160.Patel AR, Seijen-ten-Hoorn J, Velikov KP. Colloidal complexes from associated water soluble cellulose derivative (methylcellulose) and green tea polyphenol (Epigallocatechin gallate). J Colloid Interface Sci 2011;364(2):317–23. [DOI] [PubMed] [Google Scholar]
- 161.Bhushani JA, Kurrey NK, Anandharamakrishnan C. Nanoencapsulation of green tea catechins by electrospraying technique and its effect on controlled release and in-vitro permeability. J Food Eng 2017;199(Supplement C):82–92. [Google Scholar]
- 162.Ding H, Ma Y. Controlling cellular uptake of nanoparticles with pH-sensitive polymers. Sci Rep 2013;3(9):2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Prakasha Upputuri RT, Azad Mandal AK. Sustained release of green tea polyphenols from liposomal nanoparticles; release kinetics and mathematical modelling. Iranian J Biotechnol 2017;15(4):277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Radhakrishnan R, Kulhari H, Pooja D, et al. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem Phys Lipids 2016;198:51–60. [DOI] [PubMed] [Google Scholar]
- 165.Siddiqui IA, Adhami VM, Ahmad N, et al. Nanochemoprevention: sustained release of bioactive food components for cancer prevention. Nutr Cancer 2010;62(7):883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu B, Ting Y, Yang X, et al. Nanochemoprevention by encapsulation of (−)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem Commun 2012;48(18):2421–3. [DOI] [PubMed] [Google Scholar]
- 167.Wang X, Li J, Wang Y, et al. A folate receptor-targeting nanoparticle minimizes drug resistance in a human cancer model. ACS Nano 2011;5(8):6184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm 2015;93(Supplement C):52–79. [DOI] [PubMed] [Google Scholar]
- 169.Lam WH, Kazi A, Kuhn DJ, et al. A potential prodrug for a green tea polyphenol proteasome inhibitor: evaluation of the peracetate ester of (−)-epigallocatechin gallate [(−)-EGCG]. Bioorg Med Chem 2004;12(21):5587–93. [DOI] [PubMed] [Google Scholar]
- 170.Li L, Chan TH. Enantioselective synthesis of epigallocatechin-3-gallate (EGCG), the active polyphenol component from green tea. Org Lett 2001;3(5):739–41. [DOI] [PubMed] [Google Scholar]
- 171.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem 2001;276(16):13322–30. [DOI] [PubMed] [Google Scholar]
- 172.Lambert JD, Sang S, Hong J, et al. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab Dispos 2006;34(12):2111–6. [DOI] [PubMed] [Google Scholar]
- 173.Landis-Piwowar KR, Huo C, Chen D, et al. A novel prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res 2007;67(9):4303–10. [DOI] [PubMed] [Google Scholar]
- 174.Lee SC, Chan WK, Lee TW, et al. Effect of a prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate on the growth of androgen-independent prostate cancer in vivo. Nutr Cancer 2008;60(4):483–91. [DOI] [PubMed] [Google Scholar]
- 175.Wang J, Man GCW, Chan TH, et al. A prodrug of green tea polyphenol (−)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer. Cancer Lett 2018;412:10–20. [DOI] [PubMed] [Google Scholar]
- 176.Chiou YS, Ma NJ, Sang S, et al. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. J Agric Food Chem 2012;60(13):3441–51. [DOI] [PubMed] [Google Scholar]
- 177.Wang CC, Xu H, Man GC, et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis 2013;16(1):59–69. [DOI] [PubMed] [Google Scholar]
- 178.Han DW, Matsumura K, Kim B, et al. Time-dependent intracellular trafficking of FITC-conjugated epigallocatechin-3-O-gallate in L-929 cells. Bioorg Med Chem 2008;16(22):9652–9. [DOI] [PubMed] [Google Scholar]
- 179.Gu F, Langer R, Farokhzad OC. Formulation/preparation of functionalized nanoparticles for in vivo targeted drug delivery. Methods Mol Biol 2009;544(5): 589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Cancer 2008;7(9):771–82. [DOI] [PubMed] [Google Scholar]
- 181.Periasamy VS, Alshatwi AA. Tea polyphenols modulate antioxidant redox system on cisplatin-induced reactive oxygen species generation in a human breast cancer cell. Basic Clin Pharmacol Toxicol 2013;112(6):374–84. [DOI] [PubMed] [Google Scholar]


