Abstract
A sensitive and straightforward LC-IT-TOF-MS method was validated for the profiling and simultaneous quantification of anthocyanins, flavan-3-ols, flavonols, phenolic acids, and resveratrol in blueberry genotypes with fruit color ranging from deep purple (Vaccinium angustifolium) to various shades of pink (crosses of V. corymbosum, V. darrowii, and V. ashei). Standard calibration curves were linear for all analytes with correlation coefficients >0.99. The relative standard deviation for intra- and inter-day precision was lower than 10%. The method allowed an easy and selective identification and quantification of phenolics in blueberries with divergent profiles. The in vitro antioxidant assay results were strongly correlated with total phenolics and total anthocyanin content. Lowbush blueberry extracts (50 μg/mL) reduced ROS and NO production, and inhibited the transcription of the proinflammatory cytokines IL-6β, COX2, iNOS, and IL-6 in the in vitro assays at much lower concentrations than pink fruited berries (250 μg/mL).
Keywords: Pink-fruited, blueberries, LC-IT-TOF-MS, quantification, antioxidant, anti-inflammatory, PCA
1. Introduction
Phenolic compounds comprise a broad and diverse range of secondary metabolites that are naturally present in fruits and vegetables. Accumulated scientific evidence strongly suggests that long-term consumption of diets rich in plant polyphenols offers protection and management of non-communicable diseases such as cancers, cardiovascular diseases, diabetes, osteoporosis and neurodegenerative diseases (Barrajón-Catalán et al., 2014).
Blueberry fruit contains several classes of bioactive phenolic components including phenolic acids, anthocyanins, flavan-3-ols, proanthocyanidins, flavonols, and stilbenes; a wide diversity of chemical structures when the subsequent substitution by sugar groups is also included (Grace, Esposito, Dunlap, & Lila, 2014). Because of the health benefits linked to these compounds, fruit breeding has undergone a substantial shift with the adoption of metabolite profiling rapidly becoming an integral part of the process (Yuliana, Khatib, Choi, & Verpoorte, 2011). Generally, HPLC-UV-DAD technique allows easy analysis of anthocyanins, although its UV absorption spectra and overlapping chromatographic peaks may give rise to some misleading results (Grace et al., 2014). HPLC coupled with MS detection has been used mostly for compound identification purposes (Wu & Prior, 2005). Electrospray ionization techniques (ESI) are commonly used to provide molecular masses of compounds, and tandem mass to provide structural details. Anthocyanins are usually detected in the positive ion mode as their native form (positive flavylium cation), while hydroxybenzoic and hydroxycinnamic acids, catechins, and proanthocyanidins are better detected in the negative ion mode (Gavrilova, Kajdzanoska, Gjamovski, & Stefova, 2011). Few studies have utilized LC-MS for the quantification of anthocyanins and other classes of polyphenols (Cavaliere et al., 2008; Nagy, Redeuil, Bertholet, Steiling, & Kussmann, 2009), however, the research community is still in need of a simple, sensitive, and valid LC-MS method for simultaneous quantitation of a broader spectrum of phenolic compounds.
These phenolic compounds have significant modulatory effects on cellular biomarkers related to oxidative stress and inflammation, which lead to reduced risk of many chronic diseases (Zhang & Tsao, 2016). Occurrence of reactive oxygen species or oxidative stress is an outcome of several degenerative and age-related diseases, or may be a trigger for development of some conditions; for example arthritis, atherosclerosis, cancer, diabetes and others (Muriach, Flores-Bellver, Romero, & Barcia, 2014; Prior, 2015). Results from in vitro antioxidant assays may not correlate with efficacy in vivo, therefore a combination of multiple chemical antioxidant assays and a cell-based assay are suggested for robust interpretation of results (Granato et al., 2018).
In this study, a new LC/MS method was developed and validated to enable simultaneous qualitative and quantitative determination of anthocyanins, flavan-3-ols, flavonol glycosides, phenolic acids and stilbenes over a broad range of genotypes. Six pink-fruited blueberry clones were cross-compared to lowbush wild blueberry to develop a streamlined method applicable for analysis of blueberries with markedly different flavonoid profiles and concentrations. The variation in composition between genotypes was analyzed using PCA. Moreover, in vitro and cell-based antioxidant and anti-inflammatory activities of the blueberry extracts were investigated and correlated to the phenolic composition.
2. Materials and methods
2.1. Chemicals
Folin-Ciocalteu reagent, sodium carbonate, Trolox (6-hydroxy-2,5,7,8-tetramethyl chromane-2-carboxylic acid), ABTS (2,2’- azinobis-(3-ethylbenzothiazoline-6-sulfonic acid), dexamethasone (DEX), 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA) and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified eagle’s medium (DMEM), TrypLE™, fetal bovine serum (FBS), Trizol, and cDNA Reverse Transcription kit were obtained from Life Technologies, (NY, USA). The RAW 264.7 mouse macrophage cell line (ATCC TIB-71) was obtained from American Type Culture Collection (Livingstone, MT, USA). All other solvents and chemicals used in this investigation were purchased from VWR International (Suwanee, GA, USA).
The following compounds were used as reference standards in the LC-MS/MS analysis: procyanidins B1 and B2, catechin, epicatechin, cyanidin-3-galactoside, cyanidin-3-glucoside, and malvidin-3-galactoside were obtained from Chromadex (Irvine, CA, USA). Delphinidin-3-glucoside was purchased from Cayman Chemicals (Ann Arbor, MI, USA). Delphinidin-3-galactoside, malvidin-3-glucoside, petunidin-3-glucoside, myricetin-3-glucoside, kaempferol-3-glucoside, and syringetin-3-glucoside were obtained from Extrasynthese (Genay Cedex, France). Cyanidin-3-arabinoside and peonidin-3-glucoside were obtained from Polyphenols (Sandnes, Norway). Gallic acid, caffeic acid, chlorogenic acid, 2,4-dihydroxybenzoic acid, quercetin, quercetin glucoside and galactoside, quercetin arabinoside were purchased from Sigma-Aldrich (St. Louis, MO, USA). Phlorizin and daidzin were used as internal standards and were also obtained from Sigma-Aldrich. The actual concentration of all these standards was calculated based on their purity.
2.2. Plant material
Pink-fruited blueberry clones (Vaccinium x hybrids) used in this study were grown at USDA-ARS plots at the Marucci Center for Blueberry and Cranberry Research and Extension at Chatsworth, NJ, USA. Six blueberry hybrids, derived from V. corymbosum, V. virgatum, and V. darrowii crosses were investigated in this study. Table S1 (Supplementary material) lists the six clones; Pink Lemonade (PLE), Pink Champagne (PCH), Florida Rose (FLR), US 2117, US 2211 and US 2235. The ripe berries were harvested in late June-early July, and immediately frozen and stored at −70 °C until they were shipped to North Carolina on dry ice. A uniform composite individually quick frozen (IQF) sample (from harvested sites in Maine [USA] and Quebec, Nova Scotia, Prince Edward Island and New Brunswick [Canada]) of commercially grown lowbush blueberries (V. angustifolium, Aiton), obtained from the Wild Blueberry Association of North America (Old Town, ME, USA) and stored as above. All blueberry samples were freeze-dried before extraction.
2.3. Extraction of blueberries
Extraction of blueberries was conducted following our standardized procedure with minor modification (Grace et al., 2014). Freeze-dried berries (0.75 g freeze-dried blueberries × 3 replicates) were placed in 30 mL centrifuge tubes embedded in ice and homogenized in 8 mL of cold 70% aqueous methanol (0.5% acetic acid) using a Pro 250 homogenizer (Pro Scientific Inc. Oxford, CT, USA) for 4 minutes. The obtained slurry was centrifuged (Sorvall RC-6 plus, Asheville, NC, USA) for 10 minutes at 3452 g-force at 4 °C. The supernatant was collected in a 25-mL volumetric flask. The residue was then extracted two more times each by sonication with 8 mL methanol for 5 min, centrifuged as above, and supernatants were collected all together and brought to a final volume of 25 mL. The hydro-methanol extract was used for all phytochemical investigations and in vitro radical scavenging assays. An aliquot was evaporated to a dry residue and used for in vitro cell-based assays.
2.4. Total phenolics, anthocyanins, proanthocyanidins, and radical scavenging assays
The number of total phenolics was determined spectrophotometrically according to the Folin-Ciocalteu procedure (Singleton et al., 1999). Concentrations were expressed as mg gallic acid equivalent per g sample (DW) based on a standard curve created with gallic acid. Total anthocyanins were determined by HPLC and quantified as mg cyanidin-3-glucoside equivalent/g sample. Total proanthocyanidins were determined by DMAC assay against a standard curve created with procyanidin B1 reference compound. Results were expressed as procyanidin B1 equivalent (Prior, Lazarus, Cao, Muccitelli, & Hammerstone, 2001).
The radical scavenging activity was measured using the stable ABTS radical cation assay (Re et al., 1999) and Trolox as reference antioxidant. Results were reported as Trolox equivalents (µmol TE/g DW). The ferric reducing power of berry extracts was performed in a 96-well microplate using the FRAP assay (Grace et al., 2014). The reducing capacity of the extracts was calculated with reference to the reaction signal given by a FeSO4 solution; FRAP values were expressed as µmoles of Fe2+/g of dried berry.
2.5. Preparation of samples and standard solutions for LC-MS analysis
Each of the twenty-four phenolic reference compounds was accurately weighed and dissolved individually in a solvent mix (methanol-water-formic acid, 65:35:5%) at a concentration of 2.5 mg/mL. The individual standard solutions were stored at −20 °C freezer. Prior to LC-MS analysis, equal volumes from each standard solution were mixed and diluted with the solvent mix to prepare standard stock mix solution (50 µg/mL). The standard working solutions, used for calibration curves, were made by appropriate dilution of the stock solution (0.02–40 µg/mL) in methanol-water-formic acid (65:35:5%). A stock solution mix of internal standards phlorizin and daidzin was prepared at a concentration of 0.2 mg/mL; it was added to all samples at a final concentration of 5 µg/mL.
LC-MS samples of pink fruited blueberries were prepared at 95% concentration by adding 950 µL sample extract + 25 µL internal standard mix + 25 µL solvent. Lowbush blueberry LCMS solution was prepared at 70% concentration by mixing 700 µL extract + 25 µL internal standard mix + 275 µL solvent. The dilutions were considered in the calculations for final concentrations.
2.6. LC-MS/MS instrument and conditions
The analysis was performed on a hybrid IT-TOF mass spectrometer (Shimadzu LC-MS-IT-TOF, Kyoto, Japan) equipped with two LC-20AD pumps, a SIL-20AC autosampler, a CTO-20A column oven, an SPD-M20A PDA detector, a CBM-20A system controller coupled to an IT-TOF-MS through an ESI interface. All data were processed by Shimadzu software, specifically, LCMS Solution Version 3.60, Formula Predictor Version 1.2, and Accurate Mass Calculator (Shimadzu).
The chromatography was performed on a Shim-pack XR-ODS column (50 mm × 3.0 mm ×2.2 μm) (Shimadzu, Japan), and the temperature of the column oven was maintained at 50 ºC. The eluents were water (formic acid 0.5%, v/v) (A) and methanol (B), with a gradient of 5–20% B (0– 30 min), 20–35% B (30–35 min), 35–40% B (35–37 min), 40–90% B (37–38 min), 90–5% (38–40 min), then the column was re-equilibrated for 3 min at 5% B, at the flow rate of 0.6 mL/min.
The mass spectrometer was programmed to carry out a full scan over m/z 70–700 (MS1) and m/z 70–500 (MS2) in both positive and negative ionization modes. The heat block and curved desolvation line (CDL) temperature was maintained at 200 ºC; nitrogen was used as the nebulizing gas at a flow rate of 1.5 L/min, and as the drying gas at 75 kPa; the interface voltage was (+), 4.5 kV; (−), −3.5 kV; and the detector voltage was 1.61 kV. Argon was used as the collision gas for CID experiments, and the collision energy was set at 50% for MS2. In the MS1 mode, a 10 ms ion accumulation time was used, while in MS2 mode, instead, a 20 ms was used, and the window used for precursor ion isolation corresponds to a width of 3 amu. For quantitative analysis, the CID was disabled, and ion accumulation was set at 80 ms. In the method for quantitative analysis, a table of selected ions was set up based on accurate mass and retention time of each phenolic compound. Anthocyanin and flavonol glycosides were quantified as their extracted-ion chromatograms (EIC) in the positive ion mode with daidzin as an internal standard. Flavan-3-ols, phenolic acids, and resveratrol were quantified in the negative ion mode using phlorizin as an internal standard. Anthocyanin compounds that have no available reference standards were semi-quantified using the standard curves of anthocyanins having the same aglycone.
2.7. Method validation
Specificity, linearity, accuracy, precision, range, quantitation limits, and detection limits were conducted following the FDA Guidance for Industry (US Food and Drug Administration, 2015).
Standard compound calibration curves consisted of 10 concentration levels (0.01–40 µg/mL), and each concentration was prepared and assayed in three runs on three separate days. The ratios of the peak area of the analytes to that of internal standard were plotted against nominal concentrations of the analytes, and standard curves were in the form of Y = aX + b. The linearity ranges of the compounds, regression coefficients (r2), and the linear regression equations are given separately in Table 2.
Table 2.
Method validation for simultaneous quantification of phenolic compounds in blueberries
| Phenolic standards* | Regression equations | r2 | Linear range (ppm) | LLOQ/LLOD (ppm) | Precision RSD (%) Intra-day | Inter-day | Repeatability RSD (%) |
|---|---|---|---|---|---|---|---|
| Anthocyanins | |||||||
| Dp-3-gal | Y = 1.1111X – 0.0038 | 0.9997 | 0.4–40 | 1.20/0.40 | 0.60 | 3.89 | 0.34 |
| Dp-3-glc | Y = 0.6237X – 0.0554 | 0.9998 | 0.2–40 | 0.60/0.20 | 1.15 | 5.94 | 3.10 |
| Cyn-3-gal | Y = 1.0376X + 0.0311 | 0.9998 | 0.06–40 | 0.18/0.06 | 2.35 | 4.74 | 2.26 |
| Cyn-3-glc | Y = 1.9267X + 0.0190 | 1.0000 | 0.04–40 | 0.12/0.04 | 1.22 | 8.73 | 0.53 |
| Cyn-3-ara | Y = 1.1997X – 0.0159 | 0.9996 | 0.06–40 | 0.18/0.06 | 1.81 | 2.79 | 5.53 |
| Pet-3-glc | Y = 1.8492X + 0.0353 | 0.9998 | 0.06–40 | 0.18/0.06 | 0.21 | 5.54 | 1.10 |
| Peo-3-glc | Y = 2.5897X + 0.0744 | 0.9979 | 0.04–10 | 0.12/0.04 | 4.39 | 5.72 | 4.29 |
| Mv-3-gal | Y = 1.2713X + 0.0149 | 0.9998 | 0.04–20 | 0.12/0.04 | 0.80 | 2.15 | 0.83 |
| Mv-3-glc | Y = 1.7519X + 0.0420 | 0.9995 | 0.04–20 | 0.12/0.04 | 2.09 | 1.50 | 2.85 |
| Flavan-3-ols | |||||||
| Procyanidin B1 | Y = 0.7880X – 0.0087 | 0.9995 | 0.06–10 | 0.12/0.06 | 0.50 | 6.59 | 3.67 |
| Catechin | Y = 1.7000X + 0.0253 | 0.9991 | 0.02–10 | 0.06/0.0.2 | 5.21 | 5.95 | 0.87 |
| Procyanidin B2 | Y = 0.7561X – 0.0050 | 1.0000 | 0.04–10 | 0.12/0.04 | 0.59 | 8.90 | 4.07 |
| Epicatechin | Y = 1.8823X + 0.0374 | 0.9999 | 0.04–20 | 0.12/0.04 | 4.73 | 6.26 | 0.99 |
| Flavonoid | |||||||
| Myricetin-3-glc | Y = 0.2363X – 0.0065 | 0.9983 | 0.2–10 | 0.60/0.20 | 2.43 | 3.14 | 4.97 |
| Quercetin-3-glc | Y = 0.3534X – 0.0057 | 0.9982 | 0.04–20 | 0.12/0.04 | 5.32 | 3.32 | 0.36 |
| Quercetin-3-ara | Y = 0.4187X + 0.0040 | 0.9997 | 0.01–20 | 0.03/0.01 | 0.39 | 4.43 | 2.64 |
| Kaempferol-3-glc | Y = 0.3589X + 0.0096 | 0.9985 | 0.04–10 | 0.12/0.04 | 1.97 | 3.02 | 2.66 |
| Syringetin-3-glc | Y = 0.2655X + 0.0100 | 0.9959 | 0.02–10 | 0.06/0.02 | 0.35 | 3.04 | 5.23 |
| Quercetin | Y = 0.8367X – 0.0269 | 0.9993 | 0.01–40 | 0.03/0.01 | 1.28 | 1.67 | 5.31 |
| Phenolic acid | |||||||
| Gallic | Y = 0.5952X + 0.0057 | 0.9999 | 0.04–40 | 0.12/0.04 | 2.17 | 2.26 | 3.62 |
| 2,4 dihydroxybenzoic | Y = 1.1111X – 0.0038 | 0.9999 | 0.02–10 | 0.06/0.02 | 1.88 | 5.86 | 5.23 |
| Caffeic | Y = 1.5040X + 0.1045 | 0.9981 | 0.08–10 | 0.24/0.08 | 5.41 | 6.29 | 6.42 |
| Chlorogenic | Y = 2.0099X + 0.0391 | 0.9989 | 0.01–10 | 0.03/0.01 | 5.31 | 2.26 | 1.10 |
| Resveratrol | Y = 0.1976X + 0.0003 | 0.9972 | 0.01–10 | 0.03/0.01 | 0.64 | 5.52 | 1.53 |
Dp = delphinidin; Cyn = cyanidin; Pet = petunidin; Peo = peonidin; Mv = malvidin; gal = galactoside; glc = glucoside; ara = arabinoside, RSD = relative standard deviation
The lowest limit of detection (LOD) and the lowest limit of quantitation (LOQ) was set at a signal to noise ratio (s/n) of 3:1 and 9:1, respectively (Table 2). Intra- and inter-day variations were used to evaluate the precision of the developed method. Variations were expressed as the relative standard deviation (RSD) of the replicates. To determine repeatability, three independently prepared solutions of lowbush blueberry were analyzed in triplicate. The RSD value was taken to be the measure of reproducibility.
2.8. In vitro antioxidant and anti-inflammatory assays
Murine macrophage RAW264.7 cells (ATCC®, Rockville, MD, WI, USA) were subcultured in DMEM (10% FBS) according to the protocol previously (Esposito, Chen, Grace, Komarnytsky, & Lila, 2014).
2.8.1. Cell viability assay and dose range determination
RAW 264.7 cells were seeded in a 96-well plate for the viability assay. Cell viability was measured by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide] assay (Zheng & Wang, 2003) in triplicate and quantified spectrophotometrically at 550 nm using a microplate reader SynergyH1 (BioTek, Winooski, VT, USA). The concentrations of test samples that showed no changes in cell viability compared with that vehicle (80% ethanol) were selected for further studies.
2.8.2. Reactive oxygen species production in RAW264.7 macrophages
In vitro, reactive oxygen species (ROS) were determined using a fluorescent dye protocol (Choi et al., 2007). Cells were treated with pink blueberry extracts (250 µg/mL) and lowbush blueberry (50 µg/mL) for 1 h and stimulated with lipopolysaccharide (LPS, 1 μg/mL), then incubated for 18 h. Changes in gene expression were measured by comparing mRNA quantity relative to LPS. Vehicle values were obtained in the absence of LPS or test samples; dexamethasone (DEX) was used as positive control at a concentration of 10 μM. Results were expressed as means ± SEM, n = 2 experiments. *p < 0.05, **p < 0.01, ***p < 0.001 ****p < 0.0001 vs. the LPS treated group. One-way ANOVA, Dunnett’s post hoc test. PLE = Pink Lemonade; PCH = Pink Champagne; FLR = Florida Rose.The known antioxidant dexamethasone (DEX) was used as a positive control at 20 μM. The experiments were performed with two independent replications; each replication assayed at least in duplicate.
2.8.3. Nitric oxide radical inhibition in RAW264.7 macrophages.
The ability of test samples to inhibit nitric oxide (NO) radical formation was determined by a colorimetric assay using the Griess reagent system (Promega Corporation, WI, USA) according to manufacturer protocol. The absorbance was recorded at 540 nm. NO production levels for each treatment were normalized to lipopolysaccharide (LPS).
2.8.4. Biomarkers of inflammation by gene expression analysis
The RAW 264.7 cells treated with all blueberry extracts were harvested in the TRIzol reagent for total RNA extraction and purification, according to manufacturer protocol. cDNA synthesis and quantitative PCR were conducted, adopting a previously reported method (Esposito et al., 2014). All analyses were performed at least three times.
3. Statistical analysis
Statistical analyses and correlation analyses were performed using the software Prism 6.0 (GraphPad Software, San Diego, CA, USA). The results are expressed as means ± SEM. Data were analyzed by one-way ANOVA with treatment as a factor. Post hoc analyses of differences between individual experimental groups using Tukey multiple comparison tests, post hoc analyses of differences between LPS and tested groups were made using the Dunnett’s multiple comparison tests. Principal component analysis (PCA) was carried out on the quantitative data obtained by the LC-MS analysis for the phenolic compounds. The dataset was organized in a matrix with seven lines corresponding to the blueberry genotypes and 37 columns corresponding to the conentrations of phenolic compounds, and the PCA calculation was performed using the software SPSS 24 (SPSS Inc, Chicago, IL, USA).
4. Results and discussion
Six pink-fruited blueberry clones, PLE, PCH, FLR, US 2117, US 2211, US 2235, and a uniform composite sample of lowbush wild blueberry were investigated in this study. The information about the genotypes, pedigree, origin, and approximate color are listed in Table S1. The dry matter content ranged from 15 to 20.7% (Table 1).
Table 1.
Polyphenol content, anthocyanins and antioxidant capacities of blueberries
| Blueberry genotype | Dry matter % | Total phenolics1 | Total anthocyanins2 |
Total proanthocyanidins3 | ABTS4 | FRAP5 | |
|---|---|---|---|---|---|---|---|
| HPLC-UV | LC-MS | ||||||
| Pink Lemonade (PLE) | 19.9 | 15.0 ± 0.35b | 0.80 ± 0.06d | 1.00 | 3.51 ± 0.04b | 50.3 ± 2.7bc | 308 ± 13.5bc |
| Pink Champagne (PCH) | 20.7 | 8.43 ± 0.16e | 1.17 ± 0.01c | 1.34 | 0.87 ± 0.005d | 39.6 ± 1.9cd | 217 ± 9.5d |
| Florida Rose (FLR) | 17.0 | 14.1 ± 0.18b | 0.27 ± 0.00e | 0.35 | 3.92 ± 0.10a | 51.9 ± 1.5bc | 347 ± 12.9ab |
| US 2117 | 19.8 | 11.0 ± 0.43d | 0.83 ± 0.02d | 1.20 | 1.13 ± 0.01c | 58.5 ± 2.1b | 276 ± 9.4c |
| US 2211 | 18.5 | 7.53 ± 0.19e | 0.20 ± 0.02f | 0.17 | 0.73 ± 0.005d | 32.5 ± 1.7d | 149 ± 4.8e |
| US 2235 | 15.3 | 12.3 ± 0.22c | 2.12 ± 0.07b | 3.32 | 0.76 ± 0.02d | 45.9 ± 5.2c | 299 ± 9.4bc |
| Lowbush | 15.0 | 24.5 ± 0.69a | 12.6 ± 0.15a | 14.5 | 3.47 ± 0.06b | 127 ± 5.3a | 389 ± 19.4a |
Total phenolics quantified by Folin Ciocalteu assay as mg gallic acid equivalent/g
total anthocyanins measured by HPLC quantified as mg cyanidin-3-glucoside equivalent/g, LC-MS quantification (mg/g) refers to Table 3
total proanthocyanidins quantified by DMAC assay as mg procyanidin B2 equivalent/g
radical scavenging activity by ABTS as µmol Trolox equivalent/g
antioxidant capacity by FRAP assay as µmol FeSO4 equivalent/g. Results were expressed as mean ± SD (n=3). All concentrations were calculated based on dry weight. Means with different superscript letters within the same column are significantly different (p<0.05).
4.1. Total phenolics, anthocyanins, and proanthocyanidins
All blueberry genotypes were analyzed for their content of phenolic constituents, and final concentrations were expressed as mg/g dry weight (DW). Total phenolics (TP) measured using Folin Ciocalteu assay and expressed as gallic acid equivalent showed that lowbush blueberries had 24.5 ± 0.69 mg/g. The pink-fruited blueberry samples contained TP concentrations that varied from 7.53 ± 0.19 mg/g for US 2211 to 15.0 ± 0.35 mg/g for PLE. Total anthocyanin content, based on measurements of HPLC peak areas recorded at 520 nm against a cyanidin-3-glucoside standard curve, indicated that anthocyanin levels ranged from 0.20 ± 0.02 mg/g for US 2211 to 2.12 ± 0.07 mg/g for US 2235 for pink-fruited blueberries. Lowbush blueberry had 12.6 ± 0.15 mg/g anthocyanin (Table 1). Total proanthocyanidins, measured by DMAC spectrophotometric assay and quantified as procyanidin B2 equivalent, indicated that FLR contained the highest proanthocyanidin concentration (3.92 ± 0.10 mg/g). PLE (3.51 ± 0.04 mg/g) and lowbush blueberry (3.47 ± 0.06 mg/g) were significantly lower than FLR. Other blueberries contained proanthocyanidin concentrations ≤ 1.13 mg/g (Table 1). There was a high correlation between total phenolic content and total anthocyanin content at p ˂0.001, but no significant correlation was observed between total phenolic content and total proanthocyanidins.
4.2. HPLC profiles for anthocyanins and proanthocyanidins
The HPLC-DAD profile of lowbush blueberry showed 17 peaks corresponding to 22 anthocyanins, which represented the spectrum of mono-glycosidic and acylated conjugates of the anthocyanidins delphinidin, cyanidin, petunidin, peonidin, and malvidin. The lowbush blueberries have a relatively high anthocyanin content in contrast to the pink-fruited blueberry samples, thus afforded blueberries from both ends of the spectrum of anthocyanin content and flavonoid profile by which to validate this streamlined analytical protocol (see Fig. S1, Supplementary Material for HPLC-DAD profiles) (Wu & Prior, 2005).
Some of the pink-fruited samples (PLE and FLR) showed no detectable levels of acylated anthocyanin (30–37 min), while others showed traces. The monomeric anthocyanin peaks (#1–15) varied widely in relative intensities among the seven blueberry genotypes investigated.
Normal phase HPLC with fluorescence detection was able to separate proanthocyanidin components in the berry extracts according to their degree of polymerization (DP). FLR showed a large broad peak around 40 min indicating a high concentration of polymeric proanthocyanidins (DP>12). The monomeric and oligomeric proanthocyanidins (DP1-DP10) showed a similar profile in all investigated berries but differed significantly in intensities (see Fig. S2, Supplementary Material).
4.3. Antioxidant capacity
The ABTS method measures the ability of the antioxidant to quench ABTS•+ radical, while the FRAP assay measures the ability of the antioxidant to reduce the yellow ferric-TPTZ complex to the blue ferrous-TPTZ complex. Lately, these types of in vitro antioxidant assays received some criticism because they cannot be translated into effectiveness in vivo. However, they can still be used for screening measurements and comparison purposes, as being used here (Granato et al., 2018). Results showed that lowbush blueberry had the highest antioxidant capacity level, as measured by ABTS radical scavenging activity, and FRAP reducing power (127 ± 5.3 µmol Trolox equivalent/g and 389 ± 19.4 FeSO4 µmol equivalent/g, respectively). All of the other blueberry genotypes showed lower ranges of antioxidant activities, 32.5 – 58.5 µmol/g for ABTS, and 149 – 347 µmol/g for FRAP (Table 1). The relative antioxidant capacity measured by ABTS assay strongly correlated with total phenolic contents (r=0.965, p <0.001) and total anthocyanins (r=0.962 for both HPLC-UV and LC-MS, p <0.001, while antioxidant capacities measured by FRAP assay significantly correlated with total proanthocyanidins (r=0.764, p<0.05).
4.4. Qualitative and quantitative LC-MS analysis
4.4.1. Optimization of chromatographic and mass spectrometric parameters
To achieve the best separation and strong ion signals, the chromatographic conditions, including mobile phase, column temperature and flow rate were optimized. Different linear gradients of mobile phase A (water with formic acid (0.1–0.5%) and mobile phase B (methanol with formic acid (0 or 0.5%), different column temperatures (30, 40, 50 ºC), and different flow rates (0.35, 0.45, and 0.6 mL/min) of solvent gradient were compared (data not shown). The chromatographic conditions which led to the best base-line separation of closely related anthocyanins structures were water with 0.5% formic acid (A) and methanol (B) as the eluents, with a flow rate of 0.6 mL/min, and a column temperature of 50 ºC. Due to the complexity of the blueberry extracts, a detailed gradient program was employed (Section 2.6). Typical separation showing the extracted ion chromatograms (EIC) of standard reference mixture and the lowbush blueberry sample is shown in Fig.1.
Fig. 1.
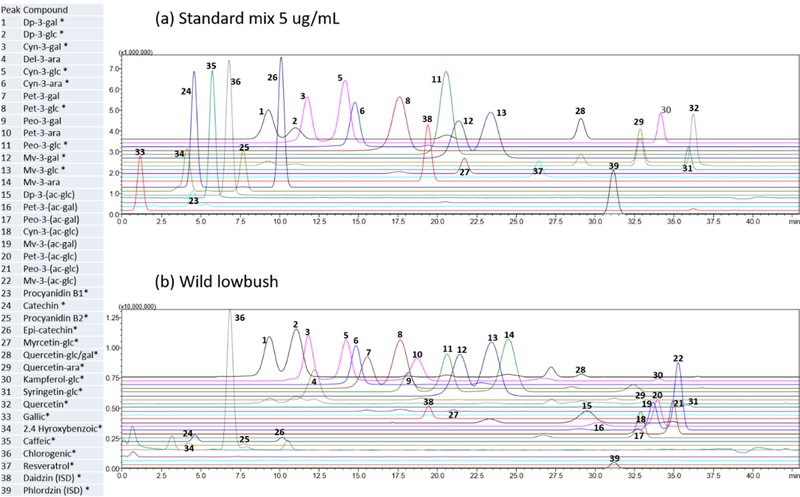
The extracted ion chromatograms (EIC) of phenolic metabolites by LC-IT-TOF-MS showing a mixture of 24 standard references (a), and of all compounds quantified in lowbush wild blueberry (b).
4.4.2. Characterization of phenolic constituents by LC-IT-TOF-MS
In this study, some anthocyanin compounds were identified by comparison with reference standards, and others were tentatively characterized based on their retention times, UV/Vis, MS and MS/MS spectra referring to the literature (Gavrilova et al., 2011; Wu & Prior, 2005), and to our previous work (Grace et al., 2014). 37 phenolic compounds including 22 anthocyanins, four flavan-3-ols, six flavonols, four phenolic acids, and resveratrol were identified in the blueberry samples. The identification data are shown in Table S2 in the Supplementary Material.
4.4.3. Quantitative analysis of phenolic components by LC-IT-TOF-MS
This method aimed to simultaneously quantify several classes of phenolic constituents with a wide range of concentrations in different blueberry genotypes. HPLC-UV analysis has been the common method for quantification of anthocyanins by measuring peak areas recorded at 520 nm against a standard curve constructed with a single anthocyanin reference standard (Grace et al., 2014; Lohachoompol, Mulholland, Srzednicki, & Craske, 2008; Yousef et al., 2013). At this wavelength, anthocyanins can be selectively detected in the presence of other flavonoids, which have a maximum absorbance at characteristic wavelengths other than 520 nm. The majority of the HPLC methods involve the use of high percentages of acids (1.0 to 15% v/v) as mobile phases to maintain a low pH (≤ 2) as a requirement for maintaining the stability of anthocyanins in solution in the form of flavylium cations (Merken & Beecher, 2000). HPLC-UV methods, however, require a long run time and consequently large volumes of solvent to achieve optimal resolution and to avoid co-elution of peaks. Also, they have limited sensitivity and are inadequate for quantification of flavonoids at low concentrations. Analytical techniques utilizing HPLC-MS have been used extensively for qualitative or semi-quantitative analyses providing information on identification of anthocyanins. There are some reports on the application of LC-tandem mass spectrometry for quantification of anthocyanin in food products, plant extracts and biological specimens (Ling et al., 2009; Lohachoompol et al., 2008; Mullen, Larcombe, Arnold, Welchman, & Crozier, 2010; Tian, Aziz, Stoner, & Schwartz, 2005; Wang, Kalt, & Sporns, 2000). Mullen et al. used a full scan high-resolution mass spectrometry with an Orbitrap analyzer for quantification of anthocyanin (as their precursor ions) in berries and berry-fed greenfinch brain tissue. Their results indicated that the Orbitrap analyzer had ca. 200-fold more sensitivity than traditional tandem MS in selected reaction monitoring (SRM) mode, and enabled both targeted and non-targeted compounds to be detected at much lower detection limits than HPLC-tandem mass. The reason for this is that anthocyanins ionize approximately 10–100 times more efficient compared to their aglycone counterparts due to the presence of the sugar moieties. This phenomenon was quantitatively reflected by lower limits of detection, quantification, and lower calibrated range for glycosylated analytes compared to their aglycones (Mullen et al., 2010).
Few previous studies utilized LC-MS for the quantification of anthocyanins alongside other classes of polyphenols. Quantitation of 12 anthocyanins (glucosides or acyl-glucosides), four flavan-3-ols, and eight flavonols in grape berries was performed previously utilizing the positive full scan MS mode, while MS/MS was used for identification purposes (Cavaliere et al., 2008). The first validated methodology describing tandem mass spectrometry-based quantification of anthocyanins and flavonols in milk-based food products was in 2009. In this study, researchers could quantify 27 compounds, including anthocyanin and flavonol isomeric compounds, by UPLC-MS/MS in the positive ion mode (Nagy et al., 2009).
The use of ion trap- top of flight mass spectrometer for quantification purposes based on EIC of their precursor ion proved to be successful and very sensitive for quantification and quality control of plant metabolites, which is attributable principally to the increased number of ions that the trap can hold. Examples include determinations of anthocyanin in commercial red and blue fruit juices (Fanali et al., 2011), phenolic acids and flavonoids in Apocynum ventum (An, Wang, Lan, Hashi, & Chen, 2013), multiple constituents in Chinese medicine (Liu et al., 2016), and monoterpenoids in peony root (Shi et al., 2016).
The method developed here used IT-TOF-MS for quantification of phenolics as their precursor ions, [M]+ for anthocyanin, [M+H]+ for flavonols, [M-H]− for flavan-3-ols, phenolic acids and resveratrol. Separation was performed using relatively low volumes of formic acid (0.5%) which was not only enough to maintain the optimum pH (2.12) for anthocyanin stability and separation, but it was also more gentle on the instrumentation for long-term use.
4.4.4. Method validation
The specificity, linear range, and sensitivity, precision, stability, reproducibility, and accuracy of the developed method were validated according to the recent FDA guidance document (US Food and Drug Administration, 2015). The specificity of the method showed a high resolution of the extracted ion chromatograms for reference standards, and in the lowbush blueberry sample as shown in Fig. 1. All calibration curves had good linearity with the regression coefficient (r2) ≥ 0.99 within the test ranges. The limits of detection (LOD) and quantitation (LOQ) of the reference compounds were determined (Table 2). Results indicated good precision for all analytes with the relative standard deviation (RSD) in intra- and inter-day of less than 10%, respectively. The reproducibility of all analytes was ˂ 5% (Table 2). These method validation results indicated that the newly developed LC-IT-TOF-MS method was acceptable for quantitative analysis of blueberry genotypes even when the selections featured considerable variability in flavonoid profiles and concentrations.
4.5. Method application for quantification of phenolics in blueberries
Sample solutions of six pink-fruited blueberry clones and lowbush blueberry were run using the LC-IT-TOF-MS validated method for simultaneous quantification of 22 anthocyanins, four flavan-3-ols, six flavonols, and four phenolic acids and resveratrol. The results of this quantification were listed in Table 3.
Table 3.
Quantitative analytical results of anthocyanins and other phenolic metabolites in six-pink fruited blueberries and lowbush blueberry by LC-MS (µg/g DW)
| Phenolic compound | Pink-Lemonade (PLE) | Pink-Champagne (PCH) | Florida Rose (FLR) | US 2117 | US 2211 | US 2235 | Lowbush | |
|---|---|---|---|---|---|---|---|---|
| Anthocyanins | ||||||||
| 1 | Dp-3-gal* | 265.1±0.9 | 163.3±0.7 | 63.6±3.1 | 194.1±3.0 | 28.4±0.5 | 223.8±6.5 | 1017±3.2 |
| 2 | Dp-3-glc* | 52.3±2.5 | 182.2±3.7 | 48.6±2.9 | 28.5±0.2 | (BQL) | 465.9±12.8 | 3134±3.6 |
| 3 | Cyn-3-gal* | 133.5±2.9 | 60.8±1.1 | 44.6±1.2 | 67.6±0.2 | (BQL) | 75.7±5.3 | 858.2±20.4 |
| 4 | Dp-3-ara | 418.7±12.5 | 381.5±10.7 | 120.8±4.3 | 397.2±0.4 | 73.7±2.5 | 458.1±5.1 | 1266±31.5 |
| 5 | Cyn-3-glc* | (BQL) | 21.3±1.5 | (BQL) | (BQL) | ----- | 33.2±1.8 | 476.0±3.8 |
| 6 | Cyn-3-ara* | 129.7±6.7 | 74.9±0.1 | 36.7±2.3 | 65.9±0.6 | (BQL) | 978.0±3.5 | 648.8±13.0 |
| 7 | Pet-3-gal | (BQL) | (BQL) | (BQL) | 88.7±0.2 | (BQL) | 109.7±0.6 | 415.7±1.6 |
| 8 | Pet-3-glc* | (BQL) | 55.8±0.6 | (BQL) | (BQL) | … | 151.4±6.1 | 820.2±6.7 |
| 9 | Peo-3-gal | (BQL) | (BQL) | (BQL) | (BQL) | (BQL) | (BQL) | 136.1±1.6 |
| 10 | Pet-3-ara | (BQL) | (BQL) | (BQL) | 92.7±0.1 | (BQL) | 136.8±1.2 | 332.3±3.0 |
| 11 | Peo-3-glc* | ----- | (BQL) | (BQL) | ----- | ----- | 29.9±1.3 | 330.3±4.0 |
| 12 | Mv-3-gal* | (BQL) | 135.7±1.1 | 13.1± | 117.9±0.7 | 67.6±3.6 | 228.8±9.1 | 1030±21.4 |
| 13 | Mv-3-glc* | ----- | 54.7±0.0 | (BQL) | (BQL) | ----- | 179.6±1.1 | 1028±26.4 |
| 14 | Mv-3-ara | (BQL) | 208.7±1.6 | (BQL) | 142.0±0.5 | (BQL) | 252.5±0.9 | 895.1±4.1 |
| 15 | Dp-3-(ac-glc) | ----- | (BQL) | ----- | ----- | ----- | (BQL) | 772.5±9.8 |
| 16 | Pet-3-(ac-gal) | ----- | ----- | ----- | ----- | ----- | ----- | (BQL) |
| 17 | Peo-3-(ac-gal) | ----- | ----- | ----- | ----- | ----- | ----- | (BQL) |
| 18 | Cyn-3-(ac-glc) | ----- | (BQL) | ----- | ----- | ----- | (BQL) | 242.0±0.7 |
| 19 | Mv-3-(ac-gal) | ----- | (BQL) | ----- | (BQL) | (BQL) | ----- | 268.7±4.4 |
| 20 | Pet-3-(ac-glc) | ----- | (BQL) | ----- | ----- | ----- | (BQL) | 268.1±5.4 |
| 21 | Peo-3-(ac-glc) | ----- | (BQL) | ----- | ----- | ----- | .. | (BQL) |
| 22 | Mv-3-(ac-glc) | ----- | (BQL) | ----- | (BQL) | ----- | (BQL) | 589.6±23.6 |
| Total | 999.3 | 1339 | 327.4 | 1195 | 169.7 | 3323 | 14529 | |
| Flavan-3-ols | ||||||||
| 23 | Procyanidin B1* | 207.2±5.2 | 82.6±3.3 | 125.2±7.0 | 177.9±1.5 | 102.2±1.9 | 98.2±3.5 | 125.1±2.5 |
| 24 | Catechin* | 259.9±5.4 | 131.1±7.1 | 202.9±1.9 | 203.9±6.3 | 179.1±3.7 | 139.3±0.7 | 117.5±3.4 |
| 25 | Procyanidin B2* | 65.9±3.3 | 54.3±1.3 | 98.7±4.5 | 51.0±0.5 | 30.0±1.0 | 25.1±0.0 | 72.0±4.1 |
| 26 | Epi-catechin* | 55.6±2.1 | 53.5±4.8 | 109.9±1.2 | 57.1±1.5 | 40.3±0.1 | 26.0±0.9 | 74.6±0.5 |
| Total | 588.6 | 321.5 | 536.7 | 489.9 | 351.6 | 288.6 | 389.2 | |
| Flavonols | ||||||||
| 27 | Myrcetin-glc* | 121.8±4.8 | 71.9±0.5 | 65.2±4.1 | 172.2±1.7 | 29.1±2.5 | 26.5±0.2 | 130.5±4.5 |
| 28 | Quercetin-glc/gal* | 27.1±0.1 | 74.0±1.5 | 15.9±1.6 | 248.9±2.0 | 75.4±2.7 | 107.1±2.0 | 189.8±5.5 |
| 29 | Quercetin-ara* | 140.4±3.3 | ----- | 82.6±1.2 | 167.4±0.3 | 60.2±0.7 | 275.4±16.9 | 53.3±1.0 |
| 30 | Kaempferol-glc* | ----- | 441.0±28.0 | 271.6±7.4 | 79.5±0.4 | 475.3±38.1 | ---- | |
| 31 | Syringetin-glc* | ----- | 10.0±0.8 | 10.9±0.5 | 5.65±0.3 | 7.9±0.5 | 4.17±0.2 | 181.8±2.4 |
| 32 | Quercetin* | 15.4±0.2 | 5.6±0.0 | 7.52±0.3 | 13.1±0.1 | 7.8±0.5 | 5.72±0.3 | 12.2±0.4 |
| Total | 547.2 | 161.5 | 623.12 | 878.85 | 259.9 | 894.19 | 567.6 | |
| Phenolic acids | ||||||||
| 33 | Gallic* | 5.4±0.3 | 3.3±0.1 | 2.80±0.3 | 2.60±0.1 | 0.05±0.0 | 1.47±0.2 | 3.51±0.5 |
| 34 | 2.4 Hyroxybenzoic* | 66.3±9.8 | 36.7±2.1 | 62.6±4.1 | 50.7±6.0 | 52.2±1.9 | 39.4±1.5 | 72.7±9.7 |
| 35 | Caffeic* | 35.8±5.0 | ----- | 29.0±2.7 | ----- | 27.1±1.5 | ----- | 23.7±4.1 |
| 36 | Chlorogenic* | 3311±34.9 | 1492±24.8 | 2698±28.8 | 2586±41.6 | 2088±26.0 | 3020±32.8 | 4150±78.7 |
| 37 | Resveratrol* | 0.4±0.0 | 0.1±0.0 | 0.47±0.2 | 0.33±0.1 | 0.12±0.0 | 0.68±0.0 | 1.15±0.4 |
| Total phenolics | 5554 | 3354 | 4280 | 5203 | 2949 | 7568 | 19737 |
Reference standards available. Dp = delphinidin; Cyn = cyanidin; Pet = petunidin; Peo = peonidin; Mv = malvidin; gal = galactoside; glc = glucoside; ara = arabinoside; Anthocyanin compounds, with no reference standard available, were quantified as the closest anthocyanin compound having the same aglycone. BQL= detected but below the quantification limit
The anthocyanin content in pink-fruited blueberries was relatively low compared to the dark blue colored lowbush blueberry. The anthocyanin content, as a sum of individual components, was 3323 (44% of the sum of phenolic component concentrations), 1339 (40%), 1195 (23%), 999.3 (18%), 327.4 (8%) and 169.7 µg/g (6%) for US 2235, PCH, US 2117, PLE, FLR, and US 2211, respectively, and 14529 µg/g (74%) for the lowbush blueberry (Table 3). These values were very well correlated (r=1, p<0.001) with results obtained by HPLC-UV measured by a calibration curve created with cyanidin-3-glucoside. The specificity of the LC-MS in selecting the ions in both positive and negative modes makes this method superior to HPLC-UV in quantifying very closely eluted or overlapping compounds. On the other hand, the comparison between the results from each technique indicated that the HPLC-UV is satisfactory for routine analysis of anthocyanins.
Four flavan-3-ol compounds were quantified in the blueberry samples. Catechin levels were higher than epicatechin in all samples (1.6 to 5.4 fold). Similarly, procyanidin B1 dimer levels exceeded the B2 procyanidin dimer in all samples (1.3 to 3.9 fold). The sum of monomeric and the sum of dimeric procyanidin was in agreement with the published data for lowbush blueberry (Gu et al., 2004). The sum of quantified flavan-3-ol showed its highest level in PLE (589 µg/g, 11%), and the lowest was in US 2235 (289 µg/g, 4%).
Six flavonol compounds were quantified in this study, quercetin and its 3-O-glucoside + galactoside and arabinoside, and the 3-O-glucosides of myricetin, kaempferol, and syringetin. Quercetin-galactoside and –glucoside eluted as one peak and were quantified together as the quercetin glucoside equivalent. The tested blueberries showed variation in the concentration of individual flavonols. Kaempferol-glucoside was not detected in PCH and lowbush blueberry. Syringetin-glucoside was not detected in PLE. Quercetin arabinoside was absent in PCH. The sum of the quantified flavonols showed highest levels in US 2235 and US 2117 (894 and 879 µg/g, respectively). FLR, PLE, and lowbush blueberry showed comparable levels of flavonols (623, 547 and 568 µg/g, respectively). PCH contained the lowest concentration of total flavonols (162 µg/g).
Chlorogenic acid represented the primary phenolic acid in all blueberries. Lowbush blueberry showed the highest level of this acid (4150 µg/g) that represented 21% of the sum of all phenolic compounds. Pink-fruited blueberries showed relatively lower concentration: 3311 µg/g (PLE), 3020 µg/g (US 2235), 2698 µg/g (FLR), 2586 µg/g (US 2117), 2088 µg/g (US 2211), and 1492 µg/g (PCH), representing 60, 40, 63, 50, 71, and 60%, respectively, of total phenolic compounds. Caffeic acid was present at comparable levels (27.1–35.8 µg/g) in PLE, FLR, US 2211, and lowbush blueberry, but was not detected in PCH, US 2235 and US 2117. 2,4-Dihydroxybenzoic acid showed a concertation range from 52.2 µg/g for US 2211 to 66.3 µg/g for PLE. Traces of gallic acid were detected in all samples. Traces of resveratrol were also detected in all samples, but at lower levels than the quantification limit.
Principle component analysis (PCA) was applied to evaluate the variability of phenolic components among the seven blueberry genotypes (Granato, Karnopp, & van Ruth, 2015). The scores and loading plots are shown in Fig 2. PCA showed separation in the scores plot between lowbush blueberry and pink-fruited blueberries in both PC1 and PC2. The main dominant features of PC1 are anthocyanins, syringetin-glc, resveratrol, and chlorogenic acid contents, while PC2 is primarily dominated by the flavan-3-ols, flavonols, and phenolic acids contents. PLE and US 2117 were differentiated from other pink-fruited blueberries in PC2.
Fig. 2.
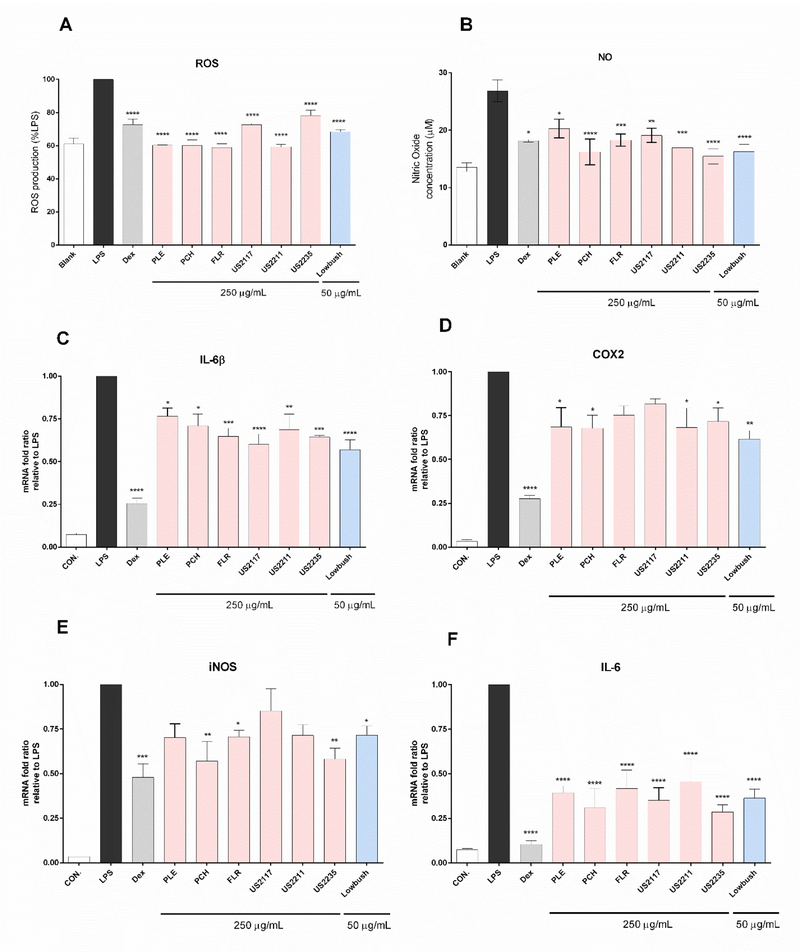
Principle Component Analysis (PCA), score plot (a) and loading plot (b) applied to blueberry genotypes using 2D projection employing the contents of phenolic compounds as variables. PC1 dominant features are anthocyanins, syringetin-glc, resveratrol, and chlorogenic acid, and PC2 dominated by flavan-3-ols, flavonols, and phenolic acid contents.
4.6. In vitro antioxidant and anti-inflammatory activities
4.6.1. Effect of blueberry extracts on ROS and NO production
Blueberry anthocyanins have been linked to lowered risk of various diseases based on antioxidant and anti-inflammatory mechanisms (Huang et al., 2018). During the inflammatory reaction, effectively controlling the cellular nitric oxide (NO) production and reactive oxygen species (ROS) levels are the essential tools for inhibiting LPS induced macrophage hyper-activation and macrophage-mediated acute inflammation responses (Esposito et al., 2014). To gauge the intracellular antioxidant and inflammatory activities, blueberry extracts were incubated with LPS-activated Raw 264.7 macrophages. When LPS was administered to macrophages, nitric oxide (NO) and reactive oxygen species (ROS) production levels almost evoked a nearly 2.0-fold induction versus the naive control (Fig. 3). When testing the seven blueberry genotypes at 50 µg/mL, only lowbush blueberry inhibited the production of ROS and NO (data not shown). Therefore, a concentration of 250 µg/mL was executed to allow detection of the activity of pink-fruited blueberries. Results showed that all pink-fruited berry extracts significantly reduced the generation of reactive ROS (p<0.001) and NO production (p<0.05) with US 2211, FLR, PCH, and PLE extracts reducing the ROS levels back to non-stimulated levels at concentration 250 μg/mL. At a concentration of 50 μg/mL, ROS and NO induction were inhibited by lowbush blueberry, to baseline levels (p<0.001) (Fig. 3). Phytochemical analysis of the blueberry genotypes (Table 1 and Table 3) indicated that lowbush blueberry has between 4- to 85-fold higher anthocyanin than the pink-fruited berries, which was not the case for the concentrations of other phenolic components. The results obtained here prove that anthocyanins play a significant role in inhibiting the production of ROS and NO in LPS induced Raw 264.7 macrophages.
Fig. 3.
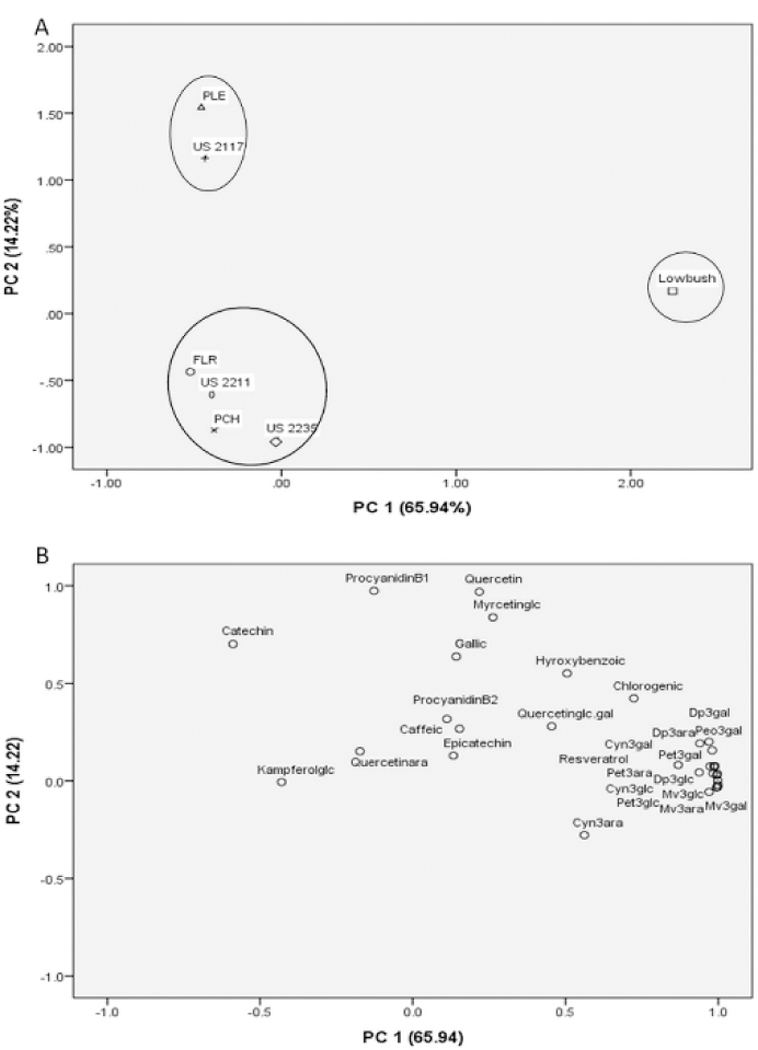
Effect of blueberry extracts on reactive oxygen species (ROS) and nitric oxide (NO) production (A and B), and on proinflammatory gene expression (C-F) in the LPS-stimulated RAW264.7 macrophage cells. Cells were treated with pink blueberry extracts (250 µg/mL) and lowbush blueberry (50 µg/mL) for 1 h and stimulated with lipopolysaccharide (LPS, one μg/mL), then incubated for 18 h for ROS & NO, incubated 4 h for gene expression, respectivly. Changes in gene expression were measured by comparing mRNA quantity relative to LPS. Vehicle values were obtained in the absence of LPS or test samples; dexamethasone (DEX) was used as positive control at a concentration of 10 μM. Results were expressed as means ± SEM, n = 2 experiments. *p < 0.05, **p < 0.01, ***p < 0.001 ****p < 0.0001 vs. the LPS treated group. One-way ANOVA, Dunnett’s post hoc test. PLE = Pink Lemonade; PCH = Pink Champagne; FLR = Florida Rose.
Examination of cytotoxicity of the extracts on macrophages by the MTT assay indicated that up to 250 μg/mL, none of the tested samples affected the viability of RAW 264.7 (data not shown)Therefore, the inhibitory effect of the blueberry extracts on the LPS induced oxidative stress (via ROS or NO) was not a result of cytotoxicity.
Blueberries are an excellent resource of anthocyanins, which can protect cells from oxidative deterioration. Malvidin-3-glucoside (Mv-3-glc) and malvidin-3-galactoside (Mv-3-gal), which are the major anthocyanins in Brightwell rabbiteye blueberry, decreased ROS level in endothelial cells at the concentration of 1 μg/mL, and variation in levels of Mv-3-glc and Mv-3-gal had a significant impact on antioxidant properties (Huang, Zhu, Li, Sui, & Min, 2016). In another study, researchers indicated that exposing human retinal capillary endothelial cells (HRCECs) to high glucose concentration for 24 h significantly decreased cell viability in comparison with normal glucose incubated cells, but, 10 μg/mL of blueberry anthocyanins, malvidin, and its galactoside or glucoside all significantly increased cell viability after 24 h of high glucose incubation. Also, their data indicated significantly reduced the levels of ROS, showing an effective attenuation of high glucose-induced oxidative damage in HRCECs (Huang et al., 2018). When blueberry extracts were tested on HepG2 human cancer cells as a model, they showed high intracellular antioxidant, and antiproliferative activities (Wang et al., 2017b).
4.6.2. Effect of blueberry extracts on inflammatory markers
Exposure of mammalian cells to LPS leads to release of pro-inflammatory cytokines and in turn activates inflammatory cascades including cytokines, lipid mediators and adhesion molecules such as interleukin-1β (IL-1β), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and interleukin-6 (IL-6), which are common genetic biomarkers involved in the LPS-stimulated murine RAW 264.7 macrophage model inflammatory response (Esposito et al., 2014).
Pink-fruited berry extracts, at a concentration of 250 μg/mL, effectively suppressed IL-1β and IL-6 (p<0.05) genes by inhibiting their expression, especially for IL-6 genes (p<0.001) based on ≥ 50% suppression relative to the LPS-stimulated controls. Among pink-fruited blueberry extracts, only PCH and US 2235 showed inhibitory effects on the expression of all genes. The latter two blueberry genotypes contained the highest levels of anthocyanin among pink-fruited clones (Table 1 and 3), indicating that anthocyanins mainly contribute to the inhibition of iNOS and IL-6. Meanwhile, the lowbush blueberry extract was able to suppress all the four tested pro-inflammatory markers significantly, at a much lower concentration of only 50 μg/mL (Fig. 3). These results are in accord with our previous work, where the blueberry anthocyanin fraction showed higher anti-inflammatory activity than crude extract, polyphenol-rich fraction, proanthocyanidin-rich fraction and ethyl acetate extract (Esposito et al., 2014). The anti-inflammatory mechanism of blueberry in LPS-stimulated RAW 264.7 macrophages was hypothesized to be due to suppression of oxidative stress and reduced expression of proinflammatory cytokines (Watson & Preedy, 2014). In addition to the above mentioned pro-inflammatory markers, blueberry extracts significantly inhibited the gene expression of TNF-a and IL-12 in mononuclear macrophage model, and MMP-2, MMP-9 in human corneal epithelial cells (Cheng et al., 2014; Li et al., 2016; Wang et al., 2017a).
Conclusion
In the present study, a validated HPLC-IT-TOF/MS method was employed for the rapid simultaneous quantitative determination of multiple classes of phenolic compounds in blueberry samples. Thirty-seven phenolic compounds were successfully separated, identified and quantified including anthocyanins, flavan-3-ols, flavonols, phenolic acids, and resveratrol. The method enabled efficient separation of anthocyanins isomers, unique selectivity based on ESI mass ions, and sensitivity for quantifying 37 analytes belonging to 5 classes of phenolics at a wide range of concentrations. Furthermore, the method was applied successfully for the investigation of six pink-fruited blueberry clones for the first time. In vitro cell bioassays for antioxidant and anti-inflammatory activities indicated that the anthocyanin group of phenolics are primarily responsible for the bioactivity. The in vitro antioxidant assay results were strongly correlated with total phenolics and total anthocyanin contents. In vitro cell-based antioxidant assays indicated that the six pink-fruited berries at concentration of 250 μg/mL, and lowbush blueberry at a concentration 50 μg/mL, were able to reduce the production ROS and NO, and inhibit the transcription of the inflammatory cytokines IL-6β, COX2, iNOS, and IL-6.
Supplementary Material
Highlights.
Validated LC-MS method was developed for quantification of 37 phenolic compounds
The method was used to study six blueberry genotypes with divergent profiles
Anthocyanins made the highest contribution to the antioxidant activities
Anthocyanins were also responsible for anti-inflammatory activity
PCA was conducted to evaluate compositional variation between blueberry genotypes
Acknowledgments
We are grateful to Charles Warlick and Lorie Beale for their excellent technical work. This research was supported by the National Institutes of Health (NIH) project (1 RO1 AT008754-01) of the National Institute for Complementary and Integrative Health (NICIH), and the Foundation for Food and Agriculture Research (FFAR) under award number 534667. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of FFAR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An H, Wang H, Lan Y, Hashi Y, & Chen S (2013). Simultaneous qualitative and quantitative analysis of phenolic acids and flavonoids for the quality control of apocynum venetum L. leaves by HPLC-DAD-ESI-IT-TOF-MS and HPLC-DAD. [] Journal of Pharmaceutical and Biomedical Analysis, 85, 295–304. [DOI] [PubMed] [Google Scholar]
- Barrajón-Catalán E, Herranz-López M, Joven J, Segura-Carretero A, Alonso-Villaverde C, Menéndez JA, & Micol V (2014). Molecular promiscuity of plant polyphenols in the management of age-related diseases: Far beyond their antioxidant properties. In Camps J (Ed.), Oxidative stress and inflammation in non-communicable diseases - molecular mechanisms and perspectives in therapeutics. advances in experimental medicine and biology , vol 824 (pp. 141–159) Springer, Cham. [DOI] [PubMed] [Google Scholar]
- Cavaliere C, Foglia P, Gubbiotti R, Sacchetti P, Samperi R, & Lagana A (2008). Rapid-resolution liquid chromatography/mass spectrometry for determination and quantitation of polyphenols in grape berries. Rapid Communications in Mass Spectrometry, 22, 3089–3099. [DOI] [PubMed] [Google Scholar]
- Cheng A, Yan H, Han C, Wang W, Tian Y, & Chen X (2014). Polyphenols from blueberries modulate inflammation cytokines in LPS-induced RAW264.7 macrophages. . International Journal of Biological Macromolecules, 69, 382–387. [DOI] [PubMed] [Google Scholar]
- Choi SY, Ko HC, Ko SY, Hwang JH, Park JG, Kang SH, … Kim SJ (2007). Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biological and Pharmaceutical Bulletin, 30, 772–778. [DOI] [PubMed] [Google Scholar]
- Esposito D, Chen A, Grace M, Komarnytsky S, & Lila M (2014). Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. Journal of Agricultural and Food Chemistry, 62, 7022–7028. [DOI] [PubMed] [Google Scholar]
- Fanali C, Dugo L, D’Orazio G, Lirangi M, Dacha M, Dugo P, & Mondello L (2011). Analysis of anthocyanins in commercial fruit juices by using nano-liquid chromatography-electrospray-mass spectrometry and high-performance liquid chromatography with UV-vis detector. Journal of Separation Science, 34, 150–159. [DOI] [PubMed] [Google Scholar]
- Gavrilova V, Kajdzanoska M, Gjamovski V, & Stefova M (2011). Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. Journal of Agriculture and Food Chemistry, 59, 4009–4018. [DOI] [PubMed] [Google Scholar]
- Grace M, Esposito D, Dunlap K, & Lila M (2014). Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild alaskan and commercial vaccinium berries. Journal of Agriculture and Food Chemistry, 62, 4007–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato D, Karnopp AR, & van Ruth SM (2015). Characterization and comparison of phenolic composition, antioxidant capacity and instrumental taste profile of juices from different botanical origins. Journal of the Science of Food and Agriculture, 95(10), 1997–2006. [DOI] [PubMed] [Google Scholar]
- Granato D, Shahidi F, Wrolstad R, Kilmartin P, Melton LD, Hidalgo FJ, Finglas P (2018). Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chemistry, 264, 471–475. [DOI] [PubMed] [Google Scholar]
- Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Prior RL (2004). Concentrations of proanthocyanidins in common foods and estimations of normal consumption. Journal of Nutrition, 134, 613–617. [DOI] [PubMed] [Google Scholar]
- Huang W, Yan Z, Li D, Ma Y, Zhou J, & Sui Z (2018). Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxidative Medicine and Cellular Longevity, 2018 [DOI] [PMC free article] [PubMed]
- Huang W, Zhu Y, Li C, Sui Z, & Min W (2016). Effect of blueberry anthocyanins malvidin and glycosides on the antioxidant properties in endothelial cells. Oxidative Medicine and Cellular Longevity, 2016 [DOI] [PMC free article] [PubMed]
- Li J, Deng R, Hua X, Zhang L, Lu F, Coursey TG, & Li DQ (2016). Blueberry component pterostilbene protects corneal epithelial cells from inflammation via anti-oxidative pathway. Scientific Reports, 6, 19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Ren C, Mallery SR, Ugalde CM, Pei P, Saradhi UV, … Liu Z (2009). A rapid and sensitive LC-MS/MS method for quantification of four anthocyanins and its application in a clinical pharmacology study of a bioadhesive black raspberry gel. Journal of Chromatography B, 877, 4027– 4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ju A, Zhou D, Li D, Kou J, Yu B, & Qi J (2016). Simultaneous qualitative and quantitative analysis of multiple chemical constituents in YiQiFuMai injection by ultra-fast liquid chromatography coupled with ion trap time-of-flight mass spectrometry. Molecules, 21, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohachoompol V, Mulholland M, Srzednicki G, & Craske J (2008). Determination of anthocyanins in various cultivars of highbush and rabbiteye blueberries. Food Chemistry, 111, 249–254. [Google Scholar]
- Merken HM, & Beecher GR (2000). Measurement of food flavonoids by high-performance liquid chromatography: A review. Journal of Agricultural and Food Chemistry, 48, 577–599. [DOI] [PubMed] [Google Scholar]
- Mullen W, Larcombe S, Arnold K, Welchman H, & Crozier A (2010). Use of accurate mass full scan mass spectrometry for the analysis of anthocyanins in berries and berry-fed tissues. Journal of Agricultural and Food Chemistry, 58, 3910–3915. [DOI] [PubMed] [Google Scholar]
- Muriach M, Flores-Bellver M, Romero FJ, & Barcia JM (2014). Diabetes and the brain: Oxidative stress, inflammation, and autophagy. Oxidative Medicine and Cellular Longevity, 2014, 102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy K, Redeuil K, Bertholet R, Steiling H, & Kussmann M (2009). Quantification of anthocyanins and flavonols in milk-based food products by ultra performance liquid chromatography-tandem mass spectrometry. Analytical Chemistry, 81, 6347–6356. [DOI] [PubMed] [Google Scholar]
- Prior RL (2015). Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. Journal of Functional Foods, 18, 797–810. [Google Scholar]
- Prior RL, Lazarus SA, Cao G, Muccitelli H, & Hammerstone JF (2001). Identification of procyanidins and anthocyanins in blueberries and cranberries (vaccinium spp.) using high-performance liquid chromatography/mass spectrometry. Journal of Agricultural and Food Chemistry, 49, 1270–1276. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, & Rice-Evans C (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26, 1231–1237. [DOI] [PubMed] [Google Scholar]
- Shi YH, Zhu S, Ge YW, Toume K, Wang Z, Batkhuu J, & Komatsu K (2016).Characterization and quantification of monoterpenoids in different types of peony root and the related paeonia species by liquid chromatography coupled with ion trap and time-of-flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 129, 581–592. [DOI] [PubMed] [Google Scholar]
- Tian Q, Aziz RM, Stoner GD, & Schwartz SJ (2005). Anthocyanin determination in bBlack raspberry (rubus occidentalis) and biological specimens using liquid chromatography-electrospray ionization tandem mass spectrometry. Journal of Food Science, 70, C43–C47. [Google Scholar]
- US Food and Drug Administration. (2015). Analytical procedures and methods validation for drugs and biologics. Guidance for Industry,
- Wang H, Guo X, Liu J, Li T, Fu X, & Liu RH (2017a). Comparative suppression of NLRP3 inflammasome activation with LPS-induced inflammation by blueberry extracts (vaccinium spp.). RSC Advances, 7, 28931–28939. [Google Scholar]
- Wang H, Guo X, Hu X, Li T, Fu X, & Liu RH (2017b). Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (vaccinium spp.). Food Chemistry, 217, 773–781. [DOI] [PubMed] [Google Scholar]
- Wang J, Kalt W, & Sporns P (2000). Comparison between HPLC and MALDI-TOF MS analysis of anthocyanins in highbush blueberries. Journal of Agricultural and Food Chemistry, 48, 3330–3335. [DOI] [PubMed] [Google Scholar]
- Watson RR, & Preedy VR (2014). Bioactive nutraceuticals and dietary supplements in neurological and brain disease: Prevention and therapy Academic Press. [Google Scholar]
- Wu X, & Prior RL (2005). Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the united states: Fruits and berries. Journal of Agricultural and Food Chemistry, 53, 2589–2599. [DOI] [PubMed] [Google Scholar]
- Yousef GG, Brown AF, Funakoshi Y, Mbeunkui F, Grace MH, Ballington JR, Lila MA(2013). Efficient quantification of the health-relevant anthocyanin and phenolic acid profiles in commercial cultivars and breeding selections of blueberries (vaccinium spp.). Journal of Agricultural and Food Chemistry, 61, 4806–4815. [DOI] [PubMed] [Google Scholar]
- Yuliana ND, Khatib A, Choi YH, & Verpoorte R (2011). Metabolomics for bioactivity assessment of natural products. Phytotherapy Research, 25, 157–169. [DOI] [PubMed] [Google Scholar]
- Zhang H, & Tsao R (2016). Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Current Opinion in Food Science, 8, 33–42. [Google Scholar]
- Zheng W, & Wang SY (2003). Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. Journal of Agricultural and Food Chemistry, 51, 502– 509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


