Summary
Peripheral neuropathy is a well-recognized treatment-related toxicity among children with cancer and is associated with exposure to neurotoxic chemotherapy agents. Acute damage, may manifest in sensory, motor, and/or autonomic neurons with symptoms that are rarely life threatening, but often severe enough to interfere with function during therapy and after treatment ends. The type of neuropathy, and specific symptoms, are associated with multiple factors, including age at time of therapy, genetic predisposition, chemotherapy type, and cumulative dose, and exposure to other agents during therapy. The aims of this review are to describe the peripheral neuropathy phenotype, among both children during cancer therapy and among survivors, to summarize host/genetic and treatment related risk factors for neuropathy, and to outline strategies to monitor and detect neuropathy during and after therapy. In addition, strategies for medical management of neuropathy during treatment are discussed and potential rehabilitation interventions to prevent or remediate functional loss are proposed.
Introduction
Modern therapy has improved survival for children with cancer;1 however, treatment has unintended consequences. These toxicities may interfere both with optimal delivery of treatment as well as daily function and quality of life. Once thought to be transient, peripheral neuropathy is a well-recognized treatment-related toxicity among children with cancer and is associated with exposure to neurotoxic chemotherapy, including vinca alkaloids and platinum compounds. Acute neuropathy is reported among 20-60% of children with acute lymphoblastic leukemia (ALL),2,3 and up to 85% of children with lymphoma and non-central nervous system (CNS) solid tumors.4 Estimates of treatment-induced neuropathy among children with CNS tumors, however, are lacking, despite common exposure to both vinca alkaloids and platinum compounds.
Exposure to neurotoxic agents during childhood cancer therapy causes acute damage, which may manifest in sensory, motor, and/or autonomic neurons.5–8 Symptoms, including dampening or loss of peripheral reflexes, pain, numbness, tingling, weakness, difficulty swallowing, altered thermoregulation, poor blood pressure control, and problems with intestinal motility, are rarely life threatening; however, they are often severe enough to interfere with function during and after completion of therapy.9 Symptoms of peripheral neuropathy usually start distally and may or may not progress proximally. They also may disappear when the offending agents are withdrawn. However, the type of neuropathy, proximal progression, and extent and persistence of symptoms in survivors after completion of therapy are associated with multiple factors, including genetic predisposition, chemotherapy type, chemotherapy cumulative dose, age at time of exposure, and exposure to other agents during therapy. Persistence into survivorship is particularly concerning due to the associated impact on function and quality of life and affects survivors across multiple tumor types. In acute lymphoblastic leukemia (ALL) survivors, long-term loss of protective sensation, vibratory detection, ankle range of motion, and distal lower extremity strength have been documented, and are associated with postural control deficits and limited walking efficiency.10 Among adult survivors of childhood onset extracranial solid tumors, 20% experience cisplatin-related sensory loss, and nearly 18% experience vincristine-associated motor neuropathy.11 Little is known about the contributions of therapy to neuropathy in adult survivors of central nervous system (CNS) tumors, as it remains a challenge to decipher whether neuropathy is secondary to tumor location, surgery, or treatment factors. However, neuropathies, secondary to treatment exposures, are common among adult survivors of childhood cancer and may be amenable to ongoing rehabilitation to improve function.
The aims of this review are to describe the peripheral neuropathy phenotype among children during cancer therapy and in survivors who have completed therapy, to summarize host/genetic and treatment related risk factors for neuropathy, and to outline strategies to monitor and detect neuropathy. In addition, we discuss strategies for medical management of neuropathy during and after treatment, and propose potential rehabilitation interventions to prevent or remediate functional loss.
Search strategy and selection criteria
Relevant peer-reviewed literature was identified by querying PubMed with the search terms “peripheral neuropathy”, “peripheral neurotoxicity”, “chemotherapy induced peripheral neuropathy”, “childhood cancer”, “leukemia”, “solid tumor”, “survivorship”, “vinca alkaloids”, “platinum”, “genetic”, “quality of life”, and “performance” from 1990–2018. Articles were also selected from authors’ personal files; and from reference lists of identified papers. Only articles in English were included. Article selection was based on relevance to the present review.
Peripheral neuropathy
Acute neuropathy presents as a single, or a combination of, motor, sensory and/or autonomic impairments (Table 1).12 Motor neuropathy can be symmetrical or asymmetrical and occurs primarily with vinca alkaloid administration. Motor neuropathy is manifested as muscle weakness, muscle atrophy, cramping, uncontrolled muscle twitching, and gradual decrement and/or loss of peripheral deep tendon reflexes.12 Loss of ankle dorsiflexion range of motion and lower extremity weakness often present as foot drop (loss of eccentric control of the ankle dorsiflexor musculature), accompanied by loss of the longitudinal arch of the foot and a characteristic “foot slapping” while walking.13,14 These changes may be best appreciated after a minute or two of walking, or when the child ascends or descends stairs. In young children, gross motor developmental milestones, for example walking, running, climbing and jumping may be significantly delayed. In the upper extremity, weakness presents as difficulty with eye-hand coordination, problems grasping small objects, difficulties with pencil grip, and difficulty with daily self-care activities such as buttoning a shirt or tying shoes.15 Sensory changes typically begin distally, manifest symmetrically, have a “glove and stocking” distribution,” starting in the fingers and toes, and moving proximally to the hands and feet.16 Children often report paresthesias (e.g. burning, tingling, or pain), numbness, temperature intolerance, or exaggerated discomfort to a stimulus resulting in less use of the affected extremities or body parts.13 Vincristine exposure is associated with jaw pain that can affect chewing;17 however, this symptom is usually observed with the first administration of the agent and is typically transient. Decreased somatosensory input, compounded by decreased motor strength and flexibility, contributes to increased risk of balance problems in affected children.18 Autonomic dysfunction, caused by chemotherapy exposure, can present as orthostatic hypotension, and gastrointestinal disturbances, including constipation or watery diarrhea.19
Table 1.
Characteristics of peripheral neuropathy by type
| Type of neuropathy | Distribution | Symptoms | Common manifestations |
|---|---|---|---|
| Motor | Symmetrical or asymmetrical | Muscle weakness, atrophy, cramping, muscle twitching, and decreased reflexes | Gross motor development lost or delayed |
| Sensory | Symmetrical, distal, “glove and stocking” | Paresthesias, numbness, temperature intolerance, and exaggerated discomfort | Jaw pain, balance problems |
| Autonomic | Orthostatic hypotension, gastrointestinal disturbance | Constipation or diarrhea |
Once present, neuropathy may persist in children with cancer during therapy because of continued exposure to neurotoxic agents, and may continue long-term post-therapy if there was more permanent nerve damage. In a study of 128 children with ALL treated according to Children’s Oncology Group (COG) protocols after 2004, 78% developed peripheral neuropathy that persisted throughout the first year of treatment, even with reductions in vincristine dose intensity.8 Progression of symptom severity after treatment is completed, or “coasting”, is also possible, and has been reported with vincristine, platinum, bortezomib, and thalidomide exposures.19,20 Permanent damage to peripheral nervous system structure and function impacts fine motor skills, balance, mobility, endurance and potentially, quality of life.5,6,10,11,18,21,22 Reinders-Messilink et al report impaired upper extremity movement fluency and difficulty with handwriting among 18 ALL survivors 2 years after treatment when compared to healthy controls; 21 and Galea et al report increased postural sway and velocity during static tandem standing with eyes closed among 32% of 79 ALL survivors (a median of 5 years off therapy), compared to only 2% of 83 age-matched controls.23 In adult survivors of childhood ALL (N=415), impaired dorsiflexion range of motion (<5 degrees) as a result of vincristine exposure is associated with a nearly 4-fold (OR 3·9; 95% CI 2·4–6·3) increase in risk for poor performance on the 6-minute walk test;10 and, in adult survivors of non-CNS solid tumors (N=531), cisplatin induced sensory impairment is associated with reduced mobility (OR 2·0; 95% CI 1·0–4·0), and with poor performance on the 6-minute walk test (OR 1·7; 95% CI 1·0–2·8).11 In a cohort of adult survivors of childhood cancer (N=1,667, median 25·5 ± 7.8 years from diagnosis), presence of persistent sensory symptoms is associated with significantly reduced physical and mental quality of life as measured by the medical outcomes survey short-form 36 (SF-36), indicating a reduction in self-reported health-related quality of life.24
Chemotherapy agents/mechanism of injury
Chemotherapy agents associated with peripheral neuropathy in the pediatric population act upon multiple sites in the nervous system (Figure 1) and may be grouped according to their action upon microtubules: (1) microtubule-targeting drugs, (2) microtubule stabilizing agents (MTSAs), and (3) non-microtubule-active drugs (Table 2). Microtubule-targeting drugs bind to one of three main classes of sites on tubulin, the vinca alkaloid domain, the paclitaxel site, and the colchicine domain (the colchicine domain will not be further discussed in this review, as drugs targeting this domain have not been utilized to treat pediatric neoplasms due to other toxicities).25 Non-microtubule-active drugs act elsewhere upon the peripheral nervous system. Chemotherapeutic regimens may use more than one agent from each class, or across classes. This makes it difficult to determine which agent, or agents, are responsible for the signs and symptoms of neuropathy experienced by a particular patient. Unfortunately, additive effects are possible.
Figure 1. Sites and mechanisms of action of neurotoxic chemotherapeutic agents.
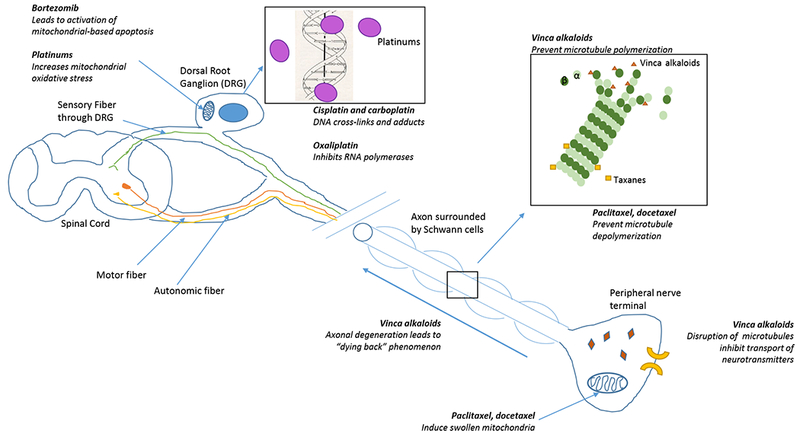
Chemotherapeutic agents can contribute to peripheral neuropathy across multiple sites of the peripheral nervous system. Agents that exert effects on the dorsal root ganglion (DRG) preferentially cause sensory neuropathies.
Table 2.
Class of chemotherapeutic agents commonly used in pediatrics, diagnoses they are used for, and primary neuropathy seen
| Class of agent | Drugs | Common diagnoses | Primary neuropathy |
|---|---|---|---|
| Vinca domain microtubule-targeting drugs | Vinca-alkyloid • Vincristine • Vinblastine • Vindesine • Vinorelbine |
• Acute lymphobastic leukemia • lymphoma • neuroblastoma • Wilms tumor • rhabdomyosarcoma • Ewing sarcoma • medulloblastoma • low-grade glioma • desmoid tumors • retinoblastoma • non-small cell lung cancer |
Motor and/or sensory |
| Microtubule stabilizing agents | Paclitaxel Docetaxel |
• ovarian cancer • breast cancer • head and neck cancer • lung cancer • Kaposi sarcoma • cervical cancer • pancreatic cancer |
Sensory |
| Non-microtubule active drugs | Bortezomib Platinum agents • Cisplatin • Carboplatin • Oxaliplatin |
• germ cell tumors • osteosarcoma • neuroblastoma • medulloblastoma • low-grade glioma • retinoblastoma • myeloma • non-Hodgkin lymphoma |
Sensory |
Vinca domain microtubule targeting drugs include the vinca alkaloid class of agents and include vincristine, vinblastine, vindesine and vinorelbine. Vinca alkaloids bind to the β-subunit of tubulin heterodimers, preventing their polymerization and incorporation into microtubules, causing cytoskeletal disorganization and microtubule disorientation within axons.26,27 Disorganization and disorientation of microtubules leads to inhibition of vesicle-mediated transport of neurotransmitters within the axoplasm and potentially axonal degeneration and denervation or “dying back neuropathy”. Vincristine, the most common agent associated with peripheral neuropathy among children with cancer, is an essential component of treatment for ALL, lymphoma, neuroblastoma, Wilms tumor, rhabdomyosarcoma, Ewing sarcoma, medulloblastoma, low-grade glioma and retinoblastoma. Vincristine exerts its cytotoxic effects by interfering with microtubule formation and mitotic spindle dynamics, leading to mitotic arrest and cell death.25 Electrophysiological testing done in children during vincristine treatment demonstrates minor changes in axonal conduction velocity but notable reductions in compound muscle action potentials (CMAPs) (Table 3).28,29 One study also noted reductions in upper extremity sensory conduction velocities and sensory nerve action potentials.29 These changes are consistent with a “dying back” neuropathy. Depending on the vincristine dose and schedule of administration, motor and/or sensory neuropathy (grade 2+) occurs in approximately 25% of patients.26 This nerve damage can persist, with motor changes notable on electrodiagnostic testing being more common than sensory abnormalities (Table 3).
Table 3.
Characteristics of electrophysiological findings among pediatric cancer populations during, and off treatment
| Article | Patient Population | Timing of Testing | Neurotoxic Agent | CV – Motor Nerves Assessed: % abnormal | CV – Sensory Nerves Assessed: % abnormal | CMAP Nerves Assessed: % abnormal | SNAP Nerves Assessed: % abnormal |
|---|---|---|---|---|---|---|---|
| On-Treatment | |||||||
| Kavcic et al 201729 | Mixed Tumor Types (67% ALL) n=39 Age: 10 ± 6·4 |
After minimum of 4 doses | Vincristine | Median, ulnar: 7% Tibial, peroneal: 11% |
Median, ulnar: 48% Sural: 7% |
Median, ulnar: 33% Tibial, peroneal: 48% |
Median, ulnar: 67% Sural: 11% |
| Yildiz & Temucin 201628 | Mixed Tumor Types with suspected neuropathy (80% ALL) n=25 Age: mean 7·2 ± 4·8 |
49·3 ± 28·6 days | Vincristine | Median, ulnar, tibial, peroneal and sural: WNL |
Median, ulnar, peroneal and sural: WNL |
Median, ulnar, tibial, peroneal and sural: Group data significantly decreased |
Median, ulnar, tibial, peroneal and sural: WNL |
| Toopchizadeh et al 200930 | Mixed Tumor Types (60% ALL) n=42 Age: mean 6·2 |
5 weeks after initiation of chemotherapy | Vincristine | Median, Tibial: WNL |
Median, Sural: WNL |
Tibial, median, and ulnar: ALL 96% Other cancers 52·9% |
Median, ulnar, Sural: WNL |
| Off Treatment | |||||||
| Kandula et al 201831 | Mixed tumor types (52% Leukemia) n= 121 Age: median 16 (range 7–47) |
Median 8.5 years (1·5 – 29) | Vincristine or Platinum | - | Sural: WNL |
Tibial and Peroneal: WNL |
Median: WNL Sural: Vinc 18% Plat 39% |
| Tay et al 201722 | ALL n=101 Age: mean 11·8 ± 3·8 |
>2 years | Vincristine | Common peroneal, tibial, median, ulnar: 61·4%* |
Sural, medial, ulnar, radial: 55%* |
Common peroneal, tibial, median, ulnar: 61·4%* |
Sural, medial, ulnar, radial: 55%* |
| Jain et al 201432 | ALL n=77 Age: mean 11·2 ± 3·9 |
within 3 years | Vincristine | Peroneal, Tibial, Median, and Ulnar: WNL |
Sural, Ulnar, Median: WNL |
Median, Ulnar: 7·8% Peroneal, Tibial: 50·7% |
Sural, Ulnar, Median: WNL |
| Ramchandren et al 20095 | ALL n=37 Age: mean 14·4 ± 2·8 |
>2 years | Vincristine | - | - | Median: 8% Peroneal: 21% |
Median: 5% Sural: WNL |
Unclear if abnormality reported was CV or amplitude, ALL Acute Lymphoblastic Leukemia, CMAP Compound Muscle Action Potential, CV Conduction Velocity, SNAP Sensory nerve action potential, WNL Result was Within Normal Limits
Despite its structural similarity to vincristine, differing by the substitution of a methyl group for the formyl group on the vindoline nucleus, vinblastine, a frequent component of pediatric chemotherapy regimens for Hodgkin disease, desmoid tumors and low-grade gliomas, is less likely to result in peripheral neuropathy than vincristine.33 Vindesine is not commonly used in pediatric protocols, though it has a neurotoxicity profile similar to that of vincristine.34 Vinorelbine, primarily used to treat non-small cell lung cancer among adults, has been used in protocols for children with relapsed or refractory leukemia, Hodgkin lymphoma, sarcomas, and brain tumors.35–39 Rates of neuropathy after vinorelbine exposure range from 3%, when delivered in conjunction with cyclophosphamide for refractory sold tumors,37 to 24% when delivered subsequent to another neurotoxic agent, bortezomib.40
MTSAs (paclitaxel and docetaxel) are often used to treat adult malignancies, including ovarian cancer, breast cancer, head and neck cancer, lung cancer, Kaposi sarcoma, cervical cancer, and pancreatic cancer, but are rarely utilized in the treatment of pediatric cancer. MTSAs cause a primary sensory neuropathy. Preclinical work has demonstrated that MTSAs induce swollen and vacuolated mitochondria in both myelinated axons and C-fibers in peripheral sensory nerves, which correlates with MTSA-induced peripheral neuropathy.41 In animal models, MTSAs also block fast axonal transport and induces microtubule aggregation without degenerative changes in peripheral nerves.42,43 However, this has not been confirmed in nerve biopsies from patients with MTSA-associated peripheral neuropathy.44
Non-microtubule-active drugs include platinum agents (cisplatin, carboplatin, and oxaliplatin), and bortezomib. Platinum agents, primarily cisplatin and carboplatin, are used in treatment regimens for germ cell tumors, osteosarcoma, neuroblastoma, medulloblastoma, low-grade glioma, and retinoblastoma. Neuropathy among patients exposed to platinum compounds is a result of injury to the dorsal root ganglions (DRG),45 where formation of intra-strand adducts and cross-links influence the structure of nuclear DNA and interfere with cell-cycle kinetics.46,47 Platinum also interacts with mitochondrial DNA, resulting in increased oxidative stress,48 and activation of apoptotic pathways.49–52 Although DRG neurons are post-mitotic, and non-dividing cells and DNA platination adducts are not lethal, the amount of DNA cross-links in DRG neurons at a given cumulative dose is significantly correlated with the degree of neurotoxicity.53 Because the pharmacokinetics of carboplatin differs from that of cisplatin, prior studies have employed a 4:1 carboplatin to cisplatin dose equivalence adjustment in assessing associations of platinum compounds to neurotoxicity.11 Most cases of platinum induced peripheral neuropathy occur after a threshold cumulative dose of 300 mg/m2 is reached,54 and almost all patients have objective evidence of nerve damage beyond a cumulative dose of 500-600 mg/m2.55 Because neuropathy following exposure to platinum agents is a result of injury to the DRG, neuropathy after platinum exposure is primarily sensory and electrophysiological evidence of reduced sensory nerve action potentials has been reported years after treatment (Table 3).31,56 Oxaliplatin, a third-generation platinum agent, also interacts with transcription factors and inhibs RNA polymerases, and is associated with immunogenic cell death.57,58 It is not commonly used in pediatric patients, primarily in the context of phase I and II trials. Oxaliplatin is associated with a cold-induced neuropathy, that does not occur with other platinum compounds.59
Bortezomib, a 20S proteasome complex inhibitor, is approved for the treatment of myeloma and non-Hodgkin lymphoma and is currently being investigated for recurrent childhood leukemia and lymphoma. Bortezomib causes a primary sensory neuropathy which appears to be more common in adults than children.60 Bortezomib leads to intracytoplasmic vacuolation, which is ascribed to mitochondrial and endoplasmic reticulum injury, and activation of the mitochondrial-based apoptotic pathway within DRG satellite cells.61
Genetic risk factors for peripheral neuropathy in children with cancer
Despite increasing knowledge regarding the mechanism of neurotoxic injury, there is a wide variation in the reported incidence, and symptom severity. Observations of inter-individual and ancestry-related differences in the prevalence of vincristine-induced neuropathy suggest that genetic variants may contribute to the incidence and/or severity of vincristine-induced neuropathy. 62 However, initial studies designed to identify genetic variants were limited to a relatively small set of candidate genes known to be involved in vincristine pharmacokinetics. These studies did not yield reproducible findings, perhaps because they were limited in scope and did not use an agnostic approach.62 Candidate gene studies designed to identify genetic variants associated with cisplatin-induced peripheral neuropathy reported associations between the development of neuropathy during treatment and drug detoxification enzymes,63,64 as well as DNA repair genes;65 similar to vincristine studies, these were not replicated in agnostic genome wide association studies (GWAS).66
Recently, an agnostic GWAS approach using two different cohorts of children with ALL treated on St. Jude Children’s Research Hospital or Children’s Oncology Group protocols identified several single nucleotide polymorphisms (SNPs) associated with increased incidence of vincristine-induced neuropathy. The top SNP associated with an increased risk and severity of neuropathy (rs924607) resides in the promoter of the centrosomal protein 72 (CEP72) gene. The T allele is associated with a 2·7 fold increased risk of vincristine-induced neuropathy, and creates a binding site for the transcriptional repressor NKX6.3, which results in lower expression of CEP72, a centrosomal protein essential for microtubule formation. Additionally, knockdown of CEP72 increases the sensitivity to vincristine of both human induced pluripotent stem cell (iPSC) neurons and primary ALL cells from patients homozygous for the CEP72 risk allele (T/T).2 This association of the CEP72 SNP with vincristine neuropathy, was validated in 2 additional adult cohorts of cancer patients.67,68 The most effective approach to mitigate chemotherapy-induced peripheral neuropathy has been dose reduction and/or discontinuation of therapy, which may ultimately decrease treatment efficacy. The identification of CEP72 variant, characterized by increased sensitivity in both neurons and leukemia cells, offers the potential to reduce vincristine dose in susceptible individuals to decrease the risk of neuropathy, without compromising its therapeutic effect, if both neurons and leukemia cells are more sensitive to vincristine resulting in decreased efficacy. However, the sensitivity of leukemia cells to genetic variants has not yet been validated.
To date, there are no GWAS studies in pediatric populations. However, a GWAS with self-reported peripheral neuropathy symptoms (using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-CIPN twenty-item scale (EORTC QLQ-CIPN20)) as the outcome in a cohort of young adult cancer survivors identified Regulation Of Nuclear Pre-MRNA Domain-Containing Protein 1B (RPRD1B) using PrediXcan,66,69 a gene-based computational method which uses reference transcriptome (genotype-gene expression) data to generate models used to ‘impute’ gene expression levels from genotype data. An association between lower expressions of RPRD1B with cisplatin induced peripheral neuropathy was identified. Defects in RPRD1B expression inhibits DNA repair mechanisms critical in resolving cisplatin-induced injury,70 and knockdown increases sensitivity to cisplatin.71
Challenges remain in the interpretation of genetic susceptibility studies. Comparison of results is difficult, due to differences in defining the neuropathy phenotype, and because not all studies control for covariates that can influence neuropathy (dose, duration of treatment, methods used to capture and quantitate the phenotype, etc.).72 Further studies are needed to understand how different gene variants predispose patients to vincristine, cisplatin- and other agent-induced neuropathy (as was done with CEP72 SNP2), and to define the influence of rare variants of CEP72 and other genes that will emerge from whole-exome and whole-genome sequencing.73 Importantly, GWAS approaches, such as those that have been used in children exposed to vincristine, which are designed to examine the modifying impact of genetics on neuropathy among children with other neurotoxic exposures, are warranted. In addition, it is not known whether the same genetic variants that predispose to acute chemotherapy-induced neuropathy also predispose to persistent neuropathy, and the contribution of other genes to this phenotype, remains to be determined.
Other pre-existing genetic conditions may increase the risk for neurotoxicity secondary to cancer therapy. Reports of severe neuropathy after vincristine exposure have led to the discovery of undiagnosed, and at times asymptomatic, conditions in pediatric patients, such as Charcot-Marie-Tooth.74 Neurofibromatoses may manifest with peripheral neuropathies and may be independent of tumor presence, as in neurofibromatosis 2.75 Children with Down syndrome are at a higher risk for developing leukemia compared to other children, and as part of their phenotype, at baseline may have neurologic impairment, including hypotonia. However, there is no information indicating an elevated risk of therapy induced peripheral neuropathy in children with Down syndrome. Further study in this population is warranted. Clinicians should consider both family history of neuropathy, and pre-existing neurological symptoms prior to initiation of neurotoxic therapy, and carefully monitor patients with histories or symptoms likely to be exacerbated by administration of neurotoxic agents.
Screening and evaluation
All children and adolescents receiving neurotoxic agents as part of their cancer therapy regimen should be screened by history and physical examination on an ongoing basis for signs and symptoms of peripheral neuropathy (Figure 2). Although some studies indicate an association between cumulative dose of neurotoxic agent and neuropathy,76 others report associations with any dose,8,77 indicating that patients should be screened regardless of agent-specific dose received. Specific query is important as relying on unsolicited patient/family report is often insufficient to detect emerging neuropathies. One study, comparing documented patient report (medical record) to specific questions and objective testing in pediatric cancer patients, found that 40% of sensory neuropathy and 15% of motor neuropathy is unrecognized.78 Optimal screening includes patient/parent interviews about changes in sensory, motor, or autonomic function, in conjunction with careful physical and neurologic exam. Signs and symptoms reflective of neuropathic pain, paresthesias and/or functional changes such as tripping while walking, regression of stair climbing ability, and difficulty with fine motor tasks may indicate emerging neuropathy and warrants further investigation.
Figure 2. Screening and evaluation of pediatric chemotherapy induced neuropathy.
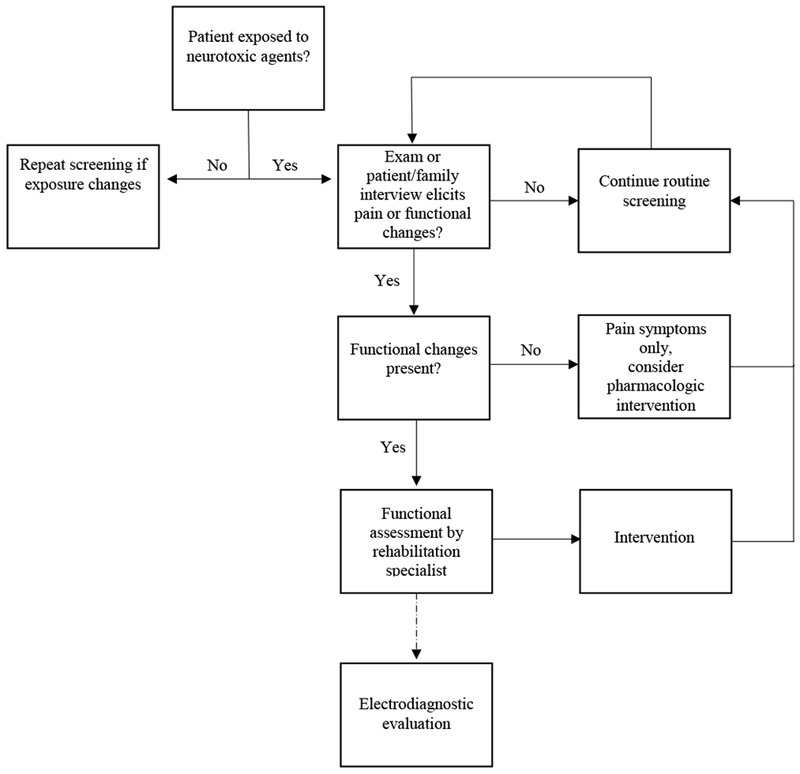
Dotted arrow indicates optional assessment pending exam and clinician suspicion.
Peripheral neuropathy is also associated with other patient factors, including age race and concomitant therapy. In patients with medulloblastoma treated in the second decade of life (10–20 years), over 70% of patients who received both chemotherapy and radiation experienced ≥grade 2 neurotoxicity.79 Compared to younger patients (>5 and <10 years), older patients (≥10 years) experienced more neurotoxicity and ototoxicity while receiving cisplatin, lomustine and vincristine.79 Measures of vincristine-induced peripheral neuropathy among 1539 patients with ALL, were positively associated with age, indicating worsening neuropathy.8 Race may also play a role in risk of chemotherapy-induced neuropathy. In children between one and 18 years of age with ALL, non-Hispanic White children had a significantly higher cumulative incidence of vincristine-related neuropathy during the first three years of therapy compared to Black, Hispanic, Asian or other children.80 Children being treated for cancer are also at risk for other conditions, including fungal infection, and may also be receiving therapy with azole antifungals. Adverse interactions between azole antifungals and vincristine have been documented, due to shared metabolism through CYP3A4. Metabolic competition increases the length of vincristine exposure.81
Once screening has indicated a potential problem, clinical assessment of peripheral neuropathy in school-aged children and adolescents with neurotoxic exposure should be completed with validated measures to identify potential need for treatment modifications or rehabilitation intervention. Variants of the Total Neuropathy Score (TNS) adapted for pediatric use include the pediatric modified TNS (ped-mTNS) and the TNS-Pediatric Vincristine (TNS-PV).76,77 These instruments include both subjective and objective measures of peripheral nerve function, and are completed in five to ten minutes by trained medical personnel (e.g. nurses, physical therapists). Both tools include assessment of reflexes, distal muscle strength, distal light touch (large fiber function), pin sensibility (small fiber function), and pain. Decreased or absent deep tendon reflexes are common in children exposed to neurotoxic agents; distal muscular weakness and sensory loss indicate more severe impairment. Currently, no validated measures are available for children younger than five years of age. Nevertheless, clinicians caring for young children should assess reflexes, distal strength, and sensation as able using developmentally appropriate techniques. Similar to young children, there are no validated measures available for children with brain and CNS tumors, highlighting one of many challenges in evaluating neuropathy in these patients. Children with CNS tumors may have pre-existing neurologic deficits or altered sensorium secondary to tumor site, surgery, or radiation. Concomitant cognitive deficits may further complicate patient reports of pain, or changes in sensation or function. Nevertheless, these patients require assessment of neuropathy and impairment, as many are treated with neurotoxic agents, including platinums and vinca alkaloids. In general, quantitative sensory testing and electrodiagnostic testing are reported in the literature,5,22,28–32,82,83 but likely do not add additional information necessary for routine clinical care, unless alternate diagnoses are being considered or for research purposes. Interestingly, electrodiagnostic testing generally reveals more extensive motor axonopathy from vincristine therapy in children than in adults.5,32
Functional testing, preferably by trained rehabilitation professionals, should be completed when significant neuropathy or functional impairment are present. In addition to refined measures of strength and sensation, these assessments include joint range of motion measurement, formal gait analysis, estimates of functional capacity (e.g. 6 minute walk test), balance testing, and standardized evaluation of manual dexterity. One widely used and standardized assessment with available age and specific normative values available for comparison is the Bruininks-Oseretsky Test of Motor Proficiency.84 These standardized assessments allow for the monitoring and response to management strategies, including rehabilitation, as well as dose reduction or cessation of neurotoxic agents.
Management of neuropathy
Management of neuropathy in cancer populations is challenging. Previous trials have investigated multiple pharmaceutical agents, including supplements such as acetylcarnitine, calcium, glutamine, omega-3 and medications such as amitriptyline, amifostine, oxycarbazepine, and venlafaxine for prevention and/or remediation of chemotherapy-induced peripheral neuropathy.85 However, studies have largely been conducted in adults and either demonstrate no benefit or are inconclusive due to insufficient sample size or difficulty in specific attribution to multi-agent treatment using neuropathic agents. The American Society of Clinical Oncology Clinical Practice Guideline (2014) concludes that no agents have demonstrated sufficient evidence to support use for the prevention of chemotherapy-induced peripheral neuropathy in adults with cancer.85 For those who develop neuropathy, data support (moderate recommendation) the use of duloxetine for paclitaxel- or oxaliplatin-induced pain.86 However, this agent has not been evaluated in pediatric patients or in persons with vincristine-induced neuropathy. Current and past randomized trials in pediatrics investigating any pharmaceutical interventions for pediatric patients are few, and are mostly limited to patients with vincristine-induced neuropathy (Table 4). Tricyclic antidepressants (e.g., nortriptyline), gabapentin, and a compounded topical gel containing baclofen, amitriptyline, and ketamine have been suggested based on their utility in other populations with neuropathic pain.85 Gabapentin is associated with pain reduction in patients with moderate or severe neuropathic pain from post-herpetic neuralgia or painful diabetic neuropathy.87 Both gabapentin and pregabalin have been used in several pediatric studies of vincristine-induced neuropathy, but their efficacy has not been unequivocally established.80,88
Table 4.
Characteristics and measured outcomes of randomized controlled trials for peripheral neuropathy or neuropathic pain in pediatric oncology patients
| NCT Number | Intervention | Diagnoses | Chemotherapy | Ages | Measured Outcomes |
|---|---|---|---|---|---|
| NCT01506453 | Gabapentin | Acute lymphoblastic leukemia | Vincristine | 1-18 years | - daily total dose of oral morphine (mg/kg/day) - current pain score - pain score in previous 24 hours - |
| NCT00369564 | Glutamic acid | Wilms tumor Rhabdomyosarcoma ALL Non-Hodgkin lymphoma |
Vincristine | 3-20 years | - Neurotoxicity - Frequency and type of neurotoxicity - Ability to receive all scheduled doses of vincristine |
| NCT00365768 | Glutamine | Leukemia Solid tumors Medulloblastoma |
Vincristine | 5-21 years | - Incidence of vincristine-induced peripheral neuropathy - Number of participants with progression of neuropathy |
| NCT00112996 | Alpha-Lipoic Acid | Solid tumors | Cisplatin Oxaliplatin |
Any | - Severity of neuropathy - Group differences in change scores - Number of courses received - Optimal tumor response |
In pediatric settings, dose reduction or treatment interruption is often considered to prevent or treat chemotherapy-induced neuropathy. For example, the maximum single dose of vincristine is typically capped to 2.0 mg, regardless of body surface area, because the incidence of neuropathy increases above this dose.20 If vocal cord paralysis, motor paralysis, severe neuropathic pain, severe abdominal cramps, typhlitis, or syndrome of inappropriate antidiuretic hormone secretion develop, vincristine is often withheld or dose-reduced.20,89 However, because vincristine is an important component of curative therapy, vincristine therapy should be resumed and/or dose-escalated as tolerated.90 Established guidelines for dose reduction or interruption are lacking, and current practices are widely variable across treatment protocols and clinician preference. The presence of underlying hereditary sensorimotor neuropathy (e.g., Charcot-Marie-Tooth disease) can lead to permanent and/or life-threatening adverse effects after administration of vincristine. These children require alternative therapy.91 Genotype-based dose adjustment,2 shorter treatment duration, and/or longer intervals between treatment may also reduce long-term toxicities. Liposomal vincristine has been marketed as a potentially less neurotoxic formulation, but this has not been established in rigorous clinical trials, and the cost of this agent is significantly greater than conventional vincristine.92
While pharmacological therapy may attenuate pain symptoms, preliminary research also supports the use of non-pharmacologic interventions in children with cancer who develop neuropathy.93 Tanner et al,94 in a pilot study, demonstrate preliminary efficacy of using ankle foot orthosis (bracing) to address loss of ankle range of motion, weakness, and associated gait abnormalities among seven children with peripheral neuropathy during cancer therapy, and suggest potential “weaning” of the orthoses after treatment ends. In addition, Casonova-Garcia et al95 provides support for use of guided imagery to control neuropathic pain among six children during cancer therapy. Several studies in adult cancer populations also suggest approaches that are likely to translate to pediatric settings. One reported a positive impact of exercise on neuropathy symptoms among 355 adults (93% female, 79% breast cancer) during neurotoxic chemotherapy exposures,96 and another, an improvement in both neuropathy symptoms and postural control as a result of balance training in 25 adults with cancer who had evidence of neuropathy.97 More work is needed in the study of these non-pharmacologic interventions in the pediatric population.
Rehabilitation strategies for children with chemotherapy-induced peripheral neuropathy should focus on remediation of impairments (postural control deficits,98,99 gait abnormalities, muscle weakness, loss of fine motor skills), support the continued development of motor control (jumping, running, stair climbing), and promote regular physical activity. In addition, loss of sensory function needs to be addressed with patient/family education. In children who develop hypersensitivity to light touch, desensitization treatments may also be helpful.
Conclusion
Peripheral neuropathy is a prevalent toxicity of chemotherapy, including vinca alkaloids and platinum agents, in children with cancer. Depending on the type of nerve tissue damaged, symptoms may manifest as motor, sensory, and/or autonomic. The recommendation to screen all patients receiving neurotoxic agents allows for the prompt initiation of management strategies. Importantly, even after cessation of therapy and optimal management, neuropathy may persist. Future research is needed to further understand how inherited genetic variants contribute to the susceptibility to and severity of peripheral neuropathy secondary to cancer therapy. Furthermore, improvements in surveillance strategies, in particular for young children and those with CNS tumors are needed. Also, further research in pharmacologic agents for prevention or treatment, and rehabilitation interventions are needed which allow for the simultaneous delivery of optimal cancer therapy and the mitigation of toxicity resulting in pain and functional impairment.
Key Messages.
Peripheral neuropathy is an adverse effect of chemotherapy that can result in dose limitations and choice of therapy, and persist into survivorship
Manifestations of neuropathy can be motor, sensory or autonomic, and include loss of reflexes, pain, weakness and gastrointestinal symptoms
Screening for neuropathy should be done on an ongoing basis in all pediatric patients receiving neurotoxic therapy
Further research is necessary to develop better strategies for treatment and remediation of peripheral neuropathy
Acknowledgments
No specific funding was used for this study.
No authors are employed by the NIH however, the following authors are recipients of NIH funding through their institutional cancer center grant or individual grants:
Kari L. Bjornard, Hiroto Inaba, Barthelemy Diouf, Marilyn J. Hockenberry, Nina S. Kadan-Lottick, Daniel C. Bowers, M. Eileen Dolan, Nicole J. Ullrich, William E. Evans, Kirsten K. Ness
Footnotes
Declaration of interests
The authors declare no competing financial or personal interests.
This manuscript has not been submitted to another journal, nor has it been published in whole or in part elsewhere.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. http://seer.cancer.gov/csr/1975_2013/ (accessed 5/18/2017).
- 2.Diouf B, Crews KR, Lew G, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA 2015; 313: 815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinders-Messelink HA, Van Weerden TW, Fock JM, et al. Mild axonal neuropathy of children during treatment for acute lymphoblastic leukaemia. Eur J Paediatr Neurol 2000; 4: 225–33. [DOI] [PubMed] [Google Scholar]
- 4.Gilchrist LS, Tanner LR, Ness KK. Short-term recovery of chemotherapy-induced peripheral neuropathy after treatment for pediatric non-CNS cancer. Pediatr Blood Cancer 2017; 64: 180–7. [DOI] [PubMed] [Google Scholar]
- 5.Ramchandren S, Leonard M, Mody RJ, et al. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J Peripher Nerv Syst 2009; 14: 184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehtinen SS, Huuskonen UE, Harila-Saari AH, Tolonen U, Vainionpaa LK, Lanning BM. Motor nervous system impairment persists in long-term survivors of childhood acute lymphoblastic leukemia. Cancer 2002; 94: 2466–73. [DOI] [PubMed] [Google Scholar]
- 7.Harila-Saari AH, Huuskonen UE, Tolonen U, Vainionpaa LK, Lanning BM. Motor nervous pathway function is impaired after treatment of childhood acute lymphoblastic leukemia: a study with motor evoked potentials. Med Pediatr Oncol 2001; 36: 345–51. [DOI] [PubMed] [Google Scholar]
- 8.Lavoie Smith EM, Li L, Chiang C, et al. Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. J Peripher Nerv Syst 2015; 20: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilchrist L Chemotherapy-induced peripheral neuropathy in pediatric cancer patients. Semin Pediatr Neurol 2012; 19: 9–17. [DOI] [PubMed] [Google Scholar]
- 10.Ness KK, Hudson MM, Pui CH, et al. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: associations with physical performance and chemotherapy doses. Cancer 2012; 118: 828–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ness KK, Jones KE, Smith WA, et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St. Jude Lifetime Cohort Study. Arch Phys Med Rehabil 2013; 94: 1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandula T, Park SB, Cohn RJ, Krishnan AV, Farrar MA. Pediatric chemotherapy induced peripheral neuropathy: A systematic review of current knowledge. Cancer Treat Rev 2016; 50: 118–28. [DOI] [PubMed] [Google Scholar]
- 13.Dropcho EJ. Neurotoxicity of cancer chemotherapy. Semin Neurol 2010; 30: 273–86. [DOI] [PubMed] [Google Scholar]
- 14.Gilchrist L, Tanner L. Gait Patterns in Children With Cancer and Vincristine Neuropathy. Pediatr Phys Ther 2016; 28: 16–22. [DOI] [PubMed] [Google Scholar]
- 15.Green JL, Knight SJ, McCarthy M, De Luca CR. Motor functioning during and following treatment with chemotherapy for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer 2013; 60: 1261–6. [DOI] [PubMed] [Google Scholar]
- 16.Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev 2014; 40: 872–82. [DOI] [PubMed] [Google Scholar]
- 17.Aytac S, Yetgin S, Tavil B. Acute and long-term neurologic complications in children with acute lymphoblastic leukemia. Turk J Pediatr 2006; 48: 1–7. [PubMed] [Google Scholar]
- 18.Varedi M, McKenna R, Lamberg EM. Balance in children with acute lymphoblastic leukemia. Pediatr Int 2017; 59: 293–302. [DOI] [PubMed] [Google Scholar]
- 19.Kandula T, Farrar MA, Kiernan MC, et al. Neurophysiological and clinical outcomes in chemotherapy-induced neuropathy in cancer. Clin Neurophysiol 2017; 128: 1166–75. [DOI] [PubMed] [Google Scholar]
- 20.Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 2013; 63: 419–37. [DOI] [PubMed] [Google Scholar]
- 21.Reinders-Messelink HA, Schoemaker MM, Hofte M, et al. Fine motor and handwriting problems after treatment for childhood acute lymphoblastic leukemia. Med Pediatr Oncol 1996; 27: 551–5. [DOI] [PubMed] [Google Scholar]
- 22.Tay CG, Lee VWM, Ong LC, Goh KJ, Ariffin H, Fong CY. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer 2017; 64. [DOI] [PubMed] [Google Scholar]
- 23.Galea V, Wright MJ, Barr RD. Measurement of balance in survivors of acute lymphoblastic leukemia in childhood. Gait Posture 2004; 19: 1–10. [DOI] [PubMed] [Google Scholar]
- 24.Huang IC, Brinkman TM, Kenzik K, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort study. J Clin Oncol 2013; 31: 4242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer 2004; 4: 253. [DOI] [PubMed] [Google Scholar]
- 26.Mora E, Smith EML, Donohoe C, Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res 2016; 6: 2416–30. [PMC free article] [PubMed] [Google Scholar]
- 27.Tanner KD, Levine JD, Topp KS. Microtubule disorientation and axonal swelling in unmyelinated sensory axons during vincristine‐induced painful neuropathy in rat. J Comp Neurol 1998; 395: 481–92. [PubMed] [Google Scholar]
- 28.Yildiz FG, Temucin CM. Vincristine-induced neurotoxicity: electrophysiological features in children. Neurol Res 2016; 38: 124–9. [DOI] [PubMed] [Google Scholar]
- 29.Kavcic M, Koritnik B, Krzan M, et al. Electrophysiological Studies to Detect Peripheral Neuropathy in Children Treated With Vincristine. J Pediatr Hematol Oncol 2017; 39: 266–71. [DOI] [PubMed] [Google Scholar]
- 30.Toopchizadeh V BM, Rezamand A, Feiz AH. Electrophysiological consequences of vincristine contained chemotherapy in children: a cohort study. J Pediatr Neurol 2009; 7: 351–6. [Google Scholar]
- 31.Kandula T, Farrar MA, Cohn RJ, et al. Chemotherapy-Induced Peripheral Neuropathy in Long-term Survivors of Childhood Cancer: Clinical, Neurophysiological, Functional, and Patient-Reported Outcomes. JAMA Neurol 2018. http://doi:10.1001/jamaneurol.2018.0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain P, Gulati S, Seth R, Bakhshi S, Toteja GS, Pandey RM. Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: prevalence and electrophysiological characteristics. J Child Neurol 2014; 29: 932–7. [DOI] [PubMed] [Google Scholar]
- 33.Lassaletta A, Scheinemann K, Zelcer SM, et al. Phase II Weekly Vinblastine for Chemotherapy-Naïve Children With Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J Clin Oncol 2016; 34: 3537–43. [DOI] [PubMed] [Google Scholar]
- 34.Joel S The comparative clinical pharmacology of vincristine and vindesine: does vindesine offer any advantage in clinical use? Cancer Treat Rev 1996; 21: 513–25. [DOI] [PubMed] [Google Scholar]
- 35.Casanova M, Ferrari A, Spreafico F, et al. Vinorelbine in previously treated advanced childhood sarcomas: evidence of activity in rhabdomyosarcoma. Cancer 2002; 94: 3263–8. [DOI] [PubMed] [Google Scholar]
- 36.Massimino M, Biassoni V, Miceli R, et al. Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol 2014; 118: 305–12. [DOI] [PubMed] [Google Scholar]
- 37.Minard-Colin V, Ichante JL, Nguyen L, et al. Phase II study of vinorelbine and continuous low doses cyclophosphamide in children and young adults with a relapsed or refractory malignant solid tumour: good tolerance profile and efficacy in rhabdomyosarcoma--a report from the Societe Francaise des Cancers et leucemies de l’Enfant et de l’adolescent (SFCE). Eur J Cancer 2012; 48: 2409–16. [DOI] [PubMed] [Google Scholar]
- 38.Shukla N, Kobos R, Renaud T, Steinherz LJ, Steinherz PG. Phase II trial of clofarabine with topotecan, vinorelbine, and thiotepa in pediatric patients with relapsed or refractory acute leukemia. Pediatr Blood Cancer 2014; 61: 431–5. [DOI] [PubMed] [Google Scholar]
- 39.Trippett TM, Schwartz CL, Guillerman RP, et al. Ifosfamide and vinorelbine is an effective reinduction regimen in children with refractory/relapsed Hodgkin lymphoma, AHOD00P1: a children’s oncology group report. Pediatr Blood Cancer 2015; 62: 60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keller S, Seipel K, Novak U, et al. Neurotoxicity of stem cell mobilization chemotherapy with vinorelbine in myeloma patients after bortezomib treatment. Leuk Res 2015; 39: 786–92. [DOI] [PubMed] [Google Scholar]
- 41.Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain 2006; 122: 245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakata T, Yorifuji H. Morphological evidence of the inhibitory effect of taxol on the fast axonal transport. Neurosci Res 1999; 35: 113–22. [DOI] [PubMed] [Google Scholar]
- 43.Cavaletti G, Cavalletti E, Montaguti P, Oggioni N, De Negri O, Tredici G. Effect on the peripheral nervous system of the short-term intravenous administration of paclitaxel in the rat. Neurotoxicology 1997; 18: 137–45. [PubMed] [Google Scholar]
- 44.Sahenk Z, Barohn R, New P, Mendell JR. Taxol neuropathy: Electrodiagnostic and sural nerve biopsy findings. Arch Neurol 1994; 51: 726–9. [DOI] [PubMed] [Google Scholar]
- 45.Cavaletti G, Tredici G, Marmiroli P, Petruccioli MG, Barajon I, Fabbrica D. Morphometric study of the sensory neuron and peripheral nerve changes induced by chronic cisplatin (DDP) administration in rats. Acta Neuropathol 1992; 84: 364–71. [DOI] [PubMed] [Google Scholar]
- 46.Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology 2006; 27: 992–1002. [DOI] [PubMed] [Google Scholar]
- 47.Gill JS, Windebank AJ. Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J Clin Invest 1998; 101: 2842–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Mizumachi T, Carcel-Trullols J, et al. Targeting human 8-oxoguanine DNA glycosylase (hOGG1) to mitochondria enhances cisplatin cytotoxicity in hepatoma cells. Carcinogenesis 2007; 28: 1629–37. [DOI] [PubMed] [Google Scholar]
- 49.McDonald ES, Windebank AJ. Cisplatin-induced apoptosis of DRG neurons involves bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol Dis 2002; 9: 220–33. [DOI] [PubMed] [Google Scholar]
- 50.Scuteri A, Galimberti A, Maggioni D, et al. Role of MAPKs in platinum-induced neuronal apoptosis. Neurotoxicology 2009; 30: 312–9. [DOI] [PubMed] [Google Scholar]
- 51.Chiorazzi A, Semperboni S, Marmiroli P. Current View in Platinum Drug Mechanisms of Peripheral Neurotoxicity. Toxics 2015; 3: 304–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meijer C, de Vries EG, Marmiroli P, Tredici G, Frattola L, Cavaletti G. Cisplatin-induced DNA-platination in experimental dorsal root ganglia neuronopathy. Neurotoxicology 1999; 20: 883–7. [PubMed] [Google Scholar]
- 53.Dzagnidze A, Katsarava Z, Makhalova J, et al. Repair capacity for platinum-DNA adducts determines the severity of cisplatin-induced peripheral neuropathy. J Neurosci 2007; 27: 9451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregg RW, Molepo JM, Monpetit VJ, et al. Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J Clin Oncol 1992; 10: 795–803. [DOI] [PubMed] [Google Scholar]
- 55.Jongen JL, Broijl A, Sonneveld P. Chemotherapy-induced peripheral neuropathies in hematological malignancies. J Neurooncol 2015; 121: 229–37. [DOI] [PubMed] [Google Scholar]
- 56.Kanat O, Ertas H, Caner B. Platinum-induced neurotoxicity: A review of possible mechanisms. World J Clin Oncol 2017; 8: 239–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Todd RC, Lippard SJ. Inhibition of transcription by platinum antitumor compounds. Metallomics : integrated biometal science 2009; 1: 280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010; 29: 482–91. [DOI] [PubMed] [Google Scholar]
- 59.Starobova H, Vetter I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front molecular neurosci 2017; 10: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Messinger YH, Gaynon PS, Sposto R, et al. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood 2012; 120: 285–90. [DOI] [PubMed] [Google Scholar]
- 61.Zheng H, Xiao WH, Bennett GJ. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. J Neuropathol Exp Neurol 2012; 238: 225–34. [DOI] [PubMed] [Google Scholar]
- 62.Egbelakin A, Ferguson MJ, MacGill EA, et al. Increased risk of vincristine neurotoxicity associated with low CYP3A5 expression genotype in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2011; 56: 361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goekkurt E, Al-Batran SE, Hartmann JT, et al. Pharmacogenetic analyses of a phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil and leucovorin plus either oxaliplatin or cisplatin: a study of the arbeitsgemeinschaft internistische onkologie. J Clin Oncol 2009; 27: 2863–73. [DOI] [PubMed] [Google Scholar]
- 64.Oldenburg J, Kraggerud SM, Brydoy M, Cvancarova M, Lothe RA, Fossa SD. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med 2007; 5: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamba JK, Fridley BL, Ghosh TM, Yu Q, Mehta G, Gupta P. Genetic variation in platinating agent and taxane pathway genes as predictors of outcome and toxicity in advanced non-small-cell lung cancer. Pharmacogenomics J 2014; 15: 1565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dolan ME, El Charif O, Wheeler HE, et al. Clinical and Genome-Wide Analysis of Cisplatin-Induced Peripheral Neuropathy in Survivors of Adult-Onset Cancer. Clin Cancer Res 2017; 23: 5757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agrawal V, Smelser DT, Carey DJ, Khan SS, Vadakara J. Association of CEP72 genotype with chemotherapy-induced neuropathy. J Clin Oncol 2016; 34: e14107–e. [Google Scholar]
- 68.Stock W, Diouf B, Crews KR, et al. An Inherited Genetic Variant in CEP72 Promoter Predisposes to Vincristine-Induced Peripheral Neuropathy in Adults With Acute Lymphoblastic Leukemia. Clin Pharmacol Ther 2017; 101: 391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gamazon ER, Wheeler HE, Shah KP, et al. A gene-based association method for mapping traits using reference transcriptome data. Nat Genet 2015; 47: 1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patidar PL, Motea EA, Fattah FJ, et al. The Kub5-Hera/RPRD1B interactome: a novel role in preserving genetic stability by regulating DNA mismatch repair. Nucleic Acids Res 2016; 44: 1718–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morales JC, Richard P, Rommel A, et al. Kub5-Hera, the human Rtt103 homolog, plays dual functional roles in transcription termination and DNA repair. Nucleic Acids Res 2014; 42: 4996–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diouf B, Crews KR, Evans WE. Vincristine pharmacogenomics: ‘winner’s curse’ or a different phenotype? Pharmacogenet Genomics 2016; 26: 51–2. [DOI] [PubMed] [Google Scholar]
- 73.Wijmenga C, Zhernakova A. The importance of cohort studies in the post-GWAS era. Nat Genet 2018; 50: 322–8. [DOI] [PubMed] [Google Scholar]
- 74.Neumann Y, Toren A, Rechavi G, et al. Vincristine treatment triggering the expression of asymptomatic Charcot-Marie-Tooth disease. Med Pediatr Oncol 1996; 26: 280–3.76. [DOI] [PubMed] [Google Scholar]
- 75.Schulz A, Grafe P, Hagel C, et al. Neuropathies in the setting of Neurofibromatosis tumor syndromes: Complexities and opportunities. Exp Neurol 2018; 299: 334–44. [DOI] [PubMed] [Google Scholar]
- 76.Lavoie Smith EM, Li L, Hutchinson RJ, et al. Measuring vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. Cancer Nurs 2013; 36: E49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gilchrist LS, Tanner L. The pediatric-modified total neuropathy score: a reliable and valid measure of chemotherapy-induced peripheral neuropathy in children with non-CNS cancers. Support Care Cancer 2013; 21: 847–56. [DOI] [PubMed] [Google Scholar]
- 78.Gilchrist LS, Marais L, Tanner L. Comparison of two chemotherapy-induced peripheral neuropathy measurement approaches in children. Support Care Cancer 2014; 22: 359–66. [DOI] [PubMed] [Google Scholar]
- 79.Tabori U, Sung L, Hukin J, et al. Medulloblastoma in the second decade of life: a specific group with respect to toxicity and management: a Canadian Pediatric Brain Tumor Consortium Study. Cancer 2005; 103: 1874–80. [DOI] [PubMed] [Google Scholar]
- 80.Anghelescu DL, Faughnan LG, Jeha S, et al. Neuropathic pain during treatment for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 2011; 57: 1147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moriyama B, Henning SA, Leung J, et al. Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses 2012; 55: 290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Courtemanche H, Magot A, Ollivier Y, et al. Vincristine-induced neuropathy: Atypical electrophysiological patterns in children. Muscle Nerve 2015; 52: 981–5. [DOI] [PubMed] [Google Scholar]
- 83.Lieber S, Blankenburg M, Apel K, Hirschfeld G, Hernaiz Driever P, Reindl T. Small-fiber neuropathy and pain sensitization in survivors of pediatric acute lymphoblastic leukemia. Eur J Paediatr Neurol 2018; 22: 457–69. [DOI] [PubMed] [Google Scholar]
- 84.Bruininks RH, Bruininks BD. BOT=2: Bruininks-Oseretsky Test of Motor Proficiency. 2nd ed: Pearson Assessments; 2005. [Google Scholar]
- 85.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014; 32: 1941–67. [DOI] [PubMed] [Google Scholar]
- 86.Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA 2013; 309: 1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moore A, Derry S, Wiffen P. Gabapentin for Chronic Neuropathic Pain. JAMA 2018; 319: 818–9. [DOI] [PubMed] [Google Scholar]
- 88.Vondracek P, Oslejskova H, Kepak T, et al. Efficacy of pregabalin in neuropathic pain in paediatric oncological patients. Eur J Paediatr Neurol 2009; 13: 332–6. [DOI] [PubMed] [Google Scholar]
- 89.Mora E, Smith EM, Donohoe C, Hertz DL. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res 2016; 6: 2416–30. [PMC free article] [PubMed] [Google Scholar]
- 90.van de Velde ME, Kaspers GL, Abbink FCH, Wilhelm AJ, Ket JCF, van den Berg MH. Vincristine-induced peripheral neuropathy in children with cancer: A systematic review. Crit Rev Oncol Hematol 2017; 114: 114–30. [DOI] [PubMed] [Google Scholar]
- 91.Naumann R, Mohm J, Reuner U, Kroschinsky F, Rautenstrauss B, Ehninger G. Early recognition of hereditary motor and sensory neuropathy type 1 can avoid life-threatening vincristine neurotoxicity. Br J Haematol 2001; 115: 323–5. [DOI] [PubMed] [Google Scholar]
- 92.O’Brien S, Schiller G, Lister J, et al. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. J Clin Oncol 2013; 31: 676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wacker K, Tanner L, Ovans J, Mason J, Gilchrist L. Improving Functional Mobility in Children and Adolescents Undergoing Treatment for Non-Central Nervous System Cancers: A Systematic Review. Am J Phys Med Rehabil 2017; 9: S385–S97. [DOI] [PubMed] [Google Scholar]
- 94.Tanner LR, Hooke MC, Hinshon S, Hansen CR. Effect of an Ankle Foot Orthosis Intervention for Children With Non-Central Nervous System Cancers: A Pilot Study. Pediatr Phys Ther 2015; 27: 425–31. [DOI] [PubMed] [Google Scholar]
- 95.Casanova-Garcia C, Lerma Lara S, Perez Ruiz M, Ruano Dominguez D, Santana Sosa E. Non-pharmacological treatment for neuropathic pain in children with cancer. Med Hypotheses 2015; 85: 791–7. [DOI] [PubMed] [Google Scholar]
- 96.Kleckner IR, Kamen C, Gewandter JS, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer 2018; 26: 1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fernandes J, Kumar S. Effect of lower limb closed kinematic chain exercises on balance in patients with chemotherapy-induced peripheral neuropathy: a pilot study. Int J Rehabil Res 2016; 39: 368–71. [DOI] [PubMed] [Google Scholar]
- 98.Gilchrist LS, Tanner LR. Short-Term Recovery of Balance Control: Association With Chemotherapy-Induced Peripheral Neuropathy in Pediatric Oncology. Pediatr Phys Ther 2018; 30: 119–24. [DOI] [PubMed] [Google Scholar]
- 99.Reinders-Messelink H, Schoemaker M, Snijders T, et al. Motor performance of children during treatment for acute lymphoblastic leukemia. Med Pediatr Oncol 1999; 33: 545–50. [DOI] [PubMed] [Google Scholar]


