Abstract
Objective:
To examine whether abnormal subcutaneous (SC) abdominal adipose stem cell (ASC) development to adipocytes in polycystic ovary syndrome (PCOS) correlates with hyperandrogenism.
Design:
Prospective cohort study
Setting:
Academic medical center
Patients:
Eight normal-weight PCOS women and eight age-and body mass index-matched normoandrogenic ovulatory (control) women.
Intervention(s):
All women underwent circulating hormone and metabolic measurements, intravenous glucose tolerance testing, total body dual-energy x-ray absorptiometry and SC abdominal fat biopsy.
Main Outcome Measure(s):
In vitro ASC commitment to preadipocytes (ZFP423 protein expression, day 0.5), preadipocyte differentiation to adipocytes (PPARγ gene expression, day 3) and adipocyte lipid content (Oil-Red-O fluorescence, day 12) were compared between PCOS and control women and correlated with clinical outcomes.
Results:
SC abdominal ASCs from PCOS compared to control women showed exaggerated commitment to preadipocytes and had greater lipid content in newly-formed adipocytes following in vitro maturation. In all women combined, ZFP423 protein expression negatively correlated with fasting plasma glucose levels, while lipid content of newly-formed adipocytes positively correlated with both PPARγ gene expression and serum free testosterone (T) levels.
Conclusion:
In normal-weight PCOS vs control women, exaggerated SC abdominal ASC commitment to preadipocytes and enhanced adipocyte lipid content during maturation in vitro negatively and positively correlate with circulating fasting glucose and androgen levels, respectively, as a possible mechanism to maintain glucose-insulin homeostasis when fat accretion is accelerated.
Keywords: Stem cell, polycystic ovary syndrome, hyperandrogenism, adipocyte, abdominal adiposity
CAPSULE:
Subcutaneous abdominal adipose stem cells of normal-weight polycystic ovary syndrome women show exaggerated development to newly-formed adipocytes in vitro vs age-and body mass index-matched controls.
Polycystic ovary syndrome (PCOS), affecting 6-10% of reproductive-aged women, is characterized by hyperandrogenism, menstrual irregularity and polycystic ovarian morphology (1). Women with PCOS by 1990 National Institutes of Health (NIH) criteria also preferentially accumulate abdominal fat with weight gain (1-3), promoting insulin resistance through intraabdominal fat deposition as a risk factor for metabolic syndrome (3-5). Specifically, radiographic studies in PCOS women confirm increased abdominal body fat underlying insulin resistance over a wide body mass index (BMI) range (6), with greater intra-abdominal fat mass in normal-weight PCOS women compared to age-and BMI-matched control women (7).
This interrelationship of hyperandrogenism, preferential abdominal fat deposition and insulin resistance also involves subcutaneous (SC) abdominal adiposity. Normally, when caloric intake exceeds energy utilization, SC adipose increases lipid storage capacity through adipocyte enlargement and new adipocyte formation to buffer fatty acid influx (8, 9). SC abdominal fat in PCOS, however, exhibits abnormal adipocyte size (7, 10), inhibition of lipolysis by androgen (11) and lipolytic catecholamine resistance (12). Moreover, overweight/obese PCOS women have metabolic inflexibility, as evidenced in vivo by diminished fasting fat oxidation from impaired lipolysis and reduced insulin-induced carbohydrate oxidation (13, 14). Such abnormal lipid metabolism, combined with testosterone (T) inhibition of early-stage SC abdominal adipogenesis, whereby multipotent adipose stem cells (ASCs) undergo commitment to preadipocytes and differentiate into adipocytes (15), could promote ectopic lipid deposition, leading to insulin resistance through lipotoxicity (16,17).
In this regard, important cellular and molecular markers of adipogenesis include ZFP423 protein, a recognized cellular marker of ASC commitment to preadipocytes (18); PPARγ, the master adipogenic gene responsible for regulating early adipogenic differentiation (19); and adipocyte lipid content (20). Therefore, the present study examines these cellular and molecular characteristics of SC abdominal adipogenesis in normal-weight PCOS and age-/BMI-matched normoandrogenic ovulatory (control) women to determine whether female type differences exist during ASC development to newly-formed adipocyte formation in vitro and, if so, whether such differences correlate with clinical outcomes. The hypothesis of this study is that SC abdominal ASCs of normal-weight PCOS women with hyperandrogenism exhibit abnormal development to adipocytes in vitro that alters the balance between adipogenesis and glucose-insulin homeostasis.
MATERIALS AND METHODS
Study Participants
Eight normal-weight PCOS and 8 control women (18-35 years; 18.5-25 kg/m2) were selected from our NIH-funded study (P50 HD071836) that originally compared body composition and fat distribution between normal-weight PCOS and control women (7). Each PCOS woman was matched to an individual control woman who was within 1.5 ± 0.6 years (mean ± SEM) and 1.0 ± 0.3 kg/m2 (mean ± SEM) of her own age and BMI, respectively. All subjects were non-Hispanic Caucasian to avoid confounding variation by ethnicity and were in good health and recruited from the general community, although one PCOS subject had attended an outpatient clinic for hyperandrogenic symptoms. All subjects had a stable BMI for at least three months before study, which remained unchanged throughout the study. Clinical and hormonal data from all control women and 5 of the 8 PCOS women were published previously (7).
PCOS was diagnosed by 1990 NIH criteria, eliminating other endocrinopathies to avoid the phenotypic variability of Rotterdam criteria (1). Hirsutism was defined as a modified Ferriman-Gallwey (mFG) score ≥ 6 (21). Biochemical hyperandrogenism was specified as an elevated mean serum total or free T (from two independent blood samples drawn on different days) > 2 SD above the normal ranges of the age- and BMI- matched control group (7). All control women had normal menstrual cycles at 21- to 35-day intervals and a luteal phase progesterone (P4) level without hirsutism, acne or alopecia (1).
Exclusion criteria included late-onset congenital adrenal hyperplasia (basal serum 17-hydroxyprogesterone > 2 ng/mL), thyroid dysfunction, hyperprolactinemia, ovarian cyst(s) > 2 cm; present/past history of smoking (<1 year), cancer, drug addiction, alcohol abuse, depression, diabetes, hepatic or renal disease; uncontrolled hypertension (≥ 165/100) or other major medical illness. Further exclusion criteria included recent (<3 months) consumption of antidepressants, diabetes medications, weight loss medications, androgens, anabolic steroids or hormonal agents (i.e. oral contraceptives/insulin sensitizers) and beta-adrenergic blocking agents.
All studies were performed in accordance with the Declaration of Helsinki and approved by the UCLA Institutional Review Board, with informed consent obtained from each subject before study participation.
Anthropometric Analysis
The waist-to-hip circumference ratio was determined with the subject in the standing position. Waist and hip circumferences were defined as girth at the upper border of the iliac crests and the largest girth over the greater trochanters, respectively (22).
Blood sampling
Blood sampling was performed in control women during the follicular phase (days 5–10 of the menstrual cycle) and in PCOS women during documented oligo- anovulation by serum P4 level. Fasting blood samples were collected immediately before a frequently sampled intravenous glucose tolerance test (FSIGT) as previously described (7, 23) to determine total and free T, androstenedione (A4), dihydrotestosterone (DHT), dehydroepiandrosterone sulfate (DHEAS), gonadotropins, estrone (E1), estradiol (E2), glucose, insulin, sex hormone binding globulin (SHBG) and lipid (total cholesterol, high density lipoprotein [HDL], low density lipoprotein [LDL], triglycerides [TG]) levels. The modified minimal model of Bergman was used to define insulin sensitivity (Si) (23).
Hormone and metabolite assays
Serum levels of total T, A4, DHEAS, DHT, and E1 were quantified by liquid chromatography tandem mass spectrometry (Quest Diagnostics Nichols Institute, San Juan Capistrano, CA). The manufacturer intra-assay coefficient of variation (CVs) were: total T, 5.6%; A4, 3.8%; DHEAS, 4.5%; DHT, 14.8%; and E1, 3%. The manufacturer inter-assay CVs were: total T, 9.5%; A4, 5.2%; DHEAS, 5.5%; DHT, 13.6%; and E1, 14.7%. Free T was determined by the concentrations of total T, SHBG, and albumin. The intra- and inter-assay CVs for free T were 10.3% and 11.7%, respectively.
The UCLA Center for Pathology Research Services measured serum insulin, LH, FSH, and E2 by electrochemiluminescence; glucose by a hexokinase method; and fasting lipids (total cholesterol, LDL, HDL and TG) by spectrophotometry. The laboratory intra-assays CVs were: insulin, 0.6%; LH, 1.0%; FSH, 2.1%; E2, 7.0%; glucose, 0.8%; total cholesterol, 0.7%; LDL, 0.5%; HDL 0.6%; and TG, 0.6%. The laboratory inter-assays CVs were: insulin, 2.6%; LH, 2.3%; FSH, 2.8 %; E2, 10.7%; glucose, 0.9%; total cholesterol, 1.0%; LDL, 1.2%; HDL, 0.9%; and TG, 0.7%.
Total body DXA
Total body dual-energy x-ray absorptiometry (DXA) was performed as previously reported (7). The android (i.e., abdominal) region by DXA spanned from the first lumbar vertebra to the superior aspect of pelvis; the gynoid region extended from the head of the femur to mid-thigh.
SC Abdominal Fat Biopsy
A SC abdominal fat biopsy was performed in each subject to obtain 0.5-1.0 gram of tissue (7), which was either immediately stored in liquid nitrogen or used for ASC studies in all PCOS and control women (N=16).
Isolation of SC abdominal ASCs
Freshly obtained adipose tissue was finely minced and treated with 0.1% collagenase (Sigma Aldrich, St. Louis, MO) and diluted in DMEM (Corning, Corning, NY) for 45-60 minutes at 37°C. The digested material was filtered through 150-micron nylon mesh (Ted Pella Inc., Redding, CA), washed with full medium solution (containing DMEM with 10% fetal calf serum [Life Technologies, Carlsbad, CA] and 1% Antibiotic-Antimycotic [100x] solution [Thermo Fisher Scientific, Waltham, MA]) and centrifuged at 200 RPM for 2 minutes at 20°C. Adipocytes floating in the upper layer were removed by pipetting, while the lower phase underwent additional centrifugation at 1200 RPM for 5 minutes at 20°C to obtain the cell pellet containing the stromal vascular fraction (24). The cell pellet was washed twice, resuspended in full culture medium and plated into 60 mm adherent culture dishes. After one week in culture, all cells were identified as CD105 (+) ASCs (25). After reaching confluency, ASCs were uniformly expanded to a second generation of cells without androgens, which were used for the studies.
Cell Culture
Adipocyte differentiation medium (DMEM/Ham’s F-12 [1:1, v/v], HEPES pH 7.4, fetal bovine serum, biotin, pantothenate, human insulin, dexamethasone, 3-Isobutyl-1-methylxanthine [IBMX], PPARγ agonist, penicillin, streptomycin, amphotericin B [Zen-Bio, Research Triangle Park, NC]) was used to differentiate ASCs into newly-formed adipocytes (15). Cell culture medium was changed every 48 hours. At each time point (Day 0, 0.5, 3, 7, 12) cells were fixed in 4% paraformaldehyde for lipid content and immunofluorescence studies. Another aliquot of cells was used for gene expression studies.
Immunofluorescence
At the end of each time course, fixed cells were treated with 0.2% Triton-X-100 (Sigma Aldrich, St. Louis, MO) for 20 minutes, washed twice with PBS, and incubated for 1 hour at RT in blocking solution (10% normal goat serum/1% bovine serum albumin [BSA] in PBS [Thermo Fisher Scientific, Waltham, MA; Sigma Aldrich, St. Louis, MO]). Cells were initially incubated with primary antibody for CD105 (1:300 [Abcam, Cambridge, MA]) overnight at 4°C following standard protocols to confirm the mesenchymal stem cell phenotype before specific lineage commitment and differentiation (26,27) (data not shown). Primary antibody for ZFP423 (1:300 [Abcam, Cambridge, MA]) also was used to identify preadipocytes derived from ASCs (18, 28). After incubation with primary CD105 or ZFP423 antibody, cells were washed 4 times and then incubated for 1 hour with a secondary antibody labeled with Texas Red fluorescence marker (1:300 [Thermo Fisher Scientific, Waltham, MA]), followed by an additional incubation for 10 min with nuclear staining marker 4’,6-diamidino-2-phenoylidole (DAPI) (1:3000 [Invitrogen, Carlsbad, CA]). Twenty representative images were subsequently taken of the fluorescent cells with an EVOS FL Digital Inverted Fluorescence microscope (Westover Scientific Inc, Bothell, WA) and the fluorescence units per cell were quantified using ImageJ software (NIH, Bethesda, MD).
Lipid Staining
An additional aliquot of fixed cells was stained with Oil-Red-O (Sigma Aldrich, St. Louis, MO) for 20 min at RT to visualize lipid droplets following manufacturer's guidelines. Nuclei were identified by DAPI staining. After 4 washes with deionized water, lipid staining was quantified by fluorescence units as above.
Gene Expression
At each respective time point, total RNA was isolated using RNeasy kits (Qiagen, Hilden, Germany). First strand cDNA was synthesized with a PTC-200 Peltier Thermal Cycler machine (MJ Research, Waltham, MA) using the high capacity cDNA reverse transcriptase kit (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. qRT-PCR was performed with the Taq PCR Master Mix kit (Qiagen, Hilden, Germany) using primers for PPARγ (Thermo Fisher Scientific, Waltham, MA). Primers for β-actin and GAPDH (Thermo Fisher Scientific, Waltham, MA) were used as internal controls. Relative expression of target genes to β-actin or GAPDH was measured using the comparative critical threshold (Ct) method. The results were expressed as fold-change obtained from triplicate values.
Statistical analysis
A paired Student’s t-test was used to compare approximately normally distributed patient characteristics and clinical data between PCOS and age and BMI pair-matched control subjects. Due to large within-female type variation, Wilcoxon-signed rank test was used to compare in vitro stem cell characteristics between PCOS vs their matched control subjects. Laboratory measures exhibiting skewed distributions and outliers (i.e., ZFP423 protein and PPARγ gene expressions, newly-formed adipocyte lipid content, serum total T levels) were log transformed prior to statistical analysis (29). Pearson correlation coefficients were used to assess associations between stem cell characteristics and relevant clinical parameters. Partial correlation coefficients were used to determine if associations remained significant after adjustment for serum total T or fasting insulin levels.
RESULTS
Patient characteristics
Age, BMI, waist and hip measurements were similar between PCOS and control women (Table 1). Serum total and free T levels were significantly greater, while serum A4 levels tended to be higher, in PCOS than control women. Fasting plasma insulin levels tended to be slightly higher in PCOS than control women, while Si values in PCOS women were within the lower-normal range. Serum gonadotropin, DHT, DHEAS, E1, E2 and fasting glucose levels were comparable between female groups, as were fasting serum lipid levels (data not shown).
Table 1:
Patient characteristics in normal-weight PCOS versus Control women.
| Patient Characteristics | PCOS (N=8) |
Control (N=8) |
P value |
|---|---|---|---|
| Age (years) | 25.5±1.4 | 25.5±1.9 | 1.0 |
| BMI (kg/m2) | 22.7±0.7 | 22.4±0.5 | 0.56 |
| Waist size (cm) | 77.3±1.9 | 78.1±1.8 | 0.77 |
| Hip size (cm) | 89.5±1.9 | 91.6±2.1 | 0.47 |
| Serum log total T (ng/dL) | 1.8±0.04 | 1.5±0.04 | 0.002 |
| Serum free T (pg/mL) | 6.1±0.5 | 2.5±0.5 | 0.002 |
| Serum A4 (ng/dL) | 211.1±33.5 | 127.5±14.6 | 0.08 |
| Serum DHT (ng/dL) | 12.0±2.6 | 8.1±0.9 | 0.21 |
| Serum DHEAS (μg/dL) | 213.6±20.1 | 199.9±44.6 | 0.82 |
| Serum E1 (pg/mL) | 80.4±10.4 | 72.0±11.9 | 0.42 |
| Serum E2 (pg/mL) | 59.8±7.6 | 107.0±34.9 | 0.19 |
| Serum LH (mIU/mL) | 17.1±2.8 | 10.8±2.2 | 0.15 |
| Serum FSH (mIU/mL) | 5.5±0.2 | 6.8±0.9 | 0.26 |
| Fasting Plasma Glucose (mg/dL) | 85.4±1.5 | 84.9±2.3 | 0.81 |
| Fasting Serum Insulin (μU/mL) | 6.2±0.8 | 4.3±0.6 | 0.08 |
| Insulin Sensitivity (x10−4/min/U/mL) | 4.9±0.9 | 8.5±2.1 | 0.12 |
| % Android Fat/Total Body Fat | 6.7±0.5 | 5.4±0.2 | 0.016 |
| % Gynoid Fat/Total Body Fat | 20.7±0.3 | 21.6±0.6 | 0.18 |
| Total Body Fat (kg) | 20.0±1.5 | 21.2±0.9 | 0.46 |
| % Total Body Fat/Total Body Mass | 32.1±1.6 | 31.8±1.1 | 0.85 |
Mean ± SEM
Conversion to SI Units: T (X 0.0347 nmol/L), free T (X 3.47 pmol/L), A4 (X 0.0349 nmol/L), DHT (X 0.0344 nmol/L), DHEAS (X 0.0271 μmol/L), E1 (X 3.699 pmol/L), E2 (X 3.67 pmol/L), LH (X 1.0 IU/L), FSH (X 1.0 IU/L), glucose (X 0.0555 mmol/L), insulin (X 7.175 pmol/L)
Dual-energy x-ray absorptiometry showed comparable amounts of total body fat mass and percent total body fat between PCOS and control women. The percent android fat relative to total body fat, however, was significantly greater in PCOS than control women, while the percent gynoid fat relative to total body fat was not. Total body lean mass was similar between PCOS women (40.7±2.0 kgs) and controls (42.9±1.4 kgs, P=0.38).
SC abdominal ASC characteristics
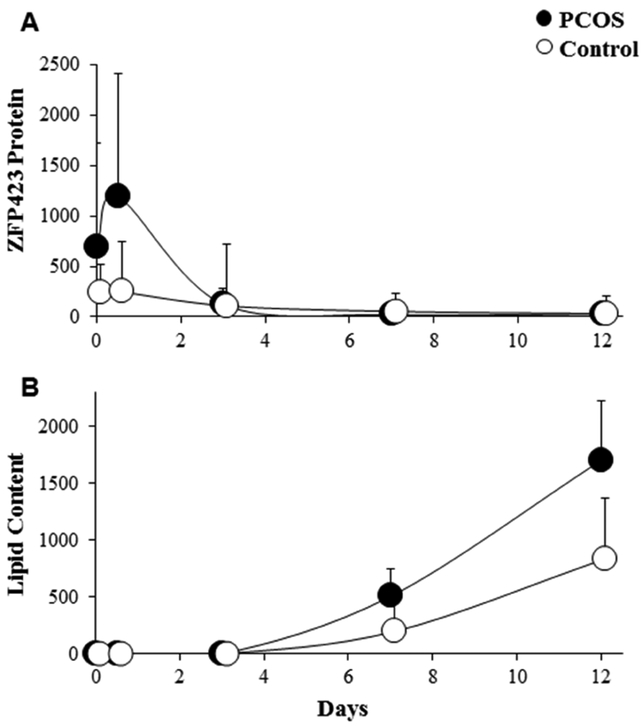
Subcutaneous abdominal ASCs cultured in adipogenic medium for 12 days showed temporal changes in stem cell phenotype that confirmed initial ASC commitment to preadipocytes followed by preadipocyte differentiation to newly-formed adipocytes. In control cells, ZFP423 protein expression reached maximal median levels at day 0.5 and then declined to lower levels by day 7 (Figures 1A and2). The lipid content of these cells was low until day 7 and then dramatically increased to reach maximal median levels in newly-formed adipocytes on day 12 (Figures 1B and2). In PCOS cells, ZFP423 protein expression rose to maximal median levels on day 0.5 (Figures 1A and2) that were significantly greater than those of control cells (P=0.012, Supplemental Figure 1A) before declining to low levels. In all pair-matched cell samples, the increase in ZFP423 protein expression in PCOS cells was either earlier in time and/or greater in magnitude than control cells on days 0 to 0.5, with 3 and 5 PCOS cell samples showing peak ZFP423 protein expressions on days 0 and 0.5, respectively (Supplemental Figure 2). The lipid content of PCOS cells increased by day 7 to reach maximal levels in newly-formed PCOS adipocytes on day 12 (Figures 1B and2) that were significantly greater than those of control adipocytes on the same day (P=0.012, Supplemental Figure 1B). In cells from all women combined, PPARγ gene expression on day 3 positively correlated with lipid content on day 12 (r=0.64, P=0.008), being slightly greater in PCOS than control cells (P=0.1). Furthermore, PPARγ gene expression on day 3 also showed a nonsignificant positive trend with serum total T (r=0.38, P=0.14) and free T (r=0.41, P=0.12) levels.
Figure 1:
Temporal changes of (A) ZFP423 protein expression and (B) lipid content in SC abdominal ASCs cultured for days 0, 0.5, 3, 7 and 12 in adipogenic medium as a function of Texas Red and Oil-Red-O fluorescence, respectively, divided by DAPI. Values are expressed as median ± 95% confidence intervals of 8 age and BMI pair-matched normal-weight PCOS and control women.
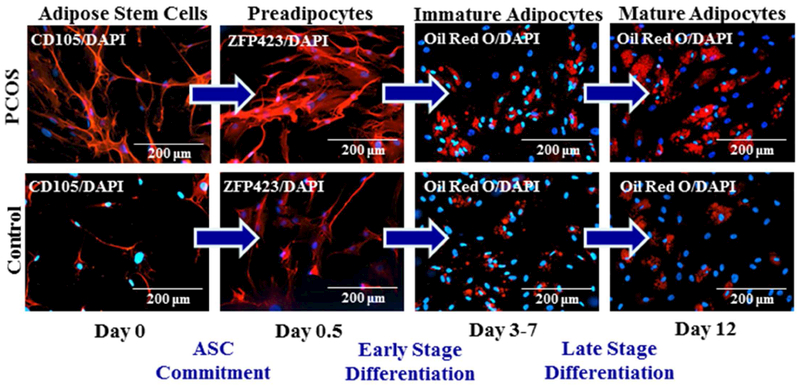
Figure 2:
Fluorescent changes of SC abdominal ASCs from a representative age and BMI pair-matched normal-weight PCOS and control woman and cultured in adipogenic medium for 12 days. Cultured ASCs underwent commitment to preadipocytes (CD105 protein expression, day 0 to ZFP423 protein expression, day 0.5) and then differentiated into immature and mature newly-formed adipocytes (Oil Red O lipid content, days 3-7 and 12, respectively).
Clinical Correlations
In SC abdominal ASCs of all women combined, ZFP423 protein expression on day 0.5 and lipid content of newly-formed adipocytes on day 12 correlated with specific clinical outcomes. On day 0.5, ZFP423 protein expression significantly negatively correlated with fasting plasma glucose levels (Figure 3A) and remained so after adjusting for serum total T levels (r=−0.78, P=0.001), suggesting that hyperandrogenemia did not influence this relationship. On day 12, lipid content of newly-formed adipocytes significantly positively correlated with serum free T levels (Figure 3B) and tended to similarly correlate with serum total T levels (Figure 3C). After adjusting for fasting plasma insulin levels, however, the significant positive correlation between lipid content of newly-formed adipocytes on day 12 and serum free T levels (r=0.55, P=0.03), was reduced to a trend (r=0.48, P=0.07), while that with total T levels also was diminished (unadjusted: r=0.49, P=0.05; adjusted: r=0.41, P=0.1), suggesting possible androgen-insulin interactions governing adipocyte lipid content. Neither cellular ZFP423 protein expression on day 0.5 nor newly-formed adipocyte lipid content on day 12 correlated with Si, fasting lipid levels or percent android fat relative to total body fat (data not shown).
Figure 3:
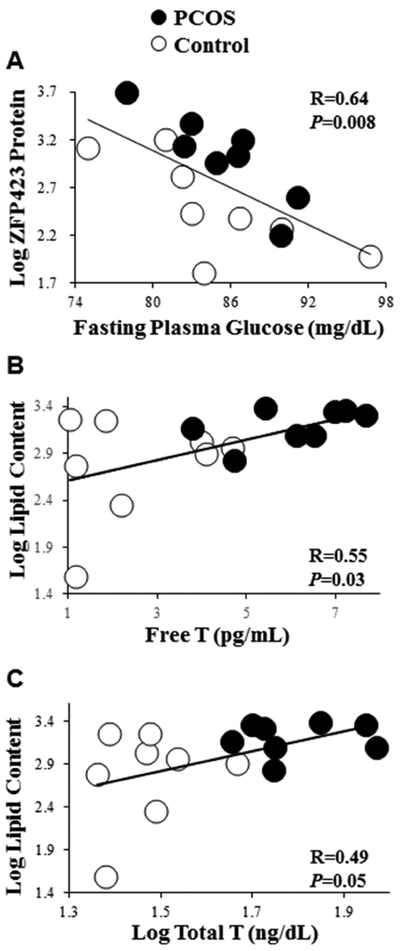
Correlations of log ZFP423 protein expression (day 0.5) with (A) fasting plasma glucose levels, and of log lipid content (day 12) with (B) serum free T and (C) log serum total T levels in 8 age and BMI pair-matched normal-weight PCOS and control women. ZFP423 protein expression and lipid content values are expressed as Texas Red and Oil-Red-O fluorescence, respectively, divided by DAPI.
DISCUSSION
Subcutaneous adipose normally increases lipid storage during fatty acid influx through adipocyte enlargement and new adipocyte formation (8, 9). This function of SC abdominal adipose appears perturbed in PCOS women, as evidenced by abnormal adipocyte size (10) and distribution (7), inhibition of lipolysis by androgen (11) and lipolytic catecholamine resistance from diminished protein levels of β2-adrenegic receptor, hormone-sensitive lipase and protein kinase A regulatory-IIβ component (12,30,31). In clinical agreement, overweight/obese PCOS women exhibit metabolic inflexibility through diminished fat oxidation from impaired fasting lipolysis and reduced insulin-induced carbohydrate oxidation (13, 14).
Using SC abdominal ASCs from normal-weight PCOS vs age- and BMI-matched control women, our study shows for the first time greater lipid content in PCOS than control adipocytes during in vitro maturation that positively correlates with serum free T levels in all women combined. Oral DHEA administration to PCOS women diminishes SC abdominal lipolysis, while DHT treatment of cultured human SC preadipocytes (i.e., the Simpson-Golabi-Behmel syndrome [SGBS] cell line) decreases beta-oxidation; increases acetyl-CoA-carboxylase mRNA expression, the rate-limiting step in lipogenesis; and enhances lipogenesis (11). Therefore, SC abdominal ASCs from hyperandrogenic PCOS women may exhibit reduced beta-oxidation and/or enhanced lipogenesis during in vitro maturation of newly-formed adipocytes, even in the absence of androgen in the culture media.
This positive relationship of lipid content in newly-formed adipocytes with serum androgen levels was partially affected by fasting plasma insulin concentrations. In this regard, T administration to female rhesus monkeys fed a Western-style diet enhances insulin-induced FFA uptake in SC abdominal adipose (32). Conversely, insulin stimulation of female SC abdominal adipocytes and human SGBS preadipocytes induces aldo-ketoreductase type 3 1C3 (AKR1C3) activity, the enzyme converting A4 to T, to promote androgen-dependent lipogenesis (11). Such androgen-insulin interactions favoring fat accretion also could affect in vitro maturation of newly-formed PCOS adipocytes and underlie metabolic inflexibility in PCOS women (13,14).
In our study, however, hyperandrogenism as a mechanism to promote fat accretion in newly-formed PCOS adipocytes is unlikely due to extant androgen actions on ASC function since several weeks elapsed between blood collection and SC abdominal fat biopsy, with additional time required for in vitro ASC generation, expansion and development to adipocytes without androgen exposure. Instead, androgen excess as the cardinal feature of PCOS could affect developmental programming of ASCs by hyperandrogenism in utero, as present in female fetuses of PCOS mothers, assuming a critical second trimester time interval when a susceptible fetus is exposed to androgen excess (33). Prenatal androgenization of female rhesus monkeys impairs maternal-fetal glucose-insulin homeostasis (34) as an antecedent to an adult PCOS-like phenotype, consisting of disordered subcutaneous abdominal (15, 35, 36) and visceral adipogenesis (37). Moreover, fetal hyperglycemia from gestational diabetes in women enhances the lipid content of SC abdominal preadipocytes in the adult offspring, independent of BMI or fat mass (38). These findings suggest an altered intrinsic reprogramming of the newly-formed PCOS adipocytes in our study, which may represent epigenetic modifications of genes influencing susceptibility to PCOS (39).
As a marker of preadipocyte differentiation, cellular PPARγ gene expression positively predicted the lipid content of newly-formed adipocytes without a significant female type effect, perhaps due to sustained blunted in vitro PPARγ gene expression in PCOS cells by the previous effect of androgens on SC abdominal ASC commitment to preadipocytes (15). In PCOS, therefore, androgen-constrained SC abdominal preadipocyte differentiation that restricts a population of mature adipocytes with reduced lipolysis and/or enhanced lipogenesis could theoretically promote ectopic lipid deposition to induce insulin resistance through lipotoxicity (16, 17, 40).
ZFP423 protein expression on day 0.5, a marker of ASC commitment to preadipocytes, was greater in PCOS than control cells and negatively correlated with fasting glucose levels in all women combined, adjusting for serum total T levels. Epigenetic changes within the ZFP423 promoter region have been shown to accompany ZFP423 gene up-regulation and enhanced small adipocyte formation to protect against insulin resistance in obese individuals (41), consistent with an increased proportion of small SC abdominal adipocytes in normal-weight PCOS women (7). In addition, prenatal ZFP423 knockout in mice fed a high fat diet induces obesity, ectopic fat deposition and insulin resistance (28), while ZFP423 up-regulation appears to compensate for impaired preadipocyte differentiation in SC abdominal adipose of PCOS-like female rhesus monkeys with insulin resistance (35). Therefore, the degree to which SC abdominal ASC commitment to preadipocytes induces hyperplasia of new-growing adipocytes during fatty acid influx might explain why some (2, 42, 43), but not all (44-46), normal-weight PCOS women maintain normal glucose-insulin homeostasis.
Important strengths of this study include the use of healthy, normal-weight PCOS women by 1990 NIH criteria who were recruited from the general population to study a less severe PCOS phenotype (47) and also were stringently matched to controls by both age and BMI to eliminate confounders affecting insulin action (1). Recruitment of non-Hispanic Caucasian subjects further eliminated ethnic differences in body fat composition and distribution (1). Consequently, in vitro differences in SC abdominal ASC development to adipocytes between PCOS and control women could be examined within a normal range of Si values and correlated with clinical reproductive and metabolic variables. Furthermore, our study is novel in investigating how various stages of SC abdominal adipogenesis, as defined by specific markers of ASC commitment to preadipocytes, preadipocyte differentiation into adipocytes and newly-formed adipocyte lipid content in vitro, differ between normal-weight PCOS women and controls in the absence of extant androgen and correlate with clinical outcomes, potentially identifying inherent PCOS-related abnormalities of stem cell function.
Limitations of our study include its small number of PCOS subjects, which diminished statistical power to examine subtle androgen-insulin interactions governing SC abdominal lipid homeostasis. Stringent subject selection in this study, however, was crucial since BMI, age and race influence adipose stem cell differentiation to adipocytes in mice and humans (48-50) and therefore was necessary to detect female type differences in adipocyte differentiation and adipocyte lipid accumulation that may not have otherwise appeared if such careful measures were not taken. Despite the small amounts of SC abdominal fat obtained at biopsy (approximately 1 gm of adipose), we were able to isolate sufficient ASCs from the adipose stromal-vascular fraction for our studies; however, insufficient number of adipocytes were obtained for additional studies of cellular metabolism and adipocyte function. Our results may not apply to other fat depots, or to individuals of other ethnic groups or who differ in PCOS phenotypic expression. Finally, the role of dexamethasone, as a glucocorticoid in adipogenic medium capable of interacting with the androgen receptor, needs to be examined in future studies that explore development of PCOS vs control stem cells to newly-formed adipocytes in vitro (51).
In conclusion, in normal-weight PCOS vs control women, exaggerated SC abdominal ASC commitment to preadipocytes and enhanced adipocyte lipid content during maturation in vitro negatively and positively correlate with circulating fasting glucose and androgen levels, respectively, as a possible mechanism to maintain glucose-insulin homeostasis when fat accretion is accelerated. Although it may be too soon to draw definitive clinical conclusions from this small study, the preliminary clinical correlates with SC abdominal adipogenesis in this pilot study provide hypotheses for possible pathophysiological mechanisms that can be tested in future studies.
Supplementary Material
Female type differences between 8 age and BMI pair-matched normal-weight PCOS and control women in (A) ZFP423 protein expression (day 0.5) and (B) lipid content (day 12) expressed as Texas Red and Oil-Red-O fluorescence, respectively, divided by DAPI.
Temporal changes of ZFP423 protein expression in SC abdominal ASCs cultured for days 0, 0.5, 3, 7 and 12 in adipogenic medium and expressed as Texas Red fluorescence divided by DAPI. Values are expressed as 8 separate age and BMI pair-matched normal-weight PCOS and control women.
ACKNOWLEDGMENTS
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health under awards P50HD071836 and P51 ODO11092 for the Endocrine Technologies Support Core (ETSC) through the Oregon National Primate Research Center, with statistical analyses supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881. Additional funding was also provided by the Santa Monica Bay Woman's Club. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Karla Largaespada, Tery Schooler and Bette Okeya for subject recruitment strategies and administrative responsibilities that were crucial for the success of these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific Statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36:487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holte J, Bergh T, Berne C, Berglund L, Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab. 1994;78:1052–58. [DOI] [PubMed] [Google Scholar]
- 3.Bjorntorp P Metabolic implications of body fat distribution. Diabet Care 1991; 14:1132–43. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN, et al. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006; 91:48–53. [DOI] [PubMed] [Google Scholar]
- 6.Tosi F, Di Sarra D, Kaufman JM, Bonin C, Moretta R, Bonora E, et al. Total body fat and central fat mass independently predict insulin resistance but not hyperandrogenemia in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100:661–9. [DOI] [PubMed] [Google Scholar]
- 7.Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, et al. Hyperandrogenism Is Accompanied by Preferential Intra-Abdominal Fat Storage In Normal Weight Polycystic Ovary Syndrome Women. J Clin Endocrinol Metab. 2016;101:4178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45:1201–10. [DOI] [PubMed] [Google Scholar]
- 9.Romacho T, Elsen M, Röhrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol. 2014;210:733–53. [DOI] [PubMed] [Google Scholar]
- 10.Mannerås-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, et al. Adipose tissue has aberrant morphology and function in PCOS: Enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96:E304–11. [DOI] [PubMed] [Google Scholar]
- 11.O’Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, et al. AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017;102:3327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faulds G, Rydén M, Ek I, Wahrenberg H, Arner P. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab. 2003; 88:2269–73. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Tfayli H, Michaliszyn SF, Arslanian S. Impaired Lipolysis, Diminished Fat Oxidation, and Metabolic Inflexibility in Obese Girls With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2018;103:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whigham LD, Butz DE, Dashti H, Tonelli M, Johnson LK, Cook ME, et al. Metabolic Evidence of Diminished Lipid Oxidation in Women With Polycystic Ovary Syndrome. Current Metabolomics. 2014;1:269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virtue S, Vidal-Puig A. It’s not how fat you are, it’s what you do with it that counts. PLoS Biol. 2008;6:e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–14. [DOI] [PubMed] [Google Scholar]
- 18.Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, et al. Transcriptional Control of Preadipocyate Determination by Zfp423. Nature. 2010;464:619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke SD, Thuillier P, Baillie RA, and Sha X Peroxisome proliferator-activated receptors: a family of lipid-activated transcription factors. Am. J. Clin. Nutr. 1999;70;566–71. [DOI] [PubMed] [Google Scholar]
- 21.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: A prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. [DOI] [PubMed] [Google Scholar]
- 22.Rosenzweig JL, Ferrannini E, Grundy SM, Haffner SM, Heine RJ, Horton ES, et al. Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008; 93:3671–89. [DOI] [PubMed] [Google Scholar]
- 23.Steil GM, Volund A, Kahn SE, Bergman RN. Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model. Suitability for use in population studies. Diabetes. 1993;42:250–6. [DOI] [PubMed] [Google Scholar]
- 24.Chazenbalk G, Chen YH, Heneidi S, Lee JM, Pall M, Chen YDI, et al. Abnormal Expression of Genes Involved in Inflammation, Lipid Metabolism, and Wnt Signaling in the Adipose Tissue of Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2012;97:E765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem Biophys Res Commun. 1999;265:134–9. [DOI] [PubMed] [Google Scholar]
- 27.Dominici MLBK, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. [DOI] [PubMed] [Google Scholar]
- 28.Shao M, Hepler C, Vishvanath L, MacPherson KA, Busbuso NC, Gupta RK. Fetal development of subcutaneous white adipose tissue is dependent on Zfp423. Mol Metab. 2017;6:111–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sokal RR, Rohlf FJ. Biometry, the principles and practice of statistics in biological research. 3rd ed New York: WH Freeman and Co., 1995. [Google Scholar]
- 30.Arner P Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie. 2005;87:39–43. [DOI] [PubMed] [Google Scholar]
- 31.Dicker A, Rydén M, Näslund E, Muehlen IE, Wirén M, Lafontan M, et al. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia. 2004;47:420–8. [DOI] [PubMed] [Google Scholar]
- 32.Varlamov O, Chu MP, McGee WK, Cameron JL, O'Rourke RW, Meyer KA, et al. Ovarian cycle-specific regulation of adipose tissue lipid storage by testosterone in female nonhuman primates. Endocrinology. 2013;154:4126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palomba S, Marotta R, Di Cello A, Russo T, Falbo A, Orio F, et al. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol. 2012;77:898–904. [DOI] [PubMed] [Google Scholar]
- 34.Abbott DH, Bruns CR, Barnett DK, Dunaif A, Goodfriend TL, Dumesic DA, et al. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. Am J Physiol Endocrinol Metab. 2010;299:E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller E, Chazenbalk GD, Aguilera P, Madrigal V, Grogan T, Elashoff D, et al. Impaired preadipocyte differentiation into adipocytes in subcutaneous abdominal adipose of PCOS-like female rhesus monkeys. Endocrinology. 2014;155:2696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heneidi S, Simerman AA, Keller E, Singh P, Li X, Dumesic DA, et al. Awakened by cellular stress: isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PLoS One. 2013;8:e64752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu N, Kwon S, Abbott DH, Geller DH, Dumesic DA, Azziz R, et al. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PLoS One. 2011;6:e27286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen NS, Strasko KS, Hjort L, Kelstrup L, Houshmand-Oregaard A, Schrolkamp M, et al. Fetal hyperglycemia changes human preadipocyte function in adult life. J Clin Endocrinol Metab. 2017;102:1141–1150. [DOI] [PubMed] [Google Scholar]
- 39.Chang RJ, Dumesic DA. Polycystic Ovary Syndrome and Hyperandrogenic States In: Strauss JF III, Barbieri RL, eds. Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management, Eighth Edition. Philadelphia: Elsevier Saunders, 2019:520–55. [Google Scholar]
- 40.de Zegher F, Lopez-Bermejo A, Ibáñez L. Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metab. 2009;20:418–23. [DOI] [PubMed] [Google Scholar]
- 41.Longo M, Raciti GA, Zatterale F, Parrillo L, Desiderio A, Spinelli R, et al. Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity. Diabetologia. 2018;61:369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vrbíková J, Cibula D, Dvoráková K, Stanická S, Sindelka G, Hill M, et al. Insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89: 2942–5. [DOI] [PubMed] [Google Scholar]
- 43.Ovesen P, Moller J, Ingerslev HJ, Jørgensen JO, Mengel A, Schmitz O, et al. Normal basal and insulin-stimulated fuel metabolism in lean women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1993;77:1636–40. [DOI] [PubMed] [Google Scholar]
- 44.Rebuffé-Scrive M, Cullberg G, Lundberg PA, Lindstedt G, Björntorp P. Anthropometric variables and metabolism in polycystic ovarian disease. Horm Metab Res. 1989;21:391–7. [DOI] [PubMed] [Google Scholar]
- 45.Diamanti-Kandarakis E, Mitrakou A, Hennes MM, Platanissiotis D, Kaklas N, Spina J, et al. Insulin sensitivity and antiandrogenic therapy in women with polycystic ovary syndrome. Metabolism. 1995;44:525–31. [DOI] [PubMed] [Google Scholar]
- 46.Morales AJ, Laughlin GA, Bützow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab. 1996;81:2854–64. [DOI] [PubMed] [Google Scholar]
- 47.Ezeh U, Yildiz BO, Azziz R. Referral bias in defining the phenotype and prevalence of obesity in polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98:E1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donato AJ, Grant GD, Hart CR, Layec G, Trinity JD, Bramwell RC, et al. The impact of ageing on adipose structure, function and vasculature in the B6D2F1 mouse: evidence of significant multisystem dysfunction. J Physiol 2014;592: 4083–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Harmelen V, Skurk T, Rohrig K, Lee YM, Halbleib M, Aprath-Husmann I, et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women of BMI and age on adipose tissue cellularity and differentiation capacity in women. International J of Obesity. 2003;27: 889–895. [DOI] [PubMed] [Google Scholar]
- 51.Hartig SM, He B, Newberg JY, Ochsner SA, Loose DS, Lanz RB, et al. Feed-forward inhibition of androgen receptor activity by glucocorticoid action in human adipocytes. Chem Biol. 2012;19:1126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Female type differences between 8 age and BMI pair-matched normal-weight PCOS and control women in (A) ZFP423 protein expression (day 0.5) and (B) lipid content (day 12) expressed as Texas Red and Oil-Red-O fluorescence, respectively, divided by DAPI.
Temporal changes of ZFP423 protein expression in SC abdominal ASCs cultured for days 0, 0.5, 3, 7 and 12 in adipogenic medium and expressed as Texas Red fluorescence divided by DAPI. Values are expressed as 8 separate age and BMI pair-matched normal-weight PCOS and control women.