Abstract
Objective
Clinical anxiety is prevalent, highly comorbid with other conditions, and associated with significant medical morbidity, disability, and public health burden. Excessive attentional deployment towards threat is a transdiagnostic dimension of anxiety seen at both initial and sustained stages of threat processing. However, group-level observations of these phenomena mask considerable within-group heterogeneity that has been linked to treatment outcomes, suggesting that a transdiagnostic, individual differences approach may capture critical, clinically relevant information.
Methods
70 clinically anxious individuals were randomized to receive 8 sessions of Attention Bias Modification (ABM; n=41 included in analysis), a computer-based mechanistic intervention that specifically targets initial stages of threat processing, or a sham control (n=21). Participants completed a mixed block/event-related fMRI task optimized to discriminate transient from sustained neural responses to threat.
Results
Larger transient responses across a wide range of cognitive-affective regions (e.g., ventrolateral prefrontal cortex, anterior cingulate cortex, amygdala) predicted better clinical outcomes following ABM, in both a priori anatomical regions and whole-brain analyses; sustained responses did not. A spatial pattern recognition algorithm using transient threat responses successfully discriminated the top quartile of ABM responders with 68% accuracy.
Conclusions
Neural alterations occurring on the relatively transient timescale that is specifically targeted by ABM predict favorable clinical outcomes. Results inform how to expand on the initial promise of neurocognitive treatments like ABM by fine-tuning their clinical indications (e.g., through personalized mechanistic intervention relevant across diagnoses) and by increasing the range of mechanisms that can be targeted (e.g., through synergistic treatment combinations and/or novel neurocognitive training protocols designed to tackle identified predictors of nonresponse).
Keywords: anxiety, attention bias modification, fMRI, threat processing
Introduction
Clinical anxiety is a prevalent and disabling condition (Kessler, 2007; Kessler, Chiu, Demler, Merikangas, & Walters, 2005) characterized by excessive attention to threat and associated prefrontal-limbic circuit disruptions. As a group, anxious individuals show threat vigilance during initial stages of processing (e.g., 16-500ms after stimulus onset)(Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). Cognitive models posit that such threat vigilance actively contributes to anxiety (Beck, Emery, & Greenberg, 1985), promoting greater awareness of threats in the environment and reinforcing catastrophic beliefs. A distinct and equally important factor in anxiety is what an individual does with threatening information once detected. Later, sustained stages of processing may include perseverative attention to threats [e.g., worry, rumination—two highly overlapping constructs prevalent in anxiety (McEvoy, Mahoney, & Moulds, 2010; McLaughlin & Nolen-Hoeksema, 2011)], which promotes sustained negative mood and has been linked to negative health consequences via physiological and immunological factors (Brosschot, Gerin, & Thayer, 2006; Brosschot, Pieper, & Thayer, 2005).
Attention Bias Modification (ABM) is a fully automated, computer-based, translational intervention rooted in the premise that the attentional patterns observed in anxious patients might point the way towards novel interventions that seek to explicitly reverse these patterns. In its typical and most widely-studied form, ABM is designed to target relatively early processes of initial vigilance to threat by modulating attentional patterns within 500ms of the appearance of a threatening stimulus. ABM has shown promise in alleviating anxiety symptoms in some patients, although results are inconsistent across individuals and studies (MacLeod & Clarke, 2015; Price, Wallace, et al., 2016). To date, ABM studies have largely focused on establishing efficacy for anxious patients fitting within narrow diagnostic categories. A dimensional, transdiagnostic approach, in which traditional diagnostic boundaries are transgressed in favor of cross-cutting, empirically derived dimensions of functioning, is increasingly viewed as important in advancing the field of clinical psychology by promoting rapid translation between basic and applied domains (Insel et al., 2010). Consistent with this viewpoint, a critical question for ABM research is whether transdiagnostic, biobehavioral dimensions of threat processing can be used as part of a neurocognitive process-based framework to classify patients according to aberrant mechanisms and then treat these mechanisms directly. Although initial and sustained bias are present simultaneously in many patients, we posited that patients relatively high on an initial threat processing dimension (relative to a more sustained processing dimension) would be ideal candidates for the present form of ABM, which is specifically designed to target initial bias.
The neural circuitry guiding attention to threat has been well-described in animals. It includes bottom-up emotional salience signals generated by the amygdala (LeDoux, 2000) to promote rapid orienting towards threat, as well as top-down ventral prefrontal signals capable of flexibly modulating amygdalar engagement (Quirk & Mueller, 2008) and altering selective attention through biasing signals (Desimone & Duncan, 1995; Pessoa, Kastner, & Ungerleider, 2002). Sustained states of anxious apprehension have partially dissociable substrates, including the bed nucleus of the stria terminalis (BNST;(Davis, Walker, Miles, & Grillon, 2010)) and the insula (Paulus & Stein, 2006). Initial human neuroimaging studies of threat processing concord well with animal models. Broadly, relevant alterations in anxious subjects’ responses to threat are apparent in the amygdala, ventromedial PFC and anterior cingulate cortex (ACC) regions implicated in automatic forms of emotion regulation (Phillips, Ladouceur, & Drevets, 2008), and lateral PFC regions implicated in voluntary emotion regulation (Banich et al., 2009; Davidson, 2003; Phillips et al., 2008) and attentional control (Nee, Wager, & Jonides, 2007; Wager & Smith, 2003). However, the directionality, temporal pattern, and specific regional location of changes may distinguish initial (Monk et al., 2006; Monk et al., 2008; Price, Eldreth, & Mohlman, 2011; Price et al., 2014) and sustained (Andreescu et al., 2011; Mohlman, Eldreth, Price, Staples, & Hanson, In press; Price et al., 2013) forms of threat processing. Using a mixed block/event-related design capable of directly comparing the two forms of processing within a single human sample, neural substrates of initial/transient responses to threatening images (e.g., amygdala, midbrain, dorsolateral PFC) were dissociated from sustained responses to blocks of threatening images (e.g., BNST, anterior insula) among healthy controls (Somerville et al., 2013).
A small extant literature has linked ABM outcomes to individual differences in patterns of behavior (Amir, Taylor, & Donohue, 2011; Price, Wallace, et al., 2016), event-related potentials (Dennis-Tiwary, Egan, Babkirk, & Denefrio, 2016), and fMRI indices during attention-to-threat tasks. Increased amygdalar responses (Britton et al., 2015) and decreased amygdalar-insular connectivity (White et al., In press) have each been associated with greater symptom reduction across anxious individuals participating in studies that compared ABM to sham training, but neither finding was specific to the active ABM condition in these relatively small imaging datasets (n=15–24 in the active ABM groups). A focus on individual differences in brain function, including simultaneous characterization of both initial and sustained processing patterns, may improve prediction by bringing predictor variables proximal to the final biobehavioral mechanisms of symptom reduction in ABM (e.g., relatively early brain responses), rather than relying on relatively indirect measures that reflect the downstream output of threat processing (e.g., reaction times). Studies in diverse clinical populations suggest that fMRI, a measure of brain function that provides both temporal and anatomical information, surpasses behavioral measures in predicting clinical outcomes, even when fMRI effect size estimates are carefully controlled (Hoeft et al., 2011; Kumari et al., 2009; Murdaugh, Cox, Cook, & Weller, 2011; Siegle, Carter, & Thase, 2006). Furthermore, fMRI may increase sensitivity to detect altered threat processing, given that altered fMRI activity in anxious participants can be observed even in the absence of detectable behavioral effects (Monk et al., 2008; Price et al., 2014). Although time course is a critical factor in attention bias, with distinct behavioral and neural profiles emerging at initial and sustained timepoints, previous studies in clinical patients have not examined whether a match between the timing of altered threat processing in an individual patient and the timing of ABM stimulus length is a critical factor in ABM outcome. ABM typically targets vigilance at a fixed interval of 500ms from stimulus onset, suggesting that threat processing alterations occurring on a brief/transient timescale are the most relevant.
Attention bias has been documented in a wide range of clinical and non-clinical anxiety (Bar-Haim et al., 2007). Thus, our randomized controlled trial included any patient with clinically elevated anxiety, providing for an appropriate dimensional examination of ABM prognosis in a sample better reflecting the heterogeneity of real-world clinical patients. Using a previously validated mixed block/event-related design (Somerville et al., 2013), we posited that larger initial/transient responses to aversive images would imply a good fit between individual patients and ABM (which specifically targets initial vigilance) and thus would track linearly with greater clinical benefit following ABM. To probe potential clinical utility of these neural patterns, we further characterized the accuracy of fMRI-based clinical prediction of favorable response using a machine learning pattern recognition approach.
ABM is a relatively mature translational intervention, with at least 7 published meta-analyses examining its clinical efficacy across numerous studies (Jones & Sharpe, 2017). Thus, our explicit focus was not on the question of overall group efficacy in relation to a control condition, which has been extensively studied in larger cohorts of anxious patients, but on understanding individual differences in outcome using neural indices of threat processing, which we expected would be proximal to the intervention’s active mechanisms. Within a broader translational framework, this approach represents an important step within an iterative process of mechanistic intervention refinement. While prior work translated a basic mechanism (attention bias) into an intervention (ABM), we now step back towards basic cognitive neuroscience in an effort to define more precisely for whom the intervention works best, and why. Ideally, this undertaking will point the way back to refined clinical practice by yielding new translational insights, including personalized intervention prescriptions, refinements to the intervention itself, and/or novel interventions or intervention combinations designed to tackle identified predictors of non-response.
Methods
Design overview
Seventy unmedicated patients reporting clinically elevated levels of trait anxiety and associated clinician-rated disability (full inclusion/exclusion criteria detailed below) were randomized to receive active ABM (n=49) or a sham control variant (n=21) (clinicaltrials.gov: (clinicaltrials.gov: NCT02303691). Uneven allocation was used to maximize sample size and statistical power in the active ABM group, as primary hypotheses concerned mechanistic predictors of ABM response. The sham sample was included to assess specificity of findings through effect size comparison, rather than to explicitly provide a well-powered examination of group-level effects of ABM vs. sham on outcomes, given that: a) the question of ABM’s group-level efficacy in relation to sham has been extensively examined in much larger primary and meta-analytic datasets (Price, Wallace, et al., 2016) (Jones & Sharpe, 2017); and b) we explicitly recruited a heterogeneous sample and anticipated that individual differences within the sample would substantially influence group-level patterns. 94.3% of patients completed the treatment and post-treatment assessment (CONSORT diagram: Figure 1). This study was approved by the Institutional Review Board.
Figure 1.
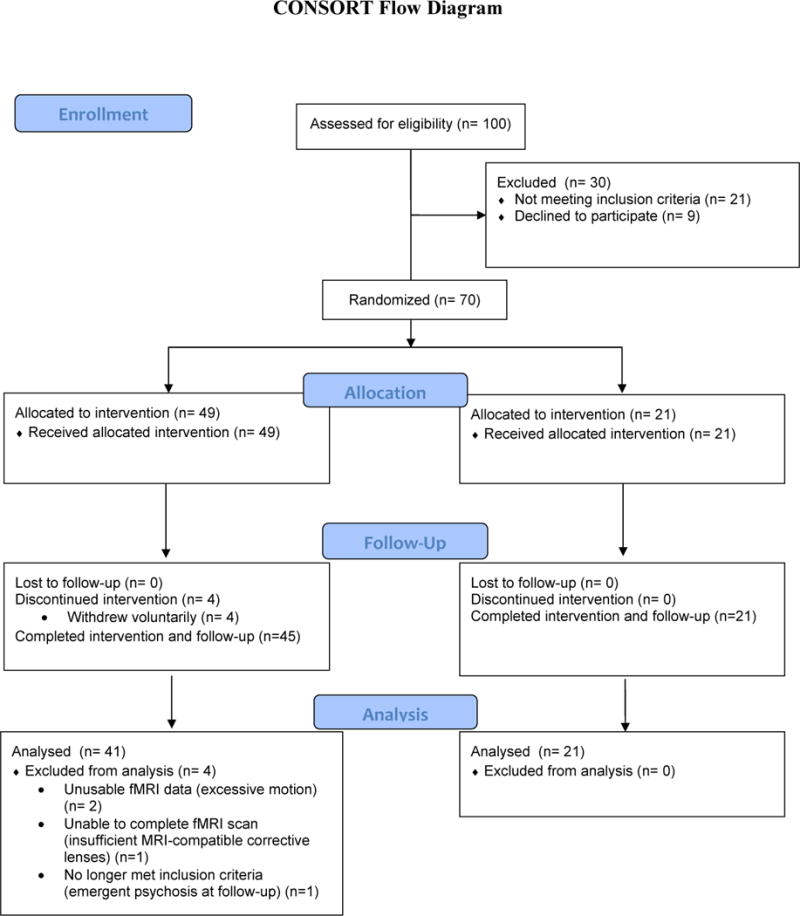
CONSORT diagram for study.
Participants
Participants were recruited and enrolled from 03/2013-11/2016, stopping when the target randomization sample of 70 participants was obtained. Participants were recruited through flyers posted in clinical settings, email announcements, and through a university-maintained research registry that matches prospective participants to relevant studies based on inclusion/exclusion criteria. Inclusion criteria specified that participants: 1) be between the ages of 18 and 55 years; 2) score >=45 on the Spielberger State-Trait Anxiety Inventory—trait form, a clinically relevant cut-point that statistically maximizes discrimination between clinical and non-clinical anxiety (Fisher & Durham, 1999) and eliminates the bottom ~84% of a typical healthy control distribution (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983); and 3) score >=75th percentile on the World Health Organization Disability Assessment Schedule 2.0—clinician-rated version, a percentile that is characteristic of individuals with one or more mental disorders (Andrews, Kemp, Sunderland, Von Korff, & Ustun, 2009). These criteria produced a study sample in which 91% of randomized participants had one or more diagnosed anxiety disorder at baseline (mean=2.10 DSM-IV-TR disorders), while the remaining 9% (n=6) captured ‘diagnostic orphans’ with clinically significant anxiety and associated impairment who did not meet full criteria for any specific DSM-IV-TR anxiety disorder (and consequently were assigned a diagnosis of Anxiety Disorder Not Otherwise Specified). Diagnoses were established by experienced master’s-level (or higher) clinicians using the MINI International Neuropsychiatric Interview. The sample met criteria for a wide range of DSM-IV-TR Anxiety Disorders, with a preponderance of distress-related disorders (Generalized Anxiety Disorder criteria met by 84% of the sample; Social Anxiety Disorder criteria met by 34% of the sample). See Table 1 for additional sample characteristics.
Table 1.
Demographic and clinical characteristics of analyzed subgroups
| ABM (n=41) | Sham (n=21) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Pre-treatment* | Post-treatment | Pre-post statistics | Pre-post effect size [95% CI] | Pre-treatment* | Post-treatment | Pre-post statistics | Pre-post effect size [95% CI] | |
| Caucasian, n (%) | 25 (61%) | – | – | – | 16 (76%) | – | – | – |
| Female, n (%) | 32 (78%) | – | – | – | 16 (76%) | – | – | – |
| Age | 30.85 (9.54) | – | – | – | 29.86 (11.84) | – | – | – |
| Anxiety diagnoses met, n (%): | – | – | – | – | – | – | ||
| GAD | 36 (88%) | 16 (76%) | ||||||
| SAD | 12 (29%) | 9 (43%) | ||||||
| Panic/agoraphobia | 4 (10%) | 3 (14%) | ||||||
| PTSD | 5 (12%) | 1 (5%) | ||||||
| Specific Phobia | 4 (10%) | 2 (10%) | ||||||
| OCD | 4 (10%) | 0 (0%) | ||||||
| Anxiety NOS | 3 (7%) | 3 (14%) | ||||||
| Comorbid depressive disorder | 14 (34%) | 6 (29%) | ||||||
| Number of anxiety diagnoses | 1.68 (1.01) | 1.12 (1.05) | t40=3.501, p=.001 | d=.54 [.22, .87] | 1.57 (0.93) | 1.33 (1.15) | t20=1.227, p=.234 | d=.22 [−.14, .58] |
| Remission of primary anxiety diagnosis, n (%) | – | 15 (37%) | – | – | – | 3 (14%) | – | – |
| Remission of all anxiety diagnoses, n (%) | – | 10 (24%) | – | – | – | 1 (5%) | – | – |
| CAPS-Vigilance | 4.54 (2.03) | 3.90 (1.97) | t40=2.500, p=.017 | d=.32 [.07, .58] | 5.24 (2.23) | 4.19 (2.16) | t20=2.796, p=.011 | d=.48 [.13, .83] |
| MASQ: Anxious Arousal | 32.20 (10.66) | 27.41 (8.59) | t40=5.136, p<.001 | d=.47 [.28, .66] | 34.05 (10.72) | 30.29 (13.92) | t20=1.642, p=.116 | d=.29 [−.06, .65] |
| MASQ: General Distress (anxiety) | 25.49 (6.98) | 22.17 (6.42) | t40=3.893, p<.001 | d=.49 [.23, .76] | 27.76 (7.28) | 25.38 (9.36) | t20=1.330, p=.199 | d=.28 [−.14, .70] |
| High Response Quartile (n=10): Post-treatment | Low Response Quartile (n=10): Post-treatment | Φ Between-group difference: High vs. Low Quartile | Sham (n=21): Post-treatment | Φ Between-group difference at post-treatment: High ABM quartile vs. all sham | |
|---|---|---|---|---|---|
| Number of anxiety diagnoses | 0.60 (0.84) | 1.60 (1.51) | t18=−1.833, p=.083 | 1.33 (1.15) | t29=1.787, p=.084 |
| Remission of all anxiety diagnoses, n (%) | 4 (40%) | 2 (20%) | X2=0.952, p=.329 | 1 (5%) | X2=6.218, p=.013 |
| CAPS-Vigilance | 1.70 (1.64) | 5.80 (1.23) | t18=−6.335, p<.001 | 4.19 (2.16) | t29=3.222, p=.003 |
| MASQ: Anxious Arousal | 23.30 (6.41) | 32.10 (11.28) | t18=−2.145, p=.046 | 30.29 (13.92) | t29=1.503, p=.144 |
| MASQ: General Distress (anxiety) | 19.40 (5.78) | 26.30 (8.38) | t18=−2.144, p=.046 | 25.38 (9.36) | t29=1.850, p=.075 |
Note: Data presented as mean (SD) unless otherwise noted. CAPS-Vigilance=Clinician-Administered PTSD Scale, hypervigilance item; MASQ=Mood and Anxiety Symptoms Questionnaire; GAD=Generalized Anxiety Disorder; SAD=Social Anxiety Disorder; PTSD=Posttraumatic Stress Disorder; OCD=Obsessive-Compulsive Disorder; NOS=Not Otherwise Specified.
No baseline differences were observed for ABM vs. sham groups on any demographic or clinical variable according to t-tests (continuous variables) or X2 (categorical variables) (p’s>.24).
Exploratory post hoc comparisons of High Response Quartile to other groups are presented to provide descriptive comparisons of whether groups defined on the basis of CAPS residual scores exhibited more generalized clinical differences at post-treatment.
Exclusion criteria included the following: 1) current medication or Cognitive-Behavioral Therapy for anxiety or depression; 2) failure to meet standard Magnetic Resonance Imaging (MRI) inclusion criteria: those who have cardiac pacemakers, neural pacemakers, surgical clips in the brain or blood vessels, surgically implanted metal plates, screws or pins, cochlear implants, Intrauterine Devices, metal braces, or other metal objects in their body, especially in the eye. If the subject reported any metal or implants in the body they had to be deemed safe by the MRI Research Center’s safety screening procedure prior to enrollment; 3) pregnancy, determined by pregnancy tests on females; 4) currently suicidal or at risk for harm to self or others; 5) visual disturbance (<20/40 as per the Snellen test, corrective lenses allowed); 6) <6th grade reading level as per the Wide Range Achievement Test; 7) presence of bipolar, psychotic, autism spectrum, substance dependence, or primary depressive disorder; 8) positive urine drug test.
The study was approved by the local institutional Internal Review Board. All participants provided informed consent prior to any study procedure.
ABM and sham conditions
Patients and clinical assessors were successfully blinded to treatment allocation (see Supplement). The ABM and sham conditions were modeled after prior studies (e.g., Amir, Beard, Burns, & Bomyea, 2009). Participants in both groups completed 8 twice-weekly sessions, in the laboratory, over 4 weeks (~10–15min/session) consisting of 300 trials of a dot-probe task. At baseline, ten idiographic threat words were selected collaboratively by the participant and clinical interviewer, immediately following the baseline clinical interview, designed to capture the full range of concepts most relevant to the participant’s daily experience of anxiety. All idiographic items were rated by the participant as -2 or -3 on a scale of pleasantness ranging from +3 (“very pleasant”) to -3 (“very unpleasant”). Each idiographic threat word was paired, on an individual (per-participant) basis, with a neutral word (drawn from a normative corpus) that was rated as neutral (score=0) by the participant and matched to the idiographic threat word on word length and the participant’s familiarity rating (on a 7-point Likert-like scale). These idiographic word pairs were supplemented by 20 threat words and 20 neutral words from a normative corpus used previously in ABM research (e.g., Amir et al., 2009). Each word was presented a total of 8 times per session in a randomized location (top/bottom).
During training trials, word pairs (80% threat-neutral; 20% neutral-neutral) were presented vertically subtending a visual angle of approximately 2 degrees, for 500ms, followed by a probe (‘E’ or ‘F’) in either the upper or lower word location which remained on-screen until the participant responded via button press to indicate the letter displayed. Participants responded via button press to indicate the probe letter displayed. Ten idiographic threat words were selected collaboratively by the participant and the clinical interviewer.
The only distinction between the ABM and control conditions was in the relationship between the probe location and the threat word in each word pair. In ABM, for 100% of threat-neutral trials (80% of all trials), the probe replaced the neutral word, thereby shaping attention away from threatening cues through practice. In the sham condition, the probe replaced either the threat or neutral word with equal likelihood.
Clinical assessment
Clinical hypervigilance to threat was assessed at pre- and post-treatment via clinical interview using the “hypervigilance” item of the well-validated Clinician-Administered PTSD Scale (CAPS-vigilance (Blake et al., 1995)), which sums two sub-items assessing frequency and intensity of vigilance, each rated on a 0–4 scale with verbal anchors. Meta-analyses suggest the use of a clinician-rated (rather than self-report) index improves sensitivity to ABM effects (Linetzky, Pergamin-Hight, Pine, & Bar-Haim, 2015; Price, Wallace, et al., 2016). The wording of the item is sufficiently broad to capture the transdiagnostic concept of vigilance to threat (e.g., “have you been especially alert or watchful” for threat-related information or “felt as if you were constantly on guard?”). Masters-level clinical assessors administered the item and were trained to provide idiographic examples of potential threat vigilance patterns based on the participant’s self-reported foci of anxiety/concern (e.g., scanning faces for signs of disapproval/rejection; being on guard for signs of deteriorating health; being on guard or watchful for phobia-related stimuli in the environment; being on guard for interoceptive panic-related cues; etc.). Of note, the latter “distress” component was explicitly quantified irrespective of frequency (i.e., “At those specific times when you were especially alert or watchful, how hard did you try to be watchful…?”) so that it could flexibly capture decreases in the severity of self-reported vigilance even for cases where exposure to triggering events may be more circumscribed (e.g., specific phobia). A random subset of videotaped interviews (15%), including both pre- and post-treatment interviews, was scored by a second evaluator; 100% reliability was obtained.
Post-treatment CAPS-vigilance scores were regressed on pre-treatment scores (within each treatment condition separately) to obtain residual CAPS-vigilance scores, a measure of pre- to-post-treatment improvement. Similar to change (difference) scores, residual scores can be both positive and negative, with lower (i.e., more negative) values indicating fewer residual vigilance symptoms (i.e., better outcome). Residual scores indicate the degree to which the improvement experienced by any given individual surpasses (negative values) or lags behind (positive values) what would be predicted on the basis of his/her pre-treatment CAPS value alone. The use of residual scores as an outcome measure generally improves power in randomized designs (Petscher & Schatschneider, 2011) and accounts for non-static relationships between pre and post (absolute) scores, although drawbacks of the approach have also been articulated (Dimitrov & Rumrill, 2003; Forbes & Carlin, 2005). See the Supplement for validation of our decision to analyze residual scores and exploration of the impact of this decision in comparison to other options (e.g., change scores).
fMRI task and data acquisition
T2*-weighted images depicting BOLD contrast (TR=2000; TE=28; flip angle=73°; 38 slices; FOV=200x200; 3.125x3.125x3.2mm voxels) were acquired on a 3T Siemens Trio approximately one week prior to the onset of ABM or sham training. Standard preprocessing steps were applied using Analysis of Functional Neuroimaging (AFNI). The following preprocessing steps were applied, as described in more detail previously (Siegle et al., 2012): slice time correction, cross-registration of functional data to a high-resolution structural scan acquired in the same fMRI session (axial MPRAGE: TR=2100; TE=3.31; 176 slices; flip angle=8°; FOV=256x208; 1mm isotropic voxels), 6-parameter motion correction, linear detrending to correct drift, conversion to percent change, temporal smoothing using a seven-point Gaussian weighted moving average filter [intended to improve signal-to-noise and reduce bias in BOLD data; (Friston et al., 2000)], 32-parameter nonlinear warping to the Montreal Neurological Institute Colin-27 brain data set, spatial smoothing [6-mm full width half maximum]. Participants with excessive motion during the task (>30% of scans showed incremental movement >1mm or incremental rotation >1°, or >30% of scans showed absolute movement from baseline >5mm or absolute rotation >5°) were excluded from analysis (n=2; 2.9%). Raw motion parameters were included as nuisance covariates in the single-subject regression models used to generate brain maps for each task condition, ensuring variance linearly related to motion did not explain findings.
The task was adapted from Somerville and colleagues; see (Somerville et al., 2013) for detailed description. Briefly, participants were alerted by a 2s text cue, presented at the start of each of eight 78s blocks, as to the nature of the upcoming block. Blocks were either “Negative”, containing a series of 10 negative images, presented for 3s each, from the International Affective Picture Set (IAPS; (Lang, Bradley, & Cuthbert, 2008)), or “Neutral,” consisting of 10 neutral IAPS images. Both negative and neutral blocks were also either “Predictable,” with each individual image being preceded by a numerical countdown (e.g., 3-2-1), or “Unpredictable,” with each image being preceded by a random string of numbers. To encourage continued attention to the images, participants completed an incidental task by responding via button press to indicate whether each image depicted an indoor or an outdoor scene (see Supplement for exploratory analyses of behavioral task data). The onsets of each transient image were jittered by presenting a pseudorandom number (varying from 1 to 8) of numerals prior to image onset, with each numeral presented for 1s, comprising a total of 44s of numeral presentations per block. Each block concluded with a 2s stop cue. Jittered onsets of each image enabled efficient estimation of transient brain responses evoked by each image type by sampling across the full hemodynamic response curve. Blocks of resting fixation cross (32s each) were interleaved with all active blocks to facilitate efficient modeling of both sustained and transient patterns.
The task design and regression analyses were optimized in previous research (Somerville et al., 2013) to allow estimation of transient responses to each image type embedded within the sustained response to each overarching block type (e.g., “Predictable Negative,” “Predictable Neutral,” etc.). The task was designed in accordance with recommendations for mixed block/event-related design (Dosenbach et al., 2006; Visscher et al., 2003) and included a number of important features that permitted transient responses to be disentangled from sustained responses. These include sufficiently variable jitter between transient stimuli, sufficient time spent in a sustained state and not experiencing a transient event (>58% total block time), sufficient time spent in resting fixation (outside of any block condition; >25% of total task time), and modeling of transients (as detailed below) using a finite impulse response (FIR) basis function rather than a canonical hemodynamic response function, which ensures that sustained condition estimates are truly maintained and not aliased by high-frequency components of the signal.
fMRI regression analyses
Both a priori anatomical region-of-interest (ROI) and whole-brain analyses were conducted. Twelve anatomical ROIs were selected a priori in order to capture a comprehensive network of regions implicated in prior literature in anxiety and threat processing, across both transient and sustained timescales. Two limbic (amygdala, BNST) and two prefrontal (VLPFC, DLPFC) regions were defined in each hemisphere, in addition to medial regions within the anterior cingulate cortex (ACC; parcellated into dorsal, perigenual, and subgenual subregions) and ventromedial PFC. Anatomical regions of interest were defined using the Automated Anatomic Labeling (AAL) atlas, which includes regional parcellations hand-drawn on the single-subject reference brain in MNI space (Tzourio-Mazoyer et al., 2002). Mean AUC and beta weight values were then extracted for each ROI using AFNI’s 3dmaskave command. The anterior cingulate cortex (ACC) was parcellated into dorsal, perigenual, and subgenual subregions using previously defined boundaries as in (Gianaros et al., 2014)]. The ventrolateral prefrontal cortex was defined as the Frontal Inferior Orbital region from the AAL atlas. The dorsolateral prefrontal cortex was defined as Brodmann’s areas 9 and 46, more lateral than |x|=19 and more dorsal than z=21. The ventromedial prefrontal cortex was defined as the Frontal Middle Orbital (medial segment) from the AAL atlas. The left and right bed nucleus of the stria terminalis (BNST) were hand-drawn onto a standardized MNI reference brain by a co-author (LB) with expertise in the structure and function of this region in animal and human research (Banihashemi & Rinaman, 2006; Banihashemi, Sheu, Midei, & Gianaros, 2015), as previously described (Banihashemi et al., 2015).
Correlations between these a priori regions and residual CAPS-vigilance scores were used to provide unbiased effect size estimates in both the ABM and control groups, with False Discovery Rate (FDR) correction applied to correct for multiple comparisons across all a priori ROIs.
A complimentary whole-brain analysis was used to identify the most predictive regions across the entire brain utilizing AFNI’s spatial autocorrelation function (‘-acf’) option implemented in 3dClustSim, with voxel-wise alpha=.001. Briefly, 3dClustSim simulates noise-only random brain maps matching the structure and smoothness of the empirical datasets from a given study, and uses these simulations to compute a cluster-size threshold that holds the probability of a false positive finding below the desired level (e.g., map-wise p<.05) for a user-defined voxel-wise p-value threshold (here, p<.001). This approach provides accurate Type I error control (Cox, Reynolds, & Taylor, 2016), but effect size estimates in the ABM group are likely to be inflated (Vul, Harris, Winkielman, & Pashler, 2009).
At the single-subject level, transient and sustained responses were modeled simultaneously using box-car regressors for each block type (Negative Predictable, Negative Unpredictable, Neutral Predictable, Neutral Unpredictable) and finite impulse response functions over 20sec to flexibly model responses to each transient image (separate Negative-Transient and Neutral-Transient regressors), along with nuisance covariates (motion parameters). Transient regressors collapsed across Predictable/Unpredictable blocks, as predictability was only manipulated at the sustained/block level (i.e., negative image sets were identical for both predictable and unpredictable blocks). As in prior work (Somerville et al., 2013), an area under the curve (AUC) was computed for each transient regressor to capture the magnitude of transient response from 2-6 TRs (4-12secs) after each image presentation and used for subsequent group analyses. As in previous validation work for the task (Somerville et al., 2013), this timeframe was selected to capture neuronal activity in the immediate aftermath of image onset (e.g., 0–500ms after image onset), after accounting for the hemodynamic delay in the BOLD signal, which generally peaks at 4–6 seconds following neuronal activity and subsides by app. 12 seconds post-neuronal event (Friston et al., 1998). AUC values were computed at every voxel as the sum of finite impulse response function beta weights derived at each of the relevant timepoints (4–12s post-stimulus onset) in response to transient images of each type (negative, neutral). For anatomical ROI analyses, AUC values as well as beta-weights for sustained (block-type) regressors were averaged for each emotional image type (negative and neutral) across all voxels in a given ROI, extracted for each participant, and analyzed in relation to CAPS-residual scores using SPSS software.
Due to the inclusion of both sustained and transient regressors in a single regression model constructed for each individual, individual differences in Negative-Transient AUC values can be conceptualized as representing the degree to which a given voxel in the brain tracked with the presentation of each transient negative image relative to the more sustained response maintained across the entirety of the block. Thus, an individual with a relatively large Negative-Transient AUC value in a given region showed a tendency for brain activity to track with each transient image presentation, rather than maintaining a consistently modulated response that tracked best with the overall sustained (block-level) manipulation.
Outliers within both voxel-wise AUC values and CAPS-vigilance scores were rescaled prior to analysis (see Supplement). Single-subject Negative-Transient AUC values were then regressed on residual CAPS-vigilance scores within the ABM group only. This within-group analysis approach was dictated by the study design to preserve maximal power for identifying brain predictors of response within the larger, active intervention group. Effect sizes and significance levels for both a priori anatomical and functional ROIs derived from the whole-brain analysis were examined in the control group as an exploratory probe of the specificity of prediction findings to active ABM. While the smaller sample size in the control group will result in reduced p-values for the same effect size, conversely, point estimates of effect size are not influenced by sample size and therefore can be used to provide a valid comparison across unequal samples, particularly when applied to the unbiased effect size estimates that are derived by using a priori anatomical ROIs. An exploratory statistical moderation analysis was undertaken for the conjoined mask of a priori anatomical ROIs, but not for the whole-brain search due to insufficient power, particularly given conservative alpha (e.g., p<.001) necessary for Type I error control in fMRI whole-brain searches (Eklund, Nichols, & Knutsson, 2016). By exploring the conjoined mask of a priori anatomical regions in a single test of moderation, Type I error could be preserved at p<.05 without the need for multiple comparisons corrections which could adversely impact power.
Exploratory Pattern Recognition Analysis
ABM participants were divided into quartiles based on CAPS residual scores in order to assess the ability to accurately make binary (yes/no) decisions about the clinical utility of ABM on an individual patient basis. Pattern Recognition of Brain Image Data (PROBID) was applied to single-subject Negative-Transient AUC maps from the quartiles in order to build and test a classifier for separating the highest/strongest ABM responders from each other quartile (partial responders, partial non-responders, and poorest responders). For post hoc exploration and descriptive validation of the clinical generalizability of these extreme quartiles, we conducted exploratory comparisons of the strong responders in relation to both the poorest responders and the entire sham sample, which generally supported more favorable clinical response among the strong CAPS responders across a range of clinical measures (see Table 1). The PROBID analysis was conducted separately for each pairwise classification task (strongest responders vs. each other quartile) because the classifier can only be built to separate two groups of equal size (i.e., make a single, binary classification decision). Briefly, the PROBID algorithm proceeds in two phases in order to identify spatially distributed patterns of transient brain responses that robustly predicted exceptionally good outcomes following ABM. During classifier training, the algorithm finds the set of regions (i.e., voxels in the whole-brain Negative Transient AUC activation map) by which the two groups (strong responders vs. another quartile) can be best distinguished from each other (i.e. a discriminating map). In the next phase, the test phase, given the brain scan from a previously unseen subject, the algorithm predicts the subject’s group. A Gaussian Process Classifier with leave-one-out cross-validation was used to determine the accuracy of predicting whether an individual would be a strong ABM responder based on this probabilistic class prediction. Finally, after determining the classifier’s accuracy (sensitivity and specificity) during the test phase, a permutation test was run to assess the statistical significance of the accuracy data by randomly permuting the datasets to assess whether the predictions the classifier provided for a particular group comparison were better than those that arise by chance.
Results
Clinical Effects
ABM participants showed pre-to-post decreases across a range of clinician-rated and self-report variables, including vigilance. Effect sizes generally favored ABM over sham, though Cis were overlapping (Table 1). See Supplement for further analyses of clinical outcomes (e.g. group*time interactions).
Regression
Primary analyses utilized Negative-Transient values to predict decreased clinical vigilance in the ABM group (see Supplement for additional task conditions, e.g., Negative-Sustained values). A priori regions. Greater Negative-Transient responses predicted greater reductions in clinical vigilance in every a priori anatomical ROIs except for one (see Table 2). The set of eleven significant findings surpassed FDR correction for multiple comparisons across the full set of twelve anatomical ROIs (family-wise FDR p<.05). Whole-brain analysis. After correcting for multiple comparisons across the whole brain, greater Negative-Transient responses predicted greater reductions in clinical vigilance across numerous regions implicated in affective processing and attentional control (see Table 2; Figure 2), including clusters encompassing a priori hypothesized regions (e.g,. dACC, pgACC, VLPFC, R amygdala) as well as novel regions (e.g., thalamus, precentral gyrus, temporal cortex). Specificity to ABM. No ROI defined by either a priori anatomical or whole-brain functional analysis was related to outcome following the sham condition; effect sizes were almost uniformly near-zero (Table 2). In an exploratory test of moderation, after summing Negative-Transient responses across a priori predictor ROIs, statistical moderation was supported (group*A-priori-Negative-Transient responses: βstandardized=−0.54;p=.048), with the significant interaction effect reflecting prediction of CAPS-vigilance residuals by the conjoined mask of a priori ROIs in the ABM group (βstandardized=−0.47;p=.001) but not the sham group (βstandardized=0.14;p=.62). In whole-brain analyses of the sham sample (Supplement), no predictors of CAPS-vigilance residual scores were found, even using lenient thresholding to equate power in the smaller sham sample.
Table 2.
Analysis of regional response to transient negative images as predictors of residual CAPS-vigilance scores
| A priori anatomical ROIs | ABM (n=41) | Sham (n=21) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Region | Brod-mann’s areas | Location of centroid voxel | x | y | z | Cluster extent (voxels) | r/R2 (unbiased) | p | r/R2 (unbiased) | p |
| L amygdala | – | L amygdala | −23 | −3 | −24 | 79 | r=−.34; R2=.116 | .032 | r=.07; R2=.005 | .78 |
| R amygdala | – | R amygdala | 26 | −1 | −22 | 77 | r=−.48; R2=.230 | .001 | r=.13; R2=.017 | .59 |
| L BNST | – | L BNST | −8 | 0 | 0 | 10 | r=−.42; R2=.176 | .007 | r=−.11; R2=.012 | .63 |
| R BNST | – | R BNST | 9 | 1 | 2 | 17 | r=−.33; R2=.109 | .036 | r=−.06; R2=.004 | .79 |
| dACC | 32, 24 | R anterior cingulate | 3 | 16 | 31 | 522 | r=−.53; R2=.281 | <.001 | r=−.03; R2=.001 | .92 |
| pgACC | 32, 24 | R anterior cingulate | 2 | 34 | 4 | 377 | r=−.42; R2=.176 | .006 | r=.06; R2=.004 | .80 |
| sgACC | 24, 25 | L anterior cingulate | 0 | 27 | 0 | 61 | r=−.10; R2=.010 | .54 | r=−.09; R2=.008 | .71 |
| VMPFC | 10, 11, 32 | R medial frontal gyrus | 2 | 48 | −4 | 507 | r=−.41; R2=.168 | .007 | r=.24; R2=.058 | .30 |
| L VLPFC | 47, 11 | L inferior frontal gyrus | −37 | 27 | −11 | 458 | r=−.48; R2=.230 | .002 | r=.10; R2=.010 | .68 |
| VLPFC | 47, 11 | R inferior frontal gyrus | 42 | 28 | −11 | 466 | r=−.49; R2=.240 | .001 | r=.25; R2=.063 | .28 |
| L DLPFC | 9, 46 | L middle frontal gyrus | −33 | 29 | 39 | 793 | r=−.38; R2=.144 | .015 | r=.12; R2=.014 | .61 |
| R DLPFC | 9, 46 | R middle frontal gyrus | 32 | 29 | 39 | 771 | r=−.37; R2=.137 | .017 | r=.08; R2=.006 | .73 |
| Whole-brain analysis | ABM (n=41) | Sham (n=21) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Region | Brod-mann’s areas | Location of centroid voxel | x | y | z | Cluster extent (voxels) | r/R2 (post hoc) |
p | r/R2 (post hoc) |
p |
| dACC/dmPFC | 32, 8, 6 | R medial frontal gyrus | 1 | 12 | 48 | 113 | r=−.61; R2=.372 | <.001 | r=.08; R2=.006 | .74 |
| L ventral postcentral gyrus/insula | 43, 13, 3 | L postcentral gyrus | −57 | −17 | 17 | 111 | r=−.67; R2=.449 | <.001 | r=−.11; R2=.012 | .63 |
| R VLPFC | 47, 13 | R inferior frontal gyrus | 38 | 13 | −19 | 86 | r=−.68; R2=.462 | <.001 | r=.05; R2=.003 | .84 |
| R DLPFC | 6, 8 | R middle frontal gyrus | 45 | 7 | 51 | 72 | r=−.64; R2=.410 | <.001 | r=−.06; R2=.004 | .80 |
| R middle/superior temporal cortex | 21, 22 | R middle temporal gyrus | 54 | −5 | −17 | 58 | r=−.61; R2=.372 | <.001 | r=.00; R2=.000 | .99 |
| L dorsal postcentral gyrus | 2, 1 | L postcentral gyrus | −52 | −30 | 51 | 50 | r=−.60; R2=.360 | <.001 | r=.25; R2=.063 | .27 |
| R insula/IFG | 44, 13 | R insula | 49 | 10 | 5 | 41 | r=−.60; R2=.360 | <.001 | r=.06; R2=.004 | .81 |
| pgACC | 32, 9 | R anterior cingulate cortex | 5 | 38 | 18 | 40 | r=−.59; R2=.348 | <.001 | r=.07; R2=.005 | .77 |
| R thalamus | – | R thalamus | 8 | −18 | 15 | 35 | r=−.60; R2=.360 | <.001 | r=−.08; R2=.006 | .72 |
| R amygdala/hippocampus | 34 | R amygdala | 17 | −4 | −25 | 33 | r=−.65; R2=.423 | <.001 | r=−.10; R2=.010 | .66 |
| L DLPFC | 6 | L precentral gyrus | −51 | −5 | 49 | 31 | r=−.63; R2=.397 | <.001 | r=.04; R2=.002 | .87 |
| L Precentral Gyrus | 6, 22 | L precentral gyrus | −56 | 0 | 6 | 29 | r=−.61; R2=.372 | <.001 | r=.04; R2=.002 | .88 |
Note: Coordinates for each cluster’s center-of-mass are presented in MNI space. Unrestricted whole brain analysis findings have voxel-wise error rate p < .001; map-wise error rate p < .05, except for italicized regions which have map-wise p < .10. “Post hoc” effect sizes from whole brain search are likely to be inflated (e.g., see Vul et al, 2009). Bolded text indicates p<.05 for a priori anatomical ROIs. dACC=dorsal anterior cingulate cortex; VLPFC= ventrolateral prefrontal cortex; DLPFC=dorsolateral prefrontal cortex; IFG=inferior frontal gyrus; pgACC=perigenual anterior cingulate cortex.
Figure 2.
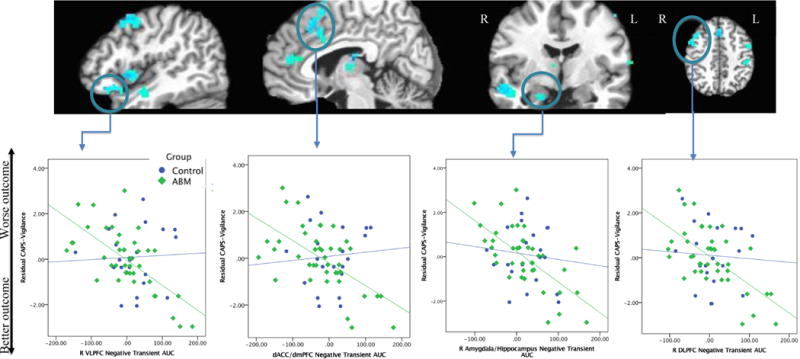
Clusters where negative transient AUC values predict CAPS-Vigilance residual scores (from whole-brain analysis). From left to right, panels display the following clusters from Table 2 whole-brain analysis: 1) R VLPFC, R insula/IFG, R middle/superior temporal cortex, R DLPFC; 2) pgACC, dACC, thalamus; 3) R middle/superior temporal cortex, R amygdala/hippocampus, thalamus; 4) R DLPFC, dACC/dmPFC, L DLPFC, L dorsal postcentral gyrus. Lower row of figures depicts representative linear relationships between negative transient AUC values from selected ROIs and residual CAPS-vigilance scores in the ABM and control groups.
Exploratory: Pattern Recognition
The group of strong ABM responders, defined as the lowest quartile on CAPS-Vigilance residual scores, generally showed superior clinical outcomes as compared to both poor (highest quartile) ABM responders and the sham group as a whole (see Table 1, bottom), suggesting that the clinical benefits seen among the strong ABM responders were not restricted to the CAPS measure but were robust across multiple clinical outcomes. Using whole-brain maps of the Negative-Transient effect, the strongest ABM responders could be discriminated by spatial patterns in Negative-Transient AUC values with 68% overall accuracy. The accuracy data for the classifier separating the strongest ABM responders from each other quartile were as follows: strongest responders vs. poorest responders (70% accuracy; 80% sensitivity; 60% specificity; p=.045; spatially distributed classifier and results shown in Figure 3); strongest responders vs. partial non-responders (70% accuracy; 80% sensitivity; 60% specificity; p=.038); strongest responders vs. partial responders (65% accuracy; 80% sensitivity; 50% specificity; p=.083). An identical pattern recognition approach failed to yield accurate prediction of better vs. worse outcomes within the sham group (n=10 per group based on median split; accuracy=15%; p>.90).
Figure 3.
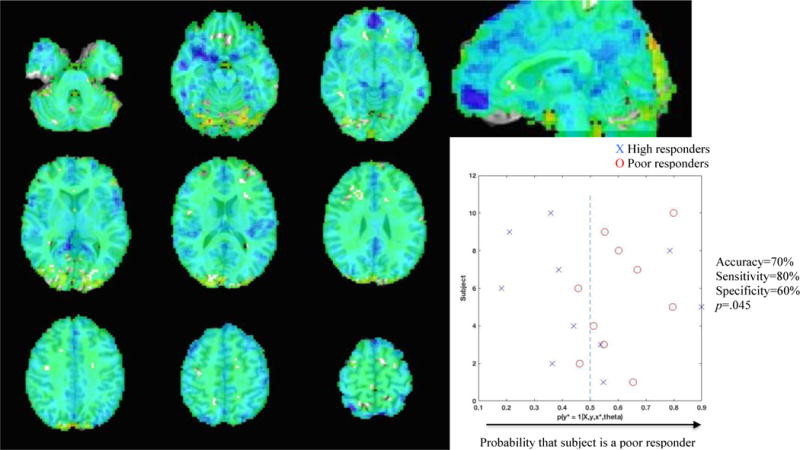
Discrimination map and classifier results from spatial pattern recognition analysis using whole-brain map of negative transient AUC values to classify individuals as high (best quartile) vs. poor (worst quartile) ABM responders. Discrimination information was strongest in vmPFC and bilateral VLFPC (dark blue shading).
Discussion
Alterations in both transient and sustained processing of threat cues are core features of clinical anxiety that cut across diagnoses, and may implicate distinct translational treatments. Efforts to translate these basic, well-defined neurocognitive mechanisms into targeted treatments will be optimized if individual differences in the relevant basic mechanisms are taken into account. For instance, while it is unlikely that the full range of heterogeneous clinical anxiety presentations can be successfully treated with a computer-based intervention targeting one unitary, highly specific mechanism (here, ABM targeting early/initial attentional responses to threat), we posited that the neural measures most relevant to this mechanism would help identify those specific anxious individuals who respond well. The present study was optimized for identifying transdiagnostic neurocognitive predictors of ABM response, rather than for explicit ABM vs. sham control comparisons. Previous studies and meta-analyses provide more conclusive information regarding ABM’s clinical efficacy (e.g., Price, Wallace, et al., 2016) and neural effects (e.g., Britton et al., 2015), and support the need for a better understanding of which patients are likely to benefit. The current study findings likewise highlight the role that individual differences—specifically, neurocognitive individual differences relevant to the intervention’s posited mechanisms of action—play in explaining why, at the group level, all individuals who receive a given treatment (such as ABM) may not show a reliable and clear benefit in relation to control/comparison conditions.
Using an fMRI task optimized to separate transient from sustained neural responses, larger transient brain responses to negative images predicted better outcomes following ABM, while sustained responses did not (see Supplement: “Analyses of other task conditions” for sustained analyses). Similar neural patterns of elevated responding were predictive across a wide range of cognitive and affective regions, in both a priori anatomical regions and whole-brain analyses with stringent Type I error control. No similar predictive patterns were observed among anxious patients receiving a sham version of the intervention. Indeed, across the network of selected a priori anatomical regions, statistical moderation findings suggested prediction of outcomes was specific to active ABM and absent for the sham condition. In an exploratory analysis using spatial pattern recognition, transient brain responses to negative images identified specific individual patients who responded exceptionally well to ABM with 65–70% accuracy, and significantly outperformed chance in spite of small sample sizes. Thus, while superior clinical effects of ABM over sham were not strongly evident in the present study, a subgroup of strong ABM responders could be identified on the basis of mechanistically relevant neural patterns. These strong responders also showed superior outcomes on several other clinical outcome measures, in relation to both other ABM recipients and to the sham group as a whole (Table 1), supporting the broader clinical relevance of the hypervigilance measure—although the generalized benefits were not statistically robust across all measures examined.
Notably, overall increased neural engagement on a transient timescale was predictive across diverse regions of the brain, including ventral affective regions previously implicated in both initial (amygdala) and more sustained (BNST, insula) forms of threat processing (Davis et al., 2010; Somerville et al., 2013), as well as numerous prefrontal and posterior regions implicated in emotional and cognitive control (Banich et al., 2009; Davidson, 2003; Nee et al., 2007; Phillips et al., 2008; Wager & Smith, 2003). These uniform patterns across much of the brain seem to argue against a strictly anatomical focus in predicting ABM response, instead highlighting the importance of joint temporal and anatomical features within the neural data. More specifically, within the overarching context of sustained blocks of anxious apprehension, having a higher ‘ceiling’ for further flexible transient responding to individual images, even among regions that likely participate in sustaining and/or regulating attention to threat, provides for a good fit for the type of training our ABM intervention provided—specifically, modification of attention to threat soon after a cue appears in the visual field. Conversely, individuals whose brain responses follow a more sustained, block-like pattern, showing little tendency towards fluctuating transient increases occurring on a relatively brief timescale, are a poor fit for ABM’s mechanistic target, and may require a distinct approach.
No regions were identified in which decreased activation predicted better outcomes. Instead, cognitive control regions and bottom-up affective regions responded similarly among ABM responders, potentially consistent with previous findings that increased activations in both PFC and amygdala (Monk et al., 2006) may index early attentional bias towards threat among anxious individuals. Furthermore, this overarching pattern across individuals held true even within the context of overall, group-level deactivations in ventromedial and ventrolateral PFC regions in response to transient negative images (see Supplement), consistent with previously published group-level findings using the current fMRI task (Somerville et al., 2013). Thus, even when neighboring and overlapping prefrontal regions exhibited an overall decrease averaging across individuals, those specific individuals who “bucked the trend” by exhibiting a relatively attenuated decrease (or, in some cases, an actual increase; see Figure 2) were more likely to respond to ABM. This pattern of relatively greater activity levels in cognitive control regions among the strongest ABM responders could reflect greater compensatory need for activation due to exaggerated stimulus-driven threat responding and/or decreased neural efficiency of top-down attentional control. Regardless, the findings suggest the best responders to ABM displayed a widely distributed pattern of increased neural engagement on the transient timescale. Consistent with a potentially widespread biomarker of the ability to engage effectively with a given intervention, we have previously reported similar evidence that increased overall neural engagement with training-relevant stimuli at baseline predicts more favorable responses across multiple forms of computer-based cognitive training paradigms, in both healthy controls (Collier & Siegle, 2015; Price, Greven, Siegle, Koster, & De Raedt, 2016) and depressed patients (Siegle et al., 2014).
As a fully automated, mechanistic intervention, ABM offers several benefits over current first-line treatments for anxiety, including cost-effectiveness, ease of dissemination, low patient burden, and high transdiagnostic relevance. However, meta-analyses suggest beneficial effects on anxiety are inconsistent across individuals and studies (e.g., Linetzky et al., 2015; Price, Wallace, et al., 2016), raising the question of which anxious patients may be most likely to benefit from ABM, and why. The present study examined mechanistic predictors of ABM response in a transdiagnostic adult sample, providing preliminary support for a process-based, neurocognitive approach to patient classification and matched treatment assignment. Current findings, including both dimensional analyses and the ability to leverage pattern recognition to predict individual patient outcomes with reasonable accuracy, suggest this approach may be fruitful for ABM and other cognitive training interventions. Though classification results are preliminary and exploratory, this approach might eventually hold the potential to surpass the ~50% response plateau pervasive in psychiatric clinical trials (Forgeard et al., 2011). Here, sensitivity was a relatively robust index—across all of the pairwise group comparisons (where strong responders were compared to other quartiles), 80% of patients who were classified at baseline as unlikely to have a strong favorable outcome did in fact fail to achieve substantial relief. Overall accuracy of the classifier (68%) was likely too low to be informative for clinical decision-making in its present form, although the classifier consistently outperformed chance. With further refinement (e.g., inclusion of multiple forms of predictive information simultaneously), this general data-driven approach could one day be helpful in informing patients regarding their odds of benefiting from interventions like ABM.
Results may have both clinical and research implications in helping to refine, personalize, and extend upon a relatively well-studied translational intervention. Matching the timescale of neural responses and intervention mechanisms may be critical to successfully alleviating anxiety with a mechanistic approach. From a precision medicine framework, mechanistic assessment may allow for identification of specific patients from within a heterogeneous pool who are a good fit for standard ABM—particularly if fMRI predictors can be translated into a clinically available form (Siegle, Steinhauer, Friedman, Thompson, & Thase, 2011). For example, given that the present fMRI task elicits reliable separation of transient from more sustained patterns of threat processing, the relevant neural patterns elicited during the task might be sufficiently captured through peripheral physiology and/or behavior. The behavioral responses collected here (incidental judgments as to whether pictures depicted indoor or outdoor scenes) proved unrelated to ABM outcomes (see Supplement), but developing alternate indices that strongly track with the neural response to transient negative images (e.g., physiological arousal measures, behavioral responses that rely on the processing of affective information within the images) is a worthwhile avenue with the potential for better clinical translation. Furthermore, given that strong anatomical distinctions were largely absent in the current neural prediction findings (i.e., transient responses across numerous regions uniformly predicted outcome), more clinically accessible methodologies that are optimized to capture the time-course (but not necessarily the anatomical source) of neural responses (e.g., pupilometry, mobile/low-cost EEG) may prove adequate for clinical translation of the current precision medicine approach. Existing neurocognitive training approaches could also be supplemented, refined, or extended to address unresolved targets identified in non-responders. For instance, alternate forms of computer-based cognitive training may successfully target sustained forms of processing, including previously developed approaches (Siegle et al., 2014). Additionally, understanding and defining the brain state that confers good outcomes following ABM could indicate a neural goal state that turns likely non-responders into responders, which might then be achievable through synergistic enhancements such as brain stimulation (Clarke, Browning, Hammond, Notebaert, & MacLeod, 2014; Heeren, Baeken, Vanderhasselt, Philippot, & de Raedt, 2015), acute pharmacology, neurofeedback, and/or other forms of cognitive training. Personalized ABM protocol development may also be indicated, such as using baseline assessment to determine the timescale of maximal neural engagement on an idiosyncratic basis [e.g., using peripheral neural indices such as pupil dilation (Price et al., 2013), or neural assessments providing fine-grained frequency information—e.g., steady-state visual evoked potentials (Woody et al., In press)] in order to fully personalize the timing of stimulus presentation during ABM.
Limitations
Prediction was applied only to acute outcomes, with a primary focus on active ABM due to the limited sample size in the sham group. Future studies should include long-term follow-up, larger ABM and control groups, and additional bona fide treatments in order to identify robust moderators of response. Though enrollment was open to all participants with clinically elevated anxiety, the sample was comprised primarily of individuals meeting criteria for one or more distress-related (as distinct from fear-related) diagnoses, with a particular preponderance of GAD. Generalizability of findings could therefore be limited to samples with similar clinical characteristics. The CAPS hypervigilance scale was selected to capture vigilance across transdiagnostic anxiety presentations, and appeared to perform as intended in the current sample (see Supplement), but the measure might capture some forms of anxiety better than others (e.g., conditions with more ubiquitous forms of threat-related cues). fMRI analyses are limited in temporal resolution due to the hemodynamic delay of the BOLD signal, which is an indirect measure that lags substantially behind neuronal activity. Nevertheless, the mixed block/event-related design utilized here was effective in this cohort at evoking separable neural substrates of transient and sustained threat processing that were consistent with previous human and animal studies (see Supplement). Preliminary pattern recognition findings were based on leave-one-out cross-validation and permutation tests of significance, but were based on small subsamples and require a more rigorous test of generalizability in a larger, independent test sample. Finally, fMRI assessments are not readily available in clinical settings. While the current study was designed to identify predictors using fMRI indices considered highly proximal to the intervention’s brain mechanisms, future work building on these findings should aim to translate these proximal brain mechanisms to more clinic-ready indices (e.g., behavioral, self-report, and peripheral psychophysiological markers).
Conclusions
The goal of the present study was to understand how individual differences in the neural mechanisms of initial and sustained threat processing relate to outcomes following a mechanistically targeted intervention (ABM). In meta-analyses of randomized controlled trials, ABM, a very low-cost, low-burden intervention, has shown both promise and limitations, producing clinically meaningful reductions in symptoms of anxiety, but only for a subset of individuals. Our results suggest that ABM’s clinical impact may be enhanced through process-based treatment-matching algorithms that take account of individual differences in the temporal pattern of neural responses to threat. While a cognitive neuroscience methodology (fMRI) was used here in an effort to precisely quantify and define mechanistic predictors of response to a translational intervention, predictive patterns of neural response will ideally be translated back to the clinic in an iterative process of intervention refinement, extension, and personalization. Current first-line treatments for clinical anxiety exhibit a ~50% response plateau, with high rates of relapse, low rates of remission, high treatment-related costs and patient burden, and little evidence to suggest which patients may benefit from which treatment options. A more efficient, effective, and personalized approach might be fostered through a greater focus on theory-driven, mechanistic predictors of treatment outcome, which cut across diagnostic boundaries, increasing generalizability; and by the use of translational treatment protocols that have clearly defined, unitary mechanisms.
Supplementary Material
Public Health Significance.
This study suggests that a translational computer-based treatment targeting attentional patterns works best for those anxiety patients showing a specific, relevant profile of brain responses. This information might help in developing a process-based framework for matching patients to treatments across many anxiety diagnoses.
Acknowledgments
This research was supported by NIH grant, K23MH100259, awarded to Dr. Rebecca Price. We gratefully acknowledge Leah Somerville for her assistance with the fMRI task.
Footnotes
Conflict of Interest Disclosures. All authors report no biomedical financial interests or potential conflicts of interest.
References
- Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with generalized anxiety disorder. Journal of Abnormal Psychology. 2009;118:28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Taylor CT, Donohue MC. Predictors of response to an attention modification program in generalized social phobia. Journal of Consulting and Clinical Psychology. 2011;79:533–541. doi: 10.1037/a0023808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Gross JJ, Lenze E, Edelman KD, Snyder S, Tanase C, et al. Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depression and anxiety. 2011;28:202–209. doi: 10.1002/da.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G, Kemp A, Sunderland M, Von Korff M, Ustun TB. Normative data for the 12 item WHO Disability Assessment Schedule 2.0. PloS one. 2009;4:e8343. doi: 10.1371/journal.pone.0008343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neuroscience and Biobehavioral Reviews. 2009;33(5):613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. The Journal of Neuroscience. 2006;26(44):11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi L, Sheu LK, Midei AJ, Gianaros PJ. Childhood physical abuse predicts stressor-evoked activity within central visceral control regions. Social Cognitive and Affective Neuroscience. 2015;10(4):474–485. doi: 10.1093/scan/nsu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Emery G, Greenberg RC. Anxiety disorders and phobias: A cognitive perspective. New York: Basic Books; 1985. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Britton J, Suway J, Clementi M, Fox N, Pine D, Bar-Haim Y. Neural changes with attention bias modification for anxiety: a randomized trial. Social Cognitive and Affective Neuroscience. 2015;10(7):913–920. doi: 10.1093/scan/nsu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Pieper S, Thayer JF. Expanding stress theory: prolonged activation and perseverative cognition. Psychoneuroendocrinology. 2005;30:1043–1049. doi: 10.1016/j.psyneuen.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Clarke PJF, Browning M, Hammond G, Notebaert L, MacLeod C. The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: Evidence from transcranial direct current stimulation. Biological Psychiatry. 2014;76(12):946–952. doi: 10.1016/j.biopsych.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Collier A, Siegle G. Individual differences in response to prediction bias training. Clinical Psychological Science. 2015;3(1):79–90. [Google Scholar]
- Cox RW, Reynolds RC, Taylor PA. AFNI and clustering: false positive rates redux. BioRXiv. 2016 doi: 10.1089/brain.2016.0475. http://dx.doi.org/10.1101/065862 [DOI] [PMC free article] [PubMed]
- Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis-Tiwary TA, Egan LJ, Babkirk S, Denefrio S. For whom the bell tolls: Neurocognitive individual differences in the acute stress-reduction effects of an attention bias modification game for anxiety. Behaviour Research and Therapy. 2016;77:105–117. doi: 10.1016/j.brat.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dimitrov DM, Rumrill PD., Jr Pretest-posttest designs and measurement of change. Work. 2003;20(2):159–165. [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erceg-Hurn DM, Mirosevich VM. Modern robust statistical methods: an easy way to maximize the accuracy and power of your research. American Psychologist. 2008;63(7):591–601. doi: 10.1037/0003-066X.63.7.591. [DOI] [PubMed] [Google Scholar]
- Fisher PL, Durham RC. Recovery rates in generalized anxiety disorder following psychological therapy: an analysis of clinically significant change in the STAI-T across outcome studies since 1990. Psychological Medicine. 1999;29:1425–1434. doi: 10.1017/s0033291799001336. [DOI] [PubMed] [Google Scholar]
- Forbes AB, Carlin JB. “Residual change” analysis is not equivalent to analysis of covariance. Journal of Clinical Epidemiology. 2005;58(5):540–541. doi: 10.1016/j.jclinepi.2004.12.002. author reply 542. [DOI] [PubMed] [Google Scholar]
- Forgeard MJC, Haigh EAP, Beck AT, Davidson RJ, Henn FA, Maier SF, et al. Beyond depression: Towards a process-based approach to research, diagnosis, and treatment. Clinical Psychology: Science & Practice. 2011;18(4):275–299. doi: 10.1111/j.1468-2850.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7(1):30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline J. To smooth or not to smooth? Bias and efficiency in fMRI time-series analysis. Neuroimage. 2000;12(2):196–208. doi: 10.1006/nimg.2000.0609. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Kuan DC, Schirda BL, Jennings JR, Sheu LK, et al. An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biological Psychiatry. 2014;75(9):738–745. doi: 10.1016/j.biopsych.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren A, Baeken C, Vanderhasselt MA, Philippot P, de Raedt R. Impact of Anodal and Cathodal Transcranial Direct Current Stimulation over the Left Dorsolateral Prefrontal Cortex during Attention Bias Modification: An Eye-Tracking Study. PLoS One. 2015;10(4):e0124182. doi: 10.1371/journal.pone.0124182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, et al. Neural systems predicting long-term outcome in dyslexia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jones EB, Sharpe L. Cognitive bias modification: A review of meta-analyses. Journal of Affective Disorders. 2017;223:175–183. doi: 10.1016/j.jad.2017.07.034. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The global burden of anxiety and mood disorders: putting the European Study of the Epidemiology of Mental Disorders (ESEMeD) findings into perspective. Journal of Clinical Psychiatry. 2007;68(Suppl 2):10–19. [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Peters ER, Fannon D, Antonova E, Premkumar P, Anilkumar AP, et al. Dorsolateral prefrontal cortex activity predicts responsiveness to cognitive-behavioral therapy in schizophrenia. Biological Psychiatry. 2009;66:594–602. doi: 10.1016/j.biopsych.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Linetzky M, Pergamin-Hight L, Pine DS, Bar-Haim Y. Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and Anxiety. 2015;32(6):383–391. doi: 10.1002/da.22344. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Clarke PJF. The attentional bias modification approach to anxiety intervention. Clinical Psychological Science. 2015;3(1):58–78. [Google Scholar]
- McEvoy PM, Mahoney AEJ, Moulds ML. Are worry, rumination, and post-event processing one and the same? Development of the repetitive thinking questionnaire. Journal of Anxiety Disorders. 2010;24:509–519. doi: 10.1016/j.janxdis.2010.03.008. [DOI] [PubMed] [Google Scholar]
- McLaughlin Ka, Nolen-Hoeksema S. Rumination as a transdiagnostic factor in depression and anxiety. Behaviour Research and Therapy. 2011;49:186–193. doi: 10.1016/j.brat.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohlman J, Eldreth DA, Price RB, Staples AM, Hanson C. Prefrontal-limbic connectivity during worry in older adults with generalized anxiety disorder. Aging and Mental Health. doi: 10.1080/13607863.2015.1109058. (In press) [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh DL, Cox, Cook EW, Weller fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, Affectective, and Behavioral Neuroscience. 2007;7(1):1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Research: Cognitive Brain Research. 2002;15(1):31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Petscher Y, Schatschneider C. A Simulation Study on the Performance of the Simple Difference and Covariance-Adjusted Scores in Randomized Experimental Designs. Journal of Education and Measurement. 2011;48(1):31–43. doi: 10.1111/j.1745-3984.2010.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Eldreth Da, Mohlman J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation. Translational Psychiatry. 2011;1:e46. doi: 10.1038/tp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Greven IM, Siegle GJ, Koster EH, De Raedt R. A novel attention training paradigm based on operant conditioning of eye gaze: Preliminary findings. Emotion. 2016;16(1):110–116. doi: 10.1037/emo0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Siegle G, Silk JS, Ladouceur CD, McFarland A, Dahl RE, et al. Sustained neural alterations in anxious youth performing an attentional bias task: A pupilometry study. Depression and Anxiety. 2013;30:22–30. doi: 10.1002/da.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Siegle GJ, Silk JS, Ladouceur CD, McFarland A, Dahl RE, et al. Looking under the hood of the dot-probe task: An fMRI study in anxious youth. Depression and Anxiety. 2014;31:178–187. doi: 10.1002/da.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Wallace M, Kuckertz JM, Amir N, Graur S, Cummings L, et al. Pooled patient-level metaanalysis of children and adults completing a computer-based anxiety intervention targeting attentional bias. Clinical Psychology Review. 2016;50:37–49. doi: 10.1016/j.cpr.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163(4):735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Price RB, Jones N, Ghinassi F, Painter T, Thase ME. You gotta work at it: Pupillary indices of task focus are prognostic for response to a neurocognitive intervention for depression. Clinical Psychological Science. 2014;2(4):455–471. [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: utility and neural correlates. Biological Psychiatry. 2011;69(8):726–733. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, et al. Towards clinically useful neuroimaging in depression treatment: Is subgenual cingulate activity robustly prognostic for depression outcome in Cognitive Therapy across studies, scanners, and patient characteristics? Archives of General Psychiatry. 2012;69(9):913–924. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral cortex. 2013;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-trait Anxiety Inventory (Form Y Self-evaluation Questionnaire) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, et al. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19(4):1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive Affective and Behavioral Neuroscience. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- White LK, Sequeira S, Britton JC, Brotman MA, Gold AL, Berman E, et al. Complementary features of attention bias modification therapy and cognitive-behavioral therapy in pediatric anxiety disorders. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2017.16070847. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ML, Miskovic V, Owens M, James KM, Feurer C, Sosoo EE, Gibb BE. Competition effects in visual cortex between emotional distractors and a primary task in remitted depression. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017;2:396–403. doi: 10.1016/j.bpsc.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


