Abstract
Background. Hemolytic uremic syndrome (HUS) is one of the common causes for acute kidney injury in childhood. Objective. The goals of our study were to identify risk factors for short-term complications and long-term outcomes of chronic kidney disease (CKD) in Shiga toxin–producing Escherichia coli (STEC)-HUS and other diarrhea positive (D+) HUS. Methods. Retrospective chart review was obtained of 58 pediatric patients treated for STEC-HUS and other D+ HUS between February 2002 and January 2011. Results. Thirty-three patients (56.9%) required dialysis. Dialysis was more likely initiated if a patient was a female (P < .012), oliguric (urine output < 0.5 mL/kg/h, P < .0005), or hemoglobin (HGB) level >10 g/dL (P = .009) at admission. Neurological complications developed only among 5 dialyzed patients (P < .042), and were more common if the patient received hemodialysis (HD) compared with peritoneal dialysis (P < .0005). CKD was noted during the subsequent follow-up clinic visits in 5 patients (8.6%). Those who developed CKD received HD (P = .002), dialysis for >10 days (P = .0004), or HGB level >10 g/dL (P = .034) at admission. Conclusions. Children with STEC-HUS/D+ HUS who may need dialysis are identified by female gender, lower urine output, higher serum creatinine level, and higher HGB at admission. They are at higher risk developing central nervous system complications especially if they needed HD. Children requiring >10 days of dialysis are at risk for development of CKD.
Keywords: STEC-HUS, D+ HUS, HGB level, CNS complications, dialysis, CKD
Introduction
Hemolytic uremic syndrome (HUS) is one of the common causes for acute kidney injury (AKI) in children. HUS is characterized by the triad of microangiopathic hemolytic anemia, nonimmune thrombocytopenia, and acute renal failure.1 Diarrhea positive (D+) HUS, especially Shiga toxin–producing Escherichia coli (STEC)-HUS receives special attention due to health services efforts to control epidemic outbreaks.2-4 Our hospital is an exclusive referral hospital for the surrounding farming/rural area, and thus admission for STEC-HUS is common. Studies that characterize demographics, clinical course, and long-term outcomes in children with STEC-HUS from our region have not been reported. Furthermore, risk factors to identify children at presentation who are at risk for having short- and long-term outcomes in STEC-HUS are still emerging.
We analyzed our experience with STEC- HUS/D+ HUS patients to identify predictors for the development of complications (need for dialysis, neurologic involvement, colonic perforation, and insulin requirement) and for the development of chronic kidney disease (CKD) at the time of presentation to potentially improve the care such of critically ill children.
Materials and Methods
This was a retrospective chart review of children admitted to the Children’s Mercy Hospital, Kansas City, MO, between February 2002 and January 2011. Fifty-eight patients were identified by using the specific International Classification of Diseases, Ninth Revision, Clinical Modification, admission code. D+ HUS was confirmed by documented diarrhea prior to presentation, documented positive stool studies for enterohemorrhagic E coli, evidence of hemolysis by positive peripheral smear for schistocytes, thrombocytopenia, elevated lactate dehydrogenase level, and abnormal renal function. Children were identified later in the cohort as STEC-HUS when Shiga toxin testing was established in the microbiology laboratory. Patients without clinical/laboratory evidence of HUS, known as atypical HUS, recurrent HUS, or secondary to Streptococcus pneumonia infection, were excluded.
Data collected from the initial admission and from the last outpatient follow-up included age, gender, home address (ZIP code), complete blood count (white blood cell count [WBC], hemoglobin [HGB], platelet, blood urea nitrogen level, serum creatinine level), urine output in the first 24 hour at admission, stool culture result, documentation of preadmission antibiotic therapy, need for dialysis, hospital day of starting dialysis, type of dialysis (peritoneal dialysis [PD]), or hemodialysis (HD) initiated, and length of dialysis. There were 6 patients who transitioned to HD from PD after a short period of time and were counted into HD group as prevailing type of renal replacement therapy. Central nervous system (CNS) complications were defined as clinical observation of presence of seizures, documentation of lethargy, reduced consciousness or altered mental status, started after initiation of dialysis as documented in the medical record. All participants were seen for regular outpatient follow-up after discharge from the hospital. Estimated glomerular filtration rate (eGFR) was calculated by using the bed-side Schwartz formula5. The diagnosis of CKD was based on having an eGFR<90 mL/min/1.73 m2 and/or having spot proteinuria >0.2 mg/mg at the 5-year follow-up.
Ethical Considerations
The study was approved by the Children’s Mercy Hospital Pediatric Institutional Reviewed Board (IRB#: 1109-149E). Due to the retrospective nature, the study involved chart review of children admitted within the study period to our institute, who have been already discharged and no personal communication were concluded between participants and the investigators as well the study proposed no more than minimal risk of harm without procedure, the study received waiver for informed consent.
Statistical Analysis
Central tendency of continuous variables (interval or ratio) was measured. The mean values were compared between the dialyzed and non-dialyzed and CKD and non-CKD groups by independent t test. Categorical variables were gender, young age (<2 years), HGB >10 g/dL, WBC >15 × 103/µL, oliguria at admission (<0.5 mL/kg/h urine over the first 24-hour period), the need for dialysis (yes/no), prolonged dialysis (>10 days), type of dialysis (HD or PD), preadmission antibiotic therapy (yes/no), and development of CKD (yes/no). The categorical variables were assessed by χ2 test for association, and when the expected frequencies of categorical variables in the 2 × 2 tables were below 5, Fisher’s exact test was used. Multivariate logistic regression model was developed to assess the role of predictive factors in the severity of acute illness. The statistical program SPSS 12.0 (SPSS Inc, Chicago, IL) was used to analyze the data. Statistical significance was defined as P < .05.
Results
We identified 82 children with the International Classification of Diseases, Ninth Revision, code of HUS during the study period. Twenty-four patients were excluded from the analysis due to wrong diagnosis, having atypical HUS or missing medical documentation, leaving 58 patients for the final analysis, that is, an average 7.3 cases/year.
Baseline Data at Admission
The mean ± SD age of our cohort was 3.7 ± 2.8 years. There gender distribution was 58.6% girls and 41.4% boys. Twenty-eight were <2 years old (48.3%). Shiga toxin was identified in 43.1% of the cases once the testing was established at our center and the remaining had shown growth of E Coli to establish the diagnosis of STEC-HUS/D+ HUS. Complement C3 was measured in 6 children and was low in 2. 28% of children who had received antibiotic prior to admission. The home address ZIP code was predominantly from Ozark and Jasper counties in the State of Missouri and Douglas counties in the State of Kansas.
Acute Kidney Injury and Need for Dialysis
The standard of care at our institute is to perform PD unless there is a concern for or an established colonic perforation, in which case we initiate HD. Dialysis was initiated in 33 cases (56.9%), 25 (75%) received just PD and 7 were transitioned to HD during the course due to extravasation of PD fluid or significant leak at the exit site, and 1 patient was started on HD. The mean duration of dialysis was 11.3 ± 10.6 days. Dialysis was required for >10 days in 8 (25.9%) children. As shown in Table 1, children who received dialysis had significantly higher HGB level at admission, 9.19 ± 2.28 g/dL, compared with the non-dialyzed group, 7.45 ± 1.96 g/dL (P = .002); had significantly lower urine output, 0.52 ± 1.16 mL/kg/h, versus the non-dialyzed group, 1.80 ± 0.86 mL/kg/h (P < .0005); and serum creatinine on admission was significantly higher in the dialyzed group, 3.45 ± 2.55 mg/dL, versus 1.71 ± 1.14 mg/dL (P = .004). There was no significant difference between age, platelet, and WBC count in the 2 groups. As shown in Table 2 on initial univariate analysis, the odds ratio (OR) for needing dialysis were female patients compared with male patients, with an OR 4.0 (95% confidence interval [CI] = 1.32-12.11, P = .012), oliguria at admission, OR 29.57 (95% CI = 5.46-159.97, P < .0005), and HGB >10 g/dL at admission, OR 5.31 (95% CI = 1.49-18.96, P = .007). Younger age (<2 years), previous antibiotic exposure, and WBC count were not associated with need for dialysis (Table 2).
Table 1.
Characteristics of Dialyzed Compared to Non-Dialyzed Groups (Independent t Test, P < .05).
| Variables | Dialyzed, n=33 (Mean ± SD) | Non-Dialyzed, n = 25 (Mean ± SD) | P |
|---|---|---|---|
| Age (years) | 3.93 ± 2.98 | 3.54 ± 2.59 | .61 |
| Urine output at admission (mL/kg/h) | 0.52 ± 1.16 | 1.81 ± 0.86 | <.0005 |
| Hemoglobin (g/dL) | 9.19 ± 1.16 | 7.45 ± 1.96 | .002 |
| Creatinine on admission (mg/dL) | 3.45 ± 2.55 | 1.71 ± 1.14 | .004 |
| WBC (103/µL) | 17.15 ± 7.1 | 18.72 ± 14.55 | .59 |
| PLT (103/µL) | 53.87 ± 39.53 | 72.31 ± 68.31 | .21 |
Abbreviations: WBC, white blood cell; PLT, platelet.
Table 2.
Predictor for Need to Initiate Dialysis in Pediatric Patients Admitted With STEC/D+ HUS (Fisher’s Exact Test and Logistic Regression, P < .05).
| Variable | Dialyzed, n = 33 (Mean ± SD) | Non-Dialyzed, n = 25 (Mean ± SD) | Univariate Odds Ratio (95% CI) | P | Multivariate Odds Ratio (95% CI) | P |
|---|---|---|---|---|---|---|
| <2 years old | 15/33 | 13/25 | 0.77 (0.27-2.18) | .62 | ||
| Female | 24/33 | 10/25 | 4 (1.32-12.11) | .01 | 3.02 (0.43-21.17) | .26 |
| Antibiotic exposure | 11/30 | 3/25 | 3.28 (0.78-13.77) | .09 | ||
| Urine output <0.5 mL/kg/h | 23/30 | 2/25 | 29.57 (5.46-159.97) | <.005 | 30.1 (3.53-255.72) | .002 |
| WBC >15 × 103/µL | 18/33 | 8/25 | 2.4 (0.81-7.14) | .112 | ||
| Hemoglobin >10 g/dL | 17/33 | 4/25 | 5.31 (1.49-18.96) | .007 | 11.16 (1.51-82.22) | .018 |
| CNS involvement | 5/33 | 0/25 | 1.17 (0.41-0.68) | .04 |
Abbreviations: WBC, white blood count; CNS, central nervous system.
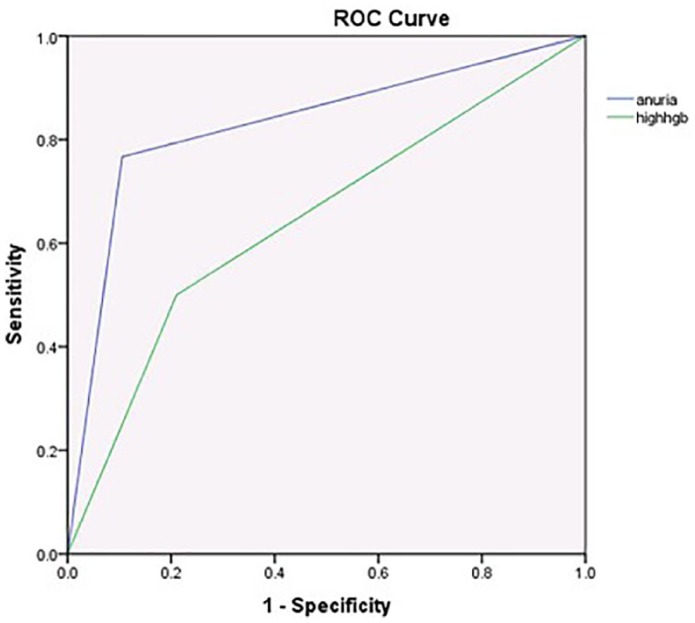
The significant risk factors for need of dialysis were entered into the multivariate regression analysis and its effect were controlled in comparison with gender, oliguria, and HGB >10 g/dL. Oliguria had an OR 30.1 (95%CI = 3.53-255.72, P = .002) and HGB level >10 g/dL had OR 11.16 (95% CI = 1.51-82.22, P = .018), remaining significant (Table 2). If we remove oliguria from the equation, HGB >10 g/dL remained the strongest indicator, OR 10.96 (P = .004). As shown in Figure 1, the receiver operating characteristic curve for oliguria and HGB >10 g/dL at admission as diagnostic test were good: oliguria AUC (area under the curve) 0.83 (95% CI = 0.71-0.95, P < .005) and HGB >10 g/dL AUC 0.67 (95% CI = 0.53-0.81, P = .02).
Figure 1.
Receiver operating characteristic (ROC) curve of urine output <0.5 mL/kg/h (blue line) and hemoglobin (HGB) >10 g/dL (green line) at admission for patients, who required dialysis. ROC for oliguria at admission = 0.83 (P < .005, 95% CI = 0.71-0.95). ROC for HGB >10 g/dL at admission = 0.67 (P = .02, 95% CI = 0.53-0.81).
Acute Nonrenal Complications
Neurological complication developed in 5 children (8.6 %). Patients who received dialysis developed severe neurological complications after initiation of dialysis significantly more often (OR = 1.18 [95% CI = 1.02-1.36], P = .042) and even more likely if they received HD (OR = 49 [95% CI = 4.37-549.25], P < .005). Neurological complications were not associated with HGB level >10 g/dL at admission, PD, younger age (<2 years), or previous antibiotic exposure (Table 1). We had no gastrointestinal perforation, need for insulin therapy, and we had 1 death in our cohort.
Long-Term Complications
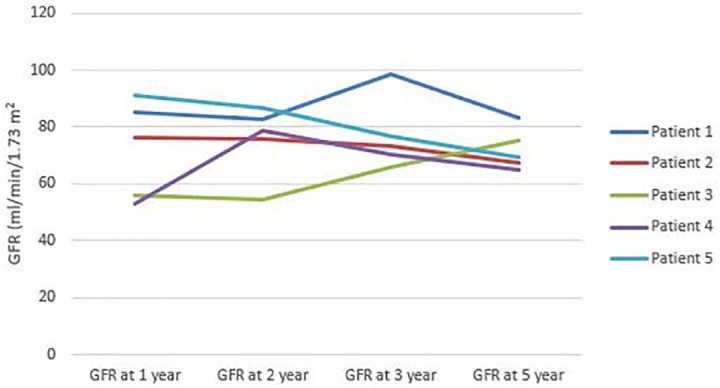
Follow-up information was available in all 58 cases from their annual clinic visits. All 5 out of 58 patients (8.6%) who were later diagnosed with CKD received dialysis during the acute phase. There was a significant difference in CKD rate between HD and PD treated patients. CKD was noted in 3 out of 8 HD (37.5%) compared with 2 out of 25 PD (8%) patients (P = .016). Patients who developed CKD had higher HGB level at admission (10.7 ± 2.64 vs 8.99 ± 2.22 g/dL, P = .034). There was no significant difference between the length of dialysis among PD and HD patients during the acute phase. However, receiving dialysis for >10 days was associated with the development of CKD regardless of dialysis modality (OR = 15.27 [95% CI = 1.55-150.76], P = .013). There was no significant association between CKD and gender, younger age (<2 year), previous antibiotic therapy, oliguria, or leukocytosis. Due to low number of patients with CKD we were not able to perform logistic regression. The changes in eGFR of CKD patients are displayed on Figure 2.
Figure 2.
Changes in glomerular filtration rate over 5 years of patients’ who developed chronic kidney disease.
Discussion
Our goal was to identify risk factors at the time of initial presentation for the development of severe acute course manifested by a need for dialysis as well for the development of chronic long-term sequelae in our STEC-HUS/D+ HUS patients. Children who eventually required dialysis had significantly higher serum HGB level, lower urine output, higher serum creatinine level at admission, and they were more likely female (Tables 1 and 2). Patients more likely developed CNS complications if they received HD compared with PD. In contrast to earlier literature, younger age, leukocytosis, and antibiotic exposure were not linked with need for dialysis in our analysis. Oliguria and HGB >10 g/dL remained to be independent predictors in multivariate analysis.
HUS is one of the most frequent causes of AKI in infants and children.1 Previous retrospective studies found several contributing factors for the development of severe AKI secondary to HUS. Antibiotic exposure during the diarrhea phase has been questioned to be associated with more likely development of HUS.6 Similarly leukocytosis and younger age have been linked with severe acute course,7 but previous meta-analysis of 49 studies found inconsistent relationship with acute and chronic sequelae.8 Though age appeared to have no significant role in our study, our study population included mainly younger patients in both dialyzed and non-dialyzed groups compared with previous reports. Similar to our finding female gender has been linked with worse outcome in other studies.8,9 The mechanism of an increased risk of HUS among females remains to be determined. Factors such as lower body surface area versus similar toxin load or increased adherence of verotoxigenic E coli have been speculated.9
Hydration has been shown to be protective prior to development of HUS in diarrhea positive E coli infection.10 Several studies demonstrated higher HGB level on admission to be associated with increased morbidity and mortality during the acute phase.7,11 It is possible that the relatively high serum HGB level despite ongoing hemolysis in HUS patients may reflect dehydration causing prerenal injury aggravating the ongoing intrinsic renal disease11 and increasing risk for rapid progression. However, recently the concept of higher HGB level was reevaluated and association was found with severe neurological complication among patients who received HD.12 Furthermore, it may be speculated that patients with higher HGB level may develop worsening hemoconcentration during HD due to fluid removal favoring more microthrombi formation causing ischemic tissue injury in other organs, including CNS system and kidneys.12 Additionally, in HUS prior to the development of renal injury, thrombin generation due to accelerated thrombogenesis and inhibition of fibrinolysis can be detected as elevated plasminogen-activator inhibitor type 1 (PAI-1)13,14 level. While PAI-1 level increases in patients who receive chronic HD13 and PD has been detected to reduce PAI-1 level in HUS patients.15
Oliguria on admission was a significant risk factor for need of dialysis in univariate and in multivariate analyses in our study. This correlates with previous finding as higher morbidity rate was identified in oliguric acute renal failure compared with nonoliguric acute renal failure.16 HGB >10 g/dL at admission was an independent and strong risk factor for need of dialysis. We feel that an elevated HGB level with an ongoing hemolytic process should raise the clinician’s attention to dehydration and also for higher risk of microthrombi formation during HD as it also correlated with increased risk for CNS complications in the HD population. As that, gentle fluid removal during dialysis in an oliguric patient with a relatively higher HGB level despite ongoing hemolysis is probably preventive of further neurological complications.11 Higher HGB level at admission additionally to hyponatremia has been recently reported as an independent risk factor for increased morbidity and mortality.17
Most of the patients are able to recover their renal function even after severe STEC-HUS/D+ HUS. In our study, we had no patients who developed end-stage renal disease yet we found prolonged dialysis (>10 days) to be significantly associated with mild form of CKD during follow-up. Per the authors’ best knowledge, modality of dialysis has not been investigated as a risk factor for CKD in HUS patients. In our small study, we observed significantly higher number of patients with CKD from the HD group compared with the PD group. In our institute, children with HUS at that time mainly received PD as the preferred dialysis modality due to patient size, and just a minority of our patients were chosen to receive primarily HD. A few patients were transitioned to HD due to PD-related complications as well. Due to our study design and low number of subjects, we cannot rule out incidental allocation of more severe cases to receive HD. However, if elevated HGB level on admission may reflect hemoconcentration as sign of existing prerenal failure, more aggressive fluid removal during HD in these patients may cause further microthrombus formation causing more extended ischemic injuries and higher risk not just for CNS complications but also for CKD.11,12,17
Our finding of severe acute illness relating to higher incidence of CKD is correlating with previous study outcomes. Oakes et al18 found an increased prevalence of CKD in their dialyzed HUS patients who had anuria for more than 5 days. Garg et al8 reviewed 49 studies to quantify the long-term prognosis of diarrhea positive pediatric HUS patients. In their meta-analysis they found 25% demonstrating long-term renal sequelae during their follow-up. The severity of acute illness, particularly CNS complications, prolonged anuria, and need for dialysis >4 weeks, was associated with worse long-term prognosis. Similarly to our finding both studies recommended long-term follow-up of HUS patient to detect late development of CKD even if the renal function was recovered by the time of hospital discharge.8,18
Conclusions
The severity of acute disease with need of dialysis in pediatric patients with STEC-HUS/D+ HUS can be predicted on admission by gender, HGB level, serum creatinine level, and urine output. Severe neurological complications maybe more likely to develop due to hemoconcentration during HD. Therefore, fluid status of anuric patients should be cautiously interpreted together with serum HGB level. The risk for development of CKD can be predicted based on the severity of the acute illness, including higher HGB level on admission potentially reflecting prerenal injury, need of dialysis, or by the length of dialysis during the acute disease.
Our study has several limitations including retrospective design and small patient number. Therefore, the authors feel the role of different dialysis modalities in preventing further complications need to be further investigated in the future.
Footnotes
Author Contributions: JSV: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
TS: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
USA: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Judith Sebestyen VanSickle  https://orcid.org/0000-0003-1937-1613
https://orcid.org/0000-0003-1937-1613
References
- 1. Siegler RL. The hemolytic uremic syndrome. Pediatr Clin North Am. 1995;42;1505-1529. [DOI] [PubMed] [Google Scholar]
- 2. Scheiring J, Andreoli SP, Zimmerhackl LB. Treatment and outcome of Shiga-toxin-associated hemolytic uremic syndrome. Pediatr Nephrol. 2008;23:1749-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lemaignen A, Ridel C, Hertig A, Rondeau E. Escherichia coli associated hemolytic and uremic syndrome: what lessons can be learned after the European epidemic of 2011 [in French]. Nephrol Ther. 2013;9:129-136. [DOI] [PubMed] [Google Scholar]
- 4. Taylor EV, Nguyen TA, Machesky KD, et al. Multistate outbreak of Escherichia coli O145 infections associated with romaine lettuce consumption, 2010. J Food Prot. 2013;76:939-944. [DOI] [PubMed] [Google Scholar]
- 5. Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gianviti A, Tozzi AE, De Petris L, et al. Risk factors for poor renal prognosis in children with hemolytic uremic syndrome. Pediatr Nephrol. 2003;18:1229-1235. [DOI] [PubMed] [Google Scholar]
- 7. Mody RK, Gu W, Jones TF, et al. Postdiarrheal hemolytic uremic syndrome in United States children: clinical spectrum and predictors of in-hospital death. J Pediatr. 2015;166:1022-1029. [DOI] [PubMed] [Google Scholar]
- 8. Garg AX, Suri RS, Barrowman N, et al. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA. 2003;290:1360-1368. [DOI] [PubMed] [Google Scholar]
- 9. Coad NA, Marshall T, Rowe B, Taylor CM. Changes in the postenteropathic form of the hemolytic uremic syndrome in children. Clin Nephrol. 1991;35:10-16. [PubMed] [Google Scholar]
- 10. Ake JA, Jelacic S, Ciol MA, et al. Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics. 2005;115:e673-e680. [DOI] [PubMed] [Google Scholar]
- 11. Balestracci A, Martin SM, Toledo I, Alvarado C, Wainsztein RE. Dehydration at admission increased the need for dialysis in hemolytic uremic syndrome children. Pediatri Nephrol. 2012;27:1407-1410. [DOI] [PubMed] [Google Scholar]
- 12. Ardissino G, Daccò V, Testa S, et al. Hemoconcentration: a major risk factor for neurological involvement in hemolytic uremic syndrome. Pediatr Nephrol. 2015;30:345-352. [DOI] [PubMed] [Google Scholar]
- 13. Chandler WL, Jelacic S, Boster DR, et al. Prothrombotic coagulation abnormalities preceding the hemolytic-uremic syndrome. N Engl J Med. 2002;346:23-32. [DOI] [PubMed] [Google Scholar]
- 14. Xu Y, Hagege J, Mougenot B, Sraer JD, Rønne E, Rondeau E. Different expression of the plasminogen activation system in renal thrombotic microangiopathy and the normal human kidney. Kidney Int. 1996;50:2011-2019. [DOI] [PubMed] [Google Scholar]
- 15. Bergstein JM, Riley M, Bang NU. Role of plasminogen-activator inhibitor type 1 in the pathogenesis and outcome of the hemolytic uremic syndrome. N Engl J Med. 1992;327:755-759. [DOI] [PubMed] [Google Scholar]
- 16. Andreoli S. Acute kidney injury in children. Pediatr Nephrol. 2009;24:253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alconcher LF, Coccia PA, Suarez ADC, et al. Hyponatremia: a new predictor of mortality in patients with Shiga toxin-producing Escherichia coli hemolytic uremic syndrome. Pediatr Nephrol. 2018;33:1791-1798. doi: 10.1007/s00467-018-3991-6 [DOI] [PubMed] [Google Scholar]
- 18. Oakes RS, Kirkhamm JK, Nelson RD, Siegler RL. Duration of oliguria and anuria as predictors of chronic renal-related sequelae in post-diarrheal hemolytic uremic syndrome. Pediatr Nephrol. 2008;23:1303-1308. [DOI] [PubMed] [Google Scholar]