Abstract
Acute kidney injury, especially early-stage disease, is a common hospital comorbidity requiring timely recognition and treatment. We investigated the effect of daily laboratory alerting of patients at risk for acute kidney injury as measured by documented International Classification of Diseases diagnoses. A quasi-experimental study was conducted at 8 New York hospitals between January 1, 2014, and June 30, 2017. Education of clinical documentation improvement specialists, physicians, and nurses was conducted from July 1, 2014, to December 31, 2014, prior to initiating daily hospital-wide laboratory acute kidney injury alerting on January 1, 2015. Incidence based on documented International Classification of Diseases diagnosis of acute kidney injury and acute tubular necrosis during the intervention periods (3 periods of 6 months each: January 1 to June 30 of 2015, 2016, and 2017) were compared to one preintervention period (January 1, 2014, to June 30, 2014). The sample consisted of 269 607 adult hospital discharges, among which there were 39 071 episodes based on laboratory estimates and 27 660 episodes of documented International Classification of Diseases diagnoses of acute kidney injury or acute tubular necrosis. Documented incidence improved significantly from the 2014 preintervention period (5.70%; 95% confidence interval: 5.52%-5.88%) to intervention periods in 2015 (9.89%; 95% confidence interval, 9.66%-10.12%; risk ratio = 1.73, P < .001), 2016 (12.76%; 95% confidence interval, 12.51%-13.01%; risk ratio = 2.24, P < .001), and 2017 (12.49%; 95% confidence interval, 12.24%-12.74%; risk ratio = 2.19, P < .001). A multifactorial intervention comprising daily laboratory alerting and education of physicians, nurses, and clinical documentation improvement specialists led to increased recognition and clinical documentation of acute kidney injury.
Keywords: acute kidney injury, clinical documentation, KDIGO: Kidney Disease Improving Global Outcomes, clinical decision support, electronic laboratory alerting
Introduction
Acute kidney injury (AKI) is a common comorbidity and affects up to 20% of all hospitalized patients.1 The KDIGO (Kidney Disease: Improving Global Outcomes) guidelines are the most widely accepted evidence-based diagnostic criteria for AKI. The diagnosis and assessment of AKI severity is dependent on incremental rise in serum creatinine (SCr) above the patient’s baseline value within a specified duration.1 Increasing severity of AKI is associated with longer length of stay (LOS), costs of care, resource utilization, and in-hospital mortality.2-4 Hence, it is imperative to diagnose and manage AKI at the earliest possible stage during an inpatient admission. Since AKI can occur on virtually every hospital service, this comorbidity must be recognized by practitioners across a broad array of clinical specialties.
Although nephrologists are well-versed with KDIGO criteria, most AKI diagnoses, especially early-stage disease, are made by nonspecialist physicians.3 Physician recognition of AKI remains poor because of inability to apply KDIGO criteria consistently in routine hospital settings, limited clinical awareness among non-nephrologists, and lack of effective clinical decision support (CDS) tools in the electronic health record (EHR).5,6 A recent study confirmed lack of recognition of early AKI by nurses.7 While the implementation of EHR alerts in bringing AKI to the attention of providers has come under intense scrutiny,6,8 the impact of such alerts has not yet been shown to be of value.9,10 Automated AKI detection algorithms using delta-checking criteria have increasingly been embedded in Laboratory Information Systems (LIS) of biochemistry laboratories,11-14 but the clinical value of LIS-generated alerts also remains unproven.
Incidence estimates based on hospital billing codes are more specific but less sensitive when compared to incidence estimates based on laboratory SCr criteria.15,16 However, billing codes tend to capture only the more severe cases of AKI (stage 2 and 3), which account for less than 30% of all AKI episodes.17 Hence, compared to laboratory data, billing codes underestimate the true disease burden and economic impact of AKI, especially early-stage disease.16,17
This study was conducted as part of a quality improvement project to standardize detection of AKI within an integrated health system in New York. The clinical aim was to introduce into routine hospital practice, especially in nonspecialist settings, a comprehensive electronic laboratory AKI alerting system based on KDIGO criteria. Besides standardizing early AKI detection, we aimed to reduce variability in diagnosis by embedding this alerting system into daily clinical workflow.18,19 The laboratory-triggered AKI alert was distributed in a patient-specific fashion to all inpatient units, with the requirement that such patients be evaluated promptly. Clinical documentation of AKI, through the International Classification of Diseases (ICD) coding system, was used as the primary end point and laboratory estimates of AKI severity were assessed as secondary end points to evaluate the effectiveness of daily laboratory alerting.
Methods
Study Design, Setting, and Population
We performed a quasi-experimental study with a pre–post study design and interrupted time-series analysis.20 The study was conducted at 8 adult hospitals within the Northwell Health System between January 1, 2014, and June 30, 2017. Although the alerting program ran in continuity, data were collected and analyzed for four 6-month intervals during this study period so as to avoid potential seasonality as a confounding factor: January 1 to June 30, 2014; January 1 to June 30, 2015; January 1 to June 30, 2016; and January 1 to June 30, 2017. For these 4 time periods; there were 65 831 discharges in 2014, 66 364 discharges in 2015, 68 889 discharges in 2016, and 68 523 discharges in 2017 at the 8 hospital sites collectively. With one exception, all sites shared the same LIS, Cerner Millennium and EHR, Sunrise Clinical Manager (Allscripts Corp, Raleigh, North Carolina). The exception was one study site which was not yet on the Cerner Millennium LIS in January to June 2014, but was by the January to June 2015 time period. This study was limited to inpatient adult medical and surgical patients greater than 18 years of age.
A health system executive committee comprising senior clinical leadership from the Department of Pathology and Laboratory Medicine, the Division of Nephrology, and the system Clinical Quality program endorsed the policy to standardize AKI detection through daily alerts.
Acute Kidney Injury Criteria and Baseline Creatinine
The KDIGO criteria rely on the ability to detect an incremental increase in SCr, compared to a baseline value, of 0.3 mg/dL within 48 hours and/or a 50% increase (1.5 times) within 7 days.1 The KDIGO definition also suggests comparing the high inpatient SCr value to a prior stable baseline, usually an outpatient SCr measurement in a patient’s normal state of health.1 But, there is no consensus on what the baseline should be, and investigators have used different surrogates.21–23 If no reliable estimate of baseline SCr can be made, the KDIGO guidelines recommend using the lowest SCr during hospitalization as the baseline.1 Although there may be risk of dilutional artefact in SCr measurements if blood samples are drawn from an intravenous line, our hospital quality metrics indicate that test cancellations due to “improper collection” are rare. The most common causes of test cancellations are clotted sample (44%) or quantity not sufficient (36%); among remaining causes, “improper collection” (wrong tube type or wrong collection methodology, including dilutional artefact) were only 6% of cancelled tests, or 0.006% of total tests (data not shown). We therefore felt that use of the lowest SCr during hospitalization as the baseline was appropriate.
Laboratory Electronic Alerting for Acute Kidney Injury
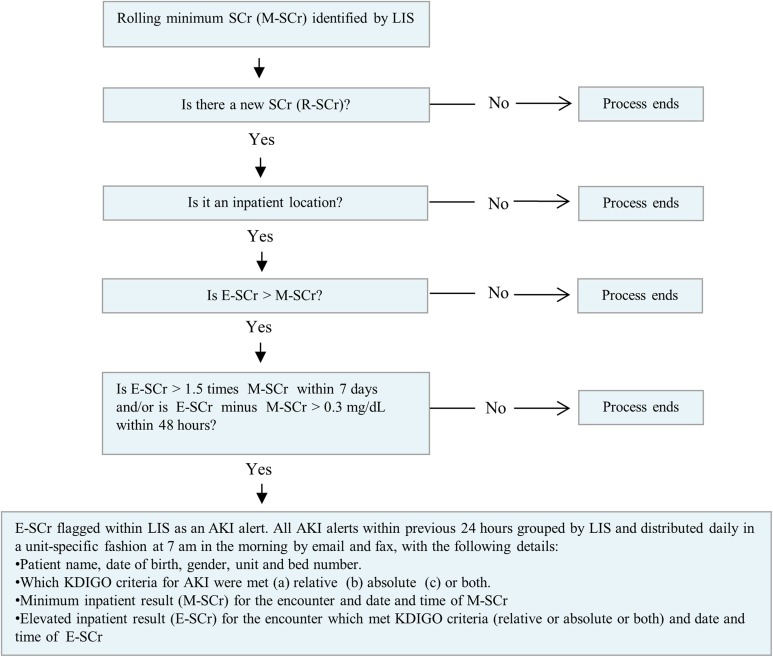
We developed a real-time alerting system based on a structured query language algorithm run on all laboratory SCr values generated during an inpatient encounter (Figure 1). Our algorithm used the delta-checking functionality within Cerner. Because of inconsistent access to prior outpatient SCr values for all hospitalized patients, the minimum inpatient SCr value was used as the baseline for this algorithm, as per KDIGO guidelines.1 Initial SCr at the time of admission was included as a potential baseline value. If the baseline value decreased during admission, then the new minimum SCr became the rolling baseline. If there was a clinically significant rise consistent with KDIGO criteria, then an alert was generated and the elevated SCr result was flagged. Patients who only had a single SCr measurement during the encounter or did not meet the KDIGO criteria were not flagged.
Figure 1.
Electronic reporting algorithm for AKI using serum creatinine measurements. AKI, acute kidney injury; E-SCr, elevated serum creatinine; KDIGO, kidney disease improving global outcomes; LIS, laboratory information system; M-SCr, minimum serum creatinine;.
All SCr measurements from emergency departments, admission units, intensive care units, or any other inpatient location were included. All SCr values throughout the study period were measured using Roche Cobas automated analyzers, based on the modified Jaffe Method. The coefficient of variation of the SCr assay ranged from 2.5% to 5% (normal creatinine range, 0.5-1.2 mg/dL).
Development of Acute Kidney Injury Rounding Report and Validation of Alerting System
To create the daily report, the project team programmed the LIS to generate an electronic report of all AKI alerts within the previous 24 hours. A unit-specific consolidated report, with patient room and bed location, was faxed and e-mailed to clinical and nursing leads of all units at 7:00 am in the morning. This “rounding” report was then discussed at morning ward rounds to ensure all members of the clinical team were aware of their patients at risk for AKI. We validated the algorithm and reporting workflow at one hospital (pilot site) from January 1, 2014, to June 30, 2014. The chief medical officer (CMO) of the pilot hospital (G.B.) conducted a provider alert awareness campaign from November to December 2013, prior to the introduction of alerting system.
Intervention
Despite daily AKI alerting at the pilot site for the first 6 months of 2014, preliminary analysis of billing data showed only a minimal improvement in provider documentation of AKI (Supplemental Figure 1). Starting in July 2014, and with the approval of the CMOs of the respective system hospitals, the Clinical Documentation Improvement (CDI) team and Department of Pathology and Laboratory Medicine created a system-wide partnership. At the pilot site, through July to December 2014, in addition to issuance of the rounding report to clinical units, a copy of the daily AKI rounding report was sent to the CDI specialists who received instructions to query physicians in case of inconsistent documentation of AKI. Supplemental Figure 1 shows that clinical documentation of AKI at the pilot site then began to increase. During this same time period (July to December 2014), physicians and nurses at all 8 hospitals received presentations regarding accurate clinical documentation of AKI based on KDIGO criteria, severity, etiology, and treatments. An awareness campaign for CDI specialists and medical coders at all 8 hospitals also was conducted from July to December 2014.
Data Collection and Statistical Analysis
We analyzed billing data for every hospital episode with a primary or secondary diagnosis-related groups (DRGs) diagnosis of AKI or acute tubular necrosis (ATN), which in turn are based on the 9th and 10th iterations of the ICD Clinical Modification (ICD-9CM, ICD-10-CM). We analyzed laboratory data on AKI alerts generated by our algorithm from the LIS database, noting that a single hospital admission can result in multiple AKI alerts. Accordingly, every hospital episode was categorized into KDIGO stages 1 to 3, as follows—stage 1: SCr increase by ≥ 0.3 mg/dL from baseline or SCr increase by 1.5 to 1.9 times baseline; stage 2: SCr increase by 2.0 to 2.9 times baseline; stage 3: SCr increase by 3.0 times baseline or SCr ≥ 4 mg/dL. Only the most severe AKI alert for each hospital episode was used for classifying laboratory data into stages 1 to 3.
Data points collected (deidentified) were age, gender, encounter number, baseline SCr result, SCr result which met the KDIGO criteria of (a) absolute rise of 0.3 mg/dL within 48 hours, (b) relative rise of 50% within 7 days, or (c) both. The institutional review board waived the need for informed patient consent because the data were collected as part of an ongoing quality improvement project.
For all hospitals, we only compared data from January 1, 2014, to June 30, 2014 (1 preintervention period) with data from January 1, 2015, to June 30, 2015, January 1, 2016 to June 30, 2016, and January 1, 2017, to June 30, 2017 (3 postintervention periods), to avoid the potential confounding effect of our educational intervention (July 1, 2014, to December 31, 2014) on data from the pilot site and to minimize the effect of seasonal variation.
All incidence estimates for billing and laboratory data were calculated using cumulative incidence methodology. The total number of hospital discharges were the denominators for incidence calculations. As noted earlier, Cerner LIS was in use at 7 of the hospitals for the full study period but was implemented in the latter half of 2014 at one of our hospitals (which was not the 2014 pilot hospital). Hence, the denominator for aggregate AKI incidence calculations from January to June 2014 for laboratory data (7 hospitals) was lower compared to billing data (8 hospitals).
The 95% confidence interval (CI) for incidence estimates was calculated assuming a normal distribution. We grouped laboratory-identified stage 1 and stage 2 AKI episodes as “early AKI” and stage 3 episodes as “late AKI.” Analysis of variance was used to assess the statistical significance of data variation of the 4 study years (2014-2017). For comparisons of individual years, risk ratio (RR) and absolute risk difference (RD) were used to compare pre- and postintervention incidence estimates. For all statistical tests, P < .05 was considered statistically significant; numerical P values are given down to P < .001. Microsoft Excel (version 2013) and Minitab (version 14) were used for all analyses.
Results
Incidence of Acute Kidney Injury Episodes
Clinical documentation
Preliminary analysis of coded ICD data for the pilot hospital site for the preintervention period (January 1, 2014, to June 30, 2014) showed that the incidence of AKI/ATN combined was 5.52% (509 episodes in 9213 discharges). After our definitive educational intervention (July 1, 2014, to December 31, 2014), incidence for documented AKI and ATN diagnosis improved steadily at the pilot site during the remainder of 2014, reaching 13.1% in December (1245 episodes in 9502 discharges). The monthly incidence rates in 2014 at the pilot site are shown in Supplemental Figure 1, documenting the steady rise in the second half of the calendar year, concurrent with the ongoing educational campaign.
For the 8 hospital sites, the study population consisted of a total of 269 607 adult hospital discharges (65 831 in 2014; 66 364 in 2015; 68 889 in 2016, and 68 523 in 2017). The episode counts for AKI, ATN, and AKI/ATN combined for all hospitals over the study period are given in Table 1. When compared to preintervention period of 2014 (2965 AKI and 789 ATN episodes), the number of coded AKI and ATN episodes increased over the postintervention periods of 2015 (5523 AKI and 1040 ATN episodes), 2016 (7589 AKI and 1198 ATN episodes), and 2017 (7383 AKI and 1173 ATN episodes). The proportion of episodes coded as AKI (versus ATN) also increased: from 79.0% in 2014, to 84.2% in 2015, 86.4% in 2016, and 86.3% in 2017; corresponding proportions for ATN were 21.0% in 2014, 15.8% in 2015, 13.6% in 2016, and 13.7% in 2017. This supports the premise that a large proportion of the increased count of documented episodes was because of better detection and treatment of early-stage AKI.
Table 1.
Study Characteristics and Incidence of AKI Episodes by Laboratory Data and Administrative (ICD) Data for 8 Hospitals by Study Period.
| Characteristic | Preintervention Period | Postintervention Periods | |||
|---|---|---|---|---|---|
| January 1, 2014, to June 30, 2014* | January 1, 2015, to June 30, 2015 | January 1, 2016, to June 30, 2016 | January 1, 2017, to June 30, 2017 | P Value† | |
| Episodes as per clinical documentation (ICD) data | |||||
| Discharges, count | 65 831 | 66 364 | 68 889 | 68 523 | NA |
| Episodes coded as AKI, count | 2965 | 5523 | 7589 | 7383 | NA |
| Proportion of episodes coded as AKI (%) | 2965/3754 (79.0) | 5523/6563 (84.2) | 7589/8787 (86.4) | 7383/8556 (86.3) | NA |
| Episodes coded as ATN, count | 789 | 1040 | 1198 | 1173 | NA |
| Proportion of episodes coded as ATN (%) | 789/3754 (21.0) | 1040/6563 (15.8) | 1198/8787 (13.6) | 1173/8556 (13.7) | NA |
| Episodes coded as AKI or ATN, count | 3754 | 6563 | 8787 | 8556 | NA |
| Incidence (95% CI) of coded AKI episodes per 100 discharges | 4.50 (4.34-4.66) | 8.32 (8.11-8.53) | 11.02 (10.79-11.25) | 10.77 (10.54-11.0) | .002 |
| Incidence (95% CI) of coded ATN episodes per 100 discharges | 1.20 (1.12-1.28) | 1.57 (1.48-1.66) | 1.74 (1.64-1.84) | 1.71 (1.61-1.81) | .0130 |
| Incidence (95% CI) of total coded AKI and ATN episodes per 100 discharges | 5.7 (5.52-5.88) | 9.89 (9.66-10.12) | 12.76 (12.51-13.01) | 12.49 (12.24-12.74) | .001 |
| Episodes as per laboratory data | |||||
| Discharges, count | 55 559‡ | 66 364 | 68 889 | 68 523 | NA |
| Stage 1 AKI episodes, count | 9061 | 10 062 | 10 891 | 11 115 | NA |
| Stage 2 AKI episodes, count | 2103 | 2381 | 2369 | 2656 | NA |
| Stage 1 and 2 AKI episodes combined (early AKI), count | 11 164 | 12 443 | 13 260 | 13 771 | NA |
| Proportion of early AKI episodes (%) | 11 164/11 821 (94.4) | 12 443/13 227 (94.1) | 13 260/14 023 (94.6) | 13 771/14 450 (95.3) | NA |
| Stage 3 AKI episodes (late AKI), count | 657 | 784 | 763 | 679 | NA |
| Proportion of late AKI episodes (%) | 657/11 821 (5.6) | 784/13 227 (5.9) | 763/14 023 (5.4) | 679/14 450 (4.7) | NA |
| All stages AKI episodes, count | 11 821 | 13 227 | 14 023 | 14 450 | NA |
| Incidence (95% CI) of AKI stage 1 episodes per 100 discharges | 16.31 (16.0-16.62) | 15.16 (14.89-15.43) | 15.81 (15.54-16.08) | 16.22 (15.94-16.5) | .903 |
| Incidence (95% CI) of AKI stage 2 episodes per 100 discharges | 3.79 (3.63-3.95) | 3.59 (3.45-3.73) | 3.44 (3.3-3.58) | 3.88 (3.74-4.02) | .514 |
| Incidence (95% CI) of AKI stage 1 and 2 episodes combined (early AKI) per 100 discharges | 20.1 (19.77-20.43) | 18.75 (18.46-19.04) | 19.25 (18.96-19.54) | 20.1 (19.8-20.4) | .832 |
| Incidence (95% CI) of AKI stage 3 (late AKI) episodes per 100 discharges | 1.18 (1.09-1.27) | 1.18 (1.09-1.27) | 1.11 (1.03-1.19) | 0.99 (0.92-1.06) | .438 |
| Incidence (95% CI) of AKI all stages combined per 100 discharges | 21.28 (20.94-21.62) | 19.93 (19.63-20.23) | 20.36 (20.06-20.66) | 21.09 (20.79-21.39) | .404 |
Abbreviations: AKI, acute kidney injury; ATN, acute tubular necrosis; CI, confidence interval; ICD, International Classification of Diseases; LIS, Laboratory Information Systems; NA, not applicable.
*All comparisons of postintervention periods (2015, 2016, and 2017) are made with the preintervention period of 2014.
†Analysis of variance was used to assess the statistical significance of data variation of the 4 study years (2014-2017).
‡The denominator for incidence calculations for laboratory data was lower for 2014 because of incomplete data: 7 of the 8 study hospitals were on the Cerner LIS at the time. For 2015 to 2017, all 8 study hospitals were on Cerner LIS.
The denominator for incidence calculations was the number of hospital discharges. For all 8 hospitals combined during the preintervention period (January to June, 2014), the incidence of AKI was 4.5% (2965 episodes in 65 831 discharges), the incidence of ATN was 1.2 % (789 episodes in 65 831 discharges), and incidence of AKI and ATN combined was 5.7% (3754 episodes in 65 831 discharges).
Beginning in January 2015, clinically documented incidence rates of AKI and ATN at all hospitals steadily increased. Documented incidence of AKI increased to 8.32% in 2015, 11.02% in 2016, and 10.77% in 2017 (P = .002). Similarly, documented incidence of ATN increased from 1.2% in 2014 to 1.57% in 2015, 1.74 % in 2016, and 1.71% in 2017 (P = .013). In aggregate (AKI and ATN), the incidence based on documented diagnoses increased significantly from 5.7% (95% CI: 5.52%-5.88%) in 2014 to 9.89% (95% CI: 9.66%-10.12%) in 2015, 12.76% (95% CI; 12.51%-13.01%) in 2016, and 12.49% (95% CI; 12.24% to 12.74%) in 2017 (P = .001).
Laboratory data
For analysis of laboratory data, we categorized all hospital episodes into KDIGO stages 1 to 3 based on the most severe laboratory AKI alert for each episode. For comparison, we grouped laboratory-identified stage 1 and 2 AKI episodes as early AKI and stage 3 episodes as late AKI. Although there is no 1:1 concordance between clinical severity of AKI cases based on KDIGO criteria, with the severity of documented ICD diagnosis of AKI or ATN, we hypothesized that most if not all stage 3 AKI episodes would be documented as ATN based on ICD coding. This distinction also allowed us to compare laboratory KDIGO criteria versus ICD coding criteria for AKI or ATN.
Table 1 shows that, compared to 2014, and allowing for capturing laboratory data for only 7 hospitals in 2014, there was no change in the distribution of laboratory-identified stages of AKI in 2015, 2016, and 2017. Specifically, stage 1 and 2 “early” AKI contributed to 94% to 95% of all laboratory-detected episodes in all 4 years (2014-2017), with only 4% to 5% being stage 3 “late” AKI episodes.
In the preintervention, baseline period (2014), the incidence of AKI by stages was 16.31 % for stage 1, 3.79 % for stage 2, 20.1% for stage 1 and 2 combined (early AKI), 1.18 % for stage 3 (late AKI), and 21.28% for all AKI stages. The incidence of stage 1 AKI episodes decreased from 16.31% (95% CI: 16.0%-16.62%) in 2014 to 15.16% (95% CI:, 14.89%-15.43%) in 2015. However, the incidence for stage 1 episodes increased to 15.81% (95% CI: 15.54%-16.08%) in 2016 and 16.22% (95% CI: 15.94%-16.5%) in 2017 (P = .903). Similarly, stage 2 AKI episodes slightly reduced in incidence from 3.79% (95% CI: 3.63%-3.95%) in 2014 to 3.59% (95% CI: 3.45%-3.73%) in 2015 and 3.44% (95% CI: 3.3%-3.58%) in 2016. Similar to stage 1, the incidence of stage 2 episodes rebounded to 3.88% (95% CI: 3.74%-4.02%) in 2017 (P = .514). Overall, laboratory-detected early AKI episodes (stage 1 and 2 combined) reduced from 20.1% (95% CI: 19.77%-20.43%) in 2014 to 18.75% (18.46%-19.04%) in 2015. However, incidence of early AKI increased to 19.25% (95% CI: 18.96%-19.54%) in 2016 and back to 20.1% (95% CI: 19.8%-20.4%) in 2017 (P = .832).
The incidence of stage 3 (late AKI) remained unchanged from 1.18% (95% CI: 1.09%-1.27%) in 2014 and 2015 to 1.11 % (95% CI: 1.03%-1.19%) in 2016. Stage 3 incidence slightly reduced to 0.99% (95% CI: 0.92%-1.06%) in 2017 (P = .438). The total incidence (all stages combined) of laboratory-detected AKI decreased from 21.28% (95% CI: 19.63%-20.23%) in 2014 to 19.93% (95% CI: 19.63%-20.23%) in 2015, but increased again to 20.36% (95% CI: 20.06%-20.66%) in 2016 and 21.09% (95% CI: 20.79%-21.39%) in 2017 (P = .404).
Comparison of Incidence Estimates Between Preintervention and Postintervention Periods
For ease of comparison of incidence estimates between preintervention and postintervention periods, we calculated the risk ratio by comparing the postintervention periods of 2015, 2016, and 2017 with the control (preintervention) period of 2014 (Table 2).
Table 2.
Comparison of Incidence Estimates Between Preintervention and Postintervention Periods.
| Variable | Postintervention Period From January 1, 2015, to June 30, 2015 | Postintervention Period From January 1, 2016, to June 30, 2016 | Postintervention Period From January 1, 2017, to June 30, 2017 | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | P Value* | RR (95% CI) | P Value† | RR (95% CI) | P Value‡ | |
| Episodes as per clinical documentation (ICD) data | ||||||
| Documentation of AKI | 1.86 (1.78-1.94) | <.001 | 2.46 (2.35-2.55) | <.001 | 2.39 (2.30-2.49) | <.001 |
| Documentation of ATN | 1.31 (1.19-1.43) | <.001 | 1.45 (1.33-1.59) | <.001 | 1.43 (1.31-1.56) | <.001 |
| Documentation of AKI and ATN combined | 1.73 (1.67-1.80) | <.001 | 2.24 (2.16-2.32) | <.001 | 2.19 (2.11-2.27) | <.001 |
| Episodes as per laboratory data | ||||||
| Laboratory episodes, AKI stage 1 | 0.93 (0.91-0.95) | <.001 | 0.97 (0.95-0.99) | .017 | 0.99 (0.97-1.02) | .6763 |
| Laboratory episodes, AKI stage 2 | 0.95 (0.89-1.0) | .0682 | 0.91 (0.86-0.96) | .0011 | 1.02 (0.97-1.08) | .4071 |
| Laboratory episodes, AKI stage 1 and 2 (early AKI) | 0.93 (0.91-0.95) | <.001 | 0.96 (0.94-0.98) | .0002 | 1.00 (0.98-1.02) | .9897 |
| Laboratory episodes, AKI stage 3 (late AKI) | 1.0 (0.9-1.11) | .9851 | 0.94 (0.84-1.04) | .216 | 0.838 (0.75-0.93) | .0012 |
| Laboratory episodes, AKI all stages | 0.94 (0.92-0.96) | <001 | 0.96 (0.94-0.98) | <.001 | 0.99 (0.97-1.02) | .4185 |
Abbreviations: AKI, acute kidney injury; ATN, acute tubular necrosis; CI, confidence interval; ICD, International Classification of Diseases; RR, risk ratio.
*Comparison between the postintervention period of 2015 with preintervention period of 2014.
†Comparison between the postintervention period of 2016 with preintervention period of 2014.
‡Comparison between the postintervention period of 2017 with preintervention period of 2014.
Clinical documentation
Compared to the preintervention period, documentation of AKI significantly increased in 2015 (RR = 1.86, P < .001), 2016 (RR = 2.46, P < .001), and 2017 (RR = 2.39, P < .001). Similarly, documentation of ATN significantly increased in 2015 (RR = 1.31, P < .001), 2016 (RR = 1.45, P < .001), and 2017 (RR = 1.43, P < .001). Overall, the documentation of AKI and ATN combined increased significantly and was sustained over the postintervention periods of 2015 (RR = 1.73, P < .001), 2016 (RR = 2.24, P < .001), and 2017 (RR = 2.19, P < .001). These RR ratios corroborate the data shown in Table 2, in which there was a >150% increase in 2015, and >200% increase in 2016 and 2017, of timely clinical documentation of AKI and ATN episodes when compared to 2014.
Laboratory data
Because of laboratory alerting and education of providers, we had hoped to see a reduction in the disease burden of AKI as calculated by laboratory incidence estimates. However, as shown in Table 2, though stage 1 episodes decreased in 2015 (RR = 0.93, P < .001) and 2016 (RR = 0.97, P = .017), this trend was not sustained in 2017 (RR = 0.99, P = .6763). Similarly, stage 2 episodes decreased in 2016 (RR = 0.91, P < .001), but this was not preceded by reduction in 2015 (RR = 0.95, P = .0682) and also not sustained in 2017 (RR = 1.02, P = .4071). The combined stage 1 and stage 2 (early AKI) incidence decreased in 2015 (RR = 0.93, P < .001) and 2016 (RR = 0.96, P = .002), but this was not sustained in 2017 (RR = 1.0, P = .9897). Stage 3 (late AKI) episodes did not decrease in 2015 (RR = 1.0, P = .9851) or 2016 (RR = 0.94, P = .216), but did decrease in 2017 (RR = 0.838, P = .0012), suggesting no consistent effect. Overall, the laboratory incidence for all stages combined decreased in 2015 (RR = 0.94, P < .001) and 2016 (RR = 0.96, P < .001), but this effect was not sustained through 2017 (RR = 0.99, P = .4185). These data do not allow distinction between whether this program was or was not effective in reducing the disease burden of AKI, since we were not examining potential risks for development of AKI in this study.
Comparison of Incidence Estimates of Acute Kidney Injury Episodes Between Laboratory Data and Coded (International Classification of Diseases) Data
Direct comparisons of incidence estimates from laboratory-identified AKI episodes versus administratively documented AKI and ATN episodes are given in Table 3 and Supplemental Figure 2. Before intervention, the incidence of laboratory-detected AKI episodes (21.28 %) was significantly different from coded ICD episodes (5.7%) in 2014 (RD, 15.57%). After intervention, this gap between laboratory incidence and documented incidence narrowed in 2015 (RD, 10.05%), improved further in 2016 (RD, 7.6%) and was sustained in 2017 (RD, 8.6%). We calculate that compared to 2014 preintervention period, an additional 2809, 5033, and 4802 episodes of AKI and ATN were documented in 2015, 2016, and 2017, respectively.
Table 3.
Comparison of Incidence Estimates of AKI Episodes Between Laboratory Data and Coded (ICD) Data, Preintervention Period, and Postintervention Periods.
| Characteristic | Preintervention Period | Postintervention Periods | ||
|---|---|---|---|---|
| January 1, 2014, to June 30, 2014 | January 1, 2015, to June 30, 2015 | January 1, 2016, to June 30, 2016 | January 1, 2017, to June 30, 2017 | |
| Incidence (95% CI) of AKI all stages combined per 100 discharges | 21.28 (20.94-21.62) | 19.93 (19.63-20.23) | 20.36 (20.06-20.66) | 21.09 (20.79-21.39) |
| Incidence (95% CI) of total coded AKI and ATN episodes per 100 discharges | 5.7 (5.52-5.88) | 9.89 (9.66-10.12) | 12.76 (12.51-13.01) | 12.49 (12.24-12.74) |
Abbreviations: AKI, acute kidney injury; ATN, acute tubular necrosis; CI, confidence interval; ICD, International Classification of Diseases.
Discussion
To our knowledge, this study is the first to show how inpatient daily laboratory alerting for AKI combined with education of clinical providers (physician and nurses), CDI specialists, and medical coders significantly improved provider recognition and documentation of AKI and ATN. As the 2014 single hospital pilot demonstrated, daily laboratory alerting alone was an insufficient intervention. Communicating the risk of AKI through daily reports in a unit-targeted fashion across the entire hospital, combined with preparatory education for both providers and the administrative teams were important factors in improved recognition and documentation of AKI. A critical factor was the collaboration between the laboratory and the CDI team. The CDI specialists were the effector arm both for reaching out to the providers regarding patients at risk for AKI and for ensuring compliance with documentation. We also had the all-important support of hospital leadership at each of the 8 hospital sites.
This study presents a workflow innovation for early AKI recognition which can be replicated in other hospital settings. First, KDIGO guidelines are automated into computable CDS, using the delta-checking functionality of the LIS.24 Delta check algorithms are highly sensitive and capture >98% of patients at risk for AKI.3,13 Second, alert fatigue is avoided by consolidating daily alerts into a single report for morning rounds.18,25,26 Third, there is an educational focus on provider behavioral change in conjunction with implementing the daily AKI alerts.9,27,28 Fourth, clinical documentation compliance is promoted by partnering with CDI professionals. Of necessity, clinical documentation demands both clinical diagnosis and appropriate clinical management. Formal AKI documentation has been associated with improved patient survival after adjusting for severity of illness.29 Fifth, linkage of administrative coding data with laboratory data enables examination of the true disease burden of AKI for registry-based clinical studies.30,31
This study has numerous strengths. First, the incidence of AKI based on laboratory estimates is similar to other investigations and confirms the reported gap between laboratory estimates and documented AKI diagnoses.4,16 Second, our alerting system is a fully automated, low-cost solution, requiring no manual laboratory intervention.15,17 Third, similar to recent reports, we show that laboratory data can be successfully used as a surveillance tool for AKI monitoring in routine hospital settings.3,32
The clinically documented rates of AKI and ATN, our primary outcome of interest, improved significantly from 5.7% in 2014 to 9.89% in 2015, 12.76% in 2016, and 12.49% in 2017. This represented a substantial increase in the assignment of hospital episodes to appropriate DRG categories of AKI (comorbidity) and ATN (major comorbidity). It is reasonable to posit that improved documentation had a significant impact on the calculated case-mix index for the 8 hospitals in the study and on expected LOS and baseline mortality for reporting to regulatory agencies.
Analysis of aggregated laboratory data for all hospitals showed a slight but significant reduction in laboratory-detected AKI incidence from 21.28% in 2014 to 19.93% in 2015 and 20.36% in 2016. However, this reduction was confined to early AKI (stage 1 and 2) and the laboratory-detected AKI incidence increased again to 21.09% in 2017. These data suggest that our intervention had some desirable impact on reducing overall disease burden in 2015 and 2016 but was not sustained through 2017. Consistent sustained reduction in disease burden and progression will require more sophisticated CDS which integrates laboratory with pharmacy data in real time to modulate dosing of nephrotoxic medications, as well as algorithmic treatment approaches and development of novel biomarkers.9,33-35
Our study has limitations. Selection of appropriate baseline SCr is an important determinant of accuracy of AKI detection algorithms. Using minimum inpatient SCr is a sensitive method for AKI detection, but it can lead to false-positives.4,36,37 However, our approach was to use laboratory alerting as a screening tool to prompt earlier clinical evaluation rather than provide a diagnosis.8 Since we were implementing this system across all units including nonspecialist areas, we accepted the trade-off of high sensitivity over lower specificity. Although some laboratory alerts could have been false-positive, the physicians were required to use their clinical judgment to attribute elevated SCr to AKI. Moreover, to satisfy documentation compliance, the provider needed to document the likely etiology, treatment, and additional nursing time spent in management of the patient. All these provider-driven steps would help ensure that the clinical diagnosis of AKI would have the requisite specificity.
Use of lowest SCr in intensive care settings could lead to overdetection of AKI. However, this has more to do with a lack of consensus on the best estimate for baseline creatinine in the literature.21 Investigators have used varying surrogates for baseline SCr, including first inpatient value, minimum inpatient value, average of first 3 values, and an average of outpatient value within 1 year prior to hospitalizations. This lack of consensus makes comparisons difficult and sometimes impractical. According to recent literature, the best value for baseline creatinine is a patient’s outpatient SCr value in a normal state of health.22,23 However, this outpatient value is not available in most in-patient settings due to lack of interoperability between outpatient and inpatient EHR systems. Consequently, the outpatient SCr value is difficult to obtain and implement consistently in AKI detection algorithms. However, KDIGO guidelines recommend that, if a reliable outpatient SCr value/estimate cannot be obtained, then the next best choice for baseline is the minimum inpatient value.
Acute kidney injury occurs most commonly as a secondary diagnosis in conjunction with other common medical and surgical diagnosis. As this study was based on laboratory and administrative coding data only, we did not assess the impact of our intervention on other clinical outcome variables such as mortality and LOS. We did not have access to granular cost data for hospital episodes. Thus, the impact of our intervention on costs-of-care was not part of this study.17,38,39
Although it can be argued that alerts in the EHR would be the ideal solution, such alerts do not provide a fail-safe for 2 reasons: the ever-present concern about “alert fatigue” and the requisite that a provider interact with the EHR in order to observe such an alert. A founding premise of this program was hospital medical leadership’s desire to guarantee medical assessment of potential AKI patients’ status at the start of every hospital day. The laboratory-based program provided the foundational data, using delta-checking functionality available within most modern LISs. Indeed, this LIS-based approach for AKI alerting is now in widespread use in the National Health Service in England.11 That being said, institutional efforts to establish real-time AKI alerts within the EHR continue.
A 24-hour rounding report could lead to an alert delay of anywhere between 1 and 23 hours. However, we believe that the enhanced sensitivity of our alerting system offsets the delay in diagnosis resulting from consolidating the alerts from the previous 24 hours into a single report. Specifically, using the minimum inpatient SCr as the baseline allows our alerting algorithm to be extremely sensitive to any significant SCr fluctuations as has been demonstrated in earlier studies.3,4 Also, this decision to use a once a day report was taken in conjunction with the CMOs’ desire to minimize alert fatigue and optimize provider focus on AKI as a clinical parameter. The fact that clinical recognition of AKI increased, as monitored by coding for this condition, provides assurance that the chosen strategy was of value.
Although the total laboratory incidence of AKI/ATN declined in 2015 and 2016, there was rebound in laboratory disease burden in 2017. This possibly could have been due to unanticipated and unwelcome provider behavioral change of not paying attention to laboratory values over the course of a day and depending solely on the daily alert. However, many studies indicate that isolated laboratory or EHR alerting is not sufficient factor to reduce inpatient AKI morbidity.27,33,40 A comprehensive approach involving algorithmic, step-wise treatment plans including proper fluid management and medication reconciliation is necessary for treatment of AKI and prevention of progression to severe stage 3 injury.
In our study, there was a reduction in severe stage 3 episodes in 2017. This leads us to believe that providers did benefit from the daily notification, countering the argument that provider inattention may have been a consequence of this alerting program. Although severe stage 3 AKI episodes can lead to chronic kidney disease (CKD) postdischarge, we do not currently have longitudinal postdischarge data to evaluate whether long-term CKD has been reduced, owing to the current lack of an electronic master patient index in our LIS and EHR systems. This prohibited accurate linkage of inpatient to outpatient laboratory data for longitudinal follow-up of patients. Patients who suffer AKI in hospitals have a much higher risk of developing long-term CKD. Similarly, patients with preexisting CKD are more likely to develop AKI when admitted to hospitals.40 In the latter instance, our data did not identify patients with preexisting CKD who may have a higher baseline SCr and may thus have been misclassified on the basis of their admission SCr values. However, it is now increasingly believed that AKI and CKD are not separate conditions but a spectrum of disorders.40 Also, there are inherent limitations in the current definitions of AKI and CKD, which categorize patients into distinct categories, when they can be superimposed on each other.
Conclusions
Laboratory data remain an underutilized resource for detection of AKI in routine hospital settings. We show how laboratory reporting of AKI can be used to augment administrative coding data in the detection of AKI and demonstrate the impact of laboratory reporting on improved clinical documentation. Simultaneously, we demonstrate the importance of linking a laboratory-based program with education of clinical providers and their administrative support personnel to achieve a sustained and significant increase in the recognition and clinical documentation of AKI.
Supplemental Material
APC-18-0034_FINAL_Supplemental_Figures for Impact of Daily Electronic Laboratory Alerting on Early Detection and Clinical Documentation of Acute Kidney Injury in Hospital Settings by Tarush Kothari, Kendal Jensen, Debbie Mallon, Gerard Brogan, and James Crawford in Academic Pathology
Acknowledgments
The authors thank Luis Eguren (Chief Information Officer, Northwell Health Laboratories) and Paul Marshall (Laboratory Information Services, Northwell Health Laboratories) for help with extracting inpatient laboratory data. The authors thank Debbie Mallon (Assistant Vice-President, Clinical Documentation Improvement, and Northwell Health) for help with extracting documentation data on primary and secondary discharge diagnoses of AKI and ATN for all the hospitals in the study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tarush Kothari, MD, MPH  https://orcid.org/0000-0002-6077-9170
https://orcid.org/0000-0002-6077-9170
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kidney Disease; Improving Global Outcomes (KDIGO) Acute Kidney Injury Workgroup. KDIGO clinical practice guideline for acute kidney injury. 2012. http://kdigo.org/clinical_practice_guidelines/pdf/KDIGO%20AKI%20Guideline.pdf. Accessed February 7, 2017.
- 2. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. [DOI] [PubMed] [Google Scholar]
- 3. Selby NM, Crowley L, Fluck RJ, et al. Use of electronic results reporting to diagnose and monitor AKI in hospitalized patients. Clin J Am Soc Nephrol. 2012;7:533–540. [DOI] [PubMed] [Google Scholar]
- 4. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kashani K, Herasevich V. Utilities of electronic medical records to improve quality of care for acute kidney injury: past, present, future. Nephron. 2015;131:92–96. [DOI] [PubMed] [Google Scholar]
- 6. Handler SM, Kane-Gill SL, Kellum JA. Optimal and early detection of acute kidney injury requires effective clinical decision support systems. Nephrol Dial Transplant. 2014;29:1802–1803. [DOI] [PubMed] [Google Scholar]
- 7. Nascimento RA, Assuncao MS, Silva JMJ, et al. Nurses’ knowledge to identify early acute kidney injury. Rev Esc Enferm USP. 2016;50:399–404. [DOI] [PubMed] [Google Scholar]
- 8. James MT, Hobson CE, Darmon M, et al. Applications for detection of acute kidney injury using electronic medical records and clinical information systems: workgroup statements from the 15th ADQI Consensus Conference. Can J Kidney Health Dis. 2016;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoste EA, Kashani K, Gibney N, et al. Impact of electronic-alerting of acute kidney injury: workgroup statements from the 15th ADQI Consensus Conference. Can J Kidney Health Dis. 2016;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lachance P, Villeneuve PM, Rewa OG, et al. Association between e-alert implementation for detection of acute kidney injury and outcomes: a systematic review. Nephrol Dial Transplant. 2017;32:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Selby NM, Hill R, Fluck RJ; NHS England “Think Kidneys” AKI Programme. Standardizing the early identification of acute kidney injury: the NHS England national patient safety alert. Nephron. 2015;131:113–117. [DOI] [PubMed] [Google Scholar]
- 12. Baron JM, Cheng XS, Bazari H, et al. Enhanced creatinine and estimated glomerular filtration rate reporting to facilitate detection of acute kidney injury. Am J Clin Pathol. 2015;143:42–49. [DOI] [PubMed] [Google Scholar]
- 13. Garner AE, Lewington AJ, Barth JH. Detection of patients with acute kidney injury by the clinical laboratory using rises in serum creatinine: comparison of proposed definitions and a laboratory delta check. Ann Clin Biochem. 2012;49:59–62. [DOI] [PubMed] [Google Scholar]
- 14. Wang HE, Jain G, Glassock RJ, Warnock DG. Comparison of absolute serum creatinine changes versus kidney disease: improving global outcomes consensus definitions for characterizing stages of acute kidney injury. Nephrol Dial Transplant. 2013;28:1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ko S, Venkatesan S, Nand K, Levidiotis V, Nelson C, Janus E. International statistical classification of diseases and related health problems coding underestimates the incidence and prevalence of acute kidney injury and chronic kidney disease in general medical patients. Intern Med J. 2018;48:310–315. [DOI] [PubMed] [Google Scholar]
- 17. Kerr M, Bedford M, Matthews B, O’Donoghue D. The economic impact of acute kidney injury in England. Nephrol Dial Transplant. 2014;29:1362–1368. [DOI] [PubMed] [Google Scholar]
- 18. Goldstein SL. Automated/integrated real-time clinical decision support in acute kidney injury. Curr Opin Crit Care. 2015;21:485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goitein L, James B. Standardized best practices and individual craft-based medicine: a conversation about quality. JAMA Intern Med. 2016;176:835–838. [DOI] [PubMed] [Google Scholar]
- 20. Harris AD, McGregor JC, Perencevich EN, et al. The use and interpretation of quasi-experimental studies in medical informatics. J Am Med Inform Assoc. 2006;13:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wald R. Predicting baseline creatinine in hospitalized patients. Clin J Am Soc Nephrol. 2012;7:697–699. [DOI] [PubMed] [Google Scholar]
- 22. Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siew ED, Peterson JF, Eden SK, Moons KG, Ikizler TA, Matheny ME. Use of multiple imputation method to improve estimation of missing baseline serum creatinine in acute kidney injury research. Clin J Am Soc Nephrol. 2013;8:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tso GJ, Tu SW, Oshiro C, et al. Automating guidelines for clinical decision support: knowledge engineering and implementation. AMIA Annu Symp Proc. 2016;2016:1189–1198. [PMC free article] [PubMed] [Google Scholar]
- 25. Connell A, Montgomery H, Morris S, et al. Service evaluation of the implementation of a digitally-enabled care pathway for the recognition and management of acute kidney injury. F1000Res. 2017;6:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kashani K, Ronco C. Acute kidney injury electronic alert for nephrologist: reactive versus proactive? Blood Purif. 2016;42:323–328. [DOI] [PubMed] [Google Scholar]
- 27. Oh J, Bia JR, Ubaid-Ullah M, Testani JM, Wilson FP. Provider acceptance of an automated electronic alert for acute kidney injury. Clin Kidney J. 2016;9:567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu G, Baines R, Westacott R, Selby N, Carr S. An educational approach to improve outcomes in acute kidney injury (AKI): report of a quality improvement project. BMJ Open. 2014;4:e004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilson FP, Bansal AD, Jasti SK, et al. The impact of documentation of severe acute kidney injury on mortality. Clin Nephrol. 2013;80:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sawhney S, Fraser SD. Epidemiology of AKI: utilizing large databases to determine the burden of AKI. Adv Chronic Kidney Dis. 2017;24:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siew ED, Basu RK, Wunsch H, et al. Optimizing administrative datasets to examine acute kidney injury in the era of big data: workgroup statement from the 15th ADQI Consensus Conference. Can J Kidney Health Dis. 2016;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Porter CJ, Juurlink I, Bisset LH, Bavakunji R, Mehta RL, Devonald MA. A real-time electronic alert to improve detection of acute kidney injury in a large teaching hospital. Nephrol Dial Transplant. 2014;29:1888–1893. [DOI] [PubMed] [Google Scholar]
- 33. Joslin J, Wilson H, Zubli D, et al. Recognition and management of acute kidney injury in hospitalised patients can be partially improved with the use of a care bundle. Clin Med (Lond). 2015;15:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kolhe NV, Staples D, Reilly T, et al. Impact of compliance with a care bundle on acute kidney injury outcomes: a prospective observational study. PLoS One. 2015;10:e0132279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Renhua L, Miaolin C, Junlin W, et al. The level of the biomarkers at the time of nephrology consultation might predict the prognosis of acute kidney injury in hospitalized patients. Blood Purif. 2014;38:89–95. [DOI] [PubMed] [Google Scholar]
- 36. Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siew ED, Matheny ME, Ikizler TA, et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int. 2010;77:536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levante C, Ronco C. The imperative for health economics assessment in acute kidney injury. Blood Purif. 2016;42:I–VI. [DOI] [PubMed] [Google Scholar]
- 39. Kolhe NV, Eldehni MT, Selby NM, McIntyre CW. The reimbursement and cost of acute kidney injury: a UK hospital perspective. Nephron Clin Pract. 2014;126:51–56. [DOI] [PubMed] [Google Scholar]
- 40. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APC-18-0034_FINAL_Supplemental_Figures for Impact of Daily Electronic Laboratory Alerting on Early Detection and Clinical Documentation of Acute Kidney Injury in Hospital Settings by Tarush Kothari, Kendal Jensen, Debbie Mallon, Gerard Brogan, and James Crawford in Academic Pathology