Abstract
Oral cancer is one of the most common cancers globally. Survival rates for patients are directly correlated with stage of diagnosis; despite this knowledge, 60% of individuals are presenting with late-stage disease. Currently, the initial evaluation of a questionable lesion is performed by a conventional visual examination with white light. If a lesion is deemed suspicious, a biopsy is taken for diagnosis. However, not all lesions present suspicious under visual white light examination, and there is limited specificity in differentiating between benign and malignant transformations. Several vital dyes, light-based detection systems, and cytology evaluation methods have been formulated to aid in the visualization process, but their lack of specific biomarkers resulted in high false-positive rates and thus limits their reliability as screening and guidance tools. In this review, we will analyze the current methodologies and demonstrate the need for specific intraoral imaging agents to aid in screening and diagnosis to identify patients earlier. Several novel molecular imaging agents will be presented as, by result of their molecular targeting, they aim to have high specificity for tumor pathways and can support in identifying dysplastic/cancerous lesions and guiding visualization of biopsy sites. Imaging agents that are easy to use, inexpensive, noninvasive, and specific can be utilized to increase the number of patients who are screened and monitored in a variety of different environments, with the ultimate goal of increasing early detection.
Keywords: oral squamous cell carcinoma (OSCC), specificity, molecular markers, conventional oral examination (COE), vital dyes, molecularly targeted approaches
Introduction
Oral cancer is one of the most common cancers globally with a high 5-year mortality rate of approximately 50%.1-4 In 2012, the global incidence for oral cancer was an estimated 529 500 cases with 292 300 deaths attributable to the disease.5 In the United States, the estimated number of incident cases in 2018 is 51 540 with 10 030 anticipated deaths.6 Oral cancer is also considered one of the most debilitating cancers, as treatment can lead to disfiguration and difficulties in speech, chewing, and swallowing.7 Statistically, oral cancer is more prevalent in developing countries, among populations with low socioeconomic status, and among the male population, likely due to increased exposure to known oral carcinogens such as tobacco and alcohol.2,3,8 Survival rates for patients are directly correlated with stage of diagnosis—if oral cancer is diagnosed at an early, localized stage (stages I and II), the disease can be more effectively managed with surgery, with or without radiation and chemotherapy.8 Unfortunately, 60% of patients present with late-stage (stages III and IV) invasive carcinomas, and, upon diagnosis, approximately half of patients have already developed regional or distant metastases, further contributing to decreased overall survival.9-12 Currently, the gold standard for evaluating the oral cavity is visual inspection and biopsy of suspicious lesions. There is a rationale for improving imaging aids to ultimately improve screening for oral cancer with the hopes of both monitoring precancerous lesions and treating new malignancies as early as possible. Ultimately, improved early detection will likely decrease the number of patients presenting with late-stage tumors and help to increase survival rates.9,13
The most common type of oral cancer is oral squamous cell carcinoma (OSCC), accounting for 90% of all oral cancer diagnoses.3 When patients present with late-stage disease, OSCC is easily recognizable due to characteristics such as the presence of nonhealing infiltrating ulcers or large exophytic lesions, bleeding, ear pain, teeth mobility, and difficulty breathing and speaking.1 Early-stage oral cancer, on the other hand, is often difficult to recognize, as it remains asymptomatic and clinically appears very similar to benign lesions adding hurdles to make early, timely diagnoses.1,7,14,15 Many patients, prior to developing malignancy, will develop precancerous lesions that are mainly leukoplakia (white patches) or erythroplakia (red patches).1,7-9 A systematic review reported that malignant transformation rates for leukoplakia ranged from 0.13% to 34.0% with a mean of 3.5%, this is contrasted with erythroplakia, which is much less common and has a significantly higher likelihood of transformation, varying from 14% to 50%.16-19 Additionally, other inflammatory disorders of the oral mucosa, such as lichen planus, could lead to an increased risk of OSCC.7 The current gold standard is a conventional oral examination (COE) using the naked eye and white light to visualize the oral cavity. Due to the subjective nature of this method, a successful examination needs to be executed by an individual with extensive training—inherently limiting the group of people who can perform them and often the environments that they can be performed in. Furthermore, despite training, the naked eye may not be able to visualize all regions of concern and often will detect both benign and malignant lesions.11 Several imaging aids have been developed, but due to lack of specificity and difficulty in usage, there is very little improvement over traditional examination. The purpose of this review is to analyze several of the current screening tools and diagnostic aids and demonstrate the need for an imaging agent that can support earlier identification of lesions. An ideal imaging agent is highly specific, painless, inexpensive, and simple to use and can be implemented in a diversity of environments. It should be able to identify, screen, and monitor high-risk patients and suspicious lesions, support in determining the necessity for biopsy and delineate margins–molecular imaging agents can fill this gap.
Current Screening Methods
Oral cancer screening has the goal of monitoring premalignant lesions and increasing the detection rate of early-stage oral cancer. Stage I and II tumors can be effectively treated and have dramatically higher survival rates compared to late-stage oral cancers, yet many dysplastic regions and early-stage malignancies are difficult to detect.1,2,7,14,15,20 The following screening methods are the most commonly discussed current screening methods (Figure 1), but others have been introduced as well.21
Figure 1.
Overview of imaging methods discussed in the article.
Conventional Oral Examination
The standard method for oral cancer screening is a COE. In a COE, a white light is used to visually inspect the oral cavity. The clinical efficacy of this method is controversial due to the reliance on the naked eye to identify initial malignant lesions that are often difficult to visualize and even harder to differentiate from benign or inflammatory lesions (Figure 2).11 Therefore, the COE is used to guide the necessity and region of biopsy, and a conclusive diagnosis is based on the histopathological evaluation.11 A meta-analysis from a systematic review found the sensitivity and specificity of COE to be 88.7% and 60.9%, respectively.22 While COE can be beneficial, this approach still has significant shortfalls. Some of the reasons for its ineffectiveness are that (1) benign oral lesions are very frequent and, even for a trained professional, visual diagnosis of superficial lesions can be difficult, leading to variable and low specificity.22 Additionally, benign oral mucosal abnormalities can be found in 5% to 15% of the population, and those could be misdiagnosed as premalignant lesions.11,23 (2) The naked eye often cannot identify dysplastic and early cancerous lesions. A study conducted by Thomson in 2002 included 26 patients who were diagnosed with OSCC or premalignant lesions. “Mirror image” biopsies were taken in anatomically comparable sites within the oral cavity from what visually appeared to be normal mucosa. Of the 26 biopsies taken, 6 patients presented with atypical cells likely due to irritation, 7 had dysplasia, and 2 had carcinoma in situ. Although the study included a small number of patients, 58% of normal appearing mucosa actually contained abnormal tissue, demonstrating that abnormal lesions may be missed by COE.13 (3) A COE’s success is dependent on the provider’s level of training and experience.
Figure 2.
Morphological appearance of different pathologies on the tongue. Malignant (a) and benign (b) oral lesions can be difficult to distinguish with white light alone.
Therefore, it has been suggested that complementation of classic COE with visual aids and specific imaging markers could improve oral cancer screening practices and early diagnosis.
Toluidine Blue
Toluidine blue is a dye that has an affinity for acidic tissue components such as nucleic acids, which are more abundant in dysplastic or malignant areas due to increased cellularity.24-28 It is used to help delineate tumor margins and aid in visual inspection for OSCC (Figure 3a).31 A meta-analysis was performed on 14 studies, and determined sensitivity and specificity for toluidine blue staining ranged from 40% to 100% and from 31% to 100%, respectively.11,32 A study that compared toluidine blue staining to visual inspection found that toluidine blue had a much higher sensitivity than a COE alone but that the specificity for both were similar—around 80%.27 Toluidine blue staining may be beneficial to high-risk patient populations, but the lack of improved specificity over COE and provider subjectivity challenges its broad clinical adoption.15, 32
Figure 3.
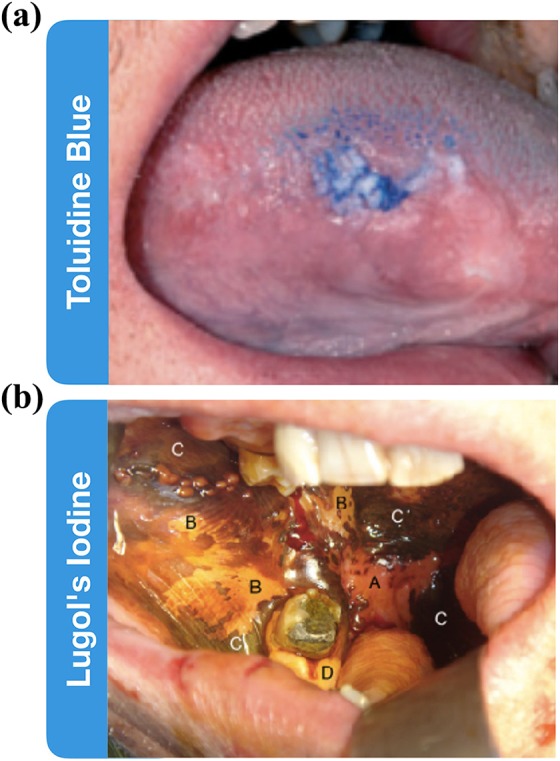
Vital dyes as diagnostic adjuncts. (a) Toluidine blue staining the oral cavity to identify leukoplakia lesion. (b) Lugol’s iodine staining oral mucosa, labels correspond to the following, A: invasive squamous cell carcinoma, B: dysplastic tissue, C: normal mucosa, D: normal orthokeratinized mucosa. Absence of staining in A indicates the malignant nature of the tissue. Adapted from McMahon et al29 and Awan et al.30
Lugol’s Iodine
Lugol's iodine, also known as Lugol's solution, is composed of elemental iodine and potassium iodide. When Lugol's iodine is applied onto normal tissue, it reacts with intracellular glycogen, resulting in a brown color change. Normal tissue, particularly superficial epithelium, contains greater amounts of glycogen than malignant tissue, which will stain to a more limited degree (Figure 3b).29,33-35 The diagnostic value of Lugol’s iodine has been described as limited due to a high degree of nonspecific staining.29 Along with tumor, Lugol’s iodine may lack staining in any regions of atrophy, areas of inflammation, and many benign, potentially never transforming histologies, such as linea alba, leukoedema, or leukoplakia—all of which are common in high-risk populations that would be the beneficiaries of screening.33,35 It has been reported that Lugol's iodine could be more useful as a tool for defining surgical margins and outlining areas for subsequent biopsy.29,33,35 Yet, as a screening and diagnostic tool, it has a high level of false positives, and expert professionals will likely be able to better discern the lesions by COE alone.35
Chemiluminescence
The application of acetic acid and chemiluminescent light is a technique that is meant to aid in screening the oral cavity by increasing OSCCs’ visual brightness and sharpness (Figure 4a).36,39 The ViziLite system (Zila Pharmaceuticals, Phoenix, Arizona) involves washing with 1% acetic acid solution for 1 minute and then subsequent examination under chemiluminescent light (wavelengths of 490-510 nm).7 The acetic acid wash dehydrates the tissue and increases the reflectance of areas with more dense nuclei. Under chemiluminescent light, malignant regions will appear “acetowhite” whereas normal epithelium will look blue.39 A study conducted by Epstein et al compared chemiluminescence to COE and demonstrated that chemiluminescence found the same amount of lesions as a COE. Yet, around half of the lesions were easier to visualize (increased brightness and/or sharpness) compared to COE.36 This finding was confirmed by several groups who concluded that the ViziLite is not useful as a diagnostic tool but can help increase visibility of lesions (reviewed in study by Farah et al21).
Figure 4.
Light-based detection systems as diagnostic adjuncts. (a) Mild dysplasia on the ventral tongue, left: white light, right: chemiluminescence. (b) Severe dysplasia on the ventral tongue, left: white light, right: wide-field autofluorescence imaging with VELscope with arrow pointing to the dysplasia. (c) Left: oral squamous cell carcinoma on the hard palate, right: reflectance confocal microscopy demonstrating disorganized cells. (a) and (b) are macroscopic techniques; (c) is a microscopic technique. Adapted from Epstein et al36, Shin et al,37 and Contaldo et al.38
Wide-Field Autofluorescence Imaging
Tissue autofluorescence is due to endogenous fluorescent material naturally occurring within cellular structures (such as collagen and keratin), metabolites (such as nicotinamide adenine dinucleotide hydride [NADH]), and flavin adenine dinucleotide (FAD).40 In lesions, changes in structure, such as hyperkeratosis, hyperchromatin, or increased concentration of FAD and NADH, can modify the autofluorescence emitted by the tissue. Malignant regions have a loss in autofluorescence when compared to normal tissue.40 Wide-field fluorescence imaging devices, such as the VELscope (LED Dental, Inc, White Rock, British Columbia, Canada), can be used to visualize this loss of autofluorescence within the oral cavity (Figure 4b).37 There are large ranges in the reported sensitivity and specificity for the VELscope, exemplified by 5 reports where this device was used to identify dysplasia or carcinoma in situ; the sensitivity and specificity ranged from 30% to 98% and 15% to 100%, respectively.41-45 Consistent readouts, however, are highly dependent on a high level of training and expertise.37,46 Additionally, it has been suggested that the VELscope may be more effectively used as a tool to support a COE by increasing sensitivity or helping to define surgical margins rather than as a diagnostic tool due to the high prevalence of benign lesions in the oral cavity that may also show a loss of fluorescence.44,47
Reflectance Confocal Microscopy
Reflectance confocal microscopy (RCM) provides for high-resolution in vivo imaging at the cellular level using focused laser illumination and detecting the back-scattered light.48 It has been described as a tool to “optically section” tissue, so the morphology of the tissue can be noninvasively visualized in thin planes. The Vivascope microscopes are used to perform RCM and can visualize 3- to 5-μm thin optical sections at a 0.5 to 1 μm resolution and up to depths of 200 to 300 μm (Caliber I.D., Rochester, New York; Figure 4c). Reflectance confocal microscopy aids in visualization of OSCC based on nuclear density and distinct, disorganized morphology of malignant cells and tissue when compared to normal oral mucosa.49-53 Currently, RCM usage has increased greatly, particularly in the dermatology field, and, since 2016 it has been approved on a per-lesion basis by Centers for Medicare and Medicaid services. Its suggested use is for diagnosis of basal cell carcinoma when suspicion is particularly high, instead of a biopsy.54 Additionally, confocal microscopy could be used in conjunction with a molecularly targeted imaging agent (eg, 5-aminolevulinic acid [5-ALA]) in order to improve contrast, simplify identification of OSCC for diagnosis, and aid in defining surgical margins.55
Brush Cytology
Brush cytology is a technique to obtain cells from the oral cavity and is a relatively painless procedure that can test multiple areas of concern in patients with a low risk of OSCC.11,23,56,57 It can be performed by rinsing the mouth, taking a sample of saliva, or scraping the surface of the mucosa, such as with a toothbrush or a cervical cytobrush.57-59 This is followed by cytopathological evaluation. The prime commercial example is the OralCDx (OralCDx Laboratories, Inc, Suffern, New York), which is a brush biopsy device with computer-assisted analysis.23,60 About 10% of all brush biopsied cases are abnormal, and while this technique may aid in identifying malignancies, the gold standard for diagnosis remains the scalpel biopsy.23 Thus, the main benefit might be in avoiding scalpel biopsies if the cytopathological examination of a region comes back as negative for malignant cells, depending on future studies of sensitivity and specificity, and especially the negative predictive value. Several studies of oral cytology brushes including the cytobrush, baby toothbrush, OralCDx, and the Orcellex have reported promising sensitivity and specificity, but standardized methodologies are needed in the future.59 However, despite the relatively low cost of the cytobrushes themselves, this technique remains resource intense due to the need of cytopathological evaluation.
Molecularly Targeted Approaches
Many of the current screening methods available lack specificity and have inconsistent evidence of their utility in clinical practice. While they may aid in diagnosis or emphasize areas of concern, their many pitfalls result in white light COE and scalpel biopsy remaining the gold standard.11 There is a clinical need for a tool that can quickly, painlessly, and accurately screen, diagnose, and delineate OSCC, and a molecularly targeted optical imaging could fill this gap. Targeted imaging agents specifically bind to certain biomarkers that are upregulated in tumors compared to surrounding normal tissue. For oral cancer detection, we consider an ideal targeted imaging agent to be fluorescent, targeted against an abundant biomarker, inexpensive, and simple to use. With this imaging tool, we expect improved early detection of tumors due to ease of visualization and increased accessibility to screening in various regions and settings.
Lectins Targeting Sialic Acid
Glycans are a promising cellular target, as it has been demonstrated that glycosylation is significantly altered in cancer cells when compared to healthy cells.21,61,62 A tool for imaging these changes is through lectins. Lectins react with the terminal, nonreducing sugars of glycoproteins and glycolipids and, therefore, have been explored for molecularly targeted imaging of malignant cells.63 Lectins are inexpensive, stable at high temperatures and low pHs, and have low toxicity.64
Sialylation is a modification of glycosylation, where sialic acid is added to the terminal ends of glycoproteins and glycolipids. These have been shown to increase in patients with oral cancer.65-67 A particular lectin that has been explored is wheat germ agglutinin (WGA), a plant lectin that binds to sialic acid.62 Despite the relatively large size of WGA (38 kDa), which might limit tissue penetration, a topical application approach has been shown by Baeten et al, where a fluorophore was conjugated to WGA and its usage tested on ex vivo patient biopsies.68 For the 7 biopsy samples, they utilized Alexa Fluor 350 conjugated WGA (AF350-WGA) and found that the tumor region was significantly brighter than normal tissue with an average signal to noise ratio of 5.88 ± 3.46 (P = .00046). Specificity was tested using an inhibitory sugar that blocked the binding sites of WGA. When preincubated with the sugar, the fluorescence decreased 3-fold indicating that AF350-WGA has a high specificity for sialic acid.68
Recently, this same group utilized a fluorescently labeled WGA with Fluorescein Isothiocyanate (FITC) (WGA-FITC) in an in vivo clinical trial (Figure 5a).71 WGA-FITC was applied topically to 64 suspicious lesions. The authors found that WGA-FITC uptake could be either increased or decreased in malignant/dysplastic areas, and they evaluated the level of differentiation between normal and malignant tissue including both positive and negative contrast. The sensitivity and specificity were found to be 89% and 82%, respectively.71 The WGA-FITC signal was also detected in ulcerated inflammatory lesions such as herpes ulcers, angular cheilitis, inflamed papilla, and a squamous papilloma lesion, causing false positives. Additionally, the sensitivity for cancerous lesions was 100% and for dysplastic lesions 81%.71
Figure 5.
Fluorescence-based diagnostic adjuncts. (a) Wheat germ agglutinin-FITC staining in the oral cavity of a patient with OSCC, left: white light, right: WGA-FITC, A: squamous cell carcinoma with a high level of differentiation between normal tissue and malignancy by increased WGA-FITC uptake, C: moderate dysplasia can display an increased or decreased WGA-FITC uptake. The example shows good differentiation from normal tissue by reduction of WGA-FITC uptake. (b) Widefield fluorescence images of 3 oral specimens obtained pre and post labeling with 2-NBDG. Neoplastic samples showed lower fluorescence pre-labeling than normal samples and a dramatic increase of fluorescence after labeling. (c) Protoporphyrin IX-based OSCC imaging in combination with autofluorescence. A: moderately differentiated squamous cell carcinoma under white light, B: wide-field autofluorescence demonstrating loss of fluorescence in the malignant area, C: 5-ALA induced PpIX fluorescence with strong red fluorescence signal from the tumor area, D: overlayed autofluorescence and PpIX images resulting in a “street light” green/red contrast of tumor to the surrounding tissue. (d) Schematic of the imaging procedure for oral cancer detection using a PARPi-FL based, orally applied solution in an ongoing phase I/II clinical study (NCT03085147) (left). Proof of principle of feasibility of tumor detection after topical application of PARPi-FL (right). Adapted from Baeten et al,68 Betz et al,69 Nitin et al,70 and Kossatz et al.105-ALA, 5-aminolevulinic acid; 2-NBDG, 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose; OSCC, oral squamous cell carcinoma; PpIX, Protoporphyrin IX; WGA, wheat germ agglutinin.
Glucose Metabolism
Glucose metabolism is a hallmark of cancer that has been studied for decades.72 Cancer cells have a tendency to increase glucose metabolism and lactate formation through anaerobic glycolysis—a process known as the Warburg effect.73 The radiolabeled glucose analogue 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) images this pathway and has become the standard radiotracer for tumor imaging via positron emission tomography (PET).74 18F-FDG is clinically useful for evaluating oral cancer in terms of assessing tumor aggressiveness, understanding tumor metabolism before and after chemotherapy, and for staging lymph node and distant metastases.75,76
However, in the specific case of early oral cancer detection, the utility of FDG-PET is limited by the spatial resolution of 0.3 cm3, complicating the search for small, early-stage tumors.70 Additionally, due to dependence on a cyclotron and the exposure to radioactivity, this approach is not feasible as a screening tool.70 Yet, the concept of imaging glucose metabolism is promising for visualizing early malignancy.21 Therefore, a fluorescent analogue of 18F-FDG, 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG), was synthesized and has been tested in oral cancer.70,77 Nitin et al demonstrated when 2-NBDG was topically applied to oral biopsy specimens, based on analysis of wide field imaging, there was a 15- to 40-fold increase in signal in tumor when compared to normal tissue (Figure 5b). Additionally, specificity was demonstrated in vitro.70 Further research needs to be conducted to determine sensitivity and specificity in vivo in order to evaluate 2-NBDGs utility as a technique for screening and diagnosing OSCC tumors.
Imaging Using Porphyrins
5-aminolevulinic acid is a precursor in the heme biosynthesis pathway which produces the fluorescent and photosensitizing Protoporphyrin IX (PpIX) as a metabolite. The administration of excess exogenous 5-ALA can increase the production of PpIX and its accumulation in highly proliferating tumor cells.78 5-ALA/PpIX has been used for both photodynamic detection (PDD) and therapy (PDT) of tumors.79-82 Photodynamic therapy is based on the ability of photosensitizers to generate cytotoxic oxygen species in the target tissue upon exposure to light of an appropriate wavelength. Interestingly, 5-ALA can be administered intravenously, intradermally, orally, or topically. The clinical use of 5-ALA PDT is excellently reviewed in studies by Baeten et al71 and Peng et al.83 Fluorescence-based imaging approaches based on 5-ALA/PpIX have been translated to the clinic in skin, brain, and bladder imaging and have also been investigated for oral cancer imaging.
A study was conducted to compare COE, wide-field autofluorescence imaging, and 5-ALA fluorescence in patients with oral cancer (n = 85). It was shown that tumor tissue demonstrated a strongly increased PpIX fluorescence after topical 5-ALA application. Tumor identification and demarcation was further improved by combining PpIX imaging with autofluorescence detection, which was reduced in tumor areas, increasing the tumor to nontumor contrast. This combined imaging method showed improved performance over traditional COE and could be especially useful in the detection of precancerous lesions, tumor borders, and areas of field cancerization (Figure 5c).69 Another study on 54 patients used incubation of 5-ALA for 1 to 2.5 hours and found red PpIX fluorescence in neoplastic tissue of all patients. The maximum contrast was detected after 1.5 hours, with tumor signals up to 12.5 times brighter than surrounding healthy tissue. Biopsies were taken from fluorescence-negative areas close to the tumor and bright fluorescent regions, and sensitivity and specificity of the 5-ALA-induced PpIX were determined to be 99% and 60%, respectively.84
Photofrin (Porfimer sodium, Quadra Logic Technologies, Vancouver, Canada) is another tool that has been initially explored as PDT agent. Photofrin injection for PDT has been approved for usage in Barrett esophagus by the Food and Drug Administration, but very few studies have been conducted regarding its usage as a diagnostic imaging agent for OSCC.85 Chang et al conducted a study using a topically applied Photofrin on suspicious oral malignancies.86 Fluorescence of Photofrin was measured macroscopically and microscopically 3 hours after application. Bright fluorescence was detected in the tumor region, which was confirmed by biopsy and histopathological analysis, and the authors reported high sensitivity (92.45%-93.75%) and specificity (95.65%-97.50% for their data set of 80 biopsies from 20 patients).86 More research needs to be conducted to determine the specificity of Photofrin-based detection for oral cancer.
Poly (ADP-ribose) Polymerase Imaging
Another biomarker that has been described for OSCC imaging is the DNA repair enzyme poly (ADP-ribose) polymerase 1 (PARP1).10,87,88 Initially, PARP1 has been described exclusively as a therapeutic target, since the inhibition of its enzymatic function can sensitize cells to radiotherapy or even lead to cell death, which has led to the development and clinical translation of several small molecule PARP inhibitors.89-91 However, the fact that PARP1 expression is upregulated in many different types of cancer92-99 makes PARP1 a good biomarker for imaging applications.10,100
A study conducted by Kossatz et al determined that the PARP1 protein is strongly expressed in OSCC biospecimens (n = 12).10 Poly (ADP-ribose) polymerase 1 expression provided for clear differentiation between tumor and normal areas, with a specificity of 97.2% and sensitivity of 97.4%. One study determined that PARP1 gene expression was increased in both leukoplakia and malignant OSCC relative to normal tissue.12,101 Interestingly, nonprogressive leukoplakia samples showed significantly lower PARP1 gene expression levels than progressive leukoplakia, which is at risk of transformation into OSCC.12 By identifying progressive leukoplakia, these could be treated prior to becoming malignant, while many other nonprogressive lesions could be observed.102
Consequently, several fluorescent PARP-targeted imaging agents have been designed (reviewed in Carney et al103). All agents are based on the clinically used PARP inhibitor olaparib but have used different dyes for fluorescent labeling, including BODIPY-FL (PARPi-FL104), Texas Red (AZD2281—Texas Red105), BODIPY650 (olaparib-BODIPY650106), photocaged BODIPY (PARPi-BODIPYc107), and silicon containing rhodamines (SiR,108). In vivo data showing successful, quick cell penetration and accumulation in PARP1-expressing nuclei are only available for PARPi-FL, which is labeled with the small, hydrophobic BODIPY-FL dye.109 PARPi-FL has been evaluated for oral cancer detection preclinically, and feasibility of specific, high contrast imaging of subcutaneous and orthotopic xenografts after intravenous injection has been reported.10,110,111 From a clinical and translational perspective, topical application of PARPi-FL would be favorable to intravenous injection; as a microdose can be administered topically, systemic toxicity is negligible, and due to minimal risks for patients, this administration route would potentially have quicker integration into the clinical routine. Additionally, OSCC originates from the mucosa and presents superficially,8 and diagnoses can be made at the epithelium.10 The feasibility of topical application for PARPi-FL has been shown,10 and subsequently PARPi-FL has entered clinical evaluation for the detection of oral cancer in a phase I/II clinical trial that is being conducted at Memorial Sloan Kettering Cancer Center (ClinicalTrials.gov identifier NCT03085147; Figure 5d).
Conclusion
In conclusion, many of the current tools, such as vital dyes, light-based detection systems, and brush cytology, have been shown to lack specificity and the general ability to discover previously unidentifiable lesions. Thus, they have not been adopted into general clinical use.11 Additionally, benign and inflammatory lesions are often almost indistinguishable from tumor, while some early malignant lesions will remain unnoticed when examined visually.3,11,23 Molecular-targeted fluorescence imaging could aid in improving early detection rates for OSCC due to a potentially high specificity and selectivity, noninvasive application, ease of use in many environments, and easy to read results. In the future, sensitivity and specificity might be increased further by novel approaches to combine a specific fluorescence signal with autofluorescence imaging or multichannel imaging. We anticipate usage of these agents in primary medical and dental care settings as well as resource-poor regions due to low cost, quick application and reading, and relative comfort for the patient. They can be used to screen and monitor high-risk patients through scanning the whole oral cavity in order to determine whether a biopsy is necessary and in which area. In the future, if high specificity is consistently shown, they could be used as diagnostic agents.112
Acknowledgment
The authors thank the Tow Foundation and Memorial Sloan Kettering Cancer Center’s Center for Molecular Imaging & Nanotechnology and the Imaging and Radiation Sciences Program for financial support (S.K.).
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. T.R. is a cofounder of Summit Biomedical Imaging, LLC. S.K., S.P., and T.R. are shareholders of Summit Biomedical Imaging, LLC. Dr. Milind Rajadhyaksha is a former employee of and owns equity in Caliber I.D. (formerly, Lucid Inc.), the company that manufactures and sells a reflectance confocal microscope (VivaScope). The VivaScope, for reflectance confocal microscopy (RCM) imaging, is the commercial version of an original laboratory prototype that was developed by Dr. Rajadhyaksha when he was at Massachusetts General Hospital, Harvard Medical School.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health grants 1 R01 CA204441, P30 CA008748, and 1 K99 CA218875 (S.K.). Received financial support from Imaging and Radiation Sciences Program (S.K.).
ORCID iD: Susanne Kossatz  https://orcid.org/0000-0002-1908-1782
https://orcid.org/0000-0002-1908-1782
Thomas Reiner, PhD  https://orcid.org/0000-0002-7819-5480
https://orcid.org/0000-0002-7819-5480
References
- 1. Bagan J, Sarrion G, Jimenez Y. Oral cancer: clinical features. Oral Oncol. 2010;46(6):414–417. [DOI] [PubMed] [Google Scholar]
- 2. Le Campion A, Ribeiro CMB, Luiz RR, et al. Low survival rates of oral and oropharyngeal squamous cell carcinoma. Int J Dent. 2017;2017:5815493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4-5):309–316. [DOI] [PubMed] [Google Scholar]
- 4. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. [DOI] [PubMed] [Google Scholar]
- 5. Shield KD, Ferlay J, Jemal A, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67(1):51–64. [DOI] [PubMed] [Google Scholar]
- 6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 7. Fedele S. Diagnostic aids in the screening of oral cancer. Head Neck Oncol. 2009;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52(4):195–215. [DOI] [PubMed] [Google Scholar]
- 9. Mishra R. Biomarkers of oral premalignant epithelial lesions for clinical application. Oral Oncol. 2012;48(7):578–584. [DOI] [PubMed] [Google Scholar]
- 10. Kossatz S, Brand C, Gutiontov S, et al. Detection and delineation of oral cancer with a PARP1 targeted optical imaging agent. Sci Rep. 2016;6: 21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44(1):10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cervigne NK, Machado J, Goswami RS, et al. Recurrent genomic alterations in sequential progressive leukoplakia and oral cancer: drivers of oral tumorigenesis? Hum Mol Genet. 2014;23(10):2618–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomson PJ. Field change and oral cancer: new evidence for widespread carcinogenesis? Int J Oral Maxillofac Surg. 2002;31(3):262–266. [DOI] [PubMed] [Google Scholar]
- 14. Silverman S., Jr Early diagnosis of oral cancer. Cancer. 1988;62(8 suppl):1796–1799. [DOI] [PubMed] [Google Scholar]
- 15. Patton LL. The effectiveness of community-based visual screening and utility of adjunctive diagnostic aids in the early detection of oral cancer. Oral Oncol. 2003;39(7):708–723. [DOI] [PubMed] [Google Scholar]
- 16. van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009;45(4-5):317–323. [DOI] [PubMed] [Google Scholar]
- 17. Villa A, Villa C, Abati S. Oral cancer and oral erythroplakia: an update and implication for clinicians. Aust Dent J. 2011;56(3):253–256. [DOI] [PubMed] [Google Scholar]
- 18. Reichart PA, Philipsen HP. Oral erythroplakia—a review. Oral Oncol. 2005;41(6):551–561. [DOI] [PubMed] [Google Scholar]
- 19. Warnakulasuriya S, Ariyawardana A. Malignant transformation of oral leukoplakia: a systematic review of observational studies. J Oral Pathol Med. 2016;45(3):155–166. [DOI] [PubMed] [Google Scholar]
- 20. Speight PM, Epstein J, Kujan O, et al. Screening for oral cancer—a perspective from the global oral cancer forum. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(6):680–687. [DOI] [PubMed] [Google Scholar]
- 21. Farah C, Bhatia N, Lalla Y, et al. Advances in early detection and diagnostic adjuncts in oral cavity cancer In: Kuriakose MA, ed. Contemporary Oral Oncology Biology, Epidemiology, Etiology, and Prevention. Switzerland: Springer International Publishing; 2017:355–421. [Google Scholar]
- 22. Fuller C, Camilon R, Nguyen S, Jennings J, Day T, Gillespie MB. Adjunctive diagnostic techniques for oral lesions of unknown malignant potential: systematic review with meta-analysis. Head Neck. 2015;37(5):755–762. [DOI] [PubMed] [Google Scholar]
- 23. Mehrotra R, Gupta DK. Exciting new advances in oral cancer diagnosis: avenues to early detection. Head Neck Oncol. 2011;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sridharan G, Shankar AA. Toluidine blue: a review of its chemistry and clinical utility. J Oral Maxillofac Pathol. 2012;16(2):251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guneri P, Epstein JB, Ergun S, Boyacioglu H. Toluidine blue color perception in identification of oral mucosal lesions. Clin Oral Investig. 2011;15(3):337–345. [DOI] [PubMed] [Google Scholar]
- 26. Rosenberg D, Cretin S. Use of meta-analysis to evaluate tolonium chloride in oral cancer screening. Oral Surg Oral Med Oral Pathol. 1989;67(5):621–627. [DOI] [PubMed] [Google Scholar]
- 27. Allegra E, Lombardo N, Puzzo L, Garozzo A. The usefulness of toluidine staining as a diagnostic tool for precancerous and cancerous oropharyngeal and oral cavity lesions. Acta Otorhinolaryngo. 2009;29(4):187–190. [PMC free article] [PubMed] [Google Scholar]
- 28. Martin IC, Kerawala CJ, Reed M. The application of toluidine blue as a diagnostic adjunct in the detection of epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(4):444–446. [DOI] [PubMed] [Google Scholar]
- 29. McMahon J, Devine JC, McCaul JA, McLellan DR, Farrow A. Use of Lugol’s iodine in the resection of oral and oropharyngeal squamous cell carcinoma. Brit J Oral Max Surg. 2010;48(2):84–87. [DOI] [PubMed] [Google Scholar]
- 30. Awan KH, Yang YH, Morgan PR, Warnakulasuriya S. Utility of toluidine blue as a diagnostic adjunct in the detection of potentially malignant disorders of the oral cavity—a clinical and histological assessment. Oral Diseases. 2012;18(8):728–733. [DOI] [PubMed] [Google Scholar]
- 31. Seoane Leston J, Diz Dios P. Diagnostic clinical aids in oral cancer. Oral Oncol. 2010;46(6):418–422. [DOI] [PubMed] [Google Scholar]
- 32. Gray M, Gold L, Burls A, Elley K. The effectiveness of toluidine blue dye as adjunct to oral cancer screening in general dental practice West Midlands Development and Evaluation Service Report. 2000, http://www.exodontia.info/files/Department_of_Public_Health_and_Epidemiology_University_of_Birmingham_2000._The_clinical_effectiveness_of_toluidine_blue_dye_as_an_adjunct_to_oral_cancer_screening_in.pdf. [Google Scholar]
- 33. Petruzzi M, Lucchese A, Baldoni E, Grassi FR, Serpico R. Use of Lugol’s iodine in oral cancer diagnosis: an overview. Oral Oncol. 2010;46(11):811–813. [DOI] [PubMed] [Google Scholar]
- 34. Kurita H, Kurashina K. Vital staining with iodine solution in delineating the border of oral dysplastic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(3):275–280. [DOI] [PubMed] [Google Scholar]
- 35. Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83(2):220–231. [PubMed] [Google Scholar]
- 36. Epstein JB, Silverman S, Jr, Epstein JD, Lonky SA, Bride MA. Analysis of oral lesion biopsies identified and evaluated by visual examination, chemiluminescence and toluidine blue. Oral Oncol. 2008;44(6):538–544. [DOI] [PubMed] [Google Scholar]
- 37. Shin D, Vigneswaran N, Gillenwater A, Richards-Kortum R. Advances in fluorescence imaging techniques to detect oral cancer and its precursors. Future Oncol. 2010;6(7):1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Contaldo M AM, Ardigò M. In Vivo Reflectance Confocal Microscopy for Oral Mucosa Assessment. Non Invasive Diagnostic Techniques in Clinical Dermatology. Berlin, Heidelberg: Springer; 2014;81–87. [Google Scholar]
- 39. Huber MA, Bsoul SA, Terezhalmy GT. Acetic acid wash and chemiluminescent illumination as an adjunct to conventional oral soft tissue examination for the detection of dysplasia: a pilot study. Quintessence Int. 2004;35(5):378–384. [PubMed] [Google Scholar]
- 40. De Veld DC, Witjes MJ, Sterenborg HJ, Roodenburg JL. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol. 2005;41(2):117–131. [DOI] [PubMed] [Google Scholar]
- 41. Lane PM, Gilhuly T, Whitehead P, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt. 2006;11(2):024006. [DOI] [PubMed] [Google Scholar]
- 42. Awan KH, Morgan PR, Warnakulasuriya S. Evaluation of an autofluorescence based imaging system (VELscope) in the detection of oral potentially malignant disorders and benign keratoses. Oral Oncol. 2011;47(4):274–277. [DOI] [PubMed] [Google Scholar]
- 43. Farah CS, McIntosh L, Georgiou A, McCullough MJ. Efficacy of tissue autofluorescence imaging (VELScope) in the visualization of oral mucosal lesions. Head Neck. 2012;34(6):856–862. [DOI] [PubMed] [Google Scholar]
- 44. Amirchaghmaghi M, Mohtasham N, Delavarian Z, Shakeri MT, Hatami M, Mozafari PM. The diagnostic value of the native fluorescence visualization device for early detection of premalignant/malignant lesions of the oral cavity. Photodiagnosis Photodyn Ther. 2017;21:19–27. [DOI] [PubMed] [Google Scholar]
- 45. Ganga RS, Gundre D, Bansal S, Shirsat PM, Prasad P, Desai RS. Evaluation of the diagnostic efficacy and spectrum of autofluorescence of benign, dysplastic and malignant lesions of the oral cavity using VELscope. Oral Oncol. 2017;75:67–74. [DOI] [PubMed] [Google Scholar]
- 46. Cicciu M, Herford AS, Cervino G, Troiano G, Lauritano F, Laino L. Tissue fluorescence imaging (VELscope) for quick non-invasive diagnosis in oral pathology. J Craniofac Surg. 2017;28(2):e112–e115. [DOI] [PubMed] [Google Scholar]
- 47. Patton LL, Epstein JB, Kerr AR. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc. 2008;139(7):896–905; quiz 93-4. [DOI] [PubMed] [Google Scholar]
- 48. Rajadhyaksha M, Anderson RR, Webb RH. Video-rate confocal scanning laser microscope for imaging human tissues in vivo. Appl Opt. 1999;38(10):2105–2115. [DOI] [PubMed] [Google Scholar]
- 49. Maitland KC, Gillenwater AM, Williams MD, et al. In vivo imaging of oral neoplasia using a miniaturized fiber optic confocal reflectance microscope. Oral Oncol. 2008;44(11):1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Contaldo M, Agozzino M, Moscarella E, Esposito S, Serpico R, Ardigo M. In vivo characterization of healthy oral mucosa by reflectance confocal microscopy: a translational research for optical biopsy. Ultrastruct Pathol. 2013;37(2):151–158. [DOI] [PubMed] [Google Scholar]
- 51. Alessi SS, Nico MM, Fernandes JD, Lourenco SV. Reflectance confocal microscopy as a new tool in the in vivo evaluation of desquamative gingivitis: patterns in mucous membrane pemphigoid, pemphigus vulgaris and oral lichen planus. Br J Dermatol. 2013;168(2):257–264. [DOI] [PubMed] [Google Scholar]
- 52. Ardigo M, Donadio C, Franceschini C, Catricala C, Agozzino M. Interest of reflectance confocal microscopy for inflammatory oral mucosal diseases. J Eur Acad Dermatol Venereol. 2015;29(9):1850–1853. [DOI] [PubMed] [Google Scholar]
- 53. Malik BH, Jabbour JM, Cheng S, et al. A novel multimodal optical imaging system for early detection of oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(3):290–300e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dinnes J, Deeks JJ, Chuchu N, et al. Reflectance confocal microscopy for the diagnosis of keratinocyte skin cancers in adults. Cochrane Database of Systematic Reviews. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maher NG, Collgros H, Uribe P, Ch’ng S, Rajadhyaksha M, Guitera P. In vivo confocal microscopy for the oral cavity: current state of the field and future potential. Oral Oncol. 2016;54:28–35. [DOI] [PubMed] [Google Scholar]
- 56. Eisen D, Frist S. The relevance of the high positive predictive value of the oral brush biopsy. Oral Oncol. 2005;41(7):753–755; author reply 6. [DOI] [PubMed] [Google Scholar]
- 57. Babshet M, Nandimath K, Pervatikar S, Naikmasur V. Efficacy of oral brush cytology in the evaluation of the oral premalignant and malignant lesions. J Cytol. 2011;28(4):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kujan O, Desai M, Sargent A, Bailey A, Turner A, Sloan P. Potential applications of oral brush cytology with liquid-based technology: results from a cohort of normal oral mucosa. Oral Oncol. 2006;42(8):810–818. [DOI] [PubMed] [Google Scholar]
- 59. H Alsarraf A, Kujan O, Farah CS. The utility of oral brush cytology in the early detection of oral cancer and oral potentially malignant disorders: a systematic review. J Oral Pathol Med. 2018;47(2):104–116. [DOI] [PubMed] [Google Scholar]
- 60. Mehrotra R, Singh MK, Pandya S, Singh M. The use of an oral brush biopsy without computer-assisted analysis in the evaluation of oral lesions: a study of 94 patients. Oral Surg Oral Med O. 2008;106(2):246–253. [DOI] [PubMed] [Google Scholar]
- 61. Wu HC, Meezan E, Black PH, Robbins PW. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry. 1969;8(6):2509–2517. [DOI] [PubMed] [Google Scholar]
- 62. Dube DH, Bertozzi CR. Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4(6):477–488. [DOI] [PubMed] [Google Scholar]
- 63. Mody R, Joshi S, Chaney W. Use of lectins as diagnostic and therapeutic tools for cancer. J Pharmacol Toxicol. 1995;33(1):1–10. [DOI] [PubMed] [Google Scholar]
- 64. Bird-Lieberman EL, Neves AA, Lao-Sirieix P, et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett’s esophagus. Nat Med. 2012;18(2):315–321. [DOI] [PubMed] [Google Scholar]
- 65. Raval GN, Patel DD, Parekh LJ, Patel JB, Shah MH, Patel PS. Evaluation of serum sialic acid, sialyltransferase and sialoproteins in oral cavity cancer. Oral Dis. 2003;9(3):119–128. [DOI] [PubMed] [Google Scholar]
- 66. Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–555. [DOI] [PubMed] [Google Scholar]
- 67. Schauer R, Kelm S, Reuter G, Roggentin P, Shaw L. Biochemistry and role of sialic acids In: Rosenberg A, ed. Biology of Sialic Acids. Boston, MA: Springer; 1995. [Google Scholar]
- 68. Baeten J, Suresh A, Johnson A, et al. Molecular imaging of oral premalignant and malignant lesions using fluorescently labeled lectins. Transl Oncol. 2014;7(2):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Betz CS, Stepp H, Janda P, et al. A comparative study of normal inspection, autofluorescence and 5-ALA-induced PPIX fluorescence for oral cancer diagnosis. Int J Cancer. 2002;97(2):245–252. [DOI] [PubMed] [Google Scholar]
- 70. Nitin N, Carlson AL, Muldoon T, El-Naggar AK, Gillenwater A, Richards-Kortum R. Molecular imaging of glucose uptake in oral neoplasia following topical application of fluorescently labeled deoxy-glucose. Int J Cancer. 2009;124(11):2634–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Baeten J, Johnson A, Sunny S, et al. Chairside molecular imaging of aberrant glycosylation in subjects with suspicious oral lesions using fluorescently labeled wheat germ agglutinin. Head Neck. 2018;40(2):292–301. [DOI] [PubMed] [Google Scholar]
- 72. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 73. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. [DOI] [PubMed] [Google Scholar]
- 74. Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42(5 suppl):1S–93S. [PubMed] [Google Scholar]
- 75. Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991;32(4):623–648; discussion 49-50. [PubMed] [Google Scholar]
- 76. Adams S, Baum RP, Stuckensen T, Bitter K, Hor G. Prospective comparison of 18F-FDG PET with conventional imaging modalities (CT, MRI, US) in lymph node staging of head and neck cancer. Eur J Nucl Med. 1998;25(9):1255–1260. [DOI] [PubMed] [Google Scholar]
- 77. Yoshioka K, Takahashi H, Homma T, et al. A novel fluorescent derivative of glucose applicable to the assessment of glucose uptake activity of Escherichia coli. Biochim Biophys Acta. 1996;1289(1):5–9. [DOI] [PubMed] [Google Scholar]
- 78. Yang X, Palasuberniam P, Kraus D, Chen B. Aminolevulinic acid-based tumor detection and therapy: molecular mechanisms and strategies for enhancement. Int J Mol Sci. 2015;16(10):25865–25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Robertson CA, Evans DH, Abraharnse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photoch Photobio B. 2009;96(1):1–8. [DOI] [PubMed] [Google Scholar]
- 81. Nokes B, Apel M, Jones C, Brown G, Lang JE. Aminolevulinic acid (ALA): photodynamic detection and potential therapeutic applications. J Surg Res. 2013;181(2):262–271. [DOI] [PubMed] [Google Scholar]
- 82. O’Connor AE, Gallagher WM, Byrne AT. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol. 2009;85(5):1053–1074. [DOI] [PubMed] [Google Scholar]
- 83. Peng Q, Warloe T, Berg K, et al. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997;79(12):2282–2308. [DOI] [PubMed] [Google Scholar]
- 84. Leunig A, Betz CS, Mehlmann M, et al. Detection of squamous cell carcinoma of the oral cavity by imaging 5-aminolevulinic acid-induced protoporphyrin IX fluorescence. Laryngoscope. 2000;110(1):78–83. [DOI] [PubMed] [Google Scholar]
- 85. Photofrin to treat precancerous lesions in Barrett’s esophagus. FDA Consum. 2003;37(6):4. [PubMed] [Google Scholar]
- 86. Chang CJ, Wilder-Smith P. Topical application of photofrin for photodynamic diagnosis of oral neoplasms. Plast Reconstr Surg. 2005;115(7):1877–1886. [DOI] [PubMed] [Google Scholar]
- 87. Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ellisen LW. PARP inhibitors in cancer therapy: promise, progress, and puzzles. Cancer cell. 2011;19(2):165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33(12):1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Michels J, Vitale I, Saparbaev M, Castedo M, Kroemer G. Predictive biomarkers for cancer therapy with PARP inhibitors. Oncogene. 2014;33(30):3894–3907. [DOI] [PubMed] [Google Scholar]
- 91. Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016;8(362):362ps17. [DOI] [PubMed] [Google Scholar]
- 92. Bieche I, de Murcia G, Lidereau R. Poly(ADP-ribose) polymerase gene expression status and genomic instability in human breast cancer. Clin Cancer Res. 1996;2(7):1163–1167. [PubMed] [Google Scholar]
- 93. Chow JPH, Man WY, Mao M, et al. PARP1 is overexpressed in nasopharyngeal carcinoma and its inhibition enhances radiotherapy. Mol Cancer Ther. 2013;12(11):2517–2528. [DOI] [PubMed] [Google Scholar]
- 94. Dziaman T, Ludwiczak H, Ciesla JM, et al. PARP-1 expression is increased in colon adenoma and carcinoma and correlates with OGG1. PLoS One. 2014;9(12):e115558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Galia A, Calogero AE, Condorelli RA, et al. PARP-1 protein expression in glioblastoma multiforme. Eur J Histochem. 2012;56:45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Salemi M, Galia A, Fraggetta F, et al. Poly (ADP-ribose) polymerase 1 protein expression in normal and neoplastic prostatic tissue. Eur J Histochem. 2013;57(2):80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ossovskaya V, Koo IC, Kaldjian EP, Alvares C, Sherman BM. Upregulation of poly (ADP-ribose) polymerase-1 (PARP1) in triple-negative breast cancer and other primary human tumor types. Genes Cancer. 2010;1(8):812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Green AR, Caracappa D, Benhasouna AA, et al. Biological and clinical significance of PARP1 protein expression in breast cancer. Breast Cancer Res Treat. 2015;149(2):353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Staibano S, Pepe S, Lo Muzio L, et al. Poly(adenosine diphosphate-ribose) polymerase 1 expression in malignant melanomas from photoexposed areas of the head and neck region. Human Pathol. 2005;36(7):724–731. [DOI] [PubMed] [Google Scholar]
- 100. Mascolo M, Ilardi G, Romano MF, et al. Overexpression of chromatin assembly factor-1 p60, poly(ADP-ribose) polymerase 1 and nestin predicts metastasizing behaviour of oral cancer. Histopathology. 2012;61(6):1089–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chiang DY, Getz G, Jaffe DB, et al. High-resolution mapping of copy-number alterations with massively parallel sequencing. Nat Methods. 2009;6(1):99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Brouns ER, Baart JA, Karagozoglu KH, Aartman IH, Bloemena E, van der Waal I. Treatment results of CO2 laser vaporisation in a cohort of 35 patients with oral leukoplakia. Oral Dis. 2013;19(2):212–216. [DOI] [PubMed] [Google Scholar]
- 103. Carney B, Kossatz S, Reiner T. Molecular imaging of PARP. J Nucl Med. 2017;58(7):1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Reiner T, Lacy J, Keliher EJ, et al. Imaging therapeutic PARP inhibition in vivo through bioorthogonally developed companion imaging agents. Neoplasia. 2012;14(3):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Reiner T, Earley S, Turetsky A, Weissleder R. Bioorthogonal small-molecule ligands for PARP1 imaging in living cells. Chembiochem. 2010;11(17):2374–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thurber GM, Reiner T, Yang KS, Kohler RH, Weissleder R. Effect of small-molecule modification on single-cell pharmacokinetics of PARP inhibitors. Mol Cancer Ther. 2014;13(4):986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Agasti SS, Laughney AM, Kohler RH, Weissleder R. A photoactivatable drug-caged fluorophore conjugate allows direct quantification of intracellular drug transport. Chem Commun (Camb). 2013;49(94):11050–11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kim E, Yang KS, Giedt RJ, Weissleder R. Red Si-rhodamine drug conjugates enable imaging in GFP cells. Chem Commun (Camb). 2014;50(34):4504–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Thurber GM, Yang KS, Reiner T, et al. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat Commun. 2013;4:1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Irwin CP, Portorreal Y, Brand C, et al. PARPi-FL—a fluorescent PARP1 inhibitor for glioblastoma imaging. Neoplasia. 2014;16(5):432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kossatz S, Weber WA, Reiner T. Optical imaging of PARP1 in response to radiation in oral squamous cell carcinoma. PLoS One. 2016;11(1):e0147752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Buchel GE, Kossatz S, Sadique A, et al. cis-Tetrachlorido-bis(indazole)osmium(IV) and its osmium(III) analogues: paving the way towards the cis-isomer of the ruthenium anticancer drugs KP1019 and/or NKP1339. Dalton Trans. 2017;46(35):11925–11941. [DOI] [PMC free article] [PubMed] [Google Scholar]






