Abstract
Background:
The effects of remnant tissue preservation on tunnel enlargement after anatomic double-bundle anterior cruciate ligament (ACL) reconstruction have not yet been established.
Hypothesis:
The preservation of ACL remnant tissue may significantly reduce the degree and incidence of tunnel enlargement after anatomic double-bundle ACL reconstruction, while the remnant-preserving procedure may not significantly increase the incidence of tunnel coalition after surgery.
Study Design:
Cohort study; Level of evidence, 2.
Methods:
A total of 79 patients underwent anatomic double-bundle ACL reconstruction. Based on the Crain classification of ACL remnant tissue, 40 patients underwent the remnant-preserving procedure (group P), and the remaining 39 patients underwent the remnant-resecting procedure (group R). There were no differences between the 2 groups concerning all background factors, including preoperative knee instability and intraoperative tunnel positions. All patients were examined using computed tomography and a standard physical examination at 2 weeks and 1 year after surgery.
Results:
During surgery, the femoral and tibial anteromedial (AM) tunnel sizes in both groups averaged 6.6 and 6.5 mm, respectively. The femoral and tibial posterolateral (PL) tunnel sizes in both groups averaged 6 and 6 mm, respectively. There were no differences in the intraoperative tunnel positions and tunnel sizes between groups. Concerning the femoral AM tunnel, the degree of tunnel enlargement in the oblique coronal and oblique axial views in group P was significantly less than that in group R (P = .0068 and .0323, respectively). Regarding the femoral AM tunnel cross-sectional area, the degree and incidence of tunnel enlargement in group P were significantly less than those in group R (P = .0086 and .0278, respectively). There were no significant differences in tunnel coalition between groups. In each group, there were no significant relationships between tunnel enlargement and each clinical outcome.
Conclusion:
Remnant preservation in anatomic double-bundle ACL reconstruction reduced enlargement of the femoral AM tunnel and did not increase the incidence of tunnel coalition. This is one of the advantages of remnant-preserving ACL reconstruction.
Keywords: anterior cruciate ligament, tunnel enlargement, anatomic double-bundle reconstruction, remnant tissue, hamstring tendon graft, clinical outcome
Bone tunnel enlargement frequently occurs, and the degree of bone tunnel enlargement is reported to be 12% to 75% after anterior cruciate ligament (ACL) reconstruction, independent of graft types and fixation methods.16,20,21,34,49,51 Although a correlation between tunnel enlargement and poor clinical results has not yet been clearly shown, the presence of enlarged tunnels severely complicates revision ACL surgery.13,23,42 Therefore, tunnel enlargement is regarded as a significant issue in the field of ACL reconstruction. There are multifactorial causes of tunnel enlargement, such as graft placement and fixation,41,58 excessive rehabilitation,16,20 micromotion at the tunnel aperture by a graft with suspensory fixation,34,50 and synovial fluid leakage within the bone tunnel.5 Although many attempts have been made to reduce tunnel enlargement,12,22,36,40 there have not been any definite solutions as of yet. Recently, it has been reported that anatomic double-bundle ACL reconstruction procedures significantly reduce the incidence and degree of postoperative tunnel enlargement compared with the single-bundle procedure.23,24 The differences between these 2 procedures in the incidence and degree of tunnel enlargement were 2% to 6% and 13% to 32%, respectively. However, the incidence and degree of tunnel enlargement after double-bundle reconstruction remain at high levels. For example, Siebold and Cafaltzis43 reported that the degree of tunnel enlargement was a mean of 34% and 46% for the femoral anteromedial (AM) and posterolateral (PL) tunnels, respectively, and 20% and 38% for the tibial AM and PL tunnels, respectively. Therefore, it is necessary to conduct studies to further reduce the incidence and degree of tunnel enlargement after anatomic double-bundle ACL reconstruction.
Recently, the preservation of ACL remnant tissue has been recognized to have several potential advantages,8 such as preservation of proprioceptive organs,1,19,32,33 graft revascularization, and graft ligamentization.2,9,14,27,39 However, less is known about the effects of remnant preservation on tunnel enlargement after ACL reconstruction. We could find only 2 studies concerned with this issue. Zhang et al59 reported that remnant preservation in single-bundle ACL reconstruction could reduce tibial tunnel enlargement. Recently, Yanagisawa et al54 reported that remnant preservation reduced the amount of femoral (14%) and tibial (10%) tunnel enlargement in double-bundle ACL reconstruction, even when residual tissue of the normal femoral attachment area was peeled off from the bony surface. In that study,54 however, tunnel enlargement was evaluated at only 6 months after surgery. In addition, Yanagisawa et al54 did not compare detailed postoperative clinical scores between the remnant-preserving and -resecting procedures. Therefore, the effect of remnant tissue preservation on tunnel enlargement after anatomic double-bundle ACL reconstruction has not yet been clearly elucidated.
We conducted a prospective comparative study using multiplanar reconstruction images on computed tomography (CT) to clarify the effect of remnant tissue preservation on tunnel enlargement at 1 year after anatomic double-bundle ACL reconstruction. We used the anatomic double-bundle ACL reconstruction procedure, which has been evaluated in many biomechanical, clinical, and arthroscopic studies,29,30,55,56 and the remnant-preserving procedure, which has also been evaluated clinically and arthroscopically in previous studies.31,57 We hypothesized that the degree and incidence of tunnel enlargement in remnant-preserving procedures may be significantly less than those in remnant-resecting procedures after the same anatomic double-bundle ACL reconstruction procedure.
Methods
Study Design
A prospective comparative study was conducted between March 2009 and May 2013 using patients who had an isolated unilateral ACL injury. Exclusion criteria from this study were patients with a combined injury in the other knee ligaments and patients who had undergone any previous knee surgery. The study design was approved by the institutional review board at our hospital before commencement. All patients were informed that if they did not want to be in this study, they could choose another ACL reconstruction procedure. Patients were also informed that CT would be conducted postoperatively.
A total of 91 patients (91 knees) were enrolled in this study. Before beginning the ACL reconstruction procedure, we arthroscopically observed the morphological status of remnant tissue in each study participant. Crain et al11 reported on an arthroscopic classification system that identified 4 different patterns of ACL morphology: type I (ACL scarring to the posterior cruciate ligament), type II (ACL healing to the roof of the notch), type III (attenuated ACL remnant healed to the lateral wall), and type IV (resorption of the torn ACL). The knees with Crain type IV remnant tissue, the proximal end of which did not attach anywhere although the distal end attached on the tibia, were categorized as group R and underwent remnant-resecting procedure (Figure 1). The knees with Crain type I, II, or III remnant tissue, the proximal end of which attached on the femur or posterior cruciate ligament, were categorized as group P and underwent remnant-preserving procedure (Figure 2).
Figure 1.
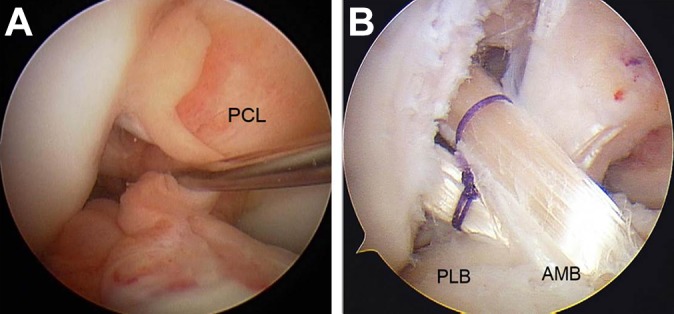
Anatomic double-bundle reconstruction without remnant tissue preservation. (A) Preoperative Crain type IV (resorption of the torn anterior cruciate ligament [ACL]) remnant tissue of the ACL. (B) A representative case: the graft surface was not covered with any remnant tissue. AMB, anteromedial bundle; PCL, posterior cruciate ligament; PLB, posterolateral bundle.
Figure 2.
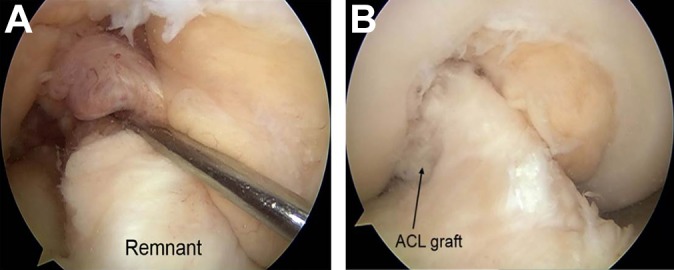
Anatomic double-bundle reconstruction with remnant tissue preservation. (A) Preoperative Crain type II (anterior cruciate ligament [ACL] healing to the roof of the notch) remnant tissue of the ACL. (B) A representative case: the graft surface was covered with remnant tissue.
Of the 91 patients, 44 were placed in group R and underwent arthroscopic anatomic double-bundle ACL reconstruction55 after the resection of remnant tissue. The other 47 patients in group P underwent arthroscopic anatomic double-bundle ACL reconstruction with the preservation of remnant tissue.57 Namely, the decision for each procedure was made according to the remnant type of each knee.
Two senior orthopaedic surgeons (E.K. and N.K.) who were sufficiently trained concerning the 2 procedures performed all operative procedures. After surgery, all patients underwent postoperative management using the same rehabilitation protocol.56 We followed up with the patients in our outpatient clinic for ≥1 year (range, 12-16 months) after surgery; 5 patients in group R and 7 patients in group P were lost to follow-up for a variety of circumstances. Thus, a total of 79 patients participated in this study and underwent clinical and radiological evaluations.
Patient Demographics
Concerning the 79 patients (38 men and 41 women), the mean age was 29 years (range, 13-66 years) at the time of surgery. There were 39 patients in group R and 40 patients in group P. The preoperative side-to-side anterior laxity measured with a KT-2000 arthrometer (MEDmetric) averaged 5.2 mm and 5.3 mm in groups R and P, respectively (Table 1). The background factors in each group are shown in Table 1. There were no significant differences between the 2 groups. The rehabilitation protocol was the same for patients undergoing meniscal repair and meniscal resection. No serious cartilage injuries occurred that needed surgical treatment.
TABLE 1.
Patient Demographicsa
| Group R | Group P | P Value | |
|---|---|---|---|
| Age, y | 29 ± 14 | 30 ± 13 | .8759 |
| Sex, male:female, n | 20:19 | 18:22 | .7387 |
| Height, cm | 163.7 ± 8.1 | 163.2 ± 7.6 | .8176 |
| Weight, kg | 61.4 ± 12.6 | 63.3 ± 10.3 | .5309 |
| Time from injury to surgery, mo | 18 ± 35 | 4 ± 3 | .1157 |
| Side-to-side anterior laxity, mm | 5.2 ± 1.4 | 5.3 ± 1.4 | .4363 |
| Meniscal tear, n | .7817 | ||
| Longitudinal tear, LM/MM | 2/6 | 3/5 | |
| Horizontal tear, LM/MM | 2/4 | 2/4 | |
| Radial tear, LM/MM | 4/1 | 3/1 | |
| Flap tear, LM/MM | 2/3 | 1/3 | |
| Partial meniscectomy, n | 10 | 7 | .5442 |
| Meniscal repair, n | 11 | 6 | .2485 |
aData are shown as mean ± SD unless otherwise indicated. Group R included patients who underwent the remnant-resecting procedure, and group P included patients who underwent the remnant-preserving procedure. LM, lateral meniscus; MM, medial meniscus.
Surgical Procedure
Arthroscopic anatomic double-bundle ACL reconstruction with/without the preservation of remnant tissue was performed using the transtibial tunnel technique according to previously described methods.55,57 Between the 2 procedures, there were no essential differences concerning the reconstruction process except for the preservation or resection of remnant tissue.
For graft preparation, the harvested semitendinosus tendon was cut in half and doubled over. A commercially available polyester tape (Leeds-Keio artificial ligament; Neoligaments) was mechanically connected at an unlooped end of the doubled tendon by the use of a previously reported technique.55 An Endobutton CL BTB (Smith & Nephew) was attached at the looped end. In group P, each graft was introduced through each tibial tunnel and remnant tissue into the femoral tunnel and was fixed with an Endobutton (Figure 3). The 2 tape portions were simultaneously secured with 2 spiked staples (Smith & Nephew) onto the tibia at the full extension position, with a 30-N load applied to each graft.
Figure 3.
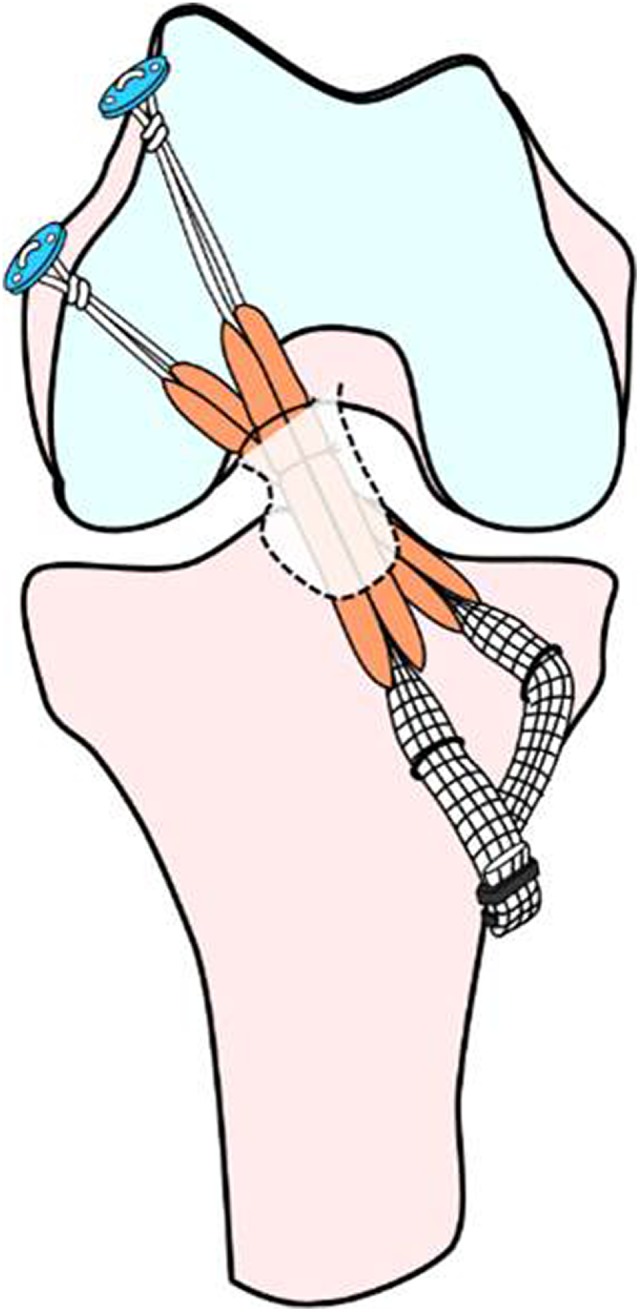
Schematic illustration of anatomic double-bundle anterior cruciate ligament reconstruction with remnant tissue preservation.
Computed Tomography
All patients underwent CT at 2 weeks and 1 year after surgery. The 2-dimensional (2D) and 3 dimensional (3D) CT scans were taken using a 64-slice multidetector CT machine (Aquilion 64; Toshiba) and were converted to multiplanar reconstruction images and observed on a monitor display using the computer software program ZioTerm2009 (Ziosoft). The knee was placed in full extension, and scanning was performed from proximal to the femoral tunnel to distal to the tibial tunnel to visualize the position of autograft fixation. The 0.5-mm sections were secondarily reconstructed with a bony algorithm to allow multiplanar reconstructions (1-mm thickness per 1-mm interval) from the axial data set. Oblique axial (OA), oblique sagittal (OS), and oblique coronal (OC) views were reconstructed based on the direction of the longitudinal axis of the femoral and tibial tunnels. The 3D scans were also reconstructed with a soft tissue algorithm using the volume-rendering technique.
The position of the femoral and tibial tunnels was evaluated by observing the AM and PL tunnel outlets on the intra-articular bone surface of the 3D CT scans using the quadrant method.6,47 On these images, surgeons who were blinded to the patient group measured the location of the intra-articular outlet center of the AM and PL tunnels using previously reported X and Y coordinates.25 The tunnel measurement was taken digitally at 10 mm from the intra-articular outlet of both the femoral and tibial tunnels without coalition in the OA, OS, and OC views according to previous studies.7,10,25,44
Measurement of the tunnel cross-sectional area was also taken digitally at 10 mm from the intra-articular outlet of both the femoral and tibial tunnels without coalition in the OA view using ImageJ software (National Institutes of Health) (Figures 4 and 5). First, a digital image file of an OA CT scan was opened using ImageJ. Then, for the measurement scale, the described scale on the image file was used as a baseline. The automated threshold included only the tunnel area. Finally, the cross-sectional area of the tunnel was calculated. All measurements were taken from the sclerotic bony margins by a blinded orthopaedic surgeon (T.M.). The degree of tunnel enlargement was defined as the percentage change in the diameter and cross-sectional area between the scans performed at 2 weeks and 1 year. Based on our previous study,25 the incidence of tunnel enlargement was determined by the number of femoral or tibial tunnels that enlarged more than 40% in area (because an increase of 20% of the diameter was equivalent to a 40% increase in the area of the cylinder tunnel).
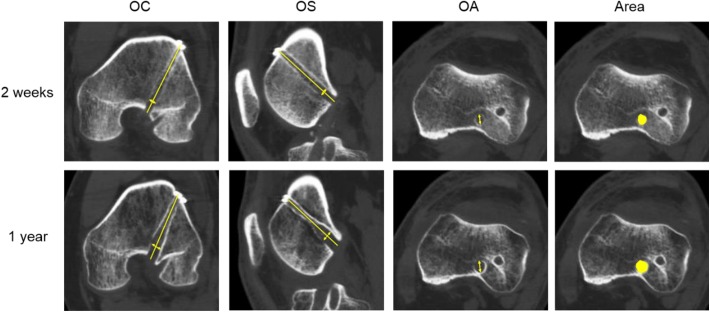
Figure 4.
Multiplanar reconstruction images from computed tomography scans of the femur at 2-week (top row) and 1-year (bottom row) follow-up. The femoral anteromedial tunnel showed a diameter at 10 mm from the intra-articular outlet of the femoral tunnels (line with arrows). OA, oblique axial; OC, oblique coronal; OS, oblique sagittal. Cross-sectional area represented by yellow circle.
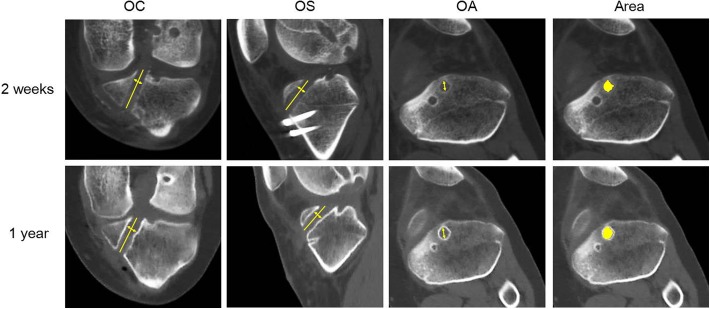
Figure 5.
Multiplanar reconstruction images from computed tomography scans of the tibia at 2-week (top row) and 1-year (bottom row) follow-up. The femoral anteromedial tunnel showed a diameter at 10 mm from the intra-articular outlet of the femoral tunnels (line with arrows). OA, oblique axial; OC, oblique coronal; OS, oblique sagittal. Cross-sectional area represented by yellow circle.
Tunnel coalition was determined by observing the AM and PL tunnel outlets on the intra-articular bone surface of the femur or tibia using 3D CT scans and measuring the width of the bony septum between the 2 tunnels according to Hantes et al.15 In addition, the thickness of the bony septum between the AM and PL tunnels was measured at 10 mm from the intra-articular outlet with coronal, sagittal, and axial 2D CT scans. When the width was zero, we defined it as tunnel coalition.
Clinical Evaluation
Each patient underwent a clinical examination ≥1 year after surgery. The side-to-side anterior laxity was measured using a KT-2000 arthrometer at 30° of knee flexion under an anterior drawer force of 133 N. An experienced knee surgeon (E.N.) obtained the KT-2000 arthrometer results postoperatively. Another experienced orthopaedic surgeon (N.K.) performed the pivot-shift test, the results of which were subjectively evaluated as “2+,” “+,” and “–” using previously reported criteria.25,30,31,56,57 For the overall evaluation, the Lysholm knee score (maximum score, 100 points),35 the International Knee Documentation Committee (IKDC) score,18 and the Tegner activity score46 were used. Peak isokinetic torque of the quadriceps and hamstring was measured at 60 deg/s of angular velocity with a Cybex II dynamometer (Lumex) in both knees ≥1 year after surgery. Muscle torque as measured postoperatively was represented as a ratio (percentage) of the uninvolved knee to the involved knee.
Statistical Analysis
Intraobserver variability for the tunnel measurements was satisfactory (mean intraclass correlation coefficient, 0.84 [range, 0.78-0.92]). An a priori power analysis was performed. The significance level was set at P = .05. In our preliminary study,25 the difference between the remnant-preserving and -resecting procedures in the incidence of tunnel enlargement was 20% to 25%, with 10 knees in each group. A sample size of 79 was calculated to have 74% to 85% power to test our hypothesis. The Pearson correlation coefficient and chi-square test were used to characterize the relationship of tunnel enlargement and coalition to clinical parameters. A statistical comparison was performed using the chi-square test, unpaired Mann-Whitney U test, and Fisher exact test for the change in tunnel enlargement and coalition. A commercially available software program, JMP 11 (SAS Institute), was used for statistical calculation.
Results
Tunnel Position
Using the grid system6,47 shown in Figure 6, we first compared the X-Y coordinates of each tunnel outlet between groups P and R (Table 2). There were no significant differences concerning each value. These results demonstrated that the 2 groups were comparable in terms of the created tunnel location and that any differences in clinical results were not caused by the difference in the tunnel outlet location but by the preservation of ACL remnant tissue.
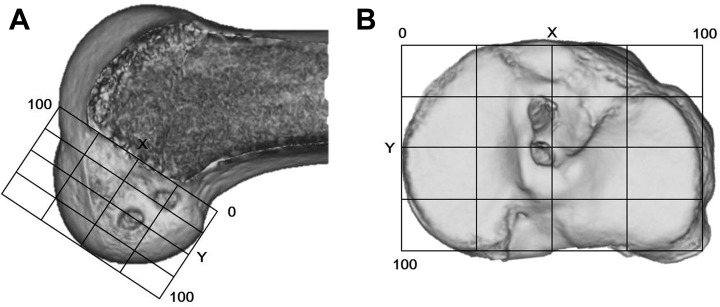
Figure 6.
The quadrant method to evaluate the position of the femoral and tibial tunnels. Evaluation of each tunnel outlet with standard and 3-dimensional computed tomography (3D CT): (A) To evaluate the center location of each femoral tunnel outlet, we drew the X and Y coordinate system on the 3D CT scan so that we could obtain a correct lateral view of the femoral condyle and determine the Blumensaat line. (B) To evaluate the center location of each tibial tunnel outlet, we drew the X and Y coordinate system on a standard CT scan.
TABLE 2.
AM and PL Tunnel Locations in the Femur and Tibiaa
| Group R | Group P | P Value | |
|---|---|---|---|
| Femur | |||
| AM tunnel | |||
| X coordinate | 31.8 ± 4.7 | 30.9 ± 4.0 | .3308 |
| Y coordinate | 16.6 ± 5.8 | 17.9 ± 5.1 | .3138 |
| PL tunnel | |||
| X coordinate | 40.3 ± 4.7 | 40.3 ± 5.3 | .9733 |
| Y coordinate | 48.0 ± 6.6 | 47.6 ± 5.9 | .7637 |
| Tibia | |||
| AM tunnel | |||
| X coordinate | 46.6 ± 2.6 | 46.8 ± 2.8 | .7776 |
| Y coordinate | 31.4 ± 6.9 | 34.3 ± 5.1 | .1964 |
| PL tunnel | |||
| X coordinate | 47.1 ± 2.2 | 47.1 ± 2.7 | .9905 |
| Y coordinate | 52.5 ± 6.7 | 52.5 ± 5.2 | .9902 |
aData are shown as mean ± SD in percentages. The center position of each tunnel outlet was expressed by the X and Y coordinates, defined in Figure 6. AM, anteromedial; PL, posterolateral.
Tunnel Size During Surgery
The femoral and tibial AM tunnel sizes in group R averaged 6.2 mm and 6.6 mm, respectively, and those in group P averaged 6.2 mm and 6.5 mm, respectively. The femoral and tibial PL tunnel sizes in group R averaged 5.8 mm and 5.9 mm, respectively, and those in group P averaged 5.8 mm and 6.0 mm, respectively. Concerning the femoral and tibial tunnel sizes, there were no significant differences between the 2 groups (Table 3).
TABLE 3.
Femoral and Tibial Tunnel Sizes During ACL Reconstructiona
| Group R | Group P | P Value | |
|---|---|---|---|
| Femoral tunnel | |||
| AM | 6.2 ± 0.3 | 6.2 ± 0.3 | .5187 |
| PL | 5.8 ± 0.2 | 5.8 ± 0.3 | .9733 |
| Tibial tunnel | |||
| AM | 6.6 ± 0.3 | 6.5 ± 0.4 | .0729 |
| PL | 5.9 ± 0.3 | 6.0 ± 0.3 | .9819 |
aData are shown as mean ± SD in millimeters. ACL, anterior cruciate ligament; AM, anteromedial; PL, posterolateral.
Degree and Incidence of Tunnel Enlargement
The degree of tunnel enlargement of the femoral AM tunnel diameter in group R averaged 20.0%, 17.8%, and 18.7% in the OC, OS, and OA views, respectively, compared with 9.1%, 11.6%, and 11.4% in group P. These differences were significantly different in the OC and OA views, with the degree of tunnel enlargement being less in group P (P = .0068 and .0323, respectively) (Table 4 and Figure 7). The degree of tunnel enlargement of the femoral PL tunnel diameter in group R averaged 4.4%, 14.1%, and 18.2% in the OC, OS, and OA views, compared with 5.7%, 5.0%, and 10.8% in group P. There were no significant differences between the 2 groups regarding tibial tunnel enlargement.
TABLE 4.
Degree of Femoral Tunnel Enlargement at 1 Year After ACL Reconstructiona
| Group R | Group P | P Value | |
|---|---|---|---|
| AM | |||
| OC | 20.0 ± 18.6 | 9.1 ± 16.3 | .0068 |
| OS | 17.8 ± 19.5 | 11.6 ± 16.4 | .1294 |
| OA | 18.7 ± 16.5 | 11.4 ± 18.9 | .0323 |
| Area | 44.0 ± 50.4 | 19.4 ± 27.1 | .0086 |
| PL | |||
| OC | 4.4 ± 21.2 | 5.7 ± 20.6 | .7880 |
| OS | 14.1 ± 31.1 | 5.0 ± 16.3 | .1107 |
| OA | 18.2 ± 27.2 | 10.8 ± 17.3 | .1510 |
| Area | 20.7 ± 44.4 | 5.0 ± 35.7 | .0857 |
aData are shown as mean ± SD in percentages. Values are the percentage change compared with the tunnel diameter and the cross-sectional area measured at the 2-week period. ACL, anterior cruciate ligament; AM, anteromedial; OA, oblique axial; OC, oblique coronal; OS, oblique sagittal; PL, posterolateral.
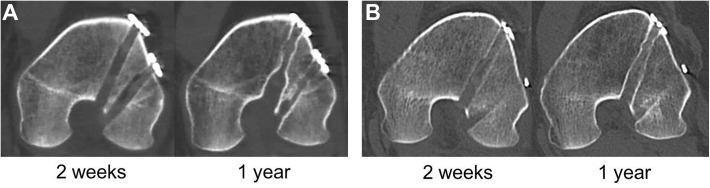
Figure 7.
Representative multiplanar reconstruction images of computed tomography of the femur: (A) Oblique coronal scans of the femoral anteromedial (AM) tunnel in group R at 2 weeks (left) and 1 year (right) after anterior cruciate ligament (ACL) reconstruction. (B) Oblique coronal scans of the femoral AM tunnel in group P at 2 weeks (left) and 1 year (right) after ACL reconstruction.
The degree of tunnel enlargement of the femoral AM tunnel cross-sectional area in group R averaged 44.0%, and that in group P averaged 19.4%. The degree of tunnel enlargement of the femoral PL tunnel area in group R averaged 20.7%, and that in group P averaged 5.0%. The degree of enlargement in the femoral AM tunnel area was significantly less in group P than in group R (P = .0086). Regarding the PL tunnel, we could find the same tendency, but the difference was not significant (Table 4). Concerning the degree of tunnel enlargement for the tibial AM and PL tunnel cross-sectional areas, there were no significant differences between the 2 groups (Table 5).
TABLE 5.
Degree of Tibial Tunnel Enlargement at 1 Year After ACL Reconstructiona
| Group R | Group P | P Value | |
|---|---|---|---|
| AM | |||
| OC | 1.7 ± 12.6 | 1.1 ± 9.4 | .1259 |
| OS | 0.7 ± 10.2 | –3.8 ± 10.2 | .1019 |
| OA | 3.6 ± 9.6 | 4.6 ± 15.2 | .0957 |
| Area | 1.5 ± 28.4 | –4.8 ± 19.0 | .2838 |
| PL | |||
| OC | 0.8 ± 16.9 | –3.1 ± 12.5 | .1693 |
| OS | –2.9 ± 15.3 | –4.3 ± 10.2 | .1529 |
| OA | –2.0 ± 15.8 | –1.4 ± 13.8 | .1583 |
| Area | –6.5 ± 39.2 | –14.1 ± 26.6 | .3918 |
aData are shown as mean ± SD in percentages. Values are the percentage change compared with the tunnel diameter and the cross-sectional area measured at the 2-week period. ACL, anterior cruciate ligament; AM, anteromedial; OA, oblique axial; OC, oblique coronal; OS, oblique sagittal; PL, posterolateral.
The incidence of femoral AM tunnel enlargement in group R was 48.7%, while that in group P was 22.5%, and the incidence of femoral PL tunnel enlargement in group R was 23.1%, while that in group P was 17.5%. Concerning the AM tunnel, the incidence of enlargement was significantly less in group P than in group R (P = .0278) (Table 6). The incidence of tibial AM tunnel enlargement in group R was 9.7%, while that in group P was 3.2%. The incidence of tibial PL tunnel enlargement in group R was 6.5%, while that in group P was 9.7% . There were no significant differences between the 2 groups.
TABLE 6.
Incidence of Tunnel Enlargement at 1 Year After ACL Reconstructiona
| Group R | Group P | P Value | |
|---|---|---|---|
| All ages | |||
| Femoral tunnel, n | 39 | 40 | |
| AM, % | 48.7 | 22.5 | .0278 |
| PL, % | 23.1 | 17.5 | .7799 |
| Tibial tunnel, n | 31 | 31 | |
| AM, % | 9.7 | 3.2 | .6052 |
| PL, % | 6.5 | 9.7 | >.999 |
| Age ≤30 y | |||
| Femoral tunnel, n | 23 | 21 | |
| AM, % | 39.1 | 4.8 | .0102 |
| PL, % | 13.0 | 9.5 | >.999 |
| Tibial tunnel, n | 19 | 16 | |
| AM, % | 0.0 | 0.0 | >.999 |
| PL, % | 0.0 | 6.3 | .4571 |
| Age >30 y | |||
| Femoral tunnel, n | 16 | 19 | |
| AM, % | 56.3 | 42.1 | .6209 |
| PL, % | 37.5 | 26.3 | .7304 |
| Tibial tunnel, n | 12 | 15 | |
| AM, % | 25.0 | 7.1 | .294 |
| PL, % | 16.7 | 14.3 | >.999 |
aACL, anterior cruciate ligament; AM, anteromedial; PL, posterolateral.
In addition, patients were divided into 2 age groups: ≤30 years and >30 years. The incidence of femoral AM tunnel enlargement was significantly greater (P = .0072) in group P patients older than 30 years compared with those 30 years and younger. Regarding the femoral AM tunnel for patients aged ≤30 years, the incidence of enlargement was significantly less in group P than in group R (P = .0102). Yet, for patients aged >30 years, there were no significant differences between the 2 groups. There was a significant difference in the postoperative Tegner score between the 2 age categories (mean score, 6.2 for ≤30 years and 5.2 for >30 years; P = .0143).
Incidence of Tunnel Coalition
On the femoral surface, tunnel outlet coalition was observed in only 1 knee (2.6% of cases) at 2 weeks in group R and none in group P. Then, at 1 year, femoral tunnel outlet coalition was observed in 2 knees (5.1%) in group R and 1 knee (2.5%) in group P. However, there were no knees with tunnel coalition at a level 10 mm from the bone surface at each period in both groups. On the tibial surface, tunnel outlet coalition was observed in 22 knees (56.4% of cases) in group R and 25 knees (62.5%) in group P at 2 weeks and then in 26 knees (66.6%) in group R and 28 knees (70.0%) in group P at 1 year after surgery. Tibial tunnel coalition at a level 10 mm from the joint surface was seen in 6 knees (15.4% of cases) in group R and 7 knees (17.5%) in group P at 2 weeks. Then, at 1 year, tibial tunnel outlet coalition was observed in 8 knees (20.5%) in group R and 9 knees (22.5%) in group P. There were no significant differences between the 2 groups, and there was no significant correlation between femoral and tibial tunnel coalition and postoperative knee laxity (Table 7).
TABLE 7.
Side-to-Side Anterior Laxity at 1 Year After ACL Reconstructiona
| Tunnel Coalition (+) | Tunnel Coalition (–) | P Value | |
|---|---|---|---|
| Group R | 1.9 ± 2.2 (n = 8) | 0.7 ± 2.6 (n = 31) | .2403 |
| Group P | 0.5 ± 2.3 (n = 9) | 0.6 ± 2.7 (n = 31) | .7985 |
aData are shown as mean ± SD. ACL, anterior cruciate ligament.
Clinical Results
There were no graft failures at final follow-up. For the side-to-side difference in anterior laxity, there were no significant differences between the 2 groups. After the side-to-side laxity values were divided into 2 categories, <2 mm and ≥2 mm, the chi-square test showed that anterior laxity in group P was significantly better than in group R (P = .0353) (Table 8). Concerning the pivot-shift test, there were no significant differences between the 2 groups (Table 8). There were also no significant differences between the 2 groups regarding postoperative results of the Lysholm knee score, IKDC score, mean isokinetic peak torque of the quadriceps and hamstring muscles, and Tegner score (Table 9).
TABLE 8.
Postoperative Knee Stability at 1 Year After ACL Reconstructiona
| Group R | Group P | P Value | |
|---|---|---|---|
| Anterior laxity, mm | 0.9 ± 2.7 | 0.6 ± 2.6 | .6335 |
| Anterior laxity, n (%) | .0353 | ||
| ≤2 mm | 24 (62) | 34 (85) | |
| >2 mm | 15 (38) | 6 (15) | |
| Pivot-shift test result, n (%) | .4225 | ||
| – | 29 (74) | 33 (83) | |
| + | 10 (26) | 7 (17) | |
| 2+ | 0 (0) | 0 (0) |
aData are shown as mean ± SD unless otherwise indicated. ACL, anterior cruciate ligament.
TABLE 9.
Clinical Outcomesa
| Group R | Group P | P Value | |
|---|---|---|---|
| Lysholm knee score | 95.2 ± 6.7 | 97.2 ± 3.9 | .1034 |
| IKDC grade, n (%) | .6919 | ||
| A (normal) | 24 (62) | 27 (68) | |
| B (nearly normal) | 12 (31) | 12 (30) | |
| C (nearly abnormal) | 3 (7) | 1 (2) | |
| D (abnormal) | 0 (0) | 0 (0) | |
| Isokinetic peak torque, % of uninjured knee torque | |||
| Quadriceps muscle | 82.2 ± 17.0 | 81.4 ± 18.1 | .8420 |
| Hamstring muscle | 83.0 ± 21.0 | 89.1 ± 17.5 | .1915 |
| Tegner activity score | 5.9 ± 1.8 | 5.7 ± 1.9 | .2432 |
aData are shown as mean ± SD unless otherwise indicated. IKDC, International Knee Documentation Committee.
Discussion
There are 2 strong points in this study. First, we used the anatomic double-bundle ACL reconstruction procedure and the remnant-preserving procedure, which have been clinically established.31 A unique feature of the remnant-preserving procedure is that the femoral attachment of remnant tissue is not detached, so as to sufficiently cover the femoral tunnel outlets with tissue.57 Second, we confirmed the similarity of the created intra-articular tunnel outlet locations and the incidence of tunnel coalition between groups P and R in this study. Thus, we believe the present study, using multiplanar reconstruction images of CT, clearly demonstrates the effects of remnant tissue preservation on tunnel enlargement after anatomic double-bundle ACL reconstruction. Namely, the remnant tissue preservation technique significantly reduced the degree of femoral AM tunnel enlargement at 1 year after surgery. In addition, the incidence of measurable femoral AM tunnel enlargement was significantly less in group P (22.5%) than in group R (48.7%), and the percentage change in the cross-sectional area of the AM tunnel was significantly less in group P (19.4%) than in group R (44.0%). Previous biomechanical studies26,28 have suggested that the most important bundle to resist tibial anterior displacement is the AM bundle.
In the present study, we could not find any significant relationship between the degree of tunnel enlargement and clinical outcomes at 1-year follow-up. This result is similar to that reported in previous studies.15,42 However, we believe that the effort to reduce tunnel enlargement is important in any type of ACL reconstruction procedure because a possibility of ACL revision surgery exists in all patients.
Remnant tissue preservation makes the creation of femoral and tibial tunnels difficult because tissue disturbs the arthroscopic observation of the bony landmarks. Therefore, it has been pointed out that malposition and coalition of the tunnels may frequently occur in remnant-preserving double-bundle reconstruction, resulting in graft malfunction and tunnel enlargement.33 However, there were no significant differences in the locations of the created tunnels and the incidence of tunnel coalition between the remnant-resecting and -preserving procedures in the current study. Kondo et al31 also reported that there were no significant differences in the intra-articular tunnel position between the remnant-resecting and -preserving procedures. This is an advantage of the remnant-preserving procedure, which includes the use of some special guide devices and an easy technique to intraoperatively confirm the guide wire location using a C-arm fluoroscope.57
In group R, the degree of tunnel enlargement at 10 mm from the intra-articular outlet was 17.8% to 20.0% in the femur and 4.4% to 18.2% in the tibia. Previously, Araki et al3 reported that the femoral bone tunnel volume of the AM and PL bundles changed to 22.3% and 12.5% in the articular third, respectively. Siebold and Cafaltzis43 reported, using magnetic resonance imaging, that at 7 months after double-bundle reconstruction, the degree of tunnel enlargement was a mean of 34% for the femoral AM tunnel, 46% for the femoral PL tunnel, 20% for the tibial AM tunnel, and 38% for the tibial PL tunnel. Jarvela et al23 reported, using magnetic resonance imaging, that at 27 months after double-bundle reconstruction, the degree of AM and PL femoral and tibial tunnel enlargement averaged 54% and 42% and 39% and 43%, respectively. Therefore, the degree of tunnel enlargement we observed in group R was comparable with that after common double-bundle ACL reconstruction procedures.
We considered the reasons why remnant tissue preservation reduces enlargement of the femoral AM tunnel after ACL reconstruction. The first possibility is related to the limitation of synovial fluid propagation within the bone tunnel by remnant tissue preservation. It is known that the levels of inflammatory cytokines are elevated in the synovial fluid for several weeks after ACL reconstruction.12,17,60 Xie et al53 reported that the synovial fluid contained high levels of proinflammatory cytokines such as TNF-alpha, IL-1beta, IL-6, and nitric oxide, which inhibited bone formation and promoted osteoclast cell activity, leading to osteolysis and tunnel enlargement. Hayward et al17 reported that the cytokines in the synovial fluid leaking into the bone tunnel disrupted healing of the bone-tendon interface. Sun et al45 suggested that the preservation of tibial remnant tissue decreased fluid leakage into the bone tunnel. Hayward et al17 also described that a reduction in synovial fluid leakage into the tibial tunnel might decrease or eliminate the adverse effects of cytokine-mediated osteolysis or bone tunnel enlargement.
A special feature of our technique31,57 is that the proximal attachment of the ACL remnant is not detached from the attachment. Generally, the femoral tunnel aperture is surrounded by synovial and fibrous tissues of Crain type I and II. Therefore, the procedure used in the present study was beneficial to sufficiently cover the tendon grafts with remnant and synovial tissues not only in the tibial tunnel but also in the femoral tunnel. We conducted a comparison in the incidence of tunnel enlargement among Crain types I, II, and III in group P patients and found no significant differences.
The second possibility for this mechanism is related to the activities of remnant tissue–derived cells.39 Nakano et al37 reported that ACL-derived cells enhanced early bone-tendon healing in rat models of ACL reconstruction. Therefore, there is a possibility that ACL remnant–derived cells also enhance intraosseous graft healing in the tunnel. Wu et al52 reported that the integration of the tendon to the bone tunnel was significantly improved by remnant preservation during ACL reconstruction. However, further basic studies are needed to clarify the mechanism in detail.
We considered the reasons why remnant tissue preservation could not reduce enlargement of the femoral PL tunnel after ACL reconstruction. Previous studies have reported that in remnant-resecting double-bundle reconstruction, the incidence and degree of femoral PL tunnel enlargement are essentially less than those of femoral AM tunnel enlargement.25,43 In addition, Kondo et al31 showed that the degree of graft coverage around the PL bundle with remnant tissue was significantly less than that around the AM bundle. Therefore, we speculate that the effect of remnant tissue preservation on femoral tunnel enlargement was not detected. Concerning tibial AM and PL bundle enlargement, some amount of ACL remnant tissue was left at the tibial attachment in our remnant-resecting procedure. Therefore, we believe that the effect of remnant tissue preservation on tibial tunnel enlargement was hardly detected in the clinical evaluation.
In the present study, the incidence of femoral AM tunnel enlargement in group P was significantly greater (P = .0072) in older patients (>30 years) than in younger patients (≤30 years). Yanagisawa et al54 reported that age was a preoperative factor associated with tunnel enlargement after double-bundle ACL reconstruction. Nishio et al38 also noted that femoral tunnel enlargement was significantly greater in older patients than in younger patients. Recently, it has been postulated that postoperative periarticular loss of bone mineral density might be associated with tunnel enlargement.4 Regarding the femoral AM tunnel, in patients aged ≤30 years, the incidence of enlargement was significantly less in group P than in group R (P = .0102). The Tegner activity score was significantly higher in younger patients than in older patients (P = .0143). Yet, there were no significant relationships between the incidence of tunnel enlargement and the postoperative Tegner score.
Previous studies have reported that excessive rehabilitation16,20 and micromotion at the tunnel aperture by a graft with suspensory fixation34,50 are the causes of tunnel enlargement. Recently, Uefuji et al48 demonstrated that younger patients have more CD34+ cells in ruptured ACLs and associated tissues, which exhibit a high proliferation and multilineage differentiation potential, especially in osteogenesis and endotheliogenesis. They suggested that when surgeons perform remnant-preserving ACL reconstruction, the age of the patients should be taken into account when predicting the healing potential. Therefore, remnant and bone quality differences may explain why AM femoral tunnel enlargement was more likely to be found in younger patients of group R than in those of group P.
There are some limitations in this study. First, because the patients were not randomized to treatment, there may be a selection bias. Second, the follow-up period was just 1 year in this study. If tunnel enlargement is more dynamic once the patient is fully active, there is the potential of enlargement later than 1 year after surgery. Third, we could not estimate bone tunnel enlargement at the tunnel outlet on the intra-articular bone surface because it was difficult to measure the tunnel diameter on 3D CT, as the diameter was changed by imaging conditions. Fourth, the follow-up rate might not be highly regarded because it was 88.6% and 85.1% in groups R and P, respectively. Fifth, we did not determine the interobserver variation in radiographic measurements. Sixth, although the between-group difference in the time from injury to surgery did not reach statistical significance, this is worth mentioning because the time intervals were not the same between groups. However, beyond these limitations, the present study provides orthopaedic surgeons with important information on anatomic double-bundle ACL reconstruction with hamstring tendons.
Conclusion
The present study showed that the preservation of ACL remnant tissue can reduce enlargement of the femoral AM tunnel after anatomic double-bundle ACL reconstruction at 1 year after surgery. This is an advantage of remnant-preserving ACL reconstruction. The present study confirmed that there were no significant differences in the locations of the created tunnels and the incidence of tunnel coalition between the remnant-resecting and remnant-preserving procedures. This is an important feature of the remnant-preserving procedure used in the present study.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the institutional review board at Hokkaido University Hospital.
References
- 1. Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002;73(3):330–334. [DOI] [PubMed] [Google Scholar]
- 2. Ahn JH, Lee SH, Choi SH, Lim TK. Magnetic resonance imaging evaluation of anterior cruciate ligament reconstruction using quadrupled hamstring tendon autografts: comparison of remnant bundle preservation and standard technique. Am J Sports Med. 2010;38(9):1768–1777. [DOI] [PubMed] [Google Scholar]
- 3. Araki D, Kuroda R, Matsumoto T, et al. Three-dimensional analysis of bone tunnel changes after anatomic double-bundle anterior cruciate ligament reconstruction using multidetector-row computed tomography. Am J Sports Med. 2014;42(9):2234–2241. [DOI] [PubMed] [Google Scholar]
- 4. Bayar A, Sarikaya S, Keser S, Ozdolap S, Tuncay I, Ege A. Regional bone density changes in anterior cruciate ligament deficient knees: a DEXA study. Knee. 2008;15(5):373–377. [DOI] [PubMed] [Google Scholar]
- 5. Berg EE, Pollard ME, Kang Q. Interarticular bone tunnel healing. Arthroscopy. 2001;17(2):189–195. [DOI] [PubMed] [Google Scholar]
- 6. Bernard M, Hertel P, Hornung H, Cierpinski T. Femoral insertion of the ACL: radiographic quadrant method. Am J Knee Surg. 1997;10(1):14–21. [PubMed] [Google Scholar]
- 7. Buelow JU, Siebold R, Ellermann A. A prospective evaluation of tunnel enlargement in anterior cruciate ligament reconstruction with hamstrings: extracortical versus anatomical fixation. Knee Surg Sports Traumatol Arthrosc. 2002;10(2):80–85. [DOI] [PubMed] [Google Scholar]
- 8. Chen T, Zhang P, Chen J, Hua Y, Chen S. Long-term outcomes of anterior cruciate ligament reconstruction using either synthetics with remnant preservation or hamstring autografts: a 10-year longitudinal study. Am J Sports Med. 2017;45(12):2739–2750. [DOI] [PubMed] [Google Scholar]
- 9. Choi S, Kim MK, Kwon YS, Kang H. Clinical and arthroscopic outcome of single bundle anterior cruciate ligament reconstruction: comparison of remnant preservation versus conventional technique. Knee. 2017;24(5):1025–1032. [DOI] [PubMed] [Google Scholar]
- 10. Clatworthy MG, Annear P, Bulow JU, Bartlett RJ. Tunnel widening in anterior cruciate ligament reconstruction: a prospective evaluation of hamstring and patella tendon grafts. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):138–145. [DOI] [PubMed] [Google Scholar]
- 11. Crain EH, Fithian DC, Paxton EW, Luetzow WF. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy. 2005;21(1):19–24. [DOI] [PubMed] [Google Scholar]
- 12. Darabos N, Haspl M, Moser C, Darabos A, Bartolek D, Groenemeyer D. Intraarticular application of autologous conditioned serum (ACS) reduces bone tunnel widening after ACL reconstructive surgery in a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2011;19(suppl 1):S36–S46. [DOI] [PubMed] [Google Scholar]
- 13. Getelman MH, Friedman MJ. Revision anterior cruciate ligament reconstruction surgery. J Am Acad Orthop Surg. 1999;7(3):189–198. [DOI] [PubMed] [Google Scholar]
- 14. Gohil S, Annear PO, Breidahl W. Anterior cruciate ligament reconstruction using autologous double hamstrings: a comparison of standard versus minimal debridement techniques using MRI to assess revascularisation. A randomised prospective study with a one-year follow-up. J Bone Joint Surg Br. 2007;89(9):1165–1171. [DOI] [PubMed] [Google Scholar]
- 15. Hantes ME, Liantsis AK, Basdekis GK, Karantanas AH, Christel P, Malizos KN. Evaluation of the bone bridge between the bone tunnels after anatomic double-bundle anterior cruciate ligament reconstruction: a multidetector computed tomography study. Am J Sports Med. 2010;38(8):1618–1625. [DOI] [PubMed] [Google Scholar]
- 16. Hantes ME, Mastrokalos DS, Yu J, Paessler HH. The effect of early motion on tibial tunnel widening after anterior cruciate ligament replacement using hamstring tendon grafts. Arthroscopy. 2004;20(6):572–580. [DOI] [PubMed] [Google Scholar]
- 17. Hayward AL, Deehan DJ, Aspden RM, Sutherland AG. Analysis of sequential cytokine release after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(10):1709–1715. [DOI] [PubMed] [Google Scholar]
- 18. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226–234. [DOI] [PubMed] [Google Scholar]
- 19. Hexter AT, Thangarajah T, Blunn G, Haddad FS. Biological augmentation of graft healing in anterior cruciate ligament reconstruction. Bone Joint J. 2018;100(3):271–284. [DOI] [PubMed] [Google Scholar]
- 20. Hoher J, Moller HD, Fu FH. Bone tunnel enlargement after anterior cruciate ligament reconstruction: fact or fiction? Knee Surg Sports Traumatol Arthrosc. 1998;6(4):231–240. [DOI] [PubMed] [Google Scholar]
- 21. Iorio R, Vadala A, Di Vavo I, et al. Tunnel enlargement after anterior cruciate ligament reconstruction in patients with post-operative septic arthritis. Knee Surg Sports Traumatol Arthrosc. 2008;16(10):921–927. [DOI] [PubMed] [Google Scholar]
- 22. Jagodzinski M, Geiges B, von Falck C, et al. Biodegradable screw versus a press-fit bone plug fixation for hamstring anterior cruciate ligament reconstruction: a prospective randomized study. Am J Sports Med. 2010;38(3):501–508. [DOI] [PubMed] [Google Scholar]
- 23. Jarvela T, Moisala AS, Paakkala T, Paakkala A. Tunnel enlargement after double-bundle anterior cruciate ligament reconstruction: a prospective, randomized study. Arthroscopy. 2008;24(12):1349–1357. [DOI] [PubMed] [Google Scholar]
- 24. Kawaguchi Y, Kondo E, Kitamura N, Kai S, Inoue M, Yasuda K. Comparisons of femoral tunnel enlargement in 169 patients between single-bundle and anatomic double-bundle anterior cruciate ligament reconstructions with hamstring tendon grafts. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1249–1257. [DOI] [PubMed] [Google Scholar]
- 25. Kawaguchi Y, Kondo E, Onodera J, et al. Tunnel enlargement and coalition after anatomic double-bundle anterior cruciate ligament reconstruction with hamstring tendon autografts: a computed tomography study. Orthop J Sports Med. 2013;1(1):2325967113486441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawaguchi Y, Kondo E, Takeda R, Akita K, Yasuda K, Amis AA. The role of fibers in the femoral attachment of the anterior cruciate ligament in resisting tibial displacement. Arthroscopy. 2015;31(3):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitamura N, Yasuda K, Yokota M, et al. The effect of intraoperative graft coverage with preserved remnant tissue on the results of the pivot-shift test after anatomic double-bundle anterior cruciate ligament reconstruction: quantitative evaluations with an electromagnetic sensor system. Am J Sports Med. 2017;45(10):2217–2225. [DOI] [PubMed] [Google Scholar]
- 28. Kondo E, Merican AM, Yasuda K, Amis AA. Biomechanical analysis of knee laxity with isolated anteromedial or posterolateral bundle-deficient anterior cruciate ligament. Arthroscopy. 2014;30(3):335–343. [DOI] [PubMed] [Google Scholar]
- 29. Kondo E, Merican AM, Yasuda K, Amis AA. Biomechanical comparisons of knee stability after anterior cruciate ligament reconstruction between 2 clinically available transtibial procedures: anatomic double bundle versus single bundle. Am J Sports Med. 2010;38(7):1349–1358. [DOI] [PubMed] [Google Scholar]
- 30. Kondo E, Yasuda K, Azuma H, Tanabe Y, Yagi T. Prospective clinical comparisons of anatomic double-bundle versus single-bundle anterior cruciate ligament reconstruction procedures in 328 consecutive patients. Am J Sports Med. 2008;36(9):1675–1687. [DOI] [PubMed] [Google Scholar]
- 31. Kondo E, Yasuda K, Onodera J, Kawaguchi Y, Kitamura N. Effects of remnant tissue preservation on clinical and arthroscopic results after anatomic double-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43(8):1882–1892. [DOI] [PubMed] [Google Scholar]
- 32. Kosy JD, Mandalia VI. Anterior cruciate ligament mechanoreceptors and their potential importance in remnant-preserving reconstruction: a review of basic science and clinical findings. J Knee Surg. 2018;31(8):736–746. [DOI] [PubMed] [Google Scholar]
- 33. Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008;24(5):560–568. [DOI] [PubMed] [Google Scholar]
- 34. L’Insalata JC, Klatt B, Fu FH, Harner CD. Tunnel expansion following anterior cruciate ligament reconstruction: a comparison of hamstring and patellar tendon autografts. Knee Surg Sports Traumatol Arthrosc. 1997;5(4):234–238. [DOI] [PubMed] [Google Scholar]
- 35. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150–154. [DOI] [PubMed] [Google Scholar]
- 36. Matsumoto T, Kuroda R, Matsushita T, et al. Reduction of tunnel enlargement with use of autologous ruptured tissue in anterior cruciate ligament reconstruction: a pilot clinical trial. Arthroscopy. 2014;30(4):468–474. [DOI] [PubMed] [Google Scholar]
- 37. Nakano N, Matsumoto T, Takayama K, et al. Age-dependent healing potential of anterior cruciate ligament remnant-derived cells. Am J Sports Med. 2015;43(3):700–708. [DOI] [PubMed] [Google Scholar]
- 38. Nishio Y, Kondo E, Onodera J, et al. Double-bundle anterior cruciate ligament reconstruction using hamstring tendon hybrid grafts in patients over 40 years of age: comparisons between different age groups. Orthop J Sports Med. 2018;6(5):2325967118773685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Novaretti JV, Astur DC, Casadio D, et al. Higher gene expression of healing factors in anterior cruciate ligament remnant in acute anterior cruciate ligament tear. Am J Sports Med. 2018;46(7):1583–1591. [DOI] [PubMed] [Google Scholar]
- 40. Robinson J, Huber C, Jaraj P, Colombet P, Allard M, Meyer P. Reduced bone tunnel enlargement post hamstring ACL reconstruction with poly-L-lactic acid/hydroxyapatite bioabsorbable screws. Knee. 2006;13(2):127–131. [DOI] [PubMed] [Google Scholar]
- 41. Segawa H, Koga Y, Omori G, Sakamoto M, Hara T. Influence of the femoral tunnel location and angle on the contact pressure in the femoral tunnel in anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31(3):444–448. [DOI] [PubMed] [Google Scholar]
- 42. Siebold R. Observations on bone tunnel enlargement after double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2007;23(3):291–298. [DOI] [PubMed] [Google Scholar]
- 43. Siebold R, Cafaltzis K. Differentiation between intraoperative and postoperative bone tunnel widening and communication in double-bundle anterior cruciate ligament reconstruction: a prospective study. Arthroscopy. 2010;26(8):1066–1073. [DOI] [PubMed] [Google Scholar]
- 44. Siebold R, Kiss ZS, Morris HG. Effect of compaction drilling during ACL reconstruction with hamstrings on postoperative tunnel widening. Arch Orthop Trauma Surg. 2008;128(5):461–468. [DOI] [PubMed] [Google Scholar]
- 45. Sun L, Zhou X, Wu B, Tian M. Inhibitory effect of synovial fluid on tendon-to-bone healing: an experimental study in rabbits. Arthroscopy. 2012;28(9):1297–1305. [DOI] [PubMed] [Google Scholar]
- 46. Tegner Y, Lysholm J, Lysholm M, Gillquist J. A performance test to monitor rehabilitation and evaluate anterior cruciate ligament injuries. Am J Sports Med. 1986;14(2):156–159. [DOI] [PubMed] [Google Scholar]
- 47. Tsuda E, Ishibashi Y, Fukuda A, Yamamoto Y, Tsukada H, Ono S. Tunnel position and relationship to postoperative knee laxity after double-bundle anterior cruciate ligament reconstruction with a transtibial technique. Am J Sports Med. 2010;38(4):698–706. [DOI] [PubMed] [Google Scholar]
- 48. Uefuji A, Matsumoto T, Matsushita T, et al. Age-related differences in anterior cruciate ligament remnant vascular-derived cells. Am J Sports Med. 2014;42(6):1478–1486. [DOI] [PubMed] [Google Scholar]
- 49. Vadala A, Iorio R, De Carli A, et al. The effect of accelerated, brace free, rehabilitation on bone tunnel enlargement after ACL reconstruction using hamstring tendons: a CT study. Knee Surg Sports Traumatol Arthrosc. 2007;15(4):365–371. [DOI] [PubMed] [Google Scholar]
- 50. Webster KE, Feller JA, Hameister KA. Bone tunnel enlargement following anterior cruciate ligament reconstruction: a randomised comparison of hamstring and patellar tendon grafts with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2001;9(2):86–91. [DOI] [PubMed] [Google Scholar]
- 51. Wilson TC, Kantaras A, Atay A, Johnson DL. Tunnel enlargement after anterior cruciate ligament surgery. Am J Sports Med. 2004;32(2):543–549. [DOI] [PubMed] [Google Scholar]
- 52. Wu B, Zhao Z, Li S, Sun L. Preservation of remnant attachment improves graft healing in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(8):1362–1371. [DOI] [PubMed] [Google Scholar]
- 53. Xie GM, Huang Fu XQ, Zhao JZ. The effect of remnant preservation on patterns of gene expression in a rabbit model of anterior cruciate ligament reconstruction. J Surg Res. 2012;176(2):510–516. [DOI] [PubMed] [Google Scholar]
- 54. Yanagisawa S, Kimura M, Hagiwara K, et al. The remnant preservation technique reduces the amount of bone tunnel enlargement following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(2):491–499. [DOI] [PubMed] [Google Scholar]
- 55. Yasuda K, Kondo E, Ichiyama H, et al. Anatomic reconstruction of the anteromedial and posterolateral bundles of the anterior cruciate ligament using hamstring tendon grafts. Arthroscopy. 2004;20(10):1015–1025. [DOI] [PubMed] [Google Scholar]
- 56. Yasuda K, Kondo E, Ichiyama H, Tanabe Y, Tohyama H. Clinical evaluation of anatomic double-bundle anterior cruciate ligament reconstruction procedure using hamstring tendon grafts: comparisons among 3 different procedures. Arthroscopy. 2006;22(3):240–251. [DOI] [PubMed] [Google Scholar]
- 57. Yasuda K, Kondo E, Kitamura N, Kawaguchi Y, Kai S, Tanabe Y. A pilot study of anatomic double-bundle anterior cruciate ligament reconstruction with ligament remnant tissue preservation. Arthroscopy. 2012;28(3):343–353. [DOI] [PubMed] [Google Scholar]
- 58. Zantop T, Petersen W. [Arthroscopic filling of misplaced and wide bone tunnels after reconstruction of the anterior cruciate ligament with bone graft in patients with recurrent instability] [published online September 18, 2011]. Oper Orthop Traumatol. doi:10.1007/s00064-011-0029-7 [Google Scholar]
- 59. Zhang Q, Zhang S, Cao X, Liu L, Liu Y, Li R. The effect of remnant preservation on tibial tunnel enlargement in ACL reconstruction with hamstring autograft: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):166–173. [DOI] [PubMed] [Google Scholar]
- 60. Zysk SP, Fraunberger P, Veihelmann A, et al. Tunnel enlargement and changes in synovial fluid cytokine profile following anterior cruciate ligament reconstruction with patellar tendon and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc. 2004;12(2):98–103. [DOI] [PubMed] [Google Scholar]