Abstract
Introduction
Gastric Carcinoid Tumors (GCT) are very rare in general population, but some studies evidenced a higher incidence among bariatric surgery patients. Laparoscopic Sleeve Gastrectomy (LSG) is a widely accepted procedure for the surgical treatment of morbid obesity. LSG acts both in reducing food intake and interfering with hormonal balance in the gut-brain axis. In these patients, incidental GCT diagnosis can occur both during pre-bariatric surgery investigation and during post-operative follow-up.
Methods
We retrospectively analyzed the database of obesity patients submitted to LSG in two different centers to find out incidence of GCT in patients treated by surgery from May 2013 to March 2018.
Results
From the 560 obese consecutive patients underwent LSG, we recorded two cases of patients with GCT (0.36%): the case 1 was a patient who had a pre-operative diagnosis of GTC receiving a curative LSG which totally included the carcinoid in the resected portion; the case 2 was a patient that received a curative endoscopic resection 42 months after LSG.
Discussion
the predisposing factors that can correlate GCT with obesity and LSG and in particular the hormonal changes have been discussed. We illustrated our experience about the management of these tumors in obese patients.
Conclusion
there are neither certain data which evidence a correlation between obesity and GCT, nor data to support the hypothesis of a higher incidence of GCT after bariatric surgery. Based on our experience in obese patients the finding of GCT in the pre-operatory phase is not an absolute contraindication for bariatric surgery.
Keywords: Obesity, Sleeve gastrectomy, Carcinoid, Gastrin, GLP-1
Highlights
-
•
Recent observations showed a high incidence of Gastric Carcinoid Tumor (GCT) in candidate patients for bariatric surgery.
-
•
From a multicenter experience with Laparoscopic Sleeve Gastrectomy.
-
•
We retrospectively recorded two GCT cases in obese patients (the first found out during pre-operative investigations and the second one detected 52 months after surgery).
-
•
The possible correlations between obesity, LSG and GTC have been discussed.
1. Introduction
Today, LSG is the most frequently used procedure for obesity [1]. Patients treated with this procedure need a careful study of the stomach by upper gastrointestinal endoscopy (UGIE) both before surgery and in the postoperative period. Recent observations showed a higher incidence of Gastric Carcinoid Tumor (GCT) in candidate patients for bariatric surgery in comparison with general population [2]. However, there are no exhaustive studies in Literature at this regard, and only few Authors [3] reported cases of GCT after LSG. From a multicenter experience with LSG, we retrospectively recorded two GCT cases in obese patients. The aims of this study were to calculate the GTC incidence in a cohort of LSG and investigate the hypothetical correlations between obesity, LSG and GTC. The available treatments of GCT in obese patients candidate or submitted to LSG was also discussed. The work has been reported in line with PROCESS criteria [4].
2. Methods
We retrospectively analyzed the database of obesity patients submitted to LSG in two different centers (Surgical Obesity Center (CCO), Clinique Saint Michel, Toulon, France, and Surgical Division of University of L'Aquila) to find out incidence of GCT in patients treated by surgery from May 2013 to March 2018. Indications and pre-intervention procedures in bariatric surgery patients followed the Guidelines of American Society for Metabolic and Bariatric Surgery [5]. This study has been registered on the Research Registry with the Unique Identifying Number 4149.
3. Results
From the 560 obese consecutive patients underwent LSG, we recorded two cases of patients with GCT (incidence approximately 0.36%): the first one was a patient in which the diagnosis was made during pre-operative investigations by endoscopy (Case 1) and the second one was a patient in which GCT was detected 42 months after surgery during endoscopic follow-up (Case 2). Both surgical and endoscopic procedures were performed by senior skilled Physicians.
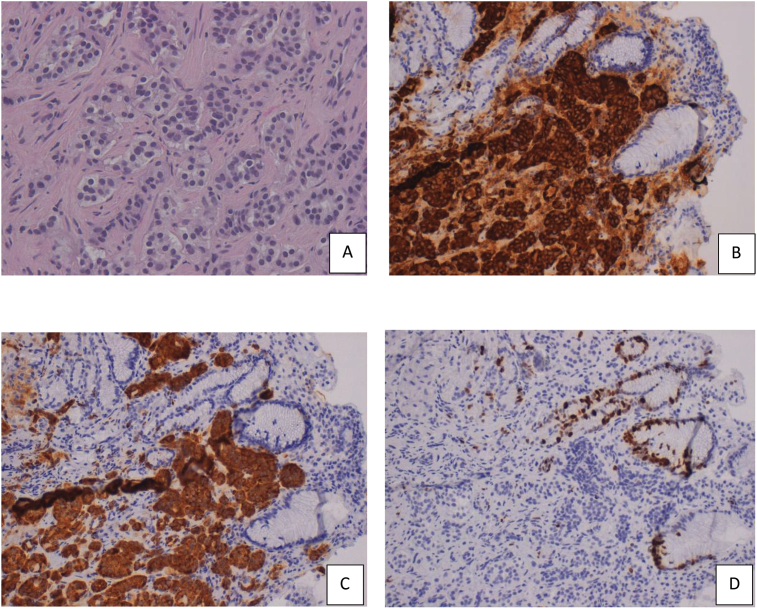
Case 1: a 40-years-old woman, candidate for LSG for morbid obesity (BMI: 41.2 kg/m2), had an UGIE before surgery. The endoscopy showed a nodule on the greater gastric curvature of 1.8 × 2 cm, and other two small polypoid lesions (<0.5 cm) in the antrum. An “en-bloc” excision of the two antral lesions have been performed, together with a biopsy of the bigger lesion. The histopathological examination evidenced a well-differentiated type I carcinoid, characterized by cells with abundant granular cytoplasm and oval nuclei. The immunohistochemical exam found out positivity for chromogranin, synaptophysin, and low Ki-67 nuclear proliferative index (<1%) (Fig. 1). The total body CT scan confirmed the masses on the greater curvature. The octreotide scan did not show further metastatic lesions. Plasma chromogranin A levels were 5.1 ng/ml (normal range 0–6 ng/ml) and gastrin levels were 862 pg/mL (normal range: 0–155). The patient, informed about the possibility of obtain by a bariatric procedure also a curative resection of the carcinoid, provided informed consent. The procedure was performed by the most skill surgeon in bariatric surgery present in our group (SC) and totally included the carcinoid in resected specimen. Post-operative course was uneventful.
Fig. 1.
Histological sections and immunohistochemistry of well-differentiated GCT A) H&E stain (x 40); B) positivity for chromogranin (x 20); C) positivity for synaptophysin (x 20); D) Ki-67 nuclear proliferative index <1% (x 20).
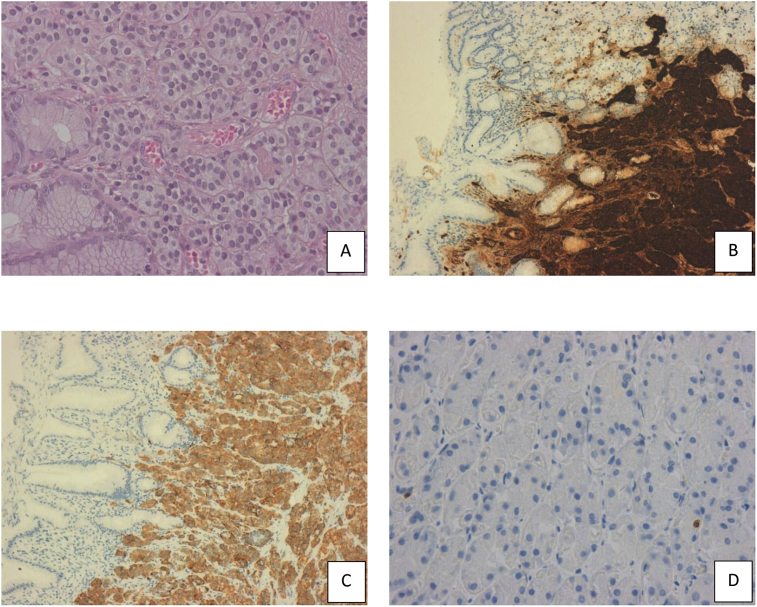
Case 2: a 45-years-old man underwent LSG for morbid obesity (BMI: 42.7 kg/m2). 42 months after surgery, he began to suffer from postprandial dyspepsia. The UGIE showed a polypoid mass of 1.5 cm in the antral region not described in the previous endoscopic exam performed 12 months after surgery. The biopsy-immunohistochemical examination showed a type I GTC, positive for chromogranin, synaptophysin and low Ki-67 nuclear proliferative index (<1%) (Fig. 2). Plasma chromogranin A levels were 7.2 ng/ml (normal range 0–6 ng/ml) and gastrin levels were 529 pg/mL (normal range 0–155). The Computed Tomography and octreoide scan confirmed the neoplasia, excluding the presence of metastasis. The Endoscopic Ultrasonography evidenced a mucosal-limited infiltration. The patient informed about the possibility of radical removal of the gastric lesion by endoscopy, provided informed consent. The endoscopic resection was performed by a skill endoscopist. The post-operative course was uneventful.
Fig. 2.
Histological sections and immunohistochemistry of well-differentiated GCT: A) H&E stain (x 40); B) positivity for chromogranin (x 10); C) positivity for synaptophysin (x 10); D) Ki-67 nuclear proliferative index <1% (x 40).
Follow-up: For both patients we had a free-from-disease survival of about three years after surgery.
4. Discussion
Carcinoid is a rare neuroendocrine neoplasm. Gastric Carcinoid represents the 4% of the total locations of this tumor and less than 1% of all gastric tumors [6]. This neoplasia is commonly classified in three types: the type I has an incidence of 70–80% associated with atrophic gastritis and pernicious anemia; the type II, having an incidence of 15–25%, is associated with Zollinger Ellison Syndrome or the MEN1 Syndrome; the type III has an incidence of 5–10% and it is the rarest. The type I and II are usually small and multiples, commonly located in mucosa and submucosa and associated with hypergastrinaemia and a low metastatic risk. The type III is associated to normal gastrinaemia and a high metastatic risk. Serum chromogranin A levels can help for the diagnosis. At the endoscopy these lesions can be nodular or polypoid. The echoendoscopy is essential to evaluate the invasion of the lower layers, and it allows the distinction with stromal tumors (GIST, lieiomyomas), which are very similar at the endoscopy [2]. In these cases, the echoendoscopy together with the histopathology is needed for the differential diagnosis. The immunohistochemistry plays a fundamental role at this regard since it allows a diagnosis of certainty, by finding out positivity for Synaptophysin, chromogranin, CD56 and NSE, with consistent immunonegativity for SMA, CD34 and CD117 (typically positive for gastric GIST) [7,8]. Carcinoid incidence in general population is 0.0001–0.0025%. This incidence is higher in patients who will undergo to bariatric surgery (0.3–0.6%) [8,9]. Our data (0.36% of incidence) are in line with the Literature. About the 83% of all cases is represented by the type I [2]. A Study on obese diabetic rats submitted to sleeve gastrectomy showed an antral G cells hyperplasia after the surgical procedure [10]. A reduction of these cells has been demonstrated after dietary restrictions, with the conclusion that hyperplasia had been provoked by hyperphagia according to unknown mechanisms. Other Authors [3,11] suggest a different explanation: all patients who have to undergo bariatric surgery need a pre-operative gastroscopy; for this reason, carcinoids are more likely to be found in these patients than in general population. Hence, obese patients could have just an increase in the incidence of diagnosis and not of the pathology itself.
If the diagnosis is done before bariatric surgery, the kind of lesion influences the treatment for gastric carcinoids. Solitary small tumors (<2 cm), especially type I and II, can be managed by endoscopic resection. Moreover, for large carcinoids (>2 cm) or multiple ones and for type III carcinoids, a high subtotal gastrectomy has been reconmended [12]. Gastric bypass, even if not contraindicated, can be a problem for subsequent endoscopic controls of the remaining stomach.
In our case we treated a type I GCT by LSG performing in a same time a bariatric procedure and a curative resection of the carcinoid. The patient was free-from-disease three years after surgery.
LSG, in selected cases, could be a procedure curative also for GCT.
Based on our knowledge, the incidence of gastric carcinoids after bariatric surgery has not been fully determined. It has been assumed that hormonal changes induced by LSG can alter gastin secretion [2,13,14]. LSG showed excellent results in glicaemic control [13,15]. Several studies [[15], [16], [17]] showed antidiabetic effects, explaining them with the increase in GLP-1 production by the terminal ileum. Basso et al. [13] evidenced a correlation between gastrin and GLP-1, defining it “Gastric Hypothesis”. According to this hypothesis, in a first phase after LSG the reduction in HCl gastric production would increase the release of antral gastrin, responsible for GLP-1 secretion by ileal L-cells. To support this theory, Grong et al. [18] evidenced a hypergastrinaemia after LSG in diabetics mices. When the GCT is diagnosed after bariatric surgery, the possible treatments are endoscopic resection, partial gastrectomy or total gastrectomy in case of multiple and widespread neoplasms or in type III carcinoid [2]. In our patient an endoscopic resection in the previous gastric tubulization was curative.
In conclusion there are neither certain data which evidence a correlation between obesity and GCT, nor data to support the hypothesis of a higher incidence of GCT after bariatric surgery. Based on our experience in obese patients [19,20], the finding of neuroendocrine tumors in the pre-operatory phase is not a contraindication for bariatric surgery.
However, the relative rarity of GCT and the low cases recorded in our series do not allow us to draw any definitive conclusions on the possible relation between obesity, LSG and GCT. Future studies on metabolic alterations induced by bariatric surgery and their possible carcinogenic implications are needed. The influence of these hormonal alterations on the risk of development of neuroendocrine tumors in the human model is a question of debate that deserves to be investigated.
Provenance and peer review
Not commissioned, peer reviewed.
Conflicts of interest
All the authors declare that they have no conflict of interest.
Sources of funding
All the authors declare that they have no source of funding.
Ethical approval
Not applicable.
Consent
There is no need for ethical approval because it is a case report. Written informed consent was obtained from the patient for publication of this case report and any accompanying imagesAuthor contribution.
Author contribution
Sista F, Abruzzese V and Clementi M contributed the original idea and steasure of the manuscript.
Carandina S contributed by conceptualization and performing the surgical procedures.
Salvatorelli A, Di Furia M, Cipolloni G and Vicentini V contributed by collecting all the data.
Guadagni S. and ClementinM contributed by conceptualization and revision of the manuscript.
The final manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Research registration number
Research Registry 4149.
Guarantor
Prof Marco Clementi, MD.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2018.09.010.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Angrisani L., Santonicola A., Iovino P. IFSO Worldwide Survey 2016: primary, endoluminal and revisional procedures. Obes. Surg. 2018 doi: 10.1007/s11695-018-3450-2. [DOI] [PubMed] [Google Scholar]
- 2.Erim T., Colak Y., Szomstein S. Gastric carcinoid tumor after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2015;11(6):e51–52. doi: 10.1016/j.soard.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Al-Harbi O., Shakir M., Al-Brahim N. Gastric carcinoid and obesity: association or coincidence? Report of two cases and literature review. Case Rep Gastrointest Med. 2013;15 doi: 10.1155/2013/848075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agha R.A., Fowler A.J., Rajmohan S., Barai I., Orgill DP for the PROCESS Group Preferred reporting of case series in surgery; the PROCESS guidelines. Int. J. Surg. 2016;36:319–323. doi: 10.1016/j.ijsu.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Mechanick J.I., Youdim A., Jones D.B. Clinical practice guidelines for the perioperative nutritional, metabolic, and non surgical support of the bariatric surgery patient-2013 update: cosponsored by American association of clinical Endocrinologists, the obesity society, and American society for metabolic and bariatric surgery. Obesity. 2013;21:s1–s27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vig T., Bindra M.S., Kumar R.M., Alexander S. Gastric glomus tumour misdiagnosed as gastric carcinoid: an unfamiliar Entity with aids to diagnosis and review of literature. J. Clin. Diagn. Res. 2017;11(5):ED32–ED33. doi: 10.7860/JCDR/2017/27968.9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee H.-W., Lee J.J., Yang D.H., Lee B.H. A clinicopathologic study of glomus tumour of the stomach. J. Clin. Gastroenterol. 2006;40(8):717–720. doi: 10.1097/00004836-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Zeni T.M., Frantzides C.T., Mahr C. Value of preoperative upper endoscopy in patients undergoing laparoscopic gastric bypass. Obes. Surg. 2006;16(2):142–146. doi: 10.1381/096089206775565177. [DOI] [PubMed] [Google Scholar]
- 9.Mong C., Van D.J., Morton J. Preoperative endoscopic screening for laparoscopic Roux-en-Y gastric bypass has a low yield for anatomic findings. Obes. Surg. 2008;18(9):1067–1073. doi: 10.1007/s11695-008-9600-1. [DOI] [PubMed] [Google Scholar]
- 10.Masuda T., Ohta M., Hirashita T. A comparative study of gastric banding and sleeve gastrectomy in obese rat model. Obes. Surg. 2011;21(11):1774–1780. doi: 10.1007/s11695-011-0512-0. [DOI] [PubMed] [Google Scholar]
- 11.Mottin C.C., Cruz R.P., Gomes Thomé G., Padoin A.V. Carcinoid tumors and morbid obesity. Obes. Surg. 2009;19(2):247–249. doi: 10.1007/s11695-008-9541-8. [DOI] [PubMed] [Google Scholar]
- 12.Moretto M., Mottin C.C., Padoin A.V. Gastric carcinoid tumor–incidental finding on endoscopy prior to bariatric surgery. Obes. Surg. 2008;18(6):747–749. doi: 10.1007/s11695-007-9342-5. [DOI] [PubMed] [Google Scholar]
- 13.Basso N., Capoccia D., Rizzello M. First-phase insulin secretion, insulin sensitivity, ghrelin, GLP-1, and PYY changes 72 h after sleeve gastrectomy in obese diabetic patients: the gastric hypothesis. Surg. Endosc. 2011;25(11):3540–3550. doi: 10.1007/s00464-011-1755-5. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen SD. The role of hormonal factors in weight loss and recidivism after bariatric surgery. Gastroenterol Res Pract Article ID 528450, doi: 10.1155/2013/528450. [DOI] [PMC free article] [PubMed]
- 15.Sista F., Abruzzese V., Clementi M., Carandina S., Amicucci G. Effect of resected gastric volume on ghrelin and GLP-1 plasma levels: a prospective study. J. Gastrointest. Surg. 2016;20(12):1931–1941. doi: 10.1007/s11605-016-3292-y. [DOI] [PubMed] [Google Scholar]
- 16.Sista F., Abruzzese V., Clementi M., Carandina S., Cecilia M., Amicucci G. The effect of sleeve gastrectomy on GLP-1 secretion and gastric emptying: a prospective study. Surg. Obes. Relat. Dis. 2017;13(1):7–14. doi: 10.1016/j.soard.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Sista F., Abruzzese V., Clementi M., Guadagni S., Montana L., Carandina S. Resolution of type 2 diabetes after sleeve gastrectomy: a 2-step hypothesis. Surg. Obes. Relat. Dis. 2018;14(3):284–290. doi: 10.1016/j.soard.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Grong E., Arbo I.B., Thu O.K., Kuhry E., Kulseng B., Mårvik R. The effect of duodenojejunostomy and sleeve gastrectomy on type2 diabetes mellitus and gastrin secretion in Goto–Kakizaki rats. Surg. Endosc. 2015;29(3):723–733. doi: 10.1007/s00464-014-3732-2. [DOI] [PubMed] [Google Scholar]
- 19.Schietroma M., Piccione F., Clementi M. Short- and long-term, 11–22 Years, results after laparoscopic nissen fundoplication in obese versus nonobese patients. 2017. [DOI] [PMC free article] [PubMed]
- 20.Sista F., Abruzzese V., Colozzi S., Schietroma M., Carlei F., Mattei A., Clementi M., Carandina S., Barrat C., Amicucci G. Int. Surg. J. 2016;3(1):11–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.