Abstract
Objective:
Although the purposes and outcomes of screening and diagnostic tests are different, they are often confused. Therefore, it is important to delineate the clinical concept of cancer screening tests to be clear in our communication not only among healthcare professionals but also with client populations. The aim of this study is to both describe and analyze the concept of cancer screening and explain their practical meaning in global contexts.
Methods:
Comparative case studies of cervical and liver cancer screening tests were used as the basis for developing an understanding of a specific concept (phenomenon) of cancer screening and for delineating the relationships between factors that cause screening to occur.
Results:
A cancer screening is defined as an action taken by both the patient and health-care provider to detect a possible pre-cancerous condition among healthy and asymptomatic individuals who are at sufficient risk of a specific disorder to warrant further investigation or treatment. The case study-based concept analysis has been shown to be useful for improving our understanding of the multi-dimensional nature of the concept in global contexts.
Conclusions:
New paradigms maximizing participation in cancer screening to detect diseases before symptoms are manifested rather than focusing on diagnosis and treatment of symptomatic infectious diseases need to be developed and implemented.
Keywords: Cancer nursing, cancer screening, global health disparities, health transition, sociocultural factor Introduction

Introduction
Although infectious diseases continue to afflict developing countries including Sub-Saharan African (SSA) countries, cancer is an increasing problem because of the aging and growth of the population as well as the presence of certain infectious agents such as human papillomavirus and the hepatitis B virus (HBV) which are important in cancer etiology.[1,2] The chances of surviving the onset of some common cancers depend largely on how early they are detected and how well they are treated.[1] In Africa, most people diagnosed with cancer are at the advanced stages of the disease, which means their prognosis for survival is poor.[3,4,5] Cervical cancer is highly preventable through screening programs that facilitate the detection and treatment of precancerous lesions. A single cervical cancer-screening test for women between the ages of 30 and 40 can reduce a woman's lifetime risk of developing cervical cancer by 25%–31%.[6] Cancer screening, however, is underutilized in SSA countries. A comprehensive review of cervical cancer screening in SSA has estimated that the screening coverage rates range from 2% to 20.2% in urban areas and from 0.4% to 14% in rural areas.[7] Reports from SSA countries including Malawi suggests that barriers to cervical cancer screening include cultural influences, religion, lack of knowledge about cervical cancer and cervical cancer prevention, low awareness of existing prevention procedures, lack of understanding between screening and diagnostic tests, fear of the stigma associated with a cancer diagnosis, and embarrassment about the screening procedure.[8,9,10]
A screening test is an important health prevention tool to detect early disease or risk factors for disease in large numbers of apparently healthy and asymptomatic individuals.[11,12,13] Screening tests are not intended to diagnose the disease, however, language used for screening and diagnostic tests is often used interchangeably among nurses and with their clients. Clarification of the clinical concept of screening tests is necessary to improve health communication among healthcare providers (HCPs) and target populations and to increase the utilization of cancer screening tests.
The World Health Organization (WHO)[14] defined screening for various cancers, as “screening is the presumptive identification of an unrecognized disease or defects using tests, examinations, or other procedures that can be applied rapidly.” The American Cancer Society[15] defined screening as “tests and exams used to find a disease, such as cancer, in people who do not have any symptoms.” The U. K. National Screening Committee[16] defined screening as “The systematic application of a test, or inquiry, to identify individuals at sufficient risk of a specific disorder to warrant further investigations or direct preventive action amongst persons who have not sought medical attention on account of symptoms of that disorder.” Maximum and others[17] have described that a screening test (sometimes termed surveillance) is a medical test or procedure performed on individuals from a defined asymptomatic population or population subgroup to assess the likelihood of individuals having a particular disease. The result of the screening test is an estimation of the suspicion of disease and determines whether a diagnostic test is warranted. Some examples of screening tests include the measurement of cholesterol levels in people who have no symptoms of cardiovascular disease or a regular Papanicolaou (Pap) smear for large numbers of asymptomatic women who are potentially at risk of cervical cancer. However, since large numbers of people need to be screened to identify a small number of potential cases, the cost and benefit of screening should be justified.
In contrast, the purpose of a diagnostic test is to establish the presence or absence of disease as a basis for intervention decisions for individuals with symptoms or who have had positive results from screening tests. Classic diagnostic algorithms are dichotomous, with two possible results; “positive” or “negative.”[18,19] Diagnostic testing is, therefore, different from screening. It is assumed that the diagnostic test is performed after a positive a screening test to establish a definitive diagnosis of the disease. Consider the example of an abnormal Pap smear. An abnormal test result leads to the diagnostic testing procedures of colposcopy, or loop electrosurgical excision procedure, or cone biopsy before initiating or recommending treatment. A diagnostic test is the sum of multiple tests and all evidence toward the cut-off score of the diagnostic tests is set toward high specificity, with weight given to precision and accuracy.[19]
In summary, screening is used when people are considered to be at high risk of developing a disease or a condition yet have no signs or symptoms. The goal of a diagnostic test is to establish a diagnosis of the disease. The diagnostic tests are done for specific indications (e.g., an abnormal screening test results or a predictive symptom) and are more reliable than screening tests.[19] However, the goal of screening is not to diagnose disease, but rather to the early detecting a disease to reduce its risk and effectively treat the disease in large numbers of asymptomatic but potentially at-risk individuals. Although the purpose and expected outcomes of screening and diagnostic tests are different, they are often confused and often used interchangeably in the literature and practice.[10,20,21,22,23] Therefore, it is important to delineate the concept of screening tests and to be clear in communication not only among health care professionals but also between HCPs and their clients. The questions to be addressed in this review include the followings: What is the concept of cancer screening test? Why is there confusion about cancer screening tests?
Concept Analysis Using Case Studies
A case of cervical cancer and a case of liver cancer are compared and contrasted to illustrate the concept of cancer screening tests. Case studies can inform the concept analysis and identify the links between problem, intervention, and outcome in this case for cancer screening action.[24,25,26,27] Yin argued[28] that using case studies is particularly helpful when researchers want to answer questions of how or why things work or not work in a real-life context.
Cervical Cancer Screening in Malawi
Cervical cancer is steadily increasing in Sub-Saharan Africa (SSA), with >75,000 new cases and 50,000 deaths occurring each year.[7,29,30] Malawi has the highest rate of cervical cancer (75.9/100,000) in the world, almost ten times higher than that reported in the US (8.1/100,000 in the US).[29,31] and it is the number one form of cancer detected in females. Cervical cancer accounts for 45.4% of all cancers in Malawi, and this rate is increasing.[5] Cervical cancer occurs in young women, and HIV-infected women have an increased risk for its occurrence.[32,33,34] In 2015, approximately 26.5% of Malawian women received cervical cancer screening, but only 15.9% of HIV-positive women were screened.[34]
Three most commonly used cervical cancer screening tests are the Pap smear, visual inspection with acetic acid (VIA) and visual inspection with Lugol's iodine (VILI).[35] The Pap smear was first used in 1928.[36] George Nicholas Pap is best known for creating the Pap test, commonly known as the Pap smear, which revolutionized the early detection of cervical cancer.[36] The Pap smear is a cytology-based screening test used to detect abnormal cells that may develop into cancer if left untreated and actual cancer cells.[36] In regularly screened populations, the Pap test identifies most abnormal cells before they become cancer. In low- and-middle-income countries, cytology-based programs are very difficult to implement, and where they are implemented, the screening coverage is low.[37,38]
Visual inspection methods have emerged as an effective screening tool in low-resource settings since it is economical and provides immediate results. VILI, also known as Schiller's test, uses Lugol's iodine[39] while VIA test uses 3%–5% acetic acid.[40] Visual inspection of the cervix procedures including the application of either Lugol's iodine or acetic acid to the cervix and viewing the cervix with the naked eye to identify color changes on the cervix and determine whether the test result is positive or negative. VIA or VILI is not based on cytology but rather on the see and treats method for precancerous lesions. It has emerged as a promising cost-effective method for women who live in resource-limited countries. Visual inspection methods are vision-based tests and are based on the fact that the majority of preinvasive and invasive cervical lesions are visible by the “naked-eye.”[37,38] The WHO has issued recommendations to use VIA when precancerous conditions are detected with cryotherapy.[41]
Case 1: Mrs. Malawi
Mrs. M. is in her 40s and living in a suburb of a large city of Malawi. She has been widowed for around 15 years and lives with her five children. She is illiterate in both Chichewa (the local Malawian language) and English. Mrs. M was diagnosed with an HIV infection and is on antiretroviral therapy. Mrs. M said her physician recommended that she received a cervical cancer-screening test but did not get the test done. She thought that, as a widow, she was not at risk for cervical cancer, and therefore the test was not necessary. Further, she did not feel ill. Recently, she came to the VIA clinic at the hospital because of continuous vaginal bleeding that she thought was a sexually transmitted disease (STD). The VIA screening test revealed advanced stage cervical cancer. Mrs. M never had a VIA screening though she visited the HIV clinic regularly. She did not go for a cervical cancer screening because she did not feel ill. However, when her vaginal bleeding did not stop, she finally underwent the VIA test. Her VIA results were positive with clinical visible bleeding on touch.
The first step in detecting cervical cancer is often an abnormal Pap test or VIA or VILI results. These screening tests are used to detect early abnormalities of the cervix, which, if untreated, could lead to cervical cancer in the future. While the screening test results are reported as either normal, inadequate, or abnormal, they cannot tell if there is a diagnosis of cervical cancer. Rather, the screening test will lead to further tests, which can provide a true diagnosis. Tests used for diagnostic purposes often use the same tests used for screening, i.e., the patient's medical history, a physical examination, colposcopy, and biopsies. Certain imaging studies may be included to find out if and where cancer has spread to establish a treatment plan.
Mrs. M. was diagnosed with cervical cancer via symptomatic presentation, which was continuous vaginal bleeding. It appears that Mrs. M. believed the purpose of the screening test was to diagnose problems, such as cervical cancer or STD. From this case, we can see that Mrs. M. was not able to differentiate between screening tests from diagnostic tests. Mrs. M's case can also provide insight on why Malawian women may delay seeking cervical cancer screening, and provides some evidence that the guidelines for managing cervical cancer screening for women with HIV infection are not being followed. Consequently, the late diagnosis of cervical cancer in Malawi makes cervical cancer management very difficult in health resources limited settings. A lack of knowledge about screening and diagnostic testing, health behavior more focus on infectious disease treatment, a lack of preventive health orientation as well as availability and affordability of adequate resources are barriers for cervical cancer screening in resource-limited settings.[3,9,20,42,43,44] This is certainly true in Mrs. M's case and is in line with reports from the SSA countries including Malawi.
Liver Cancer Screening in the U. S.
HBV is second only to tobacco as a known human carcinogen; it is the cause of up to 60% of primary hepatocellular carcinomas.[1,45] Despite a decrease in acute HBV infection, the prevalence and burden of chronic HBV infection remain substantially high in the U. S. An estimated 0.8–1.4 million people in the U. S. have chronic HBV, and of those people, 47% to 70% were born in other countries.[1,46,47] HBV infection is often imported to the U. S. as people emigrate from HBV-endemic areas. According to the report of the Institute of Medicine,[48] up to 65% of people living with HBV infection are unaware they are infected; less than one-half of the people with chronic HBV have been tested for HBV; among people who are aware of their HBV infection, only one-third report that they are receiving follow-up screening for chronic HBV. Moreover, levels of knowledge and awareness related to HBV are low among health-care providers and among high-risk populations such as Asian–Americans.[48,49,50]
The treatment for HBV infection among high-risk populations, such as Asian–American populations, should start with follow-up serology screening tests to detect indications of HBV status at a less advanced stage and before symptoms develop.[51,52] Treatment should be instituted at that time. All people who have chronic HBV infection should have regular blood screening tests every 6 months along with an annual ultrasound exam.[53,54] The blood screening tests measure HBV-directed medical evaluation and include Hepatitis B e antigen; HBV DNA (quantitative); and Alanine Aminotransferase, alpha-fetoprotein (AFP), which are potential markers for liver cancer. In addition to the clinical presentation, diagnosis of liver cancer often requires more sophisticated imaging modalities including ultrasound, CT scan, MRI, angiography, which have multiphasic contrast enhancement capabilities. Confirmation by a liver biopsy can be performed under circumstances when the diagnosis of liver cancer remains unclear.[52,54]
Case 2. Mr. Asian-American
Mr. AA is a 50-year-old man who emigrated from South Korea to the U. S. 10 years ago. Mr. AA holds an MBA from a high-ranking university in Korea, and his wife has a BA in fine arts. Mr. AA and his wife run a small laundry and dry cleaning business in a Midwestern city. They do not have health insurance and speak limited English. At one time, Mr. AA had health insurance, but he decided to discontinue his health insurance because he was not sick and the copayment for his clinical visits was too expensive. Over the last year, Mr. AA developed mild indigestion, so he purchased digestive medicine at a drug store to treat this condition. He also had general fatigue, a low appetite, and weight loss which his family thought was due to his workload or immigration-related stress. His wife encouraged him to visit family in Korea and have a medical exam. Health care in Korea would be less expensive than in the U. S. with no language barrier. When he arrived in Korea, he experienced more severe indigestion, itching in his legs, and mild jaundice. He scheduled a comprehensive physical examination that showed a chronic HBV infection with large primary liver cancer (>10 cm). Clinical history examination revealed that his mother was also infected with HBV and developed liver cirrhosis and died of liver cancer 10 years ago.
Given their high rate of HBV infection, and without appropriate intervention, mortality rates among Asian Americans with liver cancer will increase substantially in the near future. Chronic HBV infection usually does not produce symptoms, and people who have this infection feel healthy, even in the early stages of liver cancer,[48,53,54] and the disease can progress without patients even knowing they are infected. This is why HBV infection is called a silent killer and many cases of liver cancer are detected in the late stages, leading to a low survival rate after diagnosis (5%).[48,53] The CDC[53] recommends screening tests for all people born in regions of the world with high or moderate rates of HBV or who were born to parents from a country with high HBV rates. This includes all countries in Asia. It appears that Mr. AA failed to undergo screening for HBV because he did not realize that he was at risk, did not recognize his symptoms, nor did his HCP screen for HBV.
Mr. AA did have health insurance, which he canceled. Mr. AA and his family did not use the insurance; he never went to the hospital. He believed that unless he had serious health problems, it would not be necessary for him to see a health-care provider. His language barriers and unease regarding using the health care system in the U. S., including making an appointment with his HCP, were sociocultural factors that impacted his cancer screening decision making. He also worked long hours and perceived that getting tests at a hospital would take too much time. In short, getting a screening test was not a priority.
Cancer Screening Test versus Cancer Screening Behavior
A cancer screening test is defined as a medical test or procedure performed by HCPs to detect a possibly precancerous condition among healthy and asymptomatic individuals who are at sufficient risk of a specific disorder to warrant further investigation or treatment.[14,55] The subjects who test positive typically require further evaluation with subsequent diagnostic tests or procedures. There are five elements of cancer screening tests: (1) the screening tools/instruments; (2) testing performers, usually, HCPs; (3) the systematic application of the screening tools; (4) evaluation of the test; and (5) an abnormal test results leads to a diagnostic test and necessary treatment for positive or suspicious findings.
In keeping with our context-dependent knowledge and experiences with the two cases described above and from our researches,[21,42,44,49] we view cancer screening behavior as a context-bound and interactive social process that is influenced by individuals’ beliefs and knowledge, social support, and the nature of the health care system within the sociocultural context [Figure 1]. Empirical data have revealed that individuals decide to undergo cancer screening based on their past health experiences, their sociocultural context and the social influences of friends, family, and HCPs.[21,49,56,57,58] The sociocultural context, which refers to the personal characteristics of individuals, influences whether individuals recognize their risk of cervical cancer and seek cervical screening.[58] As for interpersonal factors, the case studies revealed cancer screening behavior as a function of a person's social network. The social networks of physician and wife in our case studies affected the individual's health behavior. Individual-level factors refer to knowledge and beliefs, including misunderstandings that are central to the way individuals construct a lay diagnosis as shown in our cases. Due to a lack of knowledge, misunderstanding and health beliefs, Mrs. M was not able to differentiate a screening test from a diagnostic test, and Mr. AA minimized the benefit and importance of cancer screening tests. Access to health care factors refers to factors that influence consumers’ access to a service, a provider or an institution including four dimensions as acceptability, affordability, availability, and quality.[59] Accessibility refers to the geographic distance and convenience of travel to health care; affordability refers to the ability to pay for cervical cancer screening services; availability refers to the presence of HCPs and appropriate health care facilities; quality refers to the extent to which the healthcare services provided to the population improves desired health outcomes. The influence of access to the healthcare systems in our two case studies is substantially different although conceptual overlaps are apparent between the two cases. In the case of Case Mrs. M, acceptability, affordability, and availability were the main contextual variables while in the case of Mr. AA acceptability, accessibility and quality were the important variables. The differences in these case studies points to the importance of understanding the contextually relevant factors that influence the behavior of the patient and to develop contextually relevant outreach efforts and health interventions specific to the targeted population.
Figure 1.
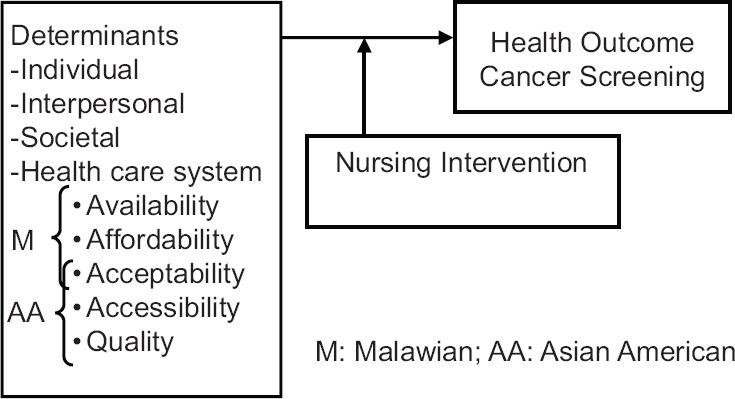
Situation-specific theory of cancer screening of Malawian and Asian American
Discussion
It seems from an analysis of these cancer case studies that although people knew that ideally, they needed to receive screening tests before they had serious symptoms, in real life, there were multiple barriers including health insurance, geographic distance from the clinic, busy schedules, lack of health resources, time, etc., that prevented them from acting on it. Moreover, there are different health transitions occurring within countries and Malawi is an example of a developing country undergoing epidemiological transition which has resulted in their health systems facing a double burden of communicable and noncommunicable disease.[2,7,34,60] Recognition, acceptance, and taking action to obtain screening tests for cancers will progress slowly in countries where communicable diseases are still prevalent. However, for countries in which infectious diseases are prevalent, it is obvious that the clinical priority is making a rapid diagnosis of an infectious disease which is accompanied with obvious acute symptoms, such as Malaria.[20,43,60] When the symptoms are less obvious, as observed in the two case studies, screening testing for prevention is not part of the health beliefs or health practice of both the HCPs and their clients.
The case study-based concept analysis was implemented to provide a framework for the observation and analysis of cancer screening test behavior among individuals with cervical cancer or liver cancer. We have used the case studies of two patients, a Malawian woman, and an Asian American man, to describe the complex situations and the social contexts that affected their attitudes and behavior concerning cancer screening. The case study-based concept analysis provides a valuable tool to identify potential antecedent factors for cancer screening behavior and the attributes of the concept of cancer screening test. Our findings have revealed that there are some factors including individual, interpersonal interactions, the health care system in which the patients and HCPs operate, and social contexts that influence patients’ cancer screening behavior [Figure 1]. The case study approach to analyze the concept of cancer screening is helpful to explain why some cancer screening behavior seems to occur effectively in some contexts but not in others. Hence, care should be taken to ensure that nursing interventions are based on contextualized knowledge rather than on purely abstract knowledge.[59,61]
Implications for Cancer Nursing
The preclinical detection of cancer is a major component of public health and oncology nursing. However, the patients and HCPs in both cases failed to use cancer screening tests. As Wilson and Jungner pointed out 50 years ago,[12] in theory screening is an admirable method of combating disease but in practice, the path to its achievement is far from simple, and there are many snags. It appears that integrating the application of cancer screening tests into existing care settings as a part of primary health care delivery can assist in managing cancers at an early stage in Malawian women with HIV infection who regularly visit the HIV clinics. As for HBV-related liver cancer screenings, the healthcare system in the U. S. is seen as uncoordinated and is driven by incentives for medical institutions rather than population needs. While outreach efforts are ongoing and critical for access to care and screening tests in the U. S., more health education programs and outreach screening programs are needed in the community, especially, for immigrant populations who face barriers related to language, lack of health insurance, and an unfamiliarity with the healthcare system in the U. S.[48,49,50,62]
The goal of cancer screening tests is to detect and prevent as well as to use the results of screening tests to make decisions about follow-up cancer diagnostic tests and subsequent treatment. Given the evidence presented here that screening failures are due to the nonparticipation of both patients and HCPs, there is an urgent need for cancer health education for both clients and HCPs including nurses and nursing students about the contextually relevant nursing intervention for cervical cancer screening and the integration of cancer screening (including cervical and liver cancer screenings) in existing health care systems and community outreach programs as well as in the nursing curriculum. With a better understanding about the multi determinants for cancer screening behavior and development of contextual nursing knowledge, (though this may require different nursing interventions across different contexts) nurses can contribute to the improvement of the use of cancer screening actions and become agents for positive change in their health care systems.
Financial support and sponsorship
This work was supported by the Fulbright Specialist Program.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Status Report on Noncommunicable Diseases. World Health Organization. 2010. [Last accessed on 2018 Aug 18]. Available from: http://www.who.int/nmh/publications/ncd_report_full_en.pdf .
- 3.Chadza E, Chirwa E, Maluwa A, Malata A, Kazembe A, Chimwaza A. Factors that contribute to delay in seeking cervical cancer diagnosis and treatment among women in Malawi. J Health Affair. 2012;4:1015–22. [Google Scholar]
- 4.Jedy-Agba E, McCormack V, Adebamowo C, Dos-Santos-Silva I. Stage at diagnosis of breast cancer in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob Health. 2016;4:e923–35. doi: 10.1016/S2214-109X(16)30259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Msyamboza KP, Manda G, Tembo B, Thambo C, Chitete L, Mindiera C, et al. Cancer survival in Malawi: A retrospective cohort study. Pan Afr Med J. 2014;19:234. doi: 10.11604/pamj.2014.19.234.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353:2158–68. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 7.Louie KS, de Sanjose S, Mayaud P. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: A comprehensive review. Trop Med Int Health. 2009;14:1287–302. doi: 10.1111/j.1365-3156.2009.02372.x. [DOI] [PubMed] [Google Scholar]
- 8.Fort VK, Makin MS, Siegler AJ, Ault K, Rochat R. Barriers to cervical cancer screening in Mulanje, Malawi: A qualitative study. Patient Prefer Adherence. 2011;5:125–31. doi: 10.2147/PPA.S17317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maseko FC, Chirwa ML, Muula AS. Health systems challenges in cervical cancer prevention program in Malawi. Glob Health Action. 2015;8:26282. doi: 10.3402/gha.v8.26282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ports KA, Reddy DM, Rameshbabu A. Cervical cancer prevention in Malawi: A qualitative study of women's perspectives. J Health Commun. 2015;20:97–104. doi: 10.1080/10810730.2014.908986. [DOI] [PubMed] [Google Scholar]
- 11.Andermann A, Blancquaert I, Beauchamp S, Déry V. Revisiting Wilson and Jungner in the genomic age: A review of screening criteria over the past 40 years. Bull World Health Organ. 2008;86:317–9. doi: 10.2471/BLT.07.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson JM, Jungner G. Principles and Practice of Screening for Disease. Geneva: WHO, Printed in France; 1968. [Google Scholar]
- 13.Morabia A, Zhang FF. History of medical screening: From concepts to action. Postgrad Med J. 2004;80:463–9. doi: 10.1136/pgmj.2003.018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. Screening for Various Cancers 2018. [Last accessed on 2018 Aug 18]. Available from: http://www.who.int/cancer/detection/variouscancer/en/
- 15.American Cancer Society. Cancer Screening Guidelines. 2018. [Last accessed on 2018 Aug 18]. Available from: https://www.cancer.org/healthy/find-cancer-early/cancer-screening-guidelines.html .
- 16.U.K. National Screening Committee. London: Department of Health; 1998. First Report of the National Screening Committee. Definitions and Classification of Population Screening Programs. Ch. 2. [Google Scholar]
- 17.Maxim LD, Niebo R, Utell MJ. Screening tests: A review with examples. Inhal Toxicol. 2014;26:811–28. doi: 10.3109/08958378.2014.955932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardinal L. Diagnostic testing in the context of high-value care: Incorporating prior probability. J Community Hosp Intern Med Perspect. 2016;6:33674. doi: 10.3402/jchimp.v6.33674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert R, Logan S, Moyer VA, Elliott EJ. Assessing diagnostic and screening tests: Part 1. Concepts. West J Med. 2001;174:405–9. doi: 10.1136/ewjm.174.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFarland DM, Gueldner SM, Mogobe KD. Integrated review of barriers to cervical cancer screening in sub-saharan africa. J Nurs Scholarsh. 2016;48:490–8. doi: 10.1111/jnu.12232. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Yang JH, Cho MO, Fawcett J. Complexity and uncertainty of living with an invisible virus of hepatitis B in Korea. J Cancer Educ. 2010;25:337–42. doi: 10.1007/s13187-010-0047-4. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen-Truong CKY, Hassouneh D, Lee-Lin F, Hsiao CY, Le TV, Tang J, et al. Health care providers’ perspectives on barriers and facilitators to cervical cancer screening in Vietnamese American women. J Transcult Nurs. 2018;29:441–8. doi: 10.1177/1043659617745135. [DOI] [PubMed] [Google Scholar]
- 23.Schleicher E. Immigrant Women and Cervical Cancer Prevention in the United States. JHU, WCHPC. 2007:1–12. [Google Scholar]
- 24.Baker GR. The contribution of case study research to knowledge of how to improve quality of care. BMJ Qual Saf. 2011;20(Suppl 1):i30–5. doi: 10.1136/bmjqs.2010.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klonoski R. The case for case studies: Deriving theory from evidence. J Business Case Stud. 2013;9:261–6. [Google Scholar]
- 26.Bennett A. Case study methods: Design, use, and comparative advantages. Case Study Method (edited Bennett & Elman) 2008. [Last accessed on 2018 Aug 18]. Available from: https://www.pdfs.semanticscholar.org/7d11/098671a75e7b289fd65adab2eb236c5cf580.pdf .
- 27.George AL. Cambridge, Massachusetts London, England: MIT Press; 2004. [Last accessed on 2018 Aug 18]. Case Studies and Theory Development in the Social Sciences. Available from: https://www.pdfs.semanticscholar.org/94e9/eec015c650880356853533c4dc9b2dac42bb.pdf . [Google Scholar]
- 28.Yin R. Case Study Research: Design and Methods. 2nd ed. Beverly Hills, CA: Sage Publishing; 1. [Google Scholar]
- 29.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 30.Barcelona (Spain): World Health Organization/Information Commissioner's Office HPV Information Centre; 2010. [Last accessed on 2018 Aug 18]. World Health Organization/Information Commissioner's Office Information Centre on HPV and Cervical Cancer (HPV Information Centre). Human Papillomavirus and Related Cancers: Summary Report 2010. Available from: http://www.screening.iarc.fr/doc/Human%20Papillomavirus%20and%20Related%20Cancers.pdf . [Google Scholar]
- 31.Msyamboza KP, Dzamalala C, Mdokwe C, Kamiza S, Lemerani M, Dzowela T, et al. Burden of cancer in malawi; common types, incidence and trends: National population-based cancer registry. BMC Res Notes. 2012;5:149. doi: 10.1186/1756-0500-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamanga NE, Hoffman GI, Ndalama B, Mapanje C, Powers KG, Chiudzu KG, et al. P5.010 prevalence and predictors of a positive cervical cancer screening test in a sexually transmitted infection clinic in Lilongwe, Malawi. Sex Transm Infect. 2013;8:338–43. [Google Scholar]
- 33.Malawi Ministry of Health. National Health Surveillance Assistant Programmer of Malawi: One Million Community Health Workers. One Million Community Health Workers. Republic of Malawi Ministry of Health. 2013 [Google Scholar]
- 34.Msyamboza KP, Phiri T, Sichali W, Kwenda W, Kachale F. Cervical cancer screening uptake and challenges in Malawi from 2011 to 2015: Retrospective cohort study. BMC Public Health. 2016;16:806. doi: 10.1186/s12889-016-3530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization, International Agency for Research on Cancer. Practical Manual on Visual Screening for Cervical Neoplasia. World Health Organization, International Agency for Research on Cancer. 2003. [Last accessed on 2018 Aug 18]. Available from: https://www.screening.iarc.fr/doc/viavilimanual.pdf .
- 36.Tan SY, Tatsumura Y. George papanicolaou (1883-1962): Discoverer of the Pap smear. Singapore Med J. 2015;56:586–7. doi: 10.11622/smedj.2015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H, Kang Y, Ju W. Cervical cancer screening in developing countries: Using visual inspection methods. Clin J Oncol Nurs. 2016;20:79–83. doi: 10.1188/16.CJON.79-83. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Geneva: World Health Organization; 2002. [Last accessed on 2018 Aug 18]. Cervical Cancer Screening in Developing Countries. Available from: http://www.who.int/cancer/media/en/cancer_cervical_37321.pdf . [Google Scholar]
- 39.Schiller W. Leucoplakia, leukokeratosis, and carcinoma of the cervix. Am J Obstet Gynecol. 1938;35:17. [Google Scholar]
- 40.World Health Organization, International Agency for Research on Cancer Handbooks on Cancer Prevention. Cervix Cancer Screening. World Health Organization, International Agency for Research on Cancer Handbooks on Cancer Prevention. 2005. [Last accessed on 2018 Aug 18]. Available from: http://www.iarc.fr/en/publications/pdfs-online/prev/handbook10/HANDBOOK10.pdf .
- 41.World Health Organization Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. World Health Organization. 2013. [Last accessed on 2018 Aug 18]. Available from: http://www.apps.who.int/iris/bitstream/handle/10665/94830/9789241548694_eng.pdf?sequence=1 . [PubMed]
- 42.Lee H, Makin MS, Mtengezo J, Malata A. VIA and challenges of a single visit approach in Malawi. Obstet Gynaecol Cases Rev. 2015;2:7–8. [Google Scholar]
- 43.Munthali AC, Ngwira BM, Taulo F. Exploring barriers to the delivery of cervical cancer screening and early treatment services in Malawi: Some views from service providers. Patient Prefer Adherence. 2015;9:501–8. doi: 10.2147/PPA.S69286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mtengezo J, Lee H. HIV-positive women's perceptions, awareness, and knowledge about cervical cancer screening in Malawi: A qualitative study. J Nurs Patient Care. 2018;3:2. [Google Scholar]
- 45.American Cancer Society. Cancer Fact & Figures. Atlanta, GA: Author; 2018. [Last accessed on 2018 Aug 18]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html . [Google Scholar]
- 46.Cohen C, Evans AA, London WT, Block J, Conti M, Block T, et al. Underestimation of chronic hepatitis B virus infection in the United States of America. J Viral Hepat. 2008;15:12–3. doi: 10.1111/j.1365-2893.2007.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee H, Levin MJ, Kim F, Warner A, Park W. Hepatitis B infection among Korean Americans in Colorado: Evidence of the need of serologic testing and vaccination. Hepat Mon. 2008;8:91–6. [Google Scholar]
- 48.Institute of Medicine. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Institute of Medicine. 2010. [Last accessed on 2018 Aug 18]. Available from: http://www.nap.edu/catalog/12793.html .
- 49.Lee H, Hann HW, Yang JH, Fawcett J. Recognition and management of HBV infection in a social context. J Cancer Educ. 2011;26:516–21. doi: 10.1007/s13187-011-0203-5. [DOI] [PubMed] [Google Scholar]
- 50.Taylor VM, Talbot J, Do HH, Liu Q, Yasui Y, Jackson JC, et al. Hepatitis B knowledge and practices among Cambodian Americans. Asian Pac J Cancer Prev. 2011;12:957–61. [PMC free article] [PubMed] [Google Scholar]
- 51.Lee H, Park W, Yang JH, You KS. Management of hepatitis B virus infection. Gastroenterol Nurs. 2010;33:120–6. doi: 10.1097/SGA.0b013e3181d72c59. [DOI] [PubMed] [Google Scholar]
- 52.Martin P, Lau DT, Nguyen MH, Janssen HL, Dieterich DT, Peters MG, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2015 update. Clin Gastroenterol Hepatol. 2015;13:2071–87.e16. doi: 10.1016/j.cgh.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. Updated CDC recommendations for the management of hepatitis B virus-infected health-care providers and students. MMWR Recomm Rep. 2012;61:1–2. [PubMed] [Google Scholar]
- 54.Terrault NA, Lok AS, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–99. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Cancer Institute. Cancer Screening Overview (PDQ®) – Health Professional Version. 2018. [Last accessed on 2018 Aug 18]. Available from: https://www.cancer.gov/about-cancer/screening/hp-screening-overview-pdq .
- 56.Juon HS, Rimal RN, Klassen A, Lee S. Social norm, family communication, and HBV screening among Asian Americans. J Health Commun. 2017;22:981–9. doi: 10.1080/10810730.2017.1388454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li S, Sim SC, Lee L, Pollack HJ, Wyatt LC, Trinh-Shevrin C, et al. Hepatitis B screening & vaccination behaviors in a community-based sample of Chinese & Korean Americans in New York city. Am J Health Behav. 2017;41:204–14. doi: 10.5993/AJHB.41.2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee H, Fawcett J, Yang JH, Hann HW. Correlates of hepatitis B virus health-related behaviors of Korean Americans: A situation-specific nursing theory. J Nurs Scholarsh. 2012;44:315–22. doi: 10.1111/j.1547-5069.2012.01468.x. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization. Global Health Force Alliance. What do We Mean by Availability, Accessibility, Acceptability and Quality of the Health Workforce? World Health Organization. 2018. [Last accessed on 2018 Aug 18]. Available from: http://www.who.int/workforcealliance/media/qa/04/en/
- 60.Lee H, Kim S, DeMarco R, Aronowitz T, Mtengezo J, Kang Y, et al. Recognizing global disparities in health and in health transitions in the 21st century: What can nurses do? Appl Nurs Res. 2015;28:60–5. doi: 10.1016/j.apnr.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Lee H, Kiang P, Kim M, Semino-Asaro S, Colten ME, Tang SS, et al. Using qualitative methods to develop a contextually tailored instrument: Lessons learned. Asia Pac J Oncol Nurs. 2015;2:192–202. doi: 10.4103/2347-5625.158018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee SY, Lee EE. Cancer screening in Koreans: A focus group approach. BMC Public Health. 2018;18:254. doi: 10.1186/s12889-018-5147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


