Abstract
Context:
Hypophosphatasia (HPP) features deficient activity of the “tissue-nonspecific” isoenzyme of alkaline phosphatase (TNSALP) due to loss-of-function mutation(s) within the TNSALP gene. Consequently, inorganic pyrophosphate, a TNSALP substrate and inhibitor of mineralization, accumulates extracellularly. This can cause rickets or osteomalacia.
Objective:
We report a 55-year-old man with HPP and chronic renal failure (CRF) requiring hemodialysis who developed severe hypercalcemia acutely after traumatic fractures and immobilization. He manifested HPP in childhood and in middle age received hemodialysis for CRF attributed to hypertension and anti-inflammatory medication. He took 2 g of calcium carbonate orally each day to bind dietary phosphorus, but never aluminum hydroxide or any form of vitamin D. Pretrauma serum levels of calcium spanned 8.4–10.7 mg/dL (normal [Nl], 8.6–10.3), inorganic phosphate 5.8–6.4 mg/dL (Nl, 2.5–4.5), and PTH 63–75 pg/mL (Nl, 10–55).
Results:
Rapid succession falls fractured multiple major bones. Six hours later, he became confused. Serum calcium was 14.9 mg/dL, ionized calcium was 7.4 mg/dL (Nl, 4.5–5.1), and PTH was 16 pg/mL. Hemodialysis quickly corrected his hypercalcemia and confusion. Low serum alkaline phosphatase persisted, and follow-up skeletal histopathology showed that his osteomalacia was severe.
Conclusion:
Hemodialysis does not heal the skeletal disease of HPP. During sudden fracture immobilization in HPP, sufficient calcium can emerge from bone, perhaps from a rapidly exchangeable calcium pool, to cause acute severe hypercalcemia if the kidneys cannot compensate for the mineral efflux. Hence, we worry that acute hypercalcemia might accompany sudden immobilization in CRF patients without HPP if they have adynamic bone disease.
Prolonged immobilization often leads to hypercalciuria, and sometimes to hypercalcemia, due to net loss of mineral from the skeleton (1–4). Immobilization hypercalcemia (IMH) is especially likely if rapid turnover has accelerated calcium egress from bone (eg, adolescence, hyperparathyroidism, hyperthyroidism, Paget disease, etc) and can be aggravated by renal failure that impairs calcium excretion (1, 5).
Hypophosphatasia (HPP) is the inborn error of metabolism caused by loss-of-function mutation(s) within the gene that encodes the “tissue-nonspecific” isoenzyme of alkaline phosphatase (TNSALP) (6). In health, TNSALP is richly expressed by chondrocytes and osteoblasts and promotes mineralization by hydrolyzing inorganic pyrophosphate (PPi), a potent inhibitor of hydroxyapatite crystal formation (7). In HPP, extracellular excesses of PPi can cause rickets or osteomalacia despite normal or sometimes elevated circulating levels of calcium and inorganic phosphate (Pi) and normal levels of vitamin D (6, 8).
We describe a potential concern for patients with chronic renal failure (CRF) and adynamic bone disease (ABD). Severe hypercalcemia occurred acutely during sudden immobilization from fractures in a man with HPP and CRF.
Patient and Methods
A 55-year-old man with HPP and CRF receiving hemodialysis was examined in our emergency department for two rapid succession falls that had broken multiple major bones. Approximately 6 hours after the trauma, he became confused without focal neurological deficits.
At age 39 years (born in 1940) in 1979 (Figure 1), he was referred to diagnose his skeletal disease that manifested in early childhood. At age 11 years, his medical record mentioned cystic changes detected radiographically in the head of both fibulas and “epiphyseal dyschondroplasia.” However, we elicited a history typical of childhood HPP (6). Premature loss of all teeth had occurred by 3 years of age (a hallmark of pediatric HPP), and he was sickly and weak during childhood. Generalized bone pain and fractures began during adolescence. At age 28 years, he started treatment for essential hypertension, and then for hyperlipidemia and hyperuricemia. We found low serum alkaline phosphatase (ALP) activity (hypophosphatasemia) of 17 IU/L (normal [Nl], 28–88), and a high urine level of the TNSALP substrate phosphoethanolamine (6) of 491 μmol/dL (Nl, 26–101) (BioScience). Radiographic skeletal survey showed characteristics of HPP in adults, including metatarsal stress fractures and a nonhealing subtrochanteric femoral pseudofracture (9), but no osteopenia (Figure 2).
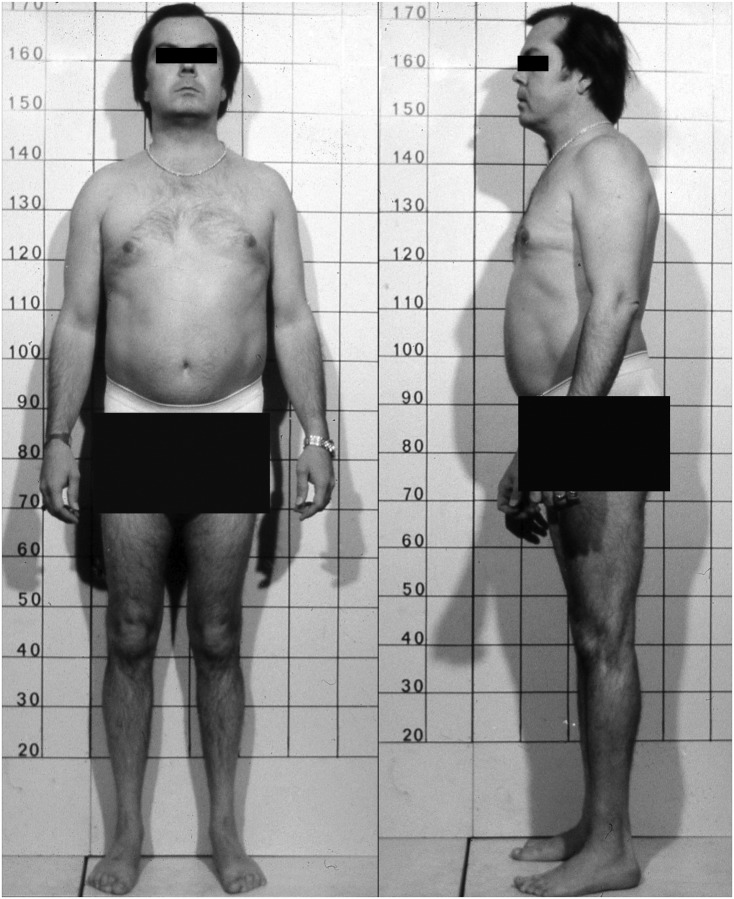
Figure 1.
Patient at diagnosis. At age 40 years and diagnosis of HPP, our patient was 170 cm (5 feet 7 inches) tall and had some apparent atrophy of the muscle of his legs.
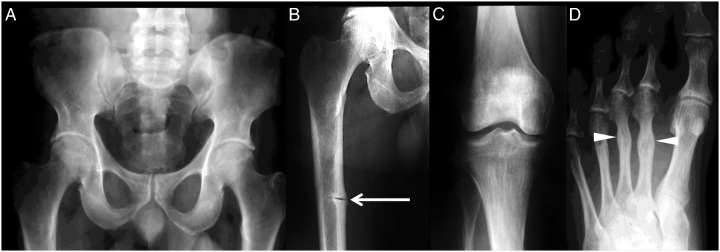
Figure 2.
Radiographic findings at diagnosis. At age 40 years, a radiographic survey of our patient's skeleton revealed findings consistent with HPP, including: unremarkable pelvis (A), yet; pseudofracture (arrow) in the medial cortex of the middle of the right femur (B); trabecular coarsening in the proximal right tibia, and to a lesser extent in the femoral condyles (note the short fibula) (C); and healed metatarsal stress fractures in the left second and third metatarsal shafts (arrowheads) (D).
At age 41 years, his femoral pseudofracture was unresponsive to vitamin D2 and calcium supplementation followed by sodium fluoride given orally. During insertion of an intramedullary rod, iliac crest biopsy confirmed osteomalacia (see Results). Six months later, the pseudofracture was only partially healed. His serum creatinine was 1.4 mg/dL (Nl, 0.8–1.3).
In 1985, when the patient was 44 years old, our discovery that pyridoxal 5′-phosphate, the major circulating form of vitamin B6, is elevated in the plasma of all HPP patients began by finding his level to be 3053 nm (Nl, 30–110) (10). His 52-year-old brother had similar clinical manifestations of HPP, serum ALP of 16 IU/L (Nl, 35–95), plasma pyridoxal 5′-phosphate of 926 nm (10), and serum creatinine of 1.6 mg/dL (Nl, 0.8–1.3).
At age 47 years, our patient underwent coronary artery bypass grafting. Nondecalcified sternum, obtained without prior tetracycline labeling, showed moderate osteomalacia but nothing to indicate secondary hyperparathyroidism.
At age 53 years, peritoneal dialysis began. At age 55 years, he received thrice weekly hemodialysis. His CRF was attributed to hypertension and nonsteroidal anti-inflammatory medication taken for bone pain. He lived independently using crutches. Calcium carbonate (Tums, 2 g/d; GlaxoSmithKline) had been prescribed by his nephrologists to bind dietary Pi, with the dose adjusted according to serum calcium levels that spanned 8.4–10.7 mg/dL. He was never given aluminum-containing compounds or any form of vitamin D. Other medications included: metoprolol 50 mg orally (po) twice a day (bid), dipyridamole 75 mg po bid, diltiazem 60 mg po three times a day, isosorbide mononitrate 60 mg po bid, lovastatin 20 mg po od, amitriptyline 20 mg po od, naproxen 250 mg po bid, polysaccharide-iron complex 150 mg po bid, and 10 000 U of epoetin alfa injected weekly. Six days before his fractures, serum calcium was 9.2 mg/dL, intact PTH was 63 pg/mL (Nl, 10–55), and aluminum was 8 ng/dL (Nl, 0–6). Three days before, he told his social worker that he felt well.
His fractures were sustained at age 55 years during two accidental rapid succession falls from standing. The first fractures included his right humerus, and then minutes later his right femur (Figure 3).
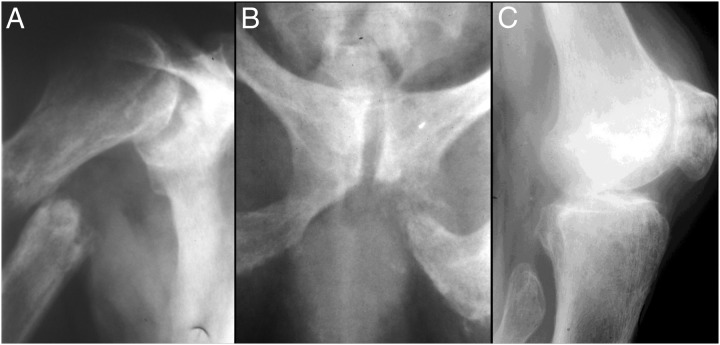
Figure 3.
Radiographs after falls and fractures. At age 55 years, radiographs showed increased osteopenia and more prominent trabecular coarsening in association with: medially displaced right proximal humeral diaphyseal fracture inferior to the surgical neck and through an area of marked focal “osteolysis” (A); fracture of the inferior left ischiopubic ramus, again through an area of focal bone lysis (B); and transverse right patellar fracture with widened irregular margins, again consistent with preexistent focal osteolysis at the location of the fracture (C). All three fractures above have irregular margins with areas of focal osteolysis. Their appearance suggests, therefore, that they are pathological fractures that occurred through preexisting lesions (likely pseudofractures). Not illustrated were an oblique fracture through the right midulnar diaphysis, oblique fracture through the right distal femoral diaphysis around his long-stem total hip prosthesis, minimally displaced transverse fractures in the right distal femoral metaphysis, and a fracture in the proximal tibial metaphysis.
Following informed written consent, TNSALP mutation analysis was performed in our laboratory by sequencing all of its coding exons and adjacent mRNA splice sites using previously reported PCR and sequencing primers and conditions (11).
Results
Six hours after the patient's fractures (which included the right humerus, ulna, femur, and tibia; left pubic ramus; and both patellas), serum calcium was 14.9 mg/dL (Nl, 8.6–10.3), creatinine was 10.7 mg/dL (Nl, 0.7–1.5), Pi was 5.0 mg/dL (Nl, 2.3–4.3), and total CO2 was 18 mmol/L (Nl, 22–32). He had become confused but without focal neurological deficits. Retesting showed serum calcium 13.5 mg/dL, ionized calcium 7.4 mg/dL (Nl, 4.5–5.1), Pi 5.6 mg/dL, intact PTH 16 pg/mL, and ALP 36 IU/L (Nl, 38–126). Serum 25-hydroxyvitamin D was not measured. Oral calcium administration was held. Additional hemodialysis using a low-calcium bath during the next week rapidly cleared his mentation and corrected his hypercalcemia within 12 hours (Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Three days after the fractures, serum calcium was 9.6 mg/dL, ionized calcium was 5.0 mg/dL, Pi was 5.3 mg/dL, intact PTH was 39 pg/mL, and calcitriol (Mayo Medical Laboratories) was 18 pg/mL (Nl, 15–60). Serum ALP activity was not increased by the skeletal trauma and was 36, 33, and 20 IU/L (Nl, 38–126) at 0, 1, and 7 days after the fractures, respectively (Supplemental Table 1). Seven days later, the patient was still unable to bear weight or transfer to a wheelchair. Our review of his skeletal radiographs spanning 15 years revealed interval development of severe diffuse osteopenia admixed with mottled sclerosis resembling renal osteodystrophy with secondary hyperparathyroidism (Figure 3). Noncontrast computed tomography showed extensive calcification of the cerebral tentorium that was somewhat increased for his age despite the inhibition of skeletal mineralization from HPP (6) (see Discussion). Nondecalcified iliac crest obtained during internal fixation of his fractured humerus showed that osteoidosis had increased, compared to his previous iliac crest biopsy. That first specimen (Figure 4, A and B), at age 41 years after two oral courses of tetracycline, documented osteomalacia including a marked increase in osteoid (osteoid surface/bone surface, 19.4%), few osteoblasts or osteoclasts, and no tetracycline fluorescence. The new specimen showed no evidence of hyperparathyroidism despite his CRF and dialysis and his radiographic changes that were in keeping with renal osteodystrophy with secondary hyperparathyroidism. Instead, the now severe osteomalacia (Figure 4, C and D) featured worse osteoidosis (osteoid surface/bone surface, 35.4%). Polarized-light microscopy documented that all bone collagen was lamellar (data not shown). Once again, low bone turnover was suggested by few osteoblasts and osteoclasts and no osteitis fibrosa. Ultraviolet microscopy demonstrated only deep “labels,” perhaps from the tetracycline taken years ago.
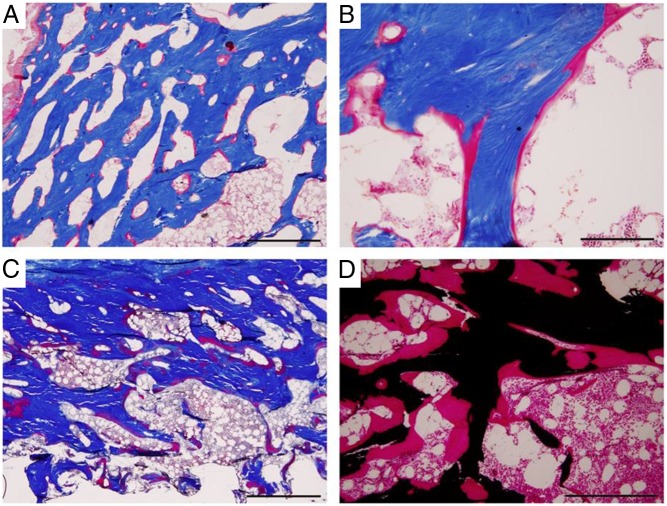
Figure 4.
Skeletal histopathology. A biopsy specimen of the patient's bone was obtained at ages 41, 47, and 55 years for histopathological assessment. Tetracycline labeling had been given for the biopsy at age 41 years. Nondecalcified sections in methyl methacrylate revealed persistent osteomalacia characterized by patchy accumulation of osteoid and a paucity of osteoclasts and osteoblasts. A and B, Iliac crest at age 41 years. A, Masson trichrome stain at low power demonstrates increased cortical porosity and osteoid surface. B, Higher magnification shows that osteoid surfaces have few osteoblasts, suggesting low turnover. C and D, Iliac crest at age 55 years. C, Masson trichrome stain again reveals increased cortical porosity and osteoid. D, Von Kossa stain demonstrates very thick osteoid with few osteoblasts. Scale bars, 1 mm, A and C; 200 μm, B; 500 μm, D.
Ten months after his fractures, the pinned humerus had not fully healed. He died at age 57 years in 1998 from recurrent sepsis. Serum ALP activity at that time was 18 IU/L (Nl, 38–126). Despite his CRF, fractures, and orthopedic procedures, hypophosphatasemia had persisted lifelong (Supplemental Table 1). His brother lived to age 67 years.
TNSALP mutation analysis showed compound heterozygosity for two mutations, an insertion/deletion in exon 4, and a missense mutation in exon 6 (c.526G>A, p.Ala176Thr). The indel mutation has not been described (12), whereas the missense mutation is common in our HPP cohort (11).
Discussion
In healthy adults, approximately 3 and 8 mg of calcium per kilogram body weight enter the extracellular space daily from the intestine and bone, respectively (13). The circulating calcium level is maintained by return to the skeleton of the amount that emerged and urinary excretion equivalent to the net amount absorbed from the diet.
In 1941, Albright et al (1) reported that loss of weight bearing can release skeletal mineral and cause IMH. It is now appreciated that hypercalciuria is especially common during prolonged immobilization (2, 14). Resorptive hypercalciuria (2) usually appears in the second week of acute immobilization and peaks in the second to third month (14). With spinal cord injury, urinary calcium is elevated by approximately the fourth week, is maximum at about 4 months, persists up to 1 year, and has resolved after 1.5 years (15). The associated trabecular and then cortical bone atrophy leads to “disuse” osteoporosis. After 3 to 6 months of immobilization, skeletal radiodensity may diminish by approximately 30% (16).
Upon paralysis, quadriplegic and paraplegic patients manifest increased bone turnover. Osteoclast numbers peak after approximately 4 months. However, in other types of immobilization, decreased bone formation and diminished osteoid thickness represent instead an ABD (16). Nevertheless, the skeletal dynamics and histopathology of all of the different types of immobilization are incompletely understood.
During immobilization, net bone resorption and release of minerals can overwhelm kidney function and cause hypercalcemia (1, 3, 4). Up to 50% of immobilized pediatric patients manifest this problem (1, 4). Remarkably, some children and adolescents develop IMH from the fracture of a single weight-bearing bone (17). Typically, accelerated bone breakdown features increased numbers of osteoclasts and disproportionate elevations of the bone turnover markers that reflect skeletal resorption vs apposition.
When renal dysfunction disables urinary calcium excretion, even minor disruptions of mineral homeostasis can cause hypercalcemia, including during the recovery phase of acute renal shutdown (5, 18), postrenal transplantation (19), and with CRF treated by dialysis (20). Years ago, CRF featured substantial secondary hyperparathyroidism with its associated skeletal manifestations (21), and before the introduction of dialysis 8% of CRF patients were hypercalcemic. However, by 1982, Evans et al (22) reported that although 14% of acute hemodialysis patients had “pure” hyperparathyroid bone disease, 25% had “pure” osteomalacia. The latter group had higher serum calcium levels. In 1986, Piraino et al (23) found that hypercalcemic dialysis patients had lower serum ALP activity, suggesting slower bone formation. In 1989, after excluding excessive calcium or vitamin D treatment, only 1% of predialysis patients were hypercalcemic (24). Approximately 20% of these patients were said to have “aplastic” or “adynamic” bone disease featuring predominantly low bone turnover with thin osteoid seams and absence of tetracycline labels (25). At first, although the radiographic findings were consistent with hyperparathyroidism, the low bone turnover was usually explained by aluminum toxicity causing osteomalacia. By 1995, ABD affected 27% and osteomalacia affected 7% of patients starting dialysis (26). Currently, hypercalcemia in CRF predicts ABD or osteomalacia, but it is now less likely from aluminum toxicity because newer Pi binders are used (27). ABD in CRF is understood to feature relatively low serum PTH levels vis-à-vis hyperparathyroid bone disease, higher serum calcium levels, and oral calcium carbonate, high-calcium dialysate, or vitamin D metabolites given to control secondary hyperparathyroidism (22, 26–28). In ABD in CRF, calcium entry into the skeleton is slower than with hyperparathyroidism, and markers of bone formation can be suppressed. Nevertheless, absorption of dietary calcium is similar (26). Currently, ABD is associated especially with diabetes, advanced age, peritoneal dialysis, and patients not yet receiving dialysis (29). In both acute and chronic renal failure, ABD seems to predispose patients to hypercalcemia (18). Now, there is concern that ABD in CRF could follow excessive doses of calcimimetics (28) and antiresorptive drugs like the bisphosphonates (30) and perhaps denosumab (31).
Despite the above understanding of renal osteodystrophy, IMH has rarely been reported in CRF, although immobilization can affect uremic bone. In 1983, Prince et al (5) described IMH “late” (ie, 10 wk) after immobilization in three men receiving dialysis. But, IMH occurred after just 3 days in one osteomalacic hemodialysis patient exposed to aluminum and taking vitamin D (5). Furthermore, immobilized burn patients have slow bone remodeling and can manifest IMH, usually after 4 weeks, but some after just 2 days, and particularly when there is renal failure (32).
Of interest, our patient's CRF, multiple fractures, and orthopedic procedures (any of which can raise serum ALP activity) did not correct his hypophosphatasemia. Increases in circulating bone ALP can follow these perturbations but did not occur, perhaps because he carried two defective TNSALP alleles. Also of interest, dialysis did not heal his severe osteomalacia. Although his serum 25-hydroxyvitamin D level was not measured at the time he fractured, his routine biochemical studies had shown adequate circulating levels of minerals and relatively low PTH levels that were against vitamin D deficiency. His worse osteomalacia at that time was instead consistent with the course of HPP during adult life (6). Any reduction of extracellular PPi levels from dialysis was apparently not sufficient to improve his skeletal mineralization. Therefore, extracellular PPi levels are probably controlled by cell surface TNSALP and not by renal excretion (6, 8). His calcification of the tentorium (a common finding with CRF) may seem paradoxical for an inborn error of metabolism that causes defective skeletal mineralization, but perhaps it reflected the enhanced mineralization of some tissues that can occur in relatively mild HPP with slight increases in extracellular PPi (7).
Our patient with HPP uniquely illustrates the importance of bone apposition together with functioning kidneys for calcium homeostasis. In severe HPP in infants and young children, hypercalcemia and hyperphosphatemia are common, and therefore, rickets might seem paradoxical (6). In adults with HPP, serum calcium levels typically are normal, but Pi levels are often elevated because of enhanced renal tubular reabsorption of Pi, possibly reflecting a role for TNSALP in renal Pi excretion (6). HPP rickets or osteomalacia would represent a type of ABD and therefore be especially vulnerable to IMH if there was CRF. In our patient with both HPP and CRF, we had no evidence that his acute hypercalcemia was due to increased intestinal absorption of calcium. We surmise that his calcium supplementation would not have been taken after his falls, fractures, and then confusion. Additionally, his hypercalcemia occurred much too quickly to have been from osteoclast-mediated resorption of his skeleton. In fact, osteoidosis in any osteomalacia such as HPP prevents osteoclasts from resorbing bone. Instead, we speculate that his novel sudden IMH associated with acute immobilization, fractures, and metabolic acidosis was derived from the hypothesized rapidly exchangeable calcium pool within the skeleton (33–35). In addition to the slowly exchangeable calcium pool principally regulated by PTH and vitamin D, a rapidly exchangeable calcium pool is postulated whereby circulating calcium levels are maintained during acute perturbations (33–35). We now know that osteocytes function in both PTH-mediated long-term calcium homeostasis and mechanical stimuli-induced rapid calcium mobilization (33–35).
Our observations should caution that oversuppression of bone remodeling in CRF (perhaps from excessive doses of potent forms of vitamin D, calcimimetics [29], bisphosphonates [30], or denosumab [31]) might lead to acute severe hypercalcemia if the patient becomes suddenly immobilized.
Supplementary Material
Acknowledgments
We dedicate this work to our patient, who taught us about HPP, and to the late Michael D. Fallon, MD, who helped to assess the histopathology. TNSALP mutation analysis was performed with the assistance of Ms Margaret Huskey. Ms Sharon McKenzie and Ms Vivienne McKenzie helped to prepare the manuscript.
This work was supported by Shriners Hospitals for Children, The Hypophosphatasia Research Fund, The Clark and Mildred Cox Inherited Metabolic Bone Disease Research Fund, The Barnes-Jewish Hospital Foundation, and The Frederick S. Upton Foundation.
Results from this work were presented in part at the Adult Bone and Mineral Working Group, 18th Annual Meeting of the American Society for Bone and Mineral Research, Seattle, Washington, September 7–11, 1996; and the Sixth International Alkaline Phosphatase Symposium, Huningue, France, May 16–19, 2012.
Disclosure Summary: M.P.W. receives research grant support and consulting fees from Alexion Pharmaceuticals, Inc, Amgen, Inc, and Ultragenyx Pharmaceutical Inc. The other authors have nothing to declare.
Abbreviations
- ABD
adynamic bone disease
- ALP
alkaline phosphatase
- bid
twice a day
- CRF
chronic renal failure
- HPP
hypophosphatasia
- IMH
immobilization hypercalcemia
- Nl
normal
- Pi
inorganic phosphate
- po
orally
- PPi
inorganic pyrophosphate
- TNSALP
tissue-nonspecific isoenzyme of ALP.
References
- 1. Albright F, Burnett CH, Cope O, Parson W. Acute atrophy of bone (osteoporosis) simulating hyperparathyroidism. J Clin Endocrinol Metab. 1941;1:711–716. [Google Scholar]
- 2. Stewart AF, Adler M, Byers CM, Segre GV, Broadus AE. Calcium homeostasis in immobilization: an example of resorptive hypercalciuria. N Engl J Med. 1982;306:1136–1140. [DOI] [PubMed] [Google Scholar]
- 3. Clouston WM, Lloyd HM. Immobilization-induced hypercalcemia and regional osteoporosis. Clin Orthop Relat Res. 1987;216:247–252. [PubMed] [Google Scholar]
- 4. Steinberg FU. The immobilized patient functional pathology and management. In: Avioli LV, ed. Topics in Bone and Mineral Disorders. New York, NY: Plenum Publishing Corporation; 1980:33–63. [Google Scholar]
- 5. Prince RL, Eisman JA, Simpson RW. Hypercalcaemia in association with renal failure: the role of immobilisation. Aust N Z J Med. 1983;13:8–10. [DOI] [PubMed] [Google Scholar]
- 6. Whyte MP. Hypophosphatasia. In: Thakker RV, Whyte MP, Eisman J, Igarashi T, eds. Genetics of Bone Biology and Skeletal Disease. San Diego, CA: Elsevier Academic Press; 2013:327–360. [Google Scholar]
- 7. Fleisch H, Russell RG, Straumann F. Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature. 1966;212:901–903. [DOI] [PubMed] [Google Scholar]
- 8. Whyte MP, Greenberg CR, Salman NJ, et al. . Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med. 2012;366:904–913. [DOI] [PubMed] [Google Scholar]
- 9. Sutton RA, Mumm S, Coburn SP, Ericson KL, Whyte MP. “Atypical femoral fractures” during bisphosphonate exposure in adult hypophosphatasia. J Bone Miner Res. 2012;27:987–994. [DOI] [PubMed] [Google Scholar]
- 10. Whyte MP, Mahuren JD, Vrabel LA, Coburn SP. Markedly increased circulating pyridoxal-5′-phosphate levels in hypophosphatasia. Alkaline phosphatase acts in vitamin B6 metabolism. J Clin Invest. 1985;76:752–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mumm S, Jones J, Finnegan P, Henthorn PS, Podgornik MN, Whyte MP. Denaturing gradient gel electrophoresis analysis of the tissue nonspecific alkaline phosphatase isoenzyme gene in hypophosphatasia. Mol Genet Metab. 2002;75:143–153. [DOI] [PubMed] [Google Scholar]
- 12. The Tissue Nonspecific Alkaline Phosphatase Gene Mutations Database. Versailles, France: SESEP Laboratory, University of Versailles-Saint Quentin; 2012. http://www.sesep.uvsq.fr/Database.html. Accessed August 14, 2012. [Google Scholar]
- 13. Auerbach GD, Marx SJ, Spiegel AM. Parathyroid hormone, calcitonin, and the calciferols. In: Williams RH, ed. Textbook of Endocrinology. 6th ed Philadelphia, PA: WB Saunders Co; 1981:922–927. [Google Scholar]
- 14. Chantraine A, Nusgens B, Lapiere CM. Bone remodeling during the development of osteoporosis in paraplegia. Calcif Tissue Int. 1986;38:323–327. [DOI] [PubMed] [Google Scholar]
- 15. Naftchi NE, Viau AT, Sell GH, Lowman EW. Mineral metabolism in spinal cord injury. Arch Phys Med Rehabil. 1980;61:139–142. [PubMed] [Google Scholar]
- 16. Minaire P, Neunier P, Edouard C, Bernard J, Courpron P, Bourret J. Quantitative histological data on disuse osteoporosis: comparison with biological data. Calcif Tissue Res. 1974;17:57–73. [DOI] [PubMed] [Google Scholar]
- 17. Rosen JF, Wolin DA, Finberg L. Immobilization hypercalcemia after single limb fractures in children and adolescents. Am J Dis Child. 1978;132:560–564. [DOI] [PubMed] [Google Scholar]
- 18. Evans RA, Bridgeman M, Hills E, Dunstan CR. Immobilisation hypercalcemia. Miner Electrolyte Metab. 1984;10:244–248. [PubMed] [Google Scholar]
- 19. Haller C, Breslau NA. Immobilization-induced hypercalcemia in a renal transplant recipient with persistent hyperparathyroidism. Am J Med. 1992;92:223–225. [DOI] [PubMed] [Google Scholar]
- 20. Drivas G, Ward M, Kerr D. Immobilization hypercalcaemia in patients on regular haemodialysis. Br Med J. 1975;3:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andress DL, Sherrard DJ. The osteodystrophy of chronic renal failure. In: Schrier R, Gottschalk C, eds. Diseases of the Kidney. 6th ed Vol. 3 Boston, MA: Little Brown; 1993:2777. [Google Scholar]
- 22. Evans RA, Flynn J, Dunstan CR, George CR, McDonnell GD. Bone metabolism in chronic renal failure. Miner Electrolyte Metab. 1982;7:207–218. [PubMed] [Google Scholar]
- 23. Piraino BM, Rault R, Greenberg A, et al. . Spontaneous hypercalcemia in patients undergoing dialysis. Etiologic and therapeutic considerations. Am J Med. 1986;80:607–615. [DOI] [PubMed] [Google Scholar]
- 24. Greenberg A, Piraino BM, Bruns FJ. Hypercalcemia in patients with advanced chronic renal failure not yet requiring dialysis. Am J Nephrol. 1989;9:205–210. [DOI] [PubMed] [Google Scholar]
- 25. Brandenburg VM, Floege J. Adynamic bone disease—bone and beyond. NDT Plus. 2008;3:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hruska KA, Teitelbaum SL. Renal osteodystrophy. N Engl J Med. 1995;333:166–174. [DOI] [PubMed] [Google Scholar]
- 27. Piraino B, Chen T, Puschett JB. Elevated bone aluminum and suppressed parathyroid hormone levels in hypercalcemic dialysis patients. Am J Nephrol. 1989;9:190–197. [DOI] [PubMed] [Google Scholar]
- 28. Malluche HH, Mawad H, Monier-Faugere MC. Effects of treatment of renal osteodystrophy on bone histology. Clin J Am Soc Nephrol. 2008;3:S157–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spasovski GB, Bervoets AR, Behets GJ, et al. . Spectrum of renal bone disease in end-stage renal failure patients not yet on dialysis. Nephrol Dial Transplant. 2003;18:1159–1166. [DOI] [PubMed] [Google Scholar]
- 30. Miller PD. Is there a role for bisphosphonates in chronic kidney disease? Semin Dial. 2007;20:186–190. [DOI] [PubMed] [Google Scholar]
- 31. McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. Am J Kidney Dis. 2012;60:626–628. [DOI] [PubMed] [Google Scholar]
- 32. Kohut B, Rossat J, Raffoul W, Lamy O, Berger MM. Hypercalcaemia and acute renal failure after major burns: an under-diagnosed condition. Burns. 2010;36:360–366. [DOI] [PubMed] [Google Scholar]
- 33. Marenzana M, Shipley AM, Squitiero P, Kunkel JG, Rubinacci A. Bone as an ion exchange organ: evidence for instantaneous cell-dependent calcium efflux from bone not due to resorption. Bone. 2005;37:545–554. [DOI] [PubMed] [Google Scholar]
- 34. Atkins GJ, Findlay DM. Osteocyte regulation of bone mineral: a little give and take. Osteoporos Int. 2012;23:2067–2079. [DOI] [PubMed] [Google Scholar]
- 35. Pirklbauer M, Mayer G. The exchangeable calcium pool: physiology and pathophysiology in chronic kidney disease. Nephrol Dial Transplant. 2011;26:2438–2444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.