Précis:
In BMI-discordant twin pairs, LF discordance is associated with larger differences in circulating metabolite profiles during a glucose tolerance test, suggesting links to cardiovascular risk.
Abstract
Context:
The associations of body mass index (BMI) and liver fat (LF) with circulating prandial metabolomic markers are incompletely understood.
Objective:
We aimed to characterize circulating metabolite excursions during an oral glucose tolerance test (OGTT) and evaluate whether the metabolomic signatures of BMI discordance coassociate with LF content.
Design, Setting, and Participants:
We measured 80 metabolite parameters by nuclear magnetic resonance, together with glucose and insulin, during a 2-hour OGTT in 64 monozygotic (MZ) and 73 dizygotic (DZ) twin pairs (aged 22.8 to 36.2 years). Metabolite excursions during the OGTT were compared within BMI-discordant (intrapair difference, BMI ≥ 3 kg/m2) cotwins separately within MZ and DZ pairs. Insulin-based indices were calculated from the OGTT. LF was measured by magnetic resonance spectroscopy in 25 BMI-discordant MZ pairs. Metabolite profiles were compared with respect to LF discordance (ΔLF% ≥ 2%).
Results:
We replicated many previously reported OGTT-induced metabolite excursions in all 274 individuals and report novel lipoprotein excursions. The associations between some metabolite excursions and BMI differed in MZ and DZ twins. In BMI-discordant MZ pairs (mean ΔBMI = 4.9 kg/m2) who were concordant for LF (Δ0.2%), few metabolites differed between the cotwins: very-low-density lipoprotein (VLDL) cholesterol and apolipoprotein B were elevated, and high-density lipoprotein size and concentration were decreased in the cotwins with higher BMI. In contrast, in BMI-discordant MZ pairs (ΔBMI = 6.1 kg/m2) who were discordant for LF (Δ6.8%), cotwins with higher BMI exhibited lower insulin sensitivity and widespread metabolomic differences: elevations in small VLDL and low-density lipoprotein particles, fatty acids (FAs), and isoleucine. Within all 64 MZ twin pairs, lower insulin sensitivity associated with higher levels of VLDLs, triglycerides, FAs, and isoleucine.
Conclusions:
BMI-discordant MZ twin pairs who also are discordant for LF have more pronounced within-pair differences in metabolomics profiles during an OGTT than BMI-discordant pairs without LF discordance.
Obesity predisposes to type 2 diabetes, cardiovascular disease, and nonalcoholic fatty liver disease (1). Nevertheless, a subset of obese individuals appears to remain insulin sensitive and metabolically healthy, suggesting underlying variation in pathogenesis (2). Recently, deposition of fat within the liver rather than subcutaneous or even visceral compartments has been linked with the development of dyslipidemia and insulin resistance (IR) in obesity (2–5).
Analysis of large-scale fasting metabolomic signatures associated with body mass index (BMI) has highlighted several cardiovascular risk markers (6). Aberrations in the constituents of these signatures have also been examined in detail using the oral glucose tolerance test (OGTT). The levels of multiple circulating metabolites react to an OGTT, and many of these responses are different in nondiabetic obese and insulin-resistant subjects, suggesting early defects in insulin action along multiple pathways (7–10). However, these studies have not controlled for genetic influences or examined associations with liver fat (LF).
Phenotypic differences between obese and normal-weight subjects may be due to genetic alterations with effects on both obesity and associated phenotypes (pleiotropy). Obesity, diabetes, and nonalcoholic fatty liver disease all have large, at least partly overlapping, heritable components (11–14). Studies in monozygotic (MZ) twins (genetically almost identical) provide a way to control for genetic factors and, when used in parallel with dizygotic (DZ) twins (sharing 50% of genetic material), to assess the contribution of genes and familial factors on traits of interest. Comparison of MZ twins discordant for BMI has provided insights into the metabolic effects of acquired factors that differ between cotwins (15). Further, discordance for LF within BMI-discordant MZ twin pairs has been linked to greater aberrations in lipid profiles, blood pressure, insulin sensitivity, and low-grade inflammation than BMI discordance alone (3, 16)
We hypothesized that the metabolomic signatures of BMI discordance would coassociate with LF discordance rather than LF concordance. To study this, we expand upon the earlier cotwin design and incorporate 80 metabolite measures, ascertained using nuclear magnetic resonance (NMR). Uniquely, we measure the same panel at 4 time points during an OGTT. We show that BMI discordance in the context of LF discordance is associated with more widespread and larger differences in metabolite profiles.
Research Design and Methods
Study sample and assessment
Participants for the study were enrolled based on their responses to questions on weight and height in young adulthood (age range: 20.0 to 36.7 years) of two Finnish population-based twin cohorts (FinnTwin12 and FinnTwin16, combined total N = 10,384). We aimed to cover a wide range of BMIs in individuals and BMI differences within pairs. We first identified all MZ pairs who were discordant for BMI (intrapair difference, ΔBMI ≥ 3 kg/m2) from FinnTwin12 and FinnTwin16, resulting in 30 BMI discordant pairs (11 male and 19 female) after exclusion of pairs in which either cotwin had major concomitant diseases, regular medication (except contraceptives), anemia, psychiatric diseases, eating disorders, or weight cycling (±5 kg within the past 3 months). We also recruited 34 BMI-concordant (ΔBMI < 3 kg/m2) MZ pairs (22 female and 12 male), 38 DZ BMI-discordant pairs (22 male and 16 female), and 35 BMI-concordant DZ pairs (19 male and 16 female) from FinnTwin16, using a random generator and matching exclusion criteria. Oral contraceptives were used by 43% of female study subjects. All invited twins were healthy, with the exception of 1 twin who had inactive ulcerative colitis and used mesalazine and azathioprine. In total, 137 twin pairs were included in the study (age range: 22.8 to 36.2, 54% male), with a BMI of 25.3 ± 4.6 (mean ± standard deviation; range: 16.3 to 48.6 kg/m2), comparable with the whole cohort at 23.7 ± 3.4 (range: 14.0 to 49.4 kg/m2).
Whole body composition was measured using a dual-energy X-ray absorptiometry scan (Lunar Prodigy, software version 8.8; see Supplemental Methods section) in all participants (17). For a subsample of 25 BMI-discordant MZ pairs (age range: 22.8 to 35.8, 60% female), LF was measured by proton magnetic resonance spectroscopy and subcutaneous and intra-abdominal fat volumes by magnetic resonance imaging (1.5 Tesla scanner, Avanto, Siemens, Germany, Erlangen) as described previously (16, 18). We divided these pairs into LF-discordant (N = 12) and LF-concordant (N = 13) pairs based on a median cutoff of >2% intrapair difference (heavier – leaner cotwin’s value) in LF content. A subset of these twins (8 LF-discordant pairs and 7 to 8 LF-concordant pairs) has been previously described, with reports of blood pressure, sports activity, insulin responses during an OGTT, C-reactive protein, branched-chain amino acids (BCAAs), and 22 biochemically measured fasting lipoprotein and lipid parameters (3, 16).
Subjects were instructed to avoid alcohol and heavy exercise for 2 days and to fast overnight for 12 hours prior to the OGTT. Venous blood samples were drawn at 4 time points (0, 30, 60, and 120 minutes during the OGTT) and inoculated into fluoride citrate tubes (for glucose), serum (for insulin and metabolites), and Li-heparine (for alanine aminotransferase, aspartate aminotransferase, and gamma-glytamyltransferase) measurements. Blood plasma and serum were obtained by centrifugation at 2000 g for 10 minutes. Plasma glucose, alanine aminotransferase, aspartate aminotransferase, and gamma-glytamyltransferase concentrations were measured using standard methodology from fresh samples, as was serum insulin [time-resolved immunofluorometric assay (Perkin Elmer, Waltham, MA) with an intra- and interassay coefficient of variation of 2.6%].
Serum samples were aliquoted after centrifugation and immediately frozen (–80°) for metabolomics analyses. After thawing, samples were analyzed using a high-throughput NMR metabolomics platform, providing quantitative information on lipoprotein subclass distributions, fats and lipids, tricarboxylic acid cycle intermediates, amino acids, carbohydrates, and glycoproteins as detailed previously (19). Eighty measures were used for this study to minimize the number of tests while still including representative measures from different biological pathways.
Data collection and analysis were approved by the ethics committee of the Helsinki University Hospital. All subjects provided written informed consent.
Statistical analyses
We first investigated in the whole study sample whether different NMR measures changed significantly during the OGTT. We compared baseline levels with the peak levels that most differed from baseline during the OGTT. We used survey regression (R package “survey” v version 3.30) to correct for the clustered sampling of cotwins, with sex and age as covariates (20). Due to the shared variance of many NMR measurements, we used principal component analysis to calculate the number of independent factors that explained 95% of observed variance. The resulting value of 20 factors was used to establish a multiple comparison corrected significance threshold of P < 0.0025 (0.05/20). Power analysis for linear regression with the R package “pwr” version 1.1-3 (used for all power calculations) suggested a power of 0.99 for intermediate effects (n = 274, α = 0.0025, f2 = 0.15).
Next, we compared peak percentage excursions between cotwins with higher and lower BMI within BMI-discordant twin pairs with paired t tests. Only metabolites with significant excursions were analyzed. We separately analyzed and compared results in 30 MZ and 38 DZ twins to assess the contributions of genetic factors, based on an established twin framework that has recently been discussed by Kujala et al. (21, 22). MZ twins share close to 100% of genetic variation, whereas DZ twins share only 50% of segregating genes on average, although both share early familial environments. If genetic factors affect both BMI and the magnitude of metabolite excursions, associations between BMI and excursions may be observable in DZ twins but weaker or absent in MZ twins. Further, if associations are seen in (earlier) population studies but not in DZ or MZ twins, familial confounding may play a role in the association. Adequate sample size and replication in other data sets is required before strong inference from such comparisons can be made. We estimated a power of 0.86 for large effects (α = 0.0031, d = 0.8) for the 30 MZ twins.
To estimate the general effects of BMI discordance on metabolite profiles, we compared the area under the curve (AUC) values of the cotwins with higher and lower BMI within BMI-discordant MZ pairs with paired Student t test, separately for LF-discordant and LF-concordant pairs. We also compared the twins with higher BMI between the two sets of twins using Student t test. We estimated a power of 0.71 to 0.75 for large effects (n = 12 to 13, α = 0.05, d = 0.8) in intrapair comparisons using a nominal significance threshold.
Last, we analyzed the relationships between OGTT-derived insulin-based indices [see Table 1 footnote (23–29)] and the AUCs of the NMR measures during the OGTT. To study the effects of acquired factors, we calculated Pearson correlation coefficients between intrapair differences in the indices and AUC measures within the MZ pairs, where associations are independent of genetic effects. All values were rank-normalized and adjusted for age and sex. We estimated a power of 0.90 for large effects.
Table 1.
Characteristics of Cotwins Within BMI Discordant MZ Pairs Stratified by Liver Fat Discordance
| Cotwins With Lower BMI Within LF-Discordant MZ Pairs | Cotwins With Higher BMI Within LF-Discordant MZ Pairs | t Test P Value | Cotwins With Lower BMI Within LF-Concordant MZ Pairs | Cotwins With Higher BMI Within LF-Concordant MZ Pairs | t Test P Value | t Test P Value (Comparing Twins With Higher BMI From Both Groups) | |
|---|---|---|---|---|---|---|---|
| Number (men/women) | 12 (7/5) | 12 (7/5) | 13 (3/10) | 13 (3/10) | |||
| BMI (kg/m3) | 25.9 ± 1.13 | 32 ± 1.13 | 5.65e−07a | 24.8 ± 1.42 | 29.7 ± 1.42 | 3.63e−06a | 0.181 |
| Weight (kg) | 81.4 ± 6.66 | 98.5 ± 6.3 | 1.82e−06a | 71 ± 4.42 | 87.9 ± 4.99 | 0.000239a | 0.2062 |
| Waist (cm) | 88.2 ± 4.71 | 104 ± 4.5 | 0.000168a | 79.3 ± 2.61 | 94.3 ± 1.75 | 0.000792a | 0.06105 |
| Fat (%) | 29.3 ± 3.12 | 38.7 ± 1.85 | 0.000367a | 32.6 ± 2.63 | 41.5 ± 2.01 | 8.13e−05a | 0.3248 |
| Android fat (%) | 34.9 ± 4.71 | 48.8 ± 2.04 | 0.00236a | 38.8 ± 3.39 | 50.3 ± 1.93 | 0.0031a | 0.6086 |
| Gynoid fat (%) | 35.8 ± 4.16 | 43.7 ± 2.64 | 0.0044a | 41.6 ± 2.64 | 47.9 ± 2.25 | 0.00221a | 0.2506 |
| Subcutaneous fat (cm3) | 3690 ± 620 | 5920 ± 606 | 8.34e−06a | 3740 ± 604 | 6070 ± 652 | 2.14e−05a | 0.8631 |
| Intra-abdominal fat (cm3) | 1100 ± 361 | 2270 ± 419 | 9.02e−05a | 591 ± 125 | 1010 ± 135 | 0.000422a | 0.002722a |
| Liver fat (%) | 1.78 ± 0.643 | 8.64 ± 1.42 | 7.23e−06a | 0.717 ± 0.255 | 0.891 ± 0.199 | 0.0605 | 7.158e−09a |
| Fasting glucose (mmol/L) | 5.27 ± 0.129 | 5.38 ± 0.155 | 0.278 | 5.02 ± 0.0732 | 5.15 ± 0.133 | 0.334 | 0.2723 |
| Fasting insulin (pmol/L) | 33.2 ± 4.88 | 68.1 ± 12.7 | 0.00205a | 27.2 ± 4.19 | 37.8 ± 5.84 | 0.145 | 0.0154a |
| HOMA-IR | 1.32 ± 0.206 | 2.81 ± 0.606 | 0.00307a | 1.02 ± 0.167 | 1.48 ± 0.245 | 0.0981 | 0.02641a |
| Matsuda index | 8.82 ± 1.69 | 4.07 ± 0.493 | 0.00253a | 9.62 ± 1.22 | 7.59 ± 0.945 | 0.296 | 0.004969a |
| Insulin secretion index | 100 ± 19.8 | 144 ± 24.8 | 0.109 | 166 ± 65.7 | 248 ± 125 | 0.324 | 0.8035 |
| Oral disposition index | 86.1 ± 13.4 | 65.5 ± 12.7 | 0.141 | 231 ± 130 | 250 ± 118 | 0.785 | 0.0846 |
| Baecke sport index | 3.32 ± 0.25 | 2.16 ± 0.2 | 0.00835a | 2.58 ± 0.227 | 2.56 ± 0.268 | 0.796 | 0.2425 |
| ASAT (U/L) | 26.4 ± 1.05 | 30.5 ± 1.86 | 0.0359a | 22 ± 1.52 | 21.9 ± 1.03 | 0.846 | 0.00031a |
| ALAT (U/L) | 24 ± 2.31 | 39.3 ± 6.02 | 0.00879a | 19.8 ± 2.49 | 19.6 ± 1.82 | 0.842 | 0.001033a |
| GT (U/L) | 21 ± 3.62 | 41.3 ± 9.71 | 0.0516 | 14.1 ± 2.81 | 14 ± 1.5 | 0.719 | 0.02139a |
| Systolic BP (mm HG) | 125 ± 3.34 | 128 ± 3.88 | 0.183 | 118 ± 2.71 | 119 ± 2.63 | 0.729 | 0.0715 |
| Diastolic BP (mm HG) | 78.8 ± 2.67 | 82.3 ± 2.43 | 0.143 | 78 ± 2.17 | 80 ± 2.22 | 0.279 | 0.4783 |
| Pulse (L/min) | 66.9 ± 4.14 | 73.8 ± 4.25 | 0.0464a | 64.8 ± 2.84 | 67 ± 3.56 | 0.377 | 0.2305 |
| Weekly alcohol consumption (doses) | 4.92 ± 2.32 | 8.34 ± 3.3 | 0.557 | 7 ± 4.84 | 5.85 ± 2.61 | 0.579 | 0.5944 |
| Smokers | 2 | 2 | 3 | 4 | |||
| Oral contraceptive users | 2 | 1 | 5 | 5 |
Values are mean ± SE. Differences between cotwins with higher compared with lower BMI within BMI-discordant MZ pairs were estimated with paired t tests, and differences between the two groups of twins with higher BMI with a t test for unrelated samples (final column). The formulas used for calculation of the used indices are as follows:
HOMA-IR (23): FPG(mmol/L) × FPI(μU/mL)/22.5
QUICKI (24): 1 / [FPI(μU/mL)log + FPG(mg/dL)log]
Matsuda index (25): 10.000 / √[FPG × FPI(μU/mL) × Glucose(mg/dL)OGTT mean × Insulin(μU/mL)OGTT mean]
Insulin secretion index (26): [Insulin(pmol/L)30 min – Insulin(pmol/L)0 min] / [Glucose(mmol/L)30 min – Glucose(mmol/L)0 min]
Oral disposition index (27): Insulin secretion index / HOMA-IR
Liver IR (28): Glucose(mmol/L)AUC0–30 × Insulin(μU/mL)AUC0–30
Adipocyte IR (29): Fasting fatty acids × FPI(μU/mL)
Abbreviations: ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; BP, blood pressure; CRP, C-reactive protein; FPG, fasting plasma glucose; FPI, fasting plasma insulin; GT, gamma-glutamyltransferase; QUICKI, quantitative insulin sensitivity check index.
a P < 0.05.
Variables with skewness >1 were natural logarithm transformed before t tests and survey regression. Analyses were performed using StataSE version 12.1 and R version 3.0.2.
Results
Ingested glucose induces excursions along axes of insulin action
To characterize how circulating metabolites react to a transition from a fasting state into a milieu with elevated insulin and glucose, and to validate the used NMR measures, we began with an overview of excursions induced by an OGTT in the whole study population (274 individual twins). There were significant excursions from baseline in 54/80 measures (using the multiple-corrected P-value threshold P < 0.0025; Fig. 1 and Supplemental Figs. 1–3), replicating many earlier OGTT results from different metabolomics platforms (7–10). In line with insulin-stimulated amino acid intake into muscle, serum amino acid concentrations were suppressed significantly—as much as 7% to 47%—from baseline to 120 minutes (Supplemental Fig. 1). Insulin also suppresses lipolysis and fatty acid (FA) release from adipose tissue; accordingly, the levels of glycerol (Supplemental Fig. 1) and most FAs (Supplemental Fig. 2) decreased. The levels of ketone bodies (3-hydroxybutyrate, acetoacetate, and acetate) decreased by 32% to 40% (Supplemental Fig. 1), consistent with insulin’s action to inhibit ketone body generation and increase their clearance (30).
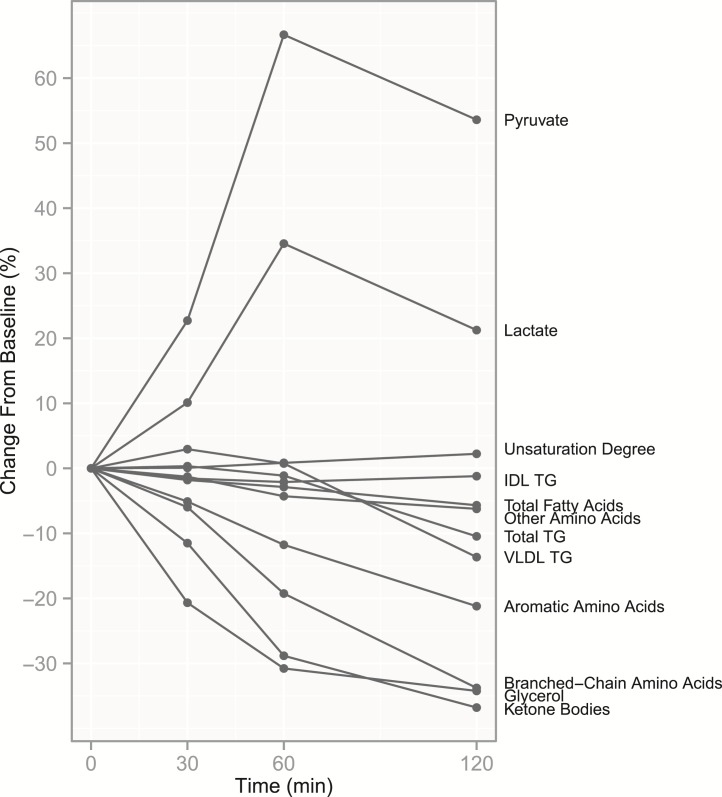
Figure 1.
Excursions during the OGTT for key metabolite measures. Median values of percentual excursions from baseline to each time point in the whole study sample (n = 274) for key metabolites. Only measures with significant changes from baseline to peak change (P < 0.0025) are included. Individual measures were summed for ketone bodies, FAs, and structural amino acid subgroups, and median values are presented for these sum variables. Changes are presented for each measure in more detail in Supplemental Figures 1–3.
We then focused on previously unreported excursions in 47 lipoprotein-related measures (Supplemental Fig. 3). As a possible effect of storage of lipids to tissues and inhibition of very-low-density lipoprotein (VLDL) secretion, total triglycerides (TGs) fell in serum during the last hour of the test (–10.6%), paired with a similar fall in VLDL TG content (–13.7%). TG content in very large and small high-density lipoprotein (HDL) increased, most evidently at 60 minutes (+18.3% and +8.2%). For most other measures, we noted no excursions or the excursions were minimal although statistically significant.
Acquired increase in BMI is associated with blunted TG excursions During an OGTT
Next, we estimated the effect of BMI on metabolite excursions from baseline to peak change by comparing the cotwins with lower and higher BMI within 30 BMI-discordant MZ twin pairs. Large weight (17.7 kg) and BMI (5.6 kg/m2) differences within these pairs (Supplemental Table 1) allowed us to assess how BMI in general influences metabolite levels during the OGTT, independent of genetic effects. Compared with their MZ cotwins with lower BMI, the cotwins with higher BMI exhibited blunted peak percentual decreases for total TGs and VLDL TGs (Fig. 2 and Supplemental Fig. 4), as well as for large and medium-sized VLDL particles, using P < 0.0031 as the multiple-corrected threshold (Supplemental Methods). This suggests that BMI discordance is linked with diminished insulin-induced clearance of VLDL particles or decreased inhibition of VLDL production. In contrast to VLDL and total serum triglyceride measures, cotwins with higher BMI expressed greater elevations in the TG content of very large HDL particles (P = 1.25×10−7) and greater depressions in linoleic acid (P = 0.0031).
Figure 2.
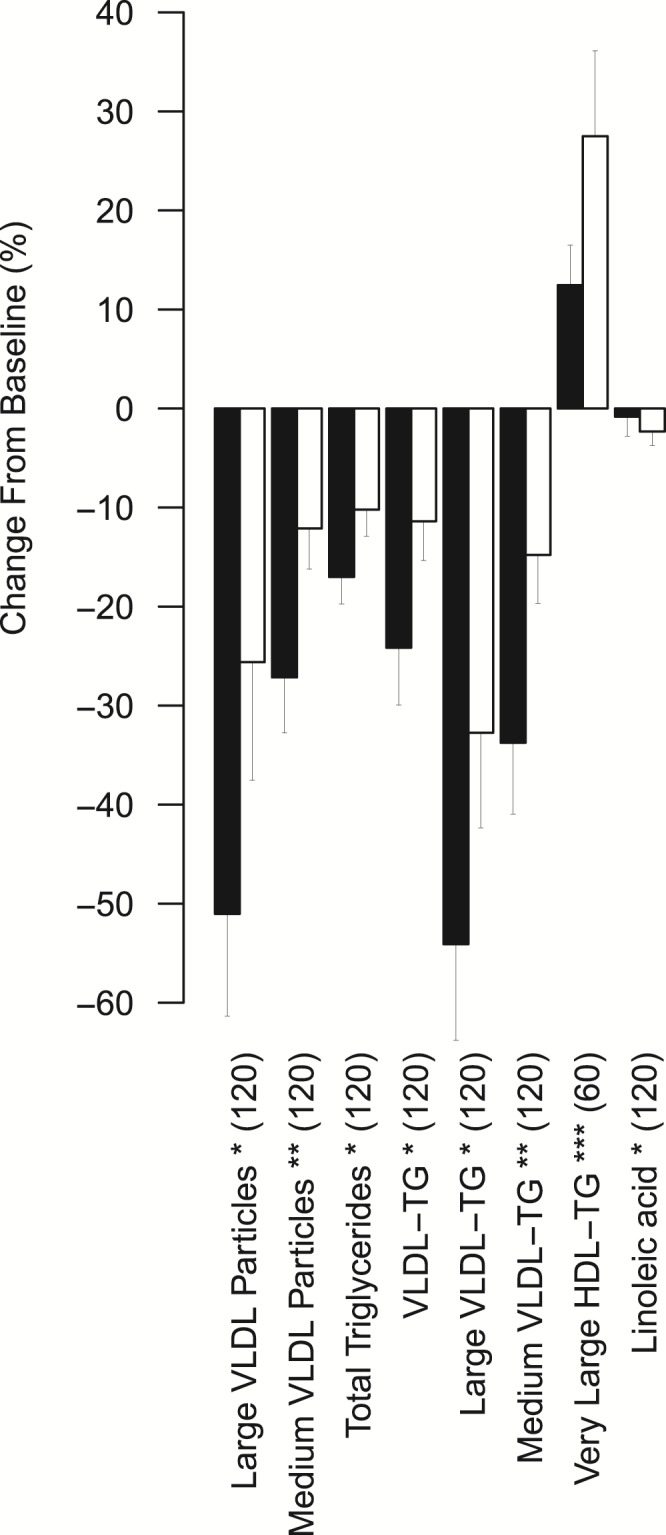
Significantly differing excursions in cotwins with higher compared with lower BMI within BMI-discordant MZ pairs during the OGTT. Black bars represent excursions in cotwins with lower BMI, and white bars represent excursions in cotwins with higher BMI within BMI-discordant MZ pairs (peak percentual excursion + SE, n = 30 pairs). Paired t tests were used to estimate significance. The peak time points are marked inside parentheses. Asterisks denote significance based on multiple-corrected P-value thresholds. *P < 0.0031; **P < 0.00063; ***P < 0.000063. LDL, low-density lipoprotein; TG, triglycerides.
We then repeated the analysis in 38 BMI-discordant DZ twin pairs. No measure reached multiple-corrected significance in this group (Supplemental Fig. 5). Excursions in large VLDL particle number and TG content appeared blunted at 120 minutes in cotwins with higher BMI, as in MZ pairs, but were only nominally significant (P = 0.015 and P = 0.012). In DZ, but not in MZ, cotwins with higher BMI, depressions in isoleucine (P = 0.023), leucine (P = 0.041), and acetoacetate (P = 0.031) levels were blunted at 120 minutes with nominal significance.
BMI discordance with or without LF discordance: two distinct MZ twin phenotypes
Because we previously demonstrated that the accumulation of LF within BMI-discordant MZ twins characterizes a metabolically unhealthy phenotype (3), we now asked whether we would see differences in the overall metabolomic signatures of BMI discordance in the two subgroups: BMI-discordant MZ pairs discordant or concordant for LF (Table 1, Supplemental Fig. 6). To validate this approach, we first examined the adiposity and metabolic characteristics of the two groups. When comparing the twins with higher BMI from the two groups, we saw significant expected differences (P < 0.05) in LF and intra-abdominal fat, but not in subcutaneous fat in magnetic resonance imaging or android vs gynoid fat distribution in dual-energy X-ray absorptiometry (Table 1). Between groups, cotwins with higher BMI in LF-discordant pairs also had higher transaminases and poorer insulin sensitivity than those in LF-concordant pairs (Table 1). In the within-pair analyses, cotwins with higher BMI, as compared with cotwins with lower BMI, had significantly higher transaminase concentrations and lower insulin sensitivity within LF-discordant but not within LF-concordant pairs (Table 1). Indices of insulin secretion did not differ between cotwins in either group.
Examining potential confounders revealed lower reported physical activity (Baecke sport index) in the cotwins with higher BMI within LF-discordant pairs when compared with their cotwins with lower BMI, but not when compared with the twins with higher BMI from LF-concordant pairs (Table 1). Differences in overall alcohol consumption were not statistically significant.
BMI discordance without LF discordance is associated with minor differences in metabolomic profiles in MZ twins
When we first restricted analyses to LF-concordant MZ twin pairs, we found mostly small within-pair differences (using P < 0.05 as the threshold) in the AUCs of lipoprotein and amino acid measures during the OGTT, despite large differences in BMI between the cotwins (Supplemental Table 2). The most important finding in these analyses was markedly decreased HDL cholesterol (HDL-C; –13.8%, P = 0.025), most evident in larger particle sizes, and consequently smaller HDL diameter (–1.44%, P = 0.015), in the cotwins with higher as compared with cotwins with lower BMI. The former also exhibited elevations in VLDL cholesterol (11.5%, P = 0.035), apolipoprotein B (ApoB) (ApoB; 7.9%, P = 0.022), glycerol (23.2%, P = 0.0091), valine (12.3, P = 0.015), and tyrosine (12.5%, P = 0.046) AUCs.
BMI discordance with LF discordance is associated with widespread differences in metabolomic profiles in MZ twins
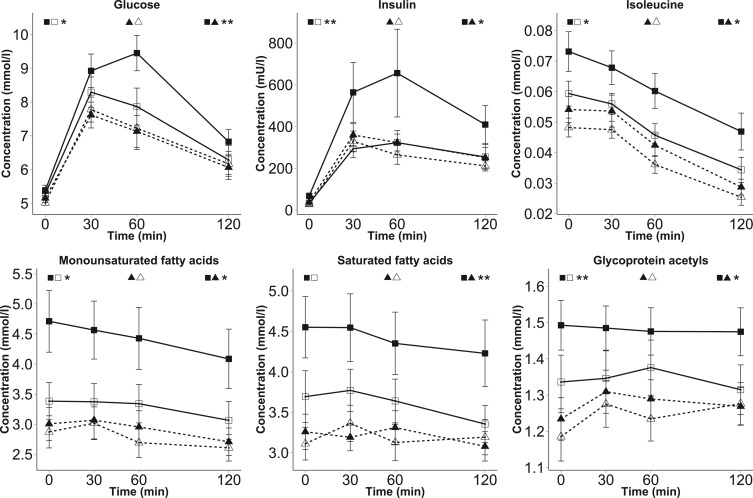
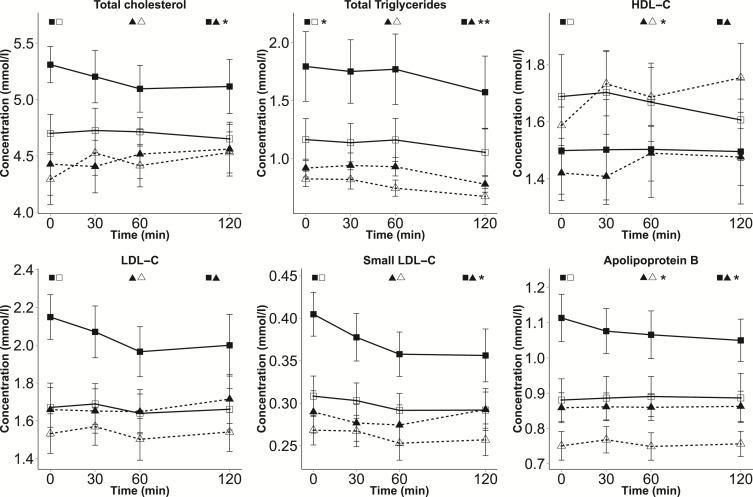
We then sought to study whether BMI discordance is associated with larger differences in the presence of LF discordance and, indeed, MZ cotwins discordant for both BMI and LF exhibited large differences in the profiles of several metabolite classes (using P < 0.05 as the threshold; Figs. 3 and 4, and Supplemental Table 2), compared with both their cotwins with lower BMI (intrapair) and with the twins with higher BMI within LF-concordant pairs (between pairs). Considerable within-pair differences were observed for the AUCs of small VLDL particles (30.5% higher for cotwins with higher BMI, P = 0.037), small low-density-lipoprotein (LDL) particles (24.7%, P = 0.039), HDL2-C (–17.9%, P = 0.045), TGs (87.5%, P = 0.033), monounsaturated FAs (37.5%, P = 0.027), isoleucine (33.2%, P = 0.028) and glycoproteins (12.0%, 0.0064). Despite decreases during the OGTT, the levels of isoleucine and monounsaturated FAs remained significantly higher in the cotwins with higher BMI within LF-discordant pairs compared with their cotwins with lower BMI even at 120 minutes (P < 0.05).
Figure 3.
Dynamic metabolite levels in subtypes of BMI discordance. Mean ± SE values of selected NMR measures are presented for 4 groups at each time point during the OGTT: cotwins with higher BMI within LF-discordant MZ pairs (solid line with black squares, n = 12), cotwins with lower BMI within LF-discordant MZ pairs (solid line with white squares, n = 12), cotwins with higher BMI within LF-concordant MZ pairs (dashed line with black triangles, n = 13), and cotwins with lower BMI within LF-concordant MZ pairs (dashed line with white triangles, n = 13). Student’s t test was used to estimate significance between the AUC values of the matched cotwins with higher and lower BMI (paired testing) and between the AUC values of the two groups of twins with higher BMI (nonpaired testing). P values are marked by asterisks next to the symbols of the corresponding groups. *P < 0.05; **P < 0.01.
Figure 4.
Dynamic metabolite levels in subtypes of BMI discordance (continued). Mean ± SE values of selected NMR measures are presented for 4 groups at each time point during the OGTT: cotwins with higher BMI within LF-discordant MZ pairs (solid line with black squares, n = 12), cotwins with lower BMI within LF-discordant MZ pairs (solid line with white squares, n = 12), cotwins with higher BMI within LF-concordant MZ pairs (dashed line with black triangles, n = 13), and cotwins with lower BMI within LF-concordant MZ pairs (dashed line with white triangles, n = 13). Student’s t test was used to estimate significance between the AUC values of the matched cotwins with higher and lower BMI (paired testing) and between the AUC values of the two groups of twins with higher BMI (nonpaired testing). P values are marked by asterisks next to the symbols of the corresponding groups. *P < 0.05; **P < 0.01.
Between twin pairs, the cotwins with higher BMI within LF-discordant pairs also differed from the cotwins with higher BMI within LF-concordant pairs: the former, in addition to many of the parameters that were highlighted in within-pair comparison, had higher AUCs for ApoB (15.9%, P = 0.021), esterified cholesterol (19.7%, P = 0.04), saturated FAs (30.2%, P = 0.0062), and omega-3 FAs (41.9%, P = 0.0019; Supplemental Table 2).
Indices of insulin sensitivity are associated with circulating metabolites in MZ twins
Lastly, because LF-discordant pairs were characterized by differences in insulin sensitivity, we asked whether OGTT-derived indices of insulin sensitivity or secretion correlate with the same metabolite levels, independent of genetic factors in all 64 MZ twin pairs. We generated intrapair differences in MZ pairs for each of the indices and metabolite AUCs during the OGTT and used these in the following correlations.
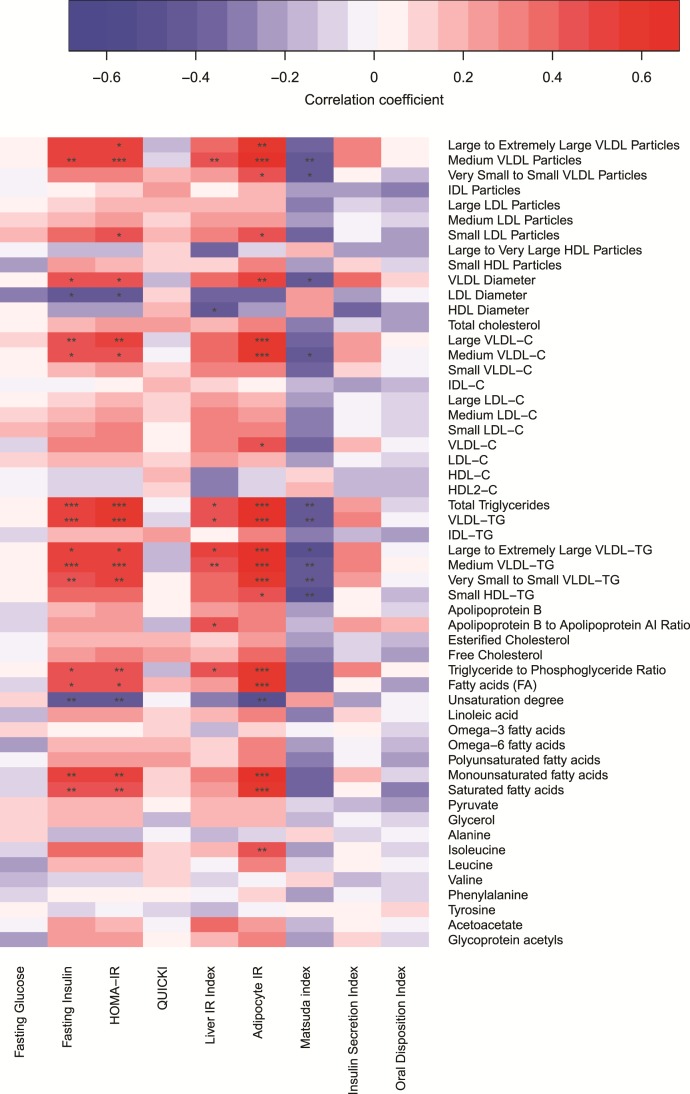
Three indices of insulin sensitivity emerged as strong overall markers of metabolite levels: fasting insulin, the Homeostasis model assessment index of insulin resistance (HOMA-IR), and adipocyte IR (Fig. 5). They are all calculated using fasting values, and were significantly associated (using a multiple-corrected threshold of P < 0.0025) with the cholesterol content of large (r = 0.13 to 0.22) and medium (r = 0.18 to 0.23)—but not smaller—VLDL particles, several TG measures such as total and VLDL-TG (r = 0.53 to 0.67), monounsaturated FAs (r = 0.48 to 0.64), and saturated FAs (r = 0.46 to 0.60). Although isoleucine was associated with adipocyte IR (r = 0.47), leucine and valine did not reach significance (r = 0.28 and r = –0.02 for adipocyte IR). Matsuda insulin sensitivity index and the Liver IR index were mostly associated with VLDL and TG measures (rMatsuda = –0.47 and rLiverIR = 0.43 to 0.44 for total and VLDL TGs, P < 0.0025). In general, associations between insulin-based indices and LDL particles were weaker than for VLDL particles, and reached significance only for smaller LDL particles (rHOMA-IR = 0.41; rAdipocyteIR = 0.44, P < 0.0025). HDL measures were not generally strongly associated with insulin-based indices.
Figure 5.
Associations between intrapair differences in insulin-based indices and the AUC values of metabolites in all 64 monozygotic twin pairs. Intrapair differences were calculated by subtracting the measurement of the cotwin with lower BMI from the measurement of the cotwin with higher BMI. Indices and AUC values were rank-normalized prior to analysis while adjusting for sex and age, and Pearson correlation coefficients were estimated. Color denotes the strength of correlations (red = positive, blue = negative) and asterisks mark their significance (*P < 0.0025; **P < 0.0005; ***P < 0.00005). IDL, intermediate-density lipoprotein.
The two indices reflective of beta cell function, insulin secretion index and the oral disposition index (a composite measure of insulin secretion and sensitivity) were not significantly associated with any AUC measures after multiple corrections.
In summary, BMI discordance with LF discordance was associated with more widespread metabolomic signatures than BMI discordance without LF discordance. Insulin sensitivity in all MZ twin pairs was associated with many, but not all of the measures composing this signature.
Discussion
In this study of young adult MZ twins, we show that, while controlling for genetic background, BMI discordance with LF discordance is associated with distinct metabolomic signatures after glucose ingestion, highlighting metabolites linked to cardiovascular disease risk, whereas the metabolomic signatures of BMI discordance without LF discordance are smaller and less widespread, though not absent.
We began by characterizing metabolomic responses to the OGTT, previously shown to induce metabolite excursions related to the suppression of ketogenesis, proteolysis, lipolysis, and VLDL production, and the promotion of glycolysis and TG uptake into peripheral tissues (30–32). In line with previous studies, we noted depressions in the blood levels of key triacylglycerol components (FAs and glycerol) (8, 9, 33), ketone bodies (10), BCAAs, and aromatic amino acids (9, 10, 33) during the OGTT. Previous studies in unrelated individuals have also shown that insulin-resistant subjects experience blunted depressions for isoleucine and ketone body levels (10), and obese subjects experience altered excursions in FAs and amino acids (34). We also saw nominally significant blunted depressions for isoleucine, leucine, and acetoacetate in the cotwins with higher BMI in DZ twin pairs, but not in MZ twin pairs where genetic effects are more fully controlled. This could reflect genetic contributions underlying both BMI and the excursions of these metabolites, or issues related to sample size. Surprisingly, BMI-related differences, especially in VLDL and TG parameters, appeared in general to be more significant in MZ than in DZ twins. Finally, we showed that unlike complex meals, which induce several-hour elevations in serum TGs (35), ingestion of pure glucose seems to decrease VLDL and TG levels (Fig. 2). This may reflect the role of insulin in inhibiting hepatic VLDL-TG secretion or directing TGs to fat tissue by increasing fat tissue lipoprotein lipase activity and inhibiting skeletal muscle lipoprotein lipase activity (31, 32).
Elevated BMI alone has been associated with widespread changes in metabolomic profiles, but how specific metabolomic classes associate with LF content is not known (6). We saw that, firstly, lipoprotein metabolism appeared markedly dissimilar in the twins with higher BMI within LF-discordant pairs, with elevations in small VLDL and LDL particles, TGs, ApoB, and esterified cholesterol during the OGTT. Our findings are in line with the concept that an insulin-resistant, fatty liver produces VLDL1-TG and ApoB in excess, with potential generation of smaller VLDL remnants and small dense LDL particles that may penetrate arterial walls easier and promote atherosclerosis (35). Nevertheless, we also saw comparable differences in HDL-C and smaller differences in VLDL cholesterol and ApoB within LF-concordant pairs, suggesting that BMI discordance in this context is not necessarily neutral with respect to cardiovascular risk.
We also saw differing levels of less conventional metabolomic risk factors in the cotwins with higher BMI within LF-discordant but not in LF-concordant pairs. They showed elevated levels of saturated and monounsaturated FAs whose fasting levels are associated with diabetes and cardiovascular risk (36, 37) and isoleucine, a BCAA predictive of future diabetes (38). Notably, though levels of isoleucine and monounsaturated FAs decreased in all groups following glucose ingestion, differences between groups were persistent. Finally, the cotwins with higher BMI within LF-discordant pairs had higher levels of glycoproteins, an inflammation-related measure associated with all-cause mortality risk in a study using the same NMR panel (39).
As the intertwined nature of LF and decreased insulin sensitivity is well established (LF content being associated with the severity of insulin resistance in liver, muscle, and adipose tissue), we examined the correlations of metabolite AUCs with indices of insulin sensitivity and secretion while controlling for genetic effects (4). Levels of metabolites associated with cardiovascular risk within MZ pairs correlated well with estimates of insulin sensitivity but poorly with estimates of beta cell function. Surprisingly, indices based on fasting values (insulin, HOMA-IR, and Adipocyte IR) exhibited the greatest correlations across several metabolite groups, highlighting the value of fasting insulin in contrast to more complex and tissue-specific indices.
Limitations of our study include its small sample size and cross-sectional design. Despite widespread differences related to LF discordance, power in this setting was lower and conclusions regarding individual measures should be approached with caution. Under- or overestimation of effect sizes is possible. Finally, though the twin design allows estimation of acquired effects (important because many measures in the NMR panel have high heritabilities, ranging between 0.23 and 0.55 for low molecular weight metabolites, 0.48 and 0.62 for lipids, and 0.50 and 0.76 for lipoproteins) (40), it does not reveal what underlying genetic structure predisposes subsets of obese subjects to different metabolomic aberrations.
Our study in young adult twins shows that differences in metabolite profiles during an OGTT depend on adiposity phenotypes. Without LF discordance, discordance in BMI appears to be associated with small metabolomic differences—especially depressions in HDL-C—and no decrease in insulin sensitivity. However, in the context of LF discordance, aberrations in lipoproteins, FAs, and isoleucine are substantially more severe and accompanied by IR. In conclusion, LF, independent of BMI and controlling for genetic background, is associated with insulin sensitivity and metabolomic signatures during an OGTT that are suggestive of changes associated with increased cardiovascular disease risk.
Supplementary Material
Acknowledgments
Author contributions: K.H.P, J.K., and A.R. participated in study conception and design. K.H.P., A.H., J.L., and N.L. acquired data. J.T.R., K.H.P., N.M., S.M.K., S.J., L.H.B., J.K., A.H., and J.L. analyzed and interpreted data. J.T.R., K.H.P., and N.M. drafted the manuscript, and all authors revised it for intellectual content and approved of the final version of the article. K.H.P. had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by Academy of Finland Grants 141054, 265240, 263278, and 264146 (to J.K.) and Grant 266286 (to K.H.P.); Centre of Excellence in Research on Mitochondria, Metabolism, and Disease (FinMIT) Grant 272376 (to K.H.P.) ; Center of Excellence in Complex Disease Genetics Grants 213506 and 129680 (to J.K.); Helsinki University Central Hospital (N.L., A.H., A.R., K.H.P., N.M.); and grants from the following foundations: Jenny and Antti Wihuri (L.H.B.), Novo Nordisk (K.H.P.), Jalmari and Rauha Ahokas (L.H.B., K.H.P.), Biomedicum Helsinki (L.H.B.), Emil Aaltonen (J.T.R., S.J.), 1.3 milj. klubi-klubben (L.H.B.), Maud Kuistila (S.M.K.), Paavo and Eila Salonen/Central Finland Health District Science Committee (S.M.K.), the Finnish Medical Foundation (J.T.R.), the Finnish Diabetes Research Foundation (K.H.P.), and the Finnish Foundation for Cardiovascular Research (S.M.K., K.H.P.). Data collection in FinnTwin16 and FinnTwin12 was supported by National Institute of Alcohol Abuse and Alcoholism Grants AA-12502 and AA-09203 (to R. J. Rose).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- ApoB
apolipoprotein B
- AUC
area under the curve
- BCAA
branched-chain amino acid
- BMI
body mass index
- DZ
dizygotic
- FA
fatty acids
- HDL
high-density lipoprotein
- HDLZ-C
high density lipoprotein cholesterol
- HOMA-IR
homeostatis model assessment of insulin resistance
- IR
insulin resistance
- LF
liver fat
- MZ
monozygotic
- NMR
nuclear magnetic resonance
- OGTT
oral glucose tolerance test
- TG
triglycerides
- VLDL
very low density lipoprotein
References
- 1. Cornier M-A, Després J-P, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge M-P, Towfighi A, Poirier P; American Heart Association Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis, and Vascular Biology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease; Stroke Council . Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124(18):1996–2019. [DOI] [PubMed] [Google Scholar]
- 2. Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609–1616. [DOI] [PubMed] [Google Scholar]
- 3. Naukkarinen J, Heinonen S, Hakkarainen A, Lundbom J, Vuolteenaho K, Saarinen L, Hautaniemi S, Rodriguez A, Frühbeck G, Pajunen P, Hyötyläinen T, Orešič M, Moilanen E, Suomalainen A, Lundbom N, Kaprio J, Rissanen A, Pietiläinen KH. Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia. 2014;57(1):167–176. [DOI] [PubMed] [Google Scholar]
- 4. Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106(36):15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Würtz P, Wang Q, Kangas AJ, Richmond RC, Skarp J, Tiainen M, Tynkkynen T, Soininen P, Havulinna AS, Kaakinen M, Viikari JS, Savolainen MJ, Kähönen M, Lehtimäki T, Männistö S, Blankenberg S, Zeller T, Laitinen J, Pouta A, Mäntyselkä P, Vanhala M, Elliott P, Pietiläinen KH, Ripatti S, Salomaa V, Raitakari OT, Järvelin M-R, Smith GD, Ala-Korpela M. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11(12):e1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wopereis S, Rubingh CM, van Erk MJ, Verheij ER, van Vliet T, Cnubben NHP, Smilde AK, van der Greef J, van Ommen B, Hendriks HFJ. Metabolic profiling of the response to an oral glucose tolerance test detects subtle metabolic changes. PLoS One. 2009;4(2):e4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao X, Peter A, Fritsche J, Elcnerova M, Fritsche A, Häring H-U, Schleicher ED, Xu G, Lehmann R. Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab. 2009;296(2):E384–E393. [DOI] [PubMed] [Google Scholar]
- 9. Spégel P, Danielsson APH, Bacos K, Nagorny CLF, Moritz T, Mulder H, Filipsson K. Metabolomic analysis of a human oral glucose tolerance test reveals fatty acids as reliable indicators of regulated metabolism. Metabolomics. 2010;6:56–66. [Google Scholar]
- 10. Ho JE, Larson MG, Vasan RS, Ghorbani A, Cheng S, Rhee EP, Florez JC, Clish CB, Gerszten RE, Wang TJ. Metabolite profiles during oral glucose challenge. Diabetes. 2013;62(8):2689–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stunkard AJ, Sørensen TI, Hanis C, Teasdale TW, Chakraborty R, Schull WJ, Schulsinger F. An adoption study of human obesity. N Engl J Med. 1986;314(4):193–198. [DOI] [PubMed] [Google Scholar]
- 12. Kaprio J, Tuomilehto J, Koskenvuo M, Romanov K, Reunanen A, Eriksson J, Stengård J, Kesäniemi YA. Concordance for Type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus in a population-based cohort of twins in Finland. Diabetologia. 1992;35(11):1060–1067. [DOI] [PubMed] [Google Scholar]
- 13. Korkeila M, Kaprio J, Rissanen A, Koskenvuo M. Effects of gender and age on the heritability of body mass index. Int J Obes. 1991;15(10):647–654. [PubMed] [Google Scholar]
- 14. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–910. [DOI] [PubMed] [Google Scholar]
- 15. Pietiläinen KH, Sysi-Aho M, Rissanen A, Seppänen-Laakso T, Yki-Järvinen H, Kaprio J, Oresic M. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects: a monozygotic twin study. PLoS One. 2007;2(2):e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaye SM, Maranghi M, Bogl LH, Kaprio J, Hakkarainen A, Lundbom J, Lundbom N, Rissanen A, Taskinen M-R, Pietiläinen KH. Acquired liver fat is a key determinant of serum lipid alterations in healthy monozygotic twins. Obesity (Silver Spring). 2013;21(9):1815–1822. [DOI] [PubMed] [Google Scholar]
- 17. Granér M, Seppälä-Lindroos A, Rissanen A, Hakkarainen A, Lundbom N, Kaprio J, Nieminen MS, Pietiläinen KH. Epicardial fat, cardiac dimensions, and low-grade inflammation in young adult monozygotic twins discordant for obesity. Am J Cardiol. 2012;109(9):1295–1302. [DOI] [PubMed] [Google Scholar]
- 18. Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, Lundbom N, Rissanen A, Ridderstråle M, Groop L, Orho-Melander M, Yki-Järvinen H. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865–872. [DOI] [PubMed] [Google Scholar]
- 19. Soininen P, Kangas AJ, Würtz P, Tukiainen T, Tynkkynen T, Laatikainen R, Järvelin M-R, Kähönen M, Lehtimäki T, Viikari J, Raitakari OT, Savolainen MJ, Ala-Korpela M. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst. 2009;134(9):1781–1785. [DOI] [PubMed] [Google Scholar]
- 20. Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645–646. [DOI] [PubMed] [Google Scholar]
- 21. Kujala UM, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all-cause mortality: the roles of genetics and childhood environment. Am J Epidemiol. 2002;156(11):985–993. [DOI] [PubMed] [Google Scholar]
- 22. Gesell A. The method of co-twin control. Science. 1942;95(2470):446–448. [DOI] [PubMed] [Google Scholar]
- 23. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 24. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–2410. [DOI] [PubMed] [Google Scholar]
- 25. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 26. Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11(3):286–292. [DOI] [PubMed] [Google Scholar]
- 27. Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med. 2009;26(12):1198–1203. [DOI] [PubMed] [Google Scholar]
- 28. Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30(1):89–94. [DOI] [PubMed] [Google Scholar]
- 29. Abdul-Ghani MA, Molina-Carrion M, Jani R, Jenkinson C, Defronzo RA. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the anti-lipolytic effect of insulin. Acta Diabetol. 2008;45(3):147–150. [DOI] [PubMed] [Google Scholar]
- 30. Keller U, Lustenberger M, Stauffacher W. Effect of insulin on ketone body clearance studied by a ketone body “clamp” technique in normal man. Diabetologia. 1988;31(1):24–29. [DOI] [PubMed] [Google Scholar]
- 31. Farese RV Jr, Yost TJ, Eckel RH. Tissue-specific regulation of lipoprotein lipase activity by insulin/glucose in normal-weight humans. Metabolism. 1991;40(2):214–216. [DOI] [PubMed] [Google Scholar]
- 32. Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes. 1993;42(6):833–842. [DOI] [PubMed] [Google Scholar]
- 33. Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, Carr SA, Thadhani R, Gerszten RE, Mootha VK. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adiels M, Taskinen M-R, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Vehkavaara S, Häkkinen A, Olofsson S-O, Yki-Järvinen H, Borén J. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49(4):755–765. [DOI] [PubMed] [Google Scholar]
- 35. Borén J, Matikainen N, Adiels M, Taskinen MR. Postprandial hypertriglyceridemia as a coronary risk factor. Clin Chim Acta. 2014;431:131–142. [DOI] [PubMed] [Google Scholar]
- 36. Mahendran Y, Cederberg H, Vangipurapu J, Kangas AJ, Soininen P, Kuusisto J, Uusitupa M, Ala-Korpela M, Laakso M. Glycerol and fatty acids in serum predict the development of hyperglycemia and type 2 diabetes in Finnish men. Diabetes Care. 2013;36(11):3732–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Würtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto-Merino D, Tillin T, Ghorbani A, Artati A, Wang Q, Tiainen M, Kangas AJ, Kettunen J, Kaikkonen J, Mikkilä V, Jula A, Kähönen M, Lehtimäki T, Lawlor DA, Gaunt TR, Hughes AD, Sattar N, Illig T, Adamski J, Wang TJ, Perola M, Ripatti S, Vasan RS, Raitakari OT, Gerszten RE, Casas JP, Chaturvedi N, Ala-Korpela M, Salomaa V. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 2015;131(9):774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fischer K, Kettunen J, Würtz P, Haller T, Havulinna AS, Kangas AJ, Soininen P, Esko T, Tammesoo M-L, Mägi R, Smit S, Palotie A, Ripatti S, Salomaa V, Ala-Korpela M, Perola M, Metspalu A. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: an observational study of 17,345 persons. PLoS Med. 2014;11(2):e1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kettunen J, Tukiainen T, Sarin A-P, Ortega-Alonso A, Tikkanen E, Lyytikäinen L-P, Kangas AJ, Soininen P, Würtz P, Silander K, Dick DM, Rose RJ, Savolainen MJ, Viikari J, Kähönen M, Lehtimäki T, Pietiläinen KH, Inouye M, McCarthy MI, Jula A, Eriksson J, Raitakari OT, Salomaa V, Kaprio J, Järvelin M-R, Peltonen L, Perola M, Freimer NB, Ala-Korpela M, Palotie A, Ripatti S. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet. 2012;44(3):269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.