Abstract
Context
Depending on its lipolytic activity, glucagon plays a promising role in obesity treatment. Glucagon-induced growth hormone (GH) release can promote its effect on lipid metabolism, although the underlying mechanisms have not been well-defined.
Objective
The present study highlights the glucagon effect on the GH/insulinlike growth factor 1 (IGF-1)/IGF-binding protein (IGFBP) axis in vivo and in vitro, taking into consideration insulin as a confounding factor.
Materials and Methods
In a double-blind, placebo-controlled study, we investigated changes in GH, IGFBP, and IGF-1 bioactivity after intramuscular glucagon administration in 13 lean controls, 11 obese participants, and 13 patients with type 1 diabetes mellitus (T1DM). The effect of glucagon on the transcription factor forkhead box protein O1 (FOXO1) translocation, the transcription of GH/IGF-1 system members, and phosphorylation of protein kinase B (Akt) was further investigated in vitro.
Results
Despite unchanged total IGF-1 and IGFBP-3 levels, glucagon decreased IGF-1 bioactivity in all study groups by increasing IGFBP-1 and IGFBP-2. The reduction in IGF-1 bioactivity occurred before the glucagon-induced surge in GH. In contrast to the transient increase in circulating insulin in obese and lean participants, no change was observed in those with T1DM. In vitro, glucagon dose dependently induced a substantial nuclear translocation of FOXO1 in human osteosarcoma cells and tended to increase IGFBP-1 and IGFBP-2 gene expression in mouse primary hepatocytes, despite absent Akt phosphorylation.
Conclusions
Our data point to the glucagon-induced decrease in bioactive IGF-1 levels as a mechanism through which glucagon induces GH secretion. This insulin-independent reduction is related to increased IGFBP-1 and IGFBP-2 levels, which are most likely mediated via activation of the FOXO/mTOR (mechanistic target of rapamycin) pathway.
Our data have demonstrated that the IGFBP-1 and -2–mediated insulin-independent glucagon-induced decrease in IGF-1 bioactivity is a mechanism through which glucagon induces GH secretion.
To date, the role of the endocrine pancreas in the metabolic regulation of insulinlike growth factor 1 (IGF-1) bioavailability has been described but is not fully understood. The effect of insulin on the growth hormone (GH)/IGF-1 axis has been thoroughly investigated in studies describing insulin-induced GH receptor expression, downregulation of IGF-binding protein-1 (IGFBP-1) through forkhead box protein O (FOXO) modulation, and upregulation of IGFBP-2, together with the consequent decrease in IGF-1 bioactivity (1–4). However, information regarding the effect of glucagon as a counterregulatory hormone of insulin on the GH/IGF-1 axis is scarce. Glucagon is involved in glucose and lipid metabolism, and interest has been increasing regarding the role of glucagon agonism in obesity treatment.
Furthermore, the use of the glucagon stimulation test, among other various medical tests and procedures, to assess GH and cortisol deficiency in humans has been established (5–8). Nevertheless, the mechanisms underlying the effect of glucagon on GH release, which might enhance glucagon’s lipolytic activity, are unknown.
Given that glucagon is known to increase IGFBP-1 (9), we investigated the glucagon-induced modulation of IGF-1 bioactivity as a possible mechanism underlying the well-described glucagon-induced surge in GH secretion. To exclude changes in insulin as a well-known major confounder, we also performed our investigations in patients with type 1 diabetes mellitus (T1DM) to determine the insulin-independent effect of glucagon. Moreover, we investigated in vitro the glucagon-induced changes in IGFBPs and their effect on IGF-1 bioactivity and the role of FOXO1, which interacts with the promoter of the IGFBP-1 gene (10), as a possible mediator.
Materials and Methods
Randomized controlled human study
Study participants
A total of 37 subjects participated in the present study (Supplemental Table 1), including 13 lean participants, 11 obese healthy participants, and 13 patients with T1DM, as described previously (11). Using data from a full medical history, those subjects with type 2 diabetes mellitus, cardiovascular disease, evidence of impaired hepatic and/or renal function, uncontrolled hypertension, any current inflammatory or malignant disease, pregnancy, or treatment with any medication known to interact with hypothalamic-pituitary function or glucose homeostasis were excluded. Intact hypothalamic-pituitary function was determined by measuring the fasting baseline levels of adrenocorticotropic hormone, cortisol, thyrotropin, free thyroxine, prolactin, luteinizing hormone, follicle-stimulating hormone, testosterone, estradiol, and sex hormone-binding globulin and performing the insulin tolerance test and/or growth hormone-releasing hormone–arginine test for the assessment of GH and cortisol levels. A 75-g oral glucose tolerance test was performed to exclude the presence of type 2 diabetes mellitus and impaired glucose tolerance in both lean and obese subjects.
Study design
All subjects received glucagon 1 mg (GlucaGen; Novo Nordisk Pharma, Inc., Mainz, Germany) intramuscularly. Serum samples were obtained at 0, 60, 120, 180, and 240 minutes after the injection and were kept frozen at −80°C until analysis. T1DM patients received their last dose of long-acting insulin the evening before the test.
Study protocol
The ethical committee of the Charité University Medicine Berlin approved the study, which was performed according to the Declaration of Helsinki. All study participants provided written informed consent. The trial was registered at ClinicalTrials.gov (registration no. NCT00929812).
Assay of GH, IGF-1, and IGFBPs
The plasma glucagon concentration was measured by radioimmunoassay. Plasma GH, IGF-1, and IGFBP-3 concentrations were quantified in one run using a commercially available automated chemiluminescent immunometric assay (Immunodiagnostic Systems, GmbH, Frankfurt, Germany) according to the manufacturer’s recommendations. IGFBP-1 and IGFBP-2 were measured in duplicate in one run using enzyme-linked immunosorbent assays (DSL Deutschland GmbH, Sinsheim, Germany).
In vitro studies
Assay of IGF-1 bioactivity
IGF-1 bioactivity was measured using the kinase receptor activation assay, as described previously (12, 13), with slight modifications. Human embryonic kidney cells stably transfected with the human IGF-1 receptor were provided from Professor J. Frystyk (Institute of Clinical Medicine, Aarhus University, Aarhus, Denmark). After 48 hours of cell culture maintenance, the cells were stimulated with different concentrations of human recombinant IGF-1 for 16 minutes at 37°C to obtain a standard curve. The IGF-1 bioactivity was then determined after adding the serum samples from the participants, as previously described (1).
In addition, the cells were plated in 48-well plates, and the phosphorylation of the IGF-1 receptor was determined after stimulating the cells with glucagon (10 and 100 nM), IGF-1 (2 ng/mL), or long arginine 3-IGF-1 (IGF-1 LR3; 2 ng/mL), an IGF-1 analog that binds IGF-1 receptor (IGF-1R) and induces the same IGF-1 signaling without IGFBP binding, and various concentrations of IGFBP-1, IGFBP-2, and IGFBP-3. We used 2 ng/mL IGF-1 because of its known induction of a 30- to 40-fold increase in the phosphorylation of IGF-1R (14).
Determination of glucagon’s effects on IGF-1 and IGFBP gene expression and protein kinase B phosphorylation
The effects of glucagon on the gene expression of IGF-1 and its binding proteins and on protein kinase B (Akt) phosphorylation were determined using mouse primary hepatocytes.
Isolation and treatment of mouse primary hepatocytes.
Mouse primary hepatocytes were isolated as described previously (15). The cells were incubated with glucagon (10 nM) for 5 hours in the presence or absence of GH (300 µg/L, 24 hours). Another experiment was performed using different concentrations of glucagon (0.1, 1, 10, and 100 nM) to evaluate the glucagon-mediated dose-dependent effect on the transcription of IGF-1 system members. All stimulations were performed in four independent experiments in duplicate. The glucagon-mediated dose-dependent effect experiment was performed in three independent experiments in three technical repeats.
Gene expression assay.
Total RNA was extracted from stimulated mouse primary hepatocytes using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and complementary DNAs were synthesized with TaqMan Reverse Transcription Reagents (Applied Biosystems, Darmstadt, Germany). Quantitative real time-polymerase chain reaction was performed using the Power SYBR Green PCR Master Mix (Applied Biosystems) and the primers set listed in Supplemental Table 2. The amplification data were analyzed using ViiA7 RUO software for real time polymerase chain reaction system, version 1.2, and the obtained expression was normalized to the mouse hypoxanthine phosphoribosyltransferase 1 gene as a housekeeping gene.
Western blotting.
To study its effect on Akt phosphorylation, the mouse hepatocytes were treated with glucagon (10 nM) in the presence or absence of GH (300 µg/L) for 24 hours. After treatment, the cells were collected and homogenized in 10 mM Tris-HCl (pH 7.2), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate supplemented with protease, and phosphatase inhibitors (Roche Diagnostics, Mannheim, Germany). The protein concentration was measured using the bicinchoninic acid assay kit purchased from VWR International GmbH (Darmstadt, Germany). Monoclonal antibodies against phosphorylated Akt (Ser473), Akt (pan), and secondary anti-rabbit IgG antibodies (Cell Signaling Technology, Frankfurt, Germany) were used according to the manufacturer’s recommendation. After blotting and developing the membranes with Lumi-Light Western blotting substrate, detection and quantification were performed using the Molecular Imager® Gel Doc™ XR+ System with Image Laboratory™ software (Bio-Rad Laboratories GmbH, Munich, Germany).
FOXO1 translocation assay
The human osteosarcoma cell line U-2 was purchased from the European Animal and Cell Collection and maintained in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum. The cells were transfected with pEGFP-FOXO1 for stable expression of FOXO1 tagged at the C terminus with green fluorescent protein using Lipofectamine 2000 (Invitrogen), following the manufacturer’s instructions. The transfected cells were maintained in Dulbecco’s modified Eagle medium under selective conditions and stimulated with serial dilution of insulin (1 to 1000 nM), LY294002 (an inhibitor of the phosphatidylinositol 3-kinase [PI3K] pathway; 0.01 to 200 µM), and glucagon (1 to 1000 nM) for 1 hour, followed by fixation of the cells and nuclear staining with 4′,6-diamidino-2-paraphenylindole. A fluorescence microscopic FOXO1 translocation assay was performed using the BD Pathway 435 Bioimager (Becton Dickinson, Heidelberg, Germany), as described previously (14). An induced translocation factor was determined by measuring the nuclear and cytoplasmic green fluorescent protein intensities using the BD Image Data Explorer (Becton Dickinson), calculating the nuclear/cytoplasmic ratios and normalizing them to untreated cells. Translocation factors less than one represent an export and those greater than one an import of FOXO1 into the nuclei.
Statistical analysis
Statistical analyses for the human study were performed using SPSS, version 22 (IBM Corp., Armonk, NY). All data are expressed as the mean ± standard error of the mean. Baseline characteristics were compared as previously described (11). Serial changes in glucose, insulin, glucagon, GH, total IGF-1, IGFBP-3, IGFBP-1, IGFBP-2, and IGF-1 bioactivity were analyzed using analysis of variance for repeated measures. When the results in the full model were substantial, the changes were compared with the baseline values using Student’s t test for paired analysis in the case of normally distributed data. In the case of skewed data, the nonparametric Wilcoxon test was used. All P values were two-sided and were regarded as statistically significant after Bonferroni’s correction for multiple testing. The baseline value was calculated as the mean of the −30- and 0-minute values. The areas under the curve, calculated using the trapezoid method, were used to compare the time course of each parameter.
Data from the in vitro studies were derived from at least three independent experiments, performed either in triplicate or as four independent experiments in duplicate. Differences between groups were analyzed for statistical significance using Student’s t test. Statistical significance was assumed at P < 0.05.
Results
Effect of glucagon on GH, IGF-1, and IGF-1 bioactivity
Plasma glucagon increased significantly after intramuscular glucagon administration by approximately eight- to ninefold, with a peak detected after 30 minutes in lean (355.9 ± 21.7 ng/L), obese (324.8 ± 30.2 ng/L), and T1DM (340.6 ± 25.6 ng/L) participants and had returned to baseline levels by 240 minutes. The glucagon area under the curve at 240 minutes increased in all study groups and was comparable among the three groups (P = 0.3 to 0.4).
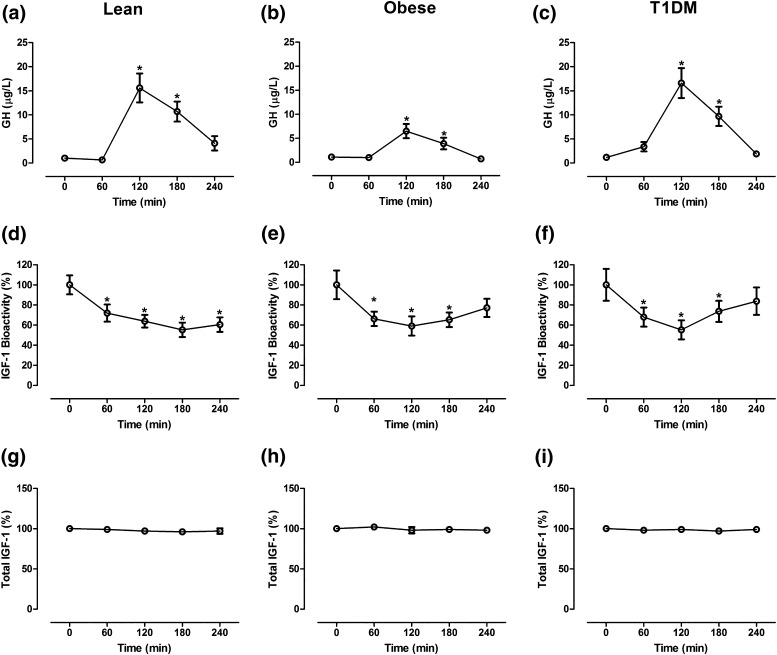
The baseline total IGF-1, IGF-1 bioactivity, and GH levels did not differ substantially in the obese or T1DM subjects compared with the lean controls. Glucagon administration induced a substantial increase in plasma GH levels in the lean [Fig. 1(a)], obese [Fig. 1(b)], and T1DM [Fig. 1(c)], participants, with a peak detected after 120 minutes. The GH levels had returned to baseline after 240 minutes. In contrast, glucagon induced a substantial reduction in serum IGF-1 bioactivity in all study groups [Fig. 1(d–f)]. The levels of circulating total IGF-1 in the lean [Fig. 1(g)], obese [Fig. 1(h)], and T1DM [Fig. 1(i)] participants showed nonsignificant changes throughout all measurement points.
Figure 1.
Changes in (a–c) GH, (d–f) IGF-1 bioactivity, and (g–i) total IGF-1 in 13 lean participants, 11 obese participants, and 13 T1DM patients after administration of glucagon. Data presented as mean ± standard error of the mean. *P < 0.01. The 0-minute value was calculated as the mean of two baseline values (−30 and 0 minutes). The bioactive and total IGF-1 values are presented relative to the baseline values.
Effect of glucagon on serum IGFBPs
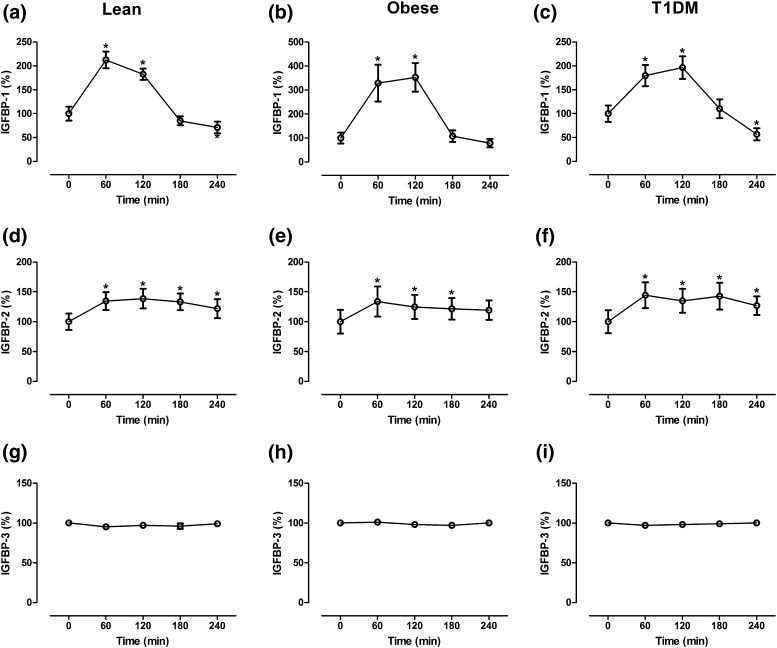
The baseline IGFBP-1 and IGFBP-2 levels were significantly lower in the obese subjects than in the lean controls (P < 0.01; Table 1). In contrast, the IGFBP-3 levels did not differ between the two groups. Similarly, the IGFBP-1, IGFBP-2, and IGFBP-3 levels were not substantially different between the T1DM patients and the lean controls. In the lean subjects, the serum IGFBP-1 levels showed a maximal increase after 60 minutes of glucagon administration, followed by a decrease toward baseline levels after 240 minutes [Fig. 2(a)]. In both obese [Fig. 2(b)] and T1DM [Fig. 2(c)] participants, the serum IGFBP-1 levels reached the maximal increase 120 minutes after glucagon administration and then decreased toward baseline levels.
Table 1.
Time Course of Total IGF-1, IGF-1 Bioactivity, IGFBP-1, IGFBP-2, IGFBP-3, and GH in Lean Participants, Patients With T1DM, and Obese Participants
| Variable | Measurement Point (min) | ||||
|---|---|---|---|---|---|
| Baseline | 60 | 120 | 180 | 240 | |
| IGF-1 (µg/L) | |||||
| LP | 181 ± 12.9 | 178.7 ± 11.9 | 178.2 ± 12.9 | 174.7 ± 12.4 | 173.7 ± 10.5 |
| T1DM | 162 ± 16.4 | 156 ± 14.8 | 161 ± 17.2 | 155 ± 14.6 | 160 ± 15.1 |
| OP | 175 ± 14.3 | 174 ± 14.6 | 170 ± 14.4 | 174 ± 14.1 | 169 ± 13.5 |
| IGF-1 bioactivity (µg/L) | |||||
| LP | 2.1 ± 0.2 | 1.51 ± 0.18a | 1.34 ± 0.13a | 1.16 ± 0.15a | 1.27 ± 0.15a |
| T1DM | 1.9 ± 0.3 | 1.29 ± 0.18a | 1.05 ± 0.18a | 1.4 ± 0.2a | 1.59 ± 0.26 |
| OP | 2.1 ± 0.3 | 1.39 ± 0.15a | 1.24 ± 0.2a | 1.37 ± 0.15a | 1.62 ± 0.19 |
| IGFBP-1 (µg/L) | |||||
| LP | 43.4 ± 6.4 | 92.2 ± 7.6a | 79.2 ± 5.1a | 36.8 ± 4 | 30.8 ± 5.3a |
| T1DM | 57.4 ± 9.9 | 103.2 ± 12.7a | 112.8 ± 13.6a | 63.3 ± 11.3 | 32.6 ± 7.3a |
| OP | 10.4 ± 2.4 | 34.2 ± 8a | 36.7 ± 6.2a | 11.2 ± 2.5 | 8.2 ± 1.8 |
| IGFBP-2 (µg/L) | |||||
| LP | 547 ± 75 | 736 ± 82a | 758 ± 89a | 729 ± 76a | 667 ± 87a |
| T1DM | 435 ± 84 | 628 ± 94a | 587 ± 87a | 621 ± 98a | 552 ± 68a |
| OP | 223 ± 44 | 298 ± 56a | 278 ± 45a | 271 ± 40a | 266 ± 36 |
| IGFBP-3 (mg/L) | |||||
| LP | 3.8 ± 0.1 | 3.6 ± 0.1 | 3.7 ± 0.1 | 3.7 ± 0.1 | 3.8 ± 0.1 |
| T1DM | 3.4 ± 0.2 | 3.3 ± 0.2 | 3.3 ± 0.2 | 3.3 ± 0.2 | 3.3 ± 0.2 |
| OP | 3.9 ± 0.1 | 3.9 ± 0.1 | 3.9 ± 0.1 | 3.9 ± 0.1 | 3.9 ± 0.1 |
| GH (µg/L) | |||||
| LP | 1.04 ± 0.4 | 0.66 ± 0.2 | 15.6 ± 3a | 10.7 ± 2.1a | 4.1 ± 1.5 |
| T1DM | 1.2 ± 0.3 | 3.4 ± 0.97 | 16.6 ± 3.1a | 9.7 ± 2a | 1.9 ± 0.4 |
| OP | 1.1 ± 0.5 | 0.98 ± 0.8 | 6.5 ± 1.5a | 3.9 ± 1.2a | 0.7 ± 0.2 |
Data presented as mean ± standard error of the mean.
P < 0.01 compared with baseline levels.
Figure 2.
Changes in (a–c) IGFBP-1, (d–f) IGFBP-2, and (g–i) IGFBP-3 in 13 lean participants, 11 obese participants, and 13 T1DM patients after administration of glucagon. Data presented as mean ± standard error of the mean. *P < 0.01. The 0-minute value was calculated as the mean of two baseline values (−30 and 0 minutes). All values are presented relative to the baseline values.
The IGFBP-2 levels showed a more sustained increase after glucagon administration in the lean [Fig. 2(d)], obese [Fig. 2(e)], and T1DM [Fig. 2(f)] participants. The circulating levels of IGFBP-3 showed nonsignificant changes in all study groups [Fig. 2(g–i)].
Effect of glucagon and IGFBPs on IGF-1 and IGF-1 LR3-induced phosphorylation of IGF-1R in vitro
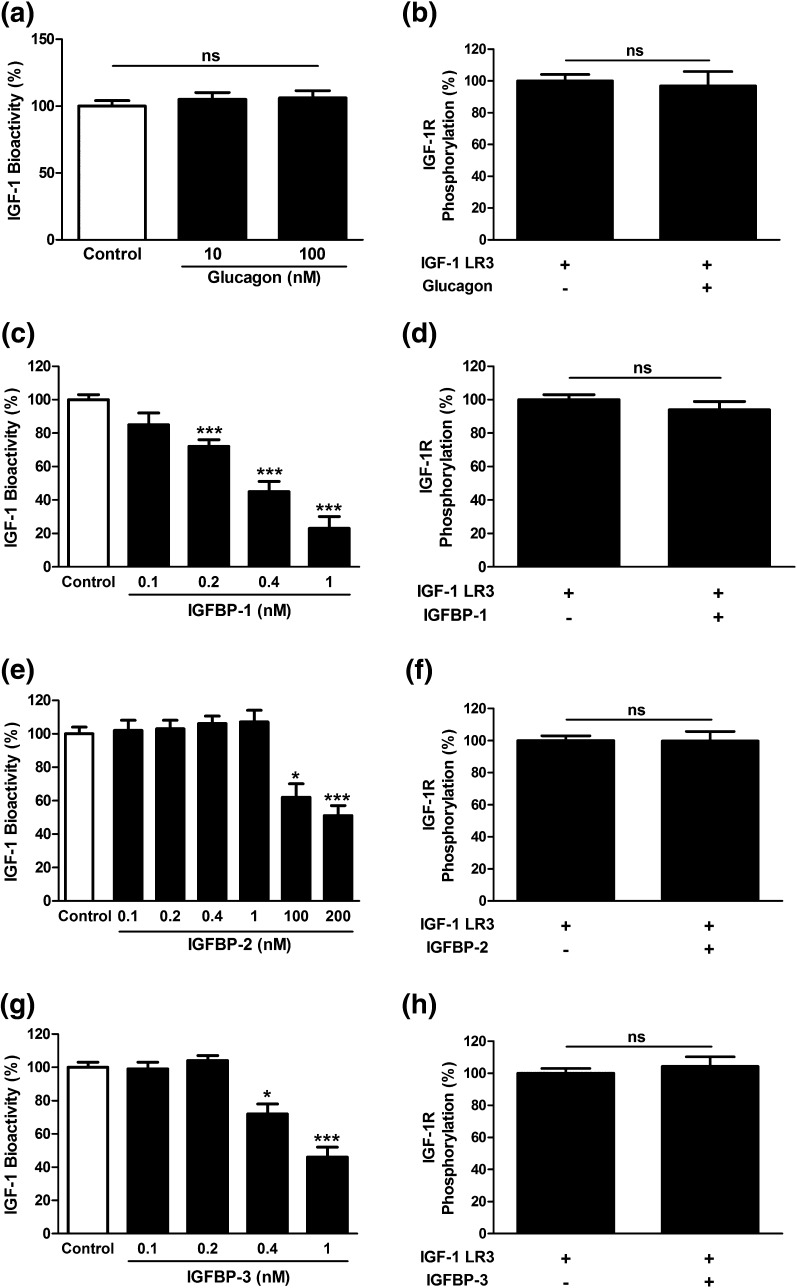
Although human embryonic kidney cells do not naturally possess the glucagon receptor (16), they were incubated with 10 and 100 nM glucagon, and IGF-1–induced phosphorylation of IGF-1R (2 ng/mL) was determined using a kinase receptor activation assay to exclude any possible interaction between glucagon and the IGF-1R. As expected, glucagon did not affect the IGF-1–induced IGF-1R phosphorylation [Fig. 3(a)]. Similarly, 100 nM of glucagon did not affect the IGF-1 LR3 (2 ng/mL)–induced phosphorylation of the IGF-1R [Fig. 3(b)].
Figure 3.
Effect of glucagon and IGFBPs on IGF-1 bioactivity and IGF-1 LR3-induced IGF-1R phosphorylation in vitro. Glucagon induced nonsignificant effects on (a) IGF-1 and (b) IGF-1 LR3-induced phosphorylation of the IGF-1R. (c) IGFBP-1 reduced IGF-1 bioactivity at dose levels of 0.2, 0.4, and 1 nM. (d) However, IGFBP-1 nonsignificantly affected IGF-1 LR3-induced phosphorylation of the IGF-1R. (e) IGFBP-2 reduced IGF-1 bioactivity at 100 and 200 nM. (f) IGFBP-2 nonsignificantly affected IGF-1 LR3-induced phosphorylation of the IGF-1R. (g) IGFBP-3 reduced the bioactivity of IGF-1, (h) with no influence on IGF-1 LR3-induced phosphorylation of the IGF-1R. Data presented as mean ± standard error of the mean, n = 8. *P < 0.05 and ***P < 0.001 versus control.
When added to IGF-1 (2 ng/mL), IGFBP-1 induced a statistically significant (P < 0.001) reduction in IGF-1 bioactivity at dose levels of 0.2, 0.4, and 1 nM [Fig. 3(c)]. In contrast, the presence of IGFBP-1 did not affect IGF-1 LR3-induced phosphorylation of the IGF-1R [Fig. 3(d)].
The inhibitory effect of IGFBP-2 on IGF-1–induced phosphorylation of the IGF-1R was first observed using concentrations of 100 and 200 nM [Fig. 3(e)], suggesting much less inhibitory potency compared with that of IGFBP-1. However, no inhibitory effect of IGFBP-2 was observed on IGF-1 LR3-induced phosphorylation of the IGF-1R [Fig. 3(f)]. IGFBP-3 significantly reduced the bioactivity of IGF-1 in vitro at concentrations of 0.4 nM (P < 0.05) and 1 nM [P < 0.001; Fig. 3(g)]. However, IGFBP-3 did not show any inhibitory influence on IGF-1 LR3 signaling [Fig. 3(h)].
Effect of glucagon on IGF-1 and IGFBP gene expression and Akt phosphorylation in vitro
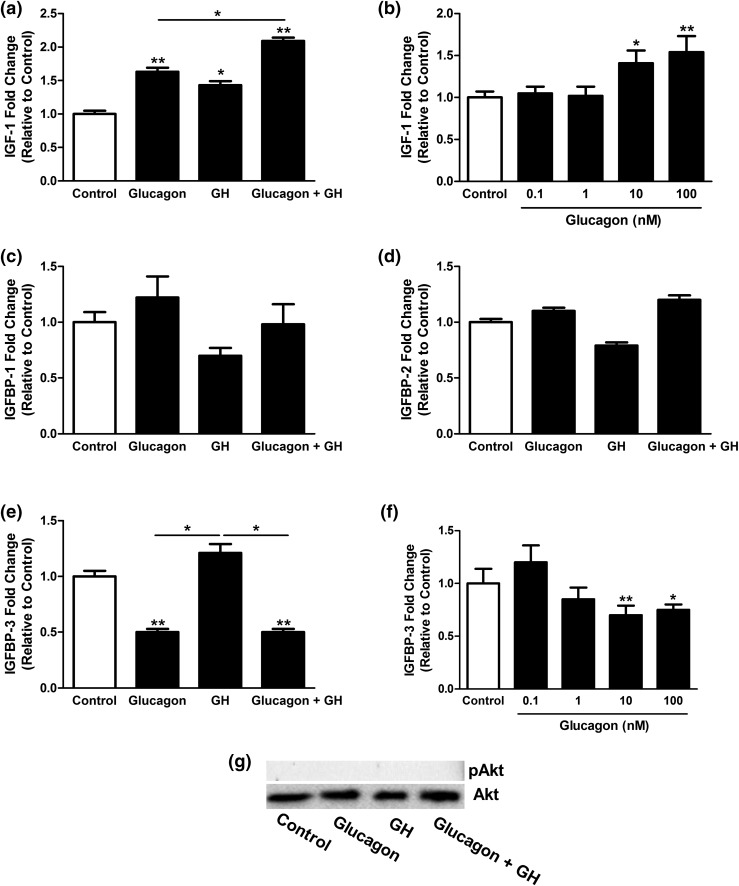
Mouse hepatocytes were isolated and treated with glucagon in the presence or absence of GH, and the expression levels of IGF-1 and IGFBP messenger RNA (mRNA) and Akt phosphorylation were determined. Treatment of mouse primary hepatocytes with GH alone for 24 hours significantly (P < 0.05) increased IGF-1 mRNA to 143% ± 5% of the control levels. The addition of 10 nM glucagon was able to increase significantly (P < 0.01) the basal and GH-stimulated IGF-1 mRNA levels further to 156% ± 7% and 208% ± 8% of the control levels, respectively [Fig. 4(a)]. Next, we tested the effect of different doses of glucagon on IGF-1 mRNA expression (Fig. 4(b)]. A statistically significant increase in the expression of IGF-1 mRNA was detected at 10 nM (P < 0.05) and 100 nM (P < 0.01) glucagon.
Figure 4.
Relative changes of (a and b) IGF-1, (c) IGFBP-1, (d) IGFBP-2, and (e) IGFBP-3 mRNA expression in mouse primary hepatocytes after treatment with glucagon and/or GH. A dose-dependent effect of glucagon was seen on (b) IGF-1 and (f) IGFBP-3 mRNA expression. (g) Akt phosphorylation after stimulation of mouse primary hepatocytes with glucagon and/or GH. Data presented as mean ± standard error of the mean, n = 8. *P < 0.05 and **P < 0.01 versus control.
Glucagon and/or GH induced nonsignificant (P > 0.05) changes in IGFBP-1 and IGFBP-2 mRNA expression levels [Fig. 4(c) and 4(d)]. However, GH tended to increase the transcription of IGFBP-3 to 118% ± 13%, although this increase was not statistically significant [Fig. 4(e)]. Glucagon (10 nM) decreased the basal and GH-stimulated IGFBP-3 mRNA expression to 49% ± 6% and 50% ± 6% of control levels, respectively. The dose-dependent effect of glucagon on IGFBP-3 mRNA abundance showed a substantial increase at doses of 10 and 100 nM [Fig. 4(f)].
Using Western blotting analysis, no phosphorylation of Akt after cell treatment with glucagon and/or GH was detected [Fig. 4(g)].
Effect of insulin and glucagon on FOXO1 translocation in U-2 osteosarcoma cells stably transfected with pEGFP-FOXO1
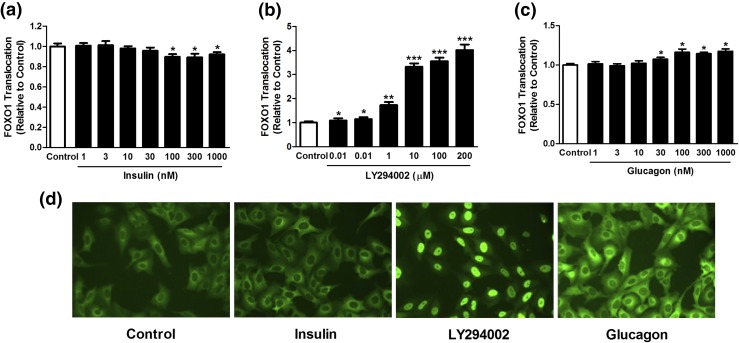
Insulin is known to modulate FOXO1 translocation through the PI3K/Akt pathway, resulting in decreased FOXO1 phosphorylation and its nuclear exclusion (17). The result of our experiments was as expected. Insulin resulted in an accumulation of FOXO1 in the cytoplasm. This effect was dose-dependent and proved to be substantial within the range of 100 to 1000 nM compared with the control cells treated with the solvent dimethyl sulfoxide only [Fig. 5(a)]. The inhibition of the PI3K pathway by 100 µM LY294002 decreased the cytoplasmic accumulation of FOXO. Again, the effect was dose-dependent within the range of 0.01 to 200 µM compared with the control cells [Fig. 5(b)]. Similarly, glucagon induced a substantial and dose-dependent accumulation of FOXO1 in the nucleus at concentrations of 30 to 1000 nM [Fig. 5(c)].
Figure 5.
Dose-dependent induction of nuclear FOXO-GFP translocation by (a) insulin, (b) LY294002, and (c) glucagon in U-2 osteosarcoma cells. (d) Representative images of control cells without stimulation mainly showing cytoplasmic localization of FOXO1-GFP, increased cytoplasmic localization of FOXO1-GFP after stimulation with insulin, LY294002-induced nuclear localization of FOXO1-GFP, and glucagon-induced nuclear localization of FOXO1-GFP. Data presented as mean ± standard error of the mean, n = 8. *P < 0.05, **P < 0.01, and **P < 0.01 versus control.
Discussion
In the present report, we have shown that glucagon induces a reduction in IGF-1 bioactivity that is independent of insulin and might serve as a mechanism to explain the well-known glucagon-induced surge in GH secretion. The reduction in IGF-1 bioactivity might be achieved by the concomitant increase in IGFBP-1 and -2. Furthermore, we have demonstrated glucagon-induced modulation of FOXO1 translocation as a possible mechanism explaining the glucagon-induced increase in IGFBP-1 levels.
Previously reported studies postulated that the glucagon-induced increase in GH secretion might result from a decrease in the plasma glucose concentrations after an initial transient surge or through a glucagon-induced increase of noradrenaline concentrations and its interaction with α-receptors in the pituitary gland (18). According to our results, glucagon induced an increase in GH levels in T1DM patients despite a sustained increase in glucose levels. In the same participants, we demonstrated that in T1DM glucose levels increased maximally after 60 minutes and thereafter returned toward baseline by 240 minutes. In contrast, only slight increases, followed by decreases toward baseline levels, were observed in the lean controls and obese participants (11). In addition, the plasma insulin concentrations did not change after glucagon or placebo administration in patients with T1DM (P = 0.2 to 0.6). In the lean and obese participants, the plasma insulin levels changed in parallel with the plasma glucose levels, with a similar increase achieving a peak after 30 minutes, followed by a decrease toward baseline level after 120 minutes (11). Furthermore, the plasma catecholamine levels remained unchanged after administration of glucagon (data not shown). These results suggest a mechanism by which glucagon might affect GH release, depending on the regulatory feedback mechanisms at both the pituitary gland and the hypothalamus. The effects were preserved in patients with T1DM patients and were, hence, insulin-independent.
IGF-1 signaling is determined by free or unbound IGF-1 and, accordingly, by the presence and interplay between IGFBPs such as IGFBP-1, IGFBP-2, and IGFBP-3 and total IGF-1 (19). The IGFBPs interact with IGF-1 and prevent it from interacting with the IGF-1R (20) and are, therefore, able to reduce IGF-1 bioactivity (21, 22). In our study, the observed decrease in IGF-1 bioactivity after glucagon administration was not due to changes in total IGF-1 or IGFBP-3 concentrations but rather was related to a remarkable increase in serum IGFBP-1 and IGFBP-2 levels. This inverse and highly dynamic relationship between bioactive IGF-1 and IGFBP-1 and IGFBP-2, respectively, is in accordance with our in vitro experiments and previous observations in patients with end-stage renal failure undergoing hemodialysis (23), patients with T1DM during insulin administration (24, 25), and healthy subjects undergoing an oral glucose tolerance test (1).
IGFBP-1 is regulated mainly by the nuclear translocation of proteins called FOXO that bind to the IGFBP-1 promoter and increase its expression. It has been reported that insulin decreases IGFBP-1 levels through the PI3K/Akt/FOXO pathway (17). In the present study, we found a mild increase in IGFBP-1 gene expression by glucagon, consistent with the findings from Hilding et al. (9, 26), who demonstrated that glucagon induces an increase in IGFBP-1 secretion in vivo and in vitro, despite the lack of detectable changes at the transcriptional level. In addition, a dose-dependent glucagon-induced increase occurred in nuclear import/activation of FOXO1. Nevertheless, FOXO activity and its nuclear-cytoplasmic shuttling is determined, in addition to its PI3K/Akt pathway-mediated phosphorylation/dephosphorylation, by its acetylation/deacetylation state, which is controlled by multiple factors such as Sir2 (silent information regulator 2), SIRT1 (mammalian ortholog sirtuin 1), and histone deacetylases class IIa (27, 28). The glucagon-induced activation of class I/IIa histone deacetylases and the consequent deacetylation and hence activation of FOXO has been previously reported (29).
IGFBP-2 is another metabolically regulated modulator of IGF-1 bioactivity (1). We previously showed that insulin reduces IGF-1 bioactivity through an increase in IGFBP-2 levels (1). Li et al. (3) reported that insulin upregulates IGFBP-2 expression through a mechanistic target of rapamycin (mTOR)-dependent pathway. In agreement with this, we have shown, to the best of our knowledge, for the first time that glucagon increases IGFBP-2, independent of changes in circulating insulin concentrations, although the exact mechanisms involved remain to be elucidated. Although we could not detect a statistically significant increase in IGFBP-2 gene expression, the levels were numerically greater after glucagon stimulation. Therefore, an effect of glucagon on IGFBP-2 expression could not be ruled out. Mothe-Satney et al. (30) demonstrated that glucagon induces mTOR phosphorylation on serine 2448. Other studies have shown that the PI3K pathway is involved in mediating some of the glucagon-induced effects (31, 32). Hence, it can be postulated that the glucagon-induced upregulation of IGFBP-2 secretion is caused by glucagon-mediated induction of PI3K signaling and the consequent activation/phosphorylation of the mTOR pathway.
Glucagon has previously been reported to be a potent stimulus of cortisol secretion (5, 33). Moreover, the IGFBP-1 promoter also contains a glucocorticoid responsive element, and glucocorticoids might have a stimulatory effect on IGFBP-1 production (34). However, the glucagon-induced surge in cortisol secretion was first seen at 120 minutes after glucagon administration (33), but glucagon was able to significantly increase the IGFBP-1 and IGFBP-2 levels within the first 60 minutes after administration. This could argue somewhat against a role of cortisol in mediating the observed increase in IGFBP-1 and IGFBP-2 levels.
In conclusion, we have shown that glucagon decreases bioactive IGF-1 levels independently of endogenous insulin. Moreover, we have demonstrated that this reduction in IGF-1 bioactivity is possibly causally related to an increase in IGFBP-1 and IGFBP-2 levels that is, in turn, most likely mediated via a glucagon-induced activation of the FOXO/mTOR pathway. Thus, our findings provide a mechanism that explains the known glucagon-induced surge in GH secretion.
Considering the well-known association between changes in GH, IGF-1, IGFBPs, and some types of cancer, our findings could have further implications in understanding the close link between pancreatic hormone levels and tumorigenesis—a safety issue that might be of special value with respect to future treatments based on glucagon agonism.
Supplementary Material
Acknowledgments
We thank Katrin Sprengel from the German Institute of Human Nutrition Potsdam-Rehbruecke for her technical assistance.
This study was supported by the graduate school from German Research Foundation Grant GK1208 (to D.F.G.). A.L.B. was funded by Grant BI 1292/4-1 from the German Research Foundation. A.M.A and A.M.M. were supported by Alexander von Humboldt Foundation Grant 1158232.
Author contributions: Z.S. and A.M.A. designed the study, acquired and analyzed the data, and wrote the manuscript. A.A., C.B.-V., B.A., A.M.M., and S.L. collected and analyzed the data and edited and reviewed the manuscript. M.O.W., V.B., J.F., M.M., J.S., A.L.B., and A.F.H.P. contributed to the study design and reviewed, edited, and critically revised the manuscript. A.M.M. reviewed, edited, and critically revised the manuscript. All authors gave final approval of the current version for publication. Z.S. and A.M.A. take full responsibility for the content of the article.
Clinical trial registry: ClinicalTrials.gov no. NCT00929812 (registered 29 June 2009).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- Akt
protein kinase B
- FOXO1
forkhead box protein O1
- GH
growth hormone
- IGF-1
insulinlike growth factor 1
- IGFBP
insulinlike growth factor-binding protein
- IGF-1 LR3
long arginine 3-insulinlike growth factor 1
- IGF-1R
insulinlike growth factor 1 receptor
- mRNA
messenger RNA
- mTOR
mechanistic target of rapamycin
- PI3K
phosphoinositide 3-kinase
- T1DM
type 1 diabetes mellitus.
References
- 1. Arafat AM, Weickert MO, Frystyk J, Spranger J, Schöfl C, Möhlig M, Pfeiffer AF. The role of insulin-like growth factor (IGF) binding protein-2 in the insulin-mediated decrease in IGF-I bioactivity. J Clin Endocrinol Metab. 2009;94(12):5093–5101. [DOI] [PubMed] [Google Scholar]
- 2. Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab. 2000;85(12):4712–4720. [DOI] [PubMed] [Google Scholar]
- 3. Li Z, Miard S, Laplante M, Sonenberg N, Picard F. Insulin stimulates IGFBP-2 expression in 3T3-L1 adipocytes through the PI3K/mTOR pathway. Mol Cell Endocrinol. 2012;358(1):63–68. [DOI] [PubMed] [Google Scholar]
- 4. Xu J, Ji S, Venable DY, Franklin JL, Messina JL. Prolonged insulin treatment inhibits GH signaling via STAT3 and STAT1. J Endocrinol. 2005;184(3):481–492. [DOI] [PubMed] [Google Scholar]
- 5. Leong KS, Walker AB, Martin I, Wile D, Wilding J, MacFarlane IA. An audit of 500 subcutaneous glucagon stimulation tests to assess growth hormone and ACTH secretion in patients with hypothalamic-pituitary disease. Clin Endocrinol (Oxf). 2001;54(4):463–468. [DOI] [PubMed] [Google Scholar]
- 6. Romero CJ, Pine-Twaddell E, Sima DI, Miller RS, He L, Wondisford F, Radovick S. Insulin-like growth factor 1 mediates negative feedback to somatotroph GH expression via POU1F1/CREB binding protein interactions. Mol Cell Biol. 2012;32(21):4258–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Secco A, di Iorgi N, Napoli F, Calandra E, Ghezzi M, Frassinetti C, Parodi S, Casini MR, Lorini R, Loche S, Maghnie M. The glucagon test in the diagnosis of growth hormone deficiency in children with short stature younger than 6 years. J Clin Endocrinol Metab. 2009;94(11):4251–4257. [DOI] [PubMed] [Google Scholar]
- 8. Yuen KC. Glucagon stimulation testing in assessing for adult growth hormone deficiency: current status and future perspectives. ISRN Endocrinol. 2011;2011:608056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hilding A, Brismar K, Thorén M, Hall K. Glucagon stimulates insulin-like growth factor binding protein-1 secretion in healthy subjects, patients with pituitary insufficiency, and patients with insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77(5):1142–1147. [DOI] [PubMed] [Google Scholar]
- 10. Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16(4):183–189. [DOI] [PubMed] [Google Scholar]
- 11. Arafat AM, Weickert MO, Adamidou A, Otto B, Perschel FH, Spranger J, Möhlig M, Pfeiffer AF. The impact of insulin-independent, glucagon-induced suppression of total ghrelin on satiety in obesity and type 1 diabetes mellitus. J Clin Endocrinol Metab. 2013;98(10):4133–4142. [DOI] [PubMed] [Google Scholar]
- 12. Chen JW, Ledet T, Orskov H, Jessen N, Lund S, Whittaker J, De Meyts P, Larsen MB, Christiansen JS, Frystyk J. A highly sensitive and specific assay for determination of IGF-I bioactivity in human serum. Am J Physiol Endocrinol Metab. 2003;284(6):E1149–E1155. [DOI] [PubMed] [Google Scholar]
- 13. Reinhard M, Frystyk J, Jespersen B, Bjerre M, Christiansen JS, Flyvbjerg A, Ivarsen P. Effect of hyperinsulinemia during hemodialysis on the insulin-like growth factor system and inflammatory biomarkers: a randomized open-label crossover study. BMC Nephrol. 2013;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bumke-Vogt C, Osterhoff MA, Borchert A, Guzman-Perez V, Sarem Z, Birkenfeld AL, Bähr V, Pfeiffer AF. The flavones apigenin and luteolin induce FOXO1 translocation but inhibit gluconeogenic and lipogenic gene expression in human cells. PLoS One. 2014;9(8):e104321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Döcke S, Lock JF, Birkenfeld AL, Hoppe S, Lieske S, Rieger A, Raschzok N, Sauer IM, Florian S, Osterhoff MA, Heller R, Herrmann K, Lindenmüller S, Horn P, Bauer M, Weickert MO, Neuhaus P, Stockmann M, Möhlig M, Pfeiffer AF, von Loeffelholz C. Elevated hepatic chemerin mRNA expression in human non-alcoholic fatty liver disease. Eur J Endocrinol. 2013;169(5):547–557. [DOI] [PubMed] [Google Scholar]
- 16. Cypess AM, Unson CG, Wu CR, Sakmar TP. Two cytoplasmic loops of the glucagon receptor are required to elevate cAMP or intracellular calcium. J Biol Chem. 1999;274(27):19455–19464. [DOI] [PubMed] [Google Scholar]
- 17. Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011; 1813:1978–1986 [DOI] [PubMed] [Google Scholar]
- 18. Glynn N, Agha A. Diagnosing growth hormone deficiency in adults. Int J Endocrinol. 2012;2012:972617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siddals KW, Westwood M, Gibson JM, White A. IGF-binding protein-1 inhibits IGF effects on adipocyte function: implications for insulin-like actions at the adipocyte. J Endocrinol. 2002;174(2):289–297. [DOI] [PubMed] [Google Scholar]
- 20. Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175(1):19–31. [DOI] [PubMed] [Google Scholar]
- 21. Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23(6):824–854. [DOI] [PubMed] [Google Scholar]
- 22. Frystyk J. Quantification of the GH/IGF-axis components: lessons from human studies. Domest Anim Endocrinol. 2012;43(2):186–197. [DOI] [PubMed] [Google Scholar]
- 23. Ivarsen P, Chen JW, Tietze I, Christiansen JS, Flyvbjerg A, Frystyk J. Marked reductions in bioactive insulin-like growth factor I (IGF-I) during hemodialysis. Growth Horm IGF Res. 2010;20(2):156–161. [DOI] [PubMed] [Google Scholar]
- 24. Ma Z, Christiansen JS, Laursen T, Lauritzen T, Frystyk J. Short-term effects of NPH insulin, insulin detemir, and insulin glargine on the GH-IGF1-IGFBP axis in patients with type 1 diabetes. Eur J Endocrinol. 2014;171(4):471–479. [DOI] [PubMed] [Google Scholar]
- 25. Ma Z, Christiansen JS, Laursen T, Wu C, Lauritzen T, Parkner T, Frystyk J. Effects of human insulin and insulin aspart preparations on levels of IGF-I, IGFBPs and IGF bioactivity in patients with type 1 diabetes. BMC Endocr Disord. 2014;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hilding A, Möller C, Hall KE. Glucagon and GLP-1 stimulate IGFBP-1 secretion in Hep G2 cells without effect on IGFBP-1 mRNA. Growth Horm IGF Res. 2002;12(1):60–68. [DOI] [PubMed] [Google Scholar]
- 27. Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7(2):104–112. [DOI] [PubMed] [Google Scholar]
- 28. von Meyenn F, Porstmann T, Gasser E, Selevsek N, Schmidt A, Aebersold R, Stoffel M. Glucagon-induced acetylation of Foxa2 regulates hepatic lipid metabolism. Cell Metab. 2013;17(3):436–447. [DOI] [PubMed] [Google Scholar]
- 29. Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, Downes M, Evans RM, Montminy M, Shaw RJ. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145(4):607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mothe-Satney I, Gautier N, Hinault C, Lawrence JC Jr, Van Obberghen E. In rat hepatocytes glucagon increases mammalian target of rapamycin phosphorylation on serine 2448 but antagonizes the phosphorylation of its downstream targets induced by insulin and amino acids. J Biol Chem. 2004;279(41):42628–42637. [DOI] [PubMed] [Google Scholar]
- 31. Gradilone SA, Carreras FI, Lehmann GL, Marinelli RA. Phosphoinositide 3-kinase is involved in the glucagon-induced translocation of aquaporin-8 to hepatocyte plasma membrane. Biol Cell. 2005;97(11):831–836. [DOI] [PubMed] [Google Scholar]
- 32. Harney JA, Rodgers RL. Insulin-like stimulation of cardiac fuel metabolism by physiological levels of glucagon: involvement of PI3K but not cAMP. Am J Physiol Endocrinol Metab. 2008;295(1):E155–E161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arafat AM, Perschel FH, Otto B, Weickert MO, Rochlitz H, Schöfl C, Spranger J, Möhlig M, Pfeiffer AF. Glucagon suppression of ghrelin secretion is exerted at hypothalamus-pituitary level. J Clin Endocrinol Metab. 2006;91(9):3528–3533. [DOI] [PubMed] [Google Scholar]
- 34. Lee PD, Giudice LC, Conover CA, Powell DR. Insulin-like growth factor binding protein-1: recent findings and new directions. Proc Soc Exp Biol Med. 1997;216:319–357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.