Abstract
Objective:
The aim of the study was to investigate the role of microRNA-21 (miR-21) in cardiomyocyte apoptosis and to determine a possible mechanism.
Methods:
H9c2 embryonic rat heart-derived cells were used in the study. Cell viability was determined using the 3-(4.5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay, and flow cytometry was used to evaluate cell apoptosis. Reverse transcription-polymerase chain reaction and western blot assays were used to detect mRNA and protein expression of the apoptosis-related proteins and miR-21. ELISA was used to detect reactive oxygen species (ROS).
Results:
Palmitate exposure greatly reduced miR-21 expression in cardiomyocytes. Apoptosis increased when miR-21 was inhibited with or without palmitate exposure. Consistently, reduced apoptosis was observed when miR-21 was overexpressed in cardiomyocytes. Caspase-3 activity was reduced after palmitate exposure. Bcl-2 protein expression was increased in H9c2 cells when transfected with the miR-21 mimic. MiR-21 overexpression alone did not induce ROS or DNA fragmentation; however, in conjunction with palmitate exposure, miR-21 mimic reduced ROS and DNA fragmentation. Moreover, palmitate administration overcame the antioxidant effect of 3 mM N-acetylcysteine to significantly inhibit apoptosis, DNA fragmentation, and caspase-3 activity. The exposure to palmitate greatly reduced p65 and p-p38 expression in the nucleus. A p38 inhibitor had no effect on the expression of Bcl-2 and cleaved caspase-3 in H9c2 cells alone; however, when combined with exposure to palmitate the p38 inhibitor induced Bcl-2 expression and inhibited caspase-3 activity. The p38 inhibitor by itself did not induce apoptosis, ROS production, or DNA fragmentation in H9c2 cells, but when palmitate was included with the p38 inhibitor, apoptosis, ROS production, and DNA fragmentation were reduced.
Conclusion:
miR-21 protects cardiomyocytes from apoptosis that is induced by palmitate through the caspase-3/NF-κB signal pathways.
Keywords: miR-21, palmitate-induced apoptosis, cardiomyocyte, caspase-3/NF-κB signal pathways
Introduction
Mature cardiomyocytes are easily influenced by fatty acid metabolism disorders caused by diseases such as diabetes, obesity, and hyperlipidemia (1). An abnormal accumulation of lipid in the myocardium results in glucose and lipid metabolism disorders, which damage heart function (2, 3). Saturated fatty acids are especially damaging and have been reported to cause apoptosis of cardiomyocytes, islet beta cells, hepatic cells, vascular smooth muscle cells, and vascular endothelial cells (4-7). Of the saturated fatty acids, palmitate has been shown to accumulate in cardiomyocytes, leading to so-called “fat toxicity,” which can result in heart dysfunction, heart failure, and apoptosis (8-12). MicroRNAs (miRs), a class of endogenous non-coding RNAs approximately 22 nucleotides long, negatively regulate gene expression by inhibiting mRNA transcription. They play an important role in cell proliferation, metabolism, differentiation, and the occurrence and development of various diseases (13-15). The changes in the levels of miRs are related to many cardiac diseases, including arrhythmia, myocardial infarction, myocardial fibrosis, and heart failure (16-19). A previous study detected abnormal expression of miR-21 in H9c2 cells when they were exposed to palmitate. The miR-21 expression level directly correlated with the apoptosis rate, indicating that miR-21 might be involved in the regulation of cell death. Palmitate was reported to induce apoptosis of cardiomyocytes through either the PI3K/AKT or AMPK signaling pathway (20, 21). In models of bacterial lipopolysaccharide induction, a reactive oxygen species (ROS)-dependent pathway was found to be regulated by miR-146a (22). Furthermore, the expression of 23 miRNAs was altered after H2O2 treatment in normal human fibroblasts (23). All these observations indicate that the expression of miRs play a significant role in fatty acid-induced apoptosis. However, few studies have focused on the exact mechanism of how certain miRs work. In the present study, we employ a model of palmitate-induced cardiomyocyte apoptosis to explore whether miR-21 is related to the regulation of cell death and to investigate a possible underlying mechanism.
Methods
Cell culture
H9c2 embryonic rat heart-derived cells were obtained from Academia Sinica (Shanghai, China). Cells were cultured in DMEM containing 15% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2.
Cell viability assay
H9c2 cells were seeded at a density of 5×103 cells/well in 96-well plates, and cell viability was determined using the MTT assay. The cells were incubated with palmitate at various concentrations (0.2, 0.4, and 0.6 mM) for 12, 18, and 24 h. Following palmitate incubation, each well was washed twice with PBS to remove the medium before 10 µL 0.5 mg/mL MTT was added to each well and incubated for an additional 4 h at 37°C. The absorbance at 490 nm was read on a microplate reader and used as a measurement of cell viability. The absorbance was normalized to cells incubated in control medium, which were considered 100% viable.
Flow cytometry
Apoptotic cells were detected using the Annexin V-FITC/propidium iodide kit (Vazyme Biotech, Nanjing, China) according to the manufacturer’s instructions. In brief, the palmitate-treated cells were washed twice with PBS and dislodged using 0.25% trypsin. Cells were centrifuged and resuspended in PBS containing 50 µg/mL propidium iodide, 0.1 g/L RNase, and 1% bovine serum albumin. The cells were then incubated at 37°C for 30 min in the dark before analysis.
Western blot analysis
Cell lysates were prepared and 20 µg of these were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Specific monoclonal anti-cleaved caspase-3 [Cell Signal Technology (CST), SN: 4380, dilution: 1:2000], monoclonal anti-Bcl-2 (CST, SN: 11988, dilution: 1:2000), monoclonal anti-p65 (CST, SN: 5741, dilution: 1:2000), monoclonal anti-p-p38 (CST, SN: 3195, dilution: 1:2000), and monoclonal anti-β-actin (CST, SN: 8457, dilution: 1:4000) antibodies were used. HRP-conjugated immunoglobulin was used as the secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). West Pico chemiluminescence was used as the substrate to visualize protein bands, which were quantified using densitometric image analysis software (Image Master VDS; Pharmacia Biotech) and normalized to β-actin expression.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using the Trizol reagent (Invitrogen, San Diego, CA, USA). The first strand of cDNA was synthesized using a reverse transcription kit (PrimeScript™ Synthesis kit, Takara Bio, Inc., Dalian, China). RT-PCR was performed using the SYBR Premix Ex Taq Kit (Takara Bio, Inc., Dalian, China) on an Applied Biosystems 7500 Real-Time PCR system (Applied Biosystems, White Plains, NY, USA). β-actin was used as an internal control. The experiment was performed in triplicates. Primers for miR-21 were designed and synthesized by Invitrogen China.
ROS detection
ROS production was measured using an ELISA kit (Vazyme Biotech) according to the manufacturer’s instructions. Measurements are given as fold changes compared with control.
Statistical analysis
Experimental results are presented as the mean ± standard deviation. Comparisons between two groups were conducted using the two-tailed Student’s t-test or the Chi square test (SPSS 18.0, Chicago, IL, USA). The comparison of multiple groups was analyzed using ANOVA with Holm–Sidak’s or Dunnett’s multiple comparisons test (GraphPad Prism 6.0, La Jolla, CA, USA). Differences were considered statistically significant when the p value was <0.05.
Results
Palmitate inhibits H9c2 proliferation and induces H9c2 apoptosis
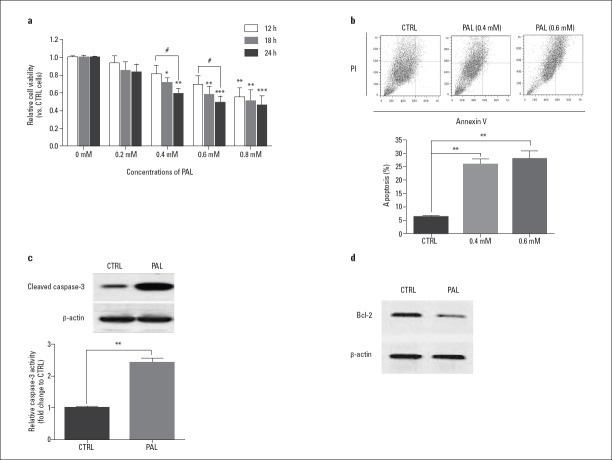
H9c2 was exposed to 0.2, 0.4, 0.6, and 0.8 mM palmitate for 12, 18, and 24 h. Significant differences in cell viability were detected at 0.4 and 0.6 mM palmitate exposure for 18 and 24 h when compared with controls, which had not been treated with palmitate (Fig. 1a, Fig. 1 Table 1-3). The apoptosis rate of H9c2 cells was measured after 24 h of exposure to 0.4 and 0.6 mM palmitate. The rate significantly increased in response to both concentrations compared with control, but there was no significant difference in the apoptosis rate between the two concentrations (Fig. 1b, Fig. 1 Table 1-3). Based on this, 0.4 mM palmitate for 24 h was used as the exposure condition in subsequent experiments. In addition, both the expression of cleaved caspase-3 and caspase-3 activity (3-fold) was increased, whereas Bcl-2 expression was inhibited, compared with the β-actin internal control after 24 h of exposure to palmitate (Fig. 1c and 1d, Table 1-3).
Figure 1.
Palmitate-induced H9c2 cardiomyocyte apoptosis.
(a) H9c2 cells treated with palmitate. (b) The apoptosis rate of H9c2 significantly increased after 24 h of exposure to 0.4 and 0.6 mM palmitate compared with the control. No significant difference in apoptosis was observed between the two concentrations. Western blot detected cleaved caspase-3 (c) and Bcl-2 (d) expression after 24 h of exposure to 0.4 mM palmitate
Table 1.
Relative cell viabilities of H9c2 treated with different concentrations of palmitate (0, 0.2, 0.4, 0.6, and 0.8 mM) for varying periods of time (12, 18, and 24 h)
| 12h | 18h | 24h | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | Mean | SD | n | |
| 0 | 1 | 0.03 | 5 | 1 | 0.04 | 5 | 1 | 0.02 | 5 |
| 0.2 | 0.93 | 0.18 | 5 | 0.81 | 0.21 | 5 | 0.83 | 0.19 | 5 |
| 0.4 | 0.81 | 0.21 | 5 | 0.71 | 0,11 | 5 | 0.59 | 0.12 | 5 |
| 0.6 | 0.69 | 0.22 | 5 | 0.58 | 0.20 | 5 | 0.49 | 0.15 | 5 |
| 0.8 | 0.55 | 0.18 | 5 | 0.51 | 0.27 | 5 | 0.46 | 0.23 | 5 |
SD - standard deviation
Table 2.
Apoptosis rate of H9c2 after 24 h of exposure to 0, 0.4, and 0.6 mM palmitate
| 0 mM | 0.4 mM | 0.6 mM | |
|---|---|---|---|
| Mean±SD | 6.220±0.2587 | 25.80±2.103 | 27.97±2.692 |
| n=3 |
SD - standard deviation
Table 3.
Relative caspase-3 activity of H9c2 cells after 24 h of exposure to 0.4 mM palmitate
| CTRL | 0.4 mM | |
|---|---|---|
| Mean±SEM | 1.000±0.007 | 2.416±0.169 |
| n=5 |
SEM - standard error of the mean
Role of miR-21 in palmitate-induced H9c2 cell apoptosis
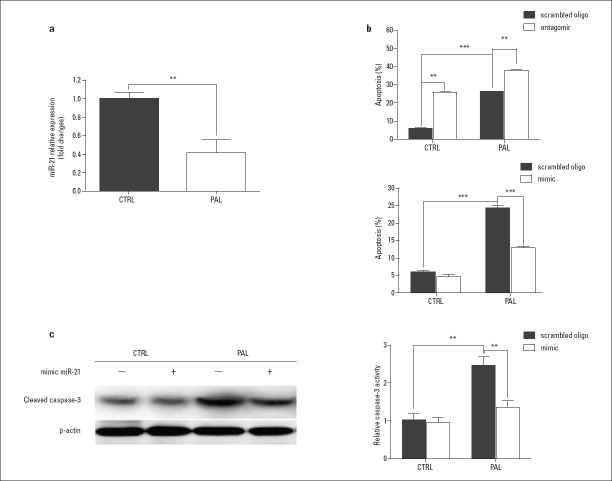
Compared with an internal control, miR-21 expression was reduced almost 3-fold after 24 h of exposure to 0.4 mM palmitate (Fig. 2a, Fig. 2 Table 4-6). The apoptosis rate was increased when miR-21 was inhibited by an antagomir (an inhibitory miR-21 mimic), both in the presence and absence of palmitate, whereas a reduction in apoptosis was observed when a miR-21 mimic was expressed by transfection (Fig. 2b, Fig. 2 Table 4-6). Moreover, cleaved caspase-3 expression was stable when transfected with the miR-21 mimic and was reduced after palmitate treatment (Fig. 2c, Fig. 2 Table 4-6).
Figure 2.
Role of miR-21 in palmitate-induced H9c2 cell apoptosis.
(a) MiR-21 expression was reduced by 60% after 24 h of exposure to 0.4 mM palmitate. (b) H9c2 cell apoptosis rate was detected with transfection of a miR-21 antagomir (a miR-21 mimic) and a scrambled oligonucleotide after 24 h of exposure to 0.4 mM palmitate. (c) Caspase-3 expression was measured with transfection of the miR-21 mimic with or without palmitate exposure
Table 4.
Relative miR-21 expression of H9c2 cells after 24 h of exposure to 0.4 mM palmitate
| CTRL | PAL | |
|---|---|---|
| Mean±SEM | 1.000±0.066 | 0.410±0.139 |
| n=5 |
SEM - standard error of the mean
Table 5.
Apoptosis rate of H9c2 cells transfected with a miR-21 antagomir (or a miR 21 mimic) and a scrambled oligonucleotide after 24 h exposure to 0.4 mM palmitate (mean±SD, n=5)
| Scrambled oligo | Antagomir | miR-21 mimic | |
|---|---|---|---|
| CTRL | 5.88±0.40 | 25.67±0.22 | 4.53±0.55 |
| PAL | 24.22±0.13 | 37.92±0.37 | 12.81±0.32 |
Table 6.
Relative caspase-3 activity of H9c2 cells transfected with a miR-21 mimic and a scrambled oligonucleotide after 24 h exposure to 0.4 mM palmitate (mean±SD, n=5)
| Scrambled oligo | miR-21 mimic | |
|---|---|---|
| CTRL | 1.00±0.34 | 0.92±0.28 |
| PAL | 2.44±0.52 | 1.33±0.41 |
Effect of miR-21 on Bcl-2 expression and ROS production
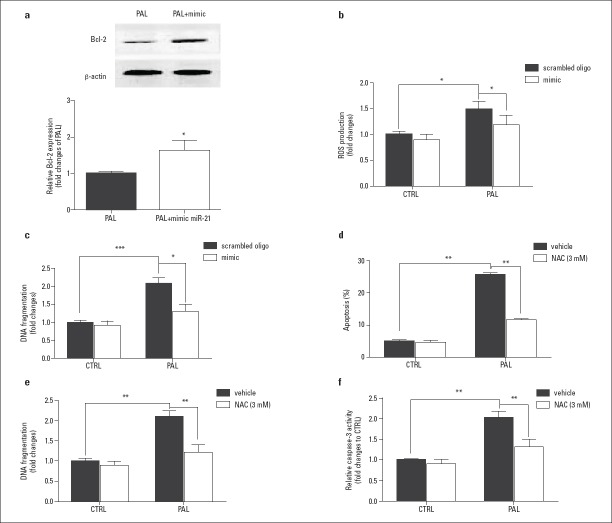
Bcl-2 protein expression was higher in H9c2 cells that were transfected with the miR-21 mimic in combination with palmitate exposure (Fig. 3a, Fig. 3 Table 7-12). The overexpressed miR 21 alone did not induce ROS and DNA fragmentation in H9c2 cells. However, when cells were treated with palmitate, the miR-21 mimic downregulated ROS and DNA fragmentation compared with a scrambled oligo group (Fig. 3b and 3c, Table 7-12). Moreover, 3 mM N acetylcysteine (NAC, an antioxidant agent) significantly inhibited apoptosis, DNA fragmentation, and cleaved caspase-3 expression in H9c2 cells exposed to palmitate (Fig. 3d, 3e, and 3f, Table 7-12).
Figure 3.
Effect of miR-21 on Bcl-2 expression and ROS production.
The expression of Bcl-2 (a), ROS (b), and DNA fragmentation (c) production were affected by the overexpression of miR-21 in H9c2 cells treated with palmitate. The effect of N acetylcysteine on apoptosis of H9c2 cells induced by palmitate (d, e, and f)
Table 7.
Relative Bcl-2 expression of H9c2 cells transfected with a miR-21 mimic after 24 h exposure to 0.4 mM palmitate
| PAL | PAL+miR-21 mimic | |
|---|---|---|
| Mean±SEM | 1.000±0.0492 | 1.621±0.2737 |
| n=5 |
Table 8.
Relative ROS production of H9c2 cells transfected with a miR-21 mimic and a scrambled oligonucleotide after 24 h exposure to 0.4 mM palmitate (mean±SD, n=5)
| Scrambled oligo | miR-21 mimic | |
|---|---|---|
| CTRL | 1.00±0.12 | 0.89±0.24 |
| PAL | 1.48±0.32 | 1.18±0.46 |
Table 9.
Relative DNA fragmentation of H9c2 cells transfected with a miR-21 mimic and a scrambled oligonucleotide after 24 h exposure to 0.4 mM palmitate (mean±SD, n=5)
| Scrambled oligo | miR-21 mimic | |
|---|---|---|
| CTRL | 1.00±0.21 | 0.92±0.32 |
| PAL | 2.09±0.36 | 1.31±0.44 |
Table 10.
Effect of N-acetylcysteine on apoptosis of H9c2 cells induced by palmitate (mean±SD, n=5)
| Vehicle | NAC (3 mM) | |
|---|---|---|
| CTRL | 5.03±0.45 | 4.43±0.65 |
| PAL | 25.73±1.81 | 11.80±0.51 |
Table 11.
Effect of N-acetylcysteine on DNA fragmentation of H9c2 cells after 24 h exposure to 0.4 mM palmitate (mean±SD, n=5)
| Vehicle | NAC (3 mM) | |
|---|---|---|
| CTRL | 1.00±0.18 | 0.88±0.31 |
| PAL | 2.09±0.31 | 1.21±0.23 |
Table 12.
Effect of N-acetylcysteine on relative caspase-3 activity of H9c2 cells after 24 h exposure to 0.4 mM palmitate (mean±SD, n=5)
| Vehicle | NAC (3 mM) | |
|---|---|---|
| CTRL | 1.00±0.13 | 0.90±0.25 |
| PAL | 2.02±0.37 | 1.31±0.49 |
MiR-21 affects nuclear factor κB pathway on palmitate-induced H9c2 cell apoptosis
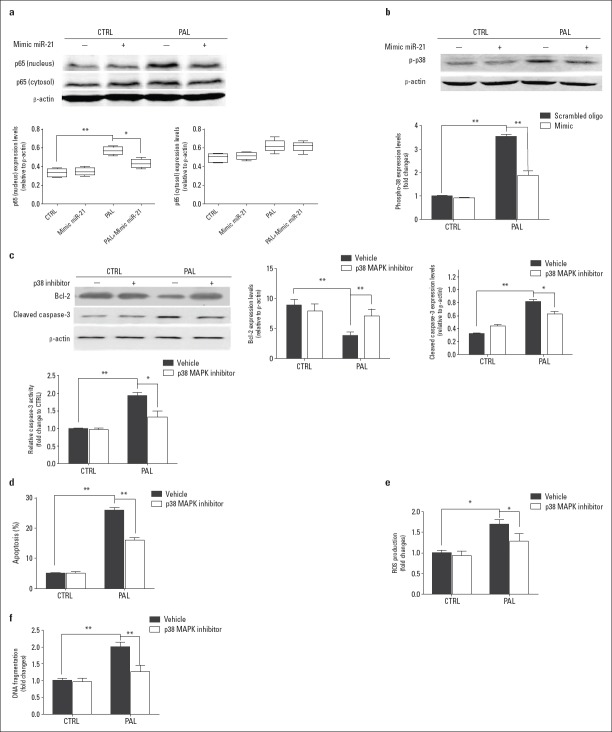
After 24 h of exposure to palmitate, the expression of p65, a key proinflammatory component of the nuclear factor κB (NF-κB) pathway in the nucleus, was significantly increased (Fig. 4a, Fig. 4 Table 13-19). The overexpression of miR-21 did not affect the expression levels of p65 either in the nucleus or in the cytoplasm but did completely suppress the increase of nuclear p65 expression after exposure to palmitate (Fig. 4a, Fig. 4 Table 13-19). No significant change in phosphorylated mitogen-activated protein kinase (MAPK) p38 (p-p38), a marker of inflammation and apoptosis, was observed in H9c2 cells transfected with the miR-21 mimic (Fig. 4b, Fig. 4 Table 13-19). However, miR-21 suppressed the upregulation of p-p38 expression after exposure to palmitate (Fig. 4b, Fig. 4 Table 13-19). Without palmitate stimulus, a p38 inhibitor had no effect on the expression of Bcl-2 or cleaved caspase-3 in H9c2 cells. When palmitate was included, the p38 inhibitor induced Bcl-2 expression and inhibited caspase-3 activity (Fig. 4c, Fig. 4 Table 13-19). The p38 inhibitor did not induce apoptosis, ROS production, or DNA fragmentation in H9c2 cells; however, in the presence of palmitate, the p38 inhibitor reduced apoptosis, ROS production, and DNA fragmentation (Fig. 4d-4f, Table 13-19).
Figure 4.
miR-21 is involved in NF-κB pathway-related apoptosis and DNA fragmentation.
miR-21 affects p65 expression (a) and phosphorylation of p38 (b) in H9c2 cells treated with palmitate. An inhibitor of p38 rescues palmitate-induced H9c2 cell apoptosis (c, d, e, and f)
Table 13.
Relative p65 expression (nucleus and cytosol) of H9c2 cells transfected with a miR-21 mimic after 24 h exposure to 0.4 mM palmitate (mean±SEM, n=5)
| CTRL | miR-21 mimic | PAL | PAL+miR-21 mimic | |
|---|---|---|---|---|
| Nucleus | 0.335±0.019 | 0.346±0.017 | 0.568±0.018 | 0.434±0.020 |
| Cytosol | 0.483±0.019 | 0.503±0.016 | 0.610±0.028 | 0.603±0.022 |
Table 14.
Relative phospho-p38 expression of H9c2 cells transfected with a miR-21 mimic after 24 h exposure to 0.4 mM palmitate
| CTRL | miR-21 mimic | PAL | PAL+miR-21 mimic | |
|---|---|---|---|---|
| Mean±SEM | 1.000±0.148 | 0.940±0.201 | 3.474±0.344 | 1.841±0.233 |
| n=5 |
SEM - standard error of the mean
Table 15.
Effect of p38 MAPK inhibitor on relative Bcl-2 and caspase-3 expression of H9c2 cells after 24 h exposure to 0.4 mM palmitate (mean±SEM, n=5)
| CTRL | p38 MAPK inhibitor | PAL | PAL+p38 MAPK inhibitor | |
|---|---|---|---|---|
| Bcl-2 | 8.849±0.452 | 7.911±0.501 | 3.802±0.237 | 7.038±0.492 |
| Caspase-3 | 0.322±0.009 | 0.431±0.011 | 0.812±0.014 | 0.623±0.015 |
Table 16.
Effect of p38 MAPK inhibitor on relative caspase-3 activity in H9c2 cells after 24 h exposure to 0.4 mM palmitate (mean±SEM, n=5)
| Vehicle | p38 MAPK inhibitor | |
|---|---|---|
| CTRL | 1.000±0.0134 | 0.960±0.063 |
| PAL | 1.920±0.098 | 1.310±0.183 |
Table 17.
Effect of p38 MAPK inhibitor on apoptosis of H9c2 cells induced by palmitate (mean±SEM, n=5)
| Vehicle | p38 MAPK inhibitor | |
|---|---|---|
| CTRL | 5.030±0.179 | 4.930±0.291 |
| PAL | 25.73±0.854 | 15.80±0.411 |
Table 18.
Effect of p38 MAPK inhibitor on relative ROS production in H9c2 cells after 24 h exposure to 0.4 mM palmitate (mean±SEM, n=5)
| Vehicle | p38 MAPK inhibitor | |
|---|---|---|
| CTRL | 1.000±0.054 | 0.932±0.107 |
| PAL | 1.687±0.112 | 1.280±0.183 |
Table 19.
Effect of p38 MAPK inhibitor on relative DNA fragmentation in H9c2 cells after 24 h exposure to 0.4 mM palmitate (mean±SEM, n=5)
| Vehicle | p38 MAPK inhibitor | |
|---|---|---|
| CTRL | 1.000±0.103 | 0.964±0.147 |
| PAL | 1.997±0.277 | 1.261±0.196 |
Discussion
It is well known that the loss of cardiomyocytes is a major factor that contributes to cardiac dysfunction and heart failure (24). Inhibition of cardiomyocyte apoptosis is a potential strategy to prevent the development of heart failure (25). In many heart diseases, saturated fatty acids were found to play a key role in myocardial injury, a consequence of cardiomyocyte apoptosis (26-28). Large amounts of palmitate, a key saturated fatty acid, can accumulate in cardiomyocytes and induce apoptosis (29); however, the exact pathway of palmitate-induced apoptosis is yet to be determined. MiRs are involved in the regulation of gene transcription. They can specifically recognize and target the 3′ non-coding region of mRNA at the transcriptional level, causing degradation or inhibition of mRNA translation, thereby regulating gene expression. An altered gene regulation plays an important role in the occurrence and development of various diseases (30). In the heart, miRs have been found to regulate various pathophysiological processes, including cardiac remodeling, cardiac development, myocardial fibrosis, angiogenesis, and cardiomyocyte apoptosis (31). In terminally differentiated cells, such as cardiomyocytes, regulation of apoptosis is crucial because inadequate or excessive apoptosis can lead to atherosclerosis, myocardial infarction, heart failure, and other cardiovascular diseases (32).
It has been found in recent years that miR-21 is highly expressed in vascular smooth muscle cells, vascular endothelial cells, cardiomyocytes, and cardiac fibroblasts. The levels of miR-21 expression have been shown to be altered in various cardiovascular diseases, indicating that miR-21 is involved in the occurrence and development of cardiovascular diseases (33-36). Studies have confirmed that miR-21 is an apoptosis-related miR, which regulates cell cycle (37, 38). Sayed et al. established a model of cardiomyocyte apoptosis by continuous hypoxia and observed the downregulation of miR-21 and upregulation of FasL, which was reversed by AKT activation. However, the overexpression of miR-21 inhibited upregulation of PTEN and FasL and increased the levels of phosphorylated AKT, leading to reduced infarct size and alleviation of heart failure (39). In the work presented here, we induced apoptosis in H9c2 cells by addition of 0.4 and 0.6 mM palmitate. The apoptosis rate was significantly higher than that in the non induced control group. Moreover, palmitate increased the expression of cleaved caspase-3 and decreased the expression of Bcl-2, indicating that the caspase and Bcl-2/Bax pathways might be involved. Bcl-2 and caspase-3 are two classic markers of apoptosis. The Bcl-2 family of genes affects mitochondrial transmembrane potential and has been extensively studied. The antiapoptotic gene Bcl-2 and the proapoptotic gene Bax are two representative members of the Bcl-2 family. Bcl-2 was the first gene found to inhibit apoptosis (40). High expression of Bcl 2 helps maintain stability of the mitochondrial membrane potential by keeping the mitochondrial permeability transition pore in a closed state, preventing the release of mitochondrial apoptotic proteins, and subsequently cell apoptosis. The caspase family of genes plays an essential role in mediating cell apoptosis, among which caspase-3 is the main executor of cell apoptosis and can be activated by various upstream factors (41-44). Activated caspase-3 induces the activation of other caspase members in a protease cascade, which ultimately leads to apoptosis (45). Studies have shown that miR-21 overexpression could inhibit H2O2-induced apoptosis of cardiomyocytes by downregulating the expression of PDCD4 (46), a protective effect also observed in hypoxia/reoxygenation-induced cell apoptosis and in rat hearts after ischemia/reperfusion injury in vivo (37). In the present study, we found that the overexpression of a miR-21 mimic could inhibit the apoptosis induced by palmitate, whereas the low expression of miR-21 accelerated the process. ROS production and DNA fragmentation, which correlate with the apoptosis, were detected in the cells. The downregulation of miR-21 in cardiomyocyte apoptosis has been reported by other groups and has been shown to correlate with increased expression of FasL protein. It was also found that the expression of miR-21 in cardiac fibroblasts was significantly higher than that in normal cardiomyocytes. In the stress state, the expression of miR-21 in cardiac fibroblasts can significantly activate extracellular signal regulated kinase (ERK)/MAPK pathway proteins and promote the proliferation of fibroblasts and fibrosis (47).
Our work has demonstrated a new pathway by which miR-21 regulates apoptosis in cardiomyocytes, namely through the caspase-3/NF-κB pathway. NF-κB is an inducible transcription factor responsible for the expression of various genes involved in inflammation, injury, apoptosis, embryonic development, and proliferation (48, 49). As the main functional element, p65 is involved in the regulation of various physiological and pathophysiological events (50-52). We showed that p65 expression level in the nucleus increased by the exposure to palmitate, which was inhibited by miR-21. The overexpression of miR-21 did not affect the expression levels of p65 either in the nucleus or in the cytoplasm but did completely suppress the increase of nuclear p65 expression after exposure to palmitate. A combination of palmitate and a p38 inhibitor induced Bcl-2 expression and reduced caspase-3 activity. In addition, the p38 inhibitor reduced palmitate-induced apoptosis, suggesting that p38 is a key factor in cardiomyocyte apoptosis. Additionally, p38 is one of the first identified transcription factors, which is regulated by phosphorylation; p38 is involved in various pathophysiological processes, including cell growth, proliferation, differentiation, and apoptosis, by regulating the expression of many downstream target genes. Phospho-p38 causes cardiomyocyte damage by promoting inflammation and cell apoptosis. Studies have shown that p38 can be activated by various inflammatory factors, including oxygen free radicals released after myocardial ischemia/reperfusion injury and calcium overload. p38 activation induces expression of some early genes, such as c-fos, c-jun, and NF-κB (53, 54), which upregulates the expression of cytokines, such as TNF-α, IL-1, and IL-8, leading to secondary myocardial damage (55-57). We also observed alterations of ROS and the amount of DNA fragmentation in H9c2 cells. ROS can activate several pro-apoptotic signaling pathways, such as MAPK p38, c-Jun N-terminal kinase, apoptosis signal regulating kinase 1, and ERK (58).
Study limitations
For this study, we used the embryonic rat heart-derived cell-line H9c2. The link between apoptosis and the miR-21/caspase-3/NF-κB pathways makes these pathways promising as therapeutic targets for heart disease; however, the findings need further study and validation in in vivo experiments and human cells to confirm the potential therapeutic benefit.
Conclusion
In summary, miR-21 protects cardiomyocytes from apoptosis induced by palmitate through the caspase-3/NF-κB pathway.
Acknowledgments
The author(s) received no financial support for the research, authorship, and/or publication of this article. We thank Hanne Gadeberg, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – X.Z., Y.G.; Design – X.Z., Y.G.; Supervision – B.C., Y.G.; Fundings – B.C., Y.G.; Materials – X.Z., B.C.; Data collection &/or processing – X.Z., Y.G.; Analysis &/or interpretation – X.Z., Y.G.; Literature search – B.C., Y.G.; Writing – X.Z., Y.G.; Critical review – B.C., Y.G.
References
- 1.Puzyrenko AM, Chekman IS, Briuzhina TS, Horchakova NO. Influence of antihypertensive and metabolic drugs on fatty acids content of lipids in cardiomyocytes of rats with spontaneous hypertension. Ukr Biokhim Zh (1999) 2013(85):67–74. doi: 10.15407/ubj85.04.067. [DOI] [PubMed] [Google Scholar]
- 2.Cacicedo J, Benjachareowong S, Chou E, Ruderman N, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–45. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- 3.Nagarajan V, Gopalan V, Kaneko M, Angeli V, Gluckman P, Richards AM, et al. Cardiac function and lipid distribution in rats fed a high-fat diet: in vivo magnetic resonance imaging and spectroscopy. Am J Physiol Heart Circ Physiol. 2013;304:H1495–504. doi: 10.1152/ajpheart.00478.2012. [DOI] [PubMed] [Google Scholar]
- 4.Chai W, Liu Z. p38 mitogen-activated protein kinase mediates palmitate-induced apoptosis but not inhibitor of nuclear factor-kappaB degradation in human coronary artery endothelial cells. Endocrinology. 2007;148:1622–8. doi: 10.1210/en.2006-1068. [DOI] [PubMed] [Google Scholar]
- 5.Staiger K, Staiger H, Weigert C, Haas C, Häring HU, Kellerer M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 2006;55:3121–6. doi: 10.2337/db06-0188. [DOI] [PubMed] [Google Scholar]
- 6.Lemaitre RN, King IB, Rice K, McKnight B, Sotoodehnia N, Rea TD, et al. Erythrocyte very long-chain saturated fatty acids associated with lower risk of incident sudden cardiac arrest. Prostaglandins Leukot Essent Fatty Acids. 2014;91:149–53. doi: 10.1016/j.plefa.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, et al. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 8.Xu S, Nam SM, Kim JH, Das R, Choi SK, Nguyen TT, et al. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis. 2015;6:e1976. doi: 10.1038/cddis.2015.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res. 2011;92:10–8. doi: 10.1093/cvr/cvr212. [DOI] [PubMed] [Google Scholar]
- 10.Falcão-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2012;17:325–44. doi: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- 11.Park M, Sabetski A, Kwan CY, Turdi S, Sweeney G. Palmitate induces ER stress and autophagy in H9c2 cells: implications for apoptosis and adiponectin resistance. J Cell Physiol. 2015;230:630–9. doi: 10.1002/jcp.24781. [DOI] [PubMed] [Google Scholar]
- 12.Ying Y, Zhu H, Liang Z, Ma X, Li S. GLP1 protects cardiomyocytes from palmitate-induced apoptosis via Akt/GSK3b/b-catenin pathway. J Mol Endocrinol. 2015;55:245–62. doi: 10.1530/JME-15-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olena AF, Patton JG. Genomic organization of microRNAs. J Cell Physiol. 2010;222:540–5. doi: 10.1002/jcp.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ. Biological functions of microRNAs: a review. J Physiol Biochem. 2011;67:129–39. doi: 10.1007/s13105-010-0050-6. [DOI] [PubMed] [Google Scholar]
- 15.Giridharan VV, Thandavarayan RA, Fries GR, Walss-Bass C, Barichello T, Justice NJ, et al. Newer insights into the role of miRNA a tiny genetic tool in psychiatric disorders: focus on post-traumatic stress disorder. Transl Psychiatry. 2016;6:e954. doi: 10.1038/tp.2016.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalucci D, Gallo P, Condorelli G. MicroRNAs in cardiovascular biology and heart disease. Circ Cardiovasc Genet. 2009;2:402–8. doi: 10.1161/CIRCGENETICS.109.857425. [DOI] [PubMed] [Google Scholar]
- 17.Yu S, Li G. MicroRNA expression and function in cardiac ischemic injury. J Cardiovasc Transl Res. 2010;3:241–5. doi: 10.1007/s12265-010-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickinson BA, Semus HM, Montgomery RL, Stack C, Latimer PA, Lewton SM, et al. Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure. Eur J Heart Fail. 2013;15:650–9. doi: 10.1093/eurjhf/hft018. [DOI] [PubMed] [Google Scholar]
- 19.Romaine SP, Tomaszewski M, Condorelli G, Samani NJ. MicroRNAs in cardiovascular disease: an introduction for clinicians. Heart. 2015;101:921–8. doi: 10.1136/heartjnl-2013-305402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, et al. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J Pharmacol Exp Ther. 2005;314:522–32. doi: 10.1124/jpet.105.083477. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Chen C, Gong W, Li Y, Edin ML, Zeldin DC, et al. Epoxyeicosatrienoic acids attenuate reactive oxygen species level, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide. J Pharmacol Exp Ther. 2011;339:451–63. doi: 10.1124/jpet.111.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmelzer C, Kitano M, Rimbach G, Niklowitz P, Menke T, Hosoe K, et al. Effects of ubiquinol-10 on microRNA-146a expression in vitro and in vivo. Mediators Inflamm. 2009;2009:415437. doi: 10.1155/2009/415437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simone NL, Soule BP, Ly D, Saleh AD, Savage JE, Degraff W, et al. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS One. 2009;4:e6377. doi: 10.1371/journal.pone.0006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandl A, Huong PL, Toth K, Zambetti G, Erhardt P. Puma deletion delays cardiac dysfunction in murine heart failure models through attenuation of apoptosis. Circulation. 2011;124:31–9. doi: 10.1161/CIRCULATIONAHA.110.988303. [DOI] [PubMed] [Google Scholar]
- 25.Hikoso S, Ikeda Y, Yamaguchi O, Takeda T, Higuchi Y, Hirotani S, et al. Progression of heart failure was suppressed by inhibition of apoptosis signal-regulating kinase 1 via transcoronary gene transfer. J Am Coll Cardiol. 2007;50:453–62. doi: 10.1016/j.jacc.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 26.Sparagna GC, Hickson-Bick DL. Cardiac fatty acid metabolism and the induction of apoptosis. Am J Med Sci. 1999;318:15–21. doi: 10.1097/00000441-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Listenberger LL, Schaffer JE. Mechanisms of lipoapoptosis: implications for human heart disease. Trends Cardiovasc Med. 2002;12:134–8. doi: 10.1016/s1050-1738(02)00152-4. [DOI] [PubMed] [Google Scholar]
- 28.de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978. doi: 10.1136/bmj.h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerner J, Minkler PE, Lesnefsky EJ, Hoppel CL. Fatty acid chain elongation in palmitate-perfused working rat heart: mitochondrial acetyl-CoA is the source of two-carbon units for chain elongation. J Biol Chem. 2014;289:10223–34. doi: 10.1074/jbc.M113.524314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dirkx E, da Costa Martins PA, De Windt LJ. Regulation of fetal gene expression in heart failure. Biochim Biophys Acta. 2013;1832:2414–24. doi: 10.1016/j.bbadis.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 31.Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol. 2009;6:419–29. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 32.Wang T, Feng Y, Sun H, Zhang L, Hao L, Shi C, et al. miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am J Pathol. 2012;181:1911–20. doi: 10.1016/j.ajpath.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–73. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 34.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–88. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 35.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, et al. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–40. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 37.Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, et al. Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovasc Res. 2010;87:431–9. doi: 10.1093/cvr/cvq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–9. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, et al. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem. 2010;285:20281–90. doi: 10.1074/jbc.M110.109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laulier C, Lopez BS. The secret life of Bcl-2: apoptosis-independent inhibition of DNA repair by Bcl-2 family members. Mutat Res. 2012;751:247–57. doi: 10.1016/j.mrrev.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Wen X, Lin ZQ, Liu B, Wei YQ. Caspase-mediated programmed cell death pathways as potential therapeutic targets in cancer. Cell Prolif. 2012;45:217–24. doi: 10.1111/j.1365-2184.2012.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox R, Aubert M. Flow cytometric detection of activated caspase-3. Methods Mol Biol. 2008;414:47–56. doi: 10.1007/978-1-59745-339-4_5. [DOI] [PubMed] [Google Scholar]
- 43.Choudhary GS, Al-Harbi S, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2015;1219:1–9. doi: 10.1007/978-1-4939-1661-0_1. [DOI] [PubMed] [Google Scholar]
- 44.Sykes MC, Mowbray AL, Jo H. Reversible glutathiolation of caspase-3 by glutaredoxin as a novel redox signaling mechanism in tumor necrosis factor-alpha-induced cell death. Circ Res. 2007;100:152–4. doi: 10.1161/01.RES.0000258171.08020.72. [DOI] [PubMed] [Google Scholar]
- 45.Guan Q, Zhang Y, Yu C, Liu Y, Gao L, Zhao J. Hydrogen sulfide protects against high-glucose-induced apoptosis in endothelial cells. J Cardiovasc Pharmacol. 2012;59:188–93. doi: 10.1097/FJC.0b013e31823b4915. [DOI] [PubMed] [Google Scholar]
- 46.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol. 2009;47:5–14. doi: 10.1016/j.yjmcc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu SX, Zhang Y, Wang YF, Li XC, Xiang MX, Bian C, et al. Upregulation of heme oxygenase-1 expression by hydroxysafflor yellow A conferring protection from anoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes. Int J Cardiol. 2012;160:95–101. doi: 10.1016/j.ijcard.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 48.DeNiro M, Al-Mohanna FA. Nuclear factor kappa-B signaling is integral to ocular neovascularization in ischemia-independent microenvironment. PLoS One. 2014;9:e101602. doi: 10.1371/journal.pone.0101602. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Dohi T, Kawashima R, Kawamura YI, Otsubo T, Hagiwara T, Amatucci A, et al. Pathological activation of canonical nuclear-factor kappaB by synergy of tumor necrosis factor alpha and TNF-like weak inducer of apoptosis in mouse acute colitis. Cytokine. 2014;69:14–21. doi: 10.1016/j.cyto.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Wu B, Iwakiri R, Tsunada S, Utsumi H, Kojima M, Fujise T, et al. iNOS enhances rat intestinal apoptosis after ischemia-reperfusion. Free Radic Biol Med. 2002;33:649–58. doi: 10.1016/s0891-5849(02)00917-6. [DOI] [PubMed] [Google Scholar]
- 51.Kim HK, Park HR, Lee JS, Chung TS, Chung HY, Chung J. Down-regulation of iNOS and TNF-alpha expression by kaempferol via NF-kappaB inactivation in aged rat gingival tissues. Biogerontology. 2007;8:399–408. doi: 10.1007/s10522-007-9083-9. [DOI] [PubMed] [Google Scholar]
- 52.Gurzov EN, Germano CM, Cunha DA, Ortis F, Vanderwinden JM, Marchetti P, et al. p53 up-regulated modulator of apoptosis (PUMA) activation contributes to pancreatic beta-cell apoptosis induced by proinflammatory cytokines and endoplasmic reticulum stress. J Biol Chem. 2010;285:19910–20. doi: 10.1074/jbc.M110.122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamekura R, Kojima T, Takano K, Go M, Sawada N, Himi T. The role of IL-33 and its receptor ST2 in human nasal epithelium with allergic rhinitis. Clin Exp Allergy. 2012;42:218–28. doi: 10.1111/j.1365-2222.2011.03867.x. [DOI] [PubMed] [Google Scholar]
- 54.Mercanoğlu GO, Pamukçu B, Safran N, Mercanoğlu F, Fici F, Güngör M. Nebivolol prevents remodeling in a rat myocardial infarction model: an echocardiographic study. Anatol J Cardiol. 2010;10:18–27. [PubMed] [Google Scholar]
- 55.Chang C, Zhang C, Zhao X, Kuang X, Tang H, Xiao X. Differential regulation of mitogen-activated protein kinase signaling pathways in human with different types of mitral valvular disease. J Surg Res. 2013;181:49–59. doi: 10.1016/j.jss.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 56.Yoon JH, Choi YJ, Lee SG. Ginsenoside Rh1 suppresses matrix metalloproteinase-1 expression through inhibition of activator protein-1 and mitogen-activated protein kinase signaling pathway in human hepatocellular carcinoma cells. Eur J Pharmacol. 2012;679:24–33. doi: 10.1016/j.ejphar.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 57.Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG, Song GY, et al. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 2011;55:594–605. doi: 10.1002/mnfr.201000292. [DOI] [PubMed] [Google Scholar]
- 58.Kuroda J, Sadoshima J. NADPH oxidase and cardiac failure. J Cardiovasc Transl Res. 2010;3:314–20. doi: 10.1007/s12265-010-9184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]