Abstract
Background:
The mutated BRAF V600E protein has been specifically detected in papillary thyroid carcinomas (PTCs) using immunohistochemical (IHC) analysis. The clonal origin of PTCs harboring BRAF mutations has recently been called into question.
Objectives:
The purpose of this study was 2-fold: (1) to compare BRAF V600E IHC expression in PTCs, poorly differentiated thyroid carcinomas (PDTCs), and anaplastic thyroid carcinomas (ATCs) with DNA mutation analysis; and (2) to study the distribution of BRAF V600E IHC staining within thyroid cancer tissues.
Methods:
Whole sections and tissue microarrays from 31 PTCs, 38 PDTCs, and 22 ATCs were subjected to both mass spectrometry genotyping for the BRAFT1799A mutation as well as IHC staining for BRAF V600E protein.
Results:
Of the 31 PTCs, 16 (52%) showed strong (3+) IHC staining and harbored BRAFT1799A, whereas the remaining 15 (48%) showed absent/faint (0/1+) staining, and were wild type for BRAF (BRAF-wt). Only 5 of 38 (13%) PDTCs harbored mutant BRAF, and these were the only ones with moderate (2+) or 3+ IHC staining. All 14 ATCs with a staining intensity of 3+ harbored BRAFT1799A, whereas the 2 ATCs with 0/1+ staining were BRAF-wt. Six ATCs showed staining of 2+, 5 of which had high background staining. Of those 6 cases, BRAFT1799A was present only in the tumor without background. Homogeneous staining was found in 13 of 14 (93%) PTCs, 3 of 3 (100%) PDTCs, and 12 of 14 ATCs (86%).
Conclusions:
First, absent/faint staining for BRAF V600E correlates perfectly with the lack of the BRAFT1799A mutation, whereas strong staining is highly specific for the BRAFT1799A mutation in PTCs, PDTCs, and ATCs. Moderate staining intensity cannot be relied on and should lead to genotypic analysis. Second, homogeneous staining occurs in the vast majority of cases, demonstrating that the BRAFT1799A mutation is a clonal event in thyroid cancer.
The BRAFV600E mutation is the most common genetic event in papillary thyroid carcinomas (PTCs) and constitutes the vast majority of BRAF mutations in thyroid cancers (1). This mutation activates the mitogen-activated protein kinase pathway and consists of a T to A transversion at nucleotide 1799, which results in a valine to glutamate substitution at residue 600 (V600E) (2–4). Pooled data on sporadic adult PTCs from 29 studies totaling 1856 patients reveal a mutation detection rate of approximately 45% in PTCs (2–7). This oncogene is also mutated in poorly differentiated thyroid carcinomas (PDTCs) and anaplastic thyroid carcinomas (ATCs) (5, 8). BRAF V600E has been shown in most (9–12), but not all (13, 14), studies to be a marker of poor outcome in PTCs. In addition, small molecule BRAF kinase inhibitors are being tested as therapies for patients with radioactive iodine–refractory BRAFT1799A mutant metastatic thyroid carcinomas.
BRAF mutation analysis has been performed using a variety of DNA-based techniques such as single-strand conformation polymorphism, direct sequencing, mutation-specific PCR, and mass spectrometry genotyping (8, 15, 16). Although robust and suitable for use in paraffin-embedded tissue, these liquid-phased assays are limited by the presence of nonneoplastic thyroid tissue, DNA degradation in formalin-fixed material, and challenges in accurately assessing the percentage of tumor cells that carry the mutation (10). A recent study used pyrosequencing to determine the percentage of mutant BRAF alleles in conventional PTCs and concluded that BRAFT1799A is a rare clonal event in PTCs (17). If confirmed, this finding would have important ramifications, since it suggests that the BRAF mutation is not an early event in PTC pathogenesis and would undermine the rationale for using therapies targeted against this oncoprotein, which, according to these authors, would only be present in a minority of tumor cells (17). The mutated BRAF V600E protein has recently been specifically detected in paraffin-embedded PTCs and malignant melanomas using immunohistochemical (IHC) analysis (15, 18). We reasoned that the IHC visualization of the BRAF V600E protein would enable us to address the issue of clonality and putative tumor heterogeneity for the BRAF mutation. Toward that goal, we analyzed a cohort of PTCs, PDTCs, and ATCs for the presence of BRAF V600E at the DNA and protein level. Since the recently published reports tested only PTCs for IHC detection of BRAF V600E, we also aimed to assess the specificity and sensitivity of this IHC technique in PDTCs and ATCs and, based on these results, to provide guidelines for the interpretation of this IHC assay in patient samples.
Materials and Methods
Patient population and histopathology
Randomly chosen cases of PTCs, PDTCs, and ATCs were selected from the pathology department files of Memorial Sloan-Kettering Cancer Center. The slides from the cases included in the study were examined by a head and neck pathologist with special interest in thyroid neoplasia (R.A.G.). The thyroid carcinomas were classified according to the last World Health Organization classification of endocrine tumors, except for the PTC tall cell variant and poorly differentiated thyroid carcinomas (19). The latter type of tumor was defined as a carcinoma displaying high mitotic activity (≥5 mitosis/10 high-power fields, ×400) and/or tumor necrosis and showing follicular cell differentiation at the morphologic or IHC level (20). The tall cell variant was characterized as a papillary carcinoma composed of >50% of tall cells. This cell type was defined as having a height at least twice its width with an oncocytic cytoplasm. The study was approved by the institutional review board of Memorial Sloan-Kettering Cancer Center.
Tissue microarray construction and IHC analysis for BRAF V600E
Tissue microarrays
Five-micrometer sections from donor blocks from each patient's tumor were stained with hematoxylin and eosin (H&E) to identify representative viable areas of the tumor. Core biopsy samples were taken manually or semiautomatically from these defined sites, with a precision instrument (Beecher Instruments, Silver Spring, Maryland) as described previously (21, 22). Three separate tissue cores with a diameter of 0.6 mm from each block were punched and arrayed in triplicate on a recipient paraffin block (21).
IHC procedure
Three tissue microarrays of PTCs, PDTCs, and ATCs (n = 74 tumors) and whole conventional tumor sections of ATCs (n = 17 tumors) were subjected to IHC analysis using the VE1 antibody directed against the BRAF V600E protein (Spring Bioscience, Pleasanton, California). Except for 1 ATC that was collected at autopsy, all 90 remaining cases originated from surgical specimens. A positive control was used with each run and consisted of a melanoma positive for BRAF V600E mutation at the DNA and protein level. Five-micrometer sections from each tissue microarray and conventional blocks were deparaffinized and pretreated with EDTA at pH 9.0. The sections were incubated with the VE1 antibody at a 1:50 dilution. The procedure was performed using the Ventana semiautomated staining system (Ventana Medical Systems, Inc., Tucson, Arizona).
IHC interpretation
Two pathologists (R.A.G., N.K.) scored the stains and were blinded to the genotyping results. There was interobserver agreement in regard to staining interpretation in 87 of 91 (96% of cases). The percentage of tumor cells displaying cytoplasmic immunopositivity was recorded. Staining intensity was scored as 0 (no staining), 1+ (faint), 2+ (moderate), and 3+ (strong). A tumor was considered immunopositive for BRAF V600E if it displayed a staining intensity of 2+ or 3+ irrespective of the number of tumor cells stained. Intratumoral heterogeneity was assessed in whole conventional sections and when at least 2 of the 3 cores from the tissue microarray contained tumor suitable for IHC scoring. For the assessment of homogeneous distribution, only 3+ areas were considered for the analysis. Homogeneous expression was defined as analogous labeling intensity of 3+ in ≥80% of the tumor cells.
Genotyping
Four sections of 10 μm from each formalin-fixed paraffin-embedded tissue block were subjected to DNA extraction using the Puregene Genomic DNA purification kit (Gentra, Inc., Minneapolis, Minnesota). The same tissue block that was used for immunostaining was also genotyped. Mutation detection was performed as described previously (8). We used a mass spectrometry Sequenom-based genotyping assay (Sequenom mass array; Sequenom, San Diego, California) to interrogate mutations in the most common thyroid oncogenes such as BRAF (including BRAFT1799A), NRAS, HRAS, KRAS, PIK3CA, and AKT1.
Results
High correlation between BRAFT1799A mutation and IHC staining for the mutated protein
Table 1 displays the correlation between the BRAFT1799A gene mutation and immunostaining intensity for BRAF V600E in PTCs, PDTCs, and ATCs. The presence of 3+ staining was always indicative of the presence of the BRAFT1799A gene mutation along the spectrum of thyroid carcinoma progression (Figure 1). Absent or faint (1+) staining correlated with wild-type BRAF (BRAF-wt) in the tumor DNA (Figure 1). A staining intensity of 2+ was encountered only in PDTCs and ATCs (Figure 1). In PDTCs, moderate (ie, 2+) staining indicated the presence of a mutated gene. Six ATCs showed staining of 2+. Five of these had high background staining (based on staining in nontumoral cells). Of those 6 cases, the BRAFT1799A mutation was present only in the tumor without background. Therefore, moderate staining, especially with a high background, does not correlate with the presence of BRAFT1799A at the DNA level. Of note, the VE1 antibody stained the colloid of neoplastic and nonneoplastic tissue. In some instances, the nonneoplastic follicular cells displayed faint staining or a coarse inhomogeneous granular staining, easily differentiated based on its pattern from the strong staining seen in tumor cells. The latter granular staining seen in nonneoplastic follicular cells was not considered as 1+ staining in the tumors.
Table 1.
Correlation Between BRAFT1799A Gene Mutation and IHC Positivity for the Mutated Protein in Thyroid Carcinomas
| Genotype Intensity | IHC Staining | |||
|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | |
| PTC BRAF T1799A (n = 16) | 0 | 0 | 0 | 16 (100) |
| PTC BRAF-wt (n = 15) | 8 (53) | 7 (47) | 0 | 0 |
| PDTC BRAF-T1799A (n = 5) | 0 | 0 | 2 (40) | 3 (60) |
| PDTC BRAF-wt (n = 33) | 8 (24) | 25 (76) | 0 | 0 |
| ATC BRAF-T1799A (n = 15) | 0 | 0 | 1 (7) | 14 (93) |
| ATC BRAF-wt (n = 7) | 1 (14.3) | 1 (14.3) | 5 (71.4)a | 0 |
Data are n (%).
Tumors with high background staining.
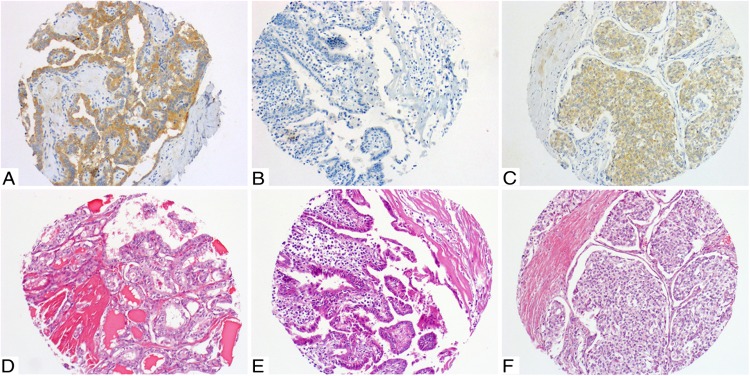
Figure 1.
A and D, Classic PTC, mutated for BRAF V600E. A, 3+ diffuse homogeneous immunostaining with VE1. D, Corresponding H&E staining. B and E, Classic PTC, wild type for BRAF V600E. B, 0 to 1+ immunostaining with VE1. E, Corresponding H&E staining. C and F, Poorly differentiated carcinoma mutated for BRAF V600E. C, 2+ diffuse homogeneous immunostaining with VE1. F, Corresponding H&E staining.
Distribution of BRAF V600E staining in subtypes of PTCs, PDTCs, and ATCs
Table 2 shows the correlation between IHC staining intensity and the BRAFT1799A mutation in the various subtypes of PTCs. All encapsulated follicular variant PTCs were BRAF-wt and had absent/faint staining, whereas 11 of 16 (69%) classic PTCs harbored BRAFT1799A and showed strong IHC staining for the mutated protein. This difference was statistically significant (P = .004) (Figure 1). All 12 PDTCs with an exclusively follicular or Hurthle cell phenotype were BRAF-wt and showed 0/1+ staining. Five of the 24 (21%) PDTCs with the PTC nuclear phenotype were BRAFT1799A mutant and had a 2+/3+ IHC labeling. Two PDTCs with a mixture of the Hurthle (ie, follicular oncocytic) and PTC phenotypes did not harbor BRAFT1799A mutations and showed faint IHC staining. In 10 BRAFT1799A ATCs with associated PTCs (2 classic and 8 tall cell), both components were strongly positive by IHC staining. In 6 of these cases, both the anaplastic and the PTC components of the tumor were genotyped and both showed the BRAFT1799A mutation and strong IHC staining (Figure 2).
Table 2.
Correlation Between BRAFT1799A Gene Mutation and IHC Positivity for the Mutant Protein in PTCs According to Tumor Subtype
| Genotype Intensity | IHC Staining | |||
|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | |
| Classic PTC BRAF-T1799A (n = 11) | 0 | 0 | 0 | 11 (100) |
| Classic PTC BRAF-wt (n = 5) | 4 (80) | 1 (20) | 0 | 0 |
| TCV PTC BRAF-T1799A (n = 2) | 0 | 0 | 0 | 2 (100) |
| FVPTC BRAF-wt (n = 9; 2 Inf and 7 Enc) | 4 (44) | 5 (56) | 0 | 0 |
| Micro PTC BRAF-T1799A (n = 3) | 0 | 0 | 0 | 3 (100) |
| Micro PTC BRAF-wt (n = 1) | 0 | 1 (100) | 0 | 0 |
Abbreviations: Enc, encapsulated; FVPTC, follicular variant of PTC; Inc, infiltrative; Micro PTC, PTC microcarcinoma variant; TCV, tall cell variant. Data are n (%).
Figure 2.
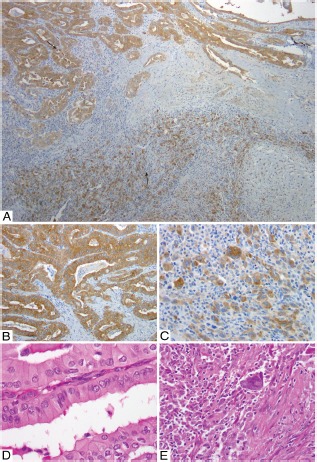
A–E, Whole section of anaplastic carcinoma with associated PTC tall cell variant (PTC TCV). Both the PTC TCV and anaplastic components have BRAF V600E mutation. A, Low-power view with diffuse homogeneous 3+ immunostaining for VE1 in PTC TCV (top arrow) and anaplastic (bottom arrow) components. B, High-power view of the PTC TCV component showing 3+ VE1 immunostaining. D, Corresponding H&E staining for B. C, High-power view of anaplastic component showing 3+ VE1 immunostaining. E, Corresponding H&E staining for C.
Intratumoral distribution of the BRAF V600E protein
Table 3 displays the intratumoral immunostaining distribution according to tumor type and BRAF mutational status in those tumors that displayed strong (3+) immunostaining. Intratumoral distribution could not be assessed in 3 of these strongly immunopositive thyroid carcinomas, either because only 1 tissue core was available for staining in the tissue microarray (n = 2 PTCs) or because of the presence of only autopsy material that is usually poorly fixed (n = 1 ATC). Overall, the homogeneous staining intensity was found in 28 of 31 (90%) of tumors carrying the BRAFT1799A mutation (Figure 2). Of the 3 cases with heterogenous staining, 3+ staining was found in 10%, 60%, and 70% of the cells.
Table 3.
Intratumor Staining Distribution of the Mutated Protein in Patients with BRAFT1799A and Strong (3+) Immunostaining According to Tumor Type
| Tumor Type | Distribution of IHC Staining in the Tumor | ||
|---|---|---|---|
| <50% + Cells Heterogeneous | 50%−79% + Cells Heterogeneous | ≥80% + Cells Homogeneous | |
| PTC (n = 14)a | 0 | 1 (7) | 13 (93) |
| PDTC (n = 3)a | 0 | 0 | 3 (100) |
| ATC (n = 14)a | 1 (7) | 1 (7) | 12 (86) |
| All thyroid carcinomas (n = 31) | 1 (3.3) | 2 (6.4) | 28 (90.3) |
Data are n (%).
In 2 PTCs and 1 ATC, intratumoral staining distribution was not assessable.
Discussion
The BRAFT1799A mutation rate found in our PTC cohort (51.6%) is consistent with previous publications on the subject (23) This genetic mutation was almost perfectly predicted by immunostaining for the novel mutation-specific anti–BRAF V600E antibody (VE1). Strong (3+) labeling of the tumor cells by IHC staining was present in all PTCs carrying the mutation, whereas negative/faint staining was seen in the wild-type neoplasms. These findings confirm those of Koperek et al (15) and Capper et al (18), who found a very strong correlation between VE1 immunostaining and BRAF genotyping in PTCs and malignant melanomas. We now report that the VE1 antibody is also useful in detecting the BRAFT1799A mutation in PDTCs and ATCs, although with some caveats. Thus, whereas strong (3+) and absent/faint (0/1+) immunostaining matched perfectly with the presence or absence of mutations in these cancer types, moderate (2+) staining was more problematic. The latter was associated with background staining in some BRAF-wt ATCs. We believe that this is due to false-positive staining rather than false-negative genotyping, because the mass spectrometry method we used accurately identifies BRAF mutations even when present in as few as 10% of the alleles, whereas the background IHC staining was seen in a homogeneous distribution across all these specimens. Hence, moderate staining intensity cannot reliably assess the mutational status of PDTCs and ATCs. Of note, moderate staining was not seen in the PTCs included in this study. We do not know the reason for such a difference between PTCs and higher grade carcinomas. As far as we know, the copy number for mutant BRAF is not lower in higher-grade tumors, and to our knowledge the stability of this protein in these histiotypes has not been explored. We also cannot exclude the possibility that oncogenic BRAF is down-regulated in more advanced carcinomas. For practical purposes, we therefore recommend genotyping all tumors that display moderate (2+) immunostaining. In contrast, BRAFT1799A mutational status can be accurately determined for all tumor types by IHC analysis if the tumor displays absent/faint (0/1+) or strong (3+) staining. In congruence with other publications (15), we found nonspecific staining of colloid material and normal follicular cells in some instances, most probably due to cross reactivity of the antibody. Therefore, in our opinion, this particular antibody cannot be used to diagnose malignancy that is not proven histologically.
As reported previously (15), there was a strong relationship between VE1 immunostaining and the subtype of PTC. The encapsulated follicular variants of PTCs were all wild type for BRAF with absent/faint staining for the mutated protein, whereas 69% of classic PTCs harbored the BRAFT1799A mutation and showed strong (3+) staining with VE1 (P = .004). These results justify the proposed reclassification of the encapsulated follicular variant of PTC as an entity closer to the follicular adenoma/carcinoma group of tumors rather than as a variant of PTC (24). Of note, the difference in the prevalence of BRAF V600E between PDTCs and ATCs in this analysis is in concordance with our previous study on the subject (8) and related to the fact that a significant portion of poorly differentiated carcinomas (34%) had a follicular phenotype, whereas none of the 22 anaplastic carcinomas had an associated follicular carcinoma component. Indeed, all ATCs and some PDTCs derived from PTCs displayed BRAF V600E expression and mutation, whereas all 12 PDTCs with an exclusively follicular/Hurthle phenotype lacked BRAF mutations and showed absent/faint staining. Moreover, in ATCs associated with PTCs, both the anaplastic and PTC components of the tumor revealed strong BRAF V600E expression. The above data confirm the notion that BRAF V600E is an early event in thyroid carcinogenesis (5).
The question of BRAF clonality has important clinical ramifications. BRAF kinase activity has been targeted pharmacologically with selective kinase inhibitors, such as vemurafenib, leading to significant clinical benefits in patients with BRAF-mutant metastatic melanoma (25, 26). A phase 2 clinical trial with this drug in patients with BRAF-mutant thyroid cancer is currently in progress. The rationale for this study would be flawed if the BRAF mutation was proven to be subclonal, and the interpretation of the results of the study would also be confounded by this hypothesis. In our series, 90% of neoplasms along the entire spectrum of thyroid carcinoma progression displayed homogeneous BRAF V600E immunostaining, defined analogous staining in ≥80% of tumor cells. These results were based on adequate sampling because we used whole sections or at least 2 tissue cores from the same tumor. Although the tissue core is only 0.6 mm and not as representative as a whole section, the cores in this study were generated from different areas of the tumor block. Furthermore, studies have shown 96%- to 98% concordance between triplicate core tissue arrays such as the one used in this endeavor and full-section IHC analyses (27). Assessment of staining intensity can be a subjective exercise. However, both pathologists who scored the stains were blinded to the genotyping results and had overall concordant results. The heterogeneity encountered in a few cases could be due to a difference in fixation and therefore antibody detection between areas of the same tumor. We cannot exclude the possibility that in rare cases BRAF may arise late in tumor evolution, but clearly this is not the dominant mechanism. Indeed, the data generated in this article argue strongly against BRAFT1799A being a subclonal event in PTC, as previously suggested by Guerra et al (17). In that study, the authors found that 27 of 41 PTCs with BRAF mutations had a mutant allelic fraction <25%, leading to their conclusion that in most cases BRAF was a subclonal event, and in 2 of those cases a coexisting KRAS mutation was noted. We cannot know for sure why the pyrosequencing methodology used in their study underestimated the BRAF mutant allelic fraction. Pyrosequencing is based on the real-time bioluminometric quantification of pyrophosphate released by dNTP incorporation. The sensitivity depends on the background level of luminescence, which in turn depends on the side reaction of adenosine 5′-phosphosulfate, an analog of ATP, with luciferase. Although the sensitivity increases in proportion to luciferase concentration, the background due to free 5′-phosphosulfate in the sequencing mixture increases as well. If this is not appropriately optimized, the sensitivity of conventional pyrosequencing chemistry is severely limited (28). Whatever the explanation for the data obtained by Guerra et al (17), the lack of intratumoral heterogeneity of BRAF V600E IHC staining in thyroid carcinoma and other malignancies is inconsistent with their findings. Of note, besides primary PTCs and melanomas, homogeneous BRAF immunostaining was also seen in various carcinomas metastatic to brain (18, 29). Moreover, genotyping of radioactive iodine refractory thyroid carcinoma reveals high concordance of BRAF mutations between multiple tumor sites within the same patient (8). If BRAF were a subclonal event, one might expect to see frequent coexistence of mutations of different known drivers of the disease in the same specimen. This is not the case. Much to the contrary, mutations of BRAF are mutually exclusive with RAS or RET/PTC in the large majority of thyroid cancers (as are coexisting BRAF and RAS in melanomas) (2, 4, 30). Moreover, none of the greater than 40 bona fide thyroid cancer cell lines derived from patient specimens harbors more than 1 mutation of these genes. The data from the article by Guerra et al (17) led us and others to postulate that BRAF mutations may not be commonly involved in tumor initiation but instead arise during tumor progression (31). However, activation of endogenous expression levels of oncogenic BRAFV600E is sufficient to induce thyroid cancers with high penetrance and short latency in murine models of the disease (32, 33), indicating that BRAF can function as a particularly virulent tumor-initiating event. The Cancer Genome Atlas program is currently undertaking the comprehensive exome sequencing of 500 PTCs. This deep sequencing study is being analyzed and has been reported in part in abstract form (34). In the near future, this analysis will provide definitive information on the BRAF mutant allelic fraction in PTCs.
In summary, immunostaining against the mutated BRAF V600E protein is a sensitive and specific tool to detect BRAFT1799A mutations in PTCs as well as in PDTCs and ATCs if used appropriately. In clinical practice, we propose the following algorithm: if staining intensity is absent (0)/faint (1+), the tumor can be regarded as BRAF-wt, and no DNA analysis is needed. Similarly, if the carcinoma shows strong (3+) staining, one can assume there is a BRAFT1799A mutation, and no genotyping is required. In contrast, moderate staining (2+) cannot be relied on and should trigger gene mutation testing. From a clinical point of view, immunostaining for mutated BRAF V600E could be particularly useful in small samples containing few tumor cells or in specimens heavily contaminated by nonneoplastic cells. It can also be helpful where clinicians do not have easy access to molecular laboratories. Finally, the data from this study clearly demonstrate that BRAFT1799A is a clonal event in well-differentiated and high-grade thyroid carcinomas.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- ATC
anaplastic thyroid carcinomas
- H&E
hematoxylin and eosin
- IHC
immunohistochemical
- PDTC
poorly differentiated thyroid carcinomas
- PTC
papillary thyroid carcinoma.
References
- 1. Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. [DOI] [PubMed] [Google Scholar]
- 2. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 3. Cohen Y, Xing M, Mambo E, . BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. [DOI] [PubMed] [Google Scholar]
- 4. Soares P, Trovisco V, Rocha AS, . BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. [DOI] [PubMed] [Google Scholar]
- 5. Nikiforova MN, Kimura ET, Gandhi M, . BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. [DOI] [PubMed] [Google Scholar]
- 6. Puxeddu E, Moretti S, Elisei R, . BRAFV599E mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:2414–2420. [DOI] [PubMed] [Google Scholar]
- 7. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. [DOI] [PubMed] [Google Scholar]
- 8. Ricarte-Filho JC, Ryder M, Chitale DA, . Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Basolo F, Torregrossa L, Giannini R, . Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: analysis of 1060 cases. J Clin Endocrinol Metab. 2010;95:4197–4205. [DOI] [PubMed] [Google Scholar]
- 10. Elisei R, Ugolini C, Viola D, . BRAFV600E mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. [DOI] [PubMed] [Google Scholar]
- 11. Namba H, Nakashima M, Hayashi T, . Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–4397. [DOI] [PubMed] [Google Scholar]
- 12. Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. [DOI] [PubMed] [Google Scholar]
- 13. Fugazzola L, Puxeddu E, Avenia N, . Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: data from a multicentric Italian study and review of the literature. Endocr Relat Cancer. 2006;13:455–464. [DOI] [PubMed] [Google Scholar]
- 14. Ito Y, Yoshida H, Maruo R, . BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J. 2009;56:89–97. [DOI] [PubMed] [Google Scholar]
- 15. Koperek O, Kornauth C, Capper D, . Immunohistochemical detection of the BRAF V600E-mutated protein in papillary thyroid carcinoma. Am J Surg Pathol. 2012;36:844–850. [DOI] [PubMed] [Google Scholar]
- 16. Lee HJ, Choi J, Hwang TS, Shong YK, Hong SJ, Gong G. Detection of BRAF mutations in thyroid nodules by allele-specific PCR using a dual priming oligonucleotide system. Am J Clin Pathol. 2010;133:802–808. [DOI] [PubMed] [Google Scholar]
- 17. Guerra A, Sapio MR, Marotta V, . The primary occurrence of BRAFV600E is a rare clonal event in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2012;97:517–524. [DOI] [PubMed] [Google Scholar]
- 18. Capper D, Preusser M, Habel A, . Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011;122:11–19. [DOI] [PubMed] [Google Scholar]
- 19. DeLellis RA, Lloyd RV, Heitz PU, Eng C, eds. Pathology & Genetics. Tumours of the Endocrine Organs, 3rd ed Lyon, France: IARC Press; 2004. World Health Organization Classification of Tumour; vol 8. [Google Scholar]
- 20. Hiltzik D, Carlson DL, Tuttle RM, . Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer 2006;106:1286–1295. [DOI] [PubMed] [Google Scholar]
- 21. Hoos A, Urist MJ, Stojadinovic A, . Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol. 2001;158:1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saltman B, Singh B, Hedvat CV, Wreesmann VB, Ghossein R. Patterns of expression of cell cycle/apoptosis genes along the spectrum of thyroid carcinoma progression. Surgery 2006;140:899–905; discussion 905–896. [DOI] [PubMed] [Google Scholar]
- 23. Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21(suppl 2):S37–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rivera M, Ricarte-Filho J, Knauf J, . Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23:1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flaherty KT, Puzanov I, Kim KB, . Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chapman PB, Hauschild A, Robert C, . Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim SH, Lewis JJ, Brennan MF, . Overexpression of cyclin D1 is associated with poor prognosis in extremity soft-tissue sarcomas. Clin Cancer Res. 1998;4:2377–2382. [PubMed] [Google Scholar]
- 28. Wu H, Wu W, Chen Z, . Highly sensitive pyrosequencing based on the capture of free adenosine 5′ phosphosulfate with adenosine triphosphate sulfurylase. Anal Chem. 2011;83:3600–3605. [DOI] [PubMed] [Google Scholar]
- 29. Capper D, Berghoff AS, Magerle M, . Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol. 2012;123:223–233. [DOI] [PubMed] [Google Scholar]
- 30. Frattini M, Ferrario C, Bressan P, . Alternative mutations of BRAF, RET and NTRK1 are associated with similar but distinct gene expression patterns in papillary thyroid cancer. Oncogene. 2004;23:7436–7440. [DOI] [PubMed] [Google Scholar]
- 31. Sarne DH. A piece of the puzzle: what does BRAF status mean in the management of patients with papillary thyroid carcinoma? J Clin Endocrinol Metab. 2012;97:3094–3096. [DOI] [PubMed] [Google Scholar]
- 32. Franco AT, Malaguarnera R, Refetoff S, . Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci USA. 2011;108:1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Charles RP, Iezza G, Amendola E, Dankort D, McMahon M. Mutationally activated BRAFV600E elicits papillary thyroid cancer in the adult mouse. Cancer Res. 2011;71:3863–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giordano T. Papillary thyroid carcinoma analysis. Paper presented at: The Cancer Genome Atlas' 2nd Annual Scientific Symposium: Enabling Cancer Research Through TCGA; November 27–28, 2012; Crystal City, VA. [Google Scholar]