AUTHOR CORRECTION
Volume 197, no. 8, p. 1386–1393, 2015, https://doi.org/10.1128/JB.02450-14. We reported a G470T mutation in the lactate dehydrogenase (ldh) gene which resulted in an R157L amino acid mutation. After review of the genome resequencing data from this strain, it appears that the mutation is in a different location in the ldh gene. The correct mutation is C483A, which results in an S161R amino acid change. This leads to the following changes.
In the abstract and throughout the paper, “R157L” should read “S161R.”
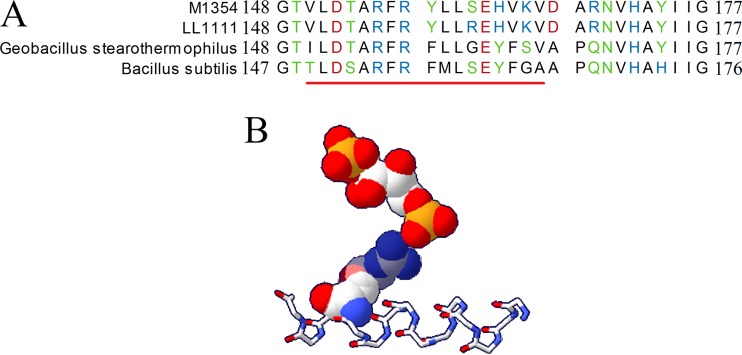
Page 1391: Figure 3 should appear as shown below. The S161R mutation does not match the previously reported mutation but is in the same alpha helix and is in close physical proximity to the fructose 1,6-bisphosphate binding site.
Page 1391: The legend to Fig. 3 should read as follows. “Potential interaction of the R161 amino acid with fructose 1,6-bisphosphate (F1,6BP). (A) Alignment of various Ldh proteins. The red line indicates a conserved helix in Bacillus subtilis and Geobacillus stearothermophilus in proximity to the F1,6BP binding site. The C. thermocellum residues pictured are 148 to 177. (B) B. subtilis lactate dehydrogenase shares the same serine residue as that of C. thermocellum, which is mutated to arginine in the LL1111 strain. The positively charged side chain of the arginine residue is in proximity to F1,6BP, potentially interacting with the negatively charged phosphate. F1,6BP and the transparent serine/arginine residue are shown as space filled, while the stick diagram shows the conserved helix. The structure was visualized using SwissPDB using the 3PQD structure (https://www.rcsb.org/structure/3PQD).”
Page 1391, column 1: Lines 26–41 should read as follows. “… The S161R mutation we found in C. thermocellum Ldh corresponds to a conserved helix in proximity to the F1,6BP (Fig. 3). The mutated serine aligns with a serine in Bacillus subtilis. Structural studies of the B. subtilis and Geobacillus stearothermophilus Ldhs show this helix interacts with the F1,6BP phosphate groups through a conserved arginine’s positively charged guanidinium group (43). An additional arginine could potentially alter the interaction with F1,6BP. Based on the observed accumulation of lactate, loss of F1,6BP activation of Ldh in enzyme assays, S161R mutation, and structural data linking the helix to F1,6BP, we believe the observed mutation in the ldh gene of strain LL1111 altered the F1,6BP allosteric regulation….”