Cyclic di-AMP is a second-messenger nucleotide that is produced by many bacteria and some archaea. Recent work has shown that c-di-AMP is unique among the signaling nucleotides, as this molecule is in many bacteria both essential on one hand and toxic upon accumulation on the other.
KEYWORDS: second messenger, phosphodiesterase, diadenylate cyclase, signal transduction
ABSTRACT
Cyclic di-AMP is a second-messenger nucleotide that is produced by many bacteria and some archaea. Recent work has shown that c-di-AMP is unique among the signaling nucleotides, as this molecule is in many bacteria both essential on one hand and toxic upon accumulation on the other. Moreover, in bacteria, like Bacillus subtilis, c-di-AMP controls a biological process, potassium homeostasis, by binding both potassium transporters and riboswitch molecules in the mRNAs that encode the potassium transporters. In addition to the control of potassium homeostasis, c-di-AMP has been implicated in many cellular activities, including DNA repair, cell wall homeostasis, osmotic adaptation, biofilm formation, central metabolism, and virulence. c-di-AMP is synthesized and degraded by diadenylate cyclases and phosphodiesterases, respectively. In the diadenylate cyclases, one type of catalytic domain, the diadenylate cyclase (DAC) domain, is coupled to various other domains that control the localization, the protein-protein interactions, and the regulation of the enzymes. The phosphodiesterases have a catalytic core that consists either of a DHH/DHHA1 or of an HD domain. Recent findings on the occurrence, domain organization, activity control, and structural features of diadenylate cyclases and phosphodiesterases are discussed in this review.
INTRODUCTION
Second messengers are signaling molecules that are used to relay primary information to an intracellular target, usually a protein or RNA molecule. The use of second messengers allows the transfer of information to multiple targets and facilitates signal transduction if the primary signal is not easily detected by the target proteins. Bacteria use specific nucleotides as second messengers (1). These include the well-studied cyclic AMP (cAMP) and guanosine tetraphosphate (ppGpp), which serve as signals for suboptimal carbon source supply and amino acid limitation, respectively (2, 3). Moreover, cyclic di-GMP is used in many bacteria to control the lifestyle decision between a unicellular motile and the multicellular sessile biofilm lifestyles (4). About 10 years ago, cyclic di-AMP was added to the list of second messengers (5). In addition, a few bacteria use also cyclic GMP and the composite dinucleotide cyclic GMP-AMP as second messengers to control cyst development and virulence, respectively (6, 7).
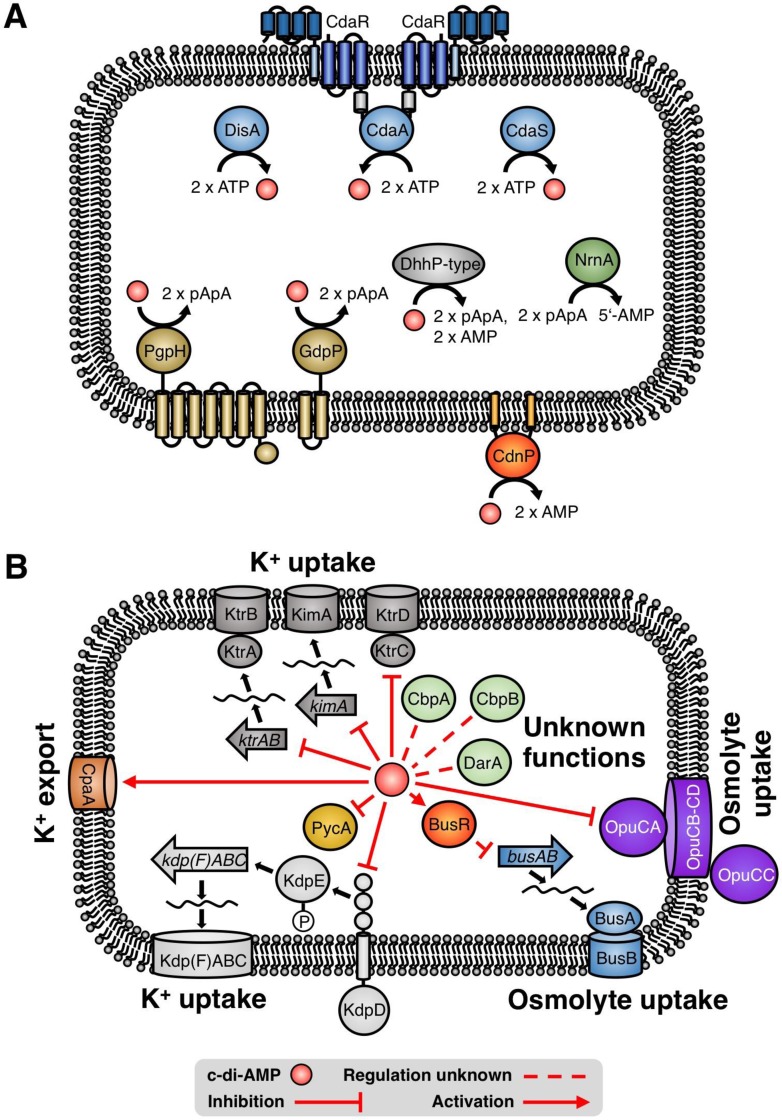
Cyclic di-AMP is synthesized and degraded by diadenylate cyclases and phosphodiesterases, respectively (see Fig. 1A for an overview). Among the second messengers, the following three features make c-di-AMP exceptional: (i) c-di-AMP is the only second messenger that is essential for many bacteria that produce it, among them the model bacterium Bacillus subtilis, the pathogens Staphylococcus aureus, Listeria monocytogenes, and Streptococcus pneumoniae, and the minimal genome bacterium Mycoplasma pneumoniae (8–12). It should be noted that the term essential is used for genes or functions that are absolutely required under standard conditions (i.e., complex medium), and that these genes or functions may become dispensable under different growth conditions. Indeed, this is the case for c-di-AMP, which is required for growth on complex medium but not for growth on defined medium (11). (ii) The accumulation of c-di-AMP poses problems for the bacteria as well; the second messenger has therefore been called “essential poison” (13). For B. subtilis and M. pneumoniae, loss of the phosphodiesterases that degrade c-di-AMP leads to cell death (13–15). (iii) c-di-AMP is the only signaling nucleotide that can regulate a single biological process by binding to two different classes of target molecules, proteins and RNA; in B. subtilis, c-di-AMP controls the activity of a potassium transporter and of the riboswitch that modulates its expression (16–18).
FIG 1.
Targets that are presumed and known to be regulated by c-di-AMP. (A) Diadenylate cyclases and phosphodiesterases, their localization, and catalyzed reactions. All diadenylate cyclases (blue) use two molecules of ATP to produce c-di-AMP with the concomitant release of two molecules of pyrophosphate. The phosphodiesterases of the PgpH and GdpP families (gold) cleave c-di-AMP to generate the linear dinucleotide pApA. The DhhP-type phosphodiesterases (gray) generate pApA, and many of the enzymes are capable of cleaving pApA to the final product AMP. For all enzymes with pApA as the final product, a nano-RNase (NrnA, green) is required to degrade the pApA to two molecules of AMP. The cell wall-anchored phosphodiesterase CdnP degrades extracellular c-di-AMP to AMP. (B) The expression of the ktrAB and kimA genes is controlled by the c-di-AMP-dependent ydaO riboswitch in B. subtilis (17, 18). c-di-AMP directly binds to the KtrC subunit and the KdpD sensor kinase of the KtrCD and KdpFABC potassium uptake systems, respectively, of S. aureus (16, 109). c-di-AMP simulates the activity of the S. aureus potassium exporter CpaA. The transport activity of the Opu osmolyte uptake system in S. aureus and L. monocytogenes is controlled by c-di-AMP (23, 24). In lactic acid bacteria, transcriptional repression of the busAB genes encoding an osmoprotectant transporter is mediated by BusR, which requires c-di-AMP for DNA binding (25, 35). The pyruvate carboxylase PycA of L. monocytogenes is inhibited by c-di-AMP (24). CbpA and CbpB from L. monocytogenes and DarA, which is conserved in several Gram-positive bacteria, are c-di-AMP targets of unknown function (16, 24, 38).
The essentiality of c-di-AMP and its requirement for the virulence of several important pathogens have attracted much interest in the investigation of its functions in the cell (see references 15 and 19 to 21 for review). Current evidence has implicated c-di-AMP in the control of potassium homeostasis and osmotic adaptation, central metabolism, cell wall metabolism, biofilm formation, DNA integrity, and virulence in Gram-positive bacteria (8, 16, 18–35). An overview of the multiple targets of c-di-AMP and their role in the cellular physiology of Gram-positive bacteria is provided in Fig. 1B. Very recently, the functions of c-di-AMP have also been studied for cyanobacteria. In these organisms, the molecule is not essential, but it plays an important role in stress adaptation, in particular during nighttime survival (36, 37). While the molecular mechanisms of c-di-AMP-mediated regulation are well understood for potassium homeostasis, osmotic regulation, and the control of central metabolism, much research is still required for the other processes that involve the second messenger. The identification of conserved target proteins of unknown function in distinct organisms, such as B. subtilis, S. aureus, and L. monocytogenes (16, 26, 38), suggests that these proteins might be involved in those additional functions.
Several reviews have addressed c-di-AMP signaling (15, 19–21). Here, we review the more recent progress in the field with a particular focus on the enzymes that make and break c-di-AMP, the diadenylate cyclases and phosphodiesterases, respectively.
DIADENYLATE CYCLASES
Types of diadenylate cyclases and their regulation.
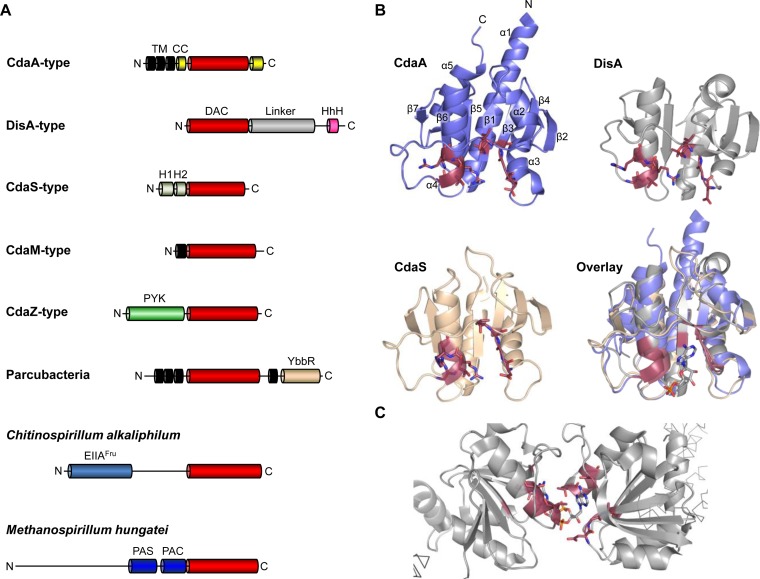
Cyclic di-AMP is synthesized from two molecules of ATP with the concomitant release of two molecules of pyrophosphate (5). All enzymes known to produce c-di-AMP share a catalytically active domain, the so-called DAC (diadenylate cyclase) domain (previously referred to as DUF147 [14, 20, 39, 40]). So far, c-di-AMP has not been detected in an organism not encoding an enzyme carrying a DAC domain. In the diadenylate cyclases, the DAC domains are accompanied by other protein domains that control the enzymatic activity of the active domains. Five classes of cyclases with different types of regulatory domains have been identified so far (14, 19, 20, 41) (see Fig. 2A). These enzymes are referred to as DisA, CdaA, CdaS, CdaM, and CdaZ. Most bacteria possess only one diadenylate cyclase, either CdaA or DisA. In contrast, the spore-forming Gram-positive model organism B. subtilis has the three enzymes, DisA, CdaA, and CdaS. None of the corresponding genes is essential, but a strain lacking both DisA and CdaA is not viable under standard laboratory conditions (8, 40). CdaS is unable to replace the other enzymes since it is expressed only late during sporulation in the forespore but not in growing cells (40, 42). The presence of three diadenylate cyclases is limited to members of the spore-forming genus Bacillus. Members of the genus Clostridium possess both CdaA and DisA. The CdaM and CdaZ cyclases are present only in few organisms, the genome-reduced Mycoplasma spp. and in the methanogenic archaeon Methanocaldococcus jannaschii. The enzymatic activity of the CdaM and CdaZ classes of enzymes has been experimentally proven (14, 41). Structural information is available for DisA, CdaA, and CdaS (see below) (5, 43, 60).
FIG 2.
Organization of diadenylate cyclases. (A) Domain structure of the different classes of diadenylate cyclases. The different domains are characterized by a color code. DAC, diadenylate cyclase domain; HhH, helix-hinge-helix domain; TM, transmembrane helix; CC, coiled-coil domain; PYK, pyruvate kinase-like domain; YbbR, YbbR domain (the unit domain of CdaR); EIIFru, PTS EII-like domain; PAS, Per-Arnt-Sim domain; PAC, PAS C-terminal domain. (B) Structures of the DAC domains of CdaA (blue, L. monocytogenes; PDB ID 4RV7), DisA (gray, T. maritima; PDB ID 3C21), and CdaS (green, B. cereus; PDB ID 2FB5), as well as an overlay of the three DAC domain structures with its bound reaction product c-di-AMP depicted in stick model. Conserved residues involved in ATP binding are highlighted in red. (C) Structure of a catalytic DAC dimer of DisA from T. maritima (PDB ID 3C21) with bound c-di-AMP depicted as a stick model. Conserved residues in the active site are shown in red.
The most widespread class of diadenylate cyclases is represented by CdaA. These enzymes are found in most Firmicutes, in the Deltaproteobacteria (but never in the Gammaproteobacteria that include E. coli), and also in cyanobacteria, in spirochaetes, in chlamydiae, and in bacteria of the Cytophaga/Flavobacterium/Bacteroides group. Moreover, CdaA is the only cyclase encoded in many pathogenic Firmicutes, such as S. aureus, L. monocytogenes, and S. pneumoniae. CdaA consists of an N-terminal domain with three transmembrane helices and the enzymatically active DAC domain, which is flanked by predicted coiled-coil domains on each side (43) (Fig. 2A). For B. subtilis CdaA, membrane localization has been proven experimentally (13). Enzymatic activity of CdaA requires a metal ion, either manganese or cobalt (43). In the Firmicutes, the cdaA gene is the first gene of a conserved operon encoding the regulatory protein CdaR (YbbR) and the phosphoglucosamine mutase GlmM. With a half-life of more than 15 min, the cdaAR portion of the mRNA belongs to the most stable mRNA molecules in B. subtilis (44). CdaR is a membrane protein that physically interacts with CdaA (see Fig. 1A) (13, 40, 45). While the coexpression of CdaA and CdaR in E. coli resulted in increased diadenylate cyclase activity, CdaR inhibits CdaA activity in the cognate cellular environment, as demonstrated for L. monocytogenes and S. aureus (40, 45, 46). In addition, B. subtilis CdaA also physically interacts with the GlmM protein. As the essential enzyme GlmM catalyzes an early step in the peptidoglycan precursor biosynthesis, this interaction is likely to link cell wall homeostasis to CdaA activity and thus c-di-AMP levels in the cell. Indeed, it has been shown that GlmM is a negative effector of CdaA activity in Lactococcus lactis (47). As mentioned above, the accumulation of c-di-AMP due to the lack of the degrading phosphodiesterases interferes with bacterial viability, and the acquisition of suppressor mutations resulting in the inactivation of CdaA has been observed in B. subtilis, L. lactis, and S. pneumoniae (13, 47, 48). In cyanobacteria, the cdaA gene is the second gene in an operon, which encodes the diaminopimelate decarboxylase LysA and the undecaprenyl pyrophosphate synthase UppS. Interestingly, both enzymes catalyze the synthesis of peptidoglycan precursors (36), suggesting a link between cell wall homeostasis and c-di-AMP synthesis in the cyanobacteria as well.
DisA is present in spore-forming Firmicutes and in actinobacteria, as well as in the hyperthermophilic bacterium Thermotoga maritima. While DisA is present in addition to CdaA (and sometimes CdaS) in the Firmicutes of the genera Bacillus and Clostridium, it is the only c-di-AMP-producing enzyme in actinobacteria and in T. maritima. This homo-octameric enzyme is composed of an N-terminal DAC domain, a linker, and a DNA-binding helix-hairpin-helix (HhH) domain (5) (Fig. 2A). For enzymatic activity, either magnesium or manganese is required (5, 49, 50). DisA moves along the DNA and was reported to scan the DNA integrity; indeed, the presence of Holliday junctions results in reduced diadenylate cyclase activity of DisA (5, 31, 51, 52). Moreover, the branch migration transferase RadA, which is encoded in a conserved operon with DisA, interacts with DisA and inhibits its enzymatic activity in both B. subtilis and Mycobacterium tuberculosis (52–55) The DisA-produced c-di-AMP was proposed to control the initiation of sporulation in B. subtilis, as DNA damage results in reduced DisA activity, and the reduced c-di-AMP levels will then result in a sporulation delay (51). In addition, a role in DNA repair in outgrowing spores was proposed for DisA to ensure that DNA lesions are eliminated during outgrowth (33, 56). Finally, DisA is required for DNA damage response also in vegetative cells of B. subtilis (57). However, it is difficult to imagine how reduced DisA activity due to the presence of single Holliday junctions might affect the global cellular c-di-AMP levels and result in a global output, such as the control of sporulation initiation. In contrast, it is conceivable that local DNA damage affects individual DisA complexes, and thus via the local c-di-AMP concentration impacts local DNA repair activity. However, the recent discovery that c-di-AMP levels in B. subtilis are controlled by the potassium concentration (18) suggests yet a different hypothesis, that the DNA is a strongly negatively charged molecule, and this negative charge is mainly buffered by potassium (58, 59). Thus, DisA might monitor the potassium concentration in the nucleoid region. At low potassium concentrations, c-di-AMP synthesis would be reduced, allowing the expression and activity of potassium transporters. In contrast, high c-di-AMP levels at high potassium concentrations would inhibit both the expression and the activity of the potassium transporters. In this way, an input that can be monitored in the nucleoid region would result in a global cellular response. Clearly, more analyses of in vivo and in vitro DisA activities are required to solve this issue.
The sporulation-specific diadenylate cyclase CdaS is only present in bacteria of the genus Bacillus and close relatives. This enzyme is required for efficient germination of the spores (60). CdaS consists of a DAC domain that is preceded by two helices that control the oligomeric state and the activity of the protein (Fig. 2A). This enzyme can exist in an active dimeric form or in an inactive hexameric form, and the transition is controlled by interactions between the N-terminal helices (40, 60). The N-terminal helices of CdaS act as an autoinhibitory domain; a protein lacking these helices is highly active (60). The molecular trigger that controls CdaS activity has so far not been identified.
For the other types of diadenylate cyclases, little or no information is available. For cyclases of the CdaM and CdaZ types, c-di-AMP synthetic activity has been proven (14, 41). In CdaM, an enzyme present in highly genome-reduced bacteria of the genus Mycoplasma, the DAC domain is fused to a transmembrane helix. CdaZ-type cyclases are present in the Euryarchaeota, including methanogens and haloarchaea. These enzymes carry an N-terminal pyruvate kinase-like domain. In addition, several other domain architectures of suspected diadenylate cyclase have been discovered (see Fig. 2A). In these proteins, the DAC domains are fused to YbbR domains that are also the building block of the regulatory protein CdaR, to regulatory domains of the sugar:phosphoenolpyruvate phosphotransferase system (PTS) or to Per-Arnt-Sim (PAS) domains. However, the activity and function of these proteins still await experimental investigation.
Structural analysis of diadenylate cyclases.
Crystal structures of diadenylate cyclases of the three major classes have been determined so far, DisA of T. maritima (5, 50), CdaS of Bacillus cereus, and CdaA of L. monocytogenes (43) (Table 1). These proteins have the conserved DAC domain of about 150 amino acids in common, but they differ largely regarding their other domains (see above) (Fig. 2A). A structure-based sequence alignment of the DAC domains of CdaA, CdaS, and DisA reveals sequence identities of 28% to 49% between these domains.
TABLE 1.
Structures of diadenylate cyclases and phosphodiesterases and their domains
| PDB ID | Enzyme | Domain(s) or mutation(s) | Ligand(s) | Metal ion | Reference |
|---|---|---|---|---|---|
| 3C1Z | DisA | DAC | None | None | 5 |
| 3C23 | DisA | DAC | 3′-dATP | None | 5 |
| 4YVZ | DisA | DAC | 3′-dATP | Mn2+ | 50 |
| 3C1Y | DisA | DAC | c-di-AMP | None | 5 |
| 4YXJ | DisA | DAC | ApCpp | None | 50 |
| 4RV7 | CdaA | DAC | ATP | Mg2+ | 43 |
| 2FB5 | CdaS | DAC | None | None | Unpublished data a |
| 2M1C | GdpP | PAS | None | None | 82 |
| 5XSI | GdpP | DHH-DHHA1 | None | Mn2+ | 107 |
| 5XSN | GdpP | DHH-DHHA1 | c-di-AMP | Mn2+ | 107 |
| 5XSP | GdpP | DHH-DHHA1 | 5′-pApA | Mn2+ | 107 |
| 5XT3 | GdpP | DHH-DHHA1 | c-di-GMP | Mn2+ | 107 |
| 5JJU | DhhP | DHH-DHHA1 | 5′-pApA and 5′-AMP | Mn2+ | 93 |
| 5O25 | DhhP | DHH-DHHA1 | None | Mn2+ | 89 |
| 5O58 | DhhP | DHH-DHHA1 | 5′-pApG | Mn2+ | 89 |
| D80N, D154N | |||||
| 5O70 | DhhP | DHH-DHHA1 | 5′-AMP | Mn2+ | 89 |
| D80N, D154N | |||||
| 5O1U | DhhP | DHH-DHHA1 | 5′-AMP | Mn2+ | 89 |
| 5O4Z | DhhP | DHH-DHHA1 | 5′-AMP | Mn2+ | 89 |
| D80N, D154N | |||||
| 5O7F | DhhP | DHH-DHHA1 | 5′-GMP | Mn2+ | 89 |
| 4S1B | PgpH | HD | c-di-AMP | Fe3+ | 96 |
| 4S1C | PgpH | HD | None | Fe3+ | 96 |
R. Zhang, M. Zhou, S. Ginell, J. Abdullah, F. Collart, and A. Joachimiak.
The DAC domain consists of a slightly twisted β-sheet formed by seven parallel and antiparallel β-strands, which are surrounded by five α-helices leading to a globular shape (Fig. 2B). The ATP-binding cavity is flanked by α-helix 4 and β-strands 1 and 5, as well as the loops that connect α1 and β1, α3 and β3, α4 and β4, and β5 and β6, respectively. In order to synthesize c-di-AMP from two ATP molecules, the active centers of two DAC domains need to assemble in a face-to-face orientation, forming a functional dimer (Fig. 2C).
Residues involved in ATP binding and catalysis of c-di-AMP formation are highly conserved among the enzymes of various organisms. These residues belong to three conserved sequence motifs. Motif I consists of Asp-Gly-Ala (DGA motif) and is located in the loop between α3 and β3, and this motif I is extended by three hydrophobic residues. Motif II is part of α-helix α4 and comprises residues Gly-Thr-Arg-His-Arg-X-Ala (RHR motif), of which the first Arg and the His bind the β- and γ-phosphate groups. Mutations in these two motifs result in a complete loss of enzymatic activity (5, 43, 50). The third motif is located in β5 and consists of two to three amino acids, with a highly conserved serine involved in β- and γ-phosphate binding and metal ion coordination. This serine of motif III is thought to polarize the γ-phosphate, which makes the nucleophilic attack of the α-phosphate easier.
The formation of c-di-AMP requires the reaction of two ATP molecules by the nucleophilic attack of each 3′-OH group on the α-phosphate of the other ATP molecule, leading to the release of two pyrophosphate molecules and concomitant ring closure. Several crystal structures of DAC domains show precatalytic or postcatalytic states, like the structures of the CdaA-ATP complex, of the DisA-ApCpp complex and of the DisA-3′-dATP complex, which correspond to the substrate-bound precatalytic state. The structure of DisA with bound c-di-AMP shows the postcatalytic state. The structures of the apo-state of CdaS and of DisA are known as well. The role of several active-site residues in substrate binding and catalysis were confirmed for DisA by site-directed mutagenesis and characterization of the mutated proteins. Each of the mutations D75N (in the DGA motif), RHR(108–110)AAA, T107V-T111V, and R130A, significantly decreased the DAC activity of DisA (50).
The reaction strictly depends on the presence of a metal ion in the active site, which is a Mg2+ or Mn2+ ion in DisA and a Mn2+ or Co2+ ion in CdaA. It has been suggested that due to the presence of the metal ion, the α-phosphate is moved more closely to the 3′-OH of the ATP bound by the neighboring subunit, hence facilitating the nucleophilic attack (50). However, it remains an enigma why CdaA is catalytically inactive in the presence of Mg2+, even though the crystal structure of CdaA was obtained with bound ATP and Mg2+ ion in the precatalytic state (43).
Inhibitors of diadenylate cyclases.
Since c-di-AMP is an essential second messenger in many bacteria, the c-di-AMP-synthesizing enzymes appear to be an attractive target for the development of novel antibiotics. Indeed, several inhibitors of DisA have been reported. As expected, the ATP analog 3′-dATP, which lacks the 3′-OH group and therefore cannot form c-di-AMP, is a competitive inhibitor, with a 50% inhibitor concentration (IC50) of 3.8 μM (50, 61). The screen of a library consisting of 1,000 compounds revealed bromophenol thiohydantoin (Br-TH) to inhibit DisA, with an IC50 of 56 μM (62). However, attempts to improve the inhibitory effect of Br-TH by modifications have failed (61). Another screen of a 2,000 compounds library identified suramin, a known antiparasitic drug, to be a more potent DisA inhibitor, with an IC50 of 1.1 μM (63). Another compound, theaflavin digallate, exhibited an IC50 of 3.4 μM and appeared to inhibit in an ATP-noncompetitive manner, as the IC50 only barely increased upon an increase in the ATP concentration (64).
c-di-AMP-DEGRADING PHOSPHODIESTERASES
Types of phosphodiesterases and their regulation.
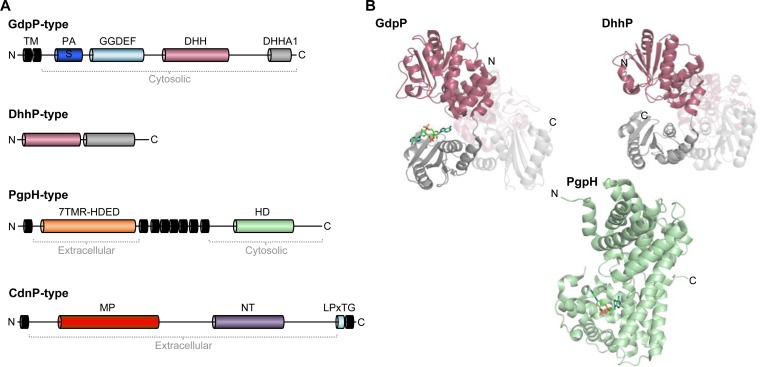
So far, four different types of phosphodiesterases that specifically degrade c-di-AMP have been identified (see Fig. 1A and 3A) (15). A GdpP-type phosphodiesterase (GGDEF domain protein-containing phosphodiesterase) was first described in L. lactis (65). For as-yet-unknown reasons, inactivation of the gdpP gene in L. lactis causes acid resistance and affects temperature sensitivity (66). Subsequently, gdpP orthologs were identified in Firmicutes, such as B. subtilis, Enterococcus faecalis, Enterococcus faecium, S. aureus, S. pneumoniae, Streptococcus mutans, Streptococcus pyogenes, and recently in the cyanobacterium Synechocystis sp. strain PCC 6803 (8, 34, 36, 67–75). In many of the Firmicutes, it has been shown that inactivation of the gdpP gene results in elevated c-di-AMP levels, which cause increased resistance toward cell wall-damaging antibiotics, such as β-lactams (76). Given the fact that c-di-AMP controls osmotic adaptation, it is reasonable to assume that elevated cellular c-di-AMP levels result in a reduced cellular turgor, which allows the bacteria to maintain the integrity of the weakened cell wall (21). Moreover, it has been observed that inactivation of the gdpP gene in S. pneumoniae, S. pyogenes, and Streptococcus suis affects the expression of virulence genes (68, 72, 77). While the link between c-di-AMP metabolism and osmotic homeostasis is well established, it is yet unclear how the nucleotide controls the virulence of pathogenic Firmicutes.
FIG 3.
Organization of c-di-AMP-degrading phosphodiesterases. (A) Domain structure of the four classes of phosphodiesterases. The different domains are characterized by a color code. TM, transmembrane helix; PAS, Per-Arnt-Sim domain; GGDEF, (degenerated) GGDEF domain; DHH, DHH domain; DHHA1, DHHA1 domain; 7TMR-HDED, seven-transmembrane helix-HDED domain; HD, HD domain; MP, metallophosphatase domain; NT, 5′-nucleotidase domain; LPxTG, surface localization motif. (B) Structures of GdpP from S. aureus (PDB ID 5XSN), the DhhP-type phosphodiesterase of T. maritima (PDB ID 5O25), and PgpH from L. monocytogenes (PDB ID 4S1B). The DHH domain (red) and the smaller DHHA1 domain (light gray) of the GdpP-type phosphodiesterases are colored as in Fig. 2A. Bound molecules are depicted in the stick model.
The GdpP-type phosphodiesterases possess two N-terminally located transmembrane helices, which are followed by a PAS domain, a degenerated GGDEF domain, and DHH and DHHA1 domains (Fig. 3A) (15). Recently, it has been reported that the transmembrane helices are essential for in vivo function (72). This finding suggests that GdpP may sense and respond to an extracellular cue. The PAS domains in prokaryotic proteins bind small-molecule metabolites, which initiates a signaling response or allows the domains to respond to a secondary cue, such as the redox potential (78). In the case of GdpP from B. subtilis and Geobacillus thermodenitrificans, the PAS domains of the phosphodiesterase bind b-type heme in a 1:1 stoichiometry (79). The binding of heme to the PAS domains inhibits the phosphodiesterase and ATPase activities of the DHH/DHHA1 and GGDEF domains, respectively (79). DHH/DHHA1 domain family proteins are named after the conserved Asp-His-His motif in their active sites, hydrolyzing a wide variety of substrates (80). While the physiological role of the ATPase function of the GGDEF domain is unknown, the DHH/DHHA1 phosphodiesterase domain is essential for cleaving c-di-AMP, its physiologically relevant substrate, to 5′-phosphoadenylyl-3′-5′-adenosine (5′-pApA) (34, 81, 82). In addition to the heme-dependent inhibition, the bacterial alarmone (p)ppGpp is a strong competitive inhibitor of the DHH/DHHA1 phosphodiesterase domain of GdpP in B. subtilis, S. aureus, and E. faecalis (46, 76, 81, 83). Interestingly, c-di-AMP also stimulates the synthesis of guanosine pentaphosphate [(p)ppGpp] by a mechanism that still needs to be elucidated (83). The (p)ppGpp-dependent control of c-di-AMP degradation suggests that the metabolisms of the two nucleotides and their signaling networks are tightly interconnected. While most of the metabolites interacting with GdpP inhibit its catalytic activity, the enzymes of B. subtilis and its close relative G. thermodenitrificans are activated in a heme- and nitric oxide (NO)-dependent manner (79). Many pathogenic bacteria, such as S. aureus and L. monocytogenes, also possess a GdpP-type phosphodiesterase (34, 75). Since the host cells of S. aureus and L. monocytogenes produce heme and NO, sensing of these metabolites may help the bacteria adjust osmotic homeostasis and their central metabolism for survival in the adverse host environment (79). However, in vivo studies are required to evaluate the role of the PAS domain in sensing heme and NO. In addition to the metabolite-dependent control of the GdpP phosphodiesterase activity, the cellular c-di-AMP levels may also be regulated by other mechanisms. In B. subtilis, an antisense transcript controlled by the alternative sigma factor σD modulates the GdpP protein levels (84). However, it remains to be elucidated why the synthesis of GdpP is modulated by σD, a sigma factor that controls motility, chemotaxis, and autolysin production.
Standalone DHH/DHHA1 proteins, here designated DhhP-type phosphodiesterases based on the nomenclature of the Borrelia burgdorferi enzyme (85), form the second group of c-di-AMP-degrading enzymes (Fig. 3A). The DhhP-type phosphodiesterases degrade c-di-AMP to AMP in a two-step process, where c-di-AMP is first converted to the linear 5′-pApA, which is then hydrolyzed to 5′-AMP (68, 85, 86). However, some of the DhhP-type phosphodiesterases have a clear preference for linear substrates compared to the cyclic dinucleotides (46, 87). DhhP-type phosphodiesterases that modulate the cellular c-di-AMP levels have been identified and characterized in several bacteria, including B. burgdorferi, Mycobacterium spp., M. pneumoniae, S. aureus, and T. maritima (14, 85, 86, 88–91). As for the GdpP-type family of phosphodiesterases, the DHH/DHHA1 domain was shown to be essential for enzyme catalysis (92). The DHH and DHHA1 motifs form the catalytic core and contribute to substrate recognition and stabilization, respectively (see below) (93). Interestingly, the DhhP orthologs from B. burgdorferi and M. pneumoniae were reported to be essential for growth, suggesting that these bacteria rely on a single phosphodiesterase for c-di-AMP degradation (14, 85). In contrast to the GdpP- and PgpH-type phosphodiesterases, the enzymes belonging to the DhhP-type family of phosphodiesterases have broader substrate specificities (14, 89, 91, 92, 94). Moreover, homologs of these enzymes are active as nano-RNases that degrade very short RNA oligonucleotides. Indeed, M. pneumoniae encodes two DhhP-type enzymes. One of them (PdeM) acts on c-di-AMP, whereas the second enzyme (NrnA) is unable to degrade c-di-AMP but functions as a nano-RNase (14, 94). Therefore, it is difficult to predict whether or not a given DhhP-type phosphodiesterase is capable of degrading c-di-AMP.
Many phenotypes can be associated with bacterial strains lacking DhhP-type phosphodiesterases that degrade c-di-AMP. For instance, inactivation of the c-di-AMP-degrading phosphodiesterase in Mycobacterium smegmatis resulted in the accumulation of fatty acids and caused abnormal cell morphology (92). The loss of phosphodiesterase activity in M. tuberculosis increased the cellular c-di-AMP levels, and the cell length was reduced (88). For S. mutans, it has been reported that a DhhP-type phosphodiesterase is involved in biofilm formation (74). Cells of an S. pneumoniae mutant lacking both the GdpP-type and the DhhP-type phosphodiesterases were significantly shorter (68). However, the molecular mechanisms by which the substrates of the DhhP-type phosphodiesterase affect diverse cellular processes remain to be elucidated.
A PgpH-type phosphodiesterase that degrades c-di-AMP was first identified in L. monocytogenes in a genetic screen aimed at the isolation of cold-sensitive mutants (15, 95). Biochemical and structural characterization of L. monocytogenes PgpH revealed that this protein is the founding member of a novel class of c-di-AMP-specific phosphodiesterases (15, 96). PgpH contains eight transmembrane helices (96, 97). One of these helices at the N terminus of PgpH is followed by an extracellular seven-transmembrane helix-HDED domain (7TMR-HDED) domain, seven transmembrane helices, and a cytosolic HD domain (Fig. 3A) (96). It has been proposed that the 7TMR-HDED domain serves as an extracellular receptor that senses a signal, which is transmitted via the seven transmembrane helices to the intracellular HD hydrolase domain (87). The HD domain is named after the conserved His-Asp motif, which is present in metal-dependent phosphohydrolases, such as RNases (80). The HD domain of PgpH binds c-di-AMP and hydrolyzes it to 5′-pApA (96). Similar to the phosphodiesterases belonging to the GdpP family, (p)ppGpp was shown to inhibit the catalytic activity of PgpH. Interestingly, c-di-AMP also seems to affect (p)ppGpp metabolism in L. monocytogenes because a pgpH mutant accumulated higher levels of the alarmone (95). Thus, cross talk between (p)ppGpp and c-di-AMP also exists in other Firmicutes. Characterization of the L. monocytogenes gdpP and pgpH mutants revealed that GdpP and PgpH are important for both intracellular and extracellular growth (96). Thus, the phosphodiesterases may sense and respond to distinct environmental signals. As described above, in the case of GdpP, it might be heme and NO, both metabolites that are present in eukaryotic host cells. In contrast, the extracellular 7TMR-HDED domain of PgpH may indicate that the protein is crucial for the adaptation of the bacteria in the environment (96). It has been suggested that the highly polar 7TMR-HDED domains may sense particular changes in ion concentrations, which is an attractive hypothesis because a major function of c-di-AMP seems to be the control of ion and osmotic homeostasis (21).
PgpH-type phosphodiesterases are widespread among bacteria, among them the majority of species belonging to the Firmicutes (13, 15, 96). Interestingly, Firmicutes, like staphylococci and streptococci, do not possess a PgpH-type phosphodiesterase (15). These bacteria synthesize another class of soluble phosphodiesterases with broader substrate specificities (see below) (15, 92). However, given the high abundance of PgpH, this phosphodiesterase seems to be the major enzyme involved in c-di-AMP degradation in bacteria.
Recently, CdnP (cyclic dinucleotide phosphodiesterase), an extracellular cell wall-anchored phosphodiesterase that hydrolyzes c-di-AMP, has been identified in the human pathogen Streptococcus agalactiae (98). CdnP is the founding member of the fourth class of c-di-AMP-degrading enzymes. It acts in concert with the phosphodiesterase NudP to degrade extracellular c-di-AMP via AMP to adenosine (99). Inactivation of the cdnP gene in S. agalactiae leads to extracellular c-di-AMP accumulation, which increases the inflammatory response of the innate immune system of infected eukaryotic host cells (98). It has been suggested that CdnP is required to promote the virulence of S. agalactiae by dampening the c-di-AMP-dependent activation of the innate immune system. CdnP consists of a 5′-nucleotidase domain and of a metallophosphoesterase domain (Fig. 3A). The metallophosphoesterase domain harbors the conserved NHE motif that is essential for enzyme activity (100, 101) (Fig. 3A). The N- and C-terminally located transmembrane helices and the LPXTG motif of CdnP are likely required for cell wall anchoring. The B. subtilis YfkN protein, which is a promiscuous nucleotide phosphoesterase (102), shares 46% overall amino acid identity with CdnP. However, YfkN consists of a CdnP-like N-terminal 2′,3′-cyclic nucleotide 2′-phosphodiesterase/3′-nucleotidase domain and a C-terminal 5′-nucleotidase domain. Interestingly, the yfkN gene was reported to be essential for growth, whereas a recent reevaluation of the essential genes in B. subtilis revealed that yfkN is in fact dispensable (103, 104). It will be interesting to test whether YfkN is involved in c-di-AMP metabolism in B. subtilis.
Structural analysis of phosphodiesterases.
Of the four known types of c-di-AMP-degrading phosphodiesterases, three have been structurally characterized (Table 1). No structure is available for CdnP.
As mentioned above, the GdpP-type phosphodiesterases carry beside their conserved DHH and DHHA1 domains two additional domains, a PAS domain and a GGDEF domain (see Fig. 3A). The PAS of the Geobacillus stearothermophilus GdpP domain shares a minimum sequence homology with heme- and nitric oxide-binding sites of other PAS domains, but it is much smaller than typical heme-binding PAS domains. A nuclear magnetic resonance (NMR) study showed a conserved fold containing a central antiparallel β-sheet surrounded by five α-helices (82). Three β-strands of the β-sheet and one α-helix are shortened in length, which explains the smaller size in comparison to the typical PAS domains, but nevertheless, it still forms a hydrophobic pocket between the β-sheet and the D-loop in the center of the protein. It was suggested that this hydrophobic pocket might bind a signaling molecule acting as a sensory domain in order to adjust the cellular c-di-AMP level in response to intra- and extracellular stimuli (82). The GGDEF domain of GdpP is degenerated, as it is actually missing the GG(D/E)EF motif (79, 89). Three-dimensional (3D) structures of GGDEF domains of other proteins are well known (for a review, see references 105 and 106), but no structure of a GGDEF domain of a GdpP-type phosphodiesterase has been reported so far.
The known crystal structures of the DHH/DHHA1 domains of GdpP from S. aureus (107) as well as of the DhhP-type phosphodiesterases from M. tuberculosis Rv2837c (93) and T. maritima (89) show a high structural similarity of the DHH/DHHA1 domains (Fig. 3B). The two distinct DHH/DHHA1-type domains are connected by a flexible loop (linker) of four amino acids. The highly conserved DHH motif in the active site is located in the loop region connecting β-strands β5 and β6. The overall structure of the DHH domain is formed by a parallel β-sheet consisting of six β-strands and is surrounded by eight α-helices. The slightly smaller DHHA1 domain is similarly shaped. It is also made of a central β-sheet consisting of six parallel and antiparallel β-strands flanked by five α-helices. This type of phosphodiesterases form dimers facing the active centers outward and a helical center.
The third type of c-di-AMP degrading phosphodiesterases, PgpH, contains the catalytically HD domain named after the highly conserved His and Asp residues in the active center. These residues play a key role in its activity because they are important for the coordination of the catalytically essential metal ions. The HD domain is formed by nine α-helices mostly ordered in an antiparallel manner with the conserved HD motif in helix α3 (Fig. 3B). Its overall shape is falcate, resulting in a metal ion (Mn2+) and c-di-AMP binding cavity. Several histidines are located in this nucleotide binding site that are responsible for the coordination of metal ions which in turn facilitate c-di-AMP binding. Additionally, all residues in the active center coordinating the c-di-AMP molecule are highly conserved in PgpH homologues (96).
CONCLUSIONS AND OPEN QUESTIONS
Even though c-di-AMP was discovered only 10 years ago, the field of c-di-AMP signaling has attracted much attention, and this research has yielded important insights, especially with respect to the essentiality and toxicity of c-di-AMP. We also have profound knowledge on the structural organization of major classes of diadenylate cyclases and c-di-AMP-degrading phosphodiesterases. However, our picture of c-di-AMP signaling is far from being complete even for well-studied model organisms. Important novel insights can be expected from the analysis of c-di-AMP metabolism and signaling in archaea, as well as in cyanobacteria, actinobacteria, and spirochaetes. For bacteria, like B. subtilis, that possess multiple diadenylate cyclases and phosphodiesterases, the interplay of these enzymes and their possible specialization in time and cellular space will be an interesting topic for future research. Finally, given the essentiality of c-di-AMP and accordingly of the synthesizing enzyme CdaA in many important pathogens, the development of drugs that target the cyclase will be a challenging task to help refill the pipeline of potential antibiotics. Moreover, initial efforts have been made to use c-di-AMP as an adjuvant to improve vaccination strategies (108). Thus, the further investigation of c-di-AMP signaling is of interest both in basic research and for the development of treatments against infectious diseases.
ACKNOWLEDGMENTS
We are grateful to the members of our labs for their contributions to the understanding of c-di-AMP signaling.
This work was supported by grants from the Deutsche Forschungsgemeinschaft via Priority Program SPP 1879 (to F.M.C., R.F., and J.S.).
REFERENCES
- 1.Gomelsky M. 2011. cAMP, c-di-GMP, c-di-AMP, and now cGMP: bacteria use them all! Mol Microbiol 79:562–565. doi: 10.1111/j.1365-2958.2010.07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Görke B, Stülke J. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 3.Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. 2015. Recent functional insights into the role of (p) ppGpp in bacterial physiology. Nat Rev Microbiol 13:298–309. doi: 10.1038/nrmicro3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hengge R, Gründling A, Jenal U, Ryan R, Yildiz F. 2015. Bacterial signal transduction by c-di-GMP and other nucleotide second messengers. J Bacteriol 197:e00331-15. doi: 10.1128/JB.00331-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witte G, Hartung S, Büttner K, Hopfner KP. 2008. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol Cell 30:167–178. doi: 10.1016/j.molcel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Marden JN, Dong Q, Roychowdhury Berleman JE, Bauer CE. 2010. Cyclic GMP controls Rhodospirillum centenum cyst development. Mol Microbiol 79:600–615. doi: 10.1111/j.1365-2958.2010.07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies BW, Bogard RW, Young TS, Mekalanos JJ. 2012. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 149:358–370. doi: 10.1016/j.cell.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo Y, Helmann JD. 2012. Analysis of the role of Bacillus subtilis σM in β-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol Microbiol 83:623–639. doi: 10.1111/j.1365-2958.2011.07953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song JH, Ko KS, Lee JY, Baek JY, Oh WS, Yoon HS, Jeong JY, Chun J. 2005. Identification of essential genes in Streptococcus pneumoniae by allelic replacement mutagenesis. Mol Cells 19:365–374. [PubMed] [Google Scholar]
- 10.Chaudhuri RR, Allen AG, Owen PJ, Shalom G, Stone K, Harrison M, Burgis TA, Lockyer M, Garcia-Lara J, Foster SJ, Pleasance SJ, Peters SE, Maskell DJ, Charles IG. 2009. Comprehensive identification of essential Staphylococcus aureus genes using transposon-mediated differential hybridization (TMDH). BMC Genomics 10:291. doi: 10.1186/1471-2164-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiteley AT, Pollock AJ, Portnoy DA. 2015. The PAMP c-di-AMP is essential for Listeria growth in macrophages and rich but not minimal medium due to a toxic increase in (p)ppGpp. Cell Host Microbe 17:788–798. doi: 10.1016/j.chom.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lluch-Senar M, Delgado J, Chen WH, Lloréns-Rico V, O'Reilly FJ, Wodke JAH, Unal EB, Yus E, Martínez S, Ferrar T, Vivancos A, Schmeisky A, Stülke J, van Noort V, Gavin AC, Bork P, Serrano L. 2015. Defining a minimal cell: essentiality of small ORFs and ncRNAs in a genome-reduced bacterium. Mol Syst Biol 11:780. doi: 10.15252/msb.20145558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gundlach J, Mehne FMP, Herzberg C, Kampf J, Valerius O, Kaever V, Stülke J. 2015. An essential poison: synthesis and degradation of cyclic di-AMP in Bacillus subtilis. J Bacteriol 197:3265–3274. doi: 10.1128/JB.00564-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blötz C, Treffon K, Kaever V, Schwede F, Hammer E, Stülke J. 2017. Identification of the components involved in cyclic di-AMP signaling in Mycoplasma pneumoniae. Front Microbiol 8:1328. doi: 10.3389/fmicb.2017.01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh TN, Woodward JJ. 2016. Too much of a good thing: regulated depletion of c-di-AMP in the bacterial cytoplasm. Curr Opin Microbiol 30:22–29. doi: 10.1016/j.mib.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corrigan RM, Campeotto I, Jeganathan T, Roelofs KG, Lee VT, Gründling A. 2013. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc Natl Acad Sci U S A 110:9084–9089. doi: 10.1073/pnas.1300595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson JW, Sudarsan N, Furukawa K, Weingerg Z, Wang JX, Breaker RR. 2013. Riboswitches in eubacteria sense the second messenger cyclic di-AMP. Nat Chem Biol 9:834–839. doi: 10.1038/nchembio.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gundlach J, Herzberg C, Kaever V, Gunka K, Hoffmann T, Weiß M, Gibhardt J, Thürmer A, Hertel D, Daniel R, Bremer E, Commichau FM, Stülke J. 2017. Control of potassium homeostasis is an essential function of the second messenger cyclic di-AMP in Bacillus subtilis. Sci Signal 10:eaal3011. doi: 10.1126/scisignal.aal3011. [DOI] [PubMed] [Google Scholar]
- 19.Corrigan RM, Gründling A. 2013. Cyclic di-AMP: another second messenger enters the fray. Nat Rev Microbiol 11:513–524. doi: 10.1038/nrmicro3069. [DOI] [PubMed] [Google Scholar]
- 20.Commichau FM, Dickmanns A, Gundlach J, Ficner R, Stülke J. 2015. A jack of all trades: the multiple roles of the unique essential second messenger cyclic di-AMP. Mol Microbiol 97:189–204. doi: 10.1111/mmi.13026. [DOI] [PubMed] [Google Scholar]
- 21.Commichau FM, Gibhardt J, Halbedel S, Gundlach J, Stülke J. 2018. A delicate connection: c-di-AMP affects cell integrity by controlling osmolyte transport. Trends Microbiol 26:175–185. doi: 10.1016/j.tim.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Zeden MS, Schuster CF, Bowman L, Zhong Q, Williams HD, Gründling A. 2018. Cyclic di-adenosine monophosphate (c-di-AMP) is required for osmotic regulation in Staphylococcus aureus but dispensable for viability in anaerobic conditions. J Biol Chem 293:3180–3200. doi: 10.1074/jbc.M117.818716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster CF, Bellows LE, Tosi T, Campeotto I, Corrigan RM, Freemont P, Gründling A. 2016. The second messenger c-di-AMP inhibits the osmolyte uptake system OpuC in Staphylococcus aureus. Sci Signal 9:ra81. doi: 10.1126/scisignal.aaf7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huynh TN, Choi PH, Sureka K, Ledvina HE, Campillo J, Tong L, Woodward JJ. 2016. Cyclic di-AMP targets the cystathione beta-synthase domain of the osmolyte transporter OpuC. Mol Microbiol 102:233–243. doi: 10.1111/mmi.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devaux L, Sleiman D, Mazzuoli MV, Gominet M, Lanotte P, Trieu-Cuot P, Kaminski PA, Firon A. 2018. Cyclic di-AMP regulation of osmotic homeostasis is essential in group B Streptococcus. PLoS Genet 14:e1007342. doi: 10.1371/journal.pgen.1007342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sureka K, Choi PH, Precit M, Delince M, Pensinger DA, Huynh TN, Jurado AR, Goo YA, Sadilek M, Iavarone AT, Sauer JD, Tong L, Woodward JJ. 2014. The cyclic dinucleotide c-di-AMP is an allosteric regulator of metabolic enzyme function. Cell 158:1389–1401. doi: 10.1016/j.cell.2014.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiteley AT, Garelis NE, Peterson BN, Choi PH, Tong L, Woodward JJ, Portnoy DA. 2017. c-di-AMP modulates Listeria monocytogenes central metabolism to regulate growth, antibiotic resistance and osmoregulation. Mol Microbiol 104:212–233. doi: 10.1111/mmi.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St-Onge RJ, Haiser HJ, Yousef MR, Sherwood E, Tschowri N, Al-Bassam M, Elliot MA. 2015. Nucleotide second messenger-mediated regulation of a muralytic enzyme in Streptomyces. Mol Microbiol 96:779–795. doi: 10.1111/mmi.12971. [DOI] [PubMed] [Google Scholar]
- 29.Gundlach J, Rath H, Herzberg C, Mäder U, Stülke J. 2016. Second messenger signaling in Bacillus subtilis: accumulation of cyclic di-AMP inhibits biofilm formation. Front Microbiol 7:804. doi: 10.3389/fmicb.2016.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Townsley L, Yannarell SM, Huynh TN, Woodward JJ, Shank EA. 2018. Cyclic di-AMP acts as an extracellular signal that impacts Bacillus subtilis biofilm formation and plant attachment. mBio 9:e00341-18. doi: 10.1128/mBio.00341-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oppenheimer-Shaanan Y, Wexselblatt E, Katzhendler J, Yavin E, Ben-Yehuda S. 2011. c-di-AMP reports DNA integrity during sporulation in Bacillus subtilis. EMBO Rep 12:594–601. doi: 10.1038/embor.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campos SS, Ibarra-Rodriguez JR, Barajas-Ornelas RC, Ramírez-Guadiana FH, Obregón-Herrera A, Setlow P, Pedraza-Reyes M. 2014. Interaction of apurinic/apyrimidinic endonucleases Nfo and ExoA with the DNA integrity scanning protein DisA in the processing of oxidative DNA damage during Bacillus subtilis spore outgrowth. J Bacteriol 196:568–578. doi: 10.1128/JB.01259-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raguse M, Torres R, Seco EM, Gándara C, Ayora S, Moeller R, Alonso JC. 2017. Bacillus subtilis DisA helps to circumvent replicative stress during spore revival. DNA Repair (Amst) 59:57–68. doi: 10.1016/j.dnarep.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Gründling A. 2011. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog 7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pham HT, Nhiep NTH, Vu TNM, Huynh TN, Zhu Y, Huynh ALD, Chakrabortti A, Marcellin E, Lo R, Howard CB, Bansal N, Woodward JJ, Liang ZX, Turner MS. 2018. Enhanced uptake of potassium or glycine betaine or export of cyclic di-AMP restores osmoresistance in a high cyclic-di-AMP Lactococcus lactis mutant. PLoS Genet 14:e1007574. doi: 10.1371/journal.pgen.1007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agostoni M, Logan-Jackson AR, Heinz ER, Severin GB, Bruger EL, Waters CM, Montgomery BL. 2018. Homeostasis of second messenger cyclic-di-AMP is critical for cyanobacterial fitness and acclimation to abiotic stress. Front Microbiol 9:1121. doi: 10.3389/fmicb.2018.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin BE, Huynh TN, Welkie DG, Diamond S, Simkovsky R, Pierce EC, Taton A, Lowe LC, Lee JJ, Rifkin SA, Woodward JJ, Golden SS. 2018. High-throughput interaction screens illuminate the role of c-di-AMP in cyanobacterial nighttime survival. PLoS Genet 14:e1007301. doi: 10.1371/journal.pgen.1007301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gundlach J, Dickmanns A, Schröder-Tittmann K, Neumann P, Kaesler J, Kampf J, Herzberg C, Hammer E, Schwede F, Kaever V, Tittmann K, Stülke J, Ficner R. 2015. Identification, characterization and structure analysis of the c-di-AMP binding PII-like signal transduction protein DarA. J Biol Chem 290:3069–3080. doi: 10.1074/jbc.M114.619619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Römling U. 2008. Great times for small molecules: c-di-AMP, a second messenger candidate in bacteria and archaea. Sci Signal 1:pe39. doi: 10.1126/scisignal.133pe39. [DOI] [PubMed] [Google Scholar]
- 40.Mehne FMP, Gunka K, Eilers H, Herzberg C, Kaever V, Stülke J. 2013. Cyclic-di-AMP homeostasis in Bacillus subtilis: both lack and high-level accumulation of the nucleotide are detrimental for cell growth. J Biol Chem 288:2004–2017. doi: 10.1074/jbc.M112.395491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kellenberger CA, Chen C, Whiteley AT, Portnoy DA, Hammond MC. 2015. RNA-based fluorescent biosensors for live cell imaging of second messenger cyclic di-AMP. J Am Chem Soc 137:6432–6435. doi: 10.1021/jacs.5b00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicolas P, Mäder U, Dervyn E, Rochat T, Leduc A, Pigeonneau N, Bidnenko E, Marchadier E, Hoebeke M, Aymerich S, Becher D, Bisicchia P, Botella E, Delumeau O, Doherty G, Denham EL, Fogg MJ, Fromion V, Goelzer A, Hansen A, Härtig E, Harwood CR, Homuth G, Jarmer H, Jules M, Klipp E, Le Chat L, Lecointe F, Lewis P, Liebermeister W, March A, Mars RA, Nannapaneni P, Noone D, Pohl S, Rinn B, Rügheimer F, Sappa PK, Samson F, Schaffer M, Schwikowski B, Steil L, Stülke J, Wiegert T, Devine KM, Wilkinson AJ, van Dijl JM, Hecker M, Völker U, Bessières P, et al. 2012. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 335:1103–1106. doi: 10.1126/science.1206848. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg J, Dickmanns A, Neumann P, Gunka K, Arens J, Kaever V, Stülke J, Ficner R, Commichau FM. 2015. Structural and biochemical analysis of the essential diadenylate cyclase CdaA from Listeria monocytogenes. J Biol Chem 290:6596–6606. doi: 10.1074/jbc.M114.630418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hambraeus G, von Wachenfeldt C, Hederstedt L. 2003. Genome-wide survey of mRNA half-lives in Bacillus subtilis identifies extremely stable mRNAs. Mol Genet Genomics 269:706–714. doi: 10.1007/s00438-003-0883-6. [DOI] [PubMed] [Google Scholar]
- 45.Rismondo J, Gibhardt J, Rosenberg J, Kaever V, Halbedel S, Commichau FM. 2016. Phenotypes associated with the essential diadenylate cyclase CdaA and its potential regulator CdaR in the human pathogen Listeria monocytogenes. J Bacteriol 198:416–426. doi: 10.1128/JB.00845-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowman L, Zeden MS, Schuster CF, Kaever V, Gründling A. 2016. New insights into the cyclic di-adenosine monophosphate (c-di-AMP) degradation pathway and the requirement of the cyclic dinucleotide for acid stress resistance in Staphylococcus aureus. J Biol Chem 291:26970–26986. doi: 10.1074/jbc.M116.747709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Y, Pham TH, Nhiep TH, Vu NM, Marcellin E, Chakrabortti A, Wang Y, Waanders J, Lo R, Huston WM, Bansal N, Nielsen LK, Liang ZX, Turner MS. 2016. Cyclic di-AMP synthesis by the diadenylate cyclase CdaA is modulated by the peptidoglycan biosynthesis enzyme GlmM in Lactococcus lactis. Mol Microbiol 99:1015–1027. doi: 10.1111/mmi.13281. [DOI] [PubMed] [Google Scholar]
- 48.Zarrella TM, Metzger DW, Bai G. 2018. Stress suppressor screening leads to detecting regulation of cyclic di-AMP homeostasis by a Trk-family effector protein in Streptococcus pneumoniae. J Bacteriol 200:e00045-18. doi: 10.1128/JB.00045-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai Y, Yang J, Zhou X, Ding X, Eisele LE, Bai G. 2012. Mycobacterium tuberculosis Rv3586 (DacA) is a diadenylate cyclase that converts ATP or ADP into c-di-AMP. PLoS One 7:e35206. doi: 10.1371/journal.pone.0035206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller M, Deimling T, Hopfner KP, Witte G. 2015. Structural analysis of the diadenylate cyclase reaction of DNA-integrity scanning protein A (DisA) and its inhibition by 3′-dATP. Biochem J 469:367–374. doi: 10.1042/BJ20150373. [DOI] [PubMed] [Google Scholar]
- 51.Bejerano-Sagie M, Oppenheimer-Shaanan Y, Berlatzky I, Rouvinski A, Meyerovich M, Ben-Yehuda S. 2006. A checkpoint protein that scans the chromosome for damage at the start of sporulation in Bacillus subtilis. Cell 125:679–690. doi: 10.1016/j.cell.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 52.Gándara C, de Lucena DKC, Torres R, Serrano E, Altenburger S, Graumann PL, Alonso JC. 2017. Activity and in vivo dynamics of Bacillus subtilis DisA are affected by RadA/Sms and by Holiday junction-processing proteins. DNA Repair (Amst) 55:17–30. doi: 10.1016/j.dnarep.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, He ZG. 2013. Radiation-sensitive gene A (RadA) targets DisA, DNA integrity scanning protein A, to negatively affect cyclic di-AMP synthesis activity in Mycobacterium smegmatis. J Biol Chem 288:22426–22436. doi: 10.1074/jbc.M113.464883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper DL, Lovett ST. 2016. Recombinational branch migration by the RadA/Sms paralog of RecA in Escherichia coli. Elife 5:e10807. doi: 10.7554/eLife.10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marie L, Rapisarda C, Morales V, Bergé M, Perry T, Soulet AL, Gruget C, Remaut H, Fronzes R, Polard P. 2017. Bacterial RadA is a DnaB-type helicase interacting with RecA to promote bidirectional D-loop extension. Nat Commun 8:15638. doi: 10.1038/ncomms15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valenzuela-García LI, Ayala-Garcia VM, Regalado-Garcia AG, Setlow P, Pedraza-Reyes M. 2018. Transcriptional coupling (Mfd) and DNA damage scanning (DisA) coordinate excision repair events for efficient Bacillus subtilis spore outgrowth. MicrobiologyOpen 7:e00593 doi: 10.1002/mbo3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gándara C, Alonso JC. 2015. DisA and c-di-AMP act at the intersection between DNA-damage response and stress homeostasis in exponentially growing Bacillus subtilis cells. DNA Repair (Amst) 27:1–8. doi: 10.1016/j.dnarep.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Gundlach J, Herzberg C, Hertel D, Thürmer A, Daniel R, Link H, Stülke J. 2017. Adaptation of Bacillus subtilis to life at extreme potassium limitation. mBio 8:e00861-17. doi: 10.1128/mBio.00861-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gundlach J, Commichau FM, Stülke J. 2018. Perspective of ions and messengers: intricate link between potassium, glutamate, and cyclic di-AMP. Curr Genet 64:191–195. doi: 10.1007/s00294-017-0734-3. [DOI] [PubMed] [Google Scholar]
- 60.Mehne FMP, Schröder-Tittmann K, Eijlander RT, Herzberg C, Hewitt L, Kaever V, Lewis RJ, Kuipers OP, Tittmann K, Stülke J. 2014. Control of the diadenylate cyclase CdaS in Bacillus subtilis: an autoinhibitory domain limits c-di-AMP production. J Biol Chem 289:21098–21107. doi: 10.1074/jbc.M114.562066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Opoku-Temeng C, Zhou J, Zheng Y, Su J, Sintim HO. 2016. Cyclic dinucleotide (c-di-GMP, c-di-AMP, and cGAMP) signaling have come of age to be inhibited by small molecules. Chem Commun (Camb.) 52:9327–9342. doi: 10.1039/C6CC03439J. [DOI] [PubMed] [Google Scholar]
- 62.Zheng Y, Zhou J, Sayre DA, Sintim HO. 2014. Identification of bromophenol thiohydantoin as an inhibitor of DisA, a c-di-AMP synthase, from a 1000 compound library, using the coralyne assay. Chem Commun (Camb) 50:11234–11237. doi: 10.1039/C4CC02916J. [DOI] [PubMed] [Google Scholar]
- 63.Opoku-Temeng C, Sintim HO. 2016. Potent inhibition of cyclic diadenylate monophosphate cyclase by the antiparasitic drug, suramin. Chem Commun (Camb) 52:3754–3757. doi: 10.1039/C5CC10446G. [DOI] [PubMed] [Google Scholar]
- 64.Opoku-Temeng C, Sintim HO. 2016. Inhibition of cyclic diadenylate cyclase, DisA, by polyphenols. Sci Rep 6:25445. doi: 10.1038/srep25445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rallu F, Gruss A, Ehrlich SD, Maguin E. 2000. Acid- and intracellular-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol Microbiol 35:517–528. doi: 10.1046/j.1365-2958.2000.01711.x. [DOI] [PubMed] [Google Scholar]
- 66.Smith WM, Pham TH, Lei L, Dou J, Soomro AH, Beatson SA, Dykes GA, Turner MS. 2012. Heat resistance and salt hypersensitivity in Lactococcus lactis due to spontaneous mutation in llmg1816 (gdpP) induced by high-temperature growth. Appl Environ Microbiol 78:7753–7759. doi: 10.1128/AEM.02316-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Argudín MA, Roisin S, Nienhaus L, Dodémont M, de Mendoca R, Nonhoff C, Deplano A, Denis O. 2018. Genetic diversity among S. aureus isolates showing oxacillin and/or cefoxitin resistance not linked to the presence of mec genes. Antimicrob Agents Chemother 62:e00091-18. doi: 10.1128/AAC.00091-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bai Y, Yang J, Eisele LE, Underwood AJ, Koestler BJ, Waters CM, Metzger DW, Bai G. 2013. Two DHH subfamily 1 proteins in Streptococcus pneumoniae possess cyclic di-AMP phosphodiesterase activity and affect bacterial growth and virulence. J Bacteriol 195:5123–5132. doi: 10.1128/JB.00769-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banerjee R, Gretes M, Harlem C, Basuino L, Chambers H. 2010. A mecA-negative strain of methicillin-resistant Staphylococcus aureus with high-level β-lactam resistance contains mutations in three genes. Antimicrob Agents Chemother 54:4900–4902. doi: 10.1128/AAC.00594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Griffiths JM, O'Neill AJ. 2011. Loss of function of the GdpP protein leads to joint β-lactam/glycopeptide tolerance in Staphylococcus aureus. Antimicrob Agents Chemother 56:579–581. doi: 10.1128/AAC.05148-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pozzi C, Waters EM, Rudkin JK, Schaeffer CR, Lohan AJ, Tong P, Loftus BJ, Pier GB, Fey PD, Massey RC, O'Gara JP. 2012. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog 8:e1002626. doi: 10.1371/journal.ppat.1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cho KH, Kang SO. 2013. Streptococcus pyogenes c-d-AMP phosphodiesterase, GdpP influences SpeB processing and virulence. PLoS One 8:e69425. doi: 10.1371/journal.pone.0069425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller C, Kong J, Tran TT, Arias CA, Saxer G, Shamoo Y. 2013. Adaptation of Enterococcus faecalis to daptomycin reveals an ordered progression of resistance. Antimicrob Agents Chemother 57:5373–5383. doi: 10.1128/AAC.01473-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peng X, Zhang Y, Bai G, Zhou X, Wu H. 2016. Cyclic di-AMP mediates biofilm formation. Mol Microbiol 99:945–959. doi: 10.1111/mmi.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Witte CE, Whiteley AT, Burke TP, Sauer JD, Portnoy DA, Woodward JJ. 2013. Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. mBio 4:e00282-13. doi: 10.1128/mBio.00282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X, Davlieva M, Reyes J, Panesso D, Arias CA, Shamoo Y. 2017. Novel phosphodiesterase of the GdpP family modulates cyclic di-AMP levels in response to cell membrane stress in daptomycin-resistant enterococci. Antimicrob Agents Chemother 61:e01422-16. doi: 10.1128/AAC.01422-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du B, Ji W, An H, Shi Y, Huang Q, Cheng Y, Fu Q, Wang H, Yan Y, Sun J. 2014. Functional analysis of c-di-AMP phosphodiesterase, GdpP, in Streptococcus suis serotype 2. Microbiol Res 169:749–758. doi: 10.1016/j.micres.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Henry JT, Crosson S. 2011. Ligand-binding PAS domains in a genomic cellular and structural context. Annu Rev Microbiol 65:261–286. doi: 10.1146/annurev-micro-121809-151631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao F, Ji Q, Soehano I, Liang ZX. 2011. Unusual heme-binding PAS domain from YybT family proteins. J Bacteriol 193:1543–1551. doi: 10.1128/JB.01364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aravind L, Koonin EV. 1998. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci 23:17–19. doi: 10.1016/S0968-0004(97)01162-6. [DOI] [PubMed] [Google Scholar]
- 81.Rao F, See RY, Zhang D, Toh DC, Ji Q, Liang ZX. 2010. YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity. J Biol Chem 285:473–482. doi: 10.1074/jbc.M109.040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan E, Rao F, Pasunooti S, Pham TH, Soehano I, Turner MS, Liew CW, Lescar J, Pervushin K, Liang ZX. 2013. Solution structure of the PAS domain of a thermophilic YybT protein homolog reveals a potential ligand-binding site. J Biol Chem 288:11949–11959. doi: 10.1074/jbc.M112.437764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Corrigan RM, Bowman L, Willis AR, Kaever V, Gründling A. 2015. Cross-talk between two nucleotide-signaling pathways in Staphylococcus aureus. J Biol Chem 290:5826–5839. doi: 10.1074/jbc.M114.598300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luo Y, Helmann JD. 2012. A σD-dependent antisense transcript modulates expression of the cyclic-di-AMP hydrolase GdpP in Bacillus subtilis. Microbiology 158:2732–2741. doi: 10.1099/mic.0.062174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ye M, Zhang JJ, Fang X, Lawlis GB, Troxell B, Zhou Y, Gomelsky M, Lou Y, Yang XF. 2014. DhhP, a cyclic di-AMP phosphodiesterase of Borrelia burgdorferi, is essential for cell growth and virulence. Infect Immun 82:1840–1849. doi: 10.1128/IAI.00030-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manikandan K, Sabareesh V, Singh N, Saigal K, Mechold U, Sinha KM. 2014. Two-step synthesis and hydrolysis of cyclic di-AMP in Mycobacterium tuberculosis. PLoS One 9:e86096. doi: 10.1371/journal.pone.0086096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anantharaman V, Aravind L. 2003. Application of comparative genomics in the identification and analysis of novel families of membrane-associated receptors in bacteria. BMC Genomics 4:34. doi: 10.1186/1471-2164-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang J, Bai Y, Zhang Y, Gabrielle VD, Jin L, Bai G. 2014. Deletion of the cyclic di-AMP phosphodiesterase gene (cnpB) in Mycobacterium tuberculosis leads to virulence in a mouse model of infection. Mol Microbiol 93:65–79. doi: 10.1111/mmi.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Drexler DJ, Müller M, Rojas-Cordova CA, Bandera AM, Witte G. 2017. Structural and biophysical analysis of the suluble DHH/DHHA1-type phosphodiesterase TM1595 from Thermotoga maritima. Structure 25:1887–1897. doi: 10.1016/j.str.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y, Yang J, Bai G. 2018. Cyclic di-AMP-mediated interaction between Mycobacterium tuberculosis ΔcnpB and macrophages implicates a novel strategy for improving BCG vaccination. Pathog Dis 76:fty008. doi: 10.1093/femspd/fty008. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Yang J, Bai G. 2018. Regulation of the CRISPR-associated genes by Rv2837c (CnpB) via Orn-like activity in TB complex mycobacteria. J Bacteriol 200:e00743-17. doi: 10.1128/JB.00743-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang Q, Luo Y, Zheng C, Yin K, Ali MK, Li X, He J. 2015. Functional analysis of a c-di-AMP-specific phosphodiesterase MsPDE from Mycobacterium smegmatis. Int J Biol Sci 11:813–824. doi: 10.7150/ijbs.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He Q, Wang F, Liu S, Zhu D, Cong H, Gao F, Li B, Wang H, Lin Z, Liao J, Gu L. 2016. Structural and biochemical insight into the mechanism of Rv2837c from Mycobacterium tuberculosis as a c-di-NMP phosphodiesterase. J Biol Chem 291:3668–3681. doi: 10.1074/jbc.M115.699801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Postic G, Danchin A, Mechold U. 2012. Characterization of NrnA homologs from Mycobacterium tuberculosis and Mycoplasma pneumoniae. RNA 18:155–165. doi: 10.1261/rna.029132.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu S, Bayles DO, Mason TM, Wilkinson BJ. 2006. A cold-sensitive Listeria monocytogenes mutant has a transposon insertion in a gene encoding a putative membrane protein and shows altered (p)ppGpp levels. Appl Environ Microbiol 72:3955–3959. doi: 10.1128/AEM.02607-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huynh TN, Luo S, Pensinger D, Sauer JD, Tong L, Woodward JJ. 2015. An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence. Proc Natl Acad Sci U S A 112:E747–E756. doi: 10.1073/pnas.1416485112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Omasits U, Ahrens C, Müller S, Wollscheid B. 2014. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 30:884–886. doi: 10.1093/bioinformatics/btt607. [DOI] [PubMed] [Google Scholar]
- 98.Andrade WA, Firon A, Schmidt T, Hornung V, Fitzgerald KA, Kurt-Jones EA, Trieu-Cuot P, Golenbock DT, Kaminski PA. 2016. Group B Streptococcus degrades cyclic-di-AMP to modulate STING-dependent type I interferon production. Cell Host Microbe 20:49–59. doi: 10.1016/j.chom.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Firon A, Dinis M, Raynal B, Poyart C, Trieu-Cout P, Kaminski PA. 2014. Extracellular nucleotide catabolism by the group B Streptococcus ectonucleotidase NudP increase bacterial survival in blood. J Biol Chem 289:5479–5489. doi: 10.1074/jbc.M113.545632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matange N, Podobnik M, Visweswariah SS. 2015. Metallophosphoesterases: structural fidelity with functional promiscuity. Biochem J 467:201–216. doi: 10.1042/BJ20150028. [DOI] [PubMed] [Google Scholar]
- 101.Zimmermann H, Zebisch M, Sträter N. 2012. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chambert R, Pereira Y, Petit-Glatron MF. 2003. Purification and characterization of YfkN, a trifunctional nucleotide phosphoesterase secreted by Bacillus subtilis. J Biochem 134:655–660. doi: 10.1093/jb/mvg189. [DOI] [PubMed] [Google Scholar]
- 103.Thomaides HB, Davison EJ, Burston L, Johnson H, Brown DR, Hunt AC, Errington J, Czaplewski L. 2007. Essential bacterial functions encoded by gene pairs. J Bacteriol 189:591–602. doi: 10.1128/JB.01381-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reuß DR, Commichau FM, Gundlach J, Zhu B, Stülke J. 2016. The blueprint of a minimal cell: MiniBacillus. Microbiol Mol Biol Rev 80:955–987. doi: 10.1128/MMBR.00029-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chou SH, Galperin MY. 2016. Diversity of cyclic di-GMP-binding proteins and mechanisms. J Bacteriol 198:32–46. doi: 10.1128/JB.00333-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schirmer T. 2016. c-di-GMP synthesis: structural aspects of evolution, catalysis and regulation. J Mol Biol 428:3683–3701. doi: 10.1016/j.jmb.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 107.Wang F, He Q, Su K, Wei T, Xu S, Gu L. 2018. Structural and biochemical characterization of the catalytic domains of GdpP reveals a unified hydrolysis mechanism for the DHH/DHHA1 phosphodiesterase. Biochem J 475:191–205. doi: 10.1042/BCJ20170739. [DOI] [PubMed] [Google Scholar]
- 108.Quintana I, Espariz M, Villar S, Gonzalez FB, Pacini F, Cabrera G, Bontempi I, Procheto E, Stülke J, Perez AR, Marcipar I, Blancato V, Magni C. 2018. Genetic engineering of Lactococcus lactis co-producing antigen and the mucosal adjuvant 3′ 5′- cyclic di adenosine monophosphate (c-di-AMP) as a design strategy to develop a mucosal vaccine prototype. Front Microbiol 9:2100. doi: 10.3389/fmicb.2018.02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moscoso JA, Schramke H, Zhang Y, Tosi T, Dehbi A, Jung K, Gründling A. 2016. Binding of cyclic di-AMP to the Staphylococcus aureus sensor kinase KdpD occurs via the universal stress protein domain and downregulates the expression of the Kdp potassium transporter. J Bacteriol 198:98–110. doi: 10.1128/JB.00480-15. [DOI] [PMC free article] [PubMed] [Google Scholar]