The bacterial cell wall acts as a primary defense against environmental insults such as changes in osmolarity. It is also a vulnerable structure, as defects in its synthesis can lead to growth arrest or cell death. The important human pathogen Staphylococcus aureus has a typical Gram-positive cell wall, which consists of peptidoglycan and the anionic polymers LTA and wall teichoic acid. Several clinically relevant antibiotics inhibit the synthesis of peptidoglycan; therefore, it and teichoic acids are considered attractive targets for the development of new antimicrobials. We show that LTA is required for efficient peptidoglycan cross-linking in S. aureus and inactivation of a peptidoglycan glycosyltransferase can partially rescue this defect, together revealing an intimate link between peptidoglycan and LTA synthesis.
KEYWORDS: Staphylococcus aureus, cell wall, lipoteichoic acid
ABSTRACT
The cell wall of Staphylococcus aureus is composed of peptidoglycan and the anionic polymers lipoteichoic acid (LTA) and wall teichoic acid. LTA is required for growth and normal cell morphology in S. aureus. Strains lacking LTA are usually viable only when grown under osmotically stabilizing conditions or after the acquisition of compensatory mutations. LTA-negative suppressor strains with inactivating mutations in gdpP, which resulted in increased intracellular c-di-AMP levels, were described previously. Here, we sought to identify factors other than c-di-AMP that allow S. aureus to survive without LTA. LTA-negative strains able to grow in unsupplemented medium were obtained and found to contain mutations in sgtB, mazE, clpX, or vraT. The growth improvement through mutations in mazE and sgtB was confirmed by complementation analysis. We also showed that an S. aureus sgtB transposon mutant, with the monofunctional peptidoglycan glycosyltransferase SgtB inactivated, displayed a 4-fold increase in the MIC of oxacillin, suggesting that alterations in the peptidoglycan structure could help bacteria compensate for the lack of LTA. Muropeptide analysis of peptidoglycans isolated from a wild-type strain and sgtB mutant strain did not reveal any sizable alterations in the peptidoglycan structure. In contrast, the peptidoglycan isolated from an LTA-negative ltaS mutant strain showed a significant reduction in the fraction of highly cross-linked peptidoglycan, which was partially rescued in the sgtB ltaS double mutant suppressor strain. Taken together, these data point toward an important function of LTA in cell wall integrity through its necessity for proper peptidoglycan assembly.
IMPORTANCE The bacterial cell wall acts as a primary defense against environmental insults such as changes in osmolarity. It is also a vulnerable structure, as defects in its synthesis can lead to growth arrest or cell death. The important human pathogen Staphylococcus aureus has a typical Gram-positive cell wall, which consists of peptidoglycan and the anionic polymers LTA and wall teichoic acid. Several clinically relevant antibiotics inhibit the synthesis of peptidoglycan; therefore, it and teichoic acids are considered attractive targets for the development of new antimicrobials. We show that LTA is required for efficient peptidoglycan cross-linking in S. aureus and inactivation of a peptidoglycan glycosyltransferase can partially rescue this defect, together revealing an intimate link between peptidoglycan and LTA synthesis.
INTRODUCTION
Staphylococcus aureus is a Gram-positive bacterium found as a commensal on the skin and in nasal passages of healthy individuals. However, this bacterium is also an important human pathogen causing hospital-acquired and community-acquired infections, such as serious skin infections, osteomyelitis, and endocarditis (1–3). Of major concern is the increasing resistance of this organism to a large number of clinically relevant antibiotics (4). A number of virulence factors contribute to successful host colonization, immune evasion, and acquisition of nutrients within the host (5). Many of these factors are either secreted proteins or other extracellular proteins closely associated with the bacterial cell envelope (6).
The cell envelope is essential for bacterial survival and pathogenesis but also is a target of a number of important antimicrobials. It functions as a barrier and protects bacteria from environmental insults but at the same time needs to allow the passage of solutes and nutrients, as well as sensing of changes in the external environment (7). S. aureus has a typical Gram-positive cell envelope, which consists of a cytoplasmic membrane surrounded by a thick peptidoglycan layer (8). The peptidoglycan layer is a dynamic macromolecular structure that undergoes constant cycles of polymerization and hydrolysis to allow bacteria to grow and to divide (7). It is composed of glycan chains made of alternating N-acetylglucosamine and N-acetylmuramic acid residues connected by peptide bridges (9). This mesh-like sacculus is able to protect the cell from environmental threats while withstanding the high internal osmotic pressure (10). The final steps of peptidoglycan synthesis are catalyzed by enzymes termed penicillin-binding proteins (PBPs), and coordinated actions of these enzymes are crucial for cell survival (11). PBPs with glycosyltransferase and transpeptidase activities polymerize the glycan chains and form peptide cross-bridges, while monofunctional transpeptidases have only the former activity (11).
S. aureus encodes four PBPs, of which PBP1, which has transpeptidase activity, and the bifunctional PBP2, which has transpeptidase and glycosyltransferase activities, are the minimal requirements for cell survival (12). In methicillin-resistant S. aureus (MRSA) strains, the alternative PBP2A, which has transpeptidase activity, is needed for β-lactam resistance, in addition to the glycosyltransferase activity of PBP2 (13, 14). Moreover, additional nonessential proteins that are involved in peptidoglycan synthesis, such as the monofunctional glycosyltransferases SgtA and SgtB (also named Mgt), have been identified (12). Previous studies have shown that, although SgtA and SgtB have glycosyltransferase activity in vitro, only SgtB can support the growth of S. aureus in the absence of the main glycosyltransferase PBP2. SgtB alone, however, cannot support the growth of S. aureus in the presence of β-lactam antibiotics when an interaction between PBP2 and PBP2A is needed (15, 16).
In Gram-positive bacteria, the peptidoglycan layer is interspersed with a plethora of proteins and cell wall polymers named teichoic acids (17). Teichoic acids are further categorized into lipoteichoic acid (LTA), which is anchored to the outer leaflet of the cytoplasmic membrane via a lipid moiety, and wall teichoic acid (WTA), which is covalently attached to the peptidoglycan (18). Teichoic acids form an important part of the cell wall and contribute to the physical and chemical properties of the cell wall and to the binding of divalent cations (19, 20). While both WTA and LTA are polyanionic cell wall polymers, they are synthesized through separate independent pathways in S. aureus and many other Gram-positive bacteria (21, 22). Consistent with this, our work on the Gram-positive pathogen Listeria monocytogenes has revealed that LTA synthesis is not abrogated in the absence of WTA and vice versa (23). Recent work using pathway-specific inhibitors and a gene interaction screen provided further evidence that the polymers not only are synthesized through separate pathways but also have distinct functions in S. aureus (24).
LTA is an anionic polymer that in S. aureus is composed of glycerolphosphate repeating units, which are further decorated with d-alanine residues and, under high salt conditions, also with N-acetylglucosamine residues, as shown recently (25, 26). Most proteins required for LTA synthesis have been identified and extensively studied over the years (22, 27). One of the key enzymes required for LTA synthesis is the LTA synthase enzyme LtaS (28, 29); this enzyme polymerizes the LTA backbone chain on the outside of the cell, using the glycerolphosphate head group of the membrane lipid phosphatidylglycerol as the substrate (29, 30). LTA is indispensable for the growth of S. aureus under standard laboratory growth conditions, which highlights its important physiological role (29, 31).
Previous studies indicated functions for LTA in helping to direct the cell division machinery (32), in controlling autolysin activity (31), in facilitating biofilm formation (33), in mediating interactions with host cell receptors (17), in controlling susceptibility and/or resistance to antimicrobial peptides, and in maintaining cation homeostasis (20). S. aureus ltaS mutants, which lack the complete LTA polymer, can be constructed in some strain backgrounds at low growth temperatures (31). It has also been shown that LTA-deficient S. aureus strains are viable when grown under osmotically stabilizing conditions in medium containing 7.5% NaCl or 40% sucrose (31, 34). However, the bacteria display severe morphological defects, including increased cell size, clustering, and cell division defects, even under conditions permissive for growth (34). Bacteria can also readily acquire compensatory mutations, allowing them to grow in unsupplemented medium and improving their morphological defects (34). The majority of compensatory mutations previously observed were in gdpP, leading to inactivation of the c-di-AMP phosphodiesterase GdpP (34). The resulting increase in cellular c-di-AMP levels allowed the bacteria to survive the cell wall stress caused by the absence of LTA, which is now thought to be due to changes in the osmotic balance in the cell (34). However, compensatory mutations that rescued the growth of an LTA-negative S. aureus strain were found not only in gdpP but also in other genes (34).
As part of the current study, we sought to identify these genes and to further characterize the encoded proteins, to gain additional insight into why LTA is essential for the growth of S. aureus and potentially to uncover novel cellular functions for proteins involved in cell wall assembly or maintenance. Using a suppressor screen approach, we found that inactivation of the monofunctional peptidoglycan glycosyltransferase SgtB allowed S. aureus to grow in the absence of LTA. We further showed that peptidoglycan cross-linking was significantly reduced in the absence of LTA and could be partially restored upon inactivation of SgtB. This might strengthen the peptidoglycan layer and thus contribute to the observed growth rescue.
RESULTS
Identification of S. aureus suppressor strains able to grow in the absence of LTA in a c-di-AMP-independent way.
LTA-deficient S. aureus strains are viable when grown under osmotically stabilizing conditions in medium containing 7.5% NaCl or 40% sucrose or in unsupplemented medium after the acquisition of compensatory mutations (31, 34). The majority of compensatory mutations observed previously were in gdpP, leading to inactivation of the c-di-AMP phosphodiesterase GdpP (34). In the same study, suppressor strains with mutations outside the gdpP gene were noted (34). In order to characterize suppressor strains with mutations in genes other than gdpP and to gain further insight into the cellular function of LTA, a larger suppressor screen was performed and the ltaS mutant LAC*ΔltaS::erm strains constructed in medium supplemented with either sucrose or NaCl were plated on unsupplemented tryptic soy agar (TSA) plates. A number of independently obtained suppressor colonies were subsequently passed four times in fresh tryptic soy broth (TSB) to further improve their growth. Next, the chromosomal DNA was isolated from 80 suppressor strains, and those lacking mutations in gdpP (coding for the c-di-AMP hydrolase) and dacA (coding for the c-di-AMP cyclase enzyme) were identified by determining the sequences of these two genes. Of 80 colonies screened, no strains had mutations in dacA and 17 strains had no mutation in gdpP, 7 of which were selected for further analysis. The absence of LTA and the presence of WTA in the suppressor strains were confirmed by Western blotting and Alcian blue/silver staining analysis, respectively (Fig. 1A and B). Next, the relative cellular c-di-AMP levels in the different strains were determined using a previously described competitive enzyme-linked immunosorbent assay (ELISA) (35, 36) (Fig. 1C). In contrast to the gdpP mutant and the LTA-negative suppressor strain with a mutation in gdpP, which showed the expected increase in c-di-AMP levels, the 7 suppressor strains chosen for further analysis did not show an increase in cellular c-di-AMP concentrations (Fig. 1C).
FIG 1.
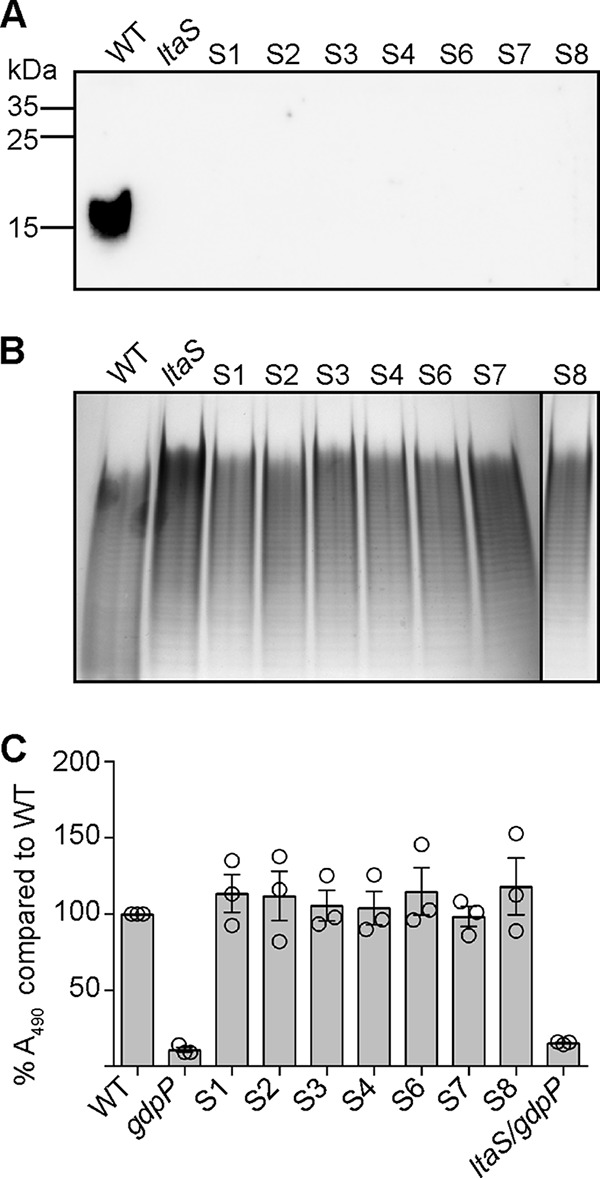
Detection of LTA by Western blotting, WTA by Alcian blue/silver staining, and c-di-AMP by using a competitive ELISA method. (A) Detection of LTA by Western blotting. Cell extracts were prepared from LAC* (WT strain), an ltaS mutant strain (strain ANG2135), and the 7 ltaS suppressor strains (strains S1 to S4 and S6 to S8) and separated on a 15% polyacrylamide gel. LTA was subsequently detected by Western blotting using a polyglycerolphosphate-specific monoclonal antibody. A representative result from three independent experiments is shown. (B) Visualization of WTA by native PAGE. WTAs were isolated from the same strains as shown in panel A and separated on a 20% native gel. Bands were visualized with Alcian blue staining and subsequently silver staining. One representative result from four independent experiments is shown. (C) c-di-AMP detection by competitive ELISA. Cytoplasmic extracts were prepared from LAC* (WT strain), the high-c-di-AMP-level control strains LAC* gdpP::kan (gdpP) and US3 (ltaS gdpP), and the 7 ltaS suppressor strains (strains S1 to S4 and S6 to S8). c-di-AMP amounts were determined and compared to those of the WT strain by ELISA. Of note, because this was a competitive ELISA, lower A490 readings were obtained for samples with higher c-di-AMP levels. The A490 reading obtained for the sample derived from the WT strain was set to 100%, and percentage values were calculated for the test strains. The average percentage values and SDs from three independent experiments (with three technical replicates) are plotted.
Identification of genomic alterations in the S. aureus suppressor strains able to grow in the absence of LTA.
Next, the genomic sequences of the 7 suppressor strains were determined and compared to that of the original ltaS mutant strains by using a whole-genome sequencing approach. Mutations were found in sgtB (SAUSA300_1855), coding for the monofunctional glycosyltransferase SgtB, and mutations in this gene arose independently in 3 suppressor strains (Table 1). Another strain had a mutation in SAUSA300_1254, coding for a hypothetical membrane protein, and SAUSA300_RS11150, encoding MazE, the antitoxin component of a type II toxin-antitoxin module (Table 1). In the original study by Corrigan et al. (34), mutations found in SAUSA300_1254 were proposed to be accessory and required to further improve the growth of the gdpP mutant suppressor strains. Consistent with a previous report (2), a large deletion in clpX (SAUSA300_1621), encoding a protein forming part of an ATP-dependent protease, was observed in one strain (Table 1). Finally, a mutation in vraT (SAUSA300_1867), coding for the membrane protein VraT and forming part of the VraRST three-component system, was identified. Using our standard genome sequence analysis workflow, an unusually large number (>300) of zero-coverage regions were obtained for suppressor strain S6, preventing us from matching a single-nucleotide polymorphism to the observed growth rescue with high confidence. Therefore, suppressor strain S6 is not listed in Table 1 and was not analyzed further. The mutations identified in the other suppressor strains were subsequently confirmed by fluorescent automated resequencing of the respective genomic region. Some of the observed mutations in sgtB, as well as the mutations in vraT, clpX, and mazE, resulted in frameshift mutations and introduction of premature stop codons, suggesting that the absence of the encoded proteins compensates for the lack of LTA.
TABLE 1.
Genomic variations detected in the suppressor strains, compared the original ltaS mutant strains
| Strain and reference position(s)a,b |
Type of mutationc |
Referenced | Allelee | Frequency (%)f | Avg quality scoreg |
Annotations | Amino acid changeh |
|---|---|---|---|---|---|---|---|
| ANG3711 (S1-sgtB) | |||||||
| 2018191 | SVN | T | A | 100 | 38.5 | SAUSA300_1855; monofunctional glycosyltransferase SgtB |
Asn11Tyr |
| 2017952–2018187 | DEL | SAUSA300_1855; monofunctional glycosyltransferase SgtB |
|||||
| ANG3712 (S2-mazE) | |||||||
| 1380637 | INS | G | 100 | 38.5 | SAUSA300_1254; hypothetical membrane protein |
Ile291FS | |
| 1380639 | SNV | C | A | 100 | 38.7 | Ser292Tyr | |
| 1380641 | REP | C | AG | 100 | 38.4 | Pro293fs | |
| 2188437–2188446 | DEL | TATCGGAAAA | SAUSA300_RS11150; antitoxin component of type II toxin-antitoxin module mazEF |
||||
| ANG3713 (S3-clpX) | |||||||
| 1775491 | SNV | T | A | 100 | 38.8 | SAUSA300_1621; ATP-dependent protease ATP-binding subunit ClpX |
Glu148Val |
| 1775495 | SNV | T | C | 100 | 38.9 | Thr147Ala | |
| 1775668 | SNV | A | C | 100 | 38.2 | Val89Gly | |
| 1775500–1775663 | DEL | ||||||
| ANG3714 (S4-sgtB): 2017704 | SNV | A | C | 100 | 38 | SAUSA300_1855; monofunctional glycosyltransferase SgtB |
Leu173* |
| ANG3717 (S7-vraT): 2027736 | SNV | A | C | 100 | 38.8 | SAUSA300_1867; membrane protein Yvfq/VraT |
Leu71* |
| ANG3718 (S8-sgtB): 2018107 | INS | T | 92.6 | 38.6 | SAUSA300_1855; monofunctional glycosyltransferase SgtB |
Ser39fs |
Suppressor strains S1, S2, S7, and S8 were derived from strain LAC*ΔltaSS (ANG2135), and suppressor strains S3, S4, and S6 were derived from strain LAC*ΔltaSN (ANG2134).
Reference positions indicate the base numbers in the LAC* reference genome (USA300_FPR3757; GenBank accession number CP000255.1).
DEL, deletion; SNV, single-nucleotide variation; INS, insertion; REP, replacement.
Base in the LAC* reference genome.
Base in the sequenced strain.
Frequency of the specific variant found in the sample reads.
Average base quality score at the indicated position.
Amino acid change in the encoded protein in LAC* versus the sequenced strain (fs, frameshift; *, premature stop codon).
Phenotypic characterization of the LTA-negative S. aureus suppressor strains.
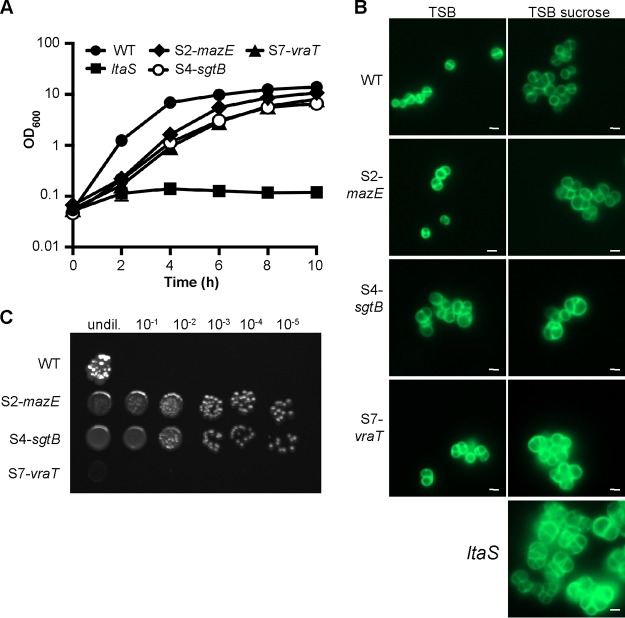
In a previous study, it was shown that S. aureus cells can grow without LTA in the absence of ClpX (2); therefore, we did not further characterize the clpX mutant suppressor strain obtained as part of this study. Instead, our analysis focused on suppressor strains with mutations in novel genes, i.e., strains with mutations in mazE, sgtB, or vraT. MazE, the antitoxin component of a type II toxin-antitoxin module and whose is part of the sigB regulon, has been shown to be essential for full activity of the alternative sigma factor SigB (37). SigB and members of its regulon enable bacteria to respond rapidly to environmental and antibiotic stresses and also play a role in cell envelope homeostasis (38, 39). Various studies have investigated the role of the VraTSR three-component regulatory system, and this system has been reported to be involved in the induction of the cell wall stressosome, mainly in the presence of cell wall-targeting antibiotics (40–42). Interestingly, the monofunctional glycosyltransferase SgtB, which was also identified as part of our screen, is one of the proteins belonging to the cell wall stressosome whose expression is regulated by VraTSR independent of the presence of cell wall-targeting antibiotics (40). To further characterize the suppressor strains, we first confirmed the growth improvement of strains S2-mazE, S4-sgtB, and S7-vraT when they were propagated in TSB. All 3 suppressor strains grew better than the original ltaS mutant strain, and their growth rate was only slightly reduced, compared to that of the wild-type (WT) LAC* strain (Fig. 2A). Next, the cell morphology of the WT strain, the original ltaS mutant, and the 3 suppressor strains was assessed by microscopy following staining of the peptidoglycan with fluorescently labeled vancomycin. The cell morphology of the suppressor strains was considerably improved; in particular, for suppressor strain S2-mazE, the division site was correctly placed in most cells (Fig. 2B). Next, the susceptibility of the suppressor strains to a number of cell wall-targeting antibiotics was determined. Although the growth and cell morphology of the suppressor strains were improved, the strains remained hypersensitive to the β-lactam antibiotic oxacillin, with MICs reduced ≥32-fold for the different suppressor strains, compared to the WT LAC* strain (Fig. 3A). The susceptibility to the cell wall- or membrane-targeting antibiotics lysostaphin, nisin, vancomycin, and daptomycin was also tested, but no drastic differences were observed (Table 2). Finally, the growth of the 3 different suppressor strains was assessed on plates containing Congo red. Congo red is an anionic azo dye traditionally used for the detection of biofilms in Staphylococcus. At higher concentrations, however, it inhibits the growth of S. aureus, and it has been used in the past to indicate differences in the cell wall integrity of different S. aureus strains (43). Recently, the target of Congo red was established as the LTA synthase enzyme LtaS (44). Therefore, our suppressor strains, which have ltaS deletions and are able to grow in the absence of LTA, should be resistant to this dye. To test the susceptibility of the suppressor strains, serial dilutions of overnight cultures were spotted on TSA plates containing 0.1% Congo red (Fig. 2C). The suppressor strains carrying mutations in mazE and sgtB were significantly more resistant to Congo red than was the WT strain, indicating that inactivation of either one of these genes is indeed sufficient to bypass the LTA requirement. However, the suppressor strain with a mutation in vraT grew poorly on the Congo red plates, suggesting that this strain might not be a true suppressor strain.
FIG 2.
Growth and cell morphology of WT and mutant S. aureus strains. (A) Bacterial growth curves. LAC* (WT strain) and the suppressor strains S2-mazE, S4-sgtB, and S7-vraT were grown overnight in TSB, and the original ltaS mutant strain (ANG2135) was grown in TSB with 40% sucrose. The next day, bacterial cells were washed and diluted in TSB to an OD600 of 0.05, and the bacterial growth was subsequently monitored over a period of 10 h. The average OD600 readings from three experiments were plotted. (B) Microscopic analysis. Bacterial cells from overnight cultures of the same strains as used in panel A were washed, back-diluted, and grown to mid-log phase in TSB or TSB with 40% sucrose, as indicated. The bacterial cells were subsequently stained with BODIPY-vancomycin and analyzed by fluorescence microscopy. (C) Analysis of bacterial growth on Congo red-containing TSA plates. Overnight cultures of the WT strain and the indicated suppressor strains were serially diluted and spotted on TSA plates supplemented with 0.1% Congo red, and the plates were incubated for 48 h at 37˚C. For panels B and C, representative results of three independent experiments are shown.
FIG 3.
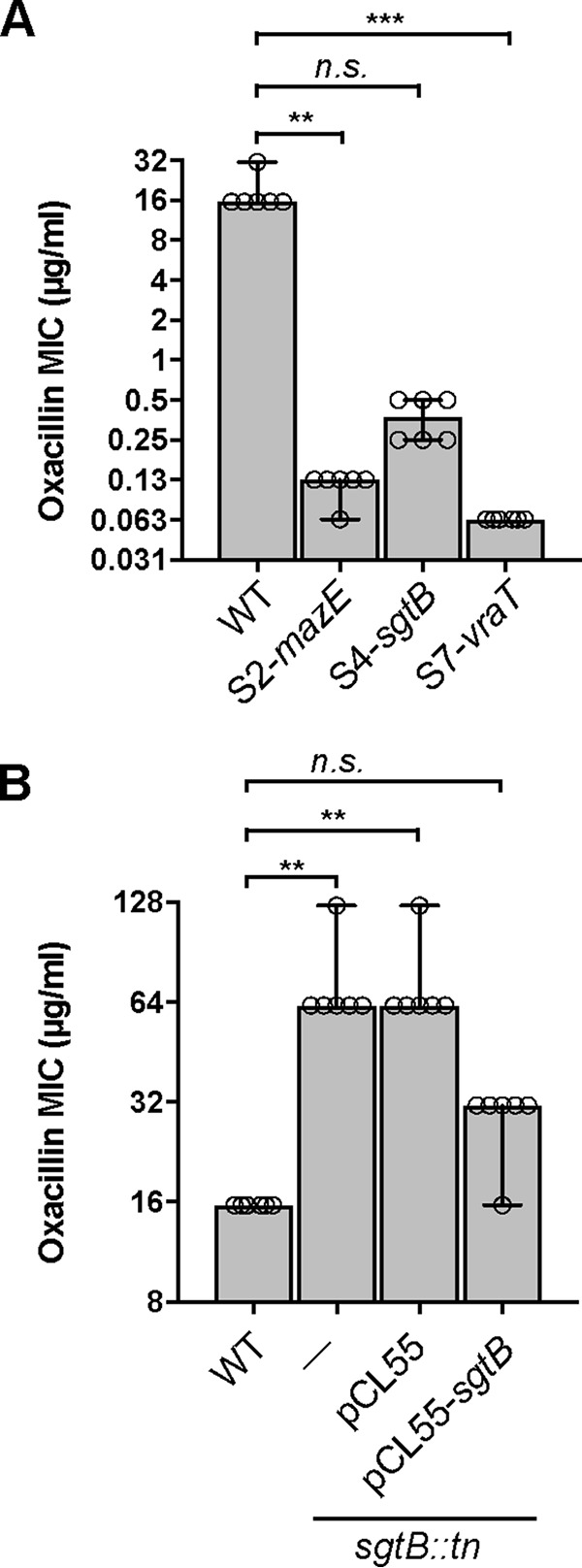
Oxacillin MICs for WT and mutant S. aureus strains. Oxacillin MICs for LAC* (WT strain) and the suppressor strains S2-mazE, S4-sgtB, and S7-vraT (A) and for LAC* (WT strain) and the LAC* sgtB::tn mutant without an integrative plasmid (−), with an integrative plasmid (pCL55), or with an integrative complementation plasmid (pCL55-sgtB) (B) are shown. Experiments were conducted with six biological replicates. Medians and 95% confidence intervals of the oxacillin MIC for all strains are plotted. A Kruskal-Wallis one-way analysis of variance test was performed, which indicated significant differences. Subsequently, Dunn’s tests were performed, and the P values were corrected for the multiple comparisons against the WT strain. **, P < 0.01; ***, P < 0.001; n.s., not significant.
TABLE 2.
MIC values for different antibiotics
| Strain | MIC (µg/ml)a of: |
|||
|---|---|---|---|---|
| Lysostaphin | Nisin | Vancomycin | Daptomycin | |
| LAC* | 0.125 (0.125, 0.125, 0.125) | 16 (16, 16, 16) | 4 (4, 4, 4) | 16 (16, 16, 16) |
| S2-mazE | 0.125 (0.125, 0.125, 0.125) | 2–4 (2, 2, 4) | 2 (2, 2, 2) | 4–8 (8, 8, 4) |
| S4-sgtB | 0.125–0.250 (0.125, 0.125, 0.25) | 4 (4, 4, 4) | 1 (1, 1, 1) | 8–16 (16, 8, 8) |
| S7-vraT | 0.500 (0.5, 0.5, 0.5) | 2–4 (2, 2, 4) | 4–8 (8, 4, 4) | 8 (8, 8, 8) |
| LAC*sgtB::tn | 0.125–0.250 (0.25, 0.125, 0.125) | 16–32 (32, 16, 32) | 4 (4, 4, 4) | 16 (16, 16, 16) |
| LAC*sgtB::tn/pCL55 | 0.125–0.250 (0.125, 0.125, 0.25) | 8–16 (16, 8, 16) | 4 (4, 4, 4) | 16 (16, 16, 16) |
| LAC*sgtB::tn/pCL55-sgtB | 0.125 (0.125, 0.125, 0.125) | 8–16 (8, 16, 16) | 4–8 (4, 8, 4) | 16 (16, 16, 16) |
The MIC was defined as the antibiotic concentration that led to >90% growth inhibition, compared to growth without antibiotic. MICs were determined in triplicate. The MIC ranges obtained are shown first, and individual MIC values of the replicate samples are shown in parentheses.
Introduction of SgtB or MazE in the respective suppressor strain results in the expected growth arrest.
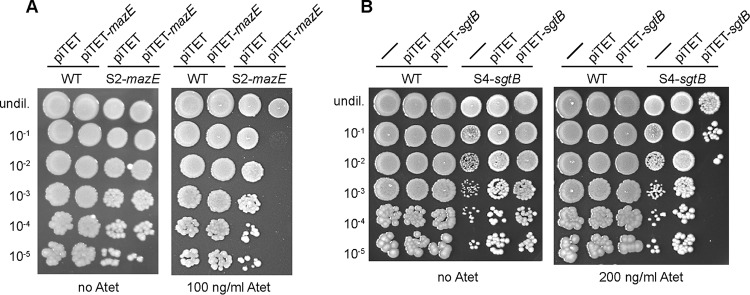
The results of the whole-genome sequencing analysis suggested that inactivation of MazE or SgtB was sufficient to allow S. aureus to grow in the absence of LTA. Introduction of a WT copy of mazE or sgtB into the respective suppressor strain should restore this phenotype and be lethal for the suppressor strains when they are grown in unsupplemented medium but should not have an effect when the bacteria are propagated in medium supplemented with 40% sucrose. In order to test this, plasmids piTET-mazE and piTET-sgtB, allowing for anhydrotetracycline (Atet)-inducible gene expression, were introduced into the respective suppressor strains. As controls, these plasmids were also introduced into the WT LAC* strain. Serial dilutions of these different strains were spotted onto TSA plates containing 100 ng/ml or 200 ng/ml Atet, for mazE or sgtB expression, respectively. As expected for successful complementation, the expression of mazE prevented the growth of the suppressor strain S2-mazE (Fig. 4A) and the expression of sgtB prevented the growth of the suppressor strain S4-sgtB (Fig. 4B) on TSA but they had no effect when the bacteria were spotted on medium supplemented with 40% sucrose (Fig. 5). Of note, the mazE suppressor strain S2-mazE contained an additional mutation in a gene coding for a membrane protein of unknown function with locus tag SAUSA300_1254. Introduction of a WT copy of this gene into the suppressor strain did not prevent the growth of this suppressor strain (data not shown). Taken together, the results of this complementation analysis support the notion that inactivation of SgtB or MazE is sufficient to allow S. aureus to grow in the absence of LTA.
FIG 4.
Growth complementation analysis using TSA plates with spot dilutions. (A) S. aureus strains LAC*/piTET, LAC*/piTET-mazE, S2-mazE/piTET, and S2-mazE/piTET-mazE were grown overnight in TSB supplemented with chloramphenicol and were washed twice with PBS, and serial dilutions were spotted on TSA plates supplemented with 7.5 μg/ml chloramphenicol, without or with 100 ng/ml Atet. (B) S. aureus strains LAC* (WT strain), LAC*/piTET, LAC*/piTET-sgtB, S4-sgtB, S4-sgtB/piTET, and S4-sgtB/piTET-sgtB were grown and samples were prepared as described for panel A but without chloramphenicol selection, and dilutions were spotted on TSA plates not supplemented or supplemented with 200 ng/ml Atet (right). Representative plate images from three independent experiments are shown.
FIG 5.
Growth complementation analysis using TSA-sucrose plates with spot dilutions. (A) S. aureus strains LAC*/piTET, LAC*/piTET-mazE, S2-mazE/piTET, and S2-mazE/piTET-mazE were grown overnight in TSB supplemented with chloramphenicol and were washed twice with PBS, and serial dilutions were spotted on TSA plates with 40% sucrose supplemented with 7.5 μg/ml chloramphenicol, without or with 100 ng/ml Atet. (B) S. aureus strains LAC* (WT strain), LAC*/piTET, LAC*/piTET-sgtB, S4-sgtB, S4-sgtB/piTET, and S4-sgtB/piTET-sgtB were grown and samples were prepared as described for panel A but without chloramphenicol selection, and dilutions were spotted on TSA plates with 40% sucrose that were not supplemented or supplemented with 200 ng/ml Atet. Representative plate images from three independent experiments are shown.
Growth characterization and antibiotic resistance of an S. aureus LAC* sgtB mutant.
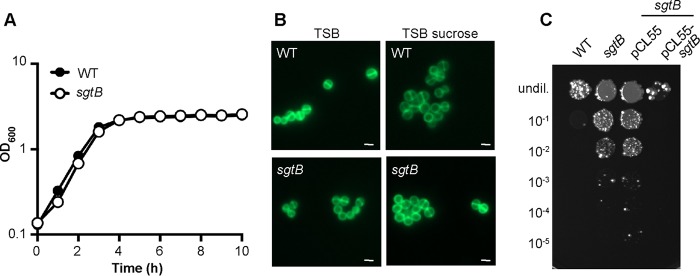
Inactivation of MazE likely has pleotropic effects due to its involvement in the activity of the alternative sigma factor SigB, and the reason why its inactivation allows S. aureus to grow in the absence of LTA could be indirect. Therefore, we next focused on trying to gain a better understanding of the cellular function of the monofunctional peptidoglycan glycosyltransferase SgtB and how its inactivation allows S. aureus to survive in the absence of LTA. To this end, the sgtB mutant strain LAC*sgtB::tn was constructed by moving the genomic region with a transposon insertion in sgtB from the Nebraska transposon mutant library strain NE596 (45) into the LAC* background. SgtB is one of two monofunctional peptidoglycan glycosyltransferases encoded in the S. aureus genome, and the protein can polymerize peptidoglycan chains in vitro (46). Although it is dispensable for the growth of S. aureus, SgtB becomes necessary for bacterial survival in the absence of the main glycosyltransferase PBP2 (12, 15, 47). Consistent with these previous observations, no differences in the cell growth and morphology of the sgtB mutant S. aureus strain LAC*sgtB::tn, compared to the WT LAC* strain, were observed (Fig. 6A and B). Our results indicate that inactivation of SgtB allows S. aureus to survive in the absence of LTA; therefore, the sgtB mutant strain should no longer be sensitive to the Congo red dye. To test this, serial dilutions of the WT strain, the sgtB mutant, and a complementation strain were spotted on TSA plates containing 0.1% Congo red, which inhibits the LtaS enzyme. Indeed, the sgtB mutant strain was considerably more resistant to Congo red than was the WT strain, and this phenotype could be complemented by introducing a functional copy of sgtB into the mutant strain (Fig. 6C). Next, we also tested the susceptibility of the sgtB mutant and the complementation strain to the cell wall-active antibiotics oxacillin, lysostaphin, nisin, vancomycin, and daptomycin. While no differences in the susceptibility of the sgtB mutant to most antibiotics, compared to the WT strain, were observed, a slight (4-fold) and statistically significant increase in resistance to oxacillin was observed (Fig. 3B and Table 2). Taken together, these results indicate that, while no growth or clearly visible morphological differences between the WT strain and the sgtB mutant strain were observed, deletion of sgtB led to increased Congo red resistance and 4-fold increased oxacillin resistance in our strain background.
FIG 6.
Growth and cell morphology of S. aureus strain LAC*sgtB::tn. (A) Bacterial growth curves. Overnight cultures of LAC* (WT strain) and strain LAC*sgtB::tn (sgtB) were diluted in TSB to an OD600 of 0.05, and growth was monitored for 10 h using a plate reader. The average OD600 values and SDs from three independent experiments are plotted. (B) Microscopic analysis. S. aureus strains LAC* (WT strain) and LAC*sgtB::tn (sgtB) were grown overnight in TSB or TSB supplemented with 40% sucrose, stained with BODIPY-vancomycin, and viewed under a fluorescence microscope. (C) Susceptibility to Congo red. Overnight cultures of LAC* (WT strain), the sgtB mutant strains LAC*sgtB::tn and LAC*sgtB::tn/pCL55, and the complementation strain LAC*sgtB::tn/pCL55-sgtB were serially diluted, and aliquots were spotted on TSA plates supplemented with 0.1% Congo red. For panels B and C, representative results from three independent experiments are shown.
Inactivation of SgtB leading to an increase in peptidoglycan cross-linking in an LTA-negative S. aureus strain.
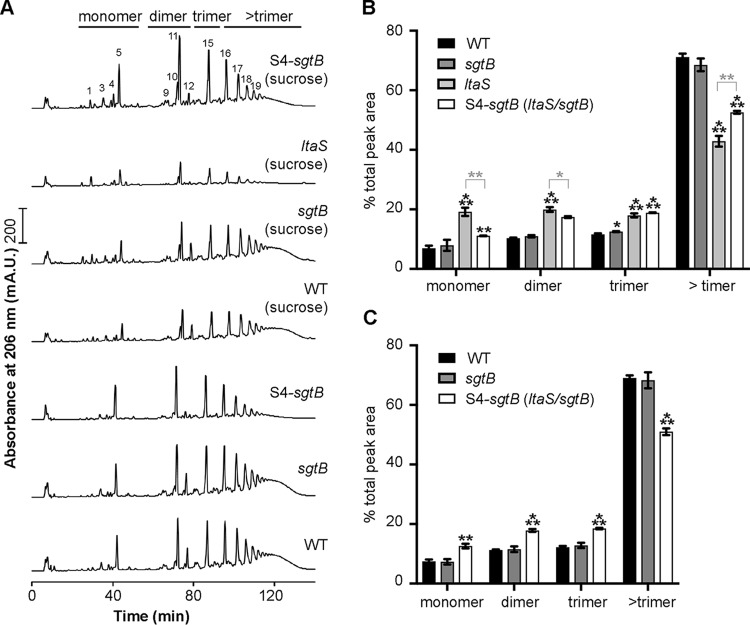
Since SgtB is involved in peptidoglycan synthesis and the sgtB mutant displayed slightly increased oxacillin resistance, we hypothesized that sgtB deletion could somehow “strengthen” the cell wall through alterations in the peptidoglycan structure. In order to investigate this, the muropeptide profiles of peptidoglycan isolated from the WT LAC* strain, the sgtB mutant strain LAC*sgtB::tn, and the ltaS sgtB mutant suppressor strain S4-sgtB were determined following the growth of these strains in TSB. In addition, the muropeptide profiles for peptidoglycan isolated from these three strains and from the original ltaS mutant strain were determined following growth in TSB supplemented with 40% sucrose (Fig. 7). The WT and sgtB mutant strains showed very similar and typical S. aureus muropeptide profiles (Fig. 7A). The chemical structures of several muropeptide fragments in a number of these peaks were determined previously in the seminal paper by de Jonge et al. (48). Where possible, we numbered the peaks as described by de Jonge et al. (48), with peaks 1 to 5 being monomeric, peaks 9 to 14 dimeric, peak 15 trimeric, and peaks 16 and above higher oligomeric muropeptide fragments. Quantification of the monomeric, dimeric, trimeric, and higher oligomeric peptidoglycan fragments showed that the peptidoglycan was highly cross-linked in both strains, with approximately 70% of the UV absorbing material being found in the higher oligomeric fraction (Fig. 7B and C). No clear differences in the muropeptide profiles of the WT strain and the sgtB mutant strain, grown either in TSB or in TSB supplemented with 40% sucrose, were found (Fig. 7). In contrast to the WT strain and the sgtB mutant strain, a decrease in the higher oligomeric peptidoglycan material was observed for the ltaS suppressor strain S4-sgtB in both TSB and TSB with sucrose (Fig. 7). Visual inspection of the chromatograms also indicated that peak 12, corresponding to a currently unknown muropeptide, was reduced in S. aureus strains unable to produce LTA, i.e., the original ltaS mutant strain and the S4-ltaS suppressor strain (Fig. 7A). Perhaps most notably, comparison of the muropeptide profiles of the peptidoglycan isolated from the original ltaS mutant strain and the ltaS sgtB suppressor strain S4-sgtB after growth in TSB with sucrose showed that, while the amount of cross-linked peptidoglycan was reduced in both strains, compared to the WT strain, the peptidoglycan in the suppressor strain was more cross-linked than that in the original ltaS mutant strain (Fig. 7A and B). Taken together, our data indicate that deletion of ltaS leads to a significant reduction in the amount of cross-linked peptidoglycan in S. aureus. Inactivation of SgtB in a WT strain does not significantly affect the amount of cross-linked peptidoglycan, as might be expected based on the observed increase in oxacillin resistance. In an LTA-negative background strain, however, inactivation of SgtB leads to an increase in peptidoglycan cross-linking, which might explain why an LTA-negative strain can grow in the absence of this monofunctional peptidoglycan glycosyltransferase.
FIG 7.
Peptidoglycan analysis of WT and mutant S. aureus strains. (A) HPLC profiles of mutanolysin-digested peptidoglycan samples. Peptidoglycan was isolated and digested with mutanolysin (as described in Materials and Methods) from the S. aureus LAC* strain (WT strain), the LAC*sgtB::tn strain (sgtB), and the LTA-negative suppressor strain S4-sgtB following growth in TSB or from the S. aureus LAC* strain (WT strain), the LAC*sgtB::tn strain (sgtB), and the LTA-negative suppressor strain S4-sgtB, as well as the original ltaS mutant strain (ANG2135), following growth in TSB with 40% sucrose. Monomeric, dimeric, trimeric, and greater than trimeric peptidoglycan fragments are indicated above the graphs, and representative profiles from three independent samples are shown. The individual muropeptide peaks were labeled, where possible, with the numbers described by de Jonge et al. (48). (B and C) Quantification of the different peptidoglycan peaks. The peaks corresponding to monomeric, dimeric, trimeric, and greater than trimeric peptidoglycan fragments were integrated and quantified for the strains used in panel A. The combined peak area was set to 100% for each strain, and the average values and SDs from the three independent peptidoglycan isolations were plotted for the strains grown in TSB with sucrose (B) and for the strains grown in TSB (C). Unpaired two-tailed Student’s t tests were used to determine statistically significant differences in monomer, dimer, trimer, or greater than trimer fractions between WT and mutant strains. The P values obtained were multiplied by Bonferroni correction factors of 3 (B) and 2 (C) because the WT peaks were compared to 3 or 2 different strains, respectively. Statistically significant differences are indicated by black asterisks. For panel B, unpaired two-tailed Student’s t tests were also used to determine statistically significant differences in monomer, dimer, trimer, or greater than trimer fractions between the ltaS mutant and the other three strains, and statistically significant differences between the ltaS mutant and the S4-ltaS suppressor strain are shown with gray asterisks. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
The anionic LTA polymer is a core component of the cell wall that is essential for survival, and a number of studies have shown its importance in various cell processes (for a review, see reference 22). Phenotypes caused by the depletion of LTA in S. aureus involve misplacement and incomplete formation of cell division septa and enlargement of the cells, together ultimately leading to cell lysis (29, 34). However, how LTA mediates these roles is still unknown. In previous work, it was shown that LtaS and other core LTA synthesis proteins physically interact with early- and late-stage cell division proteins, as well as with a number of peptidoglycan synthesis proteins (49). These findings indicate that LTA synthesis enzymes might at least transiently be part of multienzyme complexes, which might help to coordinate LTA synthesis with peptidoglycan synthesis and cell division (49).
We showed previously that S. aureus mutants with increased intracellular c-di-AMP levels could survive without LTA (34). It is now thought that, at high c-di-AMP levels and through the c-di-AMP-dependent regulation of potassium and osmolyte transporters, the internal turgor pressure in the cell might be reduced, so that the compromised LTA-depleted cell wall can sustain the internal pressure (34, 50–52). Indeed, as part of the current study, we showed that the absence of LTA led to a sizable reduction in the amount of cross-linked peptidoglycan in S. aureus (Fig. 7). This finding is consistent with the idea that, in the absence of LTA, the cell wall is likely less able to sustain the high internal turgor pressure, which might also contribute to the increased β-lactam sensitivity of LTA-negative strains observed in this study and previous studies (Table 2) (2, 34). The reason for the reduced amount of peptidoglycan cross-linking is currently not clear but could be due to mislocalization of PBPs in the absence of LTA or PBPs having reduced enzyme activity since the proper ion homeostasis cannot be maintained within the cell wall in the absence of LTA.
The aim of this study was to further elucidate the role of LTA in cell wall assembly and potentially to identify additional proteins involved in the maintenance of cell wall integrity. A suppressor screen followed by whole-genome sequencing revealed mutations in genes coding for ClpX, SgtB, MazE, and VraT (Table 1) that could bypass the requirement for LTA. Further experimentation indicated that the strain with the mutation in vraT might not be an actual suppressor strain. In contrast, complementation analysis and growth assays on agar plates containing the azo dye Congo red, which prevents the growth of S. aureus by inhibiting the LtaS enzyme (44), confirmed that inactivating mutations in sgtB and mazE could bypass the requirement for LTA. In a previous study, it was found that an S. aureus clpX mutant readily acquired inactivating mutations in ltaS, resulting in the generation of LTA-negative strains (2). Consistent with the previous work, in this study we found a large deletion in clpX in one of our LTA-negative suppressor strains (Table 1). ClpX is a protein-folding chaperon, which recognizes and targets proteins for degradation to the ClpP protease component. In the absence of ClpX, S. aureus cells become smaller, show increased production of autolysins, and have a severe growth defect at temperatures of 30˚C or lower (2). The introduction of loss-of-function mutations in the gene coding for the LTA synthase LtaS in a clpX mutant alleviates some of these effects; this is perhaps due to LTA depletion having the opposite effect, leading to increased cell size and decreased autolysis, as reported in some previous publications (2, 31).
Mutations in the monofunctional glycosyltransferase SgtB were the most prevalent mutations that arose in our suppressor screen. Previous studies showed that SgtB is not essential for cell survival and, as reported in this study and a previous study, an sgtB mutant strain does not show any obvious growth or morphological differences, compared to a WT strain, under standard growth conditions (Fig. 6) (15). Interestingly, we found that the sgtB mutant strain LAC*sgtB::tn was 4-fold more resistant to the cell wall-targeting antibiotic oxacillin and this phenotype could be complemented by introduction of a WT copy of sgtB (Table 2). These results are in accordance with previous reports, in which strains with mutations in genes that compensate for the lack of LTA, such as gdpP and clpX mutant strains, also show increased oxacillin resistance (2, 34).
The increased resistance of the sgtB mutant to oxacillin prompted us to investigate the peptidoglycan structure of an sgtB mutant in more detail, as we hypothesized that, in the absence of SgtB, changes such as increased cross-linking might be observed, which could potentially explain the increased resistance. We could not detect any obvious changes in the muropeptide profile of an sgtB mutant strain, compared to a WT strain (Fig. 7). However, the original ltaS mutant strain showed a significant reduction (around 30%) in the higher oligomeric cross-linked peptidoglycan (Fig. 7). Perhaps most importantly, the peptidoglycan isolated from the sgtB ltaS double mutant S4-sgtB suppressor strain showed an increase in peptidoglycan cross-linking, compared to the original ltaS mutant strain (Fig. 7). We speculate that this increase in peptidoglycan cross-linking could potentially strengthen the cell wall to better sustain the high internal turgor pressure and might be at least partly responsible for the observed growth improvement.
Bacterial two-hybrid studies have indicated that the S. aureus SgtB protein interacts with SgtA, PBP1, PBP2, and PBP2A (15). Therefore, SgtB and the main glycosyltransferase PBP2 might compete for substrate during peptidoglycan biosynthesis, and inactivation of the former might increase the substrate availability and activity of the latter. This might then aid in the delivery of substrate to the transpeptidase domain of the bifunctional PBP2 enzyme, resulting in increased cross-linking. Alternatively, SgtB could also affect the function of PBP4, which has been shown to be responsible for hyper-cross-linking of the staphylococcal peptidoglycan (53). Clearly, more experiments are needed to clarify the complex nature of peptidoglycan biosynthesis in S. aureus and the changes observed in the absence of LTA.
Mutations in MazE could potentially also be involved in changes in the cell wall structure of S. aureus. The MazEF type II toxin-antitoxin system is part of the sigB regulon and has been shown to be required for full activity of the alternative sigma factor SigB (37). It has also been reported that overexpression of σB causes cell wall thickening in S. aureus and increased resistance to cell wall-targeting antibiotics (38). Therefore, inactivation of MazE, as observed in one of our suppressor strains, could potentially also affect cell wall homeostasis via its effect on SigB.
In summary, our results suggest that, in the absence of LTA, peptidoglycan in S. aureus becomes less cross-linked, which might weaken the cell wall and cause it to become less able to sustain the high internal turgor pressure, ultimately leading to cell lysis. The suppressor mutations in sgtB (and perhaps also in some of the other genes observed in our screen) help the cell survive this detrimental effect by altering and strengthening the cell wall, likely allowing the cell wall to better withstand the high internal turgor pressure.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All bacterial strains used in this study are listed in Table 3. Escherichia coli strains were cultured in lysogeny broth (LB) and Staphylococcus aureus strains were cultured in TSB at 37°C with aeration, unless otherwise stated. When appropriate, the growth medium was supplemented with antibiotics and inducers as follow: for E. coli cultures, 100 μg/ml ampicillin, 10 μg/ml chloramphenicol, and 30 μg/ml kanamycin; for S. aureus cultures, 10 μg/ml chloramphenicol for plasmid selection or 7.5 μg/ml chloramphenicol for chromosomally integrated plasmid selection, 10 μg/ml erythromycin, and 90 μg/ml kanamycin. The inducer Atet was used at a concentration of 100 or 200 ng/ml in agar plates and 50 ng/ml in broth.
TABLE 3.
Bacterial strains used in this study
| Species and strain | Descriptiona | Reference or source |
|---|---|---|
| Escherichia coli | ||
| XL1-Blue | Cloning strain; Tetr; ANG127 | Stratagene |
| IM08B | E. coli-S. aureus shuttle strain; ANG3723 | 56 |
| ANG3732 | IM08B/pCL55 Ampr | 52 |
| ANG3926 | XL1-Blue/piTET-sgtB Ampr | This study |
| ANG3927 | IM08B/piTET-sgtB Ampr | This study |
| ANG3928 | IM08B/piTET Ampr | 57 |
| ANG4708 | XL1-Blue/pCL55-sgtB Ampr | This study |
| ANG4707 | IM08B/pCL55-sgtB Ampr | This study |
| ANG4727 | XL1-Blue/piTET-mazE Ampr | This study |
| ANG4728 | IM08B/piTET-mazE Ampr | This study |
| Staphylococcus aureus | ||
| LAC* | Erm-sensitive community-acquired MRSA LAC strain; ANG1575 | 58 |
| NE596 | JE2sgtB::tn, Nebraska Transposon Mutant Library strain; Ermr; ANG3930 | 45 |
| ANG1961 | LAC*gdpP::kan Kanr | 34 |
| ANG2134 | LAC*ΔltaSN::erm pCN38, isolated on 7.5% NaCl; Camr Ermr | 34 |
| ANG2135 | LAC*ΔltaSS::erm pCN38, isolated on 40% sucrose; Camr Ermr | 34 |
| ANG2137 | LAC*ΔltaSN::erm suppressor S6, original colony; Ermr | 34 |
| ANG2140 | LAC*ΔltaSS::erm suppressor S7, original colony; Ermr | 34 |
| ANG2143 | LAC*ΔltaSS::erm suppressor S8, original colony; Ermr | 34 |
| ANG2434 | LAC*ΔltaSS::erm suppressor with gdpP mutation, pass 4 (short: US3); Ermr | 34 |
| ANG3694 | LAC*ΔltaSS::erm suppressor S1, original colony; Ermr | This study |
| ANG3698 | LAC*ΔltaSS::erm suppressor S2, original colony; Ermr | This study |
| ANG3703 | LAC*ΔltaSS::erm suppressor S3, original colony; Ermr | This study |
| ANG3707 | LAC*ΔltaSS::erm suppressor S4, original colony; Ermr | This study |
| ANG3711 | LAC*ΔltaSS::erm suppressor S1, pass 4 (short: S1-sgtB); Ermr | This study |
| ANG3712 | LAC*ΔltaSS::erm suppressor S2, pass 4 (short: S2-mazE); Ermr | This study |
| ANG3713 | LAC*ΔltaSN::erm suppressor S3, pass 4 (short: S3-clpX); Ermr | This study |
| ANG3714 | LAC*ΔltaSN::erm suppressor S4, pass 4 (short: S4-sgtB); Ermr | This study |
| ANG3716 | LAC*ΔltaSN::erm suppressor S6, pass 4 (short: S6); Ermr | This study |
| ANG3717 | LAC*ΔltaSS::erm suppressor S7, pass 4 (short: S7-vraT); Ermr | This study |
| ANG3718 | LAC*ΔltaSS::erm suppressor S8, pass 4 (short: S8-sgtB); Ermr | This study |
| ANG4054 | LAC*/piTET Camr | 57 |
| ANG4056 | LAC*/piTET-sgtB Camr | This study |
| ANG4057 | S4-sgtB/piTET Ermr Camr | This study |
| ANG4058 | S4-sgtB/piTET-sgtB Ermr Camr | This study |
| ANG4059 | LAC*sgtB::tn Ermr | This study |
| ANG4710 | LAC*sgtB::tn/pCL55 Ermr Camr | This study |
| ANG4711 | LAC*sgtB::tn/pCL55-sgtB Ermr Camr | This study |
| ANG4725 | S2-mazE/piTET Ermr Camr | This study |
| ANG4729 | LAC*/piTET-mazE Camr | This study |
| ANG4730 | S2-mazE/piTET-mazE Ermr Camr | This study |
Tetr, tetracycline resistant; Ampr, ampicillin resistant; Erm, erythromycin; Ermr, erythromycin resistant; Kanr, kanamycin resistant; Camr, chloramphenicol resistant.
Plasmid and strain construction.
Strains and primers used in this study are listed in Tables 3 and 4, respectively. Strain LAC*sgtB::tn was generated by transduction using phage Φ85. In this manner, the sgtB region containing a transposon insertion in sgtB was transduced from the Nebraska transposon mutant library strain NE596 (45) into strain LAC*. Plasmids piTET-sgtB and piTET-mazE for Atet-inducible expression of sgtB and mazE in S. aureus were generated by amplifying sgtB or mazE from LAC* chromosomal DNA using the primer pair ANG2268/ANG2269 or ANG2743/ANG2744, respectively. The PCR products and plasmid piTET were digested with AvrII and SacII, ligated, and then transformed into E. coli XL1-Blue, yielding strains XL1-Blue/piTET-sgtB (ANG3926) and XL1-Blue/piTET-mazE (ANG4727). The plasmids were shuttled through E. coli strain IM08B (ANG3927 and ANG4728) and subsequently electroporated into LAC*, yielding strains LAC*/piTET-sgtB (ANG4056) and LAC*/piTET-mazE (ANG4729), or into the respective suppressor strains, yielding strains S4-sgtB/piTET-sgtB (ANG4058) and S2-mazE/piTET-mazE (ANG4730). The plasmid pCL55-sgtB for complementation and expression of sgtB from its native promoter was generated by amplifying the sgtB gene, including its native promoter region, from LAC* chromosomal DNA using primers ANG2270 and ANG2271. The PCR product and plasmid pCL55 were digested with EcoRI and BamHI, ligated, and then transformed into E. coli XL1-Blue, yielding strain XL1-Blue/pCL55-sgtB (ANG4708), shuttled through IM08B (ANG3923), and subsequently electroporated into the sgtB mutant strain, yielding strain LAC*sgtB::tn/pCL55-sgtB (ANG4711). WT LAC* and mutant S. aureus strains with the empty vectors piTET and pCL55 (Table 3) were used as control strains in several experiments. The sequences of all plasmid inserts were verified by automated fluorescence sequencing at GATC Biotechnology.
TABLE 4.
Primers used in this study
| Primer identification | Primer name | Sequencea |
|---|---|---|
| ANG2229 | sausa300_1867 Fw seq | GAAGCATTTCAAATTCAATGTGCAAG |
| ANG2230 | sausa300_1867 Rv seq | GAAGCATTTCAAATTCAATGTGCAAG |
| ANG2231 | sgtB Fw seq | GTCGTCTCTATTGGCATTTAATAGGG |
| ANG2232 | sgtB Rv seq | AATAATGAAGTACTAATCAAGTGGC |
| ANG2233 | sausa300_1254 Fw seq | CATTTTGAAGAAAGGATTAATCAA |
| ANG2234 | sausa300_1254 Rv seq | AATAATGAAGTACTAATCAAGTGGC |
| ANG2235 | clpX Fw seq | GAAGGAACTAAAGAAGATTAATCTTC |
| ANG2236 | clpX Rv seq | CTGGATATTGTTCTTCTTTTACTGCAC |
| ANG2268 | 5-AvrII-sgtB-pCL55iTet | CATG CCTAGGTTAAAAGAAGGAGCAAACGCATG |
| ANG2269 | 3-SacII-sgtB-pCL55iTet | CATG CCGCGGTTAACGATTTAATTGTGACATAGCC |
| ANG2409 | 5- EcoRI-promSgtB-pCL55 | CATG GAATTCAAGTATTGTGGTTATCGATTG |
| ANG2410 | 3- BamH1-promSgtB-pCL55 | CATG GGATCCTTAACGATTTAATTGTGACATAGCC |
| ANG2740 | 5-mazE SNP seq | AGATAATCATAGAGAAAGTCCACAGTCG |
| ANG2741 | 3-mazE SNP seq | TAAGTACGTCAGTTTTTCTTTCAATCGT |
| ANG2743 | 5-AvrII-mazE-pCL55iTet | CATG CCTAGGGGAATCAATTGGAGGTTCTCATATG |
| ANG2744 | 3-SacII-mazE-pCl55iTet | CATG CCGCGGTCATTCATTCGTTGAATTAGAAGATAAATATG |
Restriction site sequences are underlined.
Bacterial growth curves.
S. aureus LAC* and the indicated ltaS suppressor strains were grown overnight in TSB containing the relevant antibiotic. The original ltaS mutant strains were grown in TSB containing either 7.5% NaCl or 40% sucrose. Overnight cultures were washed three times in TSB and diluted to a starting optical density at 600 nm (OD600) of 0.05. Cultures were incubated at 37°C with aeration, and OD600 values were determined at 2-h intervals. WT LAC* and the sgtB mutant strain LAC*sgtB::tn were grown overnight in TSB or TSB supplemented with 10 µg/ml erythromycin. The next day, bacteria were washed three times in TSB and diluted to a starting OD600 of 0.05, and 200 μl was placed in a 96-well microtiter plate. Growth was monitored for 10 h using a SPECTROstar Nano plate reader (BMG Labtech). All growth curves were determined in triplicate, with average values and standard deviations (SDs) being plotted.
Fluorescence microscopy analysis.
Cells from 1 ml of overnight culture were collected by centrifugation and washed three times in phosphate-buffered saline (PBS) (pH 7.4), and 150 μl of the cell suspensions was applied to polylysine (0.1% [wt/vol])-treated coverslips. The coverslips coated with the bacteria were incubated for 20 min with 100 µl of a 1-μg/ml boron-dipyrromethene (BODIPY)-vancomycin (Molecular Probes) solution in water, washed, and mounted on glass slides containing 20 µl Vectashield (Vector Laboratories). Slides were viewed under a Zeiss Axio Imager A2, using a green fluorescent protein (GFP) filter set, and images were captured with an AxioCam MRc Rev.3 camera and analyzed using Zen Pro 2012 SP2 software. The experiment was performed in triplicate.
MIC determination.
Overnight cultures of the WT strain, the sgtB mutant, and the complementation strain, as well as the indicated suppressor strains, were grown overnight in TSB. The next day, cultures were adjusted to an OD600 of 0.05 in TSB, and 100-μl aliquots of the suspensions were incubated in 96-well plates with 2-fold dilutions of various antimicrobials, at the following starting concentrations: oxacillin, 500 µg/ml or 1 μg/ml as appropriate; daptomycin, 32 μg/ml; lysostaphin, 2 μg/ml; vancomycin, 32 μg/ml; nisin, 64 μg/ml. Oxacillin- and daptomycin-containing wells were supplemented with 2% (wt/vol) NaCl and 0.23 mM CaCl2, respectively. Plates were incubated for 24 h at 37°C, with shaking at 500 rpm. MICs were determined as the concentrations of antibiotic at which growth was inhibited by >90%, compared to growth without the antibiotic.
LTA detection by Western blotting.
LTA extraction and detection by Western blotting were performed as described previously (54). Briefly, samples were prepared from 1-ml overnight cultures normalized based on OD600 readings, i.e., cells from 1-ml cultures with an OD600 of 6 were suspended in 90 µl 2× SDS protein sample buffer. Ten-microliter aliquots of these samples were separated on 15% SDS-polyacrylamide gels, and the material was subsequently transferred to polyvinylidene difluoride (PVDF) membranes. LTA was detected using a polyglycerolphosphate-specific LTA monoclonal antibody (Hycult Biotechnology) at a 1:4,000 dilution and a horseradish peroxidase (HRP)-conjugated anti-mouse IgG antibody (Cell Signaling Technologies, USA) at a 1:10,000 dilution. The blots were developed by enhanced chemiluminescence (ECL) using the Clarity Western ECL blotting substrate (Bio-Rad) and were imaged using the ChemiDoc Touch imaging system (Bio-Rad). Western blots were performed in triplicate, and representative results are shown.
Detection of WTA by Alcian blue/silver staining.
Flasks with 60 ml TSB supplemented with the appropriate antibiotics were inoculated with single colonies of WT LAC* and different suppressor strains, and the cultures were incubated overnight (18 h) at 37°C, with shaking. The original ltaS mutant was grown in TSB supplemented with 40% sucrose and was incubated 6 to 8 h longer. Cells from an OD600 equivalent of 120 were harvested by centrifugation, and the bacterial pellet was stored at −20°C for further processing. WTA extraction and detection by Alcian blue/silver staining following SDS-PAGE analysis was performed as described by Covas et al. (55). Briefly, cells were washed in 20 ml buffer 1 [50 mM 2-(N-morpholino)ethanesulfonic acid (MES) (pH 6.5)], resuspended in 20 ml buffer 2 (buffer 1 with 4% [wt/vol] SDS), and boiled for 60 min. Next, the cells were washed with 20 ml buffer 2 and, after transfer to 2.0-ml reaction tubes, washed once more with 1.6 ml buffer 2, 1.6 ml buffer 3 (buffer 1 with 2% [wt/vol] NaCl), and finally 1.6 ml buffer 1. The samples were suspended in 1.6 ml buffer 4 (20 mM Tris-HCl [pH 8.0], 0.5% [wt/vol] SDS) and incubated for 4 h at 50°C, with shaking, following the addition of 2 µl of proteinase K solution (20 mg/ml) from Tritirachium album. Next, the cells were collected by centrifugation, washed once with 1.6 ml buffer 3, and washed three times with 1.6 ml of water. To release WTA, the pellets were suspended in 1 ml of 0.1 mM NaOH, and the samples were incubated for 16 h at 25°C. Next, the samples were centrifuged, 30 µl of the supernatant (containing WTA) was separated on native Tris-Tricine polyacrylamide gels, and WTA was visualized by Alcian blue/silver staining (55).
Whole-genome sequencing.
WT LAC*, LAC*ΔltaSN, LAC*ΔltaSS, and indicated suppressor strains were cultured overnight at 37°C, cells were harvested, and genomic DNA was extracted. Genome sequencing was performed by MicrobesNG (University of Birmingham), using an Illumina platform and a 250-bp paired-end read kit. Sequence analysis was performed using the CLC Genomics Workbench software package. First, the LAC* reads were aligned against the published USA300 FPR3757 genome sequence (RefSeq accession number NC_007793.1) and assembled into a reference contig, and the USA300 FPR3757 annotation was transferred onto the LAC* sequence. Next, the Illumina reads for the original ltaS mutant strains LAC*ΔltaSS and LAC*ΔltaSN, as well as the 7 different suppressor strains, were mapped onto the assembled LAC* sequence, and high-frequency (>65%) and good-quality base changes were identified using the CLC Genomics Workbench software package. Genomic alterations found in the suppressor strains but not present in the original ltaS mutant strain are summarized in Table 1.
Peptidoglycan isolation and analysis.
Overnight cultures of S. aureus strains LAC*, S4-sgtB, and LAC*sgtB::tn were prepared in TSB or TSB supplemented with 40% sucrose, and cultures of the original ltaS mutant strain LAC*ΔltaSN (ANG2134) were prepared in TSB supplemented with 40% sucrose. The next day, cells were back-diluted in 2 liters of the same growth medium, to an OD600 of 0.05. The cultures were grown at 37°C until they reached an OD600 of approximately 1.5 and were cooled on ice, and cells were collected by centrifugation. Peptidoglycan was purified and digested with mutanolysin as described previously (34, 48). High-performance liquid chromatography (HPLC) analysis of the digested peptidoglycan material was performed as described previously (48), and the muropeptide profiles were determined in triplicate for each strain and growth condition. For the quantification of monomeric, dimeric, trimeric, and higher oligomeric peptidoglycan material, the peaks were integrated. The total peak area for each muropetide profile was determined and set to 100%, the percentages of monomeric, dimeric, trimeric, and higher oligomeric peaks were calculated, and the average values and SDs from the three profiles were determined and plotted.
c-di-AMP quantification by competitive ELISA.
Five-milliliter aliquots of TSB were inoculated with single colonies of S. aureus LAC* (WT strain), strain LAC* gdpP::kan, strain US3 (LAC*ΔltaSS::erm suppressor with a mutation in gdpP), and the different LTA-negative suppressor strains, and the tubes were incubated for 8 h at 37°C. Next, the cultures were back-diluted in 10 ml TSB to an OD600 of 0.05 and were grown for 15 h at 37°C. Bacterial cells from these cultures were collected by centrifugation, cell lysates were prepared, and the cellular c-di-AMP levels were determined using a previously described competitive ELISA method (35, 36). However, instead of determining c-di-AMP levels based on a standard curve, the A490 values were directly compared. To this end, the A490 readings obtained for samples derived from the WT strain were set to 100%, and percentage values were calculated for the samples derived from the other strains. Thee independent experiments were performed, with three technical replicates, and the average values and SDs for the percentage values were determined for each strain, compared to the WT strain. Of note, since this was a competitive ELISA, decreases in A490 readings represented increases in cellular c-di-AMP levels.
Accession number(s).
The Illumina short read for the WT LAC* was published previously (35) and has been deposited in the European Nucleotide Archive under study accession number PRJEB14759. The Illumina reads for the ltaS mutants and the ltaS suppressor strains were deposited in the European Nucleotide Archive under study accession number PRJEB29420.
ACKNOWLEDGMENTS
This research was supported by the European Research Council (grant 260371 to A.G.), the Wellcome Trust (grant 100289 to A.G.), the UK Medical Research Council within the AMR Cross-council Initiative collaborative (grant MR/N002679/1 to W.V.), and the German Research Foundation (grant SCHU 3159/1-1 to C.F.S.). The Illumina sequencing was performed by MicrobesNG, which was supported by BBSRC grant BB/L024209/1.
REFERENCES
- 1.Kluytmans J, Van Belkum A, Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520. doi: 10.1128/CMR.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek KT, Bowman L, Millership C, Dupont Sogaard M, Kaever V, Siljamaki P, Savijoki K, Varmanen P, Nyman TA, Gründling A, Frees D. 2016. The cell wall polymer lipoteichoic acid becomes nonessential in Staphylococcus aureus cells lacking the ClpX chaperone. mBio 7:e01228-16. doi: 10.1128/mBio.01228-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl T, Ticehurst JR, Carroll K, Thomas DL, Nuermberger E, Bartlett JG. 2005. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis 40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 4.Hiramatsu K, Katayama Y, Matsuo M, Sasaki T, Morimoto Y, Sekiguchi A, Baba T. 2014. Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J Infect Chemother 20:593–601. doi: 10.1016/j.jiac.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Horn J, Stelzner K, Rudel T, Fraunholz M. 2017. Inside job: Staphylococcus aureus host-pathogen interactions. Int J Med Microbiol 308:607–624. [DOI] [PubMed] [Google Scholar]
- 6.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silhavy TJ, Kahne D, Walker S. 2010. The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajagopal M, Walker S. 2017. Envelope structures of Gram-positive bacteria. Curr Topics Microbiol Immunol 404:1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollmer W, Blanot D, De Pedro MA. 2008. Peptidoglycan structure and architecture. FEMS Microbiol Rev 32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 10.Archibald AL, Hancock IC, Harwood CR. 1993. Cell wall structure, synthesis, and turnover, p 381–410. In Sonenshein AL, Hoch JA, Losick R (ed), Bacillus subtilis and other Gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society of Microbiology, Washington, DC. [Google Scholar]
- 11.Höltje JV. 1998. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol Biol Rev 62:181–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed P, Atilano ML, Alves R, Hoiczyk E, Sher X, Reichmann NT, Pereira PM, Roemer T, Filipe SR, Pereira-Leal JB, Ligoxygakis P, Pinho MG. 2015. Staphylococcus aureus survives with a minimal peptidoglycan synthesis machine but sacrifices virulence and antibiotic resistance. PLoS Pathog 11:e1004891. doi: 10.1371/journal.ppat.1004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman BJ, Tomasz A. 1984. Low-affinity penicillin-binding protein associated with β-lactam resistance in Staphylococcus aureus. J Bacteriol 158:513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinho MG, de Lencastre H, Tomasz A. 2001. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc Natl Acad Sci U S A 98:10886–10891. doi: 10.1073/pnas.191260798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed P, Veiga H, Jorge AM, Terrak M, Pinho MG. 2011. Monofunctional transglycosylases are not essential for Staphylococcus aureus cell wall synthesis. J Bacteriol 193:2549–2556. doi: 10.1128/JB.01474-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang QM, Peery RB, Johnson RB, Alborn WE, Yeh WK, Skatrud PL. 2001. Identification and characterization of a monofunctional glycosyltransferase from Staphylococcus aureus. J Bacteriol 183:4779–4785. doi: 10.1128/JB.183.16.4779-4785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weidenmaier C, Peschel A. 2008. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol 6:276. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 18.Kawai Y, Marles-Wright J, Cleverley RM, Emmins R, Ishikawa S, Kuwano M, Heinz N, Bui NK, Hoyland CN, Ogasawara N, Lewis RJ, Vollmer W, Daniel RA, Errington J. 2011. A widespread family of bacterial cell wall assembly proteins. EMBO J 30:4931–4941. doi: 10.1038/emboj.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kern T, Giffard M, Hediger S, Amoroso A, Giustini C, Bui NK, Joris B, Bougault C, Vollmer W, Simorre JP. 2010. Dynamics characterization of fully hydrated bacterial cell walls by solid-state NMR: evidence for cooperative binding of metal ions. J Am Chem Soc 132:10911–10919. doi: 10.1021/ja104533w. [DOI] [PubMed] [Google Scholar]
- 20.Neuhaus FC, Baddiley J. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in Gram-positive bacteria. Microbiol Mol Biol Rev 67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown S, Santa Maria JP Jr, Walker S. 2013. Wall teichoic acids of Gram-positive bacteria. Annu Rev Microbiol 67:313–336. doi: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Percy MG, Gründling A. 2014. Lipoteichoic acid synthesis and function in Gram-positive bacteria. Annu Rev Microbiol 68:81–100. doi: 10.1146/annurev-micro-091213-112949. [DOI] [PubMed] [Google Scholar]
- 23.Percy MG, Karinou E, Webb AJ, Gründling A. 2016. Identification of a lipoteichoic acid glycosyltransferase enzyme reveals that GW-domain-containing proteins can be retained in the cell wall of Listeria monocytogenes in the absence of lipoteichoic acid or its modifications. J Bacteriol 198:2029–2042. doi: 10.1128/JB.00116-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santa Maria JP Jr, Sadaka A, Moussa SH, Brown S, Zhang YJ, Rubin EJ, Gilmore MS, Walker S. 2014. Compound-gene interaction mapping reveals distinct roles for Staphylococcus aureus teichoic acids. Proc Natl Acad Sci U S A 111:12510–12515. doi: 10.1073/pnas.1404099111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Götz F. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 26.Kho K, Meredith TC. 2018. Salt-induced stress stimulates a lipoteichoic acid-specific three-component glycosylation system in Staphylococcus aureus. J Bacteriol 200:e00017-18. doi: 10.1128/JB.00017-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reichmann NT, Gründling A. 2011. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol Lett 319:97–105. doi: 10.1111/j.1574-6968.2011.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu D, Wörmann ME, Zhang X, Schneewind O, Gründling A, Freemont PS. 2009. Structure-based mechanism of lipoteichoic acid synthesis by Staphylococcus aureus LtaS. Proc Natl Acad Sci U S A 106:1584–1589. doi: 10.1073/pnas.0809020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gründling A, Schneewind O. 2007. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc Natl Acad Sci U S A 104:8478–8483. doi: 10.1073/pnas.0701821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karatsa-Dodgson M, Wörmann ME, Gründling A. 2010. In vitro analysis of the Staphylococcus aureus lipoteichoic acid synthase enzyme using fluorescently labeled lipids. J Bacteriol 192:5341–5349. doi: 10.1128/JB.00453-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oku Y, Kurokawa K, Matsuo M, Yamada S, Lee BL, Sekimizu K. 2009. Pleiotropic roles of polyglycerolphosphate synthase of lipoteichoic acid in growth of Staphylococcus aureus cells. J Bacteriol 191:141–151. doi: 10.1128/JB.01221-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schirner K, Marles‐Wright J, Lewis RJ, Errington J. 2009. Distinct and essential morphogenic functions for wall‐ and lipo‐teichoic acids in Bacillus subtilis. EMBO J 28:830–842. doi: 10.1038/emboj.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fedtke I, Mader D, Kohler T, Moll H, Nicholson G, Biswas R, Henseler K, Götz F, Zähringer U, Peschel A. 2007. A Staphylococcus aureus ypfP mutant with strongly reduced lipoteichoic acid (LTA) content: LTA governs bacterial surface properties and autolysin activity. Mol Microbiol 65:1078–1091. doi: 10.1111/j.1365-2958.2007.05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Gründling A. 2011. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog 7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowman L, Zeden MS, Schuster CF, Kaever V, Gründling A. 2016. New insights into the cyclic di-adenosine monophosphate (c-di-AMP) degradation pathway and the requirement of the cyclic dinucleotide for acid stress resistance in Staphylococcus aureus. J Biol Chem 291:26970–26986. doi: 10.1074/jbc.M116.747709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Underwood AJ, Zhang Y, Metzger DW, Bai G. 2014. Detection of cyclic di-AMP using a competitive ELISA with a unique pneumococcal cyclic di-AMP binding protein. J Microbiol Methods 107:58–62. doi: 10.1016/j.mimet.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donegan NP, Cheung AL. 2009. Regulation of the mazEF toxin-antitoxin module in Staphylococcus aureus and its impact on sigB expression. J Bacteriol 191:2795–2805. doi: 10.1128/JB.01713-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morikawa K, Maruyama A, Inose Y, Higashide M, Hayashi H, Ohta T. 2001. Overexpression of sigma factor, ζB, urges Staphylococcus aureus to thicken the cell wall and to resist β-lactams. Biochem Biophys Res Commun 288:385–389. doi: 10.1006/bbrc.2001.5774. [DOI] [PubMed] [Google Scholar]
- 39.Guldimann C, Boor KJ, Wiedmann M, Guariglia-Oropeza V. 2016. Resilience in the face of uncertainty: sigma factor B fine-tunes gene expression to support homeostasis in Gram-positive bacteria. Appl Environ Microbiol 82:4456–4469. doi: 10.1128/AEM.00714-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuroda M, Kuroda H, Oshima T, Takeuchi F, Mori H, Hiramatsu K. 2003. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol Microbiol 49:807–821. [DOI] [PubMed] [Google Scholar]
- 41.Kuroda M, Kuwahara-Arai K, Hiramatsu K. 2000. Identification of the up- and down-regulated genes in vancomycin-resistant Staphylococcus aureus strains Mu3 and Mu50 by cDNA differential hybridization method. Biochem Biophys Res Commun 269:485–490. doi: 10.1006/bbrc.2000.2277. [DOI] [PubMed] [Google Scholar]
- 42.Boyle-Vavra S, Yin S, Jo DS, Montgomery CP, Daum RS. 2013. VraT/YvqF is required for methicillin resistance and activation of the VraSR regulon in Staphylococcus aureus. Antimicrob Agents Chemother 57:83–95. doi: 10.1128/AAC.01651-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeFrancesco AS, Masloboeva N, Syed AK, DeLoughery A, Bradshaw N, Li GW, Gilmore MS, Walker S, Losick R. 2017. Genome-wide screen for genes involved in eDNA release during biofilm formation by Staphylococcus aureus. Proc Natl Acad Sci U S A 114:E5969–E5978. doi: 10.1073/pnas.1704544114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vickery CR, Wood BM, Morris HG, Losick R, Walker S. 2018. Reconstitution of Staphylococcus aureus lipoteichoic acid synthase activity identifies Congo red as a selective inhibitor. J Am Chem Soc 140:876–879. doi: 10.1021/jacs.7b11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e0053-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terrak M, Nguyen-Distèche M. 2006. Kinetic characterization of the monofunctional glycosyltransferase from Staphylococcus aureus. J Bacteriol 188:2528–2532. doi: 10.1128/JB.188.7.2528-2532.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rebets Y, Lupoli T, Qiao Y, Schirner K, Villet R, Hooper D, Kahne D, Walker S. 2014. Moenomycin resistance mutations in Staphylococcus aureus reduce peptidoglycan chain length and cause aberrant cell division. ACS Chem Biol 9:459–467. doi: 10.1021/cb4006744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Jonge B, Chang Y-S, Gage D, Tomasz A. 1992. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain: the role of penicillin binding protein. J Biol Chem 267:11248–11254. [PubMed] [Google Scholar]
- 49.Reichmann NT, Picarra Cassona C, Monteiro JM, Bottomley AL, Corrigan RM, Foster SJ, Pinho MG, Gründling A. 2014. Differential localization of LTA synthesis proteins and their interaction with the cell division machinery in Staphylococcus aureus. Mol Microbiol 92:273–286. doi: 10.1111/mmi.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Commichau FM, Gibhardt J, Halbedel S, Gundlach J, Stülke J. 2018. A delicate connection: c-di-AMP affects cell integrity by controlling osmolyte transport. Trends Microbiol 26:175–185. doi: 10.1016/j.tim.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Corrigan RM, Campeotto I, Jeganathan T, Roelofs KG, Lee VT, Gründling A. 2013. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc Natl Acad Sci U S A 110:9084–9089. doi: 10.1073/pnas.1300595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuster CF, Bellows LE, Tosi T, Campeotto I, Corrigan RM, Freemont P, Gründling A. 2016. The second messenger c-di-AMP inhibits the osmolyte uptake system OpuC in Staphylococcus aureus. Sci Signal 9:ra81. doi: 10.1126/scisignal.aaf7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Memmi G, Filipe SR, Pinho MG, Fu Z, Cheung A. 2008. Staphylococcus aureus PBP4 is essential for β-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob Agents Chemother 52:3955–3966. doi: 10.1128/AAC.00049-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gründling A, Schneewind O. 2007. Genes required for glycolipid synthesis and lipoteichoic acid anchoring in Staphylococcus aureus. J Bacteriol 189:2521–2530. doi: 10.1128/JB.01683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Covas G, Vaz F, Henriques G, Pinho M, Filipe S. 2016. Analysis of cell wall teichoic acids in Staphylococcus aureus. Methods Mol Biol 1440:201–213. doi: 10.1007/978-1-4939-3676-2_15. [DOI] [PubMed] [Google Scholar]
- 56.Monk IR, Tree JJ, Howden BP, Stinear TP, Foster TJ. 2015. Complete bypass of restriction systems for major Staphylococcus aureus lineages. mBio 6:e00308-15. doi: 10.1128/mBio.00308-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeden MS, Schuster CF, Bowman L, Zhong Q, Williams HD, Gründling A. 2018. Cyclic-di-adenosine monophosphate (c-di-AMP) is required for osmotic regulation in Staphylococcus aureus but dispensable for viability in anaerobic conditions. J Biol Chem 293:3180–3200. doi: 10.1074/jbc.M117.818716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boles BR, Thoendel M, Roth AJ, Horswill AR. 2010. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 5:e10146. doi: 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]