Abstract
Platelets participate in the development of liver fibrosis in animal models, but little is known about the benefit of antiplatelet agents in preventing liver fibrosis in humans. We therefore explored the relationship between the use of antiplatelet agents and liver fibrosis in a prospective cohort study of patients at high risk of liver fibrosis and cardiovascular events. Consecutive patients undergoing elective coronary angiography at the University Hospital Frankfurt were prospectively included in the present study. Associations between use of antiplatelet agents (acetyl salicylic acid, P2Y12 receptor antagonists) and liver fibrosis were assessed in regression models, and the relationship between platelet‐derived growth factor beta (PDGF‐β) serum concentration, platelets, liver fibrosis, and use of antiplatelet agents was characterized. Out of 505 included patients, 337 (67%) received antiplatelet agents and 134 (27%) had liver fibrosis defined as a FibroScan transient elastography (TE) value ≥7.9 kPa. Use of antiplatelet agents was inversely associated with the presence of liver fibrosis in univariate and multivariate analyses (multivariate odds ratio [OR], 0.67; 95% confidence interval [CI], 0.51‐0.89; P = 0.006). Use of antiplatelet agents was also inversely associated with FibroTest values (beta, –0.38; SD beta, 0.15; P = 0.02). Furthermore, there was a significant correlation between platelet counts and PDGF‐β serum concentration (rho, 0.33; P < 0.0001), but PDGF‐β serum levels were not affected by antiplatelet agents. Conclusion: There is a protective association between the use of antiplatelet agents and occurrence of liver fibrosis. A randomized controlled trial is needed to explore causality and the potential of antiplatelet agents as antifibrotic therapy in patients at risk for liver fibrosis progression.
Abbreviations
- AST
aspartate aminotransferase
- BMI
body mass index
- CAD
coronary artery disease
- CAP
controlled attenuation parameter
- CI
confidence interval
- CVD
cardiovascular disease
- HSC
hepatic stellate cell
- LSEC
liver sinusoidal endothelial cell
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- PDGF‐β
platelet‐derived growth factor beta
- TE
transient elastography
Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver diseases among adults in Western countries.1 The term NAFLD covers a wide disease spectrum from varying grades of steatosis to nonalcoholic steatohepatitis, which can progress to liver cirrhosis and hepatocellular carcinoma in a relevant proportion of affected individuals.1, 2 In addition to liver‐related morbidity and mortality, there is a close relationship between NAFLD and cardiovascular disease (CVD) as NAFLD can be considered a hepatic manifestation of the metabolic syndrome.2, 3 The presence of liver fibrosis further increases the risk of developing CVD as well as mortality from CVD.4, 5, 6 In view of these facts, international guidelines recommend screening for CVDs in patients with NAFLD.7, 8
Therapeutic modalities to improve NAFLD and liver fibrosis in general are insufficient. There is emerging evidence that platelets play an important role in the establishment and progression of liver disease.9 Platelets release factors, such as PDGF‐β, chemokine (C‐X‐C motif) ligand 4, or serotonin, that participate in liver fibrosis progression either by direct activation of hepatic stellate cells (HSCs) or by recruiting inflammatory cells to the liver.9 Serotonin released by platelets could also reduce blood flow in the hepatic microcirculation by activating the contraction of HSCs or liver sinusoidal endothelial cells (LSECs).10, 11 Furthermore, platelets sequestrate in the liver sinusoids during liver damage where they license LSECs to express chemokine receptors; this further amplifies the hepatic influx of innate and adaptive immune cells.9 Although antiplatelet strategies have been shown to have a beneficial effect in animal models of chronic liver disease, little is known about the potential to ameliorate liver fibrosis of antiplatelet medications in humans.9 In the present study, we explored the relationship between the use of antiplatelet agents and the presence of liver disease in a large prospective study of patients with CVD.
Patients and Methods
Patients
The present analysis is a substudy of a prospective monocenter trial assessing the relationship between NALFD and coronary artery disease (CAD) (ClinicalTrial.gov NCT01638832). The original trial included 576 consecutive patients undergoing elective coronary angiography at the cardiology department of the University Hospital Frankfurt between January 2012 and October 2014. Indications for coronary angiography were suspected first‐time manifestation of CAD or suspected progression of known CAD in >88% of patients. Main exclusion criteria of the primary study were acute myocardial infarction, ascites, pregnancy or breastfeeding, and age younger than 18 years; details are described in Friedrich‐Rust et al.12 Clinical and demographic variables as well as laboratory test results were obtained before coronary angiography.
Patients were included in the present subanalysis if a valid TE test result to determine the degree of liver fibrosis was obtained (details below). Informed consent was obtained from all participants, and the study protocol was approved by the ethics committee of the University of Frankfurt.
Clinically significant CAD was defined as the presence of relevant coronary stenosis (i.e., ≥75% stenosis of the vessel diameter). Liver fibrosis was primarily assessed by TE using a FibroScan 502 touch device (Echosens, Paris, France) with the FibroScan M‐Probe (3.5 MHz; shear wave frequency 50 Hz; depth of measurement 25‐65 mm). To detect liver fibrosis, we chose a TE cutoff value of 7.9 kPa. In addition, serologic testing for liver fibrosis was performed using the FibroTest.13 Controlled attenuation parameter (CAP) was used to quantify hepatic steatosis. A CAP cutoff value of 234 dB/m was used to define the presence of steatosis.14 Nonalcoholic steatohepatitis was suspected in cases of a CAP value ≥324 dB/m in combination with a TE value ≥7.9 kPa in the absence of alcohol consumption >10 g/day or 20 g/day in female and male study participants, respectively.
Quantification of PDGF‐Β Serum Concentration
PDGF‐β serum quantification was performed in the subgroup of patients for whom stored serum samples were available. PDGF‐β in serum was quantified using the Human PDGF‐BB Quantikine Enzyme‐Linked Immunosorbent Assay Kit (R&D Systems) according to the manufacturer's instructions. Samples and standard curve values were measured at 450 nm on an EnVision 2104 Multilabel Plate Reader (Perkin Elmer).
Statistical Analyses
Associations of outcomes with continuous or dichotomic variables were assessed in linear and logistic regression models, respectively. After univariate analyses, multivariate analyses were performed for significant associations. Multivariate models were obtained by backward selection, using P > 0.15 for removal from the model. Group differences were assessed by means of 2 contingency tables or Wilcoxon‐Mann‐Whitney U tests, as appropriate.
Results
Study Population
A total of 505 patients were included in the present study based on the above defined inclusion criteria. Of the included patients, 134 (27%) had liver fibrosis defined as a FibroScan TE value ≥7.9 kPa. Demographic and clinical characteristics of patients according to the presence or absence of liver fibrosis are shown in Table 1. Of the entire study population, 337 (67%) were on antiplatelet therapy at the time of study inclusion, i.e., the time of assessment for liver fibrosis. Of those, 162 (48%) patients were receiving monotherapy with acetyl salicylic acid, 26 (8%) patients were receiving monotherapy with a P2Y12 receptor antagonist (clopidogrel, prasugrel, or ticagrelor), and 149 (44%) patients were receiving combination therapy with antiplatelet agents of both classes. Clinical characteristics according to the intake of number and class of antiplatelet agents are shown in Table 2.
Table 1.
Baseline Characteristics of Included Patients Based On the Presence of Liver Fibrosis (Defined as FibroScan TE Value ≥7.9 kPa)
| Characteristics | No Fibrosis (n = 371) | Fibrosis (n = 134) | P value |
|---|---|---|---|
| Age (years), mean (IQR) | 65 (56‐74) | 69 (61‐77) | 0.001 |
| Male sex, n (%) | 280 (75) | 115 (86) | 0.01 |
| BMI (kg/m2), mean (IQR) | 27 (24‐30) | 28 (24‐31) | 0.5 |
| Nicotine consumption (pack years), mean (IQR) | 22 (0‐36) | 25 (0‐40) | 0.6 |
| Alcohol consumption (g/day), mean (IQR) | 5.70 (1‐10) | 6.15 (0‐11) | 0.5 |
| Intake of statins, n (%) | 259 (70) | 95 (71) | 0.8 |
| Intake of antidiabetic medication, n (%) | 65 (18) | 39 (29) | 0.005 |
| Triglycerides (mg/dL) mean (IQR) | 141 (84‐170) | 126 (71‐158) | 0.04 |
| Cholesterin, mean (IQR) | 180 (148‐208) | 154 (120‐187) | <0.001 |
| LDL (mg/dL), mean (IQR) | 100 (75‐122) | 85 (57‐104) | <0.001 |
| HDL (mg/dL), mean (IQR) | 51 (38‐60) | 46 (34‐54) | 0.01 |
| Bilirubin (mg/dL), mean (IQR) | 0.6 (0.4‐0.7) | 0.7 (0.4‐1.0) | <0.001 |
| γGT (U/L), mean (IQR) | 42 (19‐48) | 125 (43‐137) | <0.001 |
| AST (U/L), mean (IQR) | 30 (20‐31) | 38 (22‐43) | <0.001 |
| ALT (U/L), mean (IQR) | 26 (16‐31) | 39 (17‐40) | 0.004 |
| INR (%), mean (IQR) | 1.4 (0.96‐1.1) | 1.4 (1.0‐1.39) | <0.001 |
| Hemoglobin (g/dL), mean (IQR) | 13 (12‐15) | 12.7 (11.2‐14.5) | 0.007 |
| Platelets (/nL), mean (IQR) | 232 (185‐264) | 206 (161‐240) | <0.001 |
| HbA1c (%), mean (IQR) | 5.9 (5.4‐6.1) | 6.5 (5.6‐6.7) | <0.001 |
| FLI category, mean (IQR) | 1.2 (1.0‐2.0) | 1.6 (1.0‐2.0) | <0.001 |
| CAP (dB/m), mean (IQR) | 266 (220‐308) | 280 (224‐325) | 0.05 |
| PDGF‐β, mean (IQR) | 1,430 (1,118‐1,783) | 1,353 (963‐1,656) | 0.3 |
Abbreviations: ALT, alanine aminotransferase; EtOH, ethanol; FLI, fatty liver index; γGT, gamma‐glutamyltransferase; HbA1c, hemoglobin A1c; IQR, interquartile range; HDL, high‐density lipoprotein; INR, international normalized ratio; LDL, low‐density lipoprotein.
Table 2.
Patient Characteristics of Included Patients Based On the Intake of Antiplatelet Medication
| Characteristics | No Antiplatelet Medication (n = 164) | Any Antiplatelet Medication (n = 335) | Acetyl Salicylic Acid (n = 161) | P2Y12RA (n = 26) | Combination Therapy (n = 148) | P Value* | P Value† |
|---|---|---|---|---|---|---|---|
| Age (years), mean (IQR) | 64 (55‐75) | 66 (58‐75) | 67 (59‐75) | 72 (65‐78) | 65 (55‐75) | 0.1 | 0.4 |
| Male sex, n (%) | 116 (71) | 276 (82) | 135 (84) | 19 (73) | 122 (82) | 0.003 | 0.005 |
| BMI (kg/m2), mean (IQR) | 27 (23‐30) | 27 (24‐30) | 28 (24‐31) | 26 (21‐28) | 27 (24‐30) | 0.6 | 0.3 |
| Nicotine consumption (pack years), mean (IQR) | 24 (0‐40) | 23 (0‐35) | 25 (0‐35) | 28 (0‐48) | 21 (0‐32) | 0.5 | 0.15 |
| Alcohol consumption (g/day), mean (IQR) | 5.83 (0‐9.50) | 5.84 (0‐10.25) | 6.68 (0‐12.75) | 4.25 (0‐6.75) | 5.19 (0‐8.0) | 0.7 | 0.3 |
| Intake of statins, n (%) | 60 (37) | 294 (88) | 133 (83) | 23 (88) | 138 (93) | <0.001 | <0.001 |
| Intake of antidiabetic medication, n (%) | 26 (16) | 78 (23) | 39 (24) | 2 (7.7) | 37 (25) | 0.05 | 0.12 |
| Triglycerides (mg/dL) mean (IQR) | 129 (78‐155) | 142 (82‐173) | 138 (82‐168) | 149 (73‐142) | 144 (84‐181) | 0.2 | 0.06 |
| Cholesterin, mean (IQR) | 179 (148‐208) | 169 (133‐204) | 171 (133‐206) | 170 (127‐217) | 167 (133‐203) | 0.06 | 0.04 |
| LDL (mg/dL), mean (IQR) | 104 (76‐133) | 92 (63‐116) | 93 (67‐116) | 91 (58‐119) | 91 (61‐117) | 0.008 | 0.007 |
| HDL (mg/dL), mean (IQR) | 51 (38‐59) | 49 (38‐57) | 51 (38‐59) | 48 (35‐55) | 47 (37‐56) | 0.2 | 0.07 |
| Bilirubin (mg/dL), mean (IQR) | 0.6 (0.4‐0.8) | 0.6 (0.4‐0.7) | 0.6 (0.4‐0.7) | 0.6 (0.3‐ 0.8) | 0.6 (0.4‐0.7) | 0.3 | 0.3 |
| γGT (U/L), mean (IQR) | 50 (20‐64) | 71 (22‐62) | 65 (22‐66) | 94 (24‐118) | 73 (21‐60) | 0.5 | 0.2 |
| AST (U/L), mean (IQR) | 32 (21‐33) | 33 (21‐34) | 34 (21‐34) | 30 (21‐30) | 32 (20‐ 34) | 0.8 | 0.4 |
| ALT (U/L), mean (IQR) | 31 (15‐34) | 29 (16‐34) | 31 (17‐34) | 27 (12‐29) | 28 (17‐34) | 0.5 | 0.4 |
| INR (%), mean (IQR) | 1.9 (1.0‐1.3) | 1.2 (1.0‐1.1) | 1.2 (0.9‐1.1) | 1.5 (1.0‐1.9) | 1.1 (1.0‐1.1) | <0.001 | <0.001 |
| Hemoglobin (g/dL), mean (IQR) | 13.1 (12.0‐14.4) | 13.2 (12.0‐14.6) | 13.3 (12.2‐14.7) | 12.8 (11.7‐14.2) | 13.1 (11.8‐14.4) | 0.9 | 0.4 |
| Platelets (/nL), mean (IQR) | 219 (176‐258) | 228 (180‐262) | 221 (178‐250) | 231 (177‐269) | 236 (182‐275) | 0.3 | 0.03 |
| HbA1c (%), mean (IQR) | 5.9 (5.5‐6.1) | 6.1 (5.4‐6.3) | 6.1 (5.4‐6.4) | 6.0 (5.5‐6.4) | 6.2 (5.5‐6.2) | 0.2 | 0.08 |
| FLI category, mean (IQR) | 1.3 (1.0‐2.0) | 1.4 (1.0‐2.0) | 1.4 (1.0‐2.0) | 1.1 (0‐2) | 1.4 (1.0‐2.0) | 0.3 | 0.15 |
| CAP (dB/m), mean (IQR) | 263 (215‐310) | 272 (231‐318) | 280 (243‐320) | 248 (223‐269) | 268 (224‐319) | 0.2 | 0.3 |
| PDGF‐β, mean (IQR) | 1,346 (1,024‐1,627) | 1,439 (1,117‐1,820) | 1,350 (986‐1,723) | 1,440 (1,273‐1,685) | 1,537 (1,228‐1,865) | 0.2 | 0.01 |
| FibroScan (kPa), mean (IQR) | 9.3 (4.5‐8.7) | 8.3 (4.4‐7.9) | 8.3 (4.5‐8.9) | 14.2 (5.2‐10.3) | 7.3 (4.3‐ 6.9) | 0.2 | 0.009 |
Abbreviations: ALT, alanine aminotransferase; EtOH, ethanol; FLI, fatty liver index; γGT, gamma‐glutamyltransferase; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; INR, international normalized ratio; LDL, low‐density lipoprotein.
P value for no versus any antiplatelet agent; † P value for no versus one versus two antiplatelet agents.
Association Between Use of Antiplatelet Agents and Liver Fibrosis
To assess the statistical relationship between use of antiplatelet agents and liver fibrosis, we performed logistic regression analysis of factors predicting the presence of liver fibrosis (defined as a TE value ≥7.9 kPa). In univariate analysis, age (P = 0.0006), male sex (P = 0.01), aspartate aminotransferase (AST) levels (P = 0.005), lower platelets (P = 0.0006), and the presence of diabetes (P = 0.001) were associated with liver fibrosis (Table 3). Furthermore, the use of antiplatelet agents was inversely associated with the presence of liver fibrosis in a dose‐dependent manner, i.e., a stronger association was observed for the intake of one versus two antiplatelet agents (OR, 0.76; 95% CI, 0.59‐0.98; P = 0.036) (Table 3; Fig. 1). Multivariate analyses revealed an independent inverse association between use of platelet agents and the presence of liver fibrosis (model 1: OR, 0.70; 95% CI, 0.53‐0.94; P = 0.02; model 2, in which platelets were excluded due to a possible interaction with use of antiplatelet agents: OR, 0.67; 95% CI, 0.51‐0.89; P = 0.006) (Table 3). Comparable results were obtained in linear univariate and multivariate analyses of FibroScan TE values as continuous variables (Table 4, models 1 and 2), although statistical significance was lost after inclusion of the use of statins and antidiabetic drugs in this (but not in the above described logistic) regression analysis (Table 4, model 3). Furthermore, an analysis of the use of beta blockers and liver fibrosis was performed to exclude beta blockers as a potential confounder. Of note, a positive association between the use of beta blockers and liver fibrosis was observed in contrast to the inverse association with antiplatelet agents for the association of beta blockers and liver fibrosis (adjusted OR, 3.79; 95% CI, 2.16‐6.66; P = 0.00003) and the association of antiplatelet agents and liver fibrosis after inclusion of the two variables (adjusted OR, 0.64; 95% CI, 0.49‐0.85; P = 0.002).
Table 3.
Logistic Regression Analyses of Factors Associated With the Presence of Liver Fibrosis (FibroScan TE value ≥7.9 kPa)*
| Univariate | Multivariate, Model 1 | Multivariate, Model 2 † | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age (years) | 1.03 (1.01‐1.05) | 0.0006 | 1.04 (1.02‐1.06) | 0.0002 | 1.04 (1.02‐1.06) | 0.00003 |
| Female sex | 0.48 (0.27‐0.84) | 0.01 | 0.49 (0.26‐0.91) | 0.02 | 0.38 (0.21‐0.69) | 0.001 |
| BMI (kg/m2) | 1.03 (0.99‐1.08) | 0.2 | ||||
| AST (U/L) | 1.01 (1.003‐1.02) | 0.005 | 1.02 (1.01‐1.03) | 0.001 | 1.02 (1.01‐1.89) | 0.0008 |
| Platelets (/nL) | 0.99 (0.99‐0.99) | 0.0006 | 0.99 (0.99‐0.10) | 0.02 | ||
| Antiplatelet agents, no vs. mono vs. combination therapy | 0.76 (0.59‐0.98) | 0.036 | 0.70 (0.53‐0.94) | 0.02 | 0.67 (0.51‐0.89) | 0.006 |
| Statins, use | 1.05 (0.57‐1.63) | 0.8 | ||||
| Antidiabetic drugs, use | 1.64 (0.98‐2.73) | 0.056 | ||||
| Diabetes, presence | 1.44 (1.15‐1.80) | 0.001 | 1.46 (1.15‐1.87) | 0.002 | 1.41 (1.11‐1.79) | 0.005 |
| Significant CHD, presence | 0.99 (0.93‐1.07) | 0.9 | ||||
FiboScan measurements did not meet the quality criterion of at least 10 valid TE measurements with an IQR <30% in patients with TE values >7.1 kPa in 24 patients.
Plateles were excluded from model 2 because platelets and use of antiplatelet agents might be partially dependent variables.
Figure 1.
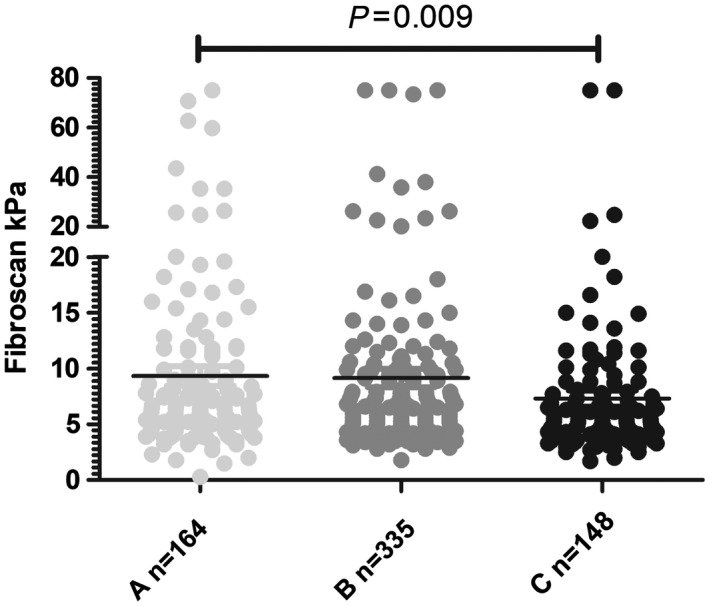
Individual FibroScan values according to the use of none (light gray), one (acetyl salicyclic acid or clopidogrel; dark gray), or two (acetyl salicylic acid + P2Y12 receptor antagonist [black]) antiplatelet agents. Black lines represent mean values.
Table 4.
Linear Regression Analyses of Factors Associated With FibroScan TE Value*
| Univariate | Multivariate, Model 1 | Multivariate, Model 2 † | ||||
|---|---|---|---|---|---|---|
| Beta (SD Beta) | P Value | Beta (SD Beta) | P Value | Beta (SD Beta) | P Value | |
| Age (years) | 0.01 (0.04) | 0.7 | ||||
| Female sex | –1.28 (1.22) | 0.3 | –2.3 (1.17) | 0.05 | ||
| BMI (kg/m2) | –0.06 (0.11) | 0.5 | ||||
| AST (U/L) | 0.06 (0.02) | 0.003 | 0.06 (0.02) | 0.003 | 0.06 (0.02) | 0.003 |
| Platelets (/nL) | –0.02 (0.01) | 0.003 | –0.02 (0.01) | 0.0007 | ||
| Antiplatelet agents, no vs. mono vs. combination therapy | –1.18 (0.61) | 0.04 | –1.01 (0.60) | 0.07 | –1.41 (0.61) | 0.02 |
| Diabetes, presence | 2.02 (0.57) | 0.0004 | 2.01 (0.56) | 0.0003 | 1.97 (0.56) | 0.0004 |
| Significant CHD, presence | 0.12 (0.16) | 0.4 | ||||
| Univariate, Model 3 ‡ | Multivariate, Model 3 ‡ | |||||
| Beta (SD Beta) | P Value | Beta (SD Beta) | P Value | |||
| Age (years) | 0.03 (0.04) | 0.5 | ||||
| Female sex | –1.42 (1.22) | 0.2 | ||||
| BMI (kg/m2) | –0.05 (0.10) | 0.7 | ||||
| AST (U/L) | 0.06 (0.02) | 0.002 | 0.06 (0.02) | 0.003 | ||
| Platelets (/nL) | –0.02 (0.01) | 0.003 | –0.02 (0.01) | 0.0004 | ||
| Antiplatelet agents, no vs. mono vs. combination therapy | –1.12 (1.84) | 0.5 | ||||
| Statins, use | –2.25 (1.23) | 0.07 | –2.52 (1.05) | 0.02 | ||
| Antidiabetic drugs, use § | –1.18 (0.61) | 0.04 | ||||
| Diabetes, presence | 2.30 (0.80) | 0.004 | 2.01 (0.55) | 0.0003 | ||
| Significant CHD, presence | 0.12 (0.16) | 0.4 | ||||
FiboScan measurements did not meet the quality criterion of at least 10 valid TE measurements with an IQR <30% in patients with TE values >7.1 kPa in 24 patients.
Platelets were excluded from model 2 because platelets and use of antiplatelet agents might be partially dependent variables.
Use of statins and antidiabetic drugs was included in model 3. §Information on antidiabetic drug use was missing in 1 patient.
Information on antidiabetic drug use was missing in 1 patient.
Due to the strong association of male sex with liver fibrosis as well as the fact that male patients were more frequently on antiplatelet agents, we performed a subanalysis of the male patients. The inverse association between antiplatelet agents remained highly significant in the subgroup of male patients, whereas statistical significance was lost in the subgroup of female patients (data not shown) (Supporting Table S1). Furthermore, the inverse association between antiplatelet agents and liver fibrosis remained significant (at least in the multivariate model) if patients with proven liver steatosis (n = 362) were analyzed exclusively (multivariate P = 0.03; Supporting Table S2) or if only patients with definite exclusion of right heart failure and FibroScan test results with interquartile ranges >30 (n = 392) were analyzed (Supporting Tables S3‐S5).
In addition to TE‐based quantification of liver fibrosis, we calculated a serologic test (FibroTest) to quantify the degree of liver fibrosis. In univariate linear regression analyses, the use of antiplatelet agents was also inversely associated with FibroTest values (beta, –0.38; SD beta, 0.15; P = 0.02). This association remained significant after adjustment for age, sex, AST levels, and presence of diabetes (multivariate beta, –0.38; SD beta, 0.15; P = 0.01) (Table 5).
Table 5.
Linear Regression Analyses of Factors Associated With FibroTest Value
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| Beta (SD Beta) | P Value | Beta (SD Beta) | P Value | |
| Age (years) | 0.09 (0.01) | <0.0001 | 0.09 (0.01) | <0.0001 |
| Female sex | –1.57 (0.30) | <0.0001 | –1.68 (0.28) | <0.0001 |
| BMI (kg/m2) | –0.01 (0.03) | 0.8 | ||
| AST (U/L) | 0.04 (0.01) | <0.0001 | 0.04 (0.01) | <0.0001 |
| Platelets (/nL) | –0.002 (0.001) | 0.2 | ||
| Antiplatelet agents, no vs. mono vs. combination therapy | –0.38 (0.15) | 0.02 | –0.38 (0.15) | 0.01 |
| Statins, use | –0.017 (0.03) | 0.5 | ||
| Antidiabetic drugs, use | 0.006 (0.04) | 0.8 | ||
| Diabetes, presence | 0.43 (0.14) | 0.001 | 0.42 (0.13) | 0.002 |
| Significant CHD, presence | 0.03 (0.04) | 0.4 | ||
Relationship Between Antiplatelet Agents, Liver Fibrosis, and PDGF‐β Serum Levels
PDGF‐β is a well‐characterized mediator of hepatic fibrogenesis in animal models and humans.15, 16, 17 Therefore, we explored the relationship between PDGF‐β, liver fibrosis, and use of antiplatelet agents. Serum for quantification of PDGF‐β concentration was available in a subgroup of 266 patients of the entire study population. There was a significant correlation between platelet counts and PDGF‐β serum concentration (rho, 0.33; P < 0.0001) (Fig. 2A). Although there was a moderate correlation between platelet counts and the degree of liver fibrosis (defined as FibroScan TE value rho, –20; P < 0.0001), there was no significant correlation between liver fibrosis and PDGF‐β serum concentration (rho, –0.07; P = 2). However, the ratio of PDGF‐β to platelets was significantly higher in patients with liver fibrosis compared to patients without liver fibrosis (7.3 versus 6.5, respectively; P = 0.048) (Fig. 2B); yet, PDGF‐β serum levels did not differ between patients who were treated or were not treated with antiplatelet agents (1,346 versus 1,439 pg/mL, respectively; P = 0.2).
Figure 2.
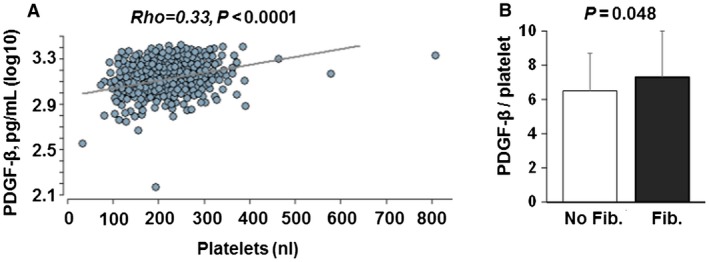
Relationship between PDGF‐β serum concentration and platelet counts. (A) PDGF‐β serum concentrations correlate with platelet counts, suggesting that platelets are a source of PDGF‐β in humans. (B) Ratio of PDGF‐β serum concentration divided through platelet count is higher in patients with liver fibrosis compared to patients without liver fibrosis. Mean values and standard deviations are shown. Abbreviation: Fib, fibrosis.
Discussion
In the present study we assessed the relationship between the use of antiplatelet agents and the presence of liver fibrosis in a large cohort of patients at risk for cardiovascular events. Our main finding was an inverse association between the use of antiplatelet agents and the presence and degree of liver fibrosis. Furthermore, there was a significant correlation between platelet levels and serum concentration of PDGF‐β, a key driver of liver fibrosis in humans and mice. However, the use of antiplatelet agents had no impact on PDGF‐β serum levels. Hence, the mechanistic basis of a possible causal protective role of antiplatelet agents in the progression of liver fibrosis remains to be elucidated.
Emerging data have revealed a pathogenic role of platelets in the pathogenesis of liver fibrosis and inflammation.9 Platelets sequestrate within hepatic sinusoids during liver injury and interact with hepatic sinusoidal epithelial cells (LSECs), which results in the release of a large number of mediators, such as chemokines, cytokines, growth factors, lipid mediators, or procoagulants.18 By releasing these mediators, platelets recruit and activate inflammatory cells, including granulocytes, macrophages, and T cells, to the liver and thereby perpetuate liver inflammation.19, 20 Furthermore, platelet‐derived mediators, such as PDGF‐β, are potent inducers of HSC transformation to profibrotic myofibroblasts.17 Additionally, platelets are fundamental regulators of plasma serotonin concentrations,21 and serotonin results by activation of HSCs and LSECs and the subsequent vasoconstriction to reduced hepatic microcirculation.10, 11 These and other data suggest that platelets could be attractive targets for antifibrotic and anti‐inflammatory therapy of liver diseases. Indeed, a significant number of studies in animal models, such as models of viral hepatitis or NALFD, platelet depletion, or application of the platelet activation inhibitors aspirin or clopidogrel, reduced infiltration of virus‐specific T cells and ameliorated hepatic inflammation, fibrosis progression, and hepatocellular carcinoma development.22, 23, 24 However, data on the impact of antiplatelet agents on liver disease in humans are scarce.25 In this regard, the results of our prospective study in a collective of patients that included a large number of individuals on antiplatelet agents contribute important data to the literature. Although results of our study are only of an associative nature and antiplatelet agents were not administered with the aim of targeting liver fibrosis, our data further substantiate the hypothesis that platelets could be attractive targets to prevent liver fibrosis progression. Nevertheless, we cannot exclude that the observed associations are due to cofactors, such as the intake of statins, as there was a strong positive correlation between the use of statins and antiplatelet agents in our cohort. Hence, randomized studies are needed and should be encouraged to definitively answer this important question.
It is known that acetyl salicylic acid and P2Y12 receptor antagonists inhibit the release of bioactive mediators, including serotonin, from the platelets’ dense granules as well as inhibit the cellular interaction between platelets and epithelial cells or inflammatory cells26; yet, our finding that PDGF‐β serum concentration is strongly dependent on platelet counts whereas the use of antiplatelet agents has no impact on PDGF‐β serum concentration suggests that acetyl salicylic acid and P2Y12 receptor antagonists may not fully reverse the profibrotic potential of platelets. It is known that other platelet receptors, such as clusters of differentiation (CD)44, can mediate platelet functions despite cyclooxygenase‐1 inhibition or P2Y12 receptor blockade.27 Furthermore, endotoxin translocation from the intestines is a hallmark of advanced liver fibrosis/cirrhosis and endotoxin has been identified is a profound activator of platelets in patients with liver cirrhosis.28 We speculate that the higher ratio of PDGF‐β serum concentration to platelet count in patients with liver fibrosis observed in our study may be explained by such a mechanism.
Our study has several limitations. First, our data cannot confirm a causal role of antiplatelet therapy in the protection from liver fibrosis. For example, patients on antiplatelet agents were more frequently on concomitant statin therapy, which may confound our observations, although no association between statin use and liver fibrosis could be detected. Nevertheless, the results of our study further substantiate the need for a randomized trial of antiplatelet agents in the prevention of liver fibrosis progression. Furthermore, the diagnosis of liver fibrosis was not based on liver biopsy results as this would have been clinically inappropriate in individuals on antiplatelet agents who are at relatively high risk of bleeding complications from liver biopsy. Moreover, the results of our study cannot be extrapolated to patients with advanced liver disease because platelets appear to play an important role in liver regeneration29 that may be lifesaving in patients with acute or acute‐on‐chronic liver failure.
In conclusion, our study reveals a protective association between the use of antiplatelet agents and the occurrence of liver fibrosis in a prospective cohort study of patients at high risk of cardiovascular events. A randomized controlled trial is needed to explore the potential of antiplatelet agents as antifibrotic therapy in patients at risk for liver fibrosis progression.
Potential conflict of interest
Dr. Zeuzem consults for, advises, and is on the speakers’ bureau for AbbVie, Gilead, Merck, and RSD. Dr. Mücke has received grants from Gilead, AbbVie, and Intercept. The other authors have nothing to report.
Supporting information
Supported by the Deutsche Forschungsgemeinschaft (LA 2806/2‐1 and LA 2806/5‐1 to C.M.L.).
References
- 1. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263‐2273. [DOI] [PubMed] [Google Scholar]
- 2. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015;62:S47‐S64. [DOI] [PubMed] [Google Scholar]
- 3. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol 2017;14:32‐42. [DOI] [PubMed] [Google Scholar]
- 4. Dogan S, Celikbilek M, Yilmaz YK, Sarikaya S, Zararsiz G, Serin HI, et al. Association between liver fibrosis and coronary heart disease risk in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2015;27:298‐304. [DOI] [PubMed] [Google Scholar]
- 5. Treeprasertsuk S, Bjornsson E, Enders F, Suwanwalaikorn S, Lindor KD. NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World J Gastroenterol 2013;19:1219‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sookoian S, Pirola CJ. Non‐alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol 2008;49:600‐607. [DOI] [PubMed] [Google Scholar]
- 7. European Association for the Study of the Liver (EASL) ; European Association for the Study of Diabetes (EASD) ; European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO clinical practice guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 8. Roeb E, Steffen HM, Bantel H, Baumann U, Canbay A, Demir M, et al. S2k guideline non‐alcoholic fatty liver disease. Z Gastroenterol 2015;53:668‐723. [DOI] [PubMed] [Google Scholar]
- 9. Chauhan A, Adams DH, Watson SP, Lalor PF. Platelets: no longer bystanders in liver disease. Hepatology 2016;64:1774‐1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruddell RG, Mann DA, Ramm GA. The function of serotonin within the liver. J Hepatol 2008;48:666‐675. [DOI] [PubMed] [Google Scholar]
- 11. Brauneis U, Gatmaitan Z, Arias IM. Serotonin stimulates a Ca2+ permeant nonspecific cation channel in hepatic endothelial cells. Biochem Biophys Res Commun 1992;186:1560‐1566. [DOI] [PubMed] [Google Scholar]
- 12. Friedrich‐Rust M, Schoelzel M, Maier S, Seeger F, Rey J, Fichtlscherer S, et al. Severity of coronary artery disease is associated with non‐alcoholic fatty liver disease: a single‐blinded prospective mono‐center study. PLoS One 2017;12:e0186720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ratziu V, Massard J, Charlotte F, Messous D, Imbert‐Bismut F, Bonyhay L, et al. LIDO Study Group; CYTOL study group. Diagnostic value of biochemical markers (FibroTest‐FibroSURE) for the prediction of liver fibrosis in patients with non‐alcoholic fatty liver disease. BMC Gastroenterol 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karlas T, Petroff D, Garnov N, Bohm S, Tenckhoff H, Wittekind C, et al. Non‐invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H‐MR spectroscopy. PLoS One 2014;9:e91987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine‐treated rats. Hepatology 2000;32:247‐255. [DOI] [PubMed] [Google Scholar]
- 16. Czochra P, Klopcic B, Meyer E, Herkel J, Garcia‐Lazaro JF, Thieringer F, et al. Liver fibrosis induced by hepatic overexpression of PDGF‐B in transgenic mice. J Hepatol 2006;45:419‐428. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida S, Ikenaga N, Liu SB, Peng ZW, Chung J, Sverdlov DY, et al. Extrahepatic platelet‐derived growth factor‐beta, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology 2014;147:1378‐1392. [DOI] [PubMed] [Google Scholar]
- 18. Lalor PF, Herbert J, Bicknell R, Adams DH. Hepatic sinusoidal endothelium avidly binds platelets in an integrin‐dependent manner, leading to platelet and endothelial activation and leukocyte recruitment. Am J Physiol Gastrointest Liver Physiol 2013;304:G469‐G478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood 2014;123:2759‐2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, et al. Platelets mediate cytotoxic T lymphocyte‐induced liver damage. Nat Med 2005;11:1167‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol Interv 2010;10:231‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujita K, Nozaki Y, Wada K, Yoneda M, Endo H, Takahashi H, et al. Effectiveness of antiplatelet drugs against experimental non‐alcoholic fatty liver disease. Gut. 2008;57:1583‐1591. [DOI] [PubMed] [Google Scholar]
- 23. Assy N, Hussein O, Khalil A, Luder A, Szvalb S, Paizi M, et al. The beneficial effect of aspirin and enoxaparin on fibrosis progression and regenerative activity in a rat model of cirrhosis. Dig Dis Sci 2007;52:1187‐1193. [DOI] [PubMed] [Google Scholar]
- 24. Sitia G, Iannacone M, Guidotti LG. Anti‐platelet therapy in the prevention of hepatitis B virus‐associated hepatocellular carcinoma. J Hepatol 2013;59:1135‐1138. [DOI] [PubMed] [Google Scholar]
- 25. Poujol‐Robert A, Boelle PY, Conti F, Durand F, Duvoux C, Wendum D, et al. Aspirin may reduce liver fibrosis progression: evidence from a multicenter retrospective study of recurrent hepatitis C after liver transplantation. Clin Res Hepatol Gastroenterol 2014;38:570‐576. [DOI] [PubMed] [Google Scholar]
- 26. Muller KA, Chatterjee M, Rath D, Geisler T. Platelets, inflammation and anti‐inflammatory effects of antiplatelet drugs in ACS and CAD. Thromb Haemost 2015;114:498‐518. [DOI] [PubMed] [Google Scholar]
- 27. Guidotti LG, Inverso D, Sironi L, Di Lucia P, Fioravanti J, Ganzer L, et al. Immunosurveillance of the liver by intravascular effector CD8(+) T cells. Cell 2015;161:486‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raparelli V, Basili S, Carnevale R, Napoleone L, Del Ben M, Nocella C, et al. Low‐grade endotoxemia and platelet activation in cirrhosis. Hepatology 2017;65:571‐581. [DOI] [PubMed] [Google Scholar]
- 29. Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, et al. Platelet‐derived serotonin mediates liver regeneration. Science 2006;312:104‐107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


