Abstract
Intestinal permeability to translocation of bacterial products is increased in cirrhosis. Regulatory T cells (Tregs) remain central to the interplay between the host and microbial milieu. We propose that Tregs are involved in promoting gut barrier integrity and a balanced interaction with gut microbiota–derived short‐chain fatty acids (SCFAs). Carbon tetrachloride cirrhosis was induced in wild‐type and recombination activating gene 1 (Rag1)‐/‐ mice. Naive T cells and Treg cells were transferred into Rag1 ‐/‐ mice. Intestinal permeability was assessed in vivo after lipopolysaccharide (LPS) oral administration, and bacterial DNA presence was evaluated in mesenteric lymph nodes. Transcript and protein levels of tight‐junction (TJ) proteins were measured in colonic tissue. Intestinal T helper profile in response to Escherichia coli (E. coli) was determined by flow cytometry. SCFAs were measured by gas chromatography–mass spectrometry in colonic content before and after E. coli challenge. Rag1 ‐/‐ mice showed significantly increased permeability to LPS and bacterial DNA translocation rate compared with control mice. Naive T and Treg cotransfer significantly reduced gut permeability to bacterial antigen translocation and restored TJ protein expression in Rag1 ‐/‐ mice. Naive T and Treg replenishment in Rag1 ‐/‐ mice restrained proinflammatory differentiation of intestinal lymphocytes in response to E. coli. The main SCFA concentration resulted in significant reduction in Rag1 ‐/‐ mice after E. coli administration but remained unaltered after naive T and Tregs cotransfer. The reduced expression of SCFA receptors induced by E. coli was reestablished following naive T and Treg reconstitution in Rag1 ‐/‐ mice. Conclusion: The restriction of gut permeability, local inflammatory differentiation, and loss of bacteria‐derived SCFAs foster the value of Tregs in preventing bacterial translocation in cirrhosis.
Abbreviations
- BT
bacterial translocation
- CCL4
carbon tetrachloride
- DCs
dendritic cells
- E. coli
Escherichia coli
- FFAR
free fatty acid receptor
- FITC
fluorescein isothiocyanate
- HBSS
Hank’s balanced salt solution
- IFN
interferon
- IHC
immunohistochemical
- IL
interleukin
- LPS
lipopolysaccharide
- MLN
mesenteric lymph node
- mRNA
messenger RNA
- PBS
phosphate‐buffered saline
- PCR
polymerase chain reaction
- Rag1
recombination activating gene 1
- SCFAs
short‐chain fatty acids
- TJ
tight‐junction
- Tregs
regulatory T cells
- WB
western blot
- WT
wild type
- ZO‐1
zonula occludens
Patients with cirrhosis are frequently threatened with bacterial infections that aggravate their prognosis.1, 2 The increased exposure to bacterial antigens is due to a pathological translocation from the gut.3, 4 Although living microorganisms may translocate and be responsible for severe infections such as spontaneous bacterial peritonitis,5 bacterial products have also been demonstrated to cross the gut barrier and be associated with poor disease outcomes.6, 7
The immunological response to increased bacterial antigen translocation rates in cirrhosis and other settings involves a decompensation toward a proinflammatory profile in which dendritic cells (DCs), monocytes, and neutrophils are recruited to the injured area.8, 9 As a consequence of this polarization, the tolerogenic response that balances out the inflammation is likely compromised. In fact, the immune homeostasis is lost during progression of cirrhosis. The so‐called cirrhosis‐associated immune dysfunction helps induce intestinal inflammation, suggesting a plausible role for regulatory immune cells and anti‐inflammatory cytokines in this context.10 Intestinal inflammation leads to increased intestinal permeability, which has been demonstrated in cirrhosis,11, 12, 13 and is key in the translocation of small bacterial antigens that, in turn, are able to induce and/or perpetuate a sustained inflammatory environment.14, 15 In this regard, tight‐junction (TJ) proteins, a family of more than 50 members, play an important role in preventing any paracellular breach16 by which these bacterial antigens may enter the sterile intraepithelial area.
It is well established that normal bacterial colonization is needed for the development of the intestinal barrier.17, 18 Short‐chain fatty acids (SCFAs) are produced by the bacterial fermentation of undigested dietary carbohydrates in the intestine and act as the main energy source of colonocytes. SCFA products of commensal microorganisms are important functional players in the maintenance of intestinal barrier integrity. In particular, butyrate is a considerable TJ regulator due to its ability to increase expression of zonula occludens (ZO‐1) and claudin‐1 as well as influence occluding redistribution in vitro. 19 These metabolic intermediaries also work as communicators between the intestinal microbiota and the immune system by promoting extrathymic differentiation of regulatory T cells (Tregs).20 SCFA receptors, G protein–coupled receptor (GPR)43/free fatty acid receptor 2 (FFAR2) and GPR42/FFAR3, are expressed on the innate immune cells and participate in regulation of the inflammatory response.21, 22
Treg cells are an essential immune population that interferes with effector T lymphocytes and DCs and provides an immunosuppressive counterpart.23, 24, 25, 26 We have described in the past that the cellular proinflammatory response in patients with cirrhosis receiving norfloxacin was modulated by an interleukin (IL)‐10‐mediated mechanism.27 Trying to understand the role of IL‐10 in the cirrhotic context, we also reported that IL‐10 was required in experimental cirrhotic models to restore normal gut barrier permeability and to lower luminal free endotoxin absorption.28 Among adaptive IL‐10‐producing cells, Treg cells are especially relevant because of their homeostatic role in the host–microbe interaction. We have identified increased rates of Treg cells in patients on selective intestinal decontamination, their correlation with serum norfloxacin concentrations, and their role as relevant IL‐10–secreting cells in these patients,29 supporting their implication in norfloxacin‐derived down‐regulation of serum proinflammatory levels in cirrhosis observed in the past.14 In addition, this cell type is implicated in gut homeostatic functions, as their role in modulating SCFAs concentration in the gut microbiota has been reported.30
In the present study, our specific aim is to evaluate whether the Tregs effect on reduced bacterial antigen translocation might be associated with an improvement in the gut barrier integrity. The purpose of the study, therefore, was to evaluate the contribution of Tregs on gut barrier integrity in the context of experimental cirrhosis. We propose that Treg cells help maintain a reduced gut permeability by promoting the barrier integrity and favoring a balanced interaction with gut microbiota anti‐inflammatory products such as SCFAs in response to episodes of bacterial antigen translocation.
We performed adoptive transfer experiments in a recombination activating gene 1 (Rag1 ‐/‐)‐deficient mice model of carbon tetrachloride (CCl4)‐induced cirrhosis to evaluate gut barrier integrity markers, gut permeability, bacterial translocation (BT) to mesenteric lymph nodes (MLNs), and the immunological status of intestinal lymphocytes as a key cell population in maintaining gut homeostasis.
Animals and Methods
Mice
Male C57Bl/6J wild‐type (WT) and Rag1 ‐/‐ mice on C57Bl/6J background (Jackson Laboratories, Bar Harbor, MN) were included in 16‐week cirrhosis‐induced protocols. Immunodeficient Rag1 ‐/‐ mice have a defective VDJ (variable, diversity, and joining) recombination, so the immune adaptive response is altered by the lack of production of mature T cells and B cells. All of the animals were subjected to a week of quarantine before the cirrhosis protocol. We selected Rag1‐/‐ mice, which are a T cell–free mouse model, to induce liver toxicity with a classical CCl4 protocol. The cirrhotic Rag‐/‐ mice let us evaluate the activation of sorted naive T cells and Tregs from WT animals. Mice were fed a standard rodent chow and kept at a constant room temperature of 21°C in a 12:12 light/dark cycle. The cirrhosis protocol began with the treatment of animals with 0.25 mmol/L phenobarbital in tap water that was maintained along the study protocol. After 4 weeks, the animals received 2 weekly weight‐controlled doses of CCl4 by oral gavage for 12 weeks. The first CCl4 dose was 100 μL/kg (2 μL per mouse) in mineral oil, and subsequent doses were adjusted based on changes in weight 48 hours after the previous dose, up to 100 μL per mouse. A group of naive WT mice not subjected to the CCl4 protocol was used as a negative control for evaluating fibrosis.
Sample size was calculated according to preliminary TJ protein gene expression levels obtained by our group and published in abstract format (Juanola et al. Hepatology 2016;64:83A). Assuming an α error of 0.017, a β error of 0.2, an equal distribution of subjects between groups (0.5 each), and an observed mean difference (E) of colonic occludin gene expression between groups of 1 with a SD of 0.3, 4 animals per group per protocol were required. Typically, mortality rates in our work are about 20%. We increased the number of Rag1 ‐/‐ mice compared with control mice to prevent possible cell‐transfer failures or an increased mortality rate. We included 40 to 45 mice in each protocol (30‐35 Rag1 ‐/‐ and 10 WT) to make sure we were able to work with at least 5 to 6 mice per group.
Rag1 ‐/‐ cirrhotic mice were subjected to adoptive transfer experiments 48 hours before laparotomies, as described.29 A group of 24 male C57Bl/6 WT mice not subjected to cirrhosis induction protocol were used for isolating spleen‐derived sorted naive T (CD4+CD25‐CD45RBhigh) cells and Treg (CD4+CD25+CD45RBlow) cells for these experiments. Postsort purity was typically more than 98%. Rag1 ‐/‐ cirrhotic animals were classified into three groups according to transferred cells: (1) mice not receiving sorted cells; (2) mice transferred with naive T cells (2 × 105 cells per mouse); and (3) mice receiving naive T and Treg cells (1 × 105 each per mouse). Twenty‐four hours prior to laparotomies, a subgroup of cirrhotic Rag1 ‐/‐ animals in each condition received oral Escherichia coli (E. coli), as described subsequently.
Laparotomies were performed under anesthesia with isoflurane. Whole blood was obtained from the cava vein for gut permeability experiments before liver perfusion. Livers were then perfused in situ with 6 mL of Hank’s balanced salt solution (HBSS) without Ca2+ and Mg2+ (Life Technologies Corp., Grand Island, NY) at 37°C at a rate of 1.5 mL per minute. Detectable MLNs were removed, and the liver, spleen, complete small intestine, colon, and content from cecum were collected.
Animals received care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals. The study was approved by the Animal Research Committee of Universidad Miguel Hernández (Alicante, Spain).
Fluorescein Isothiocyanate–LPS Intestinal Permeability Assay
A subgroup of animals in each condition was intragastrically administered 100 µg of fluorescein isothiocyanate (FITC) conjugated LPS from E. coli 0111:B4 or dextran (Sigma‐Aldrich, St. Louis, MO). Gut permeability was evaluated 2 hours after FITC‐LPS or FITC‐dextran administration as the percentage of recovery measured by fluorometry at 488 nm in 100 µL of serum samples collected from the cava vein. Dilutions of FITC‐LPS and FITC‐dextran in phosphate‐buffered saline (PBS) were used for standard curves.
Bacterial DNA Translocation Assessment in MLNs
MLNs were harvested with sterile instruments in sterile PBS at laparotomies. All tissues were disrupted by using the Tissue Lyser LT (QIAgen, Hilden, Germany), and DNA was immediately isolated using the QIAamp DNA Mini Kit. Bacterial DNA was detected by performing a broad‐range polymerase chain reaction (PCR) and partial sequencing analysis of the 16S ribosomal RNA gene, as described.14
Gene Expression Analysis
Total cellular RNA was isolated using the RNeasy Mini Kit (QIAgen). The qScript One‐Step SYBR Green quantitative real‐time PCR Kit (Quanta BioScience, Gaithesburg, MD) was used to perform the gene expression of claudin‐1, claudin‐2, ZO‐1, occludin, collagen type I alpha‐1 chain (Col1a1), tumor growth factor beta (TGF‐β), matrix metalloproteinase 2 (MMP2), tissue inhibitor of metalloproteinase 1 (TIMP1), FFAR2 and FFAR3, and IL‐10 in an IQ5 RT‐PCR (Bio‐Rad, Hercules, CA). β2‐microglobulin was used as a housekeeping gene in all gene expression analyses. Primer pairs used in the study can be followed in Supporting Table S1.
Immunohistochemistry Analysis
Immunohistochemical (IHC) assays were carried out in 4‐µm sections of paraffin‐embedded colon tissue, and processed following standard procedures. The slides were incubated with primary antibodies claudin‐1, claudin‐2, occludin, and ZO‐1 (Abcam PLC, Cambridge, United Kingdom). As secondary antibodies, we incubated the sections with the correspondent α‐goat or α‐rabbit biotinylated antibodies (Palex Medical SA, Sant Cugat del Vallés, Spain). Slides were incubated with avidin‐biotin complex (Vector Laboratories Inc., Burlingame, CA) and revealed with peroxidase substrate 3,3′‐diaminobenzidine (Vector Laboratories Inc.). Nuclei were stained by incubating the sections in Harris hematoxylin (Leica Biosystems Richmond Inc., Richmond, IL). As a negative control, staining was carried out in the absence of a primary antibody. Images were obtained in a camera‐assisted optic Leica DM2000 LED microscope (Leica Biosystems, Richmond Inc.). All represented panels are shown at original magnification ×20. A semiquantitative analysis of protein expression was performed using the ImageJ software (https://rsbweb.nih.gov).
Western Blot Analysis
Colon homogenates were lysed with radioimmunoprecipitation assay buffer and protein concentration determined by Bradford protein assay (EMD Millipore Corp., Billerica, MA). Twenty micrograms of protein extracts were resolved under reducing conditions on 6% to 15% sodium dodecyl sulfate–polyacrylamide gels and transferred to Immobilon‐P membranes (EMD Millipore Corp.). Primary antibodies used are β‐actin (Sigma‐Aldrich), ZO‐1 (Thermo Fisher Scientific, Waltham, MA), and claudin‐1, claudin‐2, and occludin (Abcam PLC). Finally, membranes were incubated with the appropriate horseradish peroxidase (HRP)–conjugated secondary antibody (Cell Signaling Technology, Leiden, Netherlands). Immobilon Western Chemilum HRP Substrate (EMD Millipore Corp.) was used to detect the activity of the membrane‐attached peroxidase, and images were obtained in ChemiDOC XRS+ operated by Image Lab software (Bio‐Rad). Protein bands were quantified by densitometry using Scion Image software (Scion Corp., Frederick, MD). Band densities were expressed relative to total β‐Actin protein.
E. Coli Culture Conditions and Administration to Mice
E. coli (CECT45) was grown in 9 mL of thioglycolate broth with resazurin (Biomérieux, Marcy l’Etoile, France) and incubated overnight at 37°C. Serial dilutions of incubated bacteria were performed before plating them in McConkey agar (Biomérieux) overnight at 37°C. Colony‐forming units (CFUs) were then counted, and cells were resuspended in PBS. Each animal was administered 1 × 106 CFUs orally.
Intestinal Lymphocyte Isolation and Th Differentiation
The whole small intestine from E. coli–challenged cirrhotic Rag1 ‐/‐ mice was obtained. Peyer’s patches, fatty tissue, and mesentery were removed from all intestines. The gut was cleaned using cold PBS without Ca2+ and Mg2+ (Euroclone, Milano, Italy), opened longitudinally, and cut into 1‐cm pieces. Intraepithelial lymphocytes (IELs) were obtained by incubating the small intestine pieces twice with HBSS Ca2+ and Mg2+ free (Life Technologies Corp.) supplemented with 5 mM ethylene diamine tetraacetic acid (Bio‐Rad), 1 mM dithiothreitol (Sigma‐Aldrich), and 1% penicillin‐streptomycin (Life Technologies Corp.) in Incubating Orbital Shake (VWR, Llinars del Vallés, Spain) for 30 minutes at 37°C. Thereafter, the tissue pieces were washed with PBS and then incubated three times in HBSS with 0.5 mg/mL collagenase D (Roche Diagnostics GmbH, Mannheim, Germany), 3 mg/mL dispase II (Sigma‐Aldrich), 1 mg/mL DNase I (Roche Diagnostics GmbH), and 1% penicillin‐streptomycin with orbital agitation for 30 minutes at 37°C to collect the lamina propria lymphocyte (LPL) cells. All supernatant fractions were filtered with 70‐µm nylon cell strainers (Corning Incorporated Life Sciences, Oneonta, NY), washed with PBS 3% fetal bovine serum (FBS) (Life Technologies Corp.), and centrifugated to harvest cell suspensions. IELs and LPLs were pooled and constituted the intestinal lymphocyte content. Live lymphocytes were obtained by preparing a 40%/80% Percoll (Sigma‐Aldrich) gradient and then cultured in advanced Roswell Park Memorial Institute (RPMI)‐1640 (Life Technologies Corp.) supplemented with 10% FBS, 1% L‐glutamine (Life Technologies), and 1% penicillin‐streptomycin. Cells were treated and marked according to manufacturer’s instructions in the Mouse Th1/Th2/Th17 Phenotyping Kit (BD Biosciences, San Diego, CA). To induce a complete T‐cell activation, E. coli was administered according to the conditions described here. Th differentiation was evaluated as intracellular production of interferon (IFN)‐γ, IL‐4, and IL‐17. Data acquisition and analysis of marked intestinal lymphocytes were performed using a FACSCanto II flow cytometer operated by FACSDiva software (BD Biosciences).
Gas Chromatography–Mass Spectrometry Analysis of SCFAs in Intestinal Content
Intestinal content samples with PBS were homogenized, and 300 µL were mixed with 300 µL of 0.5% phosphoric acid. Intestinal content suspensions were sonicated in an ultrasonic bath for 5 minutes and centrifuged for 10 minutes at 17,949g. The aqueous supernatant was extracted with the same volume of methyl tert‐buthyl ether for 5 minutes and centrifuged in the same conditions. The upper organic phase was transferred into a tube and 4‐methyl valeric acid added as internal standard at a final concentration of 500 µM. Gas chromatography–mass spectrometry (GC‐MS) system and chromatographic and mass parameter conditions were followed, as reported.31
Statistical Analysis
Continuous variables are reported as mean ± SD and categorical variables as frequency or percentages. Quantitative data were analyzed using the Mann‐Whitney U test for simple comparisons with the post hoc Bonferroni correction for multiple comparisons. Differences in qualitative variables were analyzed using the χ2 test. All reported P values are 2‐sided, and P values less than 0.05 indicate significance. All calculations were performed using SPSS Statistics 19 (IBM, Chicago, IL).
Results
Cirrhosis Induction Protocols and Mice Groups
Five independent protocols of CCl4‐induced cirrhosis were run to complete the different experiments performed. Each protocol included a group of WT mice and three groups of Rag1 ‐/‐ mice (nontransferred Rag1 ‐/‐ mice, naive T cell–transferred Rag1 ‐/‐ mice, and naive T plus Treg cell–cotransferred Rag1 ‐/‐ mice). Table 1 lists the number of mice included in each experiment distributed by groups and protocol.
Table 1.
Protocols and Number of Mice in Different Experimental Procedures Distributed by Study Groups
| Protocol | Experiments | Number of CCI4 mice/group | Total number of CCI4 mice/protocol | |||
|---|---|---|---|---|---|---|
| WT | Rag1 ‐/‐ | Rag1 ‐/‐ + naïve T | Rag1 ‐/‐ + naïve T + Treg | |||
| 1 | FITC‐LPS permeability assays | 6 | 8 | 8 | 8 | 30 |
| 2 | FITC‐dextran permeability assays | 6 | 8 | 8 | 8 | 30 |
| 3 | TJ protein IHCs in colonic tissue | 6 | 8 | 8 | 8 | 30 |
| 4 | TJ proteins mRNA and WB in colonic tissue BT in MLNs | 6 | 8 | 8 | 8 | 30 |
| FFARs mRNA in colonic tissue (except in C57BI/6) | ||||||
| SCFAs in colonic content (except in C57BI/6) | ||||||
| 5 | Th differentiation in colonic ILs in response to E. coli | ‐ | 8 | 8 | 8 | 24 |
| FFARs mRNA in colonic tissue in response to E. coli | ||||||
| SCFAs in colonic content in response to E. coli | ||||||
Gene expression levels of profibrogenic markers were evaluated in all CCl4 protocols and combined in Supporting Fig. S1. Results in Rag1 ‐/‐ mice were similar to those present in WT mice and significantly increased compared with WT mice without liver damage.
Gut Permeability to LPS Is Reduced in Cirrhotic Rag1‐/‐ Mice Cotransferred With Treg Cells
We aimed to study in vivo gut permeability to orally administered FITC‐LPS. The results of this functional test are described in Fig. 1A. Rag1 ‐/‐ mice showed a significantly increased permeability to FITC‐LPS compared with WT mice. The recovery rate of LPS in Rag1 ‐/‐ mice was not reduced after injection with spleen‐derived naive T cells. On the contrary, only the coinjection of naive T and Treg cells significantly reduced gut permeability to FITC‐LPS. In agreement with this, the translocation of bacterial DNA to MLNs of Rag1 ‐/‐ mice cotransferred with naive T and Treg cells was significantly down‐regulated to levels present in WT cirrhotic mice (Fig. 1B).
Figure 1.
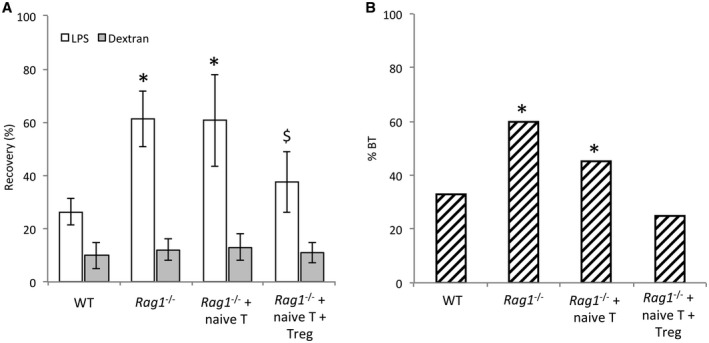
In vivo gut permeability assay in WT and Rag1 ‐/‐ cirrhotic mice study groups. (A) FITC‐LPS and FITC‐dextran recovery percentages in blood 2 hours after their intragastrical administration. (B) Bacterial DNA translocation rates in MLNs of study groups. *P < 0.05 compared with WT cirrhotic mice in both panels. § P < 0.05 compared with other Rag1 ‐/‐ groups in both panels.
Treg Cells Are Associated With a Restoration of TJ Protein Expression in Cirrhotic Rag1‐/‐ Mice
The observed reduction in gut permeability led us to study the status of TJ proteins in cirrhotic mice. The expression of TJ proteins ZO‐1, occludin, claudin 1, and claudin 2 was measured by gene expression, IHC, and western blot (WB) in all study groups (Fig. 2). Messenger RNA (mRNA) levels of all studied genes were significantly decreased in cirrhotic Rag1 ‐/‐ mice either nontransferred or transferred with naive T cells compared with cirrhotic WT mice. The cotransfer with naive T and Treg cells restored transcript expression of all genes to levels shown in cirrhotic WT mice (Fig. 2A).
Figure 2.
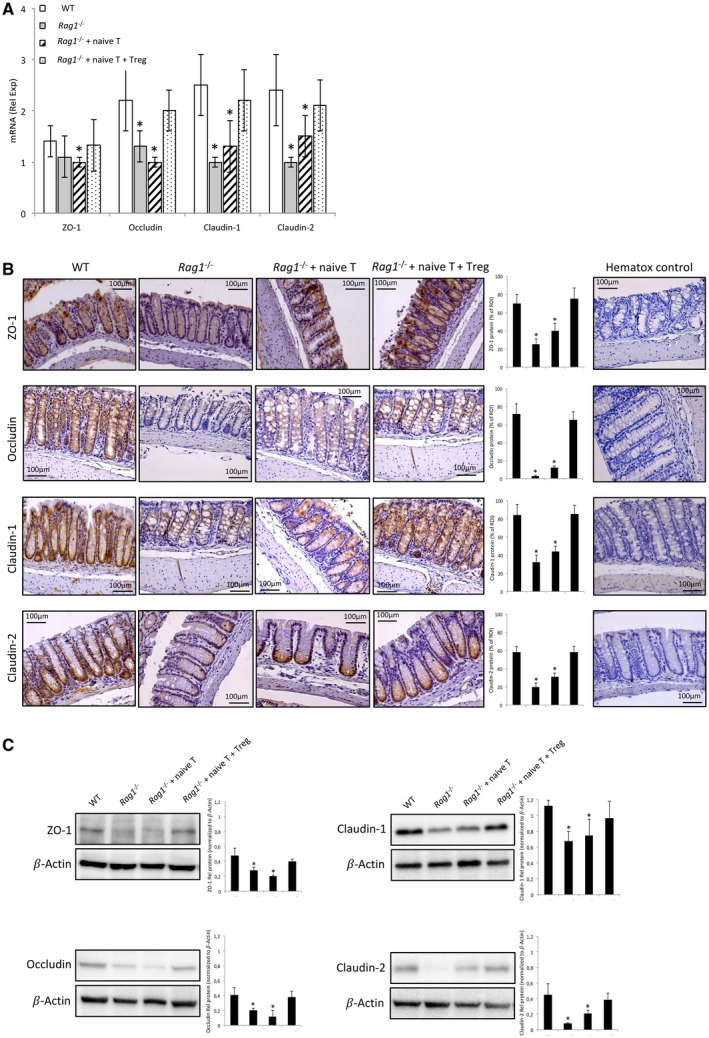
TJ protein expression in WT and Rag1 ‐/‐ cirrhotic mice study groups. (A) mRNA transcripts relative expression of ZO‐1, occludin, claudin‐1, and claudin‐2 in colon tissue homogenates. (B) Representative images of colonic tissue sections from the different study groups. Protein expression was blindly measured in user‐specified regions of interest (ROIs) as percent brown area in hematoxylin‐stained colon samples using the ImageJ software (http: //rsbweb.nih.gov). The mean and SDs from three independent ROIs in each sample are represented in bar graphs. Negative control hematoxylin‐stained sections without primary antibody are also shown. (C) Western blot analysis of ZO‐1, occludin, claudin‐1, and claudin‐2 in colon tissue homogenates. Band densitometry analysis shows the mean and SDs from four independent blots run for each protein. *P < 0.05 compared with cirrhotic WT mice in all panels.
Cirrhotic Rag1 ‐/‐ mice without any transferred cells showed a significant reduction in the protein expression of the different TJ proteins compared with the cirrhotic WT, as shown by IHC and WB analyses (Fig. 2B,C, respectively). None of the TJ protein levels significantly increased when these mice were transferred with naive T cells. However, the cotransfer with naive T and Treg cells restored all TJ protein expression to the levels shown in WT. Visually observed differences between groups in TJ protein expression were shown to be statistically significant after a semiquantitative analysis of IHC and WB images.
Transferred Treg Cells Modulate IL Proinflammatory Th1 and Th17 Responses to Oral E. Coli Administration in Cirrhotic Rag1‐/‐ Mice
Because Treg cells are functionally required to maintain gut barrier integrity and reduced permeability in CCl4‐cirrhotic mice, we were interested in evaluating whether transferred Treg cells in cirrhotic Rag1 ‐/‐ mice restrained the induced proinflammatory Th differentiation as a possible explanation for the function of Treg cells in gut barrier improvement. Figure 3A shows the flow cytometry gating strategy for determining Th subpopulations in ILs of Rag1 ‐/‐ mice in response to oral E. coli administration. Rag1 ‐/‐ mice showed a low Th differentiation from residual intestinal CD4+ cells, as expected (Fig. 3B). The injection of T naive cells induced exacerbated proinflammatory Th1 and Th17 responses that were significantly down‐regulated by the cotransfer with Treg cells. The Th2 profile was not altered in any experimental condition.
Figure 3.
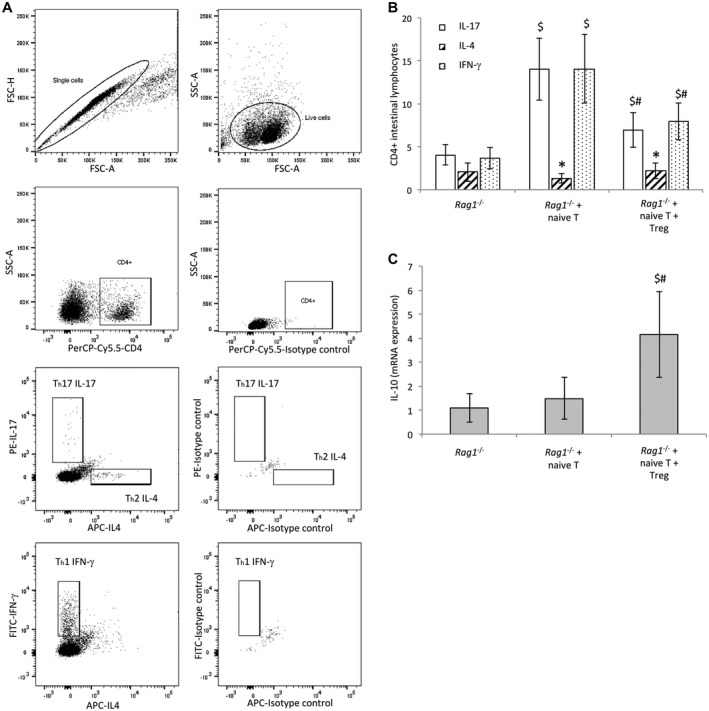
(A) Flow cytometry strategy for gating of T helper subpopulations. T helper differentiation was evaluated as intracellular production of IFN‐γ, IL‐4, and IL‐17. Isotype‐matched controls are also shown. (B) Percentage of CD4+ intestinal lymphocytes with intracellular secretion of IFN‐γ, IL‐4, or IL‐17 in response to E. coli in the different groups of Rag1 ‐/‐ cirrhotic mice. (C) mRNA expression of IL‐10 in intestinal lymphocytes isolated from the different groups of Rag1 ‐/‐ cirrhotic mice. *P < 0.01 compared with the other conditions within the same group. § P < 0.01 compared with nontransferred Rag1 ‐/‐ group. # P < 0.01 compared with naive T–transferred Rag1 ‐/‐ group. Abbreviations: APC, allophycocyanin; FSC‐A, forward scatter area; FSC‐H, forward scatter height; PE, phycoerythrin; PerCP‐Cy5.5, peridinin‐chlorophyll‐protein complex‐Cy5.5 conjugate; and SSC, side scatter.
Because Treg cells exert their action through IL‐10 production, we set out to determine transcript expression levels of IL‐10 in intestinal lymphocytes. IL‐10 levels in cirrhotic Rag1 ‐/‐ mice cotransferred with naive T and Treg cells were significantly increased in response to E. coli compared with the rest of the groups, providing a counterbalance for the observed Th proinflammatory polarization (Fig. 3C).
Treg Cells Contribute to Sustain SCFA Levels in the Intestinal Content of Cirrhotic Rag1‐/‐Mice After Exposure to E. Coli
Microbiota products such as SCFAs also play a role in maintaining gut homeostasis. We evaluated whether Treg cells are also relevant in SCFA modification in response to E. coli, as a model of induced BT, in the intestinal content of cirrhotic Rag1 ‐/‐ mice. The concentration of all measured SCFAs resulted in a significant reduction after E. coli administration, but for acetic acid in nontransferred Rag1 ‐/‐ mice. The concentration of all SCFAs remained unaltered in the intestinal content of cirrhotic Rag1 ‐/‐ mice cotransferred with naive T and Treg cells except for valeric and isovaleric acids (Fig. 4A). The gene expression levels of receptors FFAR2 and FFAR3 for these SCFAs were measured in colonic tissue homogenates (Fig. 4B). Both receptors were significantly reduced in response to E. coli in cirrhotic Rag1 ‐/‐ mice either nontransferred or transferred with naive T cells. The cotransfer with naive T and Treg cells was able to reestablish FFAR2 and FFAR3 levels shown in cirrhotic Rag1 ‐/‐ mice not challenged with E. coli.
Figure 4.
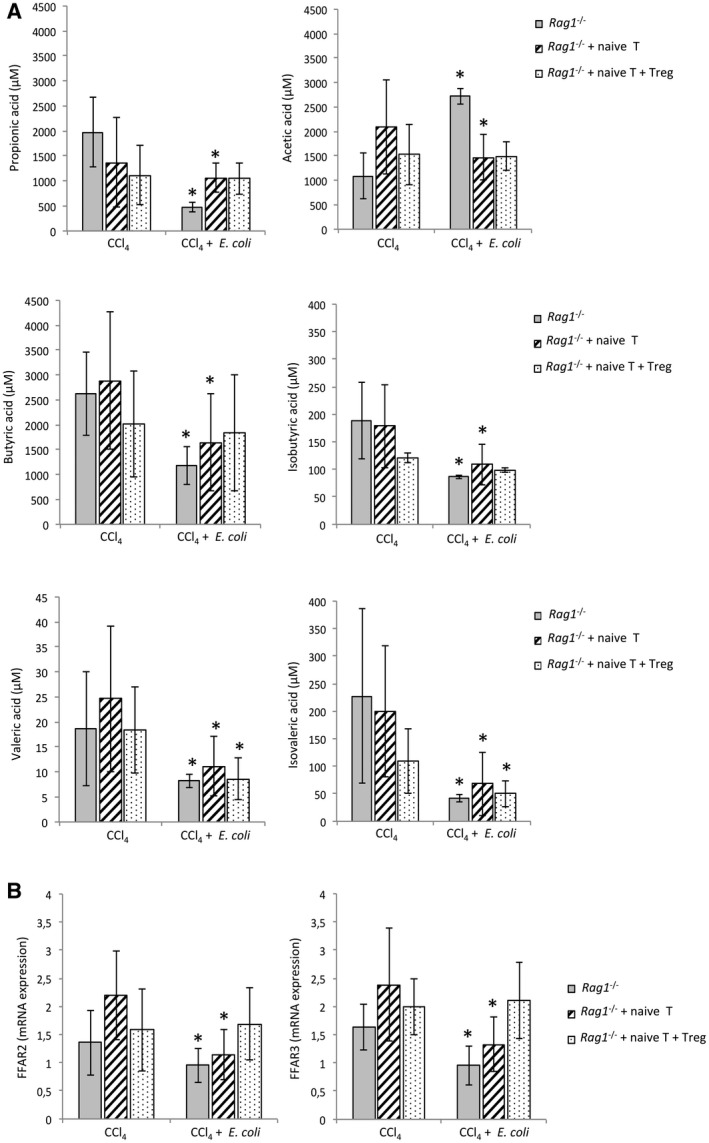
(A) SCFA concentration measured by GC‐MS analysis before and after E. coli intragastrical administration in the colonic content of different groups of Rag1 ‐/‐ cirrhotic mice. (B) mRNA expression of SCFA receptors FFAR2 and FFAR3 in colon tissue homogenates before and after E. coli intragastrical administration in the different groups of Rag1 ‐/‐ cirrhotic mice. *P < 0.01 compared with CCl4‐induced cirrhotic mice without E. coli.
Discussion
In the present study, we show that Treg cells help maintain reduced gut permeability to bacterial antigen translocation in experimental cirrhosis by improving the barrier integrity. Additionally, in response to an induced bacterial challenge, Treg cells restrict an exacerbated proinflammatory Th commitment and preserve the levels of the most representative anti‐inflammatory SCFAs and their receptors. These results give rise to the possibility of designing new interventions aimed at promoting Treg cell differentiation to prevent increased permeability and bacterial antigen translocation episodes in advanced cirrhosis.
BT is a common accepted mechanism for the development of significant bacteria‐related complications in cirrhosis, and its prevention must be considered as a permanent goal during disease progression. In the past, we have reported that Treg cells are relevant to the inverse correlation observed between bacterial antigen translocation and norfloxacin through the anti‐inflammatory cytokine IL‐10 in CCl4‐induced cirrhosis.29 In the present study, we show in vivo that gut permeability to bacterial antigens is sensitive to the Treg cell population in cirrhotic mice, as orally administered LPS recovered in blood is significantly increased in cirrhotic mice lacking adaptive immunity, and it is down‐regulated when these mice are cotransferred with spleen‐derived naive T and Treg cells from WT donors (Fig. 1). As a result, these mice also show significantly decreased rates of bacterial DNA translocation in MLNs compared with nontransferred and naive T transferred Rag1 ‐/‐ mice. It is important to outline that, although T naive transfer into Rag1 ‐/‐ mice is frequently used as an experimental model of spontaneous colitis, which normally takes several weeks to develop, our interest in this model was its intrinsic lack of adaptive immunity. We used it to study the short‐term effect (48 hours before laparotomies) of T‐cell replenishment in a liver damage–induced protocol. Under these conditions, Rag1 ‐/‐ mice do not develop colitis.
We have proposed that inflammation precedes BT, despite the fact that they feedback each other, in cirrhosis.32 In fact, systemic inflammation has been described in the absence of BT in experimental cirrhosis,33 and the reduction of inflammatory environment and improvement of gut barrier integrity markers by means of the Farnesoid X receptor agonist obeticholic acid has been associated with a significant reduction of BT in cirrhotic rats.34 Our results, in line with this evidence, would point to a progressive depletion of the intestinal Treg population that controls local proinflammatory differentiation as a possible mechanism to explain increased BT observed in advanced cirrhosis.
We provide solid data on the Treg cell involvement in the expression of TJ proteins in colon samples of cirrhotic mice (Fig. 2). The important function of these proteins in preventing paracellular movement across the barrier suggests an effect on expression and distribution of these proteins as a relevant mechanism in the association between Treg cells and a reduced permeability to small bacterial products. Although the transcellular passage of bacterial antigens cannot be discarded, the widening of intercellular spaces has been described among the structural changes of the intestinal mucosa, reviewed by Wiest et al.3 A decreased expression of ZO‐1 and occludin in colonic samples of bile duct–ligated mice has been described,35 and reduced expression of TJ proteins has also been reported in duodenal biopsies from patients with cirrhosis.36 Considering that the improved expression of different TJ proteins has been reported in experimental cirrhosis with the use of probiotic strains,37, 38 some of which are known to stimulate Treg cell expression in intestinal mucosa upon interaction with DCs,39 it is plausible that the progressive, cirrhosis‐related inflammatory environment in which Th1 and Th17 CD4+ T cells are differentiated40, 41 is accompanied by the reduction of the Treg population and the decreased expression of TJ proteins. This likely favors increased permeability and BT.
Functionally, the inflammatory control established by Treg cells in response to an oral bacterial challenge is clearly shown in cirrhotic Rag1 ‐/‐ mice (Fig. 3). The lack of Treg cells is associated with an increased percentage of intracellular IFN‐γ and IL‐17 expressing cells. Inversely, intestinal lymphocyte–derived IL‐10 mRNA expression is significantly increased in Treg‐cotransferred Rag1 ‐/‐ mice. T‐cell activation requires the expression of DCs’ costimulatory molecules.42 IL‐10‐increased levels may compromise DCs’ costimulation, switching their phenotype and decreasing effector T‐cell activation. In fact, CD80 and CD86 have shown to be down‐regulated in DCs from norfloxacin‐treated CCl4 mice only after restoration of the Treg cell population.29 The evidence that IL‐10‐deficient cirrhotic mice show increased permeability to bacterial products such as LPS or DNA28 also supports the implication of Treg cells as relevant IL‐10‐producing cells43 in providing the balanced tissue environment to prevent BT episodes in cirrhosis. Whether Tregs can modulate other immune cell populations remains to be addressed in this context and may represent a limitation in this study.
Treg cells stand central to the interplay between the host and microbial milieu.44 Cirrhosis progression is associated with intestinal bacterial overgrowth45 and the gut microbiota dysbiosis toward an increment of Proteobacteria and the reduction of SCFA‐producing species.32, 34 Accordingly, the concentration of propionic, butyric, and isobutyric acids in cirrhotic Rag1 ‐/‐ mice significantly decreased after the oral challenge with E. coli. Treg cells attenuate the SCFA reduction provoked in this condition and contribute to maintain the SCFA receptors FFAR2 and FFAR3 expression in colonic tissue (Fig. 4). Of interest, the concentration of acetic acid was increased after E. coli administration. This may likely respond to the fact that acetic acid is a fermentation product of this bacterial species.46 The induction of Treg cells by cytokine production in response to SCFAs is well established.30, 44, 47 Butyrate and propionate have also been described to promote Treg differentiation. Specifically, butyrate induces repression of LPS‐response genes in DCs and confers them with a higher capacity to promote Treg cell polarization.20 In contrast, Treg cells have also been described to shape the intestinal microbial community, promoting the diversification of commensal microbiota and the generation of immunoglobulin A.44, 48 Our results suggest that Treg cells are important contributors to this regulatory loop, restricting the loss of SCFAs in experimental cirrhosis and supporting the mutual regulation between microbiota and the immune system in the gut.
In summary, increased gut permeability to bacterial antigens in cirrhosis is lessened by Treg cells, which are associated with the expression of TJ proteins in colonic tissue. The improvement of the intercellular sealing likely reduces paracellular translocation of antigenic bacterial products. Treg cell participation in controlling local inflammatory differentiation and bacterial‐derived metabolic products such as SCFAs, which help provide homeostatic conditions, fosters the value of this population in restricting BT episodes in cirrhosis. More studies are needed to establish whether restoring the number of Tregs may have any advantages such as fewer BT episodes or less portal hypertension and liver failure in cirrhosis.
Potential conflict of interest
Nothing to report.
Authors’ contributions
O.J., P.P., I.G.H., E.C.: experimental work, data acquisition, and manuscript writing; R.G.V., A.M., F.T.: short‐chain fatty acids measurements; P.Z., J.M.G.N.: statistical analysis and interpretation of results; F.T.: flow cytometry analysis; and R.F.: study concept and design, manuscript writing.
Supporting information
Supported by Instituto de Salud Carlos III, Madrid, Spain (PI16/0967); Consellería Educación, Generalitat Valenciana, Valencia, Spain (PROMETEO/2016/001); and FEDER funds (EU).
References
- 1. Fernandez J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol 2012;56(Suppl 1):S1‐S12. [DOI] [PubMed] [Google Scholar]
- 2. Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, et al. Bacterial infections in cirrhosis. A position statement based on the EASL Special Conference 2013. J Hepatol 2014;60:1310‐1324. [DOI] [PubMed] [Google Scholar]
- 3. Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol 2014;60:197‐209. [DOI] [PubMed] [Google Scholar]
- 4. Wiest R, Garcia‐Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology 2005;41:422‐433. [DOI] [PubMed] [Google Scholar]
- 5. Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut 2012;61:297‐310. [DOI] [PubMed] [Google Scholar]
- 6. Zapater P, Frances R, Gonzalez‐Navajas JM, de la Hoz MA, Moreu R, Pascual S, et al. Serum and ascitic fluid bacterial DNA: a new independent prognostic factor in noninfected patients with cirrhosis. Hepatology 2008;48:1924‐1931. [DOI] [PubMed] [Google Scholar]
- 7. Bellot P, Garcia‐Pagan JC, Frances R, Abraldes JG, Navasa M, Perez‐Mateo M, et al. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology 2010;52:2044‐2052. [DOI] [PubMed] [Google Scholar]
- 8. Shimizu T, Tani T, Hanasawa K, Endo Y, Kodama M. The role of bacterial translocation on neutrophil activation during hemorrhagic shock in rats. Shock 2001;16:59‐63. [DOI] [PubMed] [Google Scholar]
- 9. Garcia Tsao G, Lee FY, Barden GE, Cartun R, West AB. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology 1995;108:1835‐1841. [DOI] [PubMed] [Google Scholar]
- 10. Albillos A, Lario M, Alvarez‐Mon M. Cirrhosis‐associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014;61:1385‐1396. [DOI] [PubMed] [Google Scholar]
- 11. Campillo B, Pernet P, Bories PN, Richardet JP, Devanlay M, Aussel C. Intestinal permeability in liver cirrhosis: relationship with severe septic complications. Eur J Gastroenterol Hepatol 1999;11:755‐759. [DOI] [PubMed] [Google Scholar]
- 12. Zuckerman MJ, Menzies IS, Ho H, Gregory GG, Casner NA, Crane RS, et al. Assessment of intestinal permeability and absorption in cirrhotic patients with ascites using combined sugar probes. Dig Dis Sci 2004;49:621‐626. [DOI] [PubMed] [Google Scholar]
- 13. Norman K, Pirlich M, Schulzke JD, Smoliner C, Lochs H, Valentini L, et al. Increased intestinal permeability in malnourished patients with liver cirrhosis. Eur J Clin Nutr 2012;66:1116‐1119. [DOI] [PubMed] [Google Scholar]
- 14. Frances R, Zapater P, Gonzalez‐Navajas JM, Munoz C, Cano R, Moreu R, et al. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology 2008;47:978‐985. [DOI] [PubMed] [Google Scholar]
- 15. Caro E, Frances R, Zapater P, Pascual S, Bellot P, Such J. Grade of soluble inflammatory response is mainly affected by circulating bacterial DNA concentrations in cirrhosis. Liver Int 2016;36:1473‐1480. [DOI] [PubMed] [Google Scholar]
- 16. Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol 2010;5:119‐144. [DOI] [PubMed] [Google Scholar]
- 17. Schumann A, Nutten S, Donnicola D, Comelli EM, Mansourian R, Cherbut C, et al. Neonatal antibiotic treatment alters gastrointestinal tract developmental gene expression and intestinal barrier transcriptome. Physiol Genomics 2005;23:235‐245. [DOI] [PubMed] [Google Scholar]
- 18. Kansagra K, Stoll B, Rognerud C, Niinikoski H, Ou CN, Harvey R, et al. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol 2003;285:G1162‐G1170. [DOI] [PubMed] [Google Scholar]
- 19. Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up‐regulation of tight junction protein Claudin‐1 transcription. Dig Dis Sci 2012;57:3126‐3135. [DOI] [PubMed] [Google Scholar]
- 20. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T‐cell generation. Nature 2013;504:451‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009;461:1282‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short‐chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013;145:396‐406.e10. [DOI] [PubMed] [Google Scholar]
- 23. Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol 2000;164:183‐190. [DOI] [PubMed] [Google Scholar]
- 24. Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down‐regulate co‐stimulatory molecules on antigen‐presenting cells. Eur J Immunol 2000;30:1538‐1543. [DOI] [PubMed] [Google Scholar]
- 25. Serra P, Amrani A, Yamanouchi J, Han B, Thiessen S, Utsugi T, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity 2003;19:877‐889. [DOI] [PubMed] [Google Scholar]
- 26. Misra N, Bayry J, Lacroix‐Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen‐presenting function of dendritic cells. J Immunol 2004;172:4676‐4680. [DOI] [PubMed] [Google Scholar]
- 27. Gomez‐Hurtado I, Zapater P, Bellot P, Pascual S, Perez‐Mateo M, Such J, et al. Interleukin‐10‐mediated heme oxygenase 1‐induced underlying mechanism in inflammatory down‐regulation by norfloxacin in cirrhosis. Hepatology 2011;53:935‐944. [DOI] [PubMed] [Google Scholar]
- 28. Gomez‐Hurtado I, Moratalla A, Moya‐Perez A, Peiro G, Zapater P, Gonzalez‐Navajas JM, et al. Role of interleukin 10 in norfloxacin prevention of luminal free endotoxin translocation in mice with cirrhosis. J Hepatol 2014;61:799‐808. [DOI] [PubMed] [Google Scholar]
- 29. Juanola O, Gomez‐Hurtado I, Zapater P, Moratalla A, Caparros E, Pinero P, et al. Selective intestinal decontamination with norfloxacin enhances a regulatory T cell‐mediated inflammatory control mechanism in cirrhosis. Liver Int 2016;36:1811‐1820. [DOI] [PubMed] [Google Scholar]
- 30. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia‐Villalba R, Gimenez‐Bastida JA, Garcia‐Conesa MT, Tomas‐Barberan FA, Carlos Espin J, Larrosa M. Alternative method for gas chromatography‐mass spectrometry analysis of short‐chain fatty acids in faecal samples. J Sep Sci 2012;35:1906‐1913. [DOI] [PubMed] [Google Scholar]
- 32. Gomez‐Hurtado I, Santacruz A, Peiro G, Zapater P, Gutierrez A, Perez‐Mateo M, et al. Gut microbiota dysbiosis is associated with inflammation and bacterial translocation in mice with CCl4‐induced fibrosis. PLoS One 2011;6:e23037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ubeda M, Lario M, Munoz L, Diaz D, Borrero MJ, Garcia‐Bermejo L, et al. Systemic inflammation in absence of gut bacterial translocation in C57BL/6 mice with cirrhosis. Gut 2013;62:330‐331. [DOI] [PubMed] [Google Scholar]
- 34. Ubeda M, Lario M, Munoz L, Borrero MJ, Rodriguez‐Serrano M, Sanchez‐Diaz AM, et al. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J Hepatol 2016;64:1049‐1057. [DOI] [PubMed] [Google Scholar]
- 35. Hartmann P, Haimerl M, Mazagova M, Brenner DA, Schnabl B. Toll‐like receptor 2‐mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology 2012;143:1330‐1340.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, et al. Altered intestinal tight junctions' expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest 2012;42:439‐446. [DOI] [PubMed] [Google Scholar]
- 37. Sanchez E, Nieto JC, Boullosa A, Vidal S, Sancho FJ, Rossi G, Sancho‐Bru P, et al. VSL#3 probiotic treatment decreases bacterial translocation in rats with carbon tetrachloride‐induced cirrhosis. Liver Int 2015;35:735‐745. [DOI] [PubMed] [Google Scholar]
- 38. Moratalla A, Gomez‐Hurtado I, Santacruz A, Moya A, Peiro G, Zapater P, et al. Protective effect of Bifidobacterium pseudocatenulatum CECT7765 against induced bacterial antigen translocation in experimental cirrhosis. Liver Int 2014;34:850‐858. [DOI] [PubMed] [Google Scholar]
- 39. Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut 2012;61:354‐366. [DOI] [PubMed] [Google Scholar]
- 40. Munoz L, Albillos A, Nieto M, Reyes E, Lledo L, Monserrat J, et al. Mesenteric Th1 polarization and monocyte TNF‐alpha production: first steps to systemic inflammation in rats with cirrhosis. Hepatology 2005;42:411‐419. [DOI] [PubMed] [Google Scholar]
- 41. Lemmers A, Moreno C, Gustot T, Marechal R, Degre D, Demetter P, et al. The interleukin‐17 pathway is involved in human alcoholic liver disease. Hepatology 2009;49:646‐657. [DOI] [PubMed] [Google Scholar]
- 42. Bayry J, Triebel F, Kaveri SV, Tough DF. Human dendritic cells acquire a semimature phenotype and lymph node homing potential through interaction with CD4+CD25+ regulatory T cells. J Immunol 2007;178:4184‐4193. [DOI] [PubMed] [Google Scholar]
- 43. Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin‐10‐secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 2006;212:28‐50. [DOI] [PubMed] [Google Scholar]
- 44. Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends Immunol 2015;36:3‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol 1997;26:1372‐1378. [DOI] [PubMed] [Google Scholar]
- 46. Kirkpatrick C, Maurer LM, Oyelakin NE, Yoncheva YN, Maurer R, Slonczewski JL. Acetate and formate stress: opposite responses in the proteome of Escherichia coli. J Bacteriol 2001;183:6466‐6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang M, Zhou Q, Dorfman RG, Huang X, Fan T, Zhang H, et al. Butyrate inhibits interleukin‐17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol 2016;16:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kawamoto S, Maruya M, Kato LM, Suda W, Atarashi K, Doi Y, et al. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 2014;41:152‐165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


