Paragangliomas (PGLs) are neuroendocrine neoplasms arising from neural crest tissues and belong to the most hereditary endocrine tumors with 27% having known, PGL susceptibility gene [1]. The pioneering work of Dahia et al. underlying the importance of hypoxia-inducible factors (HIFs) in PGL pathogenesis [2], the existence of mutated HIF2A in these tumors [3], oncometabolites role on HIFs stabilization, and a recent concept proposing how hereditary PGLs converge on the hypoxia signaling pathway [4], brought solid evidence of the existence of PGL hypoxiom.
HIF-2α: The Achilles’ heel
Krebs cycle (SDHx, FH, MDH2) and hypoxia signaling pathway (VHL, HIF2A, PHD1, PHD2) PGL-related genes (Figure 1), all belonging to Cluster 1 PGLs, function as poisoned arrows that hit Achilles’ heel and HIFs, promoting chromaffin/paraganglionic cell tumorigenesis. For example, SDHx and FH mutations result in the accumulation of either succinate or fumarate, both acting as oncometabolites. Oncometabolites act as competitive inhibitors of PHDs, causing a “pseudohypoxic” environment leading to HIFs stabilization. These oncometabolites also cause DNA hypermethylation, leading to epigenetic silencing of genes involved in catecholaminergic cell differentiation, promoting PGL formation. In contrast, mutations in hypoxia signaling pathway genes related to PGL development lead to direct HIFs stabilization, preferentially HIF-2α [4]. In a pseudohypoxic subtype of PGL, VHL mutations prevent degradation of HIF, causing unregulated angiogenesis and tumor formation [4]. Zhuang et al. [3] reported mutations occurring close to HIF-2α hydroxylation site that resulted in decreased HIF-2α prolyl hydroxylation, decreased VHL binding to HIF-2α, and its stabilization resulting in PGLs, somatostatinomas, and polycythemia. In chronic hypoxic conditions, hypoxia activating factor (HAF) switches the transcription of HIF-1α to HIF-2α, which plays an important role in tumor adaptation and proliferation. Due to this switch, HIF-2α expression increases leading to tumor progression.
Figure 1: Some of the metabolic pathways involved in HIF-α activation in pseudohypoxic PGLs and related tumors.
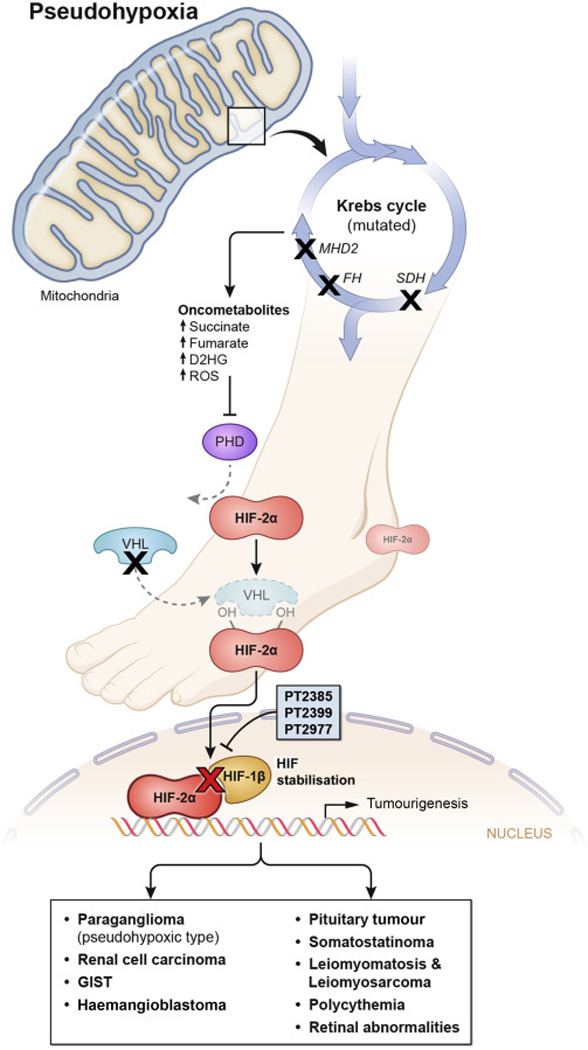
Figure depicts the generation of oncometabolites from the Krebs cycle and their role in HIF-2α stabilization and the role of PT2399, PT2385 and PT2977 in blocking the tumorigenesis. Red “X” indicates the inhibition of HIF dimerization by HIF-2α antagonists- PT2399, PT2385 and PT2977. Black “X” indicates blockage in the normal metabolic pathways leading to the formation of oncometabolites and thereby tumorigenesis. SDH: Succinate dehydrogenase complex, FH: Fumarate hydratase, MDH2 malate dehydrogenase 2, D2HG: D-2-Hydroxyglutarate, ROS: Reactive oxygen species, PHD: Prolyl hydroxylase, HIF-2α: Hypoxia-inducible factor 2 alpha, VHL: Von Hippel-Lindau protein, PT2399, PT2385 and PT2977: HIF-2α antagonists, HIF-1β: Hypoxia-inducible factor 1 beta (also known as Aryl hydrocarbon receptor nuclear translocator (ARNT)), GIST: Gastrointestinal stromal tumor.
Targeting the Achilles’ heel by HIF-2α antagonists
Recently, HIF-2α antagonists have become drugs of interest in treating tumors where dysfunctional hypoxia signaling pathway seems to play an important role in tumorigenesis; particularly in clear cell renal cell carcinoma (ccRCC) that is characterized by the inactivation of VHL, FH, and SDHx as seen in pseudohypoxic subtypes of PGL. In recent studies [5,6], in VHL mutated ccRCC, the HIF-2α antagonist, PT2399, decreased the dimerization of HIF-2α with HIF-1β subunit (ARNT), thereby inhibiting the transcription of HIF-2α target genes (e.g. VEGFA, GLUT1, PAI-1, or CCND1) leading to suppression of tumor growth. Moreover, PT2385 also resulted in decreased erythropoietin, making it a promising drug in HIF2A mutation-related polycythemia and PGLs. The other HIF-2α antagonist, PT2977, is currently being evaluated in a phase 1 study against solid tumors and ccRCC (NCT02974738).
The use of HIF-2α antagonists may be also beneficial in other HIF-2α related conditions such as somatostatinoma and retinal abnormalities caused by HIF2A mutations; polycythemia in HIF2A and PHDs mutations; RCC, central nervous system and retinal hemangioblastomas, pancreatic endocrine tumors in VHL syndrome; and finally, RCC, uterine and cutaneous leiomyomas/leiomyosarcomas in FH mutations. Though HIF-2α antagonists opened a new door in management of these cancers, development of other drugs that act on the HIF pathway are still in infancy and more work is clearly needed. However, with the increasing evidence of divergent and opposing functions of HIF-1α and HIF-2α on various genes including p53 and the differential regulation of HIF-1α and HIF-2α post-translationally [7], one must be cautious about HIF-2α inhibitors treatment efficacy in various cancers. For example, as seen in KRAS-driven mouse models of lung cancer, HIF-2α suppression inhibited tumor growth or sometimes promoted tumor growth in the same tumor context by inhibiting the tumor suppressor Scgb3a1, a HIF-2α target gene. This suggests that the effective targeting of HIF-α subunits in cancer management is challenging, sometimes causing opposing results.
Future Directions:
Promising outcomes of PT2399 described in the management of pseudohypoxic ccRCCs, opened a new insight of treating the above mentioned lethal cancers by targeting HIF-2α. We expect that HIF-2α antagonists could well offer effective targeted therapy for patients; particularly with pseudohypoxic metastatic PGLs and could improve their clinical outcome. Since these pseudohypoxic PGLs can exhibit accelerated glycolysis (Warburg effect), and glutaminolysis, the concept of multi-targets therapeutic approach including HIF-2α antagonist could become more beneficial. The other potential treatment options in these tumors are prolyl hydroxylase activators (R59949 and KRH102053) that promote hydroxylation of HIF making it vulnerable for degradation by VHL thereby causing anti-tumor effect. HIF-2α translational inhibitors could be the other classes of drugs to have a potential to increase the HIF-2α degradation. Histone deacetylase inhibitors (HDACi) are also an option in management of these tumors as transcription repressors of HIF and VEGF by blocking the acetylation of histone and causing chromatin condensation. Thus, long-awaiting high-throughput metabolomic studies on these tumors, using state-of-the-art equipment, and teamwork besides clinicians, including specialists in mass spectrometry, chemists, signaling pathways, and bioinformatics, will undoubtedly represent a dream team in attempt to defeat these tumors. Current and future HIF-2α antagonists represent new evolving therapeutic approaches, where Achilles’ heel of pseudohypoxic tumors and related conditions is hit and may cause their death or improvement, respectively.
Acknowledgments
Funding:
This work was supported by the Intramural Research Program of the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, MD, 20892. Grant No. Z1AHD008735.
Abbreviations:
- SDHx
Succinate dehydrogenase complex
- FH
Fumarate hydratase
- MDH2
Malate dehydrogenase 2
- VHL
Von Hippel-Lindau
- HIF2A
Hypoxia-inducible factor 2 alpha
- PHD1
Prolyl hydroxylase 1
- PHD2
Prolyl hydroxylase 2
- DNA
Deoxyribonucleic acid
- ARNT
Aryl hydrocarbon receptor nuclear translocator
- VEGFA
Vascular endothelial growth factor A
- GLUT1
Glucose transporter 1
- PAI-1
Plasminogen activator inhibitor-1
- CCND1
Cyclin D1
- Scgb3a1
Secretoglobin Family 3A Member 1
Footnotes
Conflict of interest statement: Authors have no conflicts of interest or nothing to disclose.
References:
- 1.Fishbein L, Leshchiner I, Walter V et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 2017; 31(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahia PL, Ross KN, Wright ME, et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet 2005; 1(1):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhuang Z, Yang C, Lorenzo F, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N. Engl. J. Med 2012; 367: 922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jochmanova I, Yang C, Zhuang Z, et al. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst 2013; 105:1270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Hill H, Christie A, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 2016; 539:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho H, Du X, Rizzi JP, et al. On-target efficacy of a HIF-2alpha antagonist in preclinical kidney cancer models. Nature 2016; 539:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 2011; 12(1):9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


