Abstract
Improved therapy of brain disorders can be achieved by focusing on neuronal networks, utilizing combined pharmacological and stimulation paradigms guided by neuroimaging. Neuronal networks that mediate normal brain functions, such as hearing, interact with other networks, which is important but commonly neglected. Network interaction changes often underlie brain disorders, including epilepsy. “Conditional multireceptive” (CMR) brain areas (e.g., brainstem reticular formation and amygdala) are critical in mediating neuroplastic changes that facilitate network interactions. CMR neurons receive multiple inputs but exhibit extensive response variability due to milieu and behavioral state changes and are exquisitely sensitive to agents that increase or inhibit GABA-mediated inhibition. Enhanced CMR neuronal responsiveness leads to expression of emergent properties—nonlinear events—resulting from network self-organization. Determining brain disorder mechanisms requires animals that model behaviors and neuroanatomical substrates of human disorders identified by neuroimaging. However, not all sites activated during network operation are requisite for that operation. Other active sites are ancillary, because their blockade does not alter network function. Requisite network sites exhibit emergent properties that are critical targets for pharmacological and stimulation therapies. Improved treatment of brain disorders should involve combined pharmacological and stimulation therapies, guided by neuroimaging, to correct network malfunctions by targeting specific network neurons.
Keywords: CNS disorders, neuroimaging, emergent property, CNS pharmacology, neuronal network, electrical stimulation, complexity, seizures, startle, pain
Introduction
There is an urgent need to hasten improvements in the therapy of brain disorders, because many patients afflicted by these disabling conditions may not be receiving optimal treatment. We propose that improved therapies can be achieved by more effective integration of several important facets of current neuroscience knowledge. Such an approach should be based on neuronal network methods guided by neuroimaging of the brain and involve integration of pharmacological and electrical stimulation therapies (Faingold and Blumenfeld 2014a). Focusing on neuronal networks and their interactions is vital to improving therapeutic interventions. Emphasis on networks is critical, because this avoids the enormous task of trying to assess the single cell activity of a significant population of the billions of neurons and glia and the trillions of brain synapses involved in a specific brain function or disorder. The network approach can also capture the crucial properties emerging from dynamic network interactions and takes into consideration the actions of the numerous endogenous neuroactive substances (neurotransmitters, neuromodulators) affecting these neurons as well as the operational changes that occur during each behavioral element of the function or disorder (Faingold and Blumenfeld 2014a). The term neuronal or neural network is used in a wide variety of ways and in different contexts, ranging from genome-, proteome-, to metabolome-based networks up to local circuit, long-range cortical-cortical or cortical subcortical networks, and even social networks, based on the variety of experimental approaches (Stam 2014). One of the most useful approaches for studying large-scale networks in human subjects is neuroimaging based on blood oxygen level changes in awake individuals (Guo and Blumenfeld 2014). Finally, and most important, networks defined using direct recordings of neuronal action potentials (APs) firing within network hubs guided by neuroanatomically based techniques, such as neuroimaging, provide the most definitive understanding of the mechanisms of network operation that can be targeted by drugs and stimulation therapies (Faingold and Blumenfeld 2014c). This combined approach has major advantages over existing techniques such as electroencephalography, magnetoencephalography, and neuroimaging used in isolation, because none of these individual techniques possesses the temporal or spatial resolution needed to allow targeted therapeutic effectiveness to be maximized. It has been proposed that understanding brain function and malfunction at the level of midsize (mesoscopic) neuronal networks of neurons (Freeman and others 2001) is vital to accelerating the therapeutic usefulness of our current knowledge. The operational definition of a mesoscopic neuronal network for purposes of this review is a group of functionally connected brain regions (nodes) whose principal neurons are required for the execution of a specific brain function or malfunction in the intact animal (Faingold and Blumenfeld 2014b). This definition would exclude brain regions that may also become more active during the function but are not required for the function to occur. Under this approach, proof of the importance of each proposed brain region would require experimental verification using methods that inactivate that region to observe if this procedure prevented the occurrence of the specific function (Feng and Faingold 2014). Brain regions that fulfill this criterion would be considered “requisite,” whereas other areas that show evidence of involvement in the function but whose inactivation did not prevent the function would be considered “ancillary.” An example of this important distinction has been observed in a rodent model of epilepsy, in which the requisite structures of the network reside in the brainstem (Faingold and others 2014a; N’Gouemo and others 2014). However, a limbic site that is clearly activated during network operation is only ancillary, as blockade of this structure does not affect the seizures, as discussed in greater detail below.
These mesoscopic neuronal networks include normal networks, such as the auditory and visual pathways that are “dedicated” to that function, and pathological networks that mediate CNS disorders, such as epilepsy and Parkinson’s disease. Functionally important interactions commonly occur between “dedicated” networks, but this fact is commonly ignored. A prime example of such a network interaction occurs between the auditory and loco-motor networks, which mediates the acoustic startle response, as discussed below. Epilepsy is one neuroscience research area that has recognized the importance of cross-network interactions, because such interactions are a common feature of this disorder (Faingold and others 2014a), as seen, for example, in the decrease in the function of networks that mediate consciousness following generalized seizures (Blumenfeld 2007, 2014).
We propose that effective pharmacological as well as therapeutic stimulation paradigms act by targeting specific emergent properties of specific sets of neurons in specific hubs within these networks (Fig. 1) (Faingold 2004, 2008, 2014a). The concept of emergent property originated in physics and was popularized via meteorology as the so-called “butterfly effect” (Lorenz 1972). Emergent properties are a manifestation of complexity theory and are nonlinear events that occur as a result of self-organization within the network (Freeman and others 2001). Thus, neurons in intact networks can exhibit such emergent properties that may not be expressed in the very same neurons in isolation but arise in vivo due to the interactions of the influences that affect them within the network (Faingold 2004, 2008). Emergent properties are important in network neuroscience, including the mechanisms that mediate memory, attention, chronic pain, and neuropsychiatric disorders, such as posttraumatic stress disorder, schizophrenia (Patel and others 2012), and consciousness (Del Cul and others 2007; Faingold and Blumenfeld 2014a; Laureys and Schiff 2012).
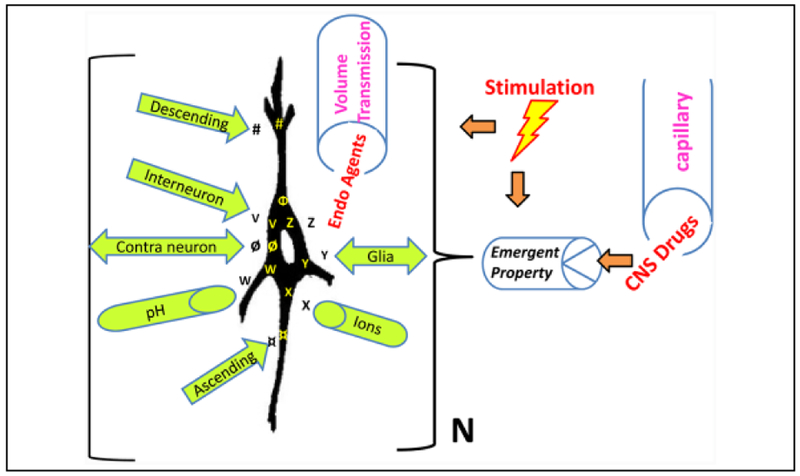
Figure 1.
Numerous influences converge on principal neurons leading to emergent properties. This is an idealized diagram of a principal neuron in a specific nucleus within a neuronal network in the brain of an awake, behaving organism that illustrates many of the influences that affect this “class” of neurons (N) in this nucleus. The neurons possess certain specific intrinsic properties (Φ), such as the propensity to exhibit burst firing or pacemaker activity. These cells also possess specific receptors (#) (metabotropic or ionotropic) onto which descending projections release a specific neuroactive substance. The neurons also possess ligand-gated receptors (V) (e.g., glutamate) (which have specific receptor subunits) onto which interneurons synaptically release a specific neuroactive substance. Projection neurons across the midline for bilaterally connected structures releasea neuroactive substance ø, which binds to its specific receptors on contralateral neurons. The principal neurons also possess the property of pH sensitivity (W). Ascending input from neuronsin nuclei in the network also release a neuroactive substance (¤), which binds to its specific receptors. The neurons possess voltage-gated ion channels (X) (e.g., K+ channels) at which local ions can act. The neurons receive input from local glial cells, which release a neuroactive substance (Y) (e.g., adenosine) that acts on specific receptors for this substance. Endogenous (Endo) neuroactive agents (Z) carried via volume transmission from nearby (spillover) or distant sites via the extracellular fluid and cerebrospinal fluid to the neurons also affect the properties of these neurons. An example would be extrasynaptic GABAA receptors that respond to the low levels of “ambient” GABA in the extracellular fluid. Finally, when an exogenous agent, such asa CNS drug or centrally acting toxin, is administered it is released via the brain blood vessels among other vectors to exert its effects on the emergent property of these principal cells to exert its effect on network function. The therapeutic effect of many CNS drugs is postulated to be mediated by a selective action on the principal cells in a specific nucleus in the neuronal network for the disorder to produce the desired effect. This selectivity is seen primarily when therapeutic doses are given. In addition, electrical, magnetic, or optogenetic neurostimulation can modulate the network and affect the emergent property. The summation of all these influences determines whether the emergent property is expressed and the actual nature of that property and its relative sensitivity in that group of neurons. This emergent property is postulated to be expressed uniquely in this network nucleus and causes this class of neurons to respond with unique sensitivityto a specific exogenous substance. Note, those influences can also be modified by brain state changes (such as sleep), whichcan significantly alter the emergent property, based on changesin milieu of the principal neuron as well as external stimulation. (Modified from Faingold 2014b, with permission.)
We propose that emergent properties in the brain arise due to the convergence of the numerous influences (Table 1) that impinge in unique combinations on the principal neurons in each specific network nucleus (Fig. 1). These influences include neurophysiological mechanisms and endogenous and exogenous neuroactive agents that act via both synaptic and volume transmissions (Faingold 2014a). These emergent properties are an important nexus for understanding network function, dysfunction, and potential therapeutic interventions, but unfortunately, they often go unrecognized. The emergent properties are the key sites of action of CNS drugs and other exogenous agents and are affected importantly by stimulation paradigms as well (Fig. 1).
Table 1.
Elements that Control Network Function and Interaction.
| A. Neuronal properties |
| 1. Burst firing |
| 2. Gap junctions |
| 3. Electrical field effects (ephaptic interactions) |
| B. Neuroactive agent (endogenous and exogenous, e.g., CNS drugs) |
| 4. Excitatory or inhibitory |
| 5. Endogenous or exogenous |
| 6. Synaptic or volume transmission |
| 7. Tonic or phasic |
| C. Neuronal milieu |
| 8. Extracellular ions and gases (O2, CO2) |
| 9. Temperature |
| 10. Buffering capacity (pH) |
| D. Network connections |
| 11. Interneuron activity |
| 12. Astrocytic integration |
| 13. External stimuli |
| 14. Multiplicity of synaptic inputs |
| E. Cyclic and “life cycle” events (development, experience, degeneration, aging, and repair) |
| 15. Brain state (circadian, sleep, coma) |
| 16. Synaptic plasticity (strength changes, synaptogenesis) |
| 17. Neurogenesis |
| 18. Neurodegeneration |
Based on Faingold and Blumenfeld (2014b), with permission.
Emergent properties can occur at many organizational levels in the brain from the receptor level to the behavioral level. A simple example is for brain neurons that possess an intrinsic property (e.g., pacemaker activity) that is not expressed when the neurons are relatively quiescent. The neurons also possess ligand-gated receptors at which a synaptically released neuroactive substance can exert its effect. An emergent property can also be expressed and alter the function of a network due to the effect of a neuroactive agent, released via volume transmission, which involves diffusion of a neuroactive substance through extracellular and cerebrospinal fluids of the CNS (Agnati and others 2014; Faingold 2014a). When this neuroactive substance is released onto these neurons it binds to the receptors and causes the neurons to exhibit pacemaker activity, which would be an emergent property of the group of similar neurons in this nucleus. An example of this is seen in the respiratory network in the medulla where an excitatory amino acid is released onto neurons in a specific respiratory network site and causes respiratory rhythm generation as an emergent property (Pace and Del Negro 2008). This emergent property may be unique to the neurons in this network nucleus under these conditions. Emergent properties are commonly seen at the mesoscopic neuronal network level in the CNS, and the nature and function of the specific property depends on the functional role of the particular network (Faingold 2004, 2014a).
Networks are not static entities, as they can undergo expansion due to repetitive activation physiologically, therapeutically, or pathophysiologically, for example, by repetitive seizures (N’Gouemo and others 2014). The expanded network may develop additional emergent properties in addition to the original emergent properties. Thus, an emergent property can be conferred on a group of neurons in a specific nucleus within the network, which makes this nucleus a target for the action of an externally presented therapy (drug or therapeutic stimulation paradigm) (Fig. 1). This emergent property can simply render those neurons more sensitive than other neurons to this exogenous substance, so that when the intact, unanesthetized organism is exposed to the lowest effective dose of this substance these neurons become a selective target for that agent’s therapeutic action. Nonrequisite sites affected by the exogenous agent may give rise to adverse effects of the agent.
An early indication that emergent properties may be targets of CNS drug action was an examination of the effects of uncompetitive and competitive N-methyl-D-aspartate (NMDA) receptor antagonists on rhythmic bursting of thalamic neurons, which led to the observation of emergent properties of the relay thalamus-nucleus reticularis network (Buzsaki 1991). A dichotomy between the effects of competitive and uncompetitive NMDA receptor antagonists on the neuronal network in a model of generalized convulsive (audiogenic) seizures was also observed (Faingold 2014b) that subsequently was resolved based on emergent property differences in different regions of this seizure network. These findings and subsequent data led to the proposal that emergent properties are generally applicable targets and mechanisms of action for CNS drugs (Faingold 2004) (as shown in the diagram in Fig. 1), which was expanded on by subsequent studies (Faingold 2014a; Margineanu 2014). Differences between the emergent properties that are the target of certain anticonvulsant drugs in excitatory versus inhibitory neuronal networks within the same brain structure have been observed recently. These property differences explain the finding that certain anticonvulsant drugs that block sodium channels in a use-dependent manner only inhibit excitatory neuronal networks and do not reduce the activity of inhibitory networks. This appears to be due to a critical emergent property difference between these networks because of a unique combination of intrinsic and synaptic properties that differs between these networks. This difference renders the inhibitory network insensitive to the therapeutic dose of the anti-convulsant, whereas the emergent properties of the excitatory network cause this network to be sensitive to the therapeutic doses of these anticonvulsant drugs (Pothmann and others 2014). Therapeutic stimulation paradigms are also proposed to alter expression of emergent properties (Fig. 1) (Chao and Xia 2013; Faingold 2008). Expression of emergent properties in specific sets of neurons in a critical network site (hub) may result from the intensification of a specific receptor or channel or result from a critical interaction of certain of the many influences affecting these neurons in that specific site (Faingold 2014a) within the intact network; this property may be absent in the same neurons when they are isolated in a brain slice or culture in vitro (Faingold 2004, 2014a). Alternatively, emergent properties can occur in vitro that are not seen in the same neurons in vivo (Faingold 2004), because the property was masked by influences, such as projections from above or below, that are absent in the isolated preparation.
Because of their nature, emergent properties are not initially predictable but can become consistent properties of brain networks under recurring conditions, as is often seen in chronic CNS disorders (Faingold and Blumenfeld 2014a). Network connections, including those with inter-neurons and glia, as well as circadian rhythms, sleep state, and neurogenesis, can also significantly affect network function and the properties of neurons in specific network sites. The only way to observe therapeutically relevant drug effects on such emergent properties is to evaluate the effect of the lowest effective dose of the drug on neurons in an intact, unanesthetized, and behaving organism during the occurrence of the function or malfunction being evaluated. An application of these concepts will be discussed below in a specific experimentally defined network in the brainstem.
Network “Silos” versus Network Interactions
Most current network research investigates a single brain function, such as vision, to try and fully explicate this function. This approach is important to understanding the details of that function but often results in a “silo” effect, in which research on one network ignores interactions with other networks, which are functionally important. Network interactions can involve additive or competitive effects. A prototypical additive network interaction is the existentially important acoustic startle response, involving brainstem auditory and locomotor network interactions (Davis and others 2008; Faingold and Tupal 2014). A prototypical competitive network interaction is the “gate control theory of pain” (Melzack and Wall 1965) wherein the somatosensory network competes with the nociceptive network to reduce pain perception. Dynamic network interactions have recently been proposed as important mechanisms that mediate the pain “connectome” (Kucyi and Davis 2015). Network interactions can be subtle, as seen in learning paradigms (Vogels and others 2011), or dramatic, as observed in epilepsy (Faingold and Tupal 2014; Schneider-Mizell and others 2010), involving interaction of several different networks. For example, a devastating outcome occurs in sudden unexpected death in epilepsy (SUDEP), in which respiratory dysfunction is commonly a precipitating factor. Animal models of SUDEP in DBA/1 and DBA/2 mice involve interactions between three different networks. This involves a positive interaction of the auditory and locomotor networks, culminating in a negative effect on the respiratory network, which causes respiratory failure and death (Faingold and Tupal 2014; Faingold and others 2015).
We propose that knowledge about the nature of network interaction mechanisms will deepen the understanding of brain function and brain disorders, as well as advance therapy of these disorders, as network interactions are common and vital for both normal function as well as CNS disorders (Faingold and Blumenfeld 2014a; Stam 2014). These interactions depend on numerous variables, including the external and internal milieu of the organism (Fig. 1). A thorough network investigation requires the use of multiple techniques (Faingold and Blumenfeld 2014a), as results from one technique invariably require validation. Thus, neuroimaging data require neuronal recording validation whenever possible, as neuroimaging can overlook important events due to temporal resolution limitations (seconds) and spatial limitations (Guo and Blumenfeld 2014), which is also a concern with magnetoencephalography and electroencephalography (Buzsaki and others 2012; Faingold and Blumenfeld 2014c). By contrast, single neuronal firing evaluates network operation over millisecond and micrometer ranges. For example, neuroimaging in hippocampal seizures that cause loss of consciousness reveals long-range network interactions between cortical and subcortical nodes (Fig. 2). It is vital that these functional maps are validated by determining the neuronal firing changes underlying the neuroimaging signal changes, as important differences between imaging changes and neuronal firing are known to occur (Englot and others 2008; Guo and Blumenfeld 2014; Mishra and others 2011; Schridde and others 2008).
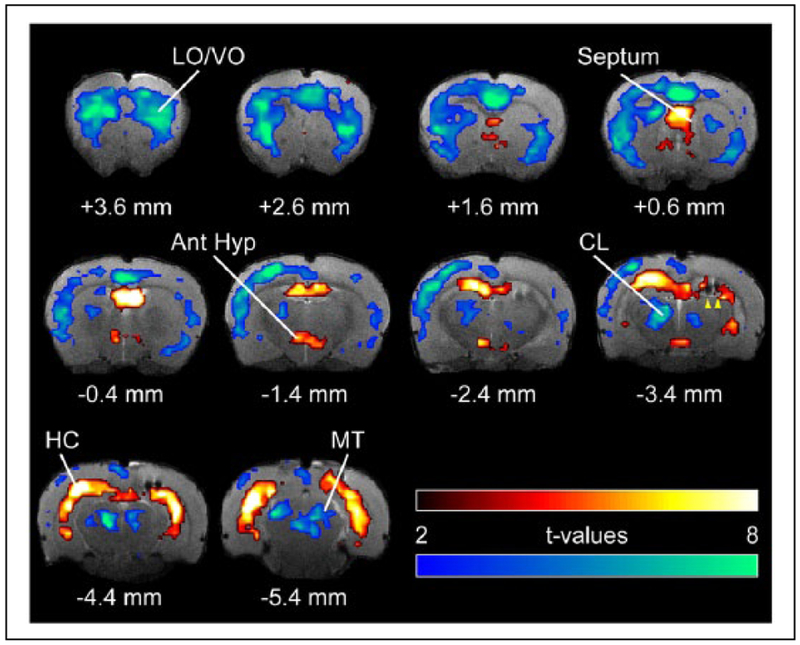
Figure 2.
Neuroimaging reveals consciousness networks. Blood oxygen level dependent (BOLD) fMRI during seizurein a rodent model of complex partial seizures demonstrates coordinated cortical and subcortical changes. Seizures are induced by hippocampal stimulation (yellow arrowheads indicate electrodes), which leads to seizure activity and an increased BOLD signal (warm colors) in the hippocampus (HC), septal nuclei, and anterior hypothalamus (Ant Hyp). This causes cortical slow oscillations (not shown) and a decreased BOLD signal (cool colors) in the orbital frontal cortex (LO/VO), as well as in subcortical arousal areas such as the intralaminar thalamic centrolateral nucleus (CL) and midbrain tegmentum (MT). Limbic seizures of this kind are associated with behavioral arrest and decreased responsiveness in both animal models and human patients with temporal lobe epilepsy. (Motelow and others 2015, with permission.)
Neuronal Network Organization and Neuroplasticity
Neuroanatomy is the structural organization of a network, which is largely fixed, but network function is dictated by neuronal physiology, which is often highly variable. Synaptic plasticity can often lead to the development of emergent properties of synaptic networks. Mechanisms related to NMDA receptors are a well-established source of long-lasting synaptic plasticity, particularly in limbic system structures such as the hippocampus where long-term potentiation is a prototypical model of learning (Larson and Munkacsy 2014). Long-term neuroplasticity also occurs commonly in epilepsy due to repetition of seizures (N’Gouemo and others 2014). Short-term plasticity is a less well recognized form of neuroplasticity, which is more difficult to study, but is nevertheless potentially extremely important to network operations. Such short-term plasticity often occurs due to high degree of response variability that is observed in a group of brain sites that have been termed “conditional multireceptive” (CMR) brain regions (Faingold 2008; Faingold and others 2014b). Brain regions that contain a significant proportion of neurons that exhibit such a high degree of response variability include the brainstem reticular formation (BRF), periaqueductal gray (PAG), amygdala, and association cortices, which will be illustrated below. These areas contain a preponderance of neurons that exhibit responses to a wide variety of input sources but exhibit extensive firing variability that is strongly influenced by the external and internal milieu as well as the behavioral state of the intact animal (Faingold 2008; Faingold and others 2014b). The variability of responses seen in CMR regions contrasts strongly with the much less variable responses of neurons seen in many other brain sites, such as the primary sensory and motor nuclei, which appears to be required in order to carry out these vital functions reliably. CMR neurons are a major source of network neuroplasticity, as they can undergo positive and negative extremes of participation in network function (Fig. 3).
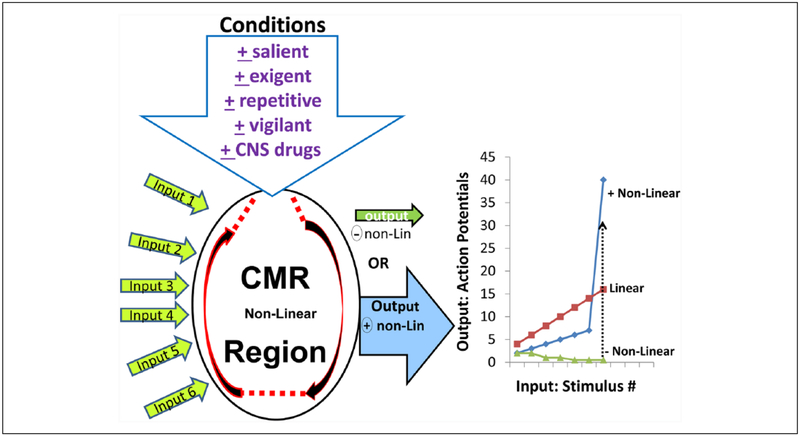
Figure 3.
Input-output relationships of a prototypical conditional multireceptive (CMR) brain region, which is capable of a significant degree of self-organization, as shown by the paired semicircular arrows. This diagram illustrates the numerous inputs that the neurons in this CMR region receive from primary networks and other CMR networks. The output of neuronsin CMR nuclei is highly dependent on the conditions that the animal is experiencing, including salient, exigent, and repetitive conditions and can also be governed by the animal’s state of vigilance as well as centrally acting pharmacological agents. The output of CMR regions is subject to nonlinearity wherein CMR neuronal responsiveness to any input can change dramatically from nonresponsiveness to hyperresponsiveness, depending on stimulus parameter type, strength, and repetition rate, as illustrated in the graph on the right. Under nonexigent (resting) conditions many CMR neurons exhibit negative (−) nonlinearity with minimal responsiveness or even nonresponsiveness to the input, as seen in neurons that exhibit response habituation. The various exigent conditions can cause CMR neurons to exhibit hyperresponsiveness and exhibit positive (+) nonlinearity, which can result in massive output, activating the self-organization characteristics of the CMR network and result in emergent properties of that network, which range, for example, from startle responses to generalized seizures. CMR neurons that exhibit negative nonlinearity under resting conditions can convert to positive nonlinearity in response to exigent changes in the animal’s behavioral state (dotted line). The nonlinear output characteristics of CMR brain regions contrast with the relatively linear responses seen in primary networks, such as sensory systems. (Based on Faingold and others 2014b, with permission.)
CMR neurons are also extremely sensitive to low doses of anesthetic, sedative, and analgesic drugs, which block GABA receptor-mediated inhibition or NMDA receptor-mediated excitation (Fig. 4). These CMR neurons are also highly sensitive to low doses of CNS stimulant and convulsant drugs, particularly agents that inhibit GABA receptor-mediated inhibition (Fig. 5). Thus, the responses of CMR neurons in the PAG to thermal stimuli are greatly reduced by very low doses of barbiturates, and the auditory responses of CMR neurons in the lateral amygdala are inhibited by very low doses of ketamine (Fig. 4), which may make an important contribution to the antidepressant effects of this uncompetitive NMDA receptor antagonist (Feng and Faingold 2013). Low doses of drugs with convulsant properties, including GABA and glycine receptor antagonists administered systemically or directly onto the neurons in vivo, produce short-term neuroplastic neuronal response increases at doses that are considerably below those that would produce seizures (Fig. 5). Thus, the responses of CMR neurons in the BRF, amygdala, and pericruciate cortex were greatly increased to sensory stimuli as well as to direct stimulation in the brain (Faingold and others 2014b). This effect is much more pronounced on the neuronal firing evoked by the various stimuli than on the spontaneous firing of these neurons, and many of these neurons become very responsive to the stimuli despite the fact that most were not clearly responsive to the same stimuli prior to drug administration. The duration of the neuroplastic effect of these agents is short-term, lasting less than 30 minutes. However, during the drug effect period the networks containing the affected CMR sites can undergo significant functional changes (Faingold and Feng 2014).
Figure 4.
Changes in conditional multireceptive (CMR) neuronal responses induced by CNS depressant drugs. Neuronal responses in the ventrolateral periaqueductal gray (PAG) and lateral amygdala (LA) are depressed by low doses of depressant drugs. Low doses of barbiturates (which enhance GABAA receptor activity) or ketamine (uncompetitive NMDA receptor antagonist) reduce CMR extracellular action potential responses to external stimuli. Panel A shows the mean (± SEM) reduction of both spontaneous (Spont.) and thermally evoked PAG neuronal firing by pentobarbital (N = 18 neurons) 15 minutes after systemic administration with recovery by 30 minutes. Panel B shows examples of rate meter histogram analysis of a PAG spontaneous and evoked neuronal firing in response to a single 30-second (bracket) noxious thermal stimulation (53°C) before (Control column), 15 minutes after pentobarbital (15 mg/kg i.p.) treatment (Drug column), and subsequent recovery (at 30 minutes) (Recovery column). Prior to pentobarbital the majority (18/35) of PAG neurons increased firing in response to the thermal stimulus in this study. (Note 10 mg/kg of pentobarbital had no significant effect on PAG neuronal firing, whereas 20 mg/kg produced greater depression of PAG firing than the 15 mg dose.) The action potential above the top histogram represents the waveform of the neuron being analyzed. The onset and duration of the thermal stimulus is illustrated by the bracket. *Significance at P < 0.01 (repeated-measures ANOVA). Panel C shows a representative example of the poststimulus time histogram (PSTH) analysis of the action of an uncompetitive NMDA antagonist (ketamine) to reversibly block the sensory responsiveness of LA neurons. In the control column the LA neuron exhibited an onset response to the auditory stimulus before ketamine treatment. LA neuronal firing was significantly reduced (P < .01, paired t-test) and almost completely suppressed 15 minutes after ketamine (30 mg/kg i.p.) treatment (drug column). Four hours after ketamine treatment, the LA neuronal response was comparable tothat prior to ketamine treatment (recovery column). The inset the control column in line C shows an example of the digital oscilloscope tracings for the PSTH. Action potential amplitude in C: 300 μV. N = number of action potentials in the PSTH. Treatment was given in unanesthetized awake, behaving rats with microwire recording electrode. (Acoustic stimulus parameters: 12 kHz tone burst, 100 ms duration, 5 ms rise-fall, 100 dB SPL, 0.5 Hz rate) (PSTH parameters 50 stimulus presentations, 1 ms bin width). All data were taken from awake, behaving rats. The data in line C is representative of the change in the mean number of action potentials/PSTH (control mean = 182.1 ± 42.0 [SEM] vs. 9.6 ± 2.0 drug mean). Note: Both drug doses were less than 30% of the anesthetic dose. (A, B: modified from Faingold and others 2014b; C: modified from Faingold 2014b).
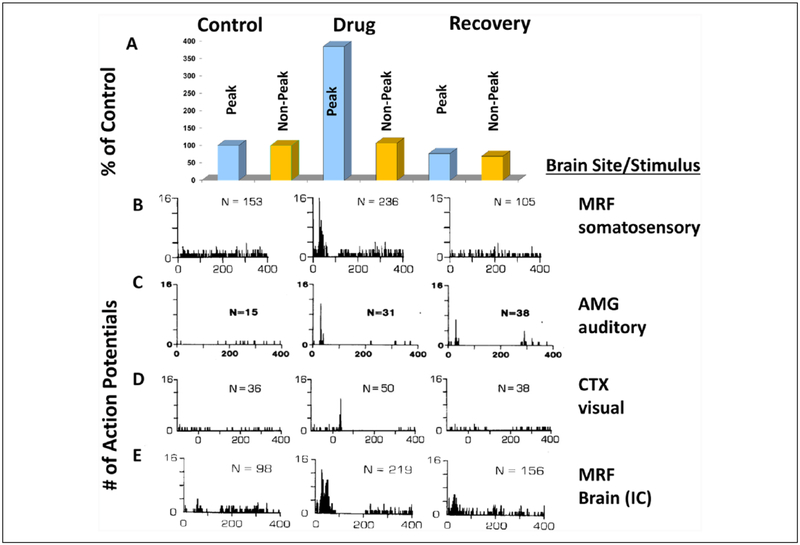
Figure 5.
Changes in extracellular action potential firing of conditional multireceptive (CMR) neurons in several brain regionsin response to sensory stimuli induced by administration of GABAA receptor antagonists (in subconvulsant doses). In predrug conditions (control column of the poststimulus histograms or PSTHs) presentations of electrical stimuli to the sciatic nervein line B to a mesencephalic reticular formation (MRF) neuron, line C amygdala (AMG) neuron response to auditory stimuli, line D response of a pericruciate cortex (CTX) neuron to visual stimuli and response of a MRF neuron to electrical stimulus within the brain to the inferior colliculus (IC) in (line E). Although spontaneous firing was observed, very little evidence of responsiveness to the stimuli is detectable, as shown by the absence of clear time-locked response peaks to the stimuli in the PSTHs (control column). After administration of a GABAA receptor antagonist these CMR neurons exhibit major responsiveness increases, which includes the induction of a very striking time-locked peak of responsiveness to each stimulus in each PSTH (drug column). These peaks indicate that the drug had induced extensive responsiveness of the CMR neurons to these stimuli. The bar graphs in A compare changes in the PSTH peak period and the rest of the recorded period for the PSTHs in line B. The peak (20–55 ms period after stimulus onset) shows a much greater percent increase as compared to the rest of the 400 ms sampling period as shown by the bar graphs for the nonpeak versus peak time points shown above each PSTH. Several of these CMR neurons showed response enhancement to more than one stimulus modality (not shown), indicating the multimodality responsiveness common in CMR neurons. This neuroplastic effect is short-term, since the firing patterns of these neurons recover to unresponsiveness with time (right column). Specific GABAA receptor antagonists and doses used are as follows: line B, pentylenetetrazol, 15 mg/kg; line C, bemegride, 3.6 mg/kg; line D, bicuculline, 0.03 mg/kg; line E, pentylenetetrazol, 5 mg/kg. Recovery times ~20 minutes after i.v. drug administration. Stimulus parameters: 0.5 Hz stimulus rate, 50 stimulus presentations (100 μs single bipolar pulses in B and E, 95 dB SPL clicks in C, and 18.5 lux visual stimulus [strobe] in D). PSTHs: bin width = 1 ms, scan length= 400 ms. Note the histograms in line D are peristimulus time histograms. N = number of action potentials per PSTH. This effect was seen in 88% of more than 700 bulbar and midbrain reticular formation neurons, 57% of more than 100 AMG, and 67% of more than 100 CTX neurons in unanesthetized cat and rat and was also seen with direct (iontophoretic) application of drug. (A, B, E: modified from Faingold and Feng 2014; C: modified from Faingold 2014b; D: modified from Faingold and others 1983.)
Primary sensory neurons recorded simultaneously with CMR neurons are minimally affected by the same doses of these convulsant drugs (~40% to 60% above control) as compared to ~300% in CMR neurons in the BRF recorded simultaneously (Faingold and others 2014b). The qualitative effects are even more dramatic. The primary sensory neurons show minor increases in peak responses to the stimuli, whereas the CMR neurons go from unresponsiveness to patterns with extensive peaks. However, this effect is strictly temporary with acute administration of these agents. The pattern of CMR neuronal firing returns to the unresponsive state gradually after drug administration is terminated. During the period of short-term plasticity major changes in network function occur, so that higher drug doses can lead to sensory induction of seizures.
The major mechanism that subserves CMR neuronal firing variability is the high incidence of subthreshold excitatory postsynaptic potential (EPSPs) that do not evoke APs. Thus, an initial stimulus may evoke an AP, but subsequent stimuli often do not, because the amplitude of the EPSP falls extensively, due to stimulus repetition. As seen in CMR neurons in the BRF, a repetitive stimulus in the auditory pathway results in a rapid decline in the EPSP amplitude (Geis and Schmid 2011), which is proposed to be important to habituation of the acoustic startle response. Tonic and/or recurrent inhibition, mediated by inhibitory amino acids, may be a key mechanism in EPSP amplitude reductions that occur in CMR neurons. The ability of low doses of agents that enhance the action of GABA (e.g., barbiturates), or inhibit NMDA receptor excitation (e.g., ketamine) to greatly inhibit CMR firing, and of GABA receptor antagonists to enhance firing (Figs. 4 and 5) supports this concept. The short period of inhibition, occurring immediately following the stimulus-evoked peak in neurons in each of the three CMR structures (Fig. 5), is consistent with recurrent inhibition.
When an organism is subjected to external stimulation, exigency, internal milieu changes or antagonists of neurotransmitter-mediated inhibition, these subthreshold neuronal responses can exceed threshold, as seen in the startle response, which is associated with enhanced EPSP amplitude response to startle-evoking stimuli (Schmid and others 2003). The conversion to suprathreshold response would enhance self-organization within the network site and result in positive nonlinear responses (Fig. 3) that result in the expression of an emergent property. This property can lead to increased functional network connections and result in short- and/or long-term neuroplasticity (Fig. 5) that mediates activation of dormant pathways within and between networks, leading to significant changes in network function. Thus, for example, cyclical changes in network function, involving short-term neuroplasticity, occur in the sleep network, which is controlled by sequential interactions of several brain regions, involving multiple neurotransmitters (Luppi and Fort 2014; Motelow and Blumenfeld 2014). In addition, large-scale nonlinear transitions in brain association cortex networks may be crucial for normal mechanisms of consciousness (Del Cul and others 2007).
Neuronal Network and Therapy of CNS Disorders: Animal Models
A better understanding of the network mechanisms of human CNS disorders requires utilization of animal models that exhibit critical elements of the disorder (Blumenfeld 2007). The major advantage of models is that invasive studies can be readily undertaken, which can rarely be done in humans. As noted above, animal models allow the ability to differentiate network structures that are requisite for expression of the disorder from those that are ancillary and not required to produce the behaviors characteristic of the function or the disorder. A recent example of this principle was observed in the network that orients the organisms spatially, which involves specific cell types in one nucleus of the network (parahippocampal region). Lesioning or stimulation of another site in this network (anterior thalamic nucleus) disrupts the function of the network (Winter and others 2015), indicating that the latter site is requisite for this network function. It is vital to determine which specific network structures are requisite, because it has been shown that not all brain sites that exhibit changes during network activation are required to produce the critical aspects of a disorder. Experiments in animals reveal that certain structures that are functionally connected to the network are ancillary, as their activity changes during the disorder, but their inactivation does not prevent the disorder, as discussed below for the amygdala in audiogenic seizures. However, ancillary sites may contribute to comorbid conditions, especially with repetitive network activation (Faingold and Blumenfeld 2014a). Neuron firing changes that subserve the function of the networks and changes induced by stimulation and drug therapies can also be evaluated most readily in animal models. Such data can yield a better understanding of the mechanisms of currently effective therapies and suggest targets for improvements (Faingold and Blumenfeld 2014a).
Proposed steps for future exploration of neuronal networks in CNS disorders and therapy (Table 2) emphasize defining the anatomical and operational elements of the network by techniques, such as neuroimaging, of changes in the brain that occur in the human CNS disorder and evaluating the changes in neuroimaging patterns that are induced by therapies that are currently effective. An example is the fMRI changes in major depression in patients, some of which are reversed with antidepressant drug therapy (Bellani and others 2011). Following establishment of neuroimaging abnormalities in a human disorder, models are needed that mimic the behaviors of the disorder and show similar neuroimaging patterns. An example is the absence (petit mal) type of generalized epilepsy in which the behavior and neuroimaging of human and animal models exhibit similar patterns (Ferris and Tenney 2014; Mishra and others 2011; Motelow and Blumenfeld 2009). When the action of therapies that effectively control the human disorder are evaluated using neuroimaging in the models, it is critical that neuroimaging is done in awake, behaving animals, because even low doses of sedative, analgesic, or anesthetic drugs induce major network changes, as illustrated above (Fig. 4) and by the striking neuroimaging changes induced by anesthesia in an absence epilepsy model (Ferris and Tenney 2014). However, requisite versus ancillary network sites cannot be differentiated using neuroimaging, magnetoencephalography, or electroencephalography, and invasive studies in models are required in which the behavioral effects of focal inactivation of each putative requisite site can be evaluated. This differentiation is critical for selective targeting of therapies. An example of this distinction is the role of the amygdala, which is an ancillary site in an epilepsy model that becomes active during network activation, but blockade of this site does not block the seizure as discussed in detail below (N’Gouemo and others 2014).
Table 2.
Steps in Network Studies for Targeted Therapy of CNS Disorders.
| 1. Network identification—Human neuroimaging—CNS disorders |
| A. Examined in untreated patients |
| B. Evaluate changes with effective therapies (drugs and stimulation paradigms) |
| 2. Network modeling—Animal neuroimaging disorder modela |
| 3. Network operation—Model |
| A. Identification of critical hubsb—focal blockade |
| B. Identify neuron firing abnormalities in hubsc |
| 4. Animal model—Therapeutics |
| A. Determine effective therapies in model—Current, new, and repurposed drugs and stimulation paradigms—Animal neuroimaging changes |
| B. Administer therapeutic doses of drugs and evaluate hub neuron firing changes based on emergent properties |
| C. Evaluate stimulation therapies effect on hub neurons |
| D. Examine combination therapies action on hub neurons |
| 5. Animal model—Mechanistic studies |
| A. Evaluate emergent property action of drugs by local application onto neurons in vivo |
| B. Evaluate intracellular mechanisms in vitro |
| 6. Human trials with new and repurposed therapies |
| 7. Human neuroimaging of effective new therapies |
Without anesthesia.
Crucial to differentiate requisite from ancillary sites, because requisite hubs are critical future targets of therapy.
Critical for identification of abnormal neuronal firing patterns that the therapy is targeted to normalize.
Based on Faingold and Blumenfeld (2014c), with permission.
Identifying the neuronal firing patterns in the requisite hubs in animal models during expression of the disorder is vital to understanding network operation, as correction of neuronal firing abnormalities is critical for establishing the most selective and effectively targeted therapy. Neuronal activity is a direct measure of network operation, and firing changes in requisite network sites induced by effective pharmacotherapies are critical to determining the mechanism of these effects. However, the use of therapeutic drug doses is vital, as excessive doses can exert actions on network sites that may be irrelevant to therapy (Faingold 2004). As noted above, drugs are proposed to act on emergent properties of specific sites in a network to produce their therapeutic effects (see Fig. 1) (Faingold 2014b).
Application of neuronal network approaches to the therapy of CNS disorders would involve combined drug and stimulation therapies targeted to specific emergent properties in a specific network site identified by neuro-imaging to correct abnormal neuronal firing patterns, diminishing network activation, and inhibiting the symptoms. Combined drug and stimulation therapies can potentially exert additive or even synergistic effects analogous to the “two-hit” hypothesis postulated for many brain disorders (e.g., Love 2005). Repetitive activation induced by an external stimulus paradigm or an internal process, such as chronic pain, may also lead to expression of an emergent property of specific network neurons on which specific drugs can exert amplified therapeutic effects. An example is seen in chronic pain syndromes, in which gabapentinoid (gabapentin and pregabalin) (Mishriky and others 2015) anticonvulsant drugs suppress neuronal firing in a requisite hub of the pain network reversing hyperalgesia. Neuroimaging and other techniques support the PAG as a critical hub in chronic pain (Pereira and others 2010). Targeting the PAG with peripheral stimulation combined with gabapentinoids, which depress pain-related PAG neuronal firing (Faingold 2014b), may improve pain control. However, the same drug dose does not affect the responses of the same neurons prior to chronic pain induction (Faingold 2014b). Gabapentinoids may also exert their anticonvulsant action by acting on the PAG as it is a requisite hub in certain epilepsy networks (Tupal and Faingold 2012).
Combination therapies ideally involve a drug that selectively affects neuronal activity in a critical requisite network hub, combined with a stimulation paradigm that affects that site or another requisite hub to exert additive therapeutic effects. In networks for CNS disorders that involve “gain of function,” such as certain forms of epilepsy, this would involve therapies that inhibit neurons in a critical network hub and reduce network activation. Alternatively, a stimulation paradigm that enhances firing could be combined with pharmacotherapy that inhibits that same site to cause “desensitization” of critical neurons. In CNS disorders involving “loss of function,” therapy would be directed to reactivating the damaged network or activating alternative pathways.
Examples of Network Explorations in Epilepsy
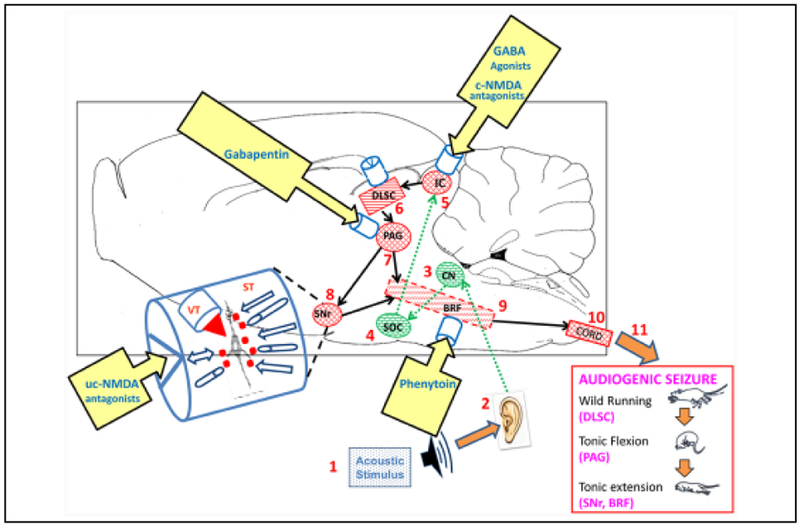
Several steps in Table 2 have evaluated the actions of competitive NMDA receptor antagonists, which are highly effective anticonvulsants in animal models (Faingold 2004). Figure 6 provides extensive details on a well-studied network for audiogenic seizures, an extremely common form of epilepsy in rodents, and details the sites of actions of several drugs with anticonvulsant properties on this network. The effects of therapeutic doses of NMDA receptor antagonists were evaluated in the seizure network of this epilepsy model. Neuronal firing in the seizure initiation site in inferior colliculus (IC), which is the most critical requisite network nucleus, was evaluated (Faingold and others 2014a). Neurons in the IC were inhibited by therapeutic (anticonvulsant) doses of competitive NMDA antagonists in vivo, and these agents were also effective with focal microinjection into IC. Direct application of NMDA antagonists onto IC neurons in the intact animal and in vitro reduced neuronal responses very effectively, indicating that IC neurons are a critical target for the anticonvulsant effects of these agents in this epilepsy model. However, brain regions in the auditory pathway rostral to the IC were not requisite to seizure induction, and in fact, no regions rostral to the level of the midbrain are requisite to these seizures (Faingold and others 2014a). It should be noted that neuronal firing in the lateral amygdala is affected during these seizures, but blockade of this region does not block seizures, rendering the amygdala as an ancillary site in this model, and illustrates this concept. The brainstem network for these audiogenic seizures operates hierarchically during the progression of seizure behaviors from wild running through tonic flexion and tonic extension convulsions. Neurons in the deep layers of superior colliculus are most active during wild running, whereas PAG neurons are most active during tonic flexion and neurons in the BRF and substantia nigra reticulata (SNr) are most active during tonic extension. Neurons in the latter two structures are the only network sites that continue to fire during the postictal depression that follows the seizure (Faingold and others 2014a).
Figure 6.
Diagram of the neuronal network for audiogenic seizures (AGSz) with emergent properties (indicated by cylinders) in each requisite site at which systemically administered drugs that block these seizures may act at therapeutic doses. The network is organized as a hierarchy beginning with the acoustic stimulus (1) input into the auditory pathway (2–4), including neurons in these nuclei (up to the level of the inferior colliculus, IC) (5), whichis the consensus seizure-initiating site. The IC projects to the brainstem locomotion network nuclei, including the deep layersof superior colliculus (DLSC) (6), projecting to the periaqueductal gray (PAG) (7) and substantia nigra reticulata (SNr) (8) and brainstem reticular formation (BRF) (9), which project to the spinal cord (10). The hierarchical activation of each requisite network nucleus produces the sequential behaviors of AGSz (wild running followed by tonic flexion and tonic extension) (11). (The critical structure that initiates each behavior is shown below the behavior.) Neurons in the BRF and SNr are the only regions that are active during the postictal behavioral depression that follows the tonic extension behavior. Therapeutic doses of several drugs with anticonvulsant properties act to inhibit neurons in the IC, including competitive (c-) NMDA antagonists, such as 2-amino-7-phosphonoheptanoate and GABA uptake inhibitor, tiagabine, as well as ethanol. Therapeutic doses of other drugs that are effectively anticonvulsant exert no effect on IC neurons, including an uncompetitive (uc-) NMDA antagonist (MK-801), gabapentin, lamotrigine, and phenytoin. Other effective anticonvulsant drugs act to reduce PAG neuronal firing such as gabapentin. Therapeutic doses of phenytoin selectively act to inhibit neurons in the BRFof the pons. The SNr is the target of MK-801, but the effect is to increase neuronal firing. The emergent property of each nucleus is seen as a confluence of influences onto the neurons in each nucleus, including neuroactive substances (red squares) released onto the neurons via synaptic transmission (ST) and from the blood vessels, cerebrospinal and extracellular fluids via volume transmission (VT), as shown in the expanded diagram of an emergent property on the left of the network. Systemically administered drugs reach each site via VT. Direct stimulation of any site within the network, either chemically or electrically, can affect seizure susceptibility and may modify the emergent properties in the affected site. (CN = cochlear nucleus; SOC = superior olivary complex, which are auditory structures important for input to the IC.) Brain regions in the auditory pathway rostral to the IC were not requisite to seizure induction, and in fact, no regions rostral to the level of the midbrain are requisite to these seizures. It should be noted that neurons in the lateral amygdala are affected by these seizures, but blockade of this region does not block seizures, rendering the amygdala as an ancillary site.
Therapeutic doses of several drugs with anticonvulsant properties have differential effects on the specific network nuclei, as illustrated in Figure 6. The lowest effective doses of agents that enhance the activation of GABAA receptors act on neurons in the IC, whereas phenytoin acts on neurons in the BRF and gabapentin acts on the PAG. As noted above, a surprising dichotomy occurs between the effects of competitive NMDA antagonists, which act on IC neurons, whereas uncompetitive NMDA antagonists do not, despite being more potent anticonvulsants. The uncompetitive NMDA antagonists actually act on SNr neurons differentially (Faingold 2014b). Many important elements of the network exploration paradigm have also been accomplished in absence and temporal lobe forms of epilepsy, examining anticonvulsant drug actions (Hughes 2009; Faingold and Blumenfeld 2014c).
Examples of Potential Future Applications
As the effectiveness of many current CNS drugs and stimulation therapies is incomplete, improved therapy may be obtained with combined treatment especially if targeting is based on network approaches guided by neuroimaging (Table 2). Once the requisite network hubs for a disorder affected by effective stimulation paradigms are identified, addition of a specific drug that also targets this network site or another requisite network site could exert additive effects. For example, additive effects of anticonvulsant drugs with stimulation paradigms are seen in chronic pain syndromes (Barbarisi and others 2010; Lu and others 2011).
Other potential applications of this combined approach include major depression and posttraumatic stress disorder (PTSD). The amygdala is proposed to be a critical neuronal network hub in both disorders (Parsons and Ressler 2013; van der Zwaag and others 2012). To improve therapy of depression a content-specific visual stimulus that activates the amygdala (van der Zwaag and others 2012) could be combined with an uncompetitive NMDA channel blocker (ketamine) that targets the amygdala (Feng and Faingold 2013; Zarate and others 2013); also see Figure 4. A recent approach to therapy of treatment-resistant depression, based on neuroimaging, is utilizing deep brain stimulation in concert with changes in electroencephalography patterns within the network to evaluate important network changes (Smart and others 2015), and these changes could potentially be enhanced by combined brain stimulation with antidepressants or other network-selective agents. Improved treatment of PTSD could potentially involve extinction of fear responses by repeated presentations of a symptom-triggering stimulus under non-fearful conditions (Parsons and Ressler 2013), plus gabapentinoids, which inhibit elements of the PTSD network (Aupperle and others 2012; Tupal and Faingold 2012) and reduce PTSD symptoms (Parsons and Ressler 2013).
In the treatment of stroke, combinations of stimulation (electrical or musical stimuli), which show promise, along with cholinesterase inhibitors or memantine, which are partially effective in stroke treatment (Allen and others 2012; Schlaug and others 2011), may also be a useful approach. Further improvements in stroke treatment could be achieved by identifying the critical connections that are damaged using neuroimaging and combining drug and stimulation strategies to activate dormant network elements to restore function or activate alternate pathways (Meinzer and others 2014).
Neuroimaging in Alzheimer’s disease (AD) has demonstrated changes affecting cognitive networks (Teipel and others 2013). Animal models show patterns analogous to those seen in patients and allow testing of potential therapies (Teng and others 2011). Approved therapies for AD have only achieved partial effectiveness (Schneider and others 2014), and stimulation therapies, including cognitive-stimulating programs and deep brain stimulation, are being utilized experimentally (Feng and others 2012; Laxton and Lozano 2013). Agents that block GABA receptors (Kleschevnikov and others 2012; Mohler 2012) as well as cholinesterase inhibitors and adenosine antagonists (Wostyn and others 2011) that improve animal cognition (Colas and others 2013; Mohler 2012) may do so because they elevate responsiveness in CMR neurons (Faingold and others 2014b); also see Figure 5. Combining these drugs with stimulation therapies and monitoring the effects with neuroimaging techniques may be able to improve the treatment of AD.
Finally, extensive research has delineated the neuronal network underlying reward, associated with drugs of abuse, and key elements in the mesolimbic reward network have been defined using various techniques (Fotros and others 2013). Several drugs that act on the major receptors in the network are able to modulate the effects of abuse-prone agents, blocking the rewarding effects and/or preventing withdrawal syndromes of the specific agents, including ethanol, opiates, and stimulants (Clapp 2012). However, the success of these drug-based therapeutic approaches is often suboptimal and variable. Stimulation-based therapies, including certain protocols and sites of electroacupuncture, have also yielded some success in reducing self-administration of abused agents, ameliorating withdrawal symptoms, and reducing drug-seeking behavior in humans and animal models of drug abuse (Li and others 2011; Meade and others 2010). Combined electroacupuncture and antagonist therapy have shown improved treatment for opiate withdrawal (Lee and others 2011). A recent study evaluated the effect of low-frequency electrical stimulation in a key nucleus in the reward network combined with administration of a dopamine (D1) receptor antagonist and observed that combined therapy was more effective in reversing cocaine “addiction” in rodents than either treatment alone, further supporting the usefulness of the combined approach based on previous network mapping of the reward network (Creed and others 2015).
Conclusion
An integrated approach applying our current knowledge and based on neuronal network concepts has the potential to improve therapy of CNS disorders. Few studies have combined all three approaches of stimulation, pharmacology, and neuroimaging to identify the target and verify that the targeted network abnormalities occurring in the disorder are positively affected by the therapies. The resulting targeted therapies should most effectively enable our current knowledge to most effectively facilitate correction of the specific pathophysiological mechanisms occurring in specific network nuclei that mediate brain disorders and significantly improve the therapy of these disabling CNS disorders.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors gratefully acknowledge support by NINDS, NS 13849, 21281 (CF), and NS052519, NS066974, NS083783 (HB), Epilepsy Foundation (CF), CURE (CF), and NIAAA AA08591 (CF) during the experiments from our laboratories discussed in the text.
Footnotes
Declaration of Conflicting Interests
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
References
- Agnati LF, Genedani S, Spano P, Guidolin D, Fuxe K. 2014. Volume transmission and the Russian-doll organization of brain cell networks: aspects of their integrative actions In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 103–19. [Google Scholar]
- Allen L, Mehta S, McClure JA, Teasell R. 2012. Therapeutic interventions for aphasia initiated more than six months post stroke: a review of the evidence. Top Stroke Rehabil 19(6):523–35. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Tankersley D, Ravindran LN, Flagan T, Stein NR, Stein MB, and others. 2012. Pregabalin effects on neural response to emotional faces. Front Hum Neurosci 6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarisi M, Pace MC, Passavanti MB, Maisto M, Mazzariello L, Pota V, and others. 2010. Pregabalin and transcutaneous electrical nerve stimulation for postherpetic neuralgia treatment. Clin J Pain 26(7):567–72. [DOI] [PubMed] [Google Scholar]
- Bellani M, Dusi N, Yeh PH, Soares JC, Brambilla P. 2011. The effects of antidepressants on human brain as detected by imaging studies. Focus on major depression. Prog Neuropsychopharmacol Biol Psychiatry 35(7):1544–52. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H 2007. Functional MRI studies of animal models in epilepsy. Epilepsia 48(Suppl 4):18–26. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H 2014. What is a seizure network? Long-range network consequences of focal seizures. Adv Exp Med Biol 813:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G 1991. The thalamic clock: emergent network properties. Neuroscience 41(2–3):351–64. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Anastassiou CA, Koch C. 2012. The origin of extra-cellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci 13(6):407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao D, Xia Y. 2013. Acupuncture treatment of epilepsy In: Xia Y, Ding G, Wu GC, editors. Current research in acu-puncture. New York, NY: Springer; p. 129–214. [Google Scholar]
- Clapp P 2012. Current progress in pharmacologic treatment strategies for alcohol dependence. Expert Rev Clin Pharmacol 5(4):427–35. [DOI] [PubMed] [Google Scholar]
- Colas D, Chuluun B, Warrier D, Blank M, Wetmore DZ, Buckmaster P, and others. 2013. Short-term treatment with the GABAA receptor antagonist pentylenetetrazole produces a sustained pro-cognitive benefit in a mouse model of Down’s syndrome. Br J Pharmacol 169(5):963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M, Pascoli VJ, Luscher C. 2015. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science 347(6222):659–64. [DOI] [PubMed] [Google Scholar]
- Davis M, Antoniadis EA, Amaral DG, Winslow JT. 2008. Acoustic startle reflex in rhesus monkeys: a review. Rev Neurosci 19(2–3):171–85. [DOI] [PubMed] [Google Scholar]
- Del Cul A, Baillet S, Dehaene S. 2007. Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol 5(10):e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. 2008. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci 28(36):9066–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold C, Blumenfeld H, editors. 2014a. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Elsevier Science. [Google Scholar]
- Faingold CL. 2004. Emergent properties of CNS neuronal networks as targets for pharmacology: application to anticonvulsant drug action. Prog Neurobiol 72(1):55–85. [DOI] [PubMed] [Google Scholar]
- Faingold CL. 2008. Electrical stimulation therapies for CNS disorders and pain are mediated by competition between different neuronal networks in the brain. Med Hypotheses 71(5):668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL. 2014a. Emergent properties of neuronal networks In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 419–28. [Google Scholar]
- Faingold CL. 2014b. Neuronal network effects of drug therapies for CNS disorders In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 443–65. [Google Scholar]
- Faingold CL, Blumenfeld H. 2014b. Introduction to neuronal networks of the brain In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 1–10. [Google Scholar]
- Faingold CL, Blumenfeld H. 2014c. Future trends in neuronal networks—selective and combined targeting of network hubs In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 467–85. [Google Scholar]
- Faingold CL, Feng HJ. 2014. Neuronal network involvement in stimulation therapies for CNS disorders In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 429–42. [Google Scholar]
- Faingold CL, Hoffmann WE, Caspary DM. 1983. Bicuculline-induced enhancement of sensory responses and cross-correlations between reticular formation and cortical neurons. Electroencephogr Clin Neurophysiol 55(3):301–13. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Raisinghani M, N’Gouemo P. 2014a. Neuronal networks in epilepsy: comparative audiogenic seizure networks In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 349–73. [Google Scholar]
- Faingold CL, Randall M, Long X, Uteshev VV, Kommajosyula SP, Tupal S. 2015. Neurotransmitters implicated in control of sudden unexpected death in epilepsy in animal models In: Lathers CM, Wannamaker BB, Schraeder PL, Leestma JE, Schacter SC, Verrier RL, editors. Sudden unexpected death in epilepsy: mechanisms and new methods for analyzing risks. Boca Raton, FL: Taylor & Francis/CRC Press; p. 251–67. [Google Scholar]
- Faingold CL, Riaz A, Stittsworth JD Jr. 2014b. Neuronal network plasticity and network interactions are critically dependent on conditional multireceptive (CMR) brain regions In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 387–406. [Google Scholar]
- Faingold CL, Tupal S. 2014. Neuronal network interactions in the startle reflex, learning mechanisms, and CNS disorders, including sudden unexpected death in epilepsy In: Faingold CL, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 407–18. [Google Scholar]
- Feng HJ, Faingold CL. 2013. Ketamine in mood disorders and epilepsy In: Costa A, Villalba E, editors. Horizons in neuroscience research. Vol. 10 New York, NY: Nova Science; p. 103–22. [Google Scholar]
- Feng HJ, Faingold CL. 2014. Network experimental approaches: inactivation, microinjection, neuronal stimulation, and recording In: Faingold CL, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 55–66. [Google Scholar]
- Feng Y, Bai L, Ren Y, Chen S, Wang H, Zhang W, and others. 2012. FMRI connectivity analysis of acupuncture effects on the whole brain network in mild cognitive impairment patients. Magn Reson Imaging 30(5):672–82. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Tenney J. 2014. Functional magnetic resonance imaging in epilepsy: methods and applications using awake animals In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 37–54. [Google Scholar]
- Fotros A, Casey KF, Larcher K, Verhaeghe JA, Cox SM, Gravel P, and others. 2013. Cocaine cue-induced dopamine release in amygdala and hippocampus: a high-resolution PET [(1)(8)F]fallypride study in cocaine dependent participants. Neuropsychopharmacology 38(9):1780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WJ, Kozma R, Werbos PJ. 2001. Biocomplexity: adaptive behavior in complex stochastic dynamical systems. Biosystems 59(2):109–23. [DOI] [PubMed] [Google Scholar]
- Geis HR, Schmid S. 2011. Glycine inhibits startle-mediating neurons in the caudal pontine reticular formation but is not involved in synaptic depression underlying short-term habituation of startle. Neurosci Res 71(2):114–23. [DOI] [PubMed] [Google Scholar]
- Guo JN, Blumenfeld H. 2014. Network imaging In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 77–89. [Google Scholar]
- Hughes JR. 2009. Absence seizures: a review of recent reports with new concepts. Epilepsy Behav 15(4):404–12. [DOI] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Faizi M, Jacobs LF, Htun K, Shamloo M, and others. 2012. Deficits in cognition and synaptic plasticity in a mouse model of Down syndrome ameliorated by GABAB receptor antagonists. J Neurosci 32(27):9217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. 2015. The dynamic pain connectome. Trends Neurosci 38(2):86–95. [DOI] [PubMed] [Google Scholar]
- Larson J, Munkacsy E. 2014. Theta-burst LTP. Brain Res. doi:10.1016/j.brainres.2014.10.034. Epub Oct 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Schiff ND. 2012. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage 61(2):478–91. [DOI] [PubMed] [Google Scholar]
- Laxton AW, Lozano AM. 2013. Deep brain stimulation for the treatment of Alzheimer disease and dementias. World Neurosurg 80(3–4):S28.e1–8. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim HY, Jang EY, Choi SH, Han CH, Lee BH, and others. 2011. Effect of acupuncture on naloxone-precipitated withdrawal syndrome in morphine-experienced rats: the mediation of GABA receptors. Neurosci Lett 504(3):301–5. [DOI] [PubMed] [Google Scholar]
- Li J, Zou Y, Ye JH. 2011. Low frequency electroacupuncture selectively decreases voluntarily ethanol intake in rats. Brain Res Bull 86(5–6):428–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz EN. 1972. Predictability: does the flap of a butterfly’s wings in Brazil set off a tornado in Texas? Available from: http://eaps4.mit.edu/research/Lorenz/Butterfly_1972.pdf.
- Love R 2005. Two hit hypothesis for temporal lobe epilepsy. Lancet Neurol 4(8):458. [DOI] [PubMed] [Google Scholar]
- Lu DP, Lu WI, Lu GP. 2011. Phenytoin (Dilantin) and acupuncture therapy in the treatment of intractable oral and facial pain. Acupunct Electrother Res 36(1–2):65–84. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Fort P. 2014. Networks of normal and disordered sleep In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 299–310. [Google Scholar]
- Margineanu DG. 2014. Systems biology, complexity, and the impact on antiepileptic drug discovery. Epilepsy Behav 38:131–42. [DOI] [PubMed] [Google Scholar]
- Meade CS, Lukas SE, McDonald LJ, Fitzmaurice GM, Eldridge JA, Merrill N, and others. 2010. A randomized trial of transcutaneous electric acupoint stimulation as adjunctive treatment for opioid detoxification. J Subst Abuse Treat 38(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Lindenberg R, Sieg MM, Nachtigall L, Ulm L, Floel A. 2014. Transcranial direct current stimulation of the primary motor cortex improves word-retrieval in older adults. Front Aging Neurosci 6:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Wall PD. 1965. Pain mechanisms: a new theory. Science 150(3699):971–9. [DOI] [PubMed] [Google Scholar]
- Mishra AM, Ellens DJ, Schridde U, Motelow JE, Purcaro MJ, DeSalvo MN, and others. 2011. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. J Neurosci 31(42):15053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishriky BM, Waldron NH, Habib AS. 2015. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 114(1):10–31. [DOI] [PubMed] [Google Scholar]
- Mohler H 2012. Cognitive enhancement by pharmacological and behavioral interventions: the murine Down syndrome model. Biochem Pharmacol 84(8):994–9. [DOI] [PubMed] [Google Scholar]
- Motelow J, Blumenfeld H. 2014. Consciousness and subcortical arousal systems In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 277–98. [Google Scholar]
- Motelow JE, Blumenfeld H. 2009. Functional neuroimaging of spike-wave seizures. Methods Mol Biol 489:189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motelow JE, Li W, Zhan Q, Mishra AM, Sachdev RN, Liu G, and others. 2015. Decreased subcortical cholinergic arousal in focal seizures. Neuron 85(3):561–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Gouemo P, Garcia-Cairasco N, Faingold CL. 2014. Physiological and pathophysiological expansion of neuronal networks In: Faingold C, Blumenfeld H, editors. Neuronal networks in brain function, CNS disorders, and therapeutics. San Diego, CA: Academic Press; p. 375–85. [Google Scholar]
- Pace RW, Del Negro CA. 2008. AMPA and metabotropic glutamate receptors cooperatively generate inspiratory-like depolarization in mouse respiratory neurons in vitro. Eur J Neurosci 28(12):2434–42. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Ressler KJ. 2013. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci 16(2):146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. 2012. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36(9):2130–42. [DOI] [PubMed] [Google Scholar]
- Pereira EA, Lu G, Wang S, Schweder PM, Hyam JA, Stein JF, and others. 2010. Ventral periaqueductal grey stimulation alters heart rate variability in humans with chronic pain. Exp Neurol 223(2):574–81. [DOI] [PubMed] [Google Scholar]
- Pothmann L, Muller C, Averkin RG, Bellistri E, Miklitz C, Uebachs M, and others. 2014. Function of inhibitory micronetworks is spared by Na+ channel-acting anticonvulsant drugs. J Neurosci 34(29):9720–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Wan CY. 2011. The use of non-invasive brain stimulation techniques to facilitate recovery from post-stroke aphasia. Neuropsychol Rev 21(3):288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S, Simons NS, Schnitzler HU. 2003. Cellular mechanisms of the trigeminally evoked startle response. Eur J Neurosci 17(7):1438–44. [DOI] [PubMed] [Google Scholar]
- Schneider-Mizell CM, Parent JM, Ben-Jacob E, Zochowski MR, Sander LM. 2010. From network structure to network reorganization: implications for adult neurogenesis. Phys Biol 7(4):046008. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, and others. 2014. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med 275(3):251–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. 2008. Negative BOLD with large increases in neuronal activity. Cereb Cortex 18(8):1814–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart OL, Tiruvadi VR, Mayberg HS. 2015. Multimodal approaches to define network oscillations in depression. Biol Psychiatry 77(12):1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ. 2014. Modern network science of neurological disorders. Nat Rev Neurosci 15(10):683–95. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Grothe M, Lista S, Toschi N, Garaci FG, Hampel H. 2013. Relevance of magnetic resonance imaging for early detection and diagnosis of Alzheimer disease. Med Clin North Am 97(3):399–424. [DOI] [PubMed] [Google Scholar]
- Teng E, Kepe V, Frautschy SA, Liu J, Satyamurthy N, Yang F, and others. 2011. [F-18]FDDNP microPET imaging correlates with brain Abeta burden in a transgenic rat model of Alzheimer disease: effects of aging, in vivo blockade, and anti-Abeta antibody treatment. Neurobiol Dis 43(3): 565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupal S, Faingold CL. 2012. The amygdala to periaqueductal gray pathway: plastic changes induced by audiogenic kindling and reversal by gabapentin. Brain Res 1475:71–9. [DOI] [PubMed] [Google Scholar]
- van der Zwaag W, Da Costa SE, Zurcher NR, Adams RB Jr, Hadjikhani N. 2012. A 7 tesla FMRI study of amygdala responses to fearful faces. Brain Topogr 25(2):125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Sprekeler H, Zenke F, Clopath C, Gerstner W. 2011. Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science 334(6062):1569–73. [DOI] [PubMed] [Google Scholar]
- Winter SS, Clark BJ, Taube JS. 2015. Spatial navigation. Disruption of the head direction cell network impairs the parahippocampal grid cell signal. Science 347(6224): 870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostyn P, Van Dam D, Audenaert K, De Deyn PP. 2011 Increased cerebrospinal fluid production as a possible mechanism underlying caffeine’s protective effect against Alzheimer’s disease. Int J Alzheimers Dis 2011: 617420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, and others. 2013. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry 74(4):257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]