Abstract
Context and Objective:
Nonalcoholic fatty liver disease (NAFLD) and its more severe form with steatohepatitis (NASH) are common in patients with type 2 diabetes mellitus (T2DM). However, they are usually believed to largely affect those with elevated aminotransferases. The aim of this study was to determine the prevalence of NAFLD by the gold standard, liver magnetic resonance spectroscopy (1H-MRS) in patients with T2DM and normal aminotransferases, and to characterize their metabolic profile.
Participants and Methods:
We recruited 103 patients with T2DM and normal plasma aminotransferases (age, 60 ± 8 y; body mass index [BMI], 33 ± 5 kg/m2; glycated hemoglobin [A1c], 7.6 ± 1.3%). We measured the following: 1) liver triglyceride content by 1H-MRS; 2) systemic insulin sensitivity (homeostasis model assessment-insulin resistance); and 3) adipose tissue insulin resistance, both fasting (as the adipose tissue insulin resistance index: fasting plasma free fatty acids [FFA] × insulin) and during an oral glucose tolerance test (as the suppression of FFA).
Results:
The prevalence of NAFLD and NASH were much higher than expected (50% and 56% of NAFLD patients, respectively). The prevalence of NAFLD was higher in obese compared with nonobese patients as well as with increasing BMI (P = .001 for trend). Higher plasma A1c was associated with a greater prevalence of NAFLD and worse liver triglyceride accumulation (P = .01). Compared with nonobese patients without NAFLD, patients with NAFLD had severe systemic (liver/muscle) and, particularly, adipose tissue (fasting/postprandial) insulin resistance (all P < .01).
Conclusions:
The prevalence of NAFLD is much higher than previously believed in overweight/obese patients with T2DM and normal aminotransferases. Moreover, many are at increased risk of NASH. Physicians should have a lower threshold for screening patients with T2DM for NAFLD/NASH.
Nonalcoholic fatty liver disease (NAFLD) is considered to be the most common cause of chronic liver disease in the United States and many parts of the world (1). However, the prevalence of NAFLD, particularly in patients with type 2 diabetes mellitus (T2DM), remains unknown. When screening by means of plasma aminotransferases (aspartate aminotransferase [AST]/alanine aminotransferase [ALT]), the prevalence has been reported to be as low as ∼15–20% (2) but is reported to be higher and more variable (20–46%) with the use of liver ultrasound (3, 4). When the gold standard magnetic resonance imaging and spectroscopy (1H-MRS) technique is used, the prevalence has been reported to be 34% in the general population (5). However, it is well established that the risk of developing NAFLD is much higher (∼2-fold) in the setting of obesity compared with healthy nonobese patients (5, 6).
There is very limited information on the prevalence of NAFLD in patients with T2DM but it is believed to be even higher. However, this information arises largely from ultrasound-based studies in highly selected populations with predominantly elevated liver transaminases (7–10). The most important limitations of such studies include the clinic-based recruitment strategy, with the risk of ascertainment bias (ie, screening primarily directed to patients with abnormal plasma aminotransferases or history of steatosis attending a liver clinic), and the low sensitivity and specificity of the liver ultrasound, particularly in obese patients. In our experience, using a 2-step approach (liver 1H-MRS followed by a liver biopsy when 1H-MRS was positive), we have found a prevalence of NAFLD > 80% in obese patients with elevated plasma aminotransferases, and even higher in the presence of T2DM (11, 12). However, the prevalence of NAFLD by 1H-MRS in patients with T2DM and normal plasma aminotransferases is unknown.
Diabetes has been recognized as an important risk factor for the presence and severity of steatohepatitis (NASH) and fibrosis (13). However, the mechanisms relating T2DM and NAFLD/NASH, especially in the setting of normal plasma aminotransferases, are unclear at the present time. Fracanzani et al (14) reported that T2DM and insulin resistance were factors closely associated with the severity of liver disease in patients with normal liver enzymes. However, this study only included a minority of patients with T2DM and normal plasma aminotransferases (n = 7; 11%). Of note, the role of glycemic control on the development of NASH and severity of liver disease has not been carefully assessed in this population before (15).
The purpose of our study was to determine the prevalence of NAFLD in patients with T2DM and normal plasma aminotransferases and to determine the role of glycemic control and other metabolic factors associated with insulin resistance on the severity of liver disease.
Research Design and Methods
Subjects
We screened a total of 170 patients with T2DM without a prior diagnosis of NAFLD and after excluding other causes of liver disease. A total of 38 patients were excluded because they did not have a liver 1H-MRS performed, and 29 patients were excluded for having elevated plasma aminotransferase levels. A total of 103 patients with T2DM were enrolled onto this study. Patients were recruited from responses to local newspaper advertisements or from people attending clinics at the University of Texas Health Science Center and Audie L. Murphy Veterans' Affairs (VA) Medical Center at San Antonio, Texas or at the University of Florida and Malcom Randall VA Medical Center at Gainesville, Florida. Forty-six patients were recruited from San Antonio, Texas, and fifty-seven from Gainesville, Florida.
Participants were in good general health without evidence of any chronic disease as determined by medical history, physical examination, routine blood chemistries, urinalysis, and electrocardiography (other than T2DM or the metabolic syndrome). No patient had evidence of clinically significant chronic kidney disease or cardiovascular disease.
Volunteers were excluded if they had a history of significant alcohol consumption (> 20 g/d in women or > 30 g/d in men), any previous diagnosis of chronic liver disease (hepatitis B or C, autoimmune hepatitis, hemochromatosis, Wilson disease, drug-induced disease, or other), type 1 diabetes, or a history of clinically significant renal, pulmonary, or heart disease (New York Heart Classification > Grade II). The study was approved by local institutional review boards, and a written informed consent was obtained from each patient prior to participation. Diabetes status was defined based upon medical history or laboratory results following standard criteria (16). Some of the patients were previously included in other reports assessing the role of ethnicity (12), and insulin resistance (11, 17, 18) on the pathogenesis of NAFLD.
Study design
Metabolic studies were performed either at the Frederic C. Bartter Clinical Research Unit (San Antonio, Texas) or at the Malcom Randall VA Medical Center (Gainesville, Florida). Baseline measurements included: 1) fasting plasma glucose, glycated hemoglobin (A1c), lipid profile, plasma aminotransferases, fasting plasma insulin (FPI), and free fatty acids (FFA); 2) total body fat by dual-energy x-ray absorptiometry (DXA); 3) liver triglyceride content by 1H-MRS; 4) 75-g oral glucose tolerance test (OGTT) to establish the diagnosis of diabetes according to current guidelines from the American Diabetes Association; and 5) liver histology to establish the diagnosis of NASH.
Measurements of total body and liver triglyceride content
Total body fat was measured by DXA (Hologic Inc.). For the quantification of liver triglyceride content a localized 1H-nuclear magnetic resonance spectra of the liver was obtained using methodology previously described (17, 18). A voxel of 30 × 30 × 30 mm was positioned in three areas of the liver, avoiding vascular and biliary structures, using an echo time/repetition time/angle of 30 milliseconds/2000 milliseconds/90 degrees. The diagnosis of NAFLD was established when liver triglyceride content was greater than 5.5% (5).
OGTT
After a 10-hour overnight fast, baseline blood samples were obtained for determination of fasting plasma glucose, insulin and FFA concentrations. After the administration of 75 g of glucose, samples were collected every 30 minutes for up to 120 minutes to determine plasma glucose, insulin, and FFA concentrations.
Liver biopsy
An ultrasound-guided liver biopsy was performed in patients diagnosed with NAFLD by 1H-MRS and at high risk of NASH due to elevated liver triglyceride content (>5.5%) in the setting of T2DM, once all other causes of liver disease were ruled out. Both are considered risk factors for NASH, particularly in the presence of obesity and insulin resistance. However, if patients had less than 5.5% liver triglyceride content by 1H-MRS, a liver biopsy was not performed to avoid this invasive procedure in cases with borderline or mild steatosis, which, in the setting of normal liver aminotransferases was believed at the time of study design to be infrequently associated with NASH and not justify performing a liver biopsy. Histopathological characteristics for the diagnosis of NASH were determined using standard criteria (19). Biopsies were evaluated by an experienced pathologist who was unaware of the patients' identity or any clinical information.
Analytical methods
Plasma glucose was measured bedside by the glucose oxidase method (Analox Glucose Analyzer; Analox Instruments). Other samples were placed on ice, processed within 15–20 minutes and frozen at −80°C until final analysis. Plasma insulin concentration was determined by RIA (Siemens), FFA by standard colorimetric methods, and A1c level was measured using high-performance liquid chromatography (Tosoh G7; Tosoh Corporation). The normal value for plasma AST and ALT levels was the clinical laboratory reference of less than 40 U/L.
Calculations
During the fasting state, we calculated an index of adipose tissue insulin resistance (Adipo-IRindex = fasting FFA × FPI [mmol/L × μU/mL]). As insulin is a strong inhibitor of lipolysis, the rationale for this index is based on the linear relationship between the increase in FPI level and inhibition of plasma FFA in healthy subjects. As extensively validated in prior reports (11, 12, 18), the implication is that the higher the fasting plasma FFA levels for a given FPI, the greater the severity of adipose tissue insulin resistance. During the postprandial state, we measured the suppression of plasma FFA concentration over the 120 minutes following the ingestion of 75 g of glucose (OGTT). The postprandial suppression of FFA was calculated as follows: ([FFA min120 − FFA min0]/FFA min0) × 100.
Statistical analysis
Normal distribution was assessed for all variables by the Shapiro-Wilk and Skewness/Kurtosis methods. Continuous variables were reported as mean ± SD or median (interquartile range) according to their distribution, whereas categorical variables were expressed as a percentage. Comparisons between groups were performed using χ2 (or Fisher's exact test) for categorical variables and an unpaired t test or a nonparametric test (ie, Mann-Whitney U test) for continuous variables according to their distribution. When comparisons were performed among more than two groups, the ANOVA and Kruskal-Wallis tests were used. Statistical significance was considered at P < .05. Statistical analyses were performed using STATA version 11.0 (StataCorp) and JMP version 11 (SAS Institute, Inc.).
Results
Patient characteristics
A total of 103 subjects with T2DM were recruited, and their baseline characteristics are summarized in Table 1. As a whole, subjects were predominantly obese, accounting for almost 70% of our participants. Patients were divided according to their body mass index (BMI) into two groups: nonobese and obese (mean BMI, 27.6 ± 1.8 kg/m2 vs 35.4 ± 3.8 kg/m2; P < .001). A statistically significant difference in total body fat by DXA was found between groups (32 ± 6 vs 38 ± 7%; P < .001). Glycemic control (and use of diabetes medications) was similar in nonobese and obese patients (A1c, 6.6% [6.1–7.6%] vs 6.9% [6.4–7.8%]; P = .24, and fasting plasma glucose, 130 [113–164] mg/dL vs 133 [116–158] mg/dL; P = .93). Compared with obese patients, insulin resistance (expressed as the homeostasis model assessment–insulin resistance [HOMA-IR]) was lower in nonobese patients (1.7 [1.1–3.2] vs 3.0 [1.9–4.6]; P = .02), as was the trend for lower liver triglyceride content measured by 1H-MRS (nonobese, 3% [2–11%] vs obese, 7% [3–14%]; P = .05).
Table 1.
Clinical Characteristics of Nonobese and Obese Patients with T2DM and Normal Liver Aminotransferases
| Nonobese (n = 31) | Obese (n = 72) | P | |
|---|---|---|---|
| Age, y | 60 ± 8 | 60 ± 8 | .72 |
| Sex (male) | 87% | 78% | .28 |
| Ethnicity | .07 | ||
| Hispanic | 23% | 31% | |
| Caucasian | 48% | 59% | |
| African American | 26% | 10% | |
| Asian | 3% | 0% | |
| BMI, kg/m2 | 27.6 ± 1.8 | 35.4 ± 3.8 | <.001 |
| Total body fat | 32 ± 6% | 38 ± 7% | <.001 |
| Plasma AST, IU/L | 20 (16–27) | 22 (19–25) | .64 |
| Plasma ALT, IU/L | 23 (17–23) | 23 (20–30) | .44 |
| Plasma alkaline phosphatase, IU/L | 61 ± 14 | 66 ± 17 | .26 |
| Liver triglyceride contenta | 3% (2–11%) | 7% (3–14%) | .05 |
| Prevalence of NAFLDa | 36% | 56% | .06 |
| Duration of T2DM, y | 8 (5–12) | 6 (2–10) | .12 |
| A1c | 6.6% (6.1–7.6%) | 6.9% (6.4–7.8%) | .24 |
| Fasting plasma glucose, mg/dL | 130 (113–164) | 133 (116–158) | .93 |
| HOMA-IR | 1.7 (1.1–3.2) | 3.0 (1.9–4.6) | .02 |
| Treatment | |||
| Metformin | 92% | 77% | .14 |
| Sulfonylurea | 58% | 44% | .23 |
| Insulin | 21% | 27% | .55 |
| Systolic BP, mm Hg | 128 ± 17 | 138 ± 17 | .02 |
| Diastolic BP, mm Hg | 78 ± 10 | 77 ± 8 | .78 |
| Antihypertensive medications | 73% | 90% | .04 |
| Plasma cholesterol, mg/dL | 156 ± 38 | 158 ± 37 | .82 |
| Plasma triglycerides, mg/dL | 104 (78–137) | 120 (86–174) | .14 |
| Plasma LDL-C, mg/dL | 89 ± 31 | 87 ± 29 | .82 |
| Plasma HDL-C, mg/dL | 43 ± 13 | 40 ± 9 | .10 |
| Statin use | 96% | 75% | .03 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Continuous variables are expressed as mean ± sd or median (interquartile range) according to their distribution.
Categorical variables are expressed as percentage.
Measured by 1H-MRS.
Prevalence of NAFLD and NASH
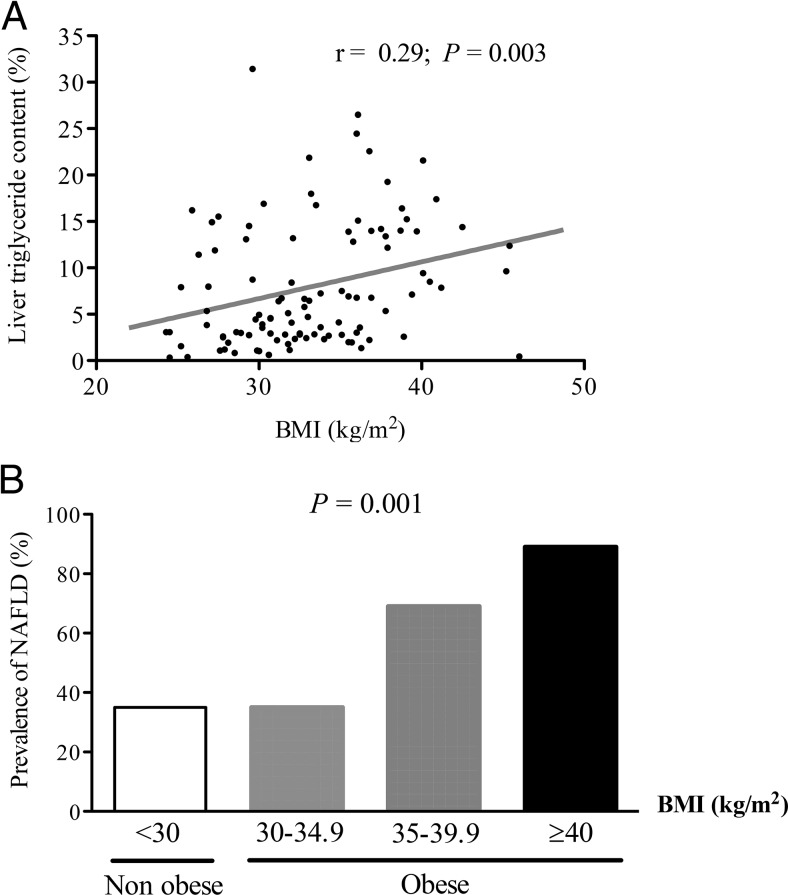
The prevalence of NAFLD (by 1H-MRS) for the entire cohort was 50%. As can be observed in Figure 1, liver triglyceride content was correlated with obesity (r = 0.29; P = .003), and this remained true after adjusting for age, sex, and diabetes control (A1c) (r = 0.20; P = .04). Also, the prevalence of NAFLD was associated with an increasing BMI per group (P = .001 for trend). Of note, this prevalence was relatively high, even in nonobese patients with T2DM and normal aminotransferase levels (36%).
Figure 1.
Relationship between BMI and liver triglyceride content measured by 1H-MRS.
A, Correlation between BMI and liver triglyceride content. B, Prevalence of NAFLD according to different BMI groups (n = 31, 34, 29, and 9, respectively).
To assess the factors that contribute to liver triglyceride accumulation, we divided patients according to the presence or absence of NAFLD. As observed in Table 2, both groups were well matched for most relevant clinical variables (ie, sex, duration of diabetes, dyslipidemia, and blood pressure [BP]), but patients with NAFLD had a trend toward a higher A1c (6.6% [6.0–7.7%] vs 6.9% [6.5–7.9%]; P = .07). A total of 66 participants did not undergo a liver biopsy: 43 because they had less than 5.5% liver triglyceride content by 1H-MRS and 23 patients who refused to a liver biopsy in the setting of normal liver transaminases. Of note, when patients who refused a liver biopsy were compared with those who underwent a liver biopsy, no significant differences were found between groups (data not shown). The mean NAFLD activity score was 3.2 ± 1.3 and the fibrosis stage was 0.5 ± 0.7 among all patients undergoing a liver biopsy (simple steatosis and NASH). The prevalence of NASH was 56%. Supplemental Table 1 summarizes the clinical characteristics of patients with and without NASH.
Table 2.
Clinical Characteristics of Patients with T2DM and Normal Liver Aminotransferases with or without NAFLD
| No-NAFLD (n = 52) | NAFLD (n = 51) | P | |
|---|---|---|---|
| Age, y | 61 ± 7 | 58 ± 8 | .02 |
| Sex, male | 83% | 78% | .58 |
| Ethnicity | .001 | ||
| Hispanic | 15% | 41% | |
| Caucasian | 58% | 55% | |
| African American | 25% | 4% | |
| Asian | 2% | 0% | |
| BMI, kg/m2 | 31.4 ± 4.1 | 34.7 ± 5.03 | <.001 |
| Plasma AST, IU/L | 20 (17– 24) | 23 (16–26) | .10 |
| Plasma ALT, IU/L | 20 (16–25) | 28 (22–33) | <.001 |
| Plasma alkaline phosphatase, IU/L | 63 ± 13 | 71 ± 24 | .43 |
| Liver triglyceride contenta | 3% (2–4%) | 14% (8–16%) | <.001 |
| Duration of T2DM, y | 7 (2–11) | 7 (3–10) | .91 |
| A1c | 6.6% (6.0–7.7%) | 6.9% (6.5–7.9%) | .07 |
| Fasting plasma glucose, mg/dL | 126 (113–160) | 137 (117–160) | .31 |
| HOMA-IR | 2.2 (1.2–3.5) | 3.8 (2.3–5.2) | <.001 |
| Treatment | |||
| Metformin | 83% | 80% | .65 |
| Sulfonylurea | 44% | 51% | .50 |
| Insulin | 23% | 28% | .55 |
| Systolic BP, mm Hg | 133 ± 16 | 137 ± 18 | .22 |
| Diastolic BP, mm Hg | 78 ± 8 | 77 ± 9 | .49 |
| Antihypertensive medications | 85% | 86% | .93 |
| Plasma cholesterol, mg/dL | 150 ± 32 | 164 ± 40 | .04 |
| Plasma triglycerides, mg/dL | 103 (78–134) | 142 (96–195) | .002 |
| Plasma LDL-C, mg/dL | 85 ± 25 | 91 ± 34 | .28 |
| Plasma HDL-C, mg/dL | 42 ± 9 | 40 ± 12 | .46 |
| Statin use | 82% | 79% | .75 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Continuous variables are expressed as mean ± sd or median (interquartile range) according to their distribution.
Categorical variables are expressed as percentage.
Measured by 1H-MRS.
The role of insulin sensitivity in the development of NAFLD
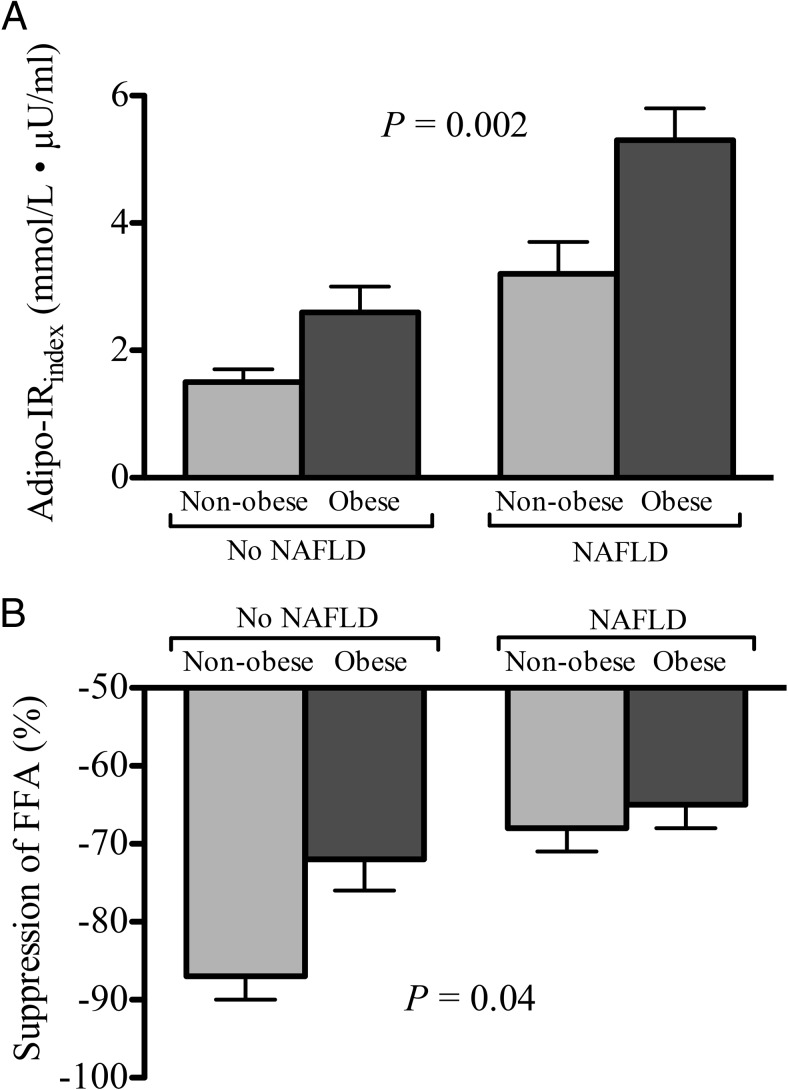
Patients with NAFLD were consistently more insulin resistant than their counterparts without NAFLD, both systemically (HOMA-IR, 2.2 [1.2–3.5] vs 3.8 [2.3–5.2]; P < .001) and at the level of adipose tissue (Adipo-IRindex, 5.8 ± 3.6 vs 2.5 ± 1.9 mmol/L ° μU/mL; P < .0001). In Figure 2 we assessed degree of adipose tissue insulin resistance during the fasting (Adipo-IRindex) and postprandial (suppression of FFA during the OGTT) states, dividing patients according to NAFLD and obesity status. In the fasting state, we found a stepwise increase in adipose tissue insulin resistance with the presence of obesity and NAFLD (Adipo-IRindex; P = .002). A similar finding was observed during the postprandial state, when we examined the suppression of plasma FFA concentration after the oral glucose load in the same groups (P = .01).
Figure 2.
The role of obesity and NAFLD on adipose tissue insulin sensitivity.
A, Adipo-IRindex (Adipo-IRindex = fasting plasma FFA × fasting plasma insulin concentration). B, Percentage suppression of plasma FFA concentration after an OGTT. Patients with NAFLD had worse adipose tissue insulin resistance when compared with those without NAFLD (No-NAFLD). Results are expressed as the mean ± SEM. P-value represents P for trend (n = 7, 10, 16, and 49, respectively).
Relationship between diabetes control and hepatic triglyceride content
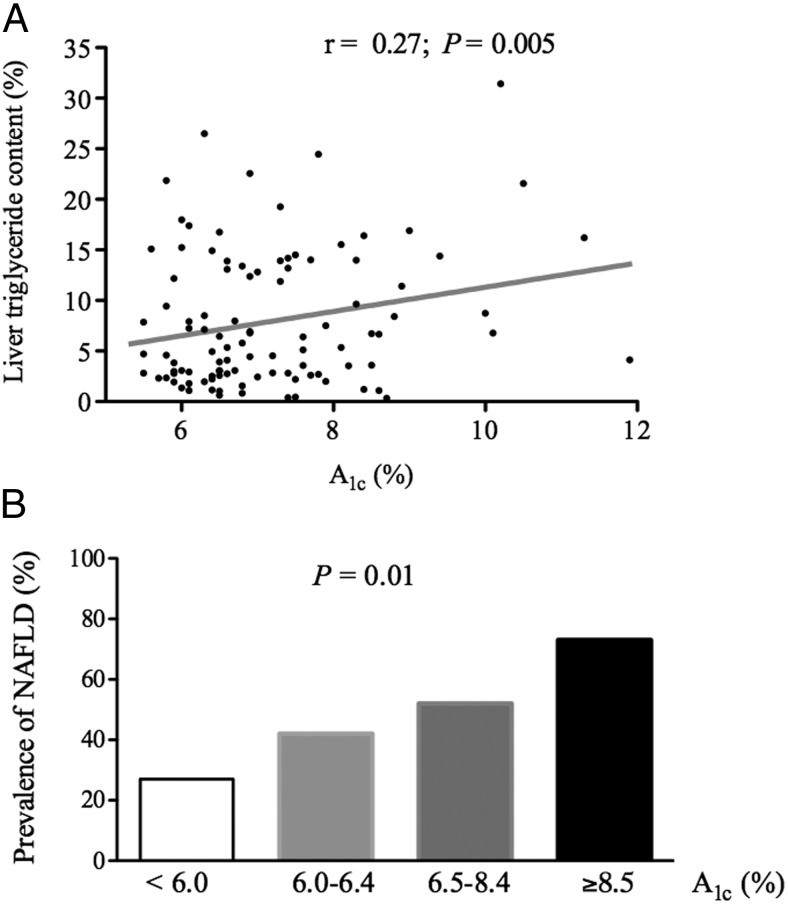
To assess whether diabetes control is related to the severity of hepatic steatosis, we examined the relationship between plasma A1c concentration and liver triglyceride content. We observed an association between them (r = 0.27; P = .005; Figure 3A), that was more evident when patients were divided into four groups according to their plasma A1c level: <6.0, 6.0–6.4, 6.5–8.4, and ≥8.5%. In doing so, there was a clear stepwise increase in the prevalence of NAFLD from 27, 42, 52, and 73%, respectively (P = .01; Figure 3B). Of note, the correlation of A1c and liver triglyceride content was independent of age, sex, and BMI when a multivariate analysis was performed. Moreover, plasma A1c was also correlated with fibrosis stage on liver histology (r = 0.37; P = .02). However, plasma A1c levels did not correlate with hepatic inflammation (r = 0.13; P = .41), ballooning of hepatocytes (r = 0.24; P = .14), or overall liver disease activity, as standardized by the NAFLD activity score (r = 0.12; P = .53). Consistent with the plasma A1c concentration findings, the fasting plasma glucose level correlated with the severity of hepatic fibrosis (r = 0.35; P = .04), but not to steatosis (r = −0.20; P = .25), inflammation (r = 0.07; P = .22), or hepatocyte ballooning (r = 0.08; P = .66).
Figure 3.
Relationship between glycemic control on liver triglyceride content measured by 1H-MRS.
A, Correlation between plasma A1c levels and liver triglyceride content. B, Prevalence of NAFLD among patients with a broad spectrum of plasma A1c levels (n = 15, 19, 54, and 15, respectively).
Discussion
Although obesity and T2DM are believed to be associated with an increased prevalence of NAFLD, no prior studies have examined this in asymptomatic patients with T2DM and normal liver aminotransferase levels, a population that most healthcare providers consider free of liver disease. However, most physicians are unaware that plasma aminotransferases, and often even liver ultrasound, the usual tests to assess liver disease in clinical practice, are not sensitive for the diagnosis of NAFLD (20). We believed that it was clinically relevant to establish the prevalence of NAFLD and NASH in this population, not only because T2DM is considered a major risk factor for the development of steatohepatitis (NASH) and liver fibrosis (21), but also because NAFLD may potentially worsen the risk of micro- (22) and macrovascular (23, 24) disease in patients with diabetes. This study is unique by screening such patients with the gold-standard technique of 1H-MRS, performing a liver biopsy when possible to assess for NASH, and establishing the prevalence of both NAFLD and NASH in a predominantly obese population with diabetes and normal aminotransferase levels. It also examined the role of insulin resistance, particularly in adipose tissue, as an underlying mechanism at play in this setting, and extending prior observations in this regard (18). The major findings are that NAFLD and NASH occur much more frequently than anticipated, and seem closely associated with dysfunctional adipose tissue. Taken together, these findings have important clinical implications to the management of patients with T2DM.
The association between diabetes and NAFLD is complex, but prospective studies have found that the presence of NAFLD has negative metabolic effects and is associated with a 3- to 5-fold increased risk of developing T2DM (25, 26). Several studies using liver ultrasound have reported that obese patients with T2DM may often have hepatic steatosis (∼60–70%) (7–10) but the real prevalence has remained uncertain given the low overall sensitivity/specificity of liver ultrasound in obese patients (27).
Studies from hepatology clinics, many of which have likely included patients with advanced liver disease or cirrhosis, have reported the presence of NASH in patients with normal plasma aminotransferases (14, 28), but none had focused on patients with T2DM, leaving the real magnitude of the problem in asymptomatic patients with diabetes unknown. Beyond these studies in highly selected populations, our goal was to establish the prevalence in overall healthy patients with T2DM with normal aminotransferases. This is a group wrongfully considered to be unaffected by liver disease, even when many of these patients have a cluster of risk factors for NAFLD. In the first study reporting on the prevalence of NAFLD in T2DM with normal aminotransferases, we were stunned by the very high prevalence of NAFLD (50%), and greater than this when obese patients alone were considered. Moreover, the very high prevalence of NAFLD in overweight subjects with normal plasma aminotransferase concentration was unexpected (36%). However, these results are consistent with the only prior study using 1H-MRS in patients with T2DM (29), in which two thirds had normal plasma aminotransferases. However, this study had no metabolic measurements or liver histology.
Of note, in our cohort, the prevalence of NASH was found to be 56%. This high prevalence, even in unsuspected patients, places all patients with T2DM at a high risk of steatohepatitis, and may help explain epidemiological studies that have reported a 2- to 3-fold increase in the risk of future end-stage liver disease and hepatocellular carcinoma in patients with diabetes (30). For instance, Casey et al (31), reported a high prevalence of fibrosis (35%) on transient elastography in 74 Australian patients with T2DM and a suspected diagnosis of NAFLD. When authors performed a liver biopsy in these patients suspected of having fibrosis, 92% had NASH and 75% advanced liver fibrosis. In a study from India, Prashanth et al (32) reported that 62% of patients with T2DM had a fatty liver when screened by liver ultrasound, of which approximately two thirds had NASH and one third had fibrosis on liver histology. In a small study from Brazil, Leite et al (33) also reported a high prevalence of NASH (78%) and fibrosis (55%) in 98 patients with T2DM who underwent a diagnostic liver biopsy. If these finding are confirmed in larger studies, it would call for proposing early screening for NAFLD/NASH of all patients with T2DM. Although at the present time clinically available biomarkers, imaging techniques (ie, ultrasound) and diagnostic panels lack ideal specificity/sensitivity for widespread clinical use, this is likely to change in the near future.
Insulin resistance and lipotoxicity play a major role in the development of NAFLD (20, 34). Prior work from our laboratory (18), established that hepatic steatosis correlates most strongly with dysfunctional/insulin-resistant adipose tissue and not obesity per se. Consistent with this, and as shown in Figure 2, we observed a strong trend (P = .002) in the fasting state for the Adipo-IRindex (fasting plasma FFA × plasma insulin), with the presence of obesity, as well as NAFLD, being major factors. As such, obese subjects with NAFLD had an almost 3-fold worse Adipo-IRindex compared with nonobese subjects without NAFLD, who were at the other end of the metabolic spectrum. A similar trend, indicative of insulin resistance and an impairment in adipose tissue lipolysis was observed by the lack of normal plasma FFA suppression during the 75-g oral glucose load, worse in those affected by obesity or NAFLD (P = .04). Taken together, it is clear that the presence of NAFLD is closely associated with a state of “sick,” dysfunctional, and insulin-resistant adipose tissue and that lipotoxicity plays a major role in the pathogenesis of NAFLD in patients with T2DM.
We also examined in this cohort of patients the role of hyperglycemia. It has been established that the prolonged exposure to elevated plasma glucose levels can cause toxicity and activate pathways that can induce apoptosis, and that diabetes has been associated with worse NASH (15, 20, 35). The relationship between plasma A1c concentration and liver triglyceride content (Figure 3A), as well as the higher prevalence of NAFLD associated with hyperglycemia (Figure 3B), are novel findings that suggests that hyperglycemia may play a significant role in the development of hepatic steatosis and necroinflammation, although there are likely a number of additional mechanisms (ie, insulin resistance/hyperinsulinemia, chronic inflammation, oxidative stress, hepatotoxic cytokines) (15, 20). Hepatic steatosis in patients with T2DM is associated with more difficult-to-control hyperglycemia, worse insulin resistance, and the need for higher insulin doses (36). Clearly, prospective studies are needed to understand the role of reversing hyperglycemia and glucotoxicity on hepatic steatosis, necroinflammation, and fibrosis in patients with T2DM and NAFLD.
Our study has some limitations. Although this is the first study looking specifically at patients with T2DM and normal liver aminotransferases, we have a relatively small sample size compared with studies examining only the presence of NAFLD and normal aminotransferases in the general population. We also acknowledge that our patient population was predominantly obese males. Therefore, results of this study should be confirmed with larger studies including a greater percentage of nonobese participants.
In summary, we have shown that the prevalence of NAFLD in patients with T2DM and normal aminotransferase levels is higher than previously believed. Moreover, approximately half the patients had NASH, the more severe form of fatty liver disease. If these results are confirmed in larger studies, it will change the current paradigm that patients with T2DM and normal liver aminotransferase levels have a low probability to develop severe liver disease. Finally, it suggests that patients with T2DM may need early screening for liver disease and that, as health care providers, we may have to reconsider current practice of guiding management for NAFLD/NASH based largely on plasma aminotransferase levels in patients with T2DM.
Supplementary Material
Acknowledgments
We thank our study volunteers and the Clinical Translational Science Institute nursing and laboratory staff for their assistance in performing the described studies.
This work was supported by grants from the American Diabetes Association (to K.C.), the Burroughs Wellcome Fund (to K.C.), and a Veterans' Affairs Merit Award (1 I01 CX000167-01; to K.C.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- 1H-MRS
magnetic resonance spectroscopy
- A1c
glycated hemoglobin
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- BP
blood pressure
- DXA
dual energy x-ray absorptiometry
- FFA
free fatty acid
- FPI
fasting plasma insulin
- HOMA-IR
homeostasis model assessment–insulin resistance
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OGTT
oral glucose tolerance test
- T2DM
type 2 diabetes mellitus.
References
- 1. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. [DOI] [PubMed] [Google Scholar]
- 2. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: The Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013;178:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am J Gastroenterol. 2007;102:2716–2717. [DOI] [PubMed] [Google Scholar]
- 4. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology. 2011;140:124–131. [DOI] [PubMed] [Google Scholar]
- 5. Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. [DOI] [PubMed] [Google Scholar]
- 6. Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40:1387–1395. [DOI] [PubMed] [Google Scholar]
- 7. Williamson RM, Price JF, Glancy S, et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic fatty liver disease in people with type 2 diabetes: The Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–119. [DOI] [PubMed] [Google Scholar]
- 9. Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–2121. [DOI] [PubMed] [Google Scholar]
- 10. Lv WS, Sun RX, Gao YY, et al. Nonalcoholic fatty liver disease and microvascular complications in type 2 diabetes. World J Gastroenterol. 2013;19:3134–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ortiz-Lopez C, Lomonaco R, Orsak B, et al. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care. 2012;35:873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Role of ethnicity in overweight and obese patients with nonalcoholic steatohepatitis. Hepatology. 2011;54:837–845. [DOI] [PubMed] [Google Scholar]
- 13. Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: Pathogenesis and treatment. Nat Rev Endocrinol. 2011;7:456–465. [DOI] [PubMed] [Google Scholar]
- 14. Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. [DOI] [PubMed] [Google Scholar]
- 15. Portillo P, Yavuz S, Bril F, Cusi K. Role of insulin resistance and diabetes in the pathogenesis and treatment of nonalcoholic fatty liver disease. Curr Hepatology Rep. 2014;13:159–170. [Google Scholar]
- 16. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 17. Bril F, Lomonaco R, Orsak B, et al. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:2178–2187. [DOI] [PubMed] [Google Scholar]
- 18. Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:1389–1397. [DOI] [PubMed] [Google Scholar]
- 19. Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012;142:711–725.e716. [DOI] [PubMed] [Google Scholar]
- 21. Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734–743. [DOI] [PubMed] [Google Scholar]
- 22. Targher G, Mantovani A, Pichiri I, et al. Nonalcoholic Fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care. 2014;37:1729–1736. [DOI] [PubMed] [Google Scholar]
- 23. Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: Causal effect or epiphenomenon? Diabetologia. 2008;51:1947–1953. [DOI] [PubMed] [Google Scholar]
- 24. Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. [DOI] [PubMed] [Google Scholar]
- 25. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57:1378–1383. [DOI] [PubMed] [Google Scholar]
- 26. Bae JC, Rhee EJ, Lee WY, et al. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes: A 4-year retrospective longitudinal study. Diabetes Care. 2011;34:727–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl 2002;1114–1122. [DOI] [PubMed] [Google Scholar]
- 28. Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. [DOI] [PubMed] [Google Scholar]
- 29. Petit JM, Guiu B, Terriat B, et al. Nonalcoholic fatty liver is not associated with carotid intima-media thickness in type 2 diabetic patients. J Clin Endocrinol Metab. 2009;94:4103–4106. [DOI] [PubMed] [Google Scholar]
- 30. El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. [DOI] [PubMed] [Google Scholar]
- 31. Casey SP, Kemp WW, McLean CA, Topliss DJ, Adams LA, Roberts SK. A prospective evaluation of the role of transient elastography for the detection of hepatic fibrosis in type 2 diabetes without overt liver disease. Scand J Gastroenterol. 2012;47:836–841. [DOI] [PubMed] [Google Scholar]
- 32. Prashanth M, Ganesh HK, Vima MV, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2009;57:205–210. [PubMed] [Google Scholar]
- 33. Leite NC, Villela-Nogueira CA, Pannain VL, et al. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: Prevalences and correlated factors. Liver Int. 2011;31:700–706. [DOI] [PubMed] [Google Scholar]
- 34. Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wong VW, Chu WC, Wong GL, Chan RS, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409–415. [DOI] [PubMed] [Google Scholar]
- 36. Ryysy L, Häkkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49:749–758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.