Abstract
Context:
Although both overt hyper- and hypothyroidism are known to lead to cognitive impairment, data on the association between subclinical thyroid dysfunction and cognitive function are conflicting.
Objective:
This study sought to determine the risk of dementia and cognitive decline associated with subclinical thyroid dysfunction among prospective cohorts.
Data Sources:
We searched in MEDLINE and EMBASE from inception until November 2014.
Study Selection:
Two physicians identified prospective cohorts that assessed thyroid function and cognitive outcomes (dementia; Mini-Mental State Examination [MMSE]).
Data Extraction:
Data were extracted by one reviewer following standardized protocols and verified by a second reviewer. The primary outcome was dementia and decline in cognitive function was the secondary outcome.
Data Synthesis:
Eleven prospective cohorts followed 16,805 participants during a median followup of 44.4 months. Five studies analyzed the risk of dementia in subclinical hyperthyroidism (SHyper) (n = 6410), six in subclinical hypothyroidism (SHypo) (n = 7401). Five studies analyzed MMSE decline in SHyper (n = 7895), seven in SHypo (n = 8960). In random-effects models, the pooled adjusted risk ratio for dementia in SHyper was 1.67 (95% confidence interval, 1.04; 2.69) and 1.14 (95% confidence interval, 0.84; 1.55) in SHypo vs euthyroidism, both without evidence of significant heterogeneity (I2 = 0.0%). The pooled mean MMSE decline from baseline to followup (mean 32 mo) did not significantly differ between SHyper or SHypo vs euthyroidism.
Conclusions:
SHyper might be associated with an elevated risk for dementia, whereas SHypo is not, and both conditions are not associated with faster decline in MMSE over time. Available data are limited, and additional large, high-quality studies are needed.
The prevalence of subclinical hypothyroidism (SHypo) reaches up to 10% in the elderly population, whereas subclinical hyperthyroidism (SHyper) has a prevalence of 2.4%, and 4.3% in the population age at least 80 years (1, 2). SHypo is biochemically defined as elevated serum TSH levels, but free thyroxine (fT4) levels within laboratory reference ranges (3), SHyper is defined as decreased serum TSH concentrations and normal fT4 and free triiodothyronine (fT3) levels. The subclinical forms of thyroid dysfunction have previously been associated with increased risk of heart failure and coronary heart disease (4–6). Furthermore, SHyper may negatively influence bone and mineral metabolism (7).
While both overt hyper- and hypothyroidism are known to lead to cognitive impairment and clinical guidelines recommend screening for thyroid dysfunction among patients with cognitive disorders (8), data on the association between subclinical thyroid dysfunction (SCTD) and cognitive function remain conflicting. In the general population, the prevalence of mild cognitive impairment is 3–22%, with a higher prevalence among adults older than 70 years (14–18%) (9–11). Mild cognitive impairment, a cognitive decline not normal for age but with essentially preserved functional activities, is believed to be the earliest clinical symptom of cognitive disorders and may be the stage to intervene with preventive therapies (11, 12). The progression rate from cognitive impairment to dementia in the general population older than 65 years is around 6–10% per year (11). SHyper has also been associated with increased risk of dementia (13), with one retrospective cohort reporting a hazard ratio of 1.6 (95% confidence interval [CI], 1.2; 2.3) for dementia (14). SHypo might also be associated with alterations in cognitive function (13, 15, 16), with one case-control study reporting a nearly 4-fold increase in the odds ratio (OR) of dementia (OR = 3.8; 95% CI, 1.6; 9.1) (17). However, data on the association between SCTD and cognitive function are conflicting (18–20).
Two recent meta-analyses yielded discrepant findings for SHypo, one showing a significant risk of cognitive alteration (composite endpoint of reduced Mini-Mental State Examination (MMSE), Wechsler Memory Scale-Revised Score, total memory quotient and Wechsler Adult Intelligence Scale [WAIS] scores) for SHypo individuals younger than 75 years with an OR of 1.56 (95% CI, 1.07; 2.27) (21), whereas the other found no decline in MMSE in SHypo patients age at least 60 years (pooled estimate [ES] 0.03; 95% CI, −0.001; 0.07) (22). As both meta-analyses were limited by pooling heterogeneous study designs (prospective and retrospective data), and did neither examine the risk of dementia nor cognitive function associated with SHyper, we conducted a meta-analysis to determine whether SHyper and SHypo were associated with an increased risk of dementia or decline in cognitive function in prospective cohorts, the gold standard for observational data.
Materials and Methods
Data sources and searches
To perform this systematic review, we followed a predefined protocol registered on PROSPERO (Record: CRD42015019819). We conducted a systematic literature search in MEDLINE and EMBASE databases from inception until November 2014 searching for articles related to SCTD and cognitive decline and dementia. The Medical Subject Headings (MeSH) in Ovid MEDLINE included “thyroid disease”, “hypothyroidism”, “hyperthyroidism”, “thyroid hormones”, “TSH”, “subclinical hyperthyroidism”, “subclinical hypothyroidism”, “subclinical dysthyroidism”, “subclinical thyroid”, “cognition”, “dementia”, “memory”, “Alzheimer”, “cognitive”, “cohort studies”, “cohort”, “controlled clinical trial”, “epidemiologic methods”, and “review.” We applied a cohort filter designed by the British Medical Journal knowledge information specialists (23) but did not use any other filters or restrictions including year limitations or language restrictions. A similar strategy with similar terms was used for EMBASE. In addition, we searched the bibliographies of included articles, as well as key articles in the field, and contacted several authors for unpublished subgroup data.
Study selection
Two reviewers (C.R., D.S.) independently screened titles and abstracts of the search results and selected publications. In a second step, the same two reviewers independently evaluated the full-text publications of the retrieved studies according to the following prespecified eligibility criteria: prospective cohort studies among participants at least 18 years old, including a SCTD and a euthyroid control group with thyroid function tests at baseline and assessment of cognitive function during followup, with published risk estimates or sufficient information to calculate them (Figure 1). We excluded studies examining solely participants with overt thyroid disease. Disagreements were resolved by an independent third author (N.R.). SHyper was defined as decreased or undetectable TSH and normal fT4, and SHypo as elevated TSH and normal fT4. Cohort-specific TSH- and fT4-cutoff levels were used to determine thyroid status. For dementia definition, we accepted all validated assessments of memory and cognitive function, and did not exclude studies that reported scales other than MMSE. For our analyses, we also collected information on clinical dementia (Supplemental Table 1). In addition, we gathered data on MMSE results at baseline and follow-up visits. Studies were included if they provided information on either dementia or MMSE outcomes, or both.
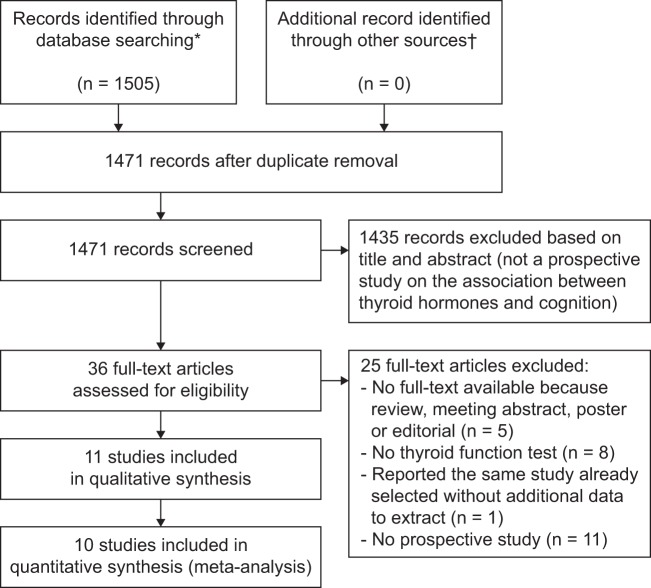
Figure 1.
PRISMA Flow Diagram for Study Evaluation for Inclusion in the Meta-analysis ‡ * Data from MEDLINE from inception to November 11, 2014 and data from EMBASE from inception to November 14, 2014. † From key articles in the field and contacts with authors. ‡ Information on the PRISMA statement can be found at http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000097.
Data extraction and quality assessment
Standardized data extraction forms were used to collect information from the included cohorts concerning patient characteristics, thyroid hormone levels, and scales for tests or criteria used to define memory function, dementia or Alzheimer’s disease (AD). Data were extracted by one reviewer (C.R.) and verified by a second independent reviewer (D.S.). Discrepancies were solved by a third author (N.R.). Two reviewers (C.R., D.S.) independently assessed study quality using key indicators of cohort study quality (24, 25): origin of population (convenience vs population based, the latter defined as a random sample of the general population), methods of outcome ascertainment and adjudication (considered as adequate if in each potential case performed by an expert panel blinded regarding the thyroid status and following defined outcome criteria), completeness of followup, assessment of the proportional hazard assumption, and adjustment for confounders.
Data synthesis and statistical analysis
We performed four main analyses on the association of 1) SHyper and dementia, 2) SHypo and dementia, 3) SHyper and MMSE, and 4) SHypo and MMSE. We expressed the estimates of the association between SCTD (ie, SHyper or SHypo) and outcomes as risk ratios (RRs) for dementia or as between-group differences in mean changes (MD) from baseline for MMSE scores. Only prospective data were analyzed. A RR greater than 1 suggests a higher risk of an event in SCTD compared with euthyroids and a MD greater than 0 suggests higher decline of MMSE in SCTD compared with euthyroids. To account for the different lengths of follow-up time across studies, we standardized MD per 1 year unit. We used most adjusted estimates provided by the studies as primary analysis. We used an inverse variance random-effects meta-analysis to pool estimates across studies. Estimates of the association between SCTD and dementia were pooled on a log scale and later exponentiated to be reported as RR. To evaluate heterogeneity across the studies, we used the Q statistic with a conservative P = .10 (26). Furthermore, we calculated the I2 statistic, indicating the proportion of variability in estimates of effects across studies that is not due to chance alone (<25% low, 25–75% increasing, >75% high heterogeneity between studies) (24). We visually evaluated publication bias through funnel plots and, statistically, with the Egger’s test (27, 28). To explore the sources of heterogeneity, we conducted several sensitivity analyses. Due to the small number of studies in each group, subgroup analysis with interaction tests could not be meaningfully performed. All P-values were two sided. All analyses were conducted using STATA software, version 13.1 (StataCorp).
Results
Study selection
Of the 1505 reports initially identified, 1471 remained after removing duplicates. We excluded 1435 records on the basis of their abstracts and 25 after full-text examination (Figure 1). Eleven studies met prespecified eligibility criteria and were included in the analyses. Among those, three studies provided information on both dementia and MMSE outcomes (Supplemental Table 1, Section A), four studies reported information on dementia outcomes only (Supplemental Table 1, Section B), and four assessed MMSE outcomes only (Supplemental Table 1, Section C). The agreement among the reviewers was 98.63% for the first screen of abstracts (κ = 0.75) and 89.74% for the full-text search (κ = 0.71).
Study characteristics
Eleven cohorts reported data on 16 805 participants (Table 1). Two cohorts only included men (29, 30). Mean age was 70 years or higher, except for one study (31). The follow-up time ranged from 12 to 152 months (median followup 44.4 mo). Eight cohorts excluded participants treated with thyroid hormones or medications altering thyroid hormone levels, whereas three excluded the participants taking thyroid hormones or thyroid altering medication in sensitivity analysis (32–34).
Table 1.
Description of Included Studies for the Effect of Subclinical Thyroid Dysfunction on Dementia/Mini-Mental State Examination (MMSE)
| Study, Year of Publication | Population, N | Women, % | Mean Age; sd, y | Followup Time, Months | Age, Min–Max, y | TSH Cutoff Level, mU/L | fT4 Measured | Thyroid Hormone Recipients Excluded? | |
|---|---|---|---|---|---|---|---|---|---|
| SHypo | SHyper | ||||||||
| Rotterdam (31), 2000c | 1843 | 61.9 | 68.8; 7.5 | 25.2 | 55–93 | >4.0 | <0.4 | Yes | Yes |
| Leiden 85-Plus Study (33), 2004 | 558 | 66.0 | 85.0; 0.0 | 44.4 | 85 | >4.8 | <0.3 | Yes | In SA |
| Rotterdam Scan (38), 2006 | 1077 | 51.2 | 72.3a; 7.4 | 66.0 | 60–90 | >4.3 | <0.4 | Yes | Yes |
| Health Ageing (36), 2008 | 1047 | 51.0 | 73.6; 6.2 | 24.0 | 64–94 | >4.8b | <0.3b | Yes | Yes |
| Framingham (34), 2008b | 1864 | 59.0 | 71.0; 7.0 | 152.4 | No | In SA | |||
| HAAS (30), 2009 | 665 | 0.0 | 78.0 | 56.4 | 71–93 | >4.3 | <0.4 | Yes | Yes |
| Japanese Study (35), 2010 | 229 | 65.0 | 80.9; 4.7 | 12.0 | >4.0 | NR | Yes | Yes | |
| Conselice (32), 2012c | 660 | 52.9 | 73.3; 6.0 | 45.6 | 65–91 | >4.5 | <0.45 | Yes | In SA |
| HIMS (29), 2012 | 3401 | 0.0 | 76.8; 3.5 | 70.8a | 70–89 | >4.0 | <0.4 | Yes | Yes |
| PROSPER (20), 2013 | 5154 | 49.4 | 75.0 | 38.4 | 80–82 | >4.5 | <0.45 | Yes | Yes |
| OCTABAIX (37), 2014d | 307 | 54.6 | 85.0; 0.0 | 36.0 | 85 | >5 | <0.25 | Yes | Yes |
Abbreviations: Conselice, Conselice Study of Brain Ageing; Framingham, The Framingham Study; HAAS, Honolulu-Asia Aging Study; Health Ageing, Health Ageing Study; HIMS, The Health in Men Study; Japanese Study, Cognitive function with SHypo in elderly people without dementia: One year follow up; Leiden 85+, Leiden 85-plus Study; MMSE, Mini-Mental State Examination; NR, not reported; OCTABAIX, OCTABAIX Study; PROSPER, The PROSPER Study; Rotterdam Scan, Rotterdam Scan Study; Rotterdam, The Rotterdam Study; SA, sensitivity analysis; SCTD, subclinical thyroid dysfunction.
Median.
The Framingham Study did not use TSH cut-offs for SCTD, but rather tertiles. Tertile 1: 0.1–1.08 mU/L for women, 0.10–0.90 mU/L for men; tertile 2: 1.10–2.03 mU/L for women, 0.99–1.80 mU/L for men; tertile 3: 2.10–9.90 mU/L for women, 1.09–9.90 mU/L for men. Therefore, this study could not be included in the meta-analysis but was added to a SA.
Due to additional unpublished data provided by the authors, the studies could be incorporated in the meta-analysis on SCTD and MMSE; unadjusted data.
Due to additional unpublished data provided by the authors, the study could be incorporated in the meta-analysis on SCTD and dementia.
Description and quality of studies
The quality of studies was heterogeneous. Nine cohorts were population based and two were convenience samples (Supplemental Table 1). All the cohorts used third-generation TSH assays, except one using second generation tests and one that did not report test details (35, 36). Four studies had a formal adjudication committee for dementia diagnosis (29–32). Seven studies provided information on attrition during followup (20, 29, 30, 32, 33, 36, 37). Six studies provided information on nonviolation of the proportional hazard assumption (29, 30, 33, 34, 37, 38). All studies reported adjusted data with various confounders, except one study that provided us unadjusted data (32).
Subclinical hyperthyroidism and dementia
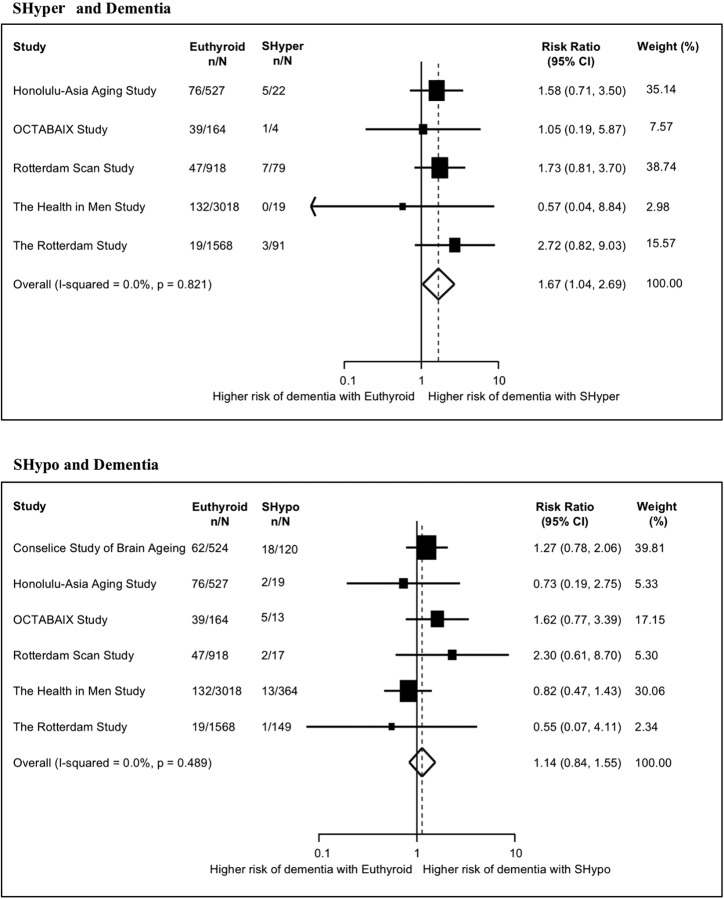
Among five cohorts analyzing the association between SHyper and dementia (n = 6410, 329 cases of dementia, mean followup, 68.3 mo) (29–31, 37, 38), the pooled RR of dementia was 1.67 (95% CI, 1.04; 2.69; I2 = 0.0%, P for heterogeneity = .82) among SHyper patients compared with euthyroidism (Figure 2). Sensitivity analyses (Table 2) excluding one study with a convenience-based sample, one study that followed both patients with and without thyroid hormone replacement, or studies without or not reported formal adjudication for dementia, yielded similar results. As the Framingham study only analyzed the relationship with dementia using TSH tertiles (highest tertile: TSH 1.9–9.9 mU/L) and did not measure fT4 (34), we added this study in a sensitivity analysis and found comparable results. A sensitivity analysis excluding 475 overlapping patients between two cohorts (31, 38) yielded similar results; we did not include these data in the main analysis, given that they examined different follow-up duration and were not based on peer-reviewed published results (the investigators sent us these data separately). The relationship between SHyper and AD was assessed by three studies only (n = 3186, 108 AD cases, mean followup, 75.0 mo) (30, 31, 38). The pooled RR for AD was 1.67 (95% CI, 0.79; 3.51; I2 = 16.8%; P for heterogeneity = .30).
Figure 2.
Association between subclinical thyroid dysfunction and dementia. RR >1 suggests higher risk of an event in SCTD than in euthyroidism. Abbreviations: Euthyroid, euthyroidism; n, number of patients with dementia per group; N, total number of patients per group; SHyper, subclinical hyperthyroidism; SHypo, subclinical hypothyroidism. Note: Weights are from random effects analysis.
Table 2.
Sensitivity Analyses on the Association between Subclinical Thyroid Dysfunction (SCTD) and Dementia
| RRa | 95% CI | P for Heterogeneity | No. of Studies | |
|---|---|---|---|---|
| Subclinical hyperthyroidism and dementia | ||||
| Main analysis (29–31, 37, 38) | 1.67 | 1.04–2.69 | .82 | 5 |
| Exclusion of one study using a convenience-based sample (30, 31, 37, 38) | 1.73 | 1.07–2.80 | .82 | 4 |
| Exclusion of one study enrolling patients with and without thyroid hormone replacement (29, 31, 37, 38) | 1.73 | 0.96–3.11 | .68 | 4 |
| Exclusion of studies without formal adjudication (30, 31) | 1.86 | 0.96–3.62 | .46 | 2 |
| Exclusion of overlapping 475 participants from two studies (29–31, 37, 38)b | 1.60 | 0.92–2.78 | .82 | 5 |
| Main analysis adding the Framingham Study (29–31, 34, 37, 38)c | 1.46 | 1.08–1.97 | .84 | 6 |
| Subclinical hypothyroidism and dementia | ||||
| Main analysis (29–32, 37, 38) | 1.14 | 0.84–1.55 | .49 | 6 |
| Exclusion of one study using a convenience-based population (29–32, 37, 38) | 1.31 | 0.91–1.89 | .65 | 5 |
| Exclusion of studies with TSH cut-off <4.5 mU/L (32, 37) | 1.36 | 0.91–2.05 | .59 | 2 |
| Exclusion of two studies enrolling patients with and without thyroid hormone replacement (29, 31, 37, 38) | 1.14 | 0.69–1.90 | .29 | 4 |
| Exclusion of studies without formal adjudication process (30–32) | 1.14 | 0.73–1.78 | .57 | 3 |
| Exclusion of one study with unadjusted data (29–31, 37, 38) | 1.06 | 0.71–1.60 | .39 | 5 |
| Exclusion of overlapping 475 participants from two studies (29–32, 37, 38)b | 1.10 | 0.80–1.50 | .66 | 6 |
| Main analysis adding the Framingham Study (29–32, 34, 37, 38)c | 1.18 | 0.91–1.52 | .60 | 7 |
Abbreviations: CI, confidence interval; N, number; RR, risk ratio; SCTD, subclinical thyroid dysfunction.
RR >1 indicates higher risk of an event in SCTD than in euthyroidism. A positive mean difference indicates larger decrease in cognitive function in SCTD than in euthyroidism.
Performed on data additionally provided by the author; we did not include these data in the main analysis, as they examined different follow-up duration and were not based on peer-reviewed published results (the investigators sent us these data separately).
Given that the Framingham Study did not use TSH cut-off for SCTD, we compared lowest vs highest tertiles (lowest tertile: 0.10–1.08 for women, 0.10–0.90 for men; highest tertile: 2.10–9.90 for women, 1.90–9.90 for men).34
Subclinical hypothyroidism and dementia
Among six studies analyzing the relationship between SHypo and dementia (n = 7401, 416 cases of dementia, mean followup, 64.6 mo) (29–32, 37, 38), the pooled RR for dementia was 1.14 (95% CI, 0.84; 1.55; I2 = 0.0%; P for heterogeneity = .49) (Figure 2). No individual study showed a statistically significant association. Sensitivity analyses (Table 2) excluding a study with a convenience-based sample, studies with TSH cut-off less than 4.5 mU/L and potentially including individuals in the euthyroid range, two studies that followed both patients with and without thyroid hormone replacement, studies without or not reported formal adjudication process for dementia, one study with additional unadjusted data, or 475 overlapping participants between two cohorts (31, 38) yielded similar results. The addition of the Framingham study (34) to the main analysis yielded similar results. Four studies analyzed the relationship between SHypo and AD (n = 3823, 151 AD cases, mean followup, 69.36 mo) (30–32, 38). The pooled RR for AD was 0.95 (95% CI, 0.52; 1.71; I2 = 0.0%, P for heterogeneity = .89).
Subclinical hyperthyroidism and MMSE
Among five studies reporting change in MMSE among participants with SHyper (n = 7895, mean followup, 33.6 mo) (20, 31, 33, 36, 37), the pooled mean MMSE decline in cognitive function per year was 0.01 points difference from baseline (95% CI, −0.14; 0.15; I2 = 23.5%; P for heterogeneity = .27; Supplemental Figure 1). Results remained similar after excluding one study using a convenience-based sample or one study that followed both patients with and without thyroid hormone replacement (Supplemental Table 2). Because the results of the main analyses between SHyper and dementia did not seem concordant with the results of the meta-analysis looking at the decrease of MMSE in SHyper participants, we undertook a sensitivity analysis including the two studies examining the relationship of SHyper and both MMSE and dementia (31, 37), which also showed no larger decline of MMSE among SHyper.
Subclinical hypothyroidism and MMSE
Among seven studies reporting change in MMSE in SHypo (n = 8960; mean followup, 32.2 mo) (20, 31–33, 35–37), pooled mean MMSE decline per year did not significantly differ between SHypo and euthyroid groups (ES, 0.01 points difference from baseline; 95% CI, −0.10; 0.12; I2 = 27.6%; P for heterogeneity = .22; Supplemental Figure 1). Sensitivity analyses (Supplemental Table 2) excluding one study with a convenience-based sample, studies using TSH cut-offs less than 4.5 mU/L, one study that followed both patients with and without thyroid hormone replacement, one study that might have subclinical hyperthyroid participants in the control group (35), or one study using unadjusted data yielded similar results.
Publication bias
Both visual inspection and Egger’s tests suggest little evidence of publication bias for all associations, although the number of available studies was small (Supplemental Figure 2) (39).
Discussion and Conclusion
In this meta-analysis of 11 prospective cohorts, we found that SHyper, but not SHypo, might be associated with an elevated risk for dementia, whereas decline in MMSE over time was minimal for both conditions. SHyper showed also a similar pattern of higher risk for AD. Results for the association between SHyper and dementia remained similar when we pooled higher-quality studies in sensitivity analyses, such as studies with formal adjudication process for the outcome assessment or population-based studies.
Our results for SHyper and risk for dementia are consistent with a nonsystematic literature review summarizing results from 13 cross-sectional or case-control, and 10 cohort studies that found supportive evidence of an association between SHyper and cognitive impairment or dementia (40). Of these 10 cohort studies, four did not meet the eligibility criteria for our systematic review: one due to missing subgroups of thyroid dysfunction (41), two analyzed only euthyroid participants (42, 43) and one had a retrospective design (14). Several other individual studies reported an association between SHyper and an elevated risk for dementia as well (14, 44, 45): a retrospective nested case-control study including 2004 patients with SHyper reported a hazard ratio for dementia of 1.79 (95% CI, 1.28; 2.51), and a cross-sectional study found a positive association between SHyper and dementia in 1276 participants (33 with SHyper) age at least 65 years (OR for dementia, 4.1; 95% CI, 1.3; 13.1) (14, 44). Van Osch et al (45) prospectively studied 178 patients with AD and 291 community-dwelling controls without objective cognitive impairment, and found an adjusted OR for AD of 2.36 (95% CI, 1.19; 4.67) in participants in the lowest (TSH < 1.3 mU/L) vs highest TSH tertile (TSH >2.1 mU/L). Another population-based prospective cohort of 313 elderly adults with normal TSH found that those with a decline of cognitive dysfunction had a mean TSH of 1.78 mU/L, whereas those without decline had a mean TSH of 2.24 mU/L (P = .001) (46).
Our findings might be consistent with the hypothesis that SHyper increases the risk of dementia, although decline in MMSE over time did not differ between SHyper and euthyroidism. In our meta-analysis, only two of 11 studies published results on both dementia and MMSE in SHyper. Analyzing only these two studies showed no larger decline of MMSE among participants with SHyper. This discrepancy might be explained by several factors: the length of followup of studies on SCTD and dementia was twice the duration of studies on SCTD and MMSE (mean follow-up time, 66 vs 33 mo), different population investigated, the modest sensitivity of MMSE as a diagnostic tool (79%) (47, 48), as well as for detecting mild cognitive impairment and subtle changes in specific cognitive domains, and the multimodal approach needed to diagnose dementia (49). Furthermore, different scores were used as gold standard depending on the type and version of diagnostic criteria (Supplemental Table 1). Factors increasing the plausibility of the association between SHyper and dementia were that results remained similar when we pooled higher quality studies in sensitivity analysis, such as studies with formal adjudication process for the outcome assessment or population-based studies, and that SHyper also showed a pattern of higher risk for AD. However, higher-quality studies are needed.
Several pathways could explain the association of thyroid dysfunction with cognition and dementia. Thyroid hormone has distinct effects on the cardiovascular system and thyroid dysfunction has been associated with several cardiovascular risk factors, including hypertension, dyslipidemia, and atrial fibrillation (4, 6). In turn, these cardiovascular risk factors are associated with a higher risk of dementia and AD (50). Most studies included in our meta-analysis adjusted for cardiovascular risk factors. However, the number and type of variables that were adjusted for differed for each study. Other explanations for the association include direct effects of thyroid hormone, such as neurotoxicity and altered gene expression in pathways relevant for dementia. The exact pathophysiological link between thyroid dysfunction and dementia remains unclear and requires more research.
Recently, two meta-analyses on SHypo and cognitive impairment were published, yielding discrepant results (21, 22). The first review included 13 studies and found a significantly higher risk for cognitive alteration (composite endpoint of incidence or prevalence of dementia or difference in MMSE, WAIS, and Wechsler Memory-Revised score) in SHypo individuals younger than 75 years (OR, 1.56; 95% CI, 1.07; 2.27; P = .02), and for dementia (OR, 1.81; 95% CI, 1.43; 2.28; P < .01) (21). However, the authors pooled different designs (cross-sectional, case-control, cohort studies), used a composite endpoint of clinical events and scales as primary outcome, and found a significant risk for the primary endpoint only in SHypo individuals younger than 75 years. As results were calculated on the basis of mean age, without availability of individual participant data, they may have been subject to potential aggregation bias (ecological fallacy) (24, 51). Contrary to that meta-analysis, all studies in our meta-analysis but one (included only in a sensitivity analysis) measured fT4 to define SCTD. The second meta-analysis analyzed 15 studies (nine cross-sectional, six prospective cohort studies) and found no association between SHypo and decline in cognitive function among people older than 60 years (cross-sectional analysis: pooled ES for MMSE −0.01 points difference from baseline [95% CI, −0.09; 0.08]; prospective analysis, pooled MMSE change: 0.03 [95% CI, −0.001; 0.07] P = .055, with heterogeneity [I2] of <0.001%) (22), which is consistent with our findings. In comparison with these two meta-analyses, we included only prospective cohorts (n = 11) allowing us to reduce the bias that could arise due to differing study designs. To keep the literature search broad enough, we excluded studies examining solely participants with overt thyroid disease but added no other exclusion criteria.
Two small placebo-controlled trials (n = 89; n = 94) found no evidence that treatment of SHypo with levothyroxine was associated with improved cognitive function (18, 52). However, these trials had several limitations. In the trial by Parle et al (52), recruitment was based on a single thyroid function test, so that euthyroid participants with transiently elevated TSH may have been included (50% in the placebo group were euthyroid at 12 months), which may have underpowered the trial to detect an effect of hormone replacement (52). Thyroxine substitution lasted only for 12 months, which may have been too short to affect cognitive function. In the trial by Jorde et al (18), one third of participants did not attend followup. Because of numerous exclusion criteria, the study population was unusually healthy, with 57% of the participants having a TSH value between 3.50 and 4.99 mU/L, so that it probably included many euthyroid participants. The ongoing TRUST (Thyroid Hormone Replacement for Subclinical Hypothyroidism) trial (ClinicalTrials.gov No. NCT01660126) may clarify whether treatment with levothyroxine in SHypo is associated with better cognitive outcomes over time (53).
There are several strengths of our meta-analysis. By combining the results of 11 prospective cohorts, we analyzed a total of 432 cases of dementia and 160 cases of AD in more than 15 000 participants. By contacting several authors of these studies, we obtained additional data that allowed us to derive more uniform subgroup and sensitivity analyses. In comparison with the two other meta-analyses (21, 22), our results are less prone to bias due to pooling of heterogeneous study design and quality, because we only included prospective cohorts. We also conducted a detailed literature search in several electronic databases with as few limitations as possible to retrieve the maximum number of studies available on the topic, and were able to include a larger number of prospective cohorts than previous meta-analyses (21, 22).
Our meta-analysis has also several limitations. Except for two studies (30, 35), studies only examined Caucasians, limiting the generalizability to other populations. All studies were performed in participants with a mean age at least 65 years and the time of followup was relatively short, ranging between 12 and 70.8 months [152.4 mo in the Framingham Study (34), added in a sensitivity analysis]. All but two studies (20, 33) assessed thyroid function tests only at baseline, which is a limitation of most previously published large cohort studies on the risks of thyroid dysfunction (54, 55). Some participants with SCTD at baseline may have normalized to euthyroidism or progressed to overt thyroid disease over time. Regarding the elderly participants in the included studies, we cannot exclude a certain degree of overdiagnosis of SHypo due to the physiological increase of TSH toward upper limits during normal ageing (56). All these nondifferential misclassification of thyroid status might bias the results toward no difference. The limited sensitivity of MMSE for detecting subtle changes in specific cognitive domains (57) may further limit our ability to detect a possible decline in cognitive function. A meta-analysis of observational studies requires cautious interpretation of the results and potential biases, and confounding and heterogeneity must be carefully investigated (58, 59). The quality of the incorporated studies was variable. We performed several sensitivity analyses to address differences between the studies, as recommended (58), although they should be interpreted with caution given the small number of studies. In study-level meta-analysis, interpretation of subgroup data should be performed with caution. Because of the small amount of studies, no meaningful subgroup analysis could be performed. There are multiple confounders for cognitive decline and dementia, the most important is age, others are depression/mood or cardiometabolic risk factors. All cohorts adjusted for age and several other confounders, but there was heterogeneity in the choice of confounders, which may lead to residual confounding. Bias in the selection of included studies cannot be excluded. To limit selection bias, we conducted a detailed literature search in several electronic databases with broad inclusion. We performed visual and statistical assessment to assess selective reporting, but these analyses were not very sensitive considering the small number of studies included (25, 28). Although included cohorts enrolled community-dwelling adults in ambulatory visits, who are therefore less likely to have an acute disease, some participants with nonthyroidal illness may have been analyzed. Included studies addressed this problem differently: two repeatedly measured thyroid values (20, 33), one assessed and adjusted for rT3 (reverse T3) (38), and others adjusted for comorbidities. We cannot exclude that some participants had nonthyroidal illness.
What are the potential clinical and research implication of our findings? Our data suggest that SHyper might represent a potentially treatable risk factor for dementia. Given the relatively high prevalence of both SCTD and cognitive dysfunction in the aging population, even a modest increase of dementia incidence among individuals with SCTD might have public health implications. Data on benefit of SCTD treatment are scarce; therefore, current guidelines do not recommend treatment for most adults with mild SCTD (serum TSH, 0.1–0.45 mU/L or 4.5–10.0 mU/L) (60, 61). Large randomized controlled trials are required to assess the efficacy of treatment in SCTD associated with dementia. For SHypo, the ongoing TRUST (Thyroid Hormone Replacement for Subclinical Hypothyroidism) trial (ClinicalTrials.gov No. NCT01660126) will clarify this issue (62).
In summary, our systematic review and meta-analysis suggests that SHyper, but not SHypo, might be associated with a modestly elevated risk of dementia. Neither SHyper nor SHypo were significantly associated with a faster decline in MMSE over time, as compared with euthyroidism. Available data were limited, and additional large, high-quality prospective cohort studies are needed.
Supplementary Material
Acknowledgments
The authors thank Francesc Formiga Pérez (Bellvitge University Hospital, Barcelona, Spain), Paola Forti (Department of Medical and Surgical Sciences, Bologna, Italy), Mohammad Arfan Ikram (Erasmus Medical Center, Rotterdam, The Netherlands), Bu Yeap (University of Western Australia and Fiona Stanley and Freemantle Hospitals, Perth, Australia) for supplying additional data from their studies.
The protocol of this meta-analysis has been published in the PROSPERO (PROSPERO Record: CRD42015019819) register.
N.R.’s work is supported by a grant from the Swiss National Science Foundation (SNSF 320030-150025). T.-H.C.’s research is supported by grants from the Swiss National Science Foundation (PBLAP3-145870, P3SMP3-155318).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- AD
Alzheimer’s disease
- CI
confidence interval
- ES
pooled estimate
- fT3
free triiodothyronine
- free thyroxine
fT4
- MMSE
Mini-Mental State Examination
- OR
odds ratio
- RR
risk ratio
- rT3
reverse triiodothyronine
- SCTD
subclinical thyroid dysfunction
- SHyper
subclinical hyperthyroidism
- SHypo
subclinical hypothyroidism
- TRUST
Thyroid Hormone Replacement for Subclinical Hypothyroidism
- WAIS
Wechsler Adult Intelligence Scale.
Reference
- 1. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76–131. [DOI] [PubMed] [Google Scholar]
- 2. Delitala AP, Pilia MG, Ferreli L, et al. Prevalence of unknown thyroid disorders in a Sardinian cohort. Eur J Endocrinol. 2014;171(1):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Helfand M. Screening for subclinical thyroid dysfunction in nonpregnant adults: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(2):128–141. [DOI] [PubMed] [Google Scholar]
- 4. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: An individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126(9):1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collet TH, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172(10):799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wirth CD, Blum MR, da Costa BR, et al. Subclinical thyroid dysfunction and the risk for fractures: A systematic review and meta-analysis. Ann Intern Med. 2014;161(3):189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waldemar G, Dubois B, Emre M, et al. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14(1):e1–e26. [DOI] [PubMed] [Google Scholar]
- 9. Ebly EM, Parhad IM, Hogan DB, Fung TS. Prevalence and types of dementia in the very old: Results from the Canadian Study of Health and Aging. Neurology. 1994;44(9):1593–1600. [DOI] [PubMed] [Google Scholar]
- 10. Di Carlo A, Baldereschi M, Amaducci L, et al. Cognitive impairment without dementia in older people: Prevalence, vascular risk factors, impact on disability. The Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2000;48(7):775–782. [DOI] [PubMed] [Google Scholar]
- 11. Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: Ten years later. Arch Neurol. 2009;66(12):1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Voisin T, Touchon J, Vellas B. Mild cognitive impairment: A nosological entity? Curr Opin Neurol. 2003;16(Suppl 2):S43–S45. [PubMed] [Google Scholar]
- 13. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142–1154. [DOI] [PubMed] [Google Scholar]
- 14. Vadiveloo T, Donnan PT, Cochrane L, Leese GP. The Thyroid Epidemiology, Audit, and Research Study (TEARS): Morbidity in patients with endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab. 2011;96(5):1344–1351. [DOI] [PubMed] [Google Scholar]
- 15. Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(6):925–935. [DOI] [PubMed] [Google Scholar]
- 16. Zhu DF, Wang ZX, Zhang DR, et al. fMRI revealed neural substrate for reversible working memory dysfunction in subclinical hypothyroidism. Brain. 2006;129(Pt 11):2923–2930. [DOI] [PubMed] [Google Scholar]
- 17. Ganguli M, Burmeister LA, Seaberg EC, Belle S, DeKosky ST. Association between dementia and elevated TSH: A community-based study. Biol Psychiatry. 1996;40(8):714–725. [DOI] [PubMed] [Google Scholar]
- 18. Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91(1):145–153. [DOI] [PubMed] [Google Scholar]
- 19. Park YJ, Lee EJ, Lee YJ, et al. Subclinical hypothyroidism (SCH) is not associated with metabolic derangement, cognitive impairment, depression or poor quality of life (QoL) in elderly subjects. Arch Gerontol Geriatr. 2010;50(3):e68–e73. [DOI] [PubMed] [Google Scholar]
- 20. Wijsman LW, de Craen AJ, Trompet S, et al. Subclinical thyroid dysfunction and cognitive decline in old age. PLoS One. 2013;8(3):e59199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(11):4240–4248. [DOI] [PubMed] [Google Scholar]
- 22. Akintola AA, Jansen SW, van Bodegom D, et al. Subclinical hypothyroidism and cognitive function in people over 60 years: A systematic review and meta-analysis. Front Aging Neurosci. 2015;7:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. BMJ Evidence Centre Study design search filters. BMJ. 2012. Available from: http://clinicalevidence.bmj.com/x/set/static/ebm/learn/665076.html.
- 24. da Costa BR, Juni P. Systematic reviews and meta-analyses of randomized trials: Principles and pitfalls. Eur Heart J. 2014;35(47):3336–3345. [DOI] [PubMed] [Google Scholar]
- 25. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323(7304):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petitti DB. Approaches to heterogeneity in meta-analysis. Stat Med. 2001;20(23):3625–3633. [DOI] [PubMed] [Google Scholar]
- 27. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–3457. [DOI] [PubMed] [Google Scholar]
- 29. Yeap BB, Alfonso H, Chubb SA, et al. Higher free thyroxine levels predict increased incidence of dementia in older men: The Health in Men Study. J Clin Endocrinol Metab. 2012;97(12):E2230–E2237. [DOI] [PubMed] [Google Scholar]
- 30. de Jong FJ, Masaki K, Chen H, et al. Thyroid function, the risk of dementia and neuropathologic changes: The Honolulu-Asia aging study. Neurobiol Aging. 2009;30(4):600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalmijn S, Mehta KM, Pols HA, Hofman A, Drexhage HA, Breteler MM. Subclinical hyperthyroidism and the risk of dementia. The Rotterdam study. Clin Endocrinol. 2000;53(6):733–737. [DOI] [PubMed] [Google Scholar]
- 32. Forti P, Olivelli V, Rietti E, et al. Serum thyroid-stimulating hormone as a predictor of cognitive impairment in an elderly cohort. Gerontology. 2012;58(1):41–49. [DOI] [PubMed] [Google Scholar]
- 33. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292(21):2591–2599. [DOI] [PubMed] [Google Scholar]
- 34. Tan ZS, Beiser A, Vasan RS, et al. Thyroid function and the risk of Alzheimer disease: The Framingham Study. Arch Intern Med. 2008;168(14):1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamamoto N, Ishizawa K, Ishikawa M, et al. Cognitive function with subclinical hypothyroidism in elderly people without dementia: One year follow up. Geriatr Gerontol Int. 2012;12(1):164–165. [DOI] [PubMed] [Google Scholar]
- 36. Hogervorst E, Huppert F, Matthews FE, Brayne C. Thyroid function and cognitive decline in the MRC Cognitive Function and Ageing Study. Psychoneuroendocrinology. 2008;33(7):1013–1022. [DOI] [PubMed] [Google Scholar]
- 37. Formiga F, Ferrer A, Padros G, et al. Thyroid status and functional and cognitive status at baseline and survival after 3 years of follow-up: The OCTABAIX study. Eur J Endocrinol. 2014;170(1):69–75. [DOI] [PubMed] [Google Scholar]
- 38. de Jong FJ, den Heijer T, Visser TJ, et al. Thyroid hormones, dementia, and atrophy of the medial temporal lobe. J Clin Endocrinol Metab. 2006;91(7):2569–2573. [DOI] [PubMed] [Google Scholar]
- 39. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 40. Gan EH, Pearce SH. Clinical review: The thyroid in mind: Cognitive function and low thyrotropin in older people. J Clin Endocrinol Metab. 2012;97(10):3438–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Annerbo S, Wahlund LO, Lökk J. The significance of thyroid-stimulating hormone and homocysteine in the development of Alzheimer’s disease in mild cognitive impairment: A 6-year follow-up study. Am J Alzheimers Dis Other Demen. 2006;21(3):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wahlin A, Bunce D, Wahlin TB. Longitudinal evidence of the impact of normal thyroid stimulating hormone variations on cognitive functioning in very old age. Psychoneuroendocrinology. 2005;30(7):625–637. [DOI] [PubMed] [Google Scholar]
- 43. Volpato S, Guralnik JM, Fried LP, Remaley AT, Cappola AR, Launer LJ. Serum thyroxine level and cognitive decline in euthyroid older women. Neurology. 2002;58(7):1055–1061. [DOI] [PubMed] [Google Scholar]
- 44. Benseñor IM, Lotufo PA, Menezes PR, Scazufca M. Subclinical hyperthyroidism and dementia: The Sao Paulo Ageing & Health Study (SPAH). BMC Public Health. 2010;10:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Osch LA, Hogervorst E, Combrinck M, Smith AD. Low thyroid-stimulating hormone as an independent risk factor for Alzheimer disease. Neurology. 2004;62(11):1967–1971. [DOI] [PubMed] [Google Scholar]
- 46. Moon JH, Park YJ, Kim TH, et al. Lower-but-normal serum TSH level is associated with the development or progression of cognitive impairment in elderly: Korean Longitudinal Study on Health and Aging (KLoSHA). J Clin Endocrinol Metab. 2014;99(2):424–432. [DOI] [PubMed] [Google Scholar]
- 47. Hancock P, Larner AJ. Test Your Memory test: Diagnostic utility in a memory clinic population. Int J Geriatr Psychiatry. 2011;26(9):976–980. [DOI] [PubMed] [Google Scholar]
- 48. Sheehan B. Assessment scales in dementia. Ther Adv Neurol Disord. 2012;5(6):349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Galvin JE, Sadowsky CH, NINCDS-ADRDA. Practical guidelines for the recognition and diagnosis of dementia. J Am Board Fam Med. 2012;25(3):367–382. [DOI] [PubMed] [Google Scholar]
- 50. de Bruijn RF, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014;12:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Egger M, Davey Smith G, Altman D eds. Systematic Reviews in Health Care: Meta-analysis in Context. London, England: BMJ Publishing Group; 2001. [Google Scholar]
- 52. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95(8):3623–3632. [DOI] [PubMed] [Google Scholar]
- 53. Quinn TJ, Gussekloo J, Kearney P, Rodondi N, Stott DJ. Subclinical thyroid disorders. Lancet. 2012;380(9839):335–336; author reply 336–337. [DOI] [PubMed] [Google Scholar]
- 54. Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295(9):1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: The EPIC-Norfolk prospective population study. Clin Endocrinol. 2010;72(3):404–410. [DOI] [PubMed] [Google Scholar]
- 56. Boucai L, Hollowell JG, Surks MI. An approach for development of age-, gender-, and ethnicity-specific thyrotropin reference limits. Thyroid. 2011;21(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatrics. 2015;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 59. Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ. 2001;323(7306):224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: Scientific review and guidelines for diagnosis and management. JAMA. 2004;291(2):228–238. [DOI] [PubMed] [Google Scholar]
- 61. Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: An evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(1):35–45. [DOI] [PubMed] [Google Scholar]
- 62. Rodondi N, Bauer DC. Subclinical hypothyroidism and cardiovascular risk: How to end the controversy. J Clin Endocrinol Metab. 2013;98(6):2267–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.