Abstract
Context:
Levothyroxine (LT4) monotherapy is the standard of care for hypothyroidism.
Objective:
To determine whether LT4 at doses that normalize the serum TSH is associated with normal markers of thyroid status.
Design:
Cross-sectional data from the US National Health and Nutrition Examination Survey (2001–2012) was used to evaluate 52 clinical parameters. LT4 users were compared to healthy controls and controls matched for age, sex, race, and serum TSH. Regression was used to evaluate for correlation with T4 and T3 levels.
Participants:
A total of 9981 participants with normal serum TSH were identified; 469 were LT4-treated.
Results:
Participants using LT4 had higher serum total and free T4 and lower serum total and free T3 than healthy or matched controls. This translated to approximately 15–20% lower serum T3:T4 ratios in LT4 treatment, as has been shown in other cohorts. In comparison to matched controls, LT4-treated participants had higher body mass index despite report of consuming fewer calories/day/kg; were more likely to be taking beta-blockers, statins, and antidepressants; and reported lower total metabolic equivalents. A serum TSH level below the mean in LT4-treated participants was associated with a higher serum free T4 but similar free and total T3; yet those with lower serum TSH levels exhibited higher serum high-density lipoprotein and lower serum low-density lipoprotein, triglycerides, and C-reactive protein. Age was negatively associated with serum free T3:free T4 ratio in all participants; caloric intake was positively associated in LT4-treated individuals.
Conclusions:
In a large population study, participants using LT4 exhibited lower serum T3:T4 ratios and differed in 12/52 objective and subjective measures.
The ideal therapeutic goal in hypothyroidism would be to restore clinical and biochemical euthyroidism via physiologic thyroid hormone replacement. This concept may seem straightforward, but there are subtleties that have only recently been recognized by the medical community (1, 2). For the past four decades, the standard approach for thyroid hormone replacement in hypothyroidism has been administration of levothyroxine (LT4) at doses that normalize the serum TSH (3). This strategy has been justified with the knowledge that, in humans, the iodothyronine deiodinases in peripheral tissues produce most of the circulating active form of thyroid hormone, T3, via conversion from T4 (4). The hypothesis that LT4 “monotherapy” will maintain an adequate serum pool of T4 and that the iodothyronine deiodinases will then provide physiologic regulation of T3 availability has been held with much conviction (5).
The dogma in clinical thyroidology that LT4 monotherapy at doses that normalize serum TSH is sufficient to restore euthyroidism (1, 2) has come into question because evidence suggests a significant proportion of patients treated with LT4 continue to experience residual symptoms of hypothyroidism, including psychological (6) and metabolic (7) effects. One hypothesis to explain this phenomenon is that serum levels of T3 might not be fully normalized (8) (ie, T4-to-T3 conversion) in these patients may be insufficient to restore levels to those achieved when thyroidal secretion of T3 is intact. A second hypothesis is based on the fact that in many tissues, intracellular T3 levels cannot be predicted based on circulating thyroid hormone levels due to the actions of the types 2 and 3 deiodinases. Thus, in some tissues a relatively higher serum T4 level could result in enhanced thyroid hormone signaling without affecting circulating T3 levels (9). In contrast, in other tissues the relatively higher serum T4 levels could impair intracellular T3 production via downregulation of a deiodinase pathway (10). In fact, an animal model of primary hypothyroidism supports the hypothesis that LT4 monotherapy does not achieve systemic euthyroidism. Thyroidectomized rodents treated with LT4 at doses that normalize serum TSH exhibit relatively lower serum T3 and higher serum T4 levels as well as markers of hypothyroidism within their brain, skeletal muscle, and liver tissues (10, 11). However, studies in humans are necessary given that interspecies differences could limit the translatability of these findings (5). Last, symptomatic differences between healthy euthyroid individuals and LT4-treated patients that have normal serum TSH could be independent of serum T4 and/or T3 levels but rather from multiple other confounders (6, 12).
Hypothyroidism is a prevalent condition (13) and levothyroxine is commonly prescribed; in 2015, levothyroxine was the single most commonly prescribed medication in the United States (14). Thus understanding whether all parameters of hypothyroidism are universally restored by LT4 monotherapy has great clinical significance. Here we used publically available data from US National Health and Nutrition Examination Survey (NHANES), a well-defined, large, cross-sectional population study to evaluate whether individuals on LT4 monotherapy were the same as those not using LT4 in terms of thyroid function tests, thyroid hormone–related markers, and to identify clinical factors associated with serum T3:T4 ratios.
Materials and Methods
Study participants
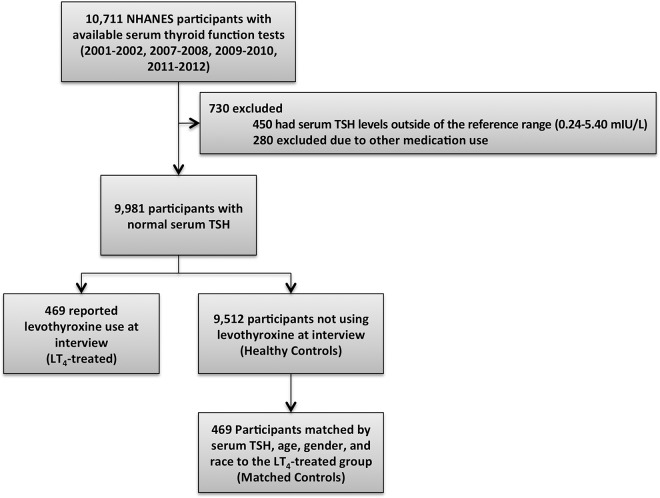
Publically available data were obtained from the NHANES, a large, multistage, survey assessing the health and nutritional status of Americans. The eligible population was restricted to individuals at least 18 years of age who had serum TSH, free T3, total T3, free T4, and total T4 measured during an NHANES cycle (2001–2002 (15), 2007–2008 (16), 2009–2010 (17), and 2011–2012 (18)). The same assays were used to measure serum TSH, free T3, total T3, free T4, and total T4 for all NHANES cycles. Participants were excluded if they had a serum TSH level outside of the reference range (0.24–5.40 mIU/liter, n = 450), were pregnant, were taking thyroid-related supplements, methimazole, propylthiouracil, liothyronine, steroids, amiodarone, lithium, desiccated thyroid preparations, antiepileptics, or dopaminergic analogues (n = 280; Figure 1). Data were obtained from demographic, questionnaire, and laboratory files; all data were collected from trained interviewers using validated procedures and questionnaires to minimize bias (19).
Figure 1.
Study profile.
Statistical methods
Analyses were completed with SPSS (version 22.0) (20). Participants were grouped based on report of LT4 use at interview. Two control groups were identified: healthy controls (remaining sample not using LT4) and matched controls, matched to 1:1 for serum TSH, age (both matched within 2 SDs of the LT4-treated population’s mean), sex, and race/ethnicity to LT4-treated individuals. Differences between groups were compared using χ2 and Student’s t test. Differences in thyroid hormones were compared between individuals below and above the entire population’s TSH mean (1.75 mIU/liter) within each group.
Pearson’s correlation coefficients were calculated to describe the relationship between serum free T3:free T4 ratio and variables. Despite known problems with measurement of serum free T3 (21), this was selected for use in these analyses in an effort to control for estrogen status between diverse study participants. Univariate linear regression was used to determine variables significantly associated with serum free T3:free T4 ratio; unstandardized regression coefficients were reported. Finally, multivariate linear regression was used to describe the association of free T3:free T4 ratio while controlling for significant variables; variables identified as significant from the both the LT4 treated and matched controls’ univariate analyses were entered into a forward selection model. The current analysis was a cross-sectional examination of serum thyroid levels among LT4-treated vs nontreated individuals and not meant to be representative of the national population. Thus, sample weights were not used to adjust for oversampling of selected groups.
Results
LT4-treated participants have a lower serum T3:T4 ratio
From NHANES years 2001–2002 (15), 2007–2008 (16), 2009–2010 (17), and 2011–2012 (18), a total of 9981 participants had a normal serum TSH level and met inclusion criteria for the present studies (Figure 1). Of these, 469 were taking LT4 (LT4-treated) and 9512 were not taking LT4 (healthy controls). In comparison to the healthy controls, LT4-treated participants had approximately 20% higher serum TSH levels, 10% lower serum free T3 levels, and 15% lower total T3 levels (Supplemental Table 1). In addition, their serum free and total T4 levels were higher than those of healthy controls by about 15%. This resulted in approximately 25% lower T3:T4 ratios in the LT4-treated participants. The LT4-treated participants also differed significantly in key demographic factors compared to these healthy “controls:” LT4-treated participants were older than those not using LT4, were more likely to be female, and had a different racial-ethnic distribution. This prompted the creation of a group of “matched controls:—469 participants who were not using LT4 and were matched by TSH, age, sex, and race were selected from the 9512 healthy controls.
When considering LT4-treated participants vs matched controls, results were consistent; LT4-treated participants exhibited 5–10% lower free and total T3 serum levels and 10–15% higher free and total T4 serum levels (Table 1). The serum T3:T4 ratios were approximately 15–20% less in LT4-treated individuals than in the matched controls.
Table 1.
Characteristics of Adult NHANES Participants With Normal Serum TSH Levels, by Levothyroxine Use
| LT4-Treated (n = 469) | Matched Controls (n = 469) | P Valuea | |
|---|---|---|---|
| Age (y) | 64.3 ± 14.1 | 64.1 ± 14.0 | .88 |
| Female (%) | 360 (77) | 360 (77) | 1.0 |
| Race (%) | |||
| Non-Hispanic white | 336 (71) | 336 (71) | 1.0 |
| Non-Hispanic black | 36 (8) | 36 (8) | |
| Hispanic | 76 (16) | 76 (16) | |
| Other | 21 (4) | 21 (4) | |
| Serum TSH (mIU/liter) | 2.13 ± 1.32 | 2.15 ± 1.29 | .83 |
| Serum free T3 (pg/ml) | 2.85 ± 0.33 | 3.01 ± 0.39 | <.0001 |
| Serum total T3 (ng/ml) | 97.56 ± 20.64 | 108.29 ± 24.89 | <.0001 |
| Serum free T4 (ng/ml) | 0.94 ± 0.21 | 0.80 ± 0.14 | <.0001 |
| Serum total T4 (μg/dL) | 9.14 ± 1.76 | 8.08 ± 1.56 | <.0001 |
| Free T3:free T4b | 3.18 ± 0.80 | 3.85 ± 0.75 | <.0001 |
| Total T3:free T4b | 109.59 ± 36.30 | 138.46 ± 38.49 | <.0001 |
| Total T3:total T4b | 11.01 ± 2.85 | 13.70 ± 3.37 | <.0001 |
Data are mean ± sd, n (%). P value by χ2 (categorical data) or Student’s t test (continuous data).
For the comparison of LT4-treated and matched controls.
Multiplied × 1000.
Not all clinical parameters are “normal” in LT4-treated participants
Fifty-two parameters possibly associated with thyroid hormone status were assessed in these groups (Table 2). The LT4-treated participants exhibited about 5% higher body mass index (BMI) than healthy and matched controls (Supplemental Table 2 and Table 2). LT4 users had slightly higher systolic and lower diastolic blood pressures than healthy controls (Supplemental Table 2), but these differences subsided in the comparison of LT4-treated participants to matched controls (Table 2). Heart rate did not differ between LT4 users and controls, although LT4 users were more likely to be taking beta-blocker medications than controls from either group.
Table 2.
Clinical Parameters of Adult NHANES Participants With Normal Serum TSH Level
| LT4-Treated (n = 469) | Matched Controls (n = 469) | P Valuea | |
|---|---|---|---|
| Objective measures | |||
| BMI (kg/m2) | 29.8 ± 6.7 | 28.2 ± 6.2 | <.001 |
| Systolic blood pressure (mm Hg) | 131 ± 22 | 131 ± 22 | .80 |
| Diastolic blood pressure (mm Hg) | 68 ± 14 | 68 ± 14 | .39 |
| Heart rate (beats per minute) | 72 ± 12 | 72 ± 12 | .59 |
| HbA1C (%) | 5.9 ± 0.9 | 5.9 ± 0.9 | .54 |
| Fasting glucose (mg/dl) | 106 ± 37 | 104 ± 33 | .21 |
| Total cholesterol (mg/dl) | 197 ± 41 | 205 ± 42 | <.01 |
| HDL (mg/dl) | 54 ± 16 | 57 ± 16 | .02 |
| LDL (mg/dl) | 115 ± 35 (n = 175) | 123 ± 37 (n = 183) | .03 |
| Triglyceride (mg/dl) | 144 ± 86 (n = 180) | 134 ± 68 (n = 1814) | .22 |
| C-reactive protein (mg/dl) | 0.50 ± 0.64 (n = 400) | 0.50 ± 1.00 (n = 402) | .94 |
| Ferritin (ng/ml) | 102 ± 106 (n = 103) | 90 ± 102 (n = 103) | .35 |
| Creatinine (mg/dl) | 0.93 ± 0.53 | 0.90 ± 0.35 | .28 |
| Creatine phosphokinase (IU/liter) | 118 ± 91 (n = 69) | 110 ± 70 (n = 67) | .54 |
| Medication use | |||
| Beta-blocker (%) | 175 (37) | 110 (24) | <.0001 |
| Statin (%) | 111 (24) | 72 (15) | <.01 |
| Insulin (%) | 20 (4) | 14 (3) | .30 |
| Oral hypoglycemic (%) | 59 (45) | 39 (38) | .25 |
| Antidepressant (%) | 101 (22) | 69 (15) | <.01 |
| Antianxiety (%) | 30 (6) | 29 (6) | .89 |
| Antipsychotic (%) | 5 (1) | 6 (1) | .76 |
| METs/physical parameters | |||
| Total METs (work and recreational activity) | 2255 ± 3464 | 3167 ± 4803 | .01 |
| Work/job requires vigorous activity (%) | 42 (10) | 27 (6) | .06 |
| Vigorous work METs | 3765 ± 4215 | 6317 ± 7196 | .07 |
| Work/job requires moderate activity (%) | 115 (27) | 128 (31) | .33 |
| Moderate work MET | 2000 ± 2429 | 3046 ± 3368 | <.01 |
| Walks/bikes for transportation (%) | 75 (18) | 82 (20) | .55 |
| Transportation MET | 826 ± 952 | 1424 ± 2033 | .02 |
| Participates in vigorous recreational activity (%) | 43 (10) | 38 (9) | .55 |
| Vigorous recreational MET | 658 ± 573 | 688 ± 795 | .84 |
| Participates in moderate recreational activity (%) | 173 (41) | 136 (32) | <.01 |
| Moderate recreational MET | 746 ± 595 | 835 ± 841 | .28 |
| Cognitive/well-being parameters | |||
| Stated health condition (%) | |||
| Excellent | 26 (6) | 39 (9) | .38 |
| Very good | 115 (26) | 111 (26) | |
| Good | 185 (42) | 181 (42) | |
| Fair | 81 (19) | 80 (19) | |
| Poor | 31 (7) | 22 (5) | |
| Number of days in the past month physical health was not good | 5.6 ± 11.5 | 4.5 ± 8.7 | .11 |
| Number of days in the past month mental health was not good | 5.1 ± 11.6 | 4.4 ± 8.8 | .32 |
| Number of days in the past month inactive due to physical or mental health | 2.5 ± 7.7 | 1.7 ± 5.7 | .12 |
| Physical, mental, or emotional limitation kept from working (%) | 82 (18) | 72 (16) | .38 |
| Experience confusion/memory problem (%) | 62 (13) | 45 (9) | .08 |
| Limited in activity due to physical, mental or emotional problem (%) | 16 (6) | 17 (5) | .91 |
| Social factors | |||
| Smoked at least 100 cigarettes in lifetime (%) | 219 (47) | 213 (47) | .67 |
| Currently smoking daily (%) | 47 (21) | 51 (24) | .54 |
| Consumed at least 12 alcoholic drinks per year (%) | 181 (64) | 178 (62) | .60 |
| Nutrient intake | |||
| Calories consumed in 24-h recall (kcal/day) | 1761 ± 715 | 1759 ± 927 | .98 |
| Calories consumed, adjusted by body weight (kcal/day/kg) | 23 ± 9 | 24 ± 13 | .05 |
| % calorie intake compared to DRI for energy | 90 ± 38 | 97 ± 54 | .05 |
| Carbohydrate consumed (g) | 217 ± 94 | 219 ± 114 | .82 |
| Carbohydrate consumed (%) | 50 ± 11 | 51 ± 11 | .39 |
| Protein consumed (g) | 67 ± 31 | 67 ± 35 | .87 |
| Protein consumed (%) | 16 ± 4 | 16 ± 5 | .95 |
| Fat consumed (g) | 68 ± 36 | 66 ± 41 | .59 |
| Fat consumed (%) | 34 ± 9 | 33 ± 9 | .33 |
| Selenium intake (mcg) | 52 ± 44 | 52 ± 45 | .91 |
Abbreviations: DRI, dietary reference intake; HbA1C, hemoglobin A1C; METS, metabolic equivalents.
Data are mean ± sd, n (%). P value by χ2 (categorical data) or Student’s t test (continuous data).
For the comparison of LT4-treated and matched controls.
Serum hemoglobin A1C levels and fasting glucose values were higher in healthy controls than LT4-treated participants, but these differences were not present in the comparison of LT4-treated participants to the matched controls (Supplemental Table 2 and Table 2). Triglyceride levels did not differ between LT4 users and either control group. Serum low-density lipoprotein (LDL), high-density lipoprotein (HDL), and total cholesterol levels were lower in the LT4-treated group than the matched controls, but more of these participants were taking statin medications (Table 2).
Antidepressant use was more prevalent in LT4-treated participants than healthy or matched controls (Supplemental Table 2 and Table 2). Although more LT4-treated participants were using anxiolytic medications than the healthy controls, there was no difference in distribution of anxiolytic, or antipsychotic use, between LT4-treated participants and matched controls.
Physical activity and metabolic equivalent assessments were also available in NHANES. In general, LT4-treated participants reported less physical activity via these measures than the healthy controls, but some of these differences were no longer significant in the comparison with matched controls (Supplemental Table 2 and Table 2). LT4 users reported significantly less total, moderate work, and transportation metabolic equivalents than matched controls (Table 2). However, LT4-treated participants reported more participation in moderate recreational activities, 41% vs 32% of matched controls.
Self-report of days in the past month where participants felt that their physical and mental health was “not good” was more frequent in LT4 users compared to healthy controls, as was report of being inactive because of physical or mental health and frequency of reported problems with confusion/memory (Supplemental Table 2). There was no significant difference in these parameters in the comparison with matched controls, although there was a general trend toward impaired well-being reports in LT4 users (Table 2).
LT4-treated participants consumed fewer calories per day in a 24-hour dietary recall than healthy controls (Supplemental Table 2). Although LT4-treated participants displayed the same calorie intake compared with matched controls, when adjusted by body weight, LT4-treated participants consumed about 5% fewer calories per day (Table 2). There were no differences in proportions of carbohydrate, protein, or fat reportedly consumed between the matched controls and LT4-treated participants.
A lower serum TSH in LT4-treated participants is associated with different metabolic profile but not higher serum T3
The mean serum TSH from the 9981 participants was 1.75 mIU/L. Each participant group (LT4-treated, healthy and matched controls) was further divided into those with serum TSH values above or below this mean (Table 3, Supplemental Tables 3 and 4). Those LT4 users with serum TSH levels below the mean have about 10% higher free and total T4 than those with serum TSH levels above the mean (Table 3). However, serum free and total T3 levels do not differ among LT4-treated participants with serum TSH levels above or below the mean. This resulted in approximately 10% lower free T3:free T4, total T3:free T4, and total T3:total T4 ratios. In other words, although serum free T4 levels are higher among LT4 users with slightly lower serum TSH levels within the normal range, serum T3 levels (free or total) are unaffected (Table 3).
Table 3.
Thyroid Hormone Levels of Participant Groups, by Serum TSH
| LT4-Treated (n = 469) | Matched Controls (n = 469) | |||
|---|---|---|---|---|
| TSH 0.24–1.74 (n = 213) | TSH 1.75–5.40 (n = 256) | TSH 0.24–1.74 (n = 210) | TSH 1.75–5.40 (n = 259) | |
| Age (y) | 64.1 ± 13.2 | 64.5 ± 14.8 | 64.0 ± 13.4 | 64.3 ± 14.35 |
| Female (%) | 178 (84) | 182 (71)b | 181 (83) | 195 (72)c |
| Race/ethnicity (%) | ||||
| Non-Hispanic white | 156 (73) | 180 (70) | 162 (74) | 186 (89) |
| Non-Hispanic black | 19 (9) | 17 (7) | 17 (8) | 21 (8) |
| Hispanic | 28 (13) | 48 (19) | 29 (13) | 51 (19) |
| Other | 10 (4) | 11 (4) | 9 (4) | 12 (4%) |
| Serum TSH (mIU/liter) | 0.95 ± 0.43 | 3.11 ± 0.97b | 0.98 ± 0.41 | 3.09 ± 0.95c |
| Serum free T3 (pg/ml) | 2.88 ± 0.34 | 2.83 ± 0.33 | 3.05 ± 0.40 | 2.98 ± 0.38 |
| Serum total T3 (ng/ml) | 98.04 ± 21.26 | 97.15 ± 20.14 | 111.96 ± 28.38 | 105.32 ± 21.25c |
| Serum free T4 (ng/ml) | 0.99 ± 0.21 | 0.90 ± 0.20b | 0.83 ± 0.14 | 0.78 ± 0.14c |
| Serum total T4 (μg/dl) | 9.52 ± 1.80 | 8.84 ± 1.67b | 8.29 ± 1.48 | 7.92 ± 1.60c |
| Free T3:free T4c | 3.03 ± 0.75 | 3.31 ± 0.81b | 3.76 ± 0.75 | 3.91 ± 0.75c |
| Total T3:free T4c | 104.07 ± 35.09 | 114.19 ± 36.71b | 138.47 ± 41.09 | 138.45 ± 36.33 |
| Total T3:total T4c | 10.60 ± 2.63 | 11.34 ± 2.99b | 13.80 ± 3.85 | 13.61 ± 2.93 |
The mean serum TSH level from the entire population was 1.75 mIU/liter. Participants within each group were then classified as having serum TSH levels above or below this mean, and then thyroid function tests reassessed for each subgroup. Data are mean ± sd, n (%). P value by χ3 (categorical data) or Student’s t test (continuous data).
For the comparison of LT4-treated and matched controls.
Multiplied × 1000.
Despite the lack of difference in serum T3 levels between LT4 users with serum TSH below and above the mean, there were several notable differences between these groups (Supplemental Table 4). LT4-treated participants with lower serum TSH levels had higher serum HDL and lower serum LDL, triglyceride, and CRP levels compared to LT4-treated participants with serum TSH levels above the mean; they were also more likely to be using statin medications.
Factors correlating and associated with the T3:T4 ratio
We next assessed correlation between the 52 clinical parameters possibly associated with thyroid hormone status and the serum free T3:free T4 ratio in participant groups (Supplemental Table 5). No parameter demonstrated a strong correlation with the serum free T3:free T4 ratio. Among the LT4-treated participants, serum triglycerides had the strongest direct correlation with serum free T3:free T4 ratio (r = 0.30) and age had the strongest inverse correlation (r = –0.41). Assessment for correlation of these 52 parameters with either free T3 (Supplemental Table 6) or free T4 (Supplemental Table 7) was also performed; some factors correlated with free T3 but not free T4 (eg, HDL), and some correlated with free T4 but not free T3 (eg, triglycerides, antidepressants).
Because these correlations were not strong, we next performed univariate regression analyses to evaluate whether any of the 52 parameters were associated with the serum free T3:free T4 ratio (Table 4 and Supplemental Table 8). In all three groups, age, creatinine, and HDL were negatively associated with the serum free T3:free T4 ratio, and BMI, triglycerides, number of calories consumed daily, and grams of fat consumed daily were positively associated. Parameters that were positively associated in both the LT4-treated participants and the healthy controls, but not the matched controls, included smoking history, alcohol consumption and carbohydrate and protein intake. beta-blocker usage (−) was associated with the serum free T3:free T4 ratio in the healthy and matched controls, but not the LT4-treated participants. Antidepressant usage was positively associated with the serum free T3:free T4 ratio in the LT4-treated participant group alone (Table 4). Many of the 52 clinical parameters were significantly associated with the serum free T3:free T4 ratio in the healthy control group only, including hemoglobin A1C (−), fasting glucose (−), LDL (+), and total cholesterol (+) (Supplemental Table 8).
Table 4.
Univariate Regression Analysis of Clinical Parameters and the Serum-Free T3:Free T4 Ratio
| LT4-Treated (n = 469) | Matched Controls (n = 469) | |||
|---|---|---|---|---|
| Regression Coefficient | P Value | Regression Coefficient | P Value | |
| Demographics variables | ||||
| Age (5-y increase) | −0.12 | <.0001 | −0.07 | <.0001 |
| Female | −0.27 | <.01 | −0.08 | .35 |
| Hispanic | 0.17 | .08 | 0.27 | <.01 |
| Non-Hispanic white | −0.16 | .05 | −0.12 | .12 |
| Non-Hispanic black | 0.16 | .25 | −0.07 | .59 |
| Other race/ethnicity | −0.05 | .80 | −0.16 | .35 |
| Objective measures | ||||
| BMI (5 kg/m2 increase) | 0.08 | <.01 | 0.09 | <.01 |
| Systolic blood pressure (mm Hg) | −0.01 | .02 | −0.01 | .41 |
| Diastolic blood pressure (mm Hg) | 0.01 | <.0001 | 0.01 | <.0001 |
| Heart rate (beats per minute) | 0.01 | .03 | 0.01 | .64 |
| HgbA1C (%) | 0.01 | .92 | 0.01 | .88 |
| Fasting glucose (50 mg/dl increase) | −0.03 | .55 | 0.02 | .74 |
| Total cholesterol (50 mg/dl increase) | 0.067 | .12 | 0.05 | .28 |
| LDL (50 mg/dl increase) | 0.04 | .64 | 0.05 | .52 |
| HDL (50 mg/dl increase) | −0.23 | .04 | −0.30 | <.01 |
| Triglyceride (50 mg/dl increase) | 0.14 | <.0001 | 0.16 | <.0001 |
| C-reactive protein (mg/dl) | −0.03 | .61 | −0.06 | .10 |
| Ferritin (50 ng/dl increase) | 0.01 | .85 | 0.07 | .12 |
| Creatinine (0.5 mg/dl increase) | −0.11 | <.01 | −0.25 | <.0001 |
| Creatinine phosphokinase (50 IU/liter increase) | 0.07 | .18 | 0.07 | .20 |
| Medication use | ||||
| Beta-blocker | −0.10 | .21 | −0.21 | <.0001 |
| Statin | 0.03 | .76 | 0.16 | .10 |
| Insulin | 0.17 | .35 | −0.06 | .78 |
| Oral hypoglycemic | 0.28 | .06 | 0.16 | .25 |
| Antidepressant | 0.23 | .01 | 0.04 | .71 |
| Antianxiety | −0.13 | .41 | −0.17 | .25 |
| Antipsychotic | −0.23 | .52 | −0.24 | .45 |
| Metabolic equivalents (METs)/physical parameters | ||||
| 1000 MET activity (work and recreational activity) | 0.01 | .46 | 0.01 | .31 |
| Work/job requires vigorous activity (%) | −0.01 | .96 | 0.06 | .71 |
| 1000 Vigorous work MET | −0.03 | .39 | 0.03 | .21 |
| Work/job requires moderate activity (%) | 0.04 | .66 | −0.06 | .43 |
| 1000 Moderate work MET | 0.07 | .03 | 0.03 | .15 |
| Walks/bikes for transportation (%) | −0.10 | .34 | −0.04 | .67 |
| 1000 Transportation MET | 0.06 | .57 | 0.01 | .80 |
| Participates in vigorous recreational activity (%) | −0.28 | .03 | −0.07 | .58 |
| 1000 Vigorous recreational MET | −0.02 | .38 | 0.02 | .15 |
| Participates in moderate recreational activity (%) | −0.05 | .54 | −0.08 | .29 |
| 1000 Moderate recreational MET | −0.09 | .33 | 0.06 | .40 |
| Cognitive/well-being parameters | ||||
| Excellent/good stated health condition | −0.03 | .70 | −0.04 | .61 |
| Poor stated health condition | 0.34 | .03 | −0.10 | .56 |
| Number of days in the past month physical health was not good | 0.01 | .04 | −0.01 | .76 |
| Number of days in the past month mental health was not good | 0.01 | .16 | 0.01 | .85 |
| Number of days in the past month inactive because of physical or mental health | 0.01 | .08 | 0.01 | .67 |
| Physical, mental, or emotional limitation kept from working | −0.06 | .55 | −0.03 | .79 |
| Experience confusion/memory problem | −0.01 | .98 | 0.26 | .03 |
| Limited in activity due to physical, mental, or emotional problem | 0.36 | .08 | −0.04 | .85 |
| Social factors | ||||
| Smoked at least 100 cigarettes | −0.02 | .82 | 0.02 | .79 |
| Currently smoking daily | 0.27 | .04 | 0.09 | .50 |
| Consumed at least 12 drinks per year | −0.20 | .04 | 0.06 | .51 |
| Number of alcoholic drinks consumed per day | 0.16 | <.0001 | 0.11 | .01 |
| Nutrient intake | ||||
| 1000 calories consumed in 24-h recall | 0.21 | <.0001 | 0.09 | .20 |
| Calories consumed, adjusted by body weight (kcal/day/kg) | 0.01 | .24 | 0.01 | .68 |
| % calorie intake compared to DRI for energy | 0.01 | .24 | 0.01 | .68 |
| 100 g carbohydrate consumed | 0.11 | <.01 | 0.05 | .09 |
| Carbohydrate consumed (%) | −0.01 | .21 | 0.01 | .35 |
| 50 g protein consumed | 0.25 | <.0001 | 0.07 | .15 |
| Protein consumed (%) | 0.01 | .87 | −0.02 | .02 |
| 50 grams fat consumed | 0.16 | <.01 | 0.08 | .06 |
| Fat consumed (%) | 0.01 | .68 | −0.01 | .38 |
| Selenium intake (mcg) | 0.01 | .98 | −0.01 | .35 |
Abbreviations: DRI, dietary reference intake; HbA1C, hemoglobin A1C.
Factors that were identified to be associated with the serum free T3:free T4 ratio in the LT4-treated participants by univariate regression analysis were then assessed in a model of multivariate regression (Table 5 and Supplemental Table 9). In this model, most clinical parameters were no longer significant among the LT4-treated and matched controls. Age was significant in all three groups; for instance in the LT4-treated participants, an age increase of 5 years was associated with a decrease in serum free T3:free T4 ratio of about 0.14 (Table 5). In the LT4-treated participants, calorie consumption was positively associated with the serum free T3:free T4 ratio, yet was not significant in either control group. In matched controls, sex was also associated (Table 5). In healthy controls, age, sex, BMI, calorie consumption, creatinine, total cholesterol, and triglycerides were also all associated with the serum free T3:free T4 ratio (Supplemental Table 9).
Table 5.
Multivariate Regression Analysis Identifies Clinical Parameters Associated With the Serum Free T3:Free T4 Ratio
| LT4-Treated (n = 469) | Matched Controls (n = 469) | |||
|---|---|---|---|---|
| Regression Coefficient | P Value | Regression Coefficient | P Value | |
| Age (5 yr increase) | −0.14 | <.0001 | −0.10 | <.0001 |
| Female | — | NS | −0.32 | <.0001 |
| BMI (5 kg/m2 increase) | — | NS | — | NS |
| Total cholesterol (50 mg/dl increase) | — | NS | — | NS |
| Triglyceride (50 mg/dl increase) | — | NS | — | NS |
| Creatinine (0.5 mg/dl increase) | — | NS | — | NS |
| 1000 MET activity (work and recreation) | — | NS | — | NS |
| 1000 calories consumed | 0.20 | .05 | — | NS |
| Beta-blocker prescription | — | NS | — | NS |
| Currently smoking daily | — | NS | — | NS |
| Number of alcoholic drinks consumed per day | — | NS | — | NS |
Abbreviation: MET, metabolic equivalent.
Age was scaled to 5 y, BMI scaled to 5 kg/m2, total cholesterol scaled to 50 mg/dl, triglyceride scaled to 50 mg/dl, creatinine scaled to 0.5 mg/dl, beta-blocker use, current smoker, number of alcohol drinks consumed per day, total METs scaled to 1000 MET, and calorie intake scaled to 1000 calories. HDL and LDL were omitted because of multicollinearity with total cholesterol.
Discussion
In comparison to euthyroid individuals not taking LT4, participants taking LT4 with a normal serum TSH exhibited 1) relatively lower serum free and total T3; 2) relatively higher serum free and total T4; and, consequently, 3) lower T3:T4 ratios; these relationships were consistent in the comparison with healthy and matched controls. Although this phenomenon has been noted in the setting of LT4 monotherapy for the past four decades (3, 8, 22–29), there are at least two prior, smaller studies showing that serum T3 levels can be normal in LT4-treated individuals with normal serum TSH (28, 30). Although this could be due to the smaller size of the study populations, it is notable that one of these studies is inconsistent with a previous publication from their own group (24).
The major strength of the present studies is the availability of biochemical data as well as markers of quality of life (QOL) in a large population sample to assess for clinical relevance. There were major differences in seven (of 21) objectives (BMI, total cholesterol, HDL, LDL; beta-blocker, statin, and antidepressant use), and five (of 31) subjective (nutrient intake, reported physical activity) clinical parameters between LT4-treated participants and matched controls. Although we recognize that these parameters are not specific markers of hypothyroidism and we cannot determine whether they were different between the groups prior to LT4 treatment, this does not mitigate that these data present a strong challenge the dogma that having a normal serum TSH equates with euthyroidism in LT4 treatment.
Although it is not clear what underlies the differences in these clinical parameters, the preclinical data indicate an important role played by the suboptimal normalization of serum T3 and/or T4 levels (10). However, the present results revealed that few of the clinical parameters were significantly associated with serum free T3, serum free T4, and/or serum free T3:free T4 ratio by univariate analysis, and the strength of the relationship was not always impressive. Furthermore, statistical significance was lost for most associations in the multivariate analysis. These observations are limited by the cross-sectional nature of this study, but may minimize the potential role for tight maintenance of serum T3 and/or T4 levels in patients with normal serum TSH. As a result, the door is open for other possible explanations, including recognized and unrecognized comorbidities, psychological implications of a chronic illness requiring long-term prescription medication, autoimmune confounders, or increased screenings and treatment in patients who do not feel well (12).
An unrelated byproduct of the present studies was the identification of demographic and biochemical variables that correlate with serum T3:T4 ratios in a large group of normal individuals. Recall that in such group serum T3 and T4 levels as well as the T3:T4 ratio is defined by thyroidal secretion as well as the deiodinase pathways (types 1 and 2). Thus, the multivariate analysis revealed that age, female sex, and serum creatinine are negatively associated with serum free T3:free T4 ratio. In contrast, BMI, total serum cholesterol, and triglycerides were positively associated with serum free T3:free T4 ratio. At the same time, in LT4-treated individuals, the type 2 deiodinase (D2) is the predominant source of circulating T3 (31), and thus any factor that affects this pathway, and thus the serum T3:T4 ratio, is of great potential clinical relevance. In this regard, in the present investigation we found that in individuals taking LT4, only two variables were significantly associated with serum T3:T4 ratio—namely age and number of calories consumed. The association with age is likely reflecting that skeletal muscle contains D2 and sarcopenia advances with age. At the same time, the association with caloric intake is reminiscent of the fact that insulin stimulates D2-mediated T3 production in the skeletal muscle (32).
There are several limitations to these studies. NHANES is cross-sectional and thus causality cannot be ascertained. It also cannot be determined whether the groups differed before treatment with respect to the measured parameters; however, it is reassuring that prevalence of LT4 use in this cohort (about 5%) resembles the prevalence of hypothyroidism that we would expect in an iodine-replete population (13). Participants in these studies were grouped based on their self-reported use of LT4 and there was no availability of records demonstrating previous diagnosis of hypothyroidism. Although hypothyroidism is a very prevalent condition (13), LT4 is not uncommonly prescribed for euthyroid individuals for other conditions such as fatigue, obesity, and depression (1), and thus it is possible that the prevalence of these conditions are different between the groups and thus represent a source of confounding. This may not be likely given that euthyroid individuals taking LT4 could exhibit low serum TSH, and thus would have been excluded from these studies. Last, there may be additional sources of recall bias or confounding as individuals taking prescription LT4 may report worse QOL because the act of taking/needing a prescription medication may influence their perception of their health; this would more likely influence reporting of subjective variables and would not explain differences in the objective parameters.
In conclusion, NHANES participants with normal serum TSH levels on LT4 monotherapy exhibit lower serum T3:T4 ratios than healthy euthyroid controls. LT4-treated individuals have higher BMIs despite reporting lower calorie intake corrected by body weight, report lower physical activity levels, and are more often taking statins, beta-blockers, and antidepressants. The mechanisms underlying these findings in the LT4-treated individuals remain undefined as we did not observe a significant association in the multivariable analysis with serum free T3, free T4, or free T3:free T4 ratio. Notwithstanding, the concept that establishing a normal serum TSH renders individuals on LT4 monotherapy clinically euthyroid should be revisited and QOL measures should be more highly prioritized in hypothyroidism research and professional guidelines.
Supplementary Material
Acknowledgments
The authors thank the National Health and Nutrition Examination Survey for providing public access to its data.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- BMI
body mass index
- D2
type 2 deiodinase
- LT4
levothyroxine
- NHANES
US National Health and Nutrition Examination Survey
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- QOL
quality of life.
Reference
- 1. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA Guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J. 2012;1:55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McAninch EA, Bianco AC. The history and future of treatment of hypothyroidism. Ann Intern Med. 2016;164:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braverman LE, Ingbar SH, Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic subjects. J Clin Invest. 1970;49:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gereben B, McAninch EA, Ribeiro MO, Bianco AC. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol. 2015;11:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol. 2002;57:577–585. [DOI] [PubMed] [Google Scholar]
- 7. Samuels MH, Kolobova I, Smeraglio A, Peters D, Purnell JQ, Schuff KG. Effects of levothyroxine replacement or suppressive therapy on energy expenditure and body composition. Thyroid. 2016;26:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PloS One. 2011;6:e22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. al-Adsani H, Hoffer LJ, Silva JE. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metabol. 1997;82:1118–1125. [DOI] [PubMed] [Google Scholar]
- 10. Werneck de Castro JP, Fonseca TL, Ueta CB, et al. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Investig. 2015;125:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Escobar-Morreale HF, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Investig. 1995;96:2828–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor PN, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med. 2014;174:32–39. [DOI] [PubMed] [Google Scholar]
- 13. Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39–51. [DOI] [PubMed] [Google Scholar]
- 14. Medicines Use and Spending in the U.S.: A Review of 2015 and Outlook to 2020. Parsippany, NJ: IMS Institute for Healthcare Informatics; 2016. [Google Scholar]
- 15. National Center for Health Statistics. National Health and Nutrition Examination Survey Data 2001–2002; http://wwwn.cdc.gov/nchs/nhanes/search/nhanes01_02.aspx Accessed October 5, 2016
- 16. National Center for Health Statistics. National Health and Nutrition Examination Survey Data 2007–2008; http://wwwn.cdc.gov/Nchs/Nhanes/Search/nhanes07_08.aspx Accessed October 5, 2016
- 17. National Center for Health Statistics. National Health and Nutrition Examination Survey Data 2009–2010; http://wwwn.cdc.gov/Nchs/Nhanes/Search/nhanes09_10.aspx Accessed October 5, 2016
- 18. National Center for Health Statistics. National Health and Nutrition Examination Survey Data 2011–2012; http://wwwn.cdc.gov/Nchs/Nhanes/Search/nhanes11_12.aspx Accessed October 5, 2016
- 19. National Center for Health Statistics. National Health and Nutrition Examination Laboratory Protocol. http://www.cdc.gov/nchs/nhanes/nhanes2001–2002/lab_methods_01_02.htm Accessed October 5, 2016
- 20. IBM SPSS Statistics for Windows. Version 22.0 Armonk, NY: IBM Corp; Released 2013. [Google Scholar]
- 21. Abdalla SM, Bianco AC. Defending plasma T3 is a biological priority. Clin Endocrinol. 2014;81:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cobb WE, Jackson IM. Drug therapy reviews: management of hypothyroidism. American journal of hospital pharmacy. 1978;35(1):51–58. [PubMed] [Google Scholar]
- 23. Jackson IM, Cobb WE. Why does anyone still use desiccated thyroid USP? Am J Med. 1978;64:284–288. [DOI] [PubMed] [Google Scholar]
- 24. Stock JM, Surks MI, Oppenheimer JH. Replacement dosage of L-thyroxine in hypothyroidism. A re-evaluation. N Engl J Med. 1974;290:529–533. [DOI] [PubMed] [Google Scholar]
- 25. Ingbar SH, Woeber KA. Thyroid hormone deficiency. In: Williams RH, ed. Textbook of Endocrinology. 5th ed W. B. Saunders Company; 1974:191–212. [Google Scholar]
- 26. Sawin CT, Surks MI, London M, Ranganathan C, Larsen PR. Oral thyroxine: variation in biologic action and tablet content. Ann Intern Med. 1984;100:641–645. [DOI] [PubMed] [Google Scholar]
- 27. Werner SC. Treatment. In: Werner SC, Ingbar SH, eds. The Thyroid a Fundamental and Clinical Text. 4th ed Baltimore, MD: Harper & Row, Publishers, Inc.; 1978:965–970. [Google Scholar]
- 28. Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764–770. [DOI] [PubMed] [Google Scholar]
- 29. Woeber KA. Levothyroxine therapy and serum free thyroxine and free triiodothyronine concentrations. J Endocrinol Investig. 2002;25:106–109. [DOI] [PubMed] [Google Scholar]
- 30. Jonklaas J, Davidson B, Bhagat S, Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA. 2008;299:769–777. [DOI] [PubMed] [Google Scholar]
- 31. Geffner DL, Azukizawa M, Hershman JM. Propylthiouracil blocks extrathyroidal conversion of thyroxine to triiodothyronine and augments thyrotropin secretion in man. J Clin Invest. 1975;55:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lartey LJ, Werneck-de-Castro JP, I OS, Unterman TG, Bianco AC. Coupling between nutrient availability and thyroid hormone activation. J Biol Chem. 2015;290:30551–30561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.