Abstract
Background
The hippocampus is vulnerable to severe damage after cerebral ischaemia–reperfusion (I/R) injury. This study aimed to explore the effect of electroacupuncture (EA) on cognitive impairment and its relationship with Ca2+neurotoxicity in a rat model of I/R injury induced by middle cerebral artery occlusion (MCAO).
Methods
60 adult male Sprague-Dawley rats were randomly divided into three groups: control (sham surgery) group, untreated MCAO group and EA-treated MCAO+EA group. Rats in the MCAO and MCAO+EA groups underwent modelling of poststroke cognitive impairment by MCAO surgery. EA was performed for 30 min daily at GV20 and GV24 (1–20 Hz) for 1 week. The Morris water maze experiment was used to assess cognitive function. 2,3,5-triphenyl tetrazolium chloride staining was used to measure infarct volume. The intracellular Ca2+content in the Cornu Ammonis (CA)1 area of the hippocampus was assessed by laser confocal scanning microscopy. ELISA was performed to evaluate the concentration of glutamate (Glu) in the hippocampus, and the protein expression of two Glu receptors (N-methyl-D-aspartic acid receptor (NMDAR) 2A and NMDAR2B) were analysed by Western blotting.
Results
Compared with the untreated MCAO group, EA effectively ameliorated cognitive impairment (P=0.01) and shrunk the infarct volume (P=0.032). The content of intracellular Ca2+, Glu and NMDAR2B in the hippocampus was significantly raised by MCAO (P=0.031-0.043), while EA abrogated these effects. NMDAR2A was decreased by MCAO (P=0.015) but increased by EA (P=0.033).
Conclusions
EA had a beneficial effect on cognitive repair after cerebral I/R, and its mechanism of action likely involves a reduction of Ca2+influx via inhibition of Glu neurotoxicity and downregulation of NMDAR2B expression.
Keywords: electroacupuncture, stroke, neuromuscular disease
Introduction
Stroke is well known to be a major cause of both physical and mental disabilities. The past few decades have witnessed growing interest in the development of new treatment methods to deal with problems resulting from stroke, such as impaired cognition. Cognition is a complex collection of mental skills, including attention, perception, comprehension, learning, memory, problem solving and reasoning.1
Cognitive dysfunction is closely related to hippocampal injury. The Cornu Ammonis (CA)1 region of the hippocampus is closely linked to memory of the temporal order of visual objects, especially over long intervals.2 Fundamental for excitatory amino acid (EAA) neurotransmission and crucial for cognitive function, N-methyl-D-aspartic acid receptors (NMDARs) are abundant and ubiquitously distributed throughout the hippocampus.3 NMDAR hyperactivity induces excess intracellular calcium concentrations and excitotoxicity. NMDARs are comprised of N-methyl-D-aspartate receptor(NR1) subunits plus at least one type of NMDAR2 subunit. Different NMDAR2 subunits confer distinct electrophysiological and pharmacological properties on the receptors and couple themselves with different signalling pathways.4 For instance, NMDAR2A-containing and NMDAR2B-containing NMDAR subtypes have opposing influences on the direction of synaptic plasticity.5 6 NMDAR2, especially NMDAR2B, plays an important role in the induction and maintenance of long-term potentiation or long-term depression, which are likely to be the underlying mechanisms of learning and memory.7
Cognitive rehabilitation has been the subject of an increasing volume of research worldwide in recent years. However, no gold standard method has been established. Acupuncture, an ancient therapy that forms a key part of traditional Chinese medicine (TCM), has enormous potential for patients with brain injury.8 Recently, a meta-analysis suggested that acupuncture has positive effects on cognitive function after stroke.9 Moreover, TCM classics report that acupuncture may alleviate stroke, particularly when administered at GV20 and GV24.8 Accordingly, it is possible that electroacupuncture (EA) at these points could improve outcomes.
The aim of the present study was to investigate the effect of acupuncture on cognitive impairment. We hypothesised that the mechanism may involve NMDAR-induced excess intracellular Ca2+ concentration in the CA1 area of the hippocampus and that excess glutamate (Glu) and its receptors—NMDAR2A and NMDAR2B—may also play an important role in this process.
Methods
Experimental animals
A total of 60 male Sprague-Dawley rats (270±20 g) were obtained from the Shanghai SLAC Laboratory Animal (Shanghai, China) and maintained in the animal centre of Fujian University of Traditional Chinese Medicine (Fuzhou, China). All animals were housed under pathogen-free conditions with a 12-hour light/dark cycle and allowed to eat and drink freely. All rats were numbered and assigned randomly to specific groups according to a previously reported protocol.7
Middle cerebral artery occlusion (MCAO)
After an overnight fast, rats were anaesthetised using an intraperitoneal injection of 10% pentobarbital sodium (3 mL/kg). Approximately 18–22 mm of nylon surgical thread was inserted into the left internal carotid artery until the blunted distal end felt resistance to block the middle cerebral artery. After 2 hours of occlusion, the thread was pulled out to allow complete blood reperfusion of the ischaemic area. A control operation was carried out as above without the occlusion of the middle cerebral artery as reported previously.10 11 The neurological status and motor function of the rats were evaluated after establishment of the MCAO model with a TSE MotoRater system,8 and rats with similar baseline neurological status and motor function underwent further experiments.
Grouping and acupuncture intervention
Sixty animals were randomly and evenly divided into three groups: control group (20 rats undergoing control surgery), MCAO group (20 rats subjected to MCAO operation) and MCAO+electroacupuncture (EA) group (20 rats undergoing both MCAO modelling and EA interventions).
After a 2-hour recovery from the MCAO surgery, rats in the MCAO+EA group received an EA intervention for 30 min for 7 days. Needles were inserted to a depth of 2–3 mm at GV20 and GV24 and connected to an EA device (Model G6805, SMIF, Shanghai, China) with an intensity wave of 20 Hz for 30 min per acupuncture point under 10% pentobarbital sodium anaesthesia. In the control group and MCAO group, needles were routinely inserted but not connected to the EA device. All experiments were performed on the 8th day post-MCAO.
Morris water maze
All rats (n=60 in total) were subjected to testing by Morris water maze to examine cognitive function. The water maze was a circular tank with a diameter of 120 cm and a height of 50 cm. It was positioned in the middle of a well-lit testing room enriched with distal visual stimuli. The bottom of the maze was raised 0.3 m above the floor of the room. At the beginning of each day, the tank was filled with a mixture of cold and hot tap water to a depth of 30 cm and maintained at a temperature of 26°C±2°C. The water was rendered opaque by adding 2 kg of milk in order to prevent the animals from seeing a submerged circular escape platform, measuring 5 cm in diameter and 28 cm in height, which was positioned 2 cm below the surface of the water, hidden from the rat’s view. Four points, equally spaced along the circumference of the pool, were arbitrarily designated N, E, S and W. These points served as the starting positions at which the rat was lowered gently into the water, with its head facing the wall of the water maze. A video camera, connected to an image analysis system that in turn was connected to a microcomputer running the maze software, was mounted above the centre of the water maze. The swim path of the animal was tracked, digested and stored for subsequent behavioural analysis using the same software. Each trial was started and ended manually by the investigator, who operated a remote switch connected to the microcomputer.12 13
2,3,5-triphenyl tetrazolium chloride (TTC) staining
Five rats from each group were deeply anaesthetised with pentobarbital sodium (concentration 3%, 30 mg/kg per rat) via intraperitoneal injection. Their brains were harvested immediately and stored at −20°C for 15 min to induce rigidity before being cut into coronal sections of 2 mm thickness at the middle of a connecting line between the prefrontal cortex and optic chiasma. Samples were immersed in 2% TTC in the dark for 15 min (at 37°C) and fixed in 4% paraformaldehyde (PFA) solution for 24 hours, before being photographed using routine protocols.
For each rat brain, analysis of ischaemic cerebral damage included total and core infarct volumes and hemispheric infarct size (calculated as the percentage of total hemispheric volume to exclude the possible contributing effect of hemispheric oedema to infarct size). Computer-assisted image analysis was used to calculate infarct volumes. Sequential integration of the respective areas yielded total and core infarct volumes. The degree of infarction was shown as the ratio of infarction volume to the whole brain volume.14 The MCAO modelling process was deemed successful if the ratio of infarction volume was more than 1% or if a significant infarction zone was observed in a specific section of the brain.
Hippocampal Glu content
Five rats from each group were deeply anaesthetised with pentobarbital sodium (concentration 3%, 30 mg/kg per rat) via intraperitoneal injection. The hippocampus was separated quickly and stored in liquid nitrogen pending measurement of the concentration of Glu using ELISA kits (Sigma, San Francisco, California, USA).
Hippocampal protein expression of NMDAR2A and NMDAR2B
Protein samples were harvested from rats that did not undergo TTC staining (five rats from each group). A total of 50 µg protein, obtained from the CA1 region of the hippocampus, was loaded onto a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel. After the electrophoresis, proteins were electrotransferred onto polyvinylidene fluoride (PVDF) membranes. Blots were blocked with 5% non-fat milk for 2 hours and then incubated with primary antibodies against NMDAR2A, NMDAR2B and β-actin (1:1000; Cell Signaling Technology) overnight at 4°C. Subsequently, blots were incubated with horse radish peroxidase (HRP)-conjugated secondary antibody (1:5000; Cell Signaling Technology) for 50 min. β-actin was used as a loading control. All blots were developed using a commercially available enhanced chemiluminescence kit and examined using a Bio-Image Analysis system (Bio-Rad, Hercules, California, USA).
Measurement of intracellular Ca2+ in the hippocampus
The primary hippocampal neurons were harvested from rats that did not undergo TTC staining (five rats from each group). The primary hippocampal neurons were cultured in artificial cerebrospinal fluid (4°C) with 5% CO2/95% air at 37°C. The samples were cut into coronal sections of 2 mm thickness from the middle of a connecting line between the prefrontal cortex and optic chiasma. The sections were loaded with 10 μmol/L of calcium indicator fluo-3/AM for 30 min at 37°C in the dark, then they were rinsed in artificial cerebrospinal fluid three times. Changes in fluorescence corresponding to changes in intracellular calcium were monitored with a laser scanning confocal microscope equipped with a 488 nm laser line (LSM 710; Carl Zeiss Microscopy, Jena, Thuringia, Germany). The fluorescence recordings of individual cells were monitored with ZEN software (Carl Zeiss Company).15
Statistical analysis
All laboratory workers were kept blind to treatment allocation during the analysis. For statistical analysis, SPSS V.18.0 for Windows was used. Data are presented as mean±SEM. The statistical analyses were performed using a one-way analysis of variance followed by the Student-Newman-Keuls test. A P value of <0.05 was considered statistically significant.
Results
Effect of EA on the degree of infarction
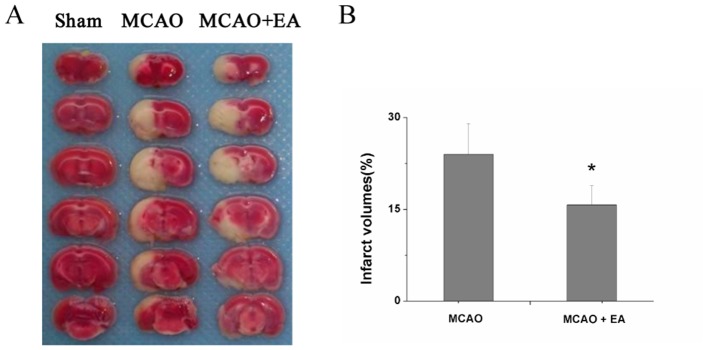
EA reduced cerebral infarct volumes after successful MCAO model establishment. As shown in figure 1, rats in the control group exhibited no cerebral infarction. MCAO was successfully performed and caused large areas of cerebral infarction. The pathological character of cerebral infarction was demonstrably alleviated by EA, reflected by decreased infarct volume (P=0.032).
Figure 1.
Effect of EA on cerebral infarction in cerebral ischaemia–reperfusion (I/R) injured rats. (A) Pathological character of cerebral ischaemia in each group, verified by TTC staining. (B) Quantitative indices of infarct volume in untreated MCAO versus EA-treated MCAO+EA groups. *P<0.05. EA, electroacupuncture; MCAO, middle cerebral artery occlusion; TTC, 2,3,5-triphenyl tetrazolium chloride.
Effect of EA on the Morris water maze
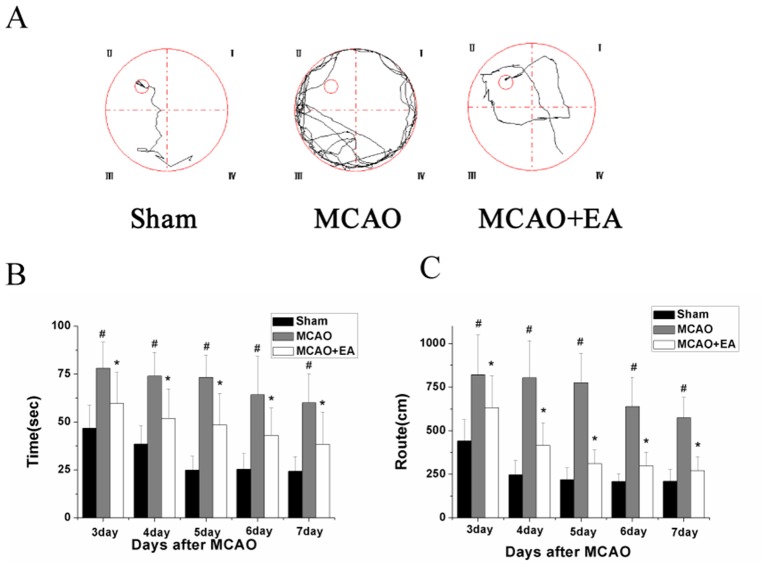
MCAO+EA significantly improved the route length (distance swum before finding the platform) compared with the untreated MCAO group (figure 2A). Compared with the sham-operated control group, the length of the route and the time taken by rats in the MCAO group to reach the hidden platform were markedly increased. Moreover, EA significantly decreased the latency and route length (P=0.014 and P=0.024; figure 2B, C). Additionally, the number of times that rats crossed the location of the platform was significantly decreased in MCAO versus sham groups (p=0.01), and increased in MCAO+EA versus MCAO groups (data not shown).
Figure 2.
Effect of EA on cognitive impairment in cerebral ischaemia–reperfusion (I/R) injured rats. The learning and memory ability of rats was determined using a Morris water maze test. (A) Tracing images from the Morris water maze test; (B) Time taken by rats to find the hidden platform in each group; (C) Distance travelled (route length) by rats before reaching the platform. #P<0.05 versus control group; *P<0.05 versus MCAO group. EA, electroacupuncture; MCAO, middle cerebral artery occlusion.
Effect of EA on Glu, NMDAR2A and NMDAR2B content in the CA1 area of the hippocampus
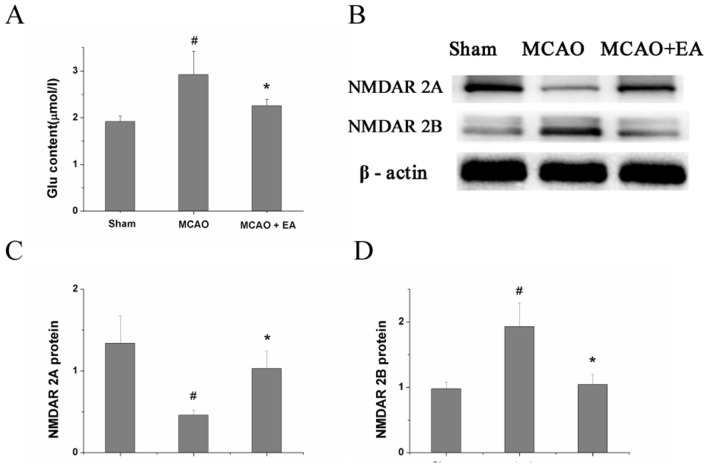
As shown in figure 3, after MCAO surgery, the content of Glu and protein expression of NMDAR2B in the MCAO group was significantly increased compared with the sham-operated control group (P=0.031), and the protein expression of NMDAR2A was decreased. EA administration effectively reversed these effects, compared with the untreated MCAO group (P=0.043). EA administration increased the protein expression of NMDAR2A. Furthermore, the upregulation of Glu content and NMDAR2B protein expression in MCAO rats was shown to be attenuated by EA administration.
Figure 3.
Effect of EA on Glu content and expression of NMDAR2A and NMDAR2B protein. (A) Glu content in the CA1 area of the hippocampus in each group, measured by ELISA. (B) Protein expression of NMDAR2A and NMDAR2B in the CA1 area of the hippocampus in each group, examined by Western blotting. (C,D) Quantitative indices of protein expression of NR2A and NR2B, derived from figure 2B. #P<0.05 versus control group; *P<0.05 versus MCAO group. EA, electroacupuncture; Glu, glutamate; MCAO, middle cerebral artery occlusion; NMDAR2A, N-methyl-D-asparticacid receptors 2A; NMDAR2B, N-methyl-D-asparticacid receptors 2B.
Effect of EA on intracellular Ca2+content in the CA1 area of the hippocampus
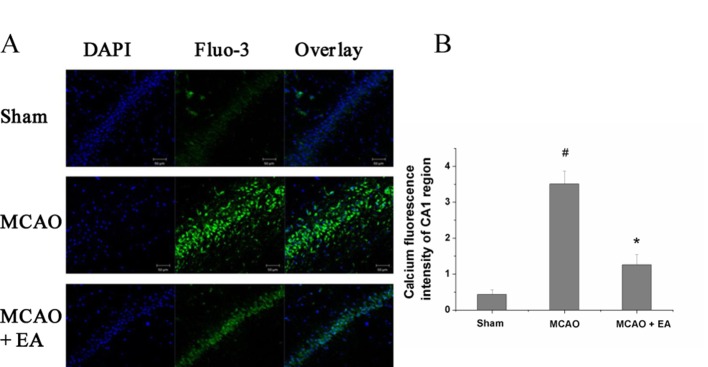
EA downregulated the intracellular Ca2+content, compared with the MCAO group. In figure 4, Ca2+, indicated by green fluorescence intensity, was stronger after MCAO without treatment (P=0.015). Compared with the MCAO group, the value of green fluorescence intensity in the MCAO+EA group showed a significant decrease (P=0.033).
Figure 4.
Effect of EA on intracellular Ca2+concentration. (A) Ca2+ concentration was evaluated by green fluorescence using a Fluo-3 probe and laser scanning confocal microscope. (B) Intracellular Ca2+ was calculated as the ratio of green Fluo-3-stained cells to the total number of blue DAPI-stained cells. #P<0.05 versus control group; *P<0.05 versus MCAO group. EA, electroacupuncture; MCAO, middle cerebral artery occlusion.
Discussion
In our study, it was shown that MCAO successfully induced cerebral infarction via upregulation of NMDAR2B and downregulation of NMDAR2A. EA improved several indices, and was associated with enhanced spatial learning and memory ability, reduced cerebral infarct volume, downregulation of related proteins (Glu, NMDAR2B) and decreased intracellular Ca2+.
EA is gaining increasing credibility as a mainstream medical therapy.15–17 Survivors of stroke frequently present with cognitive impairment that severely affects their daily life. There is growing evidence that EA is an effective treatment for stroke and its potentially beneficial effects include a narrowing of infarct volume, reduction of neuronal death and prevention of brain oedema.18 It is also emphasised that EA should be integrated into early cognitive rehabilitation.19 In our study, MCAO successfully modelled cerebral infarction and caused large volume infarcts, which were positively impacted by EA. Furthermore, we used the Morris water maze, in which an animal is required to find a submerged platform, to measure spatial reference memory. With respect to this behavioural test, MCAO impaired the spatial learning and memory ability of rats, while EA significantly alleviated it. A previous study has shown that manual acupuncture improves reference memory after cerebral infarction,20 and our study demonstrates that EA is also an effective way to induce neural functional recovery.
Ischaemia causes excessive EAA release in the hippocampus. Glu, as a member of the EAA family, also significantly increases after ischaemia injury.21 Excessive Glu persistently stimulates the postsynaptic NMDARs leading to excessive Ca2+ influx and finally impairs cognitive function.22 Theoretically, reducing Glu release and inhibiting the activity of NMDARs can impede Ca2+ influx and prevent cognitive impairment, which was preliminarily confirmed in a previous study.23 We found that MCAO significantly increased Glu content, concentration of intracellular Ca2+ and protein expression of NMDAR2B, but induced a decrease in NMDAR2A; each of these mediators plays a different role in ischaemia.23 Moreover, EA abrogated the harmful effects of MCAO. NMDAR2A activation can promote neuronal regeneration and protect neuronal function. NMDAR2B mediates free radical chain reactions that cause neurotoxicity and induce neuronal apoptosis.24 We found opposite changes in NMDAR2A and NMDAR2B after MCAO. EA seemed to alleviate ischaemic injury via multiple pathways, including downregulation of NMDAR2B and upregulation of NMDAR2A. However, the role of apoptosis in cognitive repair is attracting more and more attention worldwide, and Glu release, which results in further Ca2+-mediated neurotoxicity, is proven to be a stimulator of neuronal apoptosis. It has been reported that acupuncture improves cognitive function via regulation of Glu and intracellular Ca2+.25 Although the detailed cognitive mechanisms still need further study, our experiment indicates that the hippocampus has a close relationship with cognitive function, and EA protects the hippocampus after MCAO via regulation of Glu and intracellular Ca2+. As for the roles of NMDAR2B and NMDAR2A in ischaemic stroke, our study provides a novel insight into their differential effects. The NMDAR family is composed of an essential NMDAR1 subunit, which has complicated roles in ischaemic stroke. Moreover, the ionotropic properties of the NMDAR in stroke are fully maintained when it is coupled to at least one NMDAR2A–D. The forebrain, which is the most vulnerable to ischaemic injury, is populated by NMDAR2A and NMDAR2B subunits, and animal studies have shown that expression of NMDAR2A and NMDAR2B are associated with the occurrence of stroke. Thus, it is important to explore therapies targeting NMDAR2A and NMDAR2B for the treatment of stroke. A previous study reported that MCAO-induced ischaemic stroke upregulated the level of NMDAR2A/p-NMDAR2B (phosphorylated NMDAR2B) and that transient cerebral ischaemia resulted in incremental tyrosine phosphorylation of NMDAR2A and NMDAR2B subunits in the rat brain, suggesting that the tyrosine phosphorylation of NMDAR2 subunits plays a key role in ischaemia-induced neuronal damage.26 However, it is still controversial whether baseline expression of non-phosphorylated NMDAR2A and NMDAR2B are involved in stroke. Our study showed that decreased NMDAR2A and increased NMDAR2B were both associated with ischaemic stroke, suggesting that non-phosphorylated NMDAR2A and NMDAR2B related mechanisms are also involved in the protective effects of EA.
As reported previously, it is worth considering additional mechanisms of action that may be involved in the positive effects of EA. Liu et al 27 found that EA ameliorated cognitive impairment in rats with I/R injury and mechanisms related to autophagy were involved in this process, verified by EA-induced modulation of Bcl-2 and Bax expression. In addition, it would be worth further investigating the clinical utility of EA for acute stroke management and as an adjunctive therapy to rehabilitation. For example, a pilot study has indicated the safety of early acupuncture therapy in the acute stage of ischaemic stroke.18 Another clinical trial showed that acupuncture in combination with rehabilitation may have benefits for the treatment of acute and subacute stroke compared with rehabilitation alone.28
In conclusion, EA alleviated cerebral ischaemia/reperfusion injury and had a significant effect on cognitive repair after onset of stroke in a rat model. The mechanism of action appears to involve enhanced NMDAR2A expression, downregulation of NMDAR2B expression and decreased Ca2+-mediated neurotoxicity via inhibition of Glu release.
Footnotes
YZ and XM contributed equally.
Contributors: YZ designed the experiment. TZ, RL, ZL and XM performed the experiment and analysed the data. YZ, RL and XM wrote the manuscript and all authors read and approved the final version accepted for publication.
Funding: 2017 Scientific Funding Scheme for Young and Middle-aged Elites Training Project supported by Health Department of Fujian Province (2017-ZQN-72)
Competing interests: None declared.
Ethics approval: The experimental protocol and animal care procedures were approved by the Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine (reference no. FYZB-B1-2016026).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Moore C. Reflections on clinical applications of yoga in voice therapy with MTD. Logoped Phoniatr Vocol 2012;37:144–50. 10.3109/14015439.2012.731080 [DOI] [PubMed] [Google Scholar]
- 2. Yang S, Yang S, Moreira T, et al. Interlamellar CA1 network in the hippocampus. Proc Natl Acad Sci U S A 2014;111:12919–24. 10.1073/pnas.1405468111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenbrock H, Kramer G, Hobson S, et al. Functional interaction of metabotropic glutamate receptor 5 and NMDA-receptor by a metabotropic glutamate receptor 5 positive allosteric modulator. Eur J Pharmacol 2010;639:40–6. 10.1016/j.ejphar.2010.02.057 [DOI] [PubMed] [Google Scholar]
- 4. Russo R, Cavaliere F, Varano GP, et al. Impairment of neuronal glutamate uptake and modulation of the glutamate transporter GLT-1 induced by retinal ischemia. PLoS One 2013;8:e69250 10.1371/journal.pone.0069250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu L, Wong TP, Pozza MF, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 2004;304:1021–4. 10.1126/science.1096615 [DOI] [PubMed] [Google Scholar]
- 6. Bartlett TE, Bannister NJ, Collett VJ, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology 2007;52:60–70. 10.1016/j.neuropharm.2006.07.013 [DOI] [PubMed] [Google Scholar]
- 7. Mohamad O, Song M, Wei L, et al. Regulatory roles of the NMDA receptor GluN3A subunit in locomotion, pain perception and cognitive functions in adult mice. J Physiol 2013;591:149–68. 10.1113/jphysiol.2012.239251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao H, Wang Y, Chang D, et al. Acupuncture for vascular mild cognitive impairment: a systematic review of randomised controlled trials. Acupunct Med 2013;31:368–74. 10.1136/acupmed-2013-010363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu F, Li ZM, Jiang YJ, et al. A meta-analysis of acupuncture use in the treatment of cognitive impairment after stroke. J Altern Complement Med 2014;20:535–44. 10.1089/acm.2013.0364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xue X, You Y, Tao J, et al. Electro-acupuncture at points of Zusanli and Quchi exerts anti-apoptotic effect through the modulation of PI3K/Akt signaling pathway. Neurosci Lett 2014;558:14–19. 10.1016/j.neulet.2013.10.029 [DOI] [PubMed] [Google Scholar]
- 11. Feng X, Yang S, Liu J, et al. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep 2013;7:1516–22. 10.3892/mmr.2013.1392 [DOI] [PubMed] [Google Scholar]
- 12. Veng LM, Granholm AC, Rose GM. Age-related sex differences in spatial learning and basal forebrain cholinergic neurons in F344 rats. Physiol Behav 2003;80:27–36. 10.1016/S0031-9384(03)00219-1 [DOI] [PubMed] [Google Scholar]
- 13. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984;11:47–60. 10.1016/0165-0270(84)90007-4 [DOI] [PubMed] [Google Scholar]
- 14. Zhou H, Zhang Z, Wei H, et al. Activation of STAT3 is involved in neuroprotection by electroacupuncture pretreatment via cannabinoid CB1 receptors in rats. Brain Res 2013;1529:154–64. 10.1016/j.brainres.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 15. Rui C, Yuxiang L, Yinju H, et al. Protective effects of Lycium barbarum polysaccharide on neonatal rat primary cultured hippocampal neurons injured by oxygen-glucose deprivation and reperfusion. J Mol Histol 2012;43:535–42. 10.1007/s10735-012-9420-4 [DOI] [PubMed] [Google Scholar]
- 16. Liu W, Mukherjee M, Sun C, et al. Electroacupuncture may help motor recovery in chronic stroke survivors: a pilot study. J Rehabil Res Dev 2008;45:587–96. 10.1682/JRRD.2007.11.0181 [DOI] [PubMed] [Google Scholar]
- 17. Mukherjee M, McPeak LK, Redford JB, et al. The effect of electro-acupuncture on spasticity of the wrist joint in chronic stroke survivors. Arch Phys Med Rehabil 2007;88:159–66. 10.1016/j.apmr.2006.10.034 [DOI] [PubMed] [Google Scholar]
- 18. Liu CH, Hsieh YT, Tseng HP, et al. Acupuncture for a first episode of acute ischaemic stroke: an observer-blinded randomised controlled pilot study. Acupunct Med 2016;34:349–55. 10.1136/acupmed-2015-010825 [DOI] [PubMed] [Google Scholar]
- 19. Chou P, Chu H, Lin JG. Effects of electroacupuncture treatment on impaired cognition and quality of life in Taiwanese stroke patients. J Altern Complement Med 2009;15:1067–73. [PubMed] [Google Scholar]
- 20. Liu CZ, Yu JC, Zhang XZ, et al. Acupuncture prevents cognitive deficits and oxidative stress in cerebral multi-infarction rats. Neurosci Lett 2006;393:45–50. 10.1016/j.neulet.2005.09.049 [DOI] [PubMed] [Google Scholar]
- 21. Héja L, Barabás P, Nyitrai G, et al. Glutamate uptake triggers transporter-mediated GABA release from astrocytes. PLoS One 2009;4:e7153 10.1371/journal.pone.0007153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sasaki A, Matsubara A, Tabuchi K, et al. Immunoelectron microscopic analysis of neurotoxic effect of glutamate in the vestibular end organs during ischemia. Acta Otolaryngol 2012;132:686–92. 10.3109/00016489.2012.656322 [DOI] [PubMed] [Google Scholar]
- 23. Johnston MV. Excitotoxicity in perinatal brain injury. Brain Pathol 2005;15:234–40. 10.1111/j.1750-3639.2005.tb00526.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li XB, Yang ZX, Yang L, et al. Neuroprotective effects of flax lignan against NMDA-induced neurotoxicity in vitro. CNS Neurosci Ther 2012;18:927–33. 10.1111/cns.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leung MC, Yip KK, Ho YS, et al. Mechanisms underlying the effect of acupuncture on cognitive improvement: a systematic review of animal studies. J Neuroimmune Pharmacol 2014;9:492–507. 10.1007/s11481-014-9550-4 [DOI] [PubMed] [Google Scholar]
- 26. Yanli L, Xizhou Z, Yan W, et al. Clonidine preconditioning alleviated focal cerebral ischemic insult in rats via up-regulating p-NMDAR1 and down-regulating NMDAR2A / p-NMDAR2B. Eur J Pharmacol 2016;793:89–94. 10.1016/j.ejphar.2016.10.036 [DOI] [PubMed] [Google Scholar]
- 27. Liu F, Jiang YJ, Zhao HJ, et al. Electroacupuncture ameliorates cognitive impairment and regulates the expression of apoptosis-related genes Bcl-2 and Bax in rats with cerebral ischaemia-reperfusion injury. Acupunct Med 2015;33:478–84. 10.1136/acupmed-2014-010728 [DOI] [PubMed] [Google Scholar]
- 28. Vados L, Ferreira A, Zhao S, et al. Effectiveness of acupuncture combined with rehabilitation for treatment of acute or subacute stroke: a systematic review. Acupunct Med 2015;33:180–7. 10.1136/acupmed-2014-010705 [DOI] [PubMed] [Google Scholar]