Abstract
Objective
To examine for an opening effect on the blood–brain barrier (BBB) in intact rats and rats with experimental ischaemia-reperfusion (I/R) during the recovery period after various electroacupuncture (EA) treatments with different time courses, and to determine whether there is a time-dependent effect. An additional objective was to determine whether this method could induce the penetration of nerve growth factor (NGF) through the BBB.
Methods
A middle cerebral artery occlusion (MCAO) model was first established. We chose different stimulation time courses and observed the effects of EA treatment (100 Hz frequency; 2 mA intensity) at GV20 and GV26 on the BBB in rats recovering from MCAO 3 weeks after modelling. The rats were injected with 2% Evans blue (EB) saline. The brain water content was measured using a wet/dry weighing method. The degree of penetration of EB was detected using spectrophotometry and laser confocal microscopy. The rats were then injected with NGF, and the concentration of NGF in the brain tissues was measured using ELISA.
Results
The increase in the BBB permeability was most notable following the 8 min EA stimulation (P<0.05), which may be advantageous for the targeted delivery of drugs (such as NGF) into the brain. Additionally, this effect did not appear to cause brain oedema (P>0.05) in healthy or MCAO rats.
Conclusions
EA treatment for a certain stimulation time at GV20 and GV26 in MCAO rats can increase BBB permeability.
Keywords: electroacupuncture, stroke, neurology, electrical stimulation therapy
Introduction
Ischaemic stroke accounts for >80% of all cases of stroke and has a high morbidity and mortality worldwide, with ischaemic and haemorrhagic strokes each having caused >3 million deaths in 2013.1 Enormous progress has been achieved by scientists throughout the world in the treatment of brain related disorders; however, drug delivery to the brain remains a challenge. In fact, 98% of all therapeutic molecules cannot reach the brain parenchyma in pharmacologically relevant concentrations.2 The neurovascular unit, which acts as a blood–brain barrier (BBB), is one of the major defence mechanisms of the brain and regulates the passage of large molecules to and from the brain to maintain its delicate ionic and metabolic environment. Unless recognised by a specific transport system, hydrophilic and lipophilic molecules larger than ~500 Da show extremely limited penetration across the BBB.3
Many methods have been used to disrupt the BBB, including modifying agents to allow them to penetrate the BBB or using drug carriers, such as liposomes and nanoparticles.4 However, only a finite concentration of drugs can be delivered due to the limited number of receptors and discrete quantity of molecules that can be attached to a carrier. Therefore, exploring applicable methods to effectively, non-invasively, and selectively open the BBB to facilitate drug delivery to the brain is of great clinical interest and is an important pursuit.5 6 Electroacupuncture (EA) is widely accepted as a common complementary therapy for stroke.7 Our previous study demonstrated that EA at GV20 and GV15 can increase the permeability of the BBB to nerve growth factor (NGF), increase its level in the cerebral tissue of rats with cerebral infarction (CI), and favour the regeneration and repair of nerves.8 In another study, we also found that acupuncture point injection of camphol and EA can effectively strengthen the permeability of the BBB to Evans blue (EB). Notably, EA can induce complex effects on the central nervous system (CNS) that are influenced by multiple factors, such as the acupuncture points stimulated, approach and parameters.
Therefore, the present study was conducted to evaluate the effects of different time courses of EA stimulation in a rat model of middle cerebral artery occlusion (MCAO) on the permeability of the BBB and the penetration of exogenous NGF during the recovery period. To achieve these aims, EB (which otherwise does not cross the BBB) was permeated into the brain parenchyma and used as a tracer to depict the degree of BBB opening. Simultaneously, the brain water content was measured to determine whether EA-induced BBB opening caused adverse reactions, such as cerebral oedema. Therefore, these experiments were conducted in two parts. First, we investigated the influence of EA delivered over different time courses on the degree of penetration of EB in normal and MCAO rats during the recovery period. Second, based on the first part, we assessed the efficiency and therapeutic potential of the EA-induced BBB opening technique in vivo by determining whether the concentration of NGF in the brain was increased by EA treatment compared with NGF administration alone.
Methods
Experimental animals
All procedures in this study were performed in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Zhejiang Chinese Medical University and conformed to internationally accepted ethical standards. All efforts were made to alleviate animal suffering, minimise the number of animals used, and utilise alternatives to in vivo techniques when possible. The experiments were performed on adult male Sprague Dawley (SD) rats weighing 250–280 g. The animals were housed at 25±2°C and 50±10% humidity with a 12-hour light/dark cycle. Food and water were accessible ad libitum and supplied by the Laboratory Animal Centre of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China.
EA treatment
Part 1
The influence of different time courses of EA on the degree of penetration of EB in rats was evaluated using the following two experiments.
Experiment 1
The influence of different time courses of EA on the degree of penetration of EB in intact SD rats was investigated. Male Sprague Dawley rats were randomly divided into one of the following seven groups (n=16 rats each): normal control; EA-1min; EA-5min; EA-15min; EA-30min; EA-45min; and EA-60min groups. Each group was further randomly divided into two subgroups (n=8 each) to determine the brain water content and degree of EB penetration, respectively. Rats in the EA groups underwent insertion of acupuncture needles (length 25 mm, diameter 0.30 mm; Hwato, Suzhou Medical Supplies Factory Co, Ltd, China), at GV20 (Baihui) and GV26 (Shuigou). The needles were then stimulated using an acupuncture point nerve stimulator (HANS-200, Nanjing Jinsheng, Ltd, China) at an intensity of 2 mA and a frequency of 100 Hz. The rats were injected with 2% EB in saline via the caudal vein using an indwelling needle (4 ml/kg). The normal control group that did not undergo EA treatment received only an injection of 2% EB in saline via the caudal vein. The brain water content was measured using a wet/dry weight method. The degree of penetration of EB was detected using a spectrophotometer, and laser confocal microscopy was used to detect the EB penetration in the control, EA-5min and EA-15min groups.
Experiment 2
Further optimisation of the EA stimulation time course, based on the results of experiment 1, was performed to investigate the degree of penetration of EB in MCAO rats during their recovery period (3 weeks after the operation when the integrity of BBB was ensured). The MCAO rats were randomly divided into model, EA-4min, EA-6min, EA-8min, EA-12min, EA15 min, EA-30min and EA-45min groups. Another sham surgery group was established, for a total of nine groups (n=16 rats each). The same detection methods used in experiment 1 were employed.
Part 2
The influence of EA on the degree of penetration of NGF in MCAO rats was investigated. Based on the results of part 1, a duration of 8 min was chosen as the optimal EA stimulation time. The rats were randomly divided into MCAO model, EA and sham operation groups (n=8 rats each). Rats in the EA group underwent insertion of acupuncture needles (0.30×25 mm in size) at GV20 and GV26. The needles were then connected to a HANS-200 acupuncture point nerve stimulator and stimulated with a current of 2 mA and a frequency of 100 Hz for 8 min. The rats were injected with NGF via the caudal vein (10 µg/kg). Rats in the sham operation and model groups were injected with NGF alone. The content of NGF in the brain tissues was measured by enzyme-linked immunosorbent assay (ELISA).
MCAO model establishment
Rats in the MCAO groups were subjected to MCAO using a previously described method with some modifications. Briefly, the rats were fasted for 12 hours but were allowed free access to water before the surgery. The animals were anaesthetised with 10% chloral hydrate, then the right common carotid artery (CCA), internal carotid artery (ICA) and external carotid artery (ECA) were surgically exposed and isolated, and the ECA was ligated. The middle cerebral artery (MCA) was occluded by inserting a monofilament nylon suture with a heat-rounded tip into the ICA through the CCA stump, and advancing it further until it closed the origin of the MCA. Body temperature was monitored and maintained at 36.5–37.5°C throughout the surgery. Sham surgery control rats underwent the same surgical procedure without the insertion of a filament. After 60 min of occlusion, the suture was carefully withdrawn up to the right carotid bifurcation to restore blood flow. After recovery from anaesthesia, the rats were returned to their cages with free access to food and water.
Regional cerebral blood flow measurement
Laser Doppler flowmetry (LDF, PeriFlux 5000, Perimed, Sweden) and dynamic laser speckle were used to monitor cerebral blood flow (CBF) in the region of the MCA during pre-ischaemia and after the onset of MCAO to verify the success of the cerebral ischaemia procedure. The LDF probe was focused on the skull cranial window of the left and right temporal bone (5 mm lateral and 1 mm posterior to bregma). The Doppler flux was continuously measured until stable data were obtained from both the ischaemic and control sides. A blood flow reduction >45±10% of the baseline value was considered a successful occlusion.
Neurological deficit evaluation
Neurological deficit scores were obtained by an examiner who was blinded to the group assignments 1.5 hours after MCAO using a modified scoring system based on the system developed by Longa et al.9 The scores were as follows: 0 = no apparent neurological deficits; 1 = mild neurological deficit with difficulty fully extending the contralateral forelimb; 2 = moderate neurological deficit with mild circling to the contralateral side; 3 = severe neurological deficit with slumping towards the contralateral (paralysed) side when walking; and 4 = inability to walk autonomously without loss of consciousness. The higher the neurological deficit score, the more severe the impairment of the motor motion injury. Neurological deficit scores of 1 to 3 were indicative of a successful MCAO model. Rats that died from subarachnoid haemorrhage or pulmonary insufficiency and asphyxia were eliminated from the study (mortality rate 21.8%).
TTC staining
The infarct volume was determined by TTC staining (2,3,5-triphenyltetrazolium chloride, T8170, Solarbio, Beijing) 24 hours after MCAO. Additionally, six rats in the model group were euthanased with chloral hydrate, and their brains were rapidly collected. Their brains were then sliced into five coronal sections of 2 mm thickness and stained with a 2% solution of TTC at 37°C for 20 min, followed by fixation with 4% paraformaldehyde.
Brain water content measurement
The brain water content was measured using a standard wet/dry weighing method. Six rats from each group were deeply anaesthetised with 10% chloral hydrate and euthanased by decapitation after EA. Their brains were rapidly removed, placed on a cooled surface and divided into the ipsilateral and contralateral hemispheres. The two hemispheres were packaged with pre-weighed tin foils and immediately weighed using an electronic balance to obtain the wet weight; then the tissue was re-weighed to obtain the dry weight after drying for 72 hours in an oven at 80°C. The brain water content was calculated using the following equation: brain water content (%) = (wet weight−dry weight)/(wet weight)×100.
EB assay
The integrity of the BBB was investigated using EB extravasation as previously described with some modifications. Briefly, the EB solution (EB, Biosharp, FW:960.80, 2% in saline, 4 mL/kg) was injected into the tail vein during the final minute of EA treatment, and the dye was allowed to circulate for 30 min. The animals were then perfused with saline through the left ventricle until colourless fluid was obtained from the right atrium. After decapitation, the brains were removed, and the right cerebral cortex was weighed and homogenised in 50% trichloroacetic acid to precipitate protein by sonication. The samples were then cooled for 30 min and centrifuged at 10 000 g for 20 min at 4°C. The supernatants were obtained and diluted threefold with ethanol. The concentration of the tracer in the supernatant was measured at 620 nm and 680 nm for excitation and emission, respectively, using an ultraviolet spectrophotometer (SpectraMax M5, Molecular Devices Co, USA). The extravasated EB was expressed as μg/g of brain tissue against a standard curve (80 µg/mL of EB dissolved in saline with eight serial dilutions).
Quantification of EB under laser confocal microscopy
Three frozen slices from the brain hemispheres were selected, with a slice thickness of 30 μm and 3 mm between each slice. Then the slices were counterstained with DAPI (4', 6-diamidino-2-phenylindole; no. E607303, Sangon Biotech) for 15 min at 37°C before fluorescence microscopy. The red light was set and photographs were taken with laser confocal microscopy (LCM, Nikon Eclipse Ti). The red fluorescence integrated optical density (FIOD) values of EB in the hemispheres of the same area were measured using Image-Pro Plus software 6.0 and statistically analysed (FIOD/area*100). The scale of the FIOD was taken to reflect the deposition of EB in the brain tissue.10
NGF ELISA
The concentrations of NGF were measured using ab100757-betaNGF rat ELISA kits according to the manufacturer’s instructions. Briefly, 3 weeks after surgery, rats in the normal and model groups were injected with only the NGF solution (XF8415011, R&D Systems, 10 µg/kg) via the tail vein. Rats in the EA group were injected with the NGF solution, which was allowed to circulate for 30 min, 1 minute before completion of EA treatment (3 weeks after MCAO modelling). The animals were then perfused with saline, their brains were removed, and the right cerebral cortex was weighed. The samples were homogenised and then centrifuged at 18 000 g for 30 min at 0°C. The supernatants were obtained, and NGF standards (100 µl/well) and samples (100 µl/well) were added to 96-well ELISA plates and incubated overnight at 4°C. After washing, the plates were incubated with biotinylated β-NGF detection antibody for 60 min at room temperature. The plates were washed again and incubated with a horseradish peroxidase (HRP)-streptavidin solution for 45 min at room temperature. Then the cells were washed again and incubated in the dark with TMB (3,3′,5,5′-tetramethylbenzidine) one-step substrate reagent. Stop solution was added to the plates, and the resulting plates were read at 450 nm using a spectrophotometer. The sample values were calculated based on a standard curve in the linear range.
Statistical analysis
All data are presented as mean±SEM and were analysed using SPSS version 13.0 (Chicago, USA). One-way analysis of variance (ANOVA) was performed, followed by tests of least significant difference (LSD) for intergroup comparisons. Differences with values of P<0.05 were considered statistically significant.
Results
Establishment of the MCAO model
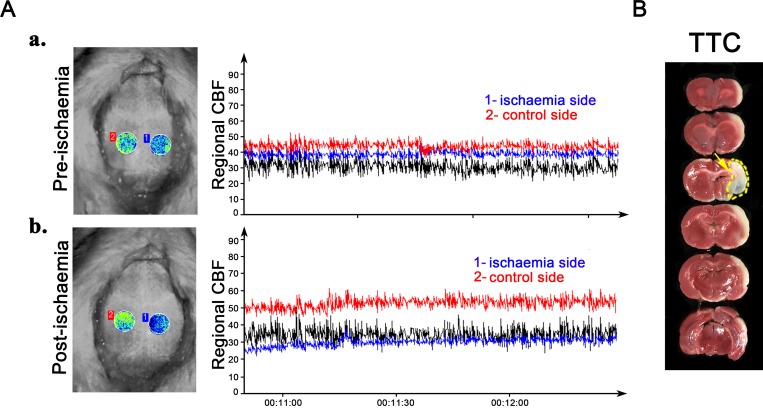
The brain infarction volume and CBF measurements are shown in figure 1. Figure 1A shows the brain imaging and CBF as measured by the laser Doppler speckle technique (parts a and b). The Doppler flux was continuously measured, and the regional CBF in the skull cranial window decreased from the baseline value by more than 45±10%, indicating successful occlusion. The blue line depicts the regional CBF of the ischaemic side (1), and the red line illustrates the control side (2). Figure 1B shows the TTC staining. In the MCAO groups, an extensive lesion developed in the lateral cortex. The normal tissue was stained deep red, while the infarct area appeared as pale grey (indicated by the yellow arrow).
Figure 1.
Establishment of the middle cerebral artery occlusion model. Brain imaging was captured using a dynamic laser speckle technique to monitor the regional cerebral blood flow (CBF) in the skull cranial window pre-ischaemia (Aa) and post-ischaemia (Ab). TTC staining shows development of an extensive lesion in the lateral cortex (indicated by yellow arrow in (B)). TTC, 2,3,5-triphenyltetrazolium chloride.
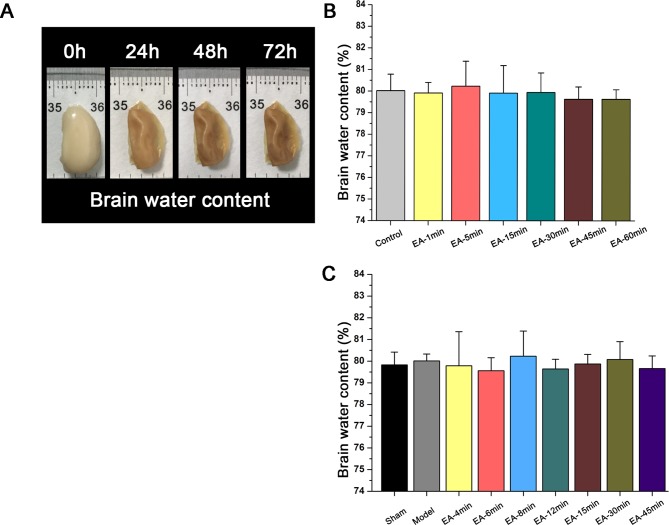
EA treatment at GV20 and GV26 did not cause brain oedema
The effects of EA on ipsilateral brain water content, an indicator of brain oedema, were considered (figure 2). Brain oedema is a major ischaemic injury that is associated with increased BBB permeability. Figure 2A shows the morphological changes that were observed in the ipsilateral hemisphere after drying in an oven for 72 hours. Figure 2B shows the brain water content for different durations of EA treatment in control Sprague Dawley rats. There were no significant differences (P>0.05) between the EA-treated healthy SD groups (1min, 5min, 15min, 30min, 45min and 60min) and the normal control group. Similarly, as shown in figure 2C there were no significant differences (P>0.05) between the EA-treated MCAO groups (4min, 6min, 8min, 12min, 15min, 30min and 45min), untreated MCAO control group and sham group, confirming that the EA treatment did not induce significant brain oedema.
Figure 2.
Effects of electroacupuncture (EA) treatment at GV20 and GV26 on brain water content in: (B)healthy Sprague Dawley rats that received electroacupuncture (EA) or remained untreated (control group); and (C) rats undergoing sham surgery or middle cerebral artery occlusion (MCAO) that received EA or no treatment (model group). Data are expressed as mean±SEM (n=8 rats per group).
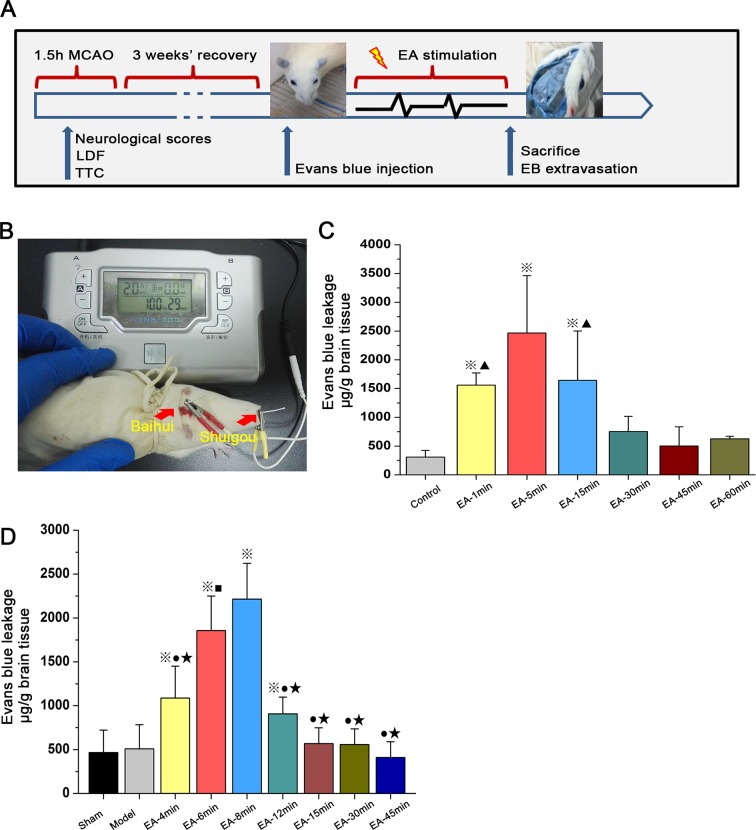
Opening of the BBB induced by EA
The effects of EA over time on BBB permeability is shown in figure 3. Figure 3A and figure 3B show the experimental protocol and procedures, respectively. Following EA stimulation (100 Hz, 2 mA) over different time courses (compared to the untreated control group), figure 3C shows the EB concentration in the right hemisphere of healthy Sprague Dawley rats. The degree of penetration of EB in the EA-1min group, EA-5min group and EA-15min group was apparently higher than those in the untreated healthy control group (P<0.01). The degree of penetration of EB in the EA-5min group was higher than those in the other groups (P<0.01). There were no statistically significant differences between the EA-1min group and EA-15min group (P>0.05). Figure 3D shows the EB concentration in the right hemisphere of the MCAO rats (3 weeks after MCAO surgery). The EA-4min group, EA-6min group, EA-8min group and EA-12min group had apparently higher EB concentrations than the untreated model and sham-operated groups (P<0.01). The degree of penetration of EB in the EA-8min and EA-6min groups was higher than those in the other groups (P<0.01). The EA-6min group had lower penetration than the EA-8min group (P<0.05).
Figure 3.
Transient breakdown of the blood–brain barrier by electroacupunture (EA) treatment as assessed by Evans blue (EB) leakage in: (C) healthy Sprague Dawley rats that received electroacupuncture (EA) or remained untreated (control group); and (D) rats undergoing sham surgery or middle cerebral artery occlusion (MCAO) that received EA or no treatment (model group). Data are expressed as mean±SEM (n=8 per group). ※P<0.01 versus Control group, ▲P<0.01 versus EA-5 min group (3C); ※P<0.01 versus Model group, ★P<0.01 versus EA-6 min group, ■P<0. 01 vs EA-8 min group (3D). LDF, laser Doppler flowmetry; TTC, 2,3,5-triphenyltetrazolium chloride.
Quantitative analysis of EB FIOD in brain parenchyma
FIOD was measured at the frozen sections of coronal brain tissue under LCM (figure 4). Figure 4A shows substantial red fluorescence found in the brain parenchyma of the EA-5min group and the EA-15min group (indicated by white arrows). Figure 4B shows the FIOD of EB was significantly higher in the EA-5min and EA-15min groups than that of the control group (three slices of each specimen; P<0.01); the difference between the EA-5min group and EA-15min group was also significant (P<0.01).
Figure 4.
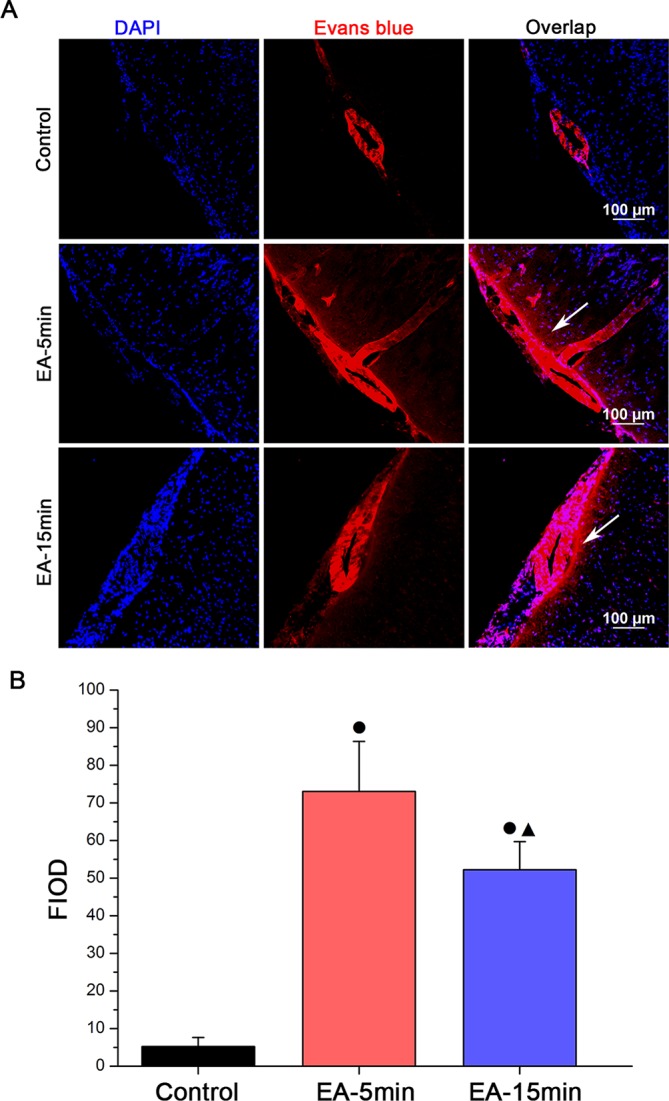
Representative images (A; scale bar 100 µm) and quantification (B) by group of Evans blue fluorescence integrated optical density (FIOD) levels (detected by immunofluorescence staining) in cerebral cortex sections from a subset of 15 healthy Sprague-Dawley rats that remained untreated (control group, n=5) or received electroacupuncture (EA) treatment for 5 or 15 minutes (EA-5min group and EA-15min group, respectively, n=5 each). Data are expressed as mean±SEM. ●P<0.01 versus control group, ▲P<0.01 versus EA-5 min group (4B). DAPI, 4', 6-diamidino-2-phenylindole.
Effects of EA treatment on the penetration of NGF
To examine the opening effects of EA on selected parameters of the BBB in the MCAO rats, the concentrations of NGF in the right hemisphere brain tissue were measured using ELISA kits. Figure 5 shows that the NGF level was significantly elevated in the EA group compared with the sham operation group and the untreated MCAO (model) group (P<0.05).
Figure 5.
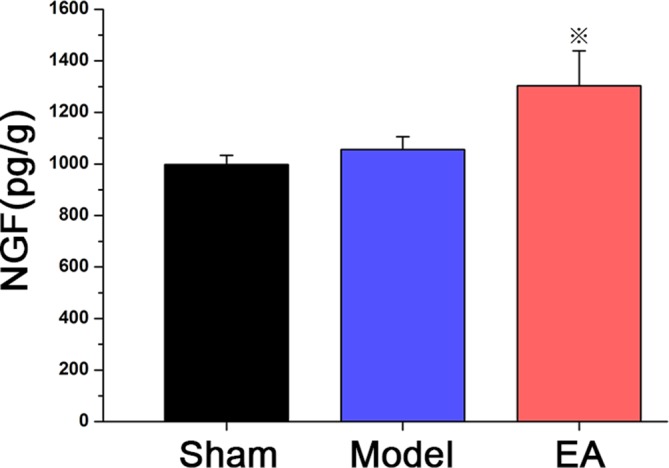
Concentration of nerve growth factor (NGF) in the brain of Sprague-Dawley rats undergoing a middle cerebral artery occlusion operation that remained untreated (model group, n=8) or received electroacupuncture (EA) for 8 min at 100 Hz frequency and 2 mA intensity (EA group, n=8) compared with a sham MCAO group receiving no treatment (sham group, n=8). Data are expressed as mean±SEM ※P<0.01 versus control group.
Discussion
In the present study, the effects of EA treatment on BBB permeability were investigated. EA pretreatment was observed to effectively induce BBB opening and increase the exogenous NGF concentrations in the cerebral cortex without leading to ischaemic damage. Furthermore, the EA stimulation-induced BBB opening effect had time-dependent differences; the 8 min duration of EA stimulation was associated with the best performance.
Ischaemic stroke remains one of the leading causes of death and disability worldwide, and the poor prognosis for patients with stroke is largely due to the lack of effective therapies.11 12 The BBB mostly consists of the endothelial cells of the microvasculature. The composition and structure of the BBB include many factors that either maintain or disturb the fluid balance in the brain during normal and pathological processes. However, the transit restrictions imposed by the BBB represent the most critical barriers to overcome with respect to the challenge of drug delivery to the CNS. Therefore, efforts are needed to not only address drug development but also to specifically design suitable vehicles for CNS drug delivery via systemic administration. Currently, transient pharmacological stimulation of BBB opening for drug delivery is tempting. However, the potential toxic effects, particularly under pathological conditions, are notable.
Therefore, dysfunction of the BBB has been well-documented in the aetiology or progression of several CNS pathologies.13 Enhancing the permeability of the BBB is necessary for the delivery of drugs into the damaged CNS. Specific BBB crossing vehicles are required to deliver drugs with CNS transit properties. However, these procedures can result in adverse side effects. Thus, researchers have attempted to use less invasive methods for NGF delivery. In the context of emerging neurological diseases, targeting drugs to the CNS is in high demand, but vehicles for BBB crossing remain in a nascent stage, and much progress is needed for their full implementation.14 EA treatments are a form of acupuncture that deliver electrical stimulation of acupuncture needles at selected points. EA has been shown to significantly promote the recovery of neurological function and thus improve quality of life. EA can alleviate neurological deficits and reduce cortical apoptosis in rats with ischaemia/reperfusion (I/R) injury. These anti-apoptotic effects may be due to upregulation of p-ERK.15 Moreover, EA can enhance recovery of neurological function, decrease cerebral infarct volume (IV) and increase HIF-1α expression in ischaemic rats.16 Another research study also suggested that the treatment of traumatic brain injury (TBI) in the acute stage with EA for 60 min may increase regional blood flow and attenuate levels of TGIF in the injured cortex, may lead to a decrease in neuronal apoptosis and cell infarction volume, and may represent one mechanism by which functional recovery may occur.17 High-frequency (100 Hz) EA stimulation improves motor symptoms in medial forebrain bundle (MFB)-transected rats by restoring homeostasis in the basal ganglia circuit.18 Previous studies have also demonstrated a correlation between the increase in certain neurotrophic factors, such as NGF, and the development, survival and maintenance of neuronal functions in both the peripheral and central nervous systems.19–21 However, EA can induce complex effects in the CNS, which is influenced by multiple factors, such as the points stimulated, method and parameters. Therefore, while EA stimulation has other effects in addition to its protection of the injured nervous system, its bidirectional regulatory effects can increase the permeability of the BBB.
The present study investigated the role of EA in opening the BBB. This study used the MCAO rat stroke model during the recovery period, and the effects of EA stimulation on the BBB permeability and the concentration of exogenous NGF were determined. The brain water content was measured using the standard wet/dry weighing method to determine whether the EA-induced opening of the BBB caused adverse reactions such as cerebral oedema. According to our results, the different time courses of the EA stimulation did not cause cerebral oedema or secondary brain injury. Furthermore, we studied the time-dependent relationship of the efficiency of EA treatment to open the BBB by investigating EB extravasation. The results confirmed the influence of EA stimulation, and there were time-dependent differences. The degree of penetration of EB in the EA-5min group was higher than those in the other groups. We then used LCM to confirm this phenomenon. Interestingly, the degree of penetration of EB in the EA-5min group remained higher than in the control group. According to our results, we hypothesise that there must be an optimal stimulation time for BBB opening. Thus, we optimised the experimental scheme, and the stimulation time was further refined (EA-4min, EA-6min, EA-8min, EA-12min, EA-15min and EA-45min). We found that the degree of penetration of EB in the EA-8min group was higher than those in the other groups. Then, we chose 8 min as the optimal EA time course to verify whether it can accelerate drug delivery across the BBB. Our studies suggest that the exogenous level of NGF was significantly elevated in the EA group compared with the sham group and (untreated MCAO) model group.
Our study has some limitations that must be acknowledged. In the modelling process, although the changes in CBF were dynamically monitored by LDF, the individual physiological responses in the rats resulted in differences in the angle and depth of nylon insertion, which particularly required high proficiency of the operators. Over the course of the EA interventions, there were certain subtle differences in the intervention times for various reasons, such as the shedding of needles and stress response of rats, which could have affected the final results. In the present study, we clarified the time-dependent effects of EA on the BBB permeability, but the exact mechanisms of action and the added benefit of electrical stimulation on BBB opening remain unclear. Thus, further observations are needed in follow-up experiments.
This study extends our earlier studies,22 in which EA was performed at GV20 and GV26 in rats to open the BBB, and the results demonstrated that rats treated with certain time courses of stimulation show an increase in BBB permeability, which is advantageous for the targeted delivery of drugs (such as NGF) into the CNS. Although the study of the relationship between EA and BBB and the exact mechanisms of its opening effect is relatively new, it is important to remember that the neurovascular unit is a complex multicellular structure with components that constantly interact to modulate each other’s functions under both physiological and pathological conditions. Therefore, the BBB not only protects and maintains homeostasis in the CNS, but also allows tightly controlled communication with the peripheral nervous system. However, the present study did not focus on these changes after EA treatment. These findings require further investigation, and we believe our study is a useful first step towards accumulating knowledge regarding the influence of EA on the BBB in brain ischaemia.
Footnotes
JZ and XL contributed equally.
Contributors: XL and ZD conceived and designed the experiments. JZ, HZ, YC, SX, YZ and JJ performed the experiments. JZ and HZ analysed the data and wrote the paper. All authors approved the final version of the manuscript accepted for publication.
Funding: This study was supported by the National Natural Science Foundation of China (NSFC) grant no. 81373758 and 81774407.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: a report from the American Heart Association. Circulation 2017;135:e146–e603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neuwelt E, Abbott NJ, Abrey L, et al. Strategies to advance translational research into brain barriers. Lancet Neurol 2008;7:84–96. 10.1016/S1474-4422(07)70326-5 [DOI] [PubMed] [Google Scholar]
- 3. Yarnitsky D, Gross Y, Lorian A, et al. Increased BBB permeability by parasympathetic sphenopalatine ganglion stimulation in dogs. Brain Res 2004;1018:236–40. 10.1016/j.brainres.2004.05.103 [DOI] [PubMed] [Google Scholar]
- 4. Gulyaev AE, Gelperina SE, Skidan IN, et al. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res 1999;16:1564–9. 10.1023/A:1018983904537 [DOI] [PubMed] [Google Scholar]
- 5. Konofagou EE. Optimization of the ultrasound-induced blood-brain barrier opening. Theranostics 2012;2:1223–37. 10.7150/thno.5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burgess A, Ayala-Grosso CA, Ganguly M, et al. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One 2011;6:e27877 10.1371/journal.pone.0027877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsui P, Leung MC. Comparison of the effectiveness between manual acupuncture and electro-acupuncture on patients with tennis elbow. Acupunct Electrother Res 2002;27:107–17. 10.3727/036012902816026040 [DOI] [PubMed] [Google Scholar]
- 8. Lin XM, Tan KP, Zhang AJ, et al. Effect of electroacupuncture on the permeability of blood-brain barrier for nerve growth factor and its relevance to PKC pathway in cerebral ischemia rats. Zhen Ci Yan Jiu 2009;34:110–3. [PubMed] [Google Scholar]
- 9. Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84–91. 10.1161/01.STR.20.1.84 [DOI] [PubMed] [Google Scholar]
- 10. Xu Y, Cui H, Zhu Q, et al. Unilateral opening of rat blood-brain barrier assisted by diagnostic ultrasound targeted microbubbles destruction. Biomed Res Int 2016;2016:1–10. 10.1155/2016/4759750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 2007;54:34–66. 10.1016/j.brainresrev.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 12. Barone FC. Ischemic stroke intervention requires mixed cellular protection of the penumbra. Curr Opin Investig Drugs 2009;10:220–3. [PubMed] [Google Scholar]
- 13. Bartanusz V, Jezova D, Alajajian B, et al. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol 2011;70:194–206. 10.1002/ana.22421 [DOI] [PubMed] [Google Scholar]
- 14. Peluffo H, Unzueta U, Negro-Demontel ML, et al. BBB-targeting, protein-based nanomedicines for drug and nucleic acid delivery to the CNS. Biotechnol Adv 2015;33:277–87. 10.1016/j.biotechadv.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 15. Wu C, Li C, Zhou G, et al. Effects of electroacupuncture on the cortical extracellular signal regulated kinase pathway in rats with cerebral ischaemia/reperfusion. Acupunct Med 201735:430–6. 10.1136/acupmed-2016-011121 [DOI] [PubMed] [Google Scholar]
- 16. Li C, Zhang T, Yu K, et al. Neuroprotective effect of electroacupuncture and upregulation of hypoxia-inducible factor-1α during acute ischaemic stroke in rats. Acupunct Med 2017;35:acupmed-2016-011148 10.1136/acupmed-2016-011148 [DOI] [PubMed] [Google Scholar]
- 17. Chuang CH, Hsu YC, Wang CC, et al. Cerebral blood flow and apoptosis-associated factor with electroacupuncture in a traumatic brain injury rat model. Acupunct Med 2013;31:395–403. 10.1136/acupmed-2013-010406 [DOI] [PubMed] [Google Scholar]
- 18. Jia J, Sun Z, Li B, et al. Electro-acupuncture stimulation improves motor disorders in Parkinsonian rats. Behav Brain Res 2009;205:214–8. 10.1016/j.bbr.2009.06.024 [DOI] [PubMed] [Google Scholar]
- 19. Isackson PJ. Trophic factor response to neuronal stimuli or injury. Curr Opin Neurobiol 1995;5:350–7. 10.1016/0959-4388(95)80048-4 [DOI] [PubMed] [Google Scholar]
- 20. Sendtner M, Pei G, Beck M, et al. Developmental motoneuron cell death and neurotrophic factors. Cell Tissue Res 2000;301:71–84. 10.1007/s004410000217 [DOI] [PubMed] [Google Scholar]
- 21. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 2001;24:677–736. 10.1146/annurev.neuro.24.1.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu B, Lü GH, Sun Y, et al. Effect of electroacupuncture combined with intragastric administration of borneol on the permeability of blood-brain barrier in the mouse. Zhen Ci Yan Jiu 2011;36:335–40. [PubMed] [Google Scholar]