Abstract
The control of parasitic nematodes impacting animal health relies on the use of broad spectrum anthelmintics. However, intensive use of these drugs has led to the selection of resistant parasites in livestock industry. In that respect, there is currently an urgent need for novel compounds able to control resistant parasites. Nicotine has also historically been used as a de-wormer but was removed from the market when modern anthelmintics became available. The pharmacological target of nicotine has been identified in nematodes as acetylcholine-gated ion channels. Nicotinic-sensitive acetylcholine receptors (N-AChRs) therefore represent validated pharmacological targets that remain largely under-exploited. In the present study, using an automated larval migration assay (ALMA), we report that nicotinic derivatives efficiently paralyzed a multiple (benzimidazoles/levamisole/pyrantel/ivermectin) resistant field isolate of H. contortus. Using C. elegans as a model we confirmed that N-AChRs are preferential targets for nornicotine and anabasine. Functional expression of the homomeric N-AChR from C. elegans and the distantly related horse parasite Parascaris equorum in Xenopus oocytes highlighted some striking differences in their respective pharmacological properties towards nicotine derivative sensitivity. This work validates the exploitation of the nicotine receptors of parasitic nematodes as targets for the development of resistance-breaking compounds.
Graphical abstract
Highlights
-
•
Monitoring of H. contortus L3 anthelmintic sensitivity with the automated larval migration assay.
-
•
Nicotinic derivatives paralyze multiple anthelmintic-resistant H. contortus.
-
•
C. elegans and Parascaris spp ACR-16 N-AChRs are targeted by nicotinic derivatives.
1. Introduction
The control of gastro-intestinal nematodes of veterinary importance is mainly based on the use of broad spectrum anthelmintics such as levamisole, benzimidazoles and avermectins. Multiple resistant isolates could therefore represent a major threat for animal health as well as for production sustainability. For example, the haematophagous parasite Haemonchus contortus (barber pole worm), that is one of the most prevalent and pathogenic trichostrongylid species affecting small ruminants worldwide, has developed multiresistance against these three main classes of anthelmintics, thus stressing the need for the development of novel resistance-breaking drugs (Van Wyk et al., 1999; Mortensen et al., 2003; Kaplan, 2004; Peter and Chandrawathani, 2005). In this respect, nicotine-sensitive acetylcholine receptors of parasitic nematodes appear to be pharmacological targets of prime interest. Acetylcholine is a major excitatory neurotransmitter in both vertebrates and invertebrates. Acetylcholine receptors are members of the cys-loop ligand-gated ion channel superfamily and consist of five subunits arranged around a central pore (Unwin, 2005). Each subunit possesses an N-terminal extracellular domain containing a dicysteine loop followed by four transmembrane regions (TM1–TM4) of which TM2 lines the ion channel. In nematodes, the muscular acetylcholine receptors fall into two pharmacological classes that are preferentially activated by the cholinergic agonist levamisole (L-type) or nicotine (N-type) respectively. These cholinergic agonists induce a prolonged activation of muscular AChR causing spastic paralysis of the worms, which are either killed as with the free-living nematode Caenorhabditis elegans or expelled from the host organism in the case of H. contortus. (Aceves et al., 1970; Aubry et al., 1970; Harrow and Gration, 1985).
The molecular composition of L-AChR and N-AChR was first deciphered in the model nematode Caenorhabditis elegans. The main C. elegans L-AChR is a heteromeric receptor composed of five subunits encoded by the unc-38, unc-63, lev-8, unc-29 and lev-1 genes respectively (Lewis et al., 1980, 1987; Fleming et al., 1997; Culetto et al., 2004; Towers et al., 2005). Co-expression of these five distinct L-AChR subunits together with three additional C. elegans ancillary proteins led to the robust expression of a functional C. elegans L-AChR in Xenopus laevis oocytes (Boulin et al., 2008). The recombinant C. elegans L-AChR was found to be sensitive to levamisole (Lev) but insensitive to nicotine (Nic). The C. elegans N-AChR is a homomeric receptor composed of five identical subunits encoded by the acr-16 gene and requires only the RIC-3 ancillary protein to enhance its functional expression in Xenopus oocytes (Ballivet et al., 1996; Halevi et al.,. 2003). In contrast with the L-AChR subtype, the recombinant C. elegans N-AChR was found to be very responsive to Nic whereas Lev did not induce any response. Similarly, the recombinant N-AChR made of the ACR-16 subunits from the pig parasitic nematode Ascaris suum presented the same differential response between Lev and Nic as observed for its C. elegans counterpart (Abongwa et al., 2016).
Whereas the L-AChRs are targets for several anti-parasitic drugs (for review Wolstenholme and Neveu, 2017) there is currently no anthelmintic on the market targeting the N-AChRs. However, several decades ago, nicotine has been used as an anthelminthic in livestock (Waller et al., 2001; McKellar and Jackson, 2004), therefore validating nicotine-sensitive AChR from nematode as potent anthelmintic targets.
In the present study, using an automated larval migration assay (ALMA), we provide evidence that nicotine and nicotine derivatives targeting AChR are able to paralyze a multiple drug-resistant isolate of H. contortus. In addition, using recombinant N-AChRs expressed in Xenopus oocytes, we deciphered their mode of action. Our results suggest that compounds targeting the N-AChR are potentially able to control levamisole, pyrantel and ivermectin-resistant parasites and need to be further explored.
2. Material and methods
2.1. Ethics statement
All animal care and experimental procedures were conducted in strict accordance with the European guidelines for the care and use of laboratory animals and were approved by the ethical committee from Indre et Loire under experimental agreement 6623 provided by the French Veterinary Services.
2.2. Nematodes
Haemonchus contortus L3 larvae from the Weybridge and Kokstad isolates were obtained as previously described (Delannoy-Normand et al., 2010). In the present study, the anthelmintic susceptible Weybridge isolate (UK) was used as a reference (Roos et al., 1990). Kokstad is a field isolate from South Africa, which is resistant to benzimidazoles, ivermectin and levamisole (Neveu et al., 2007; de Lourdes Mottier, M. & Prichard, R. K. 2008; Ménez et al., 2016). However, for Kokstad isolate, pyrantel resistance status remained to be determined. In that respect, for the present study, sheep were infected with 10 000 Kokstad L3 larvae and were subsequently treated with a full dose of Levamisole (7.5 mg/kg of bodyweight) at 14 days post infection (dpi) and a full dose of pyrantel (20 mg/kg of bodyweight) at 19 dpi and finally with a full dose of ivermectin (0,2 mg/kg of bodyweight) at 24 dpi. Host faeces were collected 35 days post infection and positive fecal egg counting after treatments confirmed the Lev/Pyr/Ivm multi-resistant status of the Kokstad isolate. L3 larvae corresponding to the multiple resistant adult's progeny were harvested from coprocultures and used for automated larval migration assays. Benzimidazole susceptibility or resistance status of the Weybridge and Kokstad isolate was investigated by performing egg hatch assays as described by Coles et al. (1992) using thiabendazole (TBZ). The test performed in triplicate confirmed the Weybridge isolate TBZ-susceptibility (ED50: 0.024 ± 0.001 μg/ml) and the Kokstad isolate TBZ-resistance (ED50: 0.437 ± 0.205 μg/ml).
Adult Parascaris equorum were obtained as described in Courtot et al., (2015). Caenorhabditis elegans experiments were carried out on the Bristol N2; acr-16 (ok789) and lev-8(ok1519) strains obtained from the Caenorhabditis Genetics Center (CGC).
2.3. Automated larval migration assay
The automated larval migration assay (ALMA) used for the present study was adapted from the technology previously designed for H. contortus L2 motility monitoring (Blanchard et al., 2018) with minor modifications. Using a Quanta Master spectrofluorometer (Horiba PTI, NJ, USA), larval motility was estimated by measuring H. contortus L3 auto-fluorescence resulting from ultraviolet excitation. . Motility assays were performed using 7500 H. contortus L3 larvae. Worms were transferred into a 5 mL glass tube and left for 15 min to concentrate by gravity. The supernatant was removed and replaced by 2 ml of tap water or anthelmintic solution. After 20 min, the tube was inverted on a 20 μm sieve. After a stabilization time of 60 s, the fluorescence accumulation (correlated to the number of larvae migrating through the sieve) was measured during 5 min . Each set of experiment was performed in triplicate.
2.4. cDNA synthesis
Total RNA was prepared from the distinct nematode species using 50 μL of pelleted L3 larvae of H. contortus or 50 adults C. elegans or cross-section (5 mm thick) from the mid body region of an individual adult worm of P. equorum. Frozen samples were ground in liquid nitrogen and homogenized in Trizol reagent (Invitrogen, Carlsbad, CA, USA) and total RNA was isolated according to the manufacturer's recommendations. RNA pellets were dissolved in 25 μL of RNA secure resuspension solution (Ambion, Austin, TX, USA) and DNase-treated using the TURBO DNA-free kit (Ambion). RNA concentrations were measured using a nanodrop spectrophotometer (Thermo Scientific, Waltham, MA, USA). First-strand cDNA synthesis was performed on 1 μg of total RNA using the superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendations.
2.5. Cloning of complete coding cDNA sequences of acr-16 from H. contortus and P. equorum
To identify the cDNA sequences from acr-16 homologs in H. contortus and P. equorum, nested polymerase chain reactions (PCR) were performed on respective first-strand cDNA templates with the Phusion High fidelity Polymerase (New England Biolabs) and PCR products were cloned into the transcription vector pTB207 (Boulin et al., 2008) using the In-Fusion®HD cloning kit (Clontech). For H. contortus, the following primers were designed based on the Hco-acr-16 mRNA sequence available in Genbank (accession number EU051823): Hc-ACR16-F-Xho1 (ATGTGGAGCTTGCTGATCGC) and Hc-ACR16-R-Apa1 (CTAGGCGACCAGATATGGAG). For P. equorum, a blast search (Altschul et al., 1997) with Asu-ACR-16 as a query against the partial P. equorum genomic sequence database (available at https://www.sanger.ac.uk/resources/downloads/helminths/) retrieved contig NODE_2631440_length_27442_2.804278 as the best hit containing an incomplete sequence for the acr-16 gene. Then, specific primers were designed to amplify the coding sequence of Peq-acr-16 (FuPeq16ptbamF TCGTGTAATTGACGCTGCGTCT, FuPeq16ptbapaR CTATGCTATCGTGTAAGGCGCA, Peq-acr-16-F0 TTCAGAGTGATAACGCATAACGG, Peq-acr-16-Z1R GCAAATACGTTAGTGTAAGTATGG). The novel complete coding sequences of acr-16 were named Hco-acr-16 for H. contortus and Peq-acr-16 for P. equorum according to Beech et al. recommendations (Beech et al., 2010) and were deposited to GenBank under the accession numbers MH806893 and MH806894, respectively.
2.6. Sequence analysis
Deduced amino-acid sequences were aligned using MUSCLE (Edgar, 2004). Signal peptide predictions were carried out using the SignalP 3.0 server (Bendtsen et al., 2004) and membrane-spanning regions were predicted using the SMART server (Schultz et al., 1998). Phylogenetic analysis was performed on deduced amino-acid sequence. Sequence from the signal peptide, the intracellular loop (between TM3 and TM4) and C-terminal tail were removed as they could not be aligned unambiguously. Maximal likelihood phylogeny reconstruction was performed using PhyML V20120412 (https://github.com/stephaneguindon/phyml-downloads/releases) and significance of internal tree branches was estimated using bootstrap resampling of the dataset 100 times. The accession numbers sequences used for the analysis are:
Caenorhabditis elegans: ACR-5 NP_498437; ACR-6 NP_491354; ACR-7 NP_495647; ACR-8 NP_509745; ACR-9 NP_510285; ACR-10 NP_508692; ACR-11 NP_491906; ACR-12 NP_510262; ACR-13 (=LEV-8) NP_509932; ACR-14 NP_495716; ACR-15 NP_505206; ACR-16 NP_505207; ACR-17 NP_001023961; ACR-18 NP_506868; ACR-19 NP_001129756; ACR-20 NP_001122627; ACR-23 NP_504024; ACR-24 NP_001255866; DEG-3 NP_505897; DES-2 NP_001256320; EAT-2 NP_496959; LEV-1 NP_001255705;; UNC-29 NP_492399; UNC-38 NP_491472; UNC-63 NP_491533. Haemonchus contortus: Hco-ACR-16 MH806893; Parascaris equorum: Peq-ACR-16 MH806894.
2.6.1. Caenorhabditis elegans experiments
Worms were maintained at 20 °C on nematode growth medium (NGM) plates and fed on a bacterial lawn (Escherichia coli OP50). Paralysis assays were performed on gravid adults as previously described (Gottschalk et al., 2005).
2.7. Electrophysiology experiments
The pTB207 containing either the C. elegans, H. contortus and P. equorum acr-16 cDNAs were linearized with the NheI restriction enzyme (Thermofisher) and used as templates for cRNA synthesis using the T7 mMessage mMachine kit (Ambion). In parallel, cRNAs for the ancillary proteins Hco-RIC-3, Hco-UNC-50 and Hco-UNC-74 were also synthesized and mixed with the respective acr-16 cRNAs. Xenopus laevis defolliculated oocytes were obtained from Ecocyte Bioscience (Germany). The oocytes were injected in the animal pole with a total volume of 36 nL of cRNA mix containing 50 ng/μL of each cRNA in RNase-free water using the Drummond nanoject II microinjector. Microinjected oocytes were incubated at 20 °C for 48H before recording. Two-electrode voltage-clamp recordings were carried out using an Oocyte Clamp OC-725C amplifier (Warner instrument) on oocytes being voltage-clamped at −60 mV. The electrophysiology experiments were performed on BAPTA-free oocytes as the previous study investigating the ACR-16 N-AChR from Ascaris suum showed a calcium permeability (PCa/PNa) of 0.4, indicating that the calcium ion is not the major ion going through the ACR-16 channel (Abongwa et al., 2016). In accordance, we found no statistical differences in the EC50 values for ACh- and Nic-elicited currents resulting from BAPTA-AM-free and BAPTA-AM-treated oocytes, thus downplaying a putative confounding effect of calcium-activated chloride channels on whole-cell current responses. Acetylcholine and nicotine were dissolved in recording buffer (100 mM NaCl, 2.5 mM KCl, 1 mM CaCl2.2H2O, 5 mM HEPES, pH 7.3). Nornicotine and anabasine were prepared first in DMSO and diluted subsequently in recording buffer so that DMSO final concentration was less than 0.1%. Currents were recorded and analyzed using the pCLAMP 10.4 package (Molecular Devices). EC50 values were determined using non-linear regression on normalized data (1 mM ACh as maximal response) using GraphPad Prism® software.
3. Materials
Acetylcholine chloride (ACh), ivermectin; (−)-tetramisole hydrochloride (levamisole), (−)-nicotine hydrogen tartrate, pyrantel citrate, (±)-nornicotine, anabasine were purchased from Sigma-Aldrich.
4. Results
4.1. Automated larval migration assay (ALMA) confirms the multidrug resistant status of the H. contortus Kokstad isolate
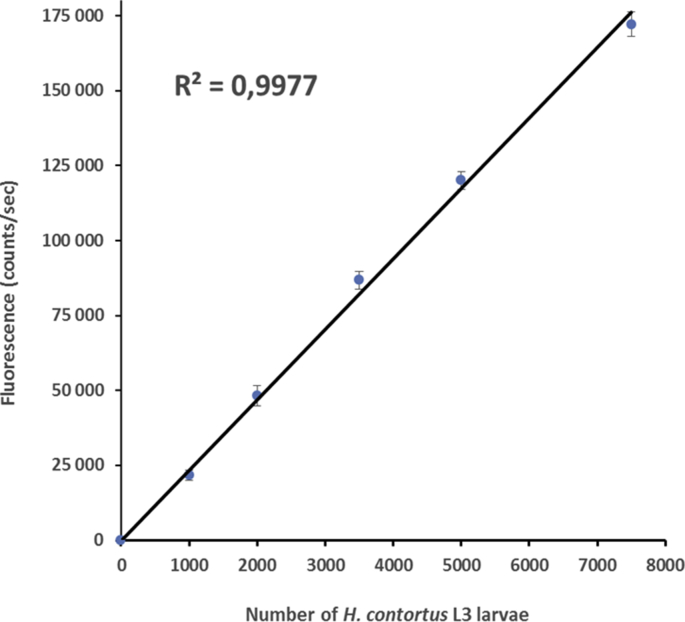
We recently reported the development of the ALMA technology (Automated Larval Migration Assay), a spectrofluorometric-based approach to quantify the migration rate of the H. contortus L2 larvae (Blanchard et al., 2018). Here, we adapted the ALMA to the L3 stage in order to use it for testing the in vitro anthelmintic activity of several drug standards. Interestingly, as previously reported for L2 larvae, we found a highly significant relationship between the fluorescence measured and the accumulation of L3 larvae that migrated into the recording chamber (R2 = 0.9977) after 5 min (S1 Fig.). Therefore, we were able to measure the L3 migration and the in vitro effect of anthelmintics by quantifying the increase in fluorescence against time in the absence or presence of drug.
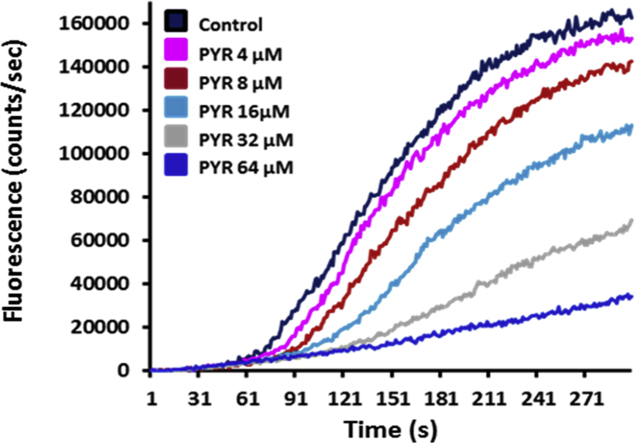
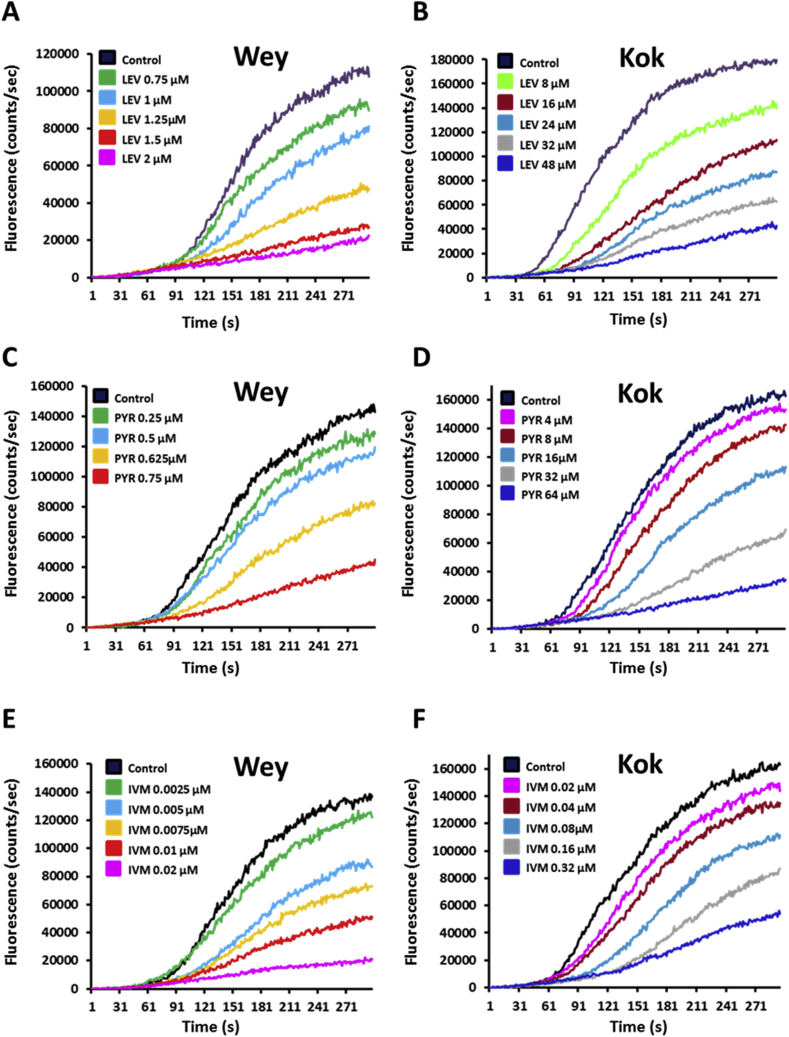
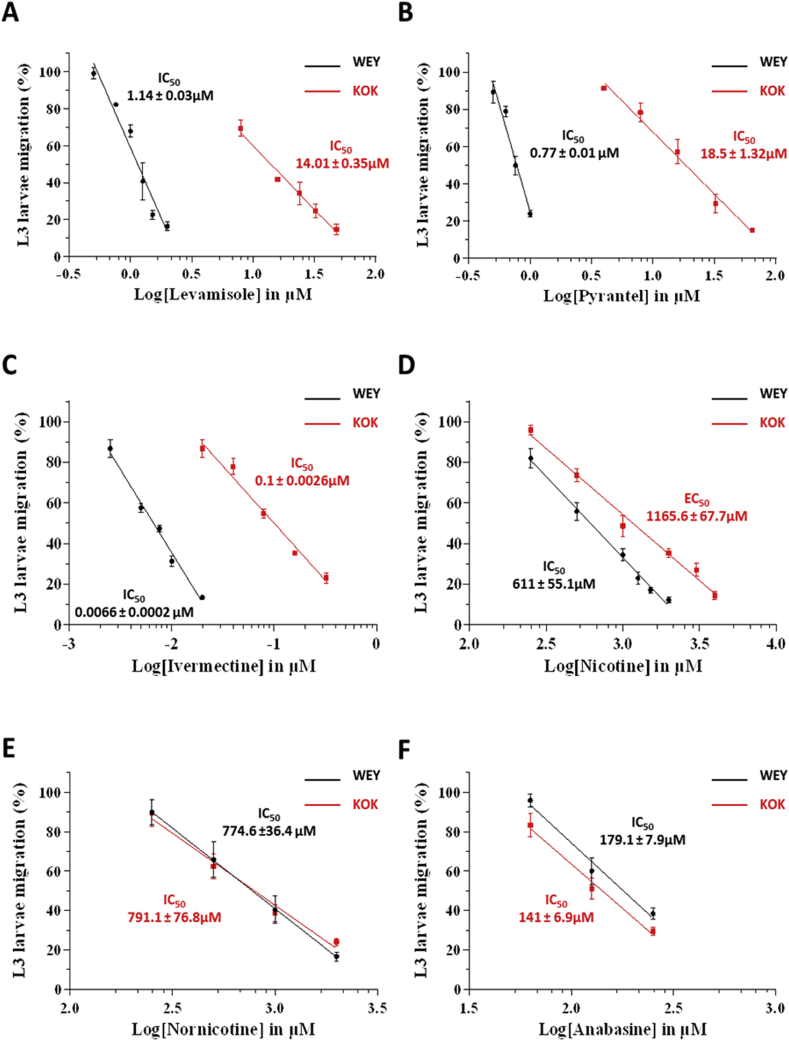
In order to determine their respective Lev, Pyr and Ivm susceptibility, ALMA assays were performed on H. contortus L3 from the anthelmintic-susceptible Weybridge (Wey) and the Lev/Pyr/Ivm multi-resistant Kokstad (Kok) isolates (Fig. 1, Fig. 2, Fig. 3; S2 Fig.). In the absence of drug application, a similar pattern of migration kinetic was observed between both isolates. In contrast, dose-response assays performed with Lev, Pyr and Ivm revealed a drastic reduction of drugs efficacy on the Kok L3 in comparison with Wey worms (Fig. 1; Fig. 3; S2 Fig.). The IC50 values of Lev, Pyr and Ivm were 1.14 ± 0.03 μM, 0.77 ± 0.01 μM and 6.6 ± 0.2 nM for Wey versus 14.01 ± 0.35 μM, 18.5 ± 1.32 μM and 100 ± 2.6 nM for Kok, respectively. Based on the respective IC50 values, the calculated resistance factors (Kelly and Hall, 1979) between Kok and Wey were 12.3, 23.9 and 15.1 confirming the Lev, Pyr and Ivm resistance status of the Kok isolate and the drug susceptibility of the Wey isolate as previously determined in vivo (see Material and methods section).
Fig. 1.
Motility modulation of H. contortus L3 larvae exposed to levamisole, pyrantel or ivermectin. The automated larval migration assay (ALMA) was used to determine dose-dependent paralysis effect of Lev, Pyr or Ivm on the H. contortus L3 from Weybridge (A; C and E) or Kokstad isolate (B; D and F). Representative recording traces of the real-time fluorescence counting relative to the L3 migration during 5 min exposed to Lev (A and B); Pyr (C and D) or Ivm (E and F). Each trace corresponds to the mean data from 3 runs performed with 7500 L3 larvae. The controls correspond to untreated L3 larvae.
Fig. 2.
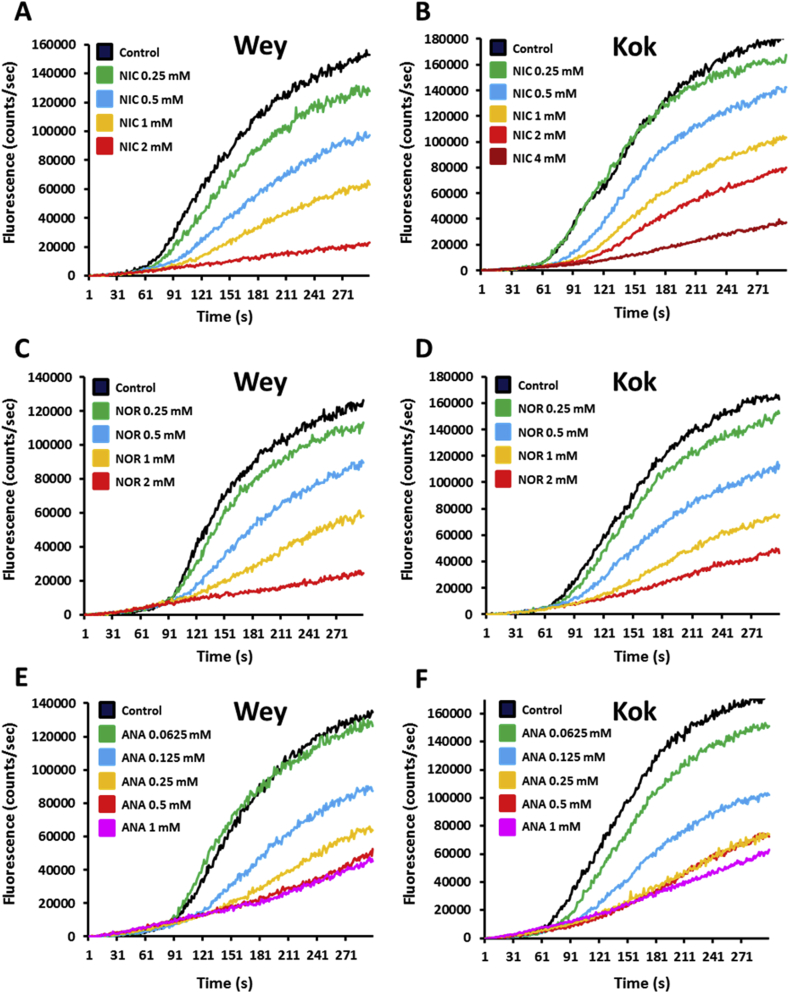
Motility modulation of H. contortus L3 larvae exposed to nicotine, nornicotine or anabasine. The automated larval migration assay (ALMA) was used to determine dose-dependent paralysis effect of Nic, Nor or Ana on the H. contortus L3 from Weybridge (A; C and E) or Kokstad isolate (B; D and F). Representative recording traces of the real-time fluorescence counting relative to the L3 migration during 5 min exposed to Nic (A and B); Nor (C and D) or Ana (E and F). Each trace corresponds to the mean data from 3 runs performed with 7500 L3 larvae. The controls correspond to untreated L3 larvae.
Fig. 3.
Determination of the dose response relationships for levamisole (A); pyrantel (B); ivermectin (C); nicotine (D); nornicotine (E) and anabasine (F) on H. contortus L3 larvae using the automated larval migration assay (ALMA). Results are shown as the mean ± se from 3 distinct ALMA assays performed with 7500 H. contortus L3 larvae from the Weybridge isolate (in black) or Kokstad isolate (in red). IC50 values are indicated on the graphs. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.2. Nicotine and nicotinic derivatives paralyze both lev-susceptible and lev-resistant isolates
The ALMA assay was then used to compare the effect of a set of nicotinic compounds including, nicotine (Nic), nornicotine (Nor) and anabasine (Ana) on H. contortus L3 from the Wey and Kok isolates (Fig. 2; Fig. 3; S2 Fig.). The application of Nic, Nor and Ana led to migration reductions of both Wey and Kok L3. Surprisingly, whereas Kok and Wey L3 presented a similar response to Nor (IC50 Wey Nor (774.6 ± 36.4 μM), IC50 Kok Nor (791.1 ± 76.8 μM)); Kok worms were less susceptible to Nic (IC50 Wey Nic (611 ± 55.1 μM), IC50 Kok Nic (1165.6 ± 67.7 μM)) but more responsive to Ana than their Wey counterparts (IC50 Wey Ana (179.1 ± 7.9 μM), IC50 Kok Ana (141 ± 6.9 μM)). These results confirmed the anthelmintic activity of the three drugs and provided a first evidence that nicotinic compounds are efficient on the Lev/Pyr/Ivm resistant worms.
4.3. N-AChRs are relevant drug targets for the control of levamisole and pyrantel resistant worms
In order to get first insights about the mode of action of nicotine and nicotinic-derivatives on Lev/Pyr resistant worms, we used the free living nematode Caenorhabditis elegans as a model. In addition to the wild type strain Bristol N2, two C. elegans mutant strains lacking respectively the L-AChR or N-AChR subtype (i.e. lev-8(oK1519), or acr-16(oK789) respectively) were used in the presents study. Note that lev-8 null mutants were chosen among other L-AChR subunit invalidated mutant, as these worms are not impaired in their locomotion and are Lev and Pyr-resistant, thus mirroring the phenotype of H. contortus Kokstad L3 larvae (Hernando et al., 2012; Blanchard et al., 2018).
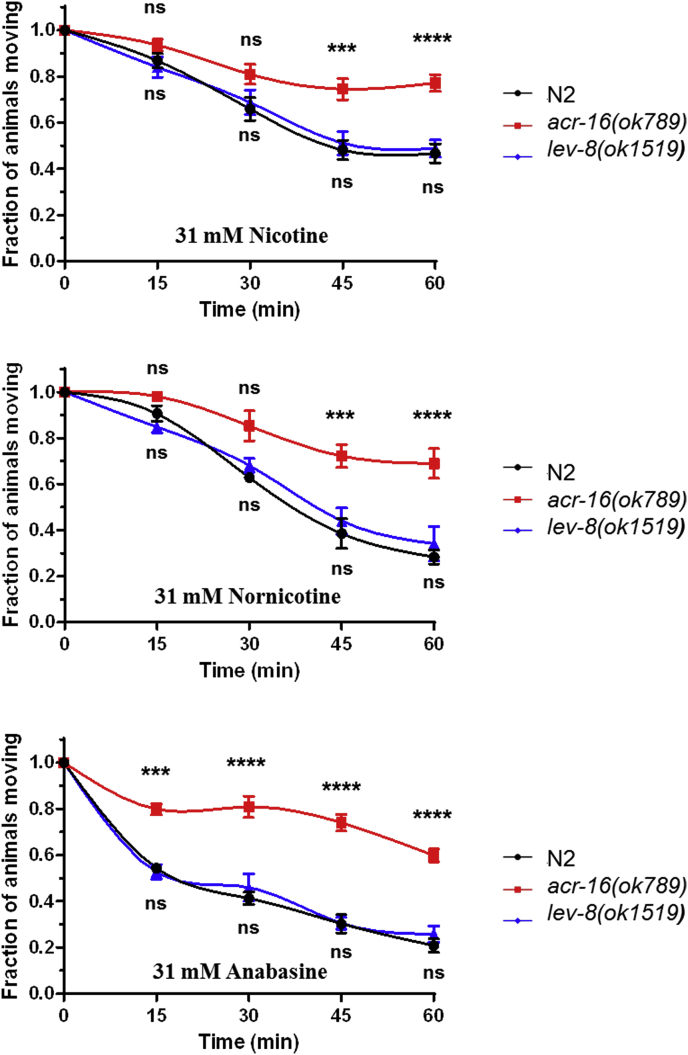
Paralysis assays were performed as described by Gottschalk et al., (2005) on agar plate containing 31 mM Nic, Nor or Ana (Fig. 4). Whereas N2 worms and lev-8 mutant motilities were affected by Nic, Nor and Ana, the acr-16 mutant lacking the N-AChR subtype was significantly less sensitive to the drugs. Taken together, these results support the hypothesis that the nematode N-AChR subtype including the ACR-16 subunit contributes to the anthelmintic effect of Nic, Nor and Ana.
Fig. 4.
Effects of nicotine, nornicotine or anabasine on the motility of C. elegans. Paralysis assays were performed on N2, acr-16 (ok789) and lev-8 (ok1519) C. elegans strains in agar plate containing 31 mM of nicotine (A), nornicotine (B) or anabasine (C) after 15, 30, 45 and 60 min drug exposure. Paralysis was scored based on the absence of worm's movement in response to prodding. Data are the mean ± SEM of n = 12, ****p < 0.0001, ***p < 0.001, **p < 0.01 and *p < 0.05, one way ANOVA with Bonferroni post-hoc test between N2 and the mutant strains.
4.4. Nicotinic derivatives activate recombinant homomeric N-AChRs expressed in Xenopus oocytes
It has been previously reported that the ACR-16 AChR subunit from C. elegans and the distantly related pig parasite Ascaris suum are able to form homomeric functional N-AChRs when expressed in Xenopus oocytes with the RIC-3 ancillary protein (Boulin et al., 2008; Abongwa et al., 2016).
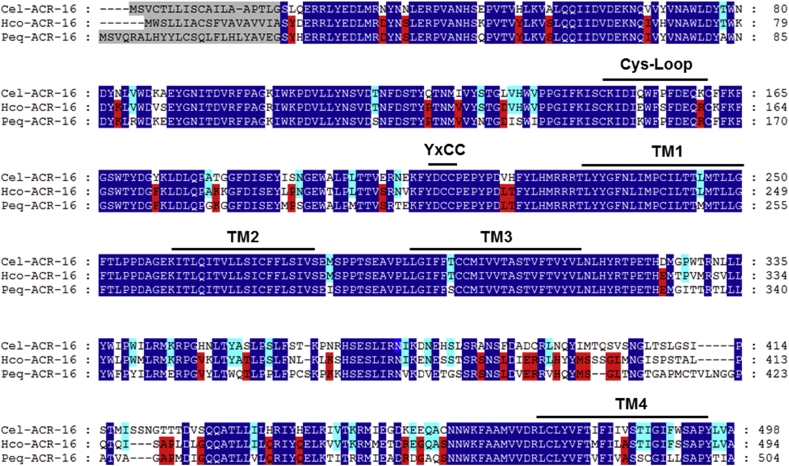
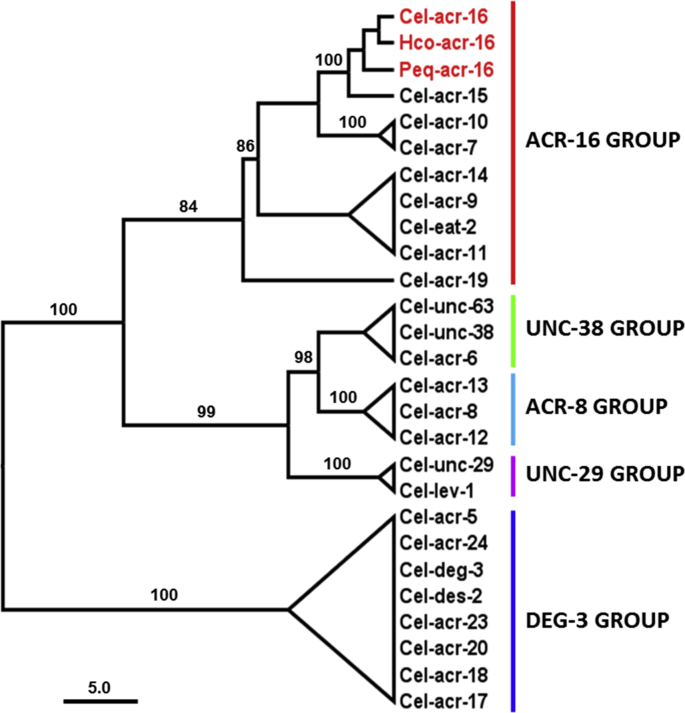
In order to further investigate the mode of action of nicotinic derivatives on nematode N-AChR, full-length cDNA sequences corresponding to acr-16 were obtained from C. elegans, H. contortus and the horse parasite Parascaris equorum. An alignment of the ACR-16 subunit sequences from the three nematode species is presented in Fig. 5. All sequences shared features of an AChR subunit including a predicted signal peptide, a “cys-loop”, four transmembrane domains and the vicinal dicysteines characteristics of alpha subunits. Protein sequences were highly conserved between the Clade V and Clade III species with identities for the mature proteins, excluding the signal peptide sequence, ranging from 76% to 89%. The orthologous relationship between the C. elegans ACR-16 subunit with its counterparts from parasitic species was confirmed by a phylogenetic analysis (S3 Fig.). Hco-acr-16 and Peq-acr-16 sequences have been deposited in Genbank with accession numbers MH806893 and MH806894 respectively.
Fig. 5.
Amino-acid alignments of ACR-16 subunit sequences from Caenorhabditis elegans, Haemonchus contortus and Parascaris equorum. acr-16 deduced amino-acid sequences were aligned using the MUSCLE algorithm (Edgar, 2004) and further processed using GeneDoc. Predicted signal peptide sequences are shaded in grey. Amino acids conserved between all the ACR-16 sequences are highlighted in dark blue. Amino acids specifically shared by ACR-16 homologs from parasitic species are highlighted in red. Amino acids specifically shared by Clade V nematode species (C. elegans and H. contortus) are highlighted in light blue. The cys-loop, the four transmembrane regions (TM1–TM4) and the primary agonist binding (YxCC) are indicated above the sequences. Cel (Caenorhabditis elegans), Hco (Haemonchus contortus), Peq (Parascaris equorum). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
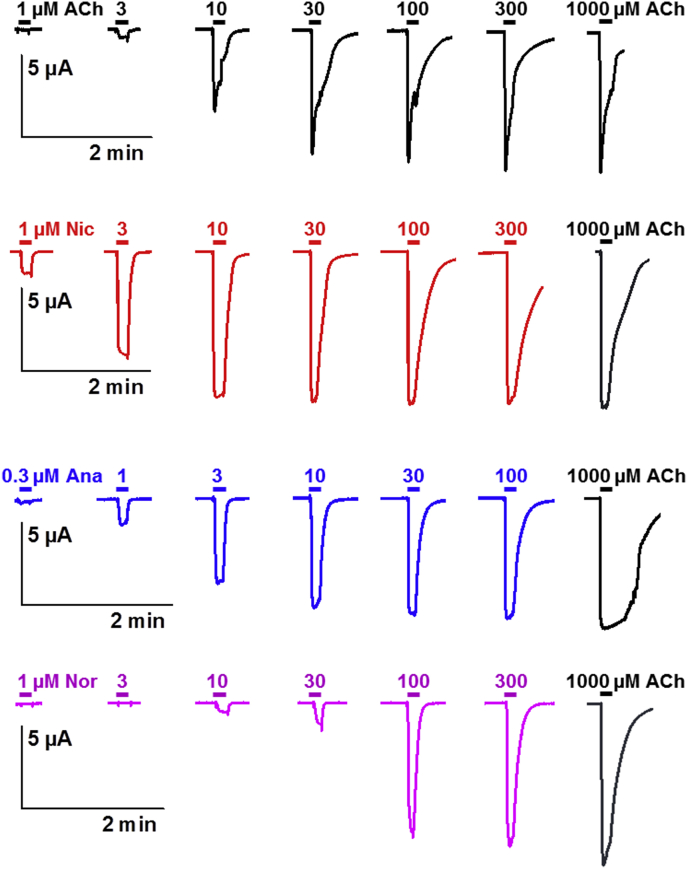
The cRNAs encoding ACR-16 from C. elegans, H. contortus or P. equorum were micro-injected in combination with cRNAs encoding the C. elegans RIC-3 ancillary protein in Xenopus oocytes. Two days after injection, we recorded robust currents in the μA range following the perfusion of 100 μM acetylcholine (ACh) in oocytes expressing ACR-16 from C. elegans and P. equorum demonstrating that the subunits assembled into functional AChRs. As previously reported for C. elegans and A. suum (Boulin et al., 2008; Abongwa et al., 2016), the P. equorum receptor made of ACR-16 displayed rapid and large activating inward currents with fast-desensitization kinetics which is a hallmark of the N-AChRs (Fig. 6). Nevertheless, Hco-ACR-16 failed to produce a functional receptor (n = 30), (S4 Fig.). Note that neither extending the expression time nor replacing RIC-3 from C. elegans by the RIC-3.1 and RIC-3.2 from H. contortus in the cRNA mix led to a functional N-AChR made of Hco-ACR-16 subunit (n = 16).
Fig. 6.
Concentration-response relationships of acetylcholine and nicotine derivatives on the P. equorum N-AChR expressed in Xenopus oocytes. Representative current traces for single oocytes perfused with acetylcholine (ACh), nicotine (Nic), anabasine (Ana) and nornicotine (Nor). The concentration of agonist (μM) is indicated above each trace.
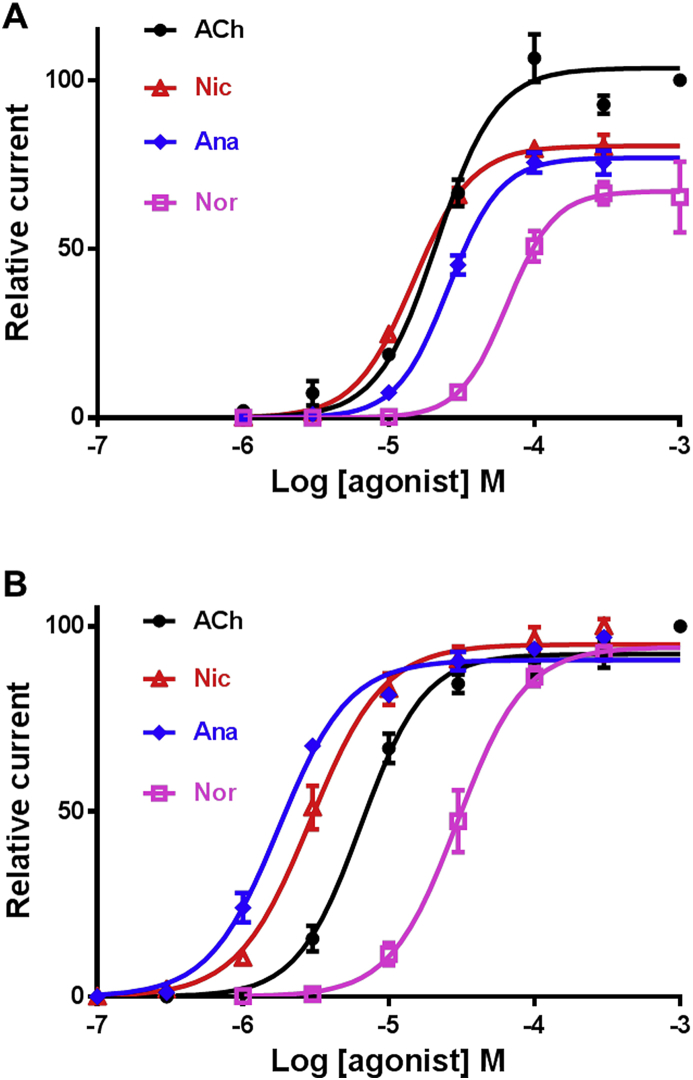
Next, we obtained the ACh concentration-response curve for Cel and Peq-N-AChR with maximal current amplitude elicited by 1 mM ACh (Fig. 7). The EC50 value of ACh was 6.4 ± 1.1 μM (n = 6) for Peq-N-AChR (Table 1) which was markedly more sensitive to ACh than the Cel-N-AChR (21.4 ± 1.1 μM) as well as the P. equorum morantel heteromeric receptor (34.9 ± 1.1 μM) made of the ACR-26 and ACR-27 subunits (Courtot et al., 2015). The pharmacological profiles of Cel-N-AChR and Peq-N-AChR were then established with Nic, Nor and Ana (Figs. 6 and 7). As expected, both receptors were highly responsive to 100 μM Nic with 79.5 ± 7.5% of ACh response (n = 16) for Cel-N-AChR and 96.6 ± 9.2% of ACh response (n = 8) for Peq-N-AChR. In addition, the perfusion of 100 μM Nor and Ana resulted in very similar currents as for Nic (Fig. 4). Interestingly, Nic and Ana were more potent than ACh in activating the P. equorum N-AChR as revealed by their respective EC50 values (2.9 ± 0.5 μM and 1.7 ± 0.1 μM, respectively), unlike Nor (34.9 ± 7.2 μM). The C. elegans N-AChR was more sensitive to Nic than ACh (15.1 ± 1.3 μM versus 22.0 ± 1.2 μM, respectively), showed a similar EC50 for Ana (27.5 ± 0.9 μM) and was less responsive to Nor (67.7 ± 6.6 μM). The Hill coefficient for the two N-AChRs ranged from 1.7 ± 0.2 (Nic, n = 8) to 2.7 ± 0.4 (Nor, n = 7) suggesting that more than one molecule must occupy the receptor to open the channel (Table 1). As a control, the nicotinic derivatives were also applied to the levamisole-sensitive receptors of C. elegans (Boulin et al., 2008) and H. contortus (Boulin et al., 2011). When perfused, Nic, Nor and Ana failed to induce significant response on oocytes expressing Cel-L-AChR and Hco-L-AChR-1 (S5 Fig.). Similarly, Nor and Ana did elicit very small currents on few oocytes while Hco-L-AChR-2 responded robustly to Nic (S5 Fig.). Altogether these results highlight some striking differences in the respective pharmacological properties of the C. elegans and P. equorum recombinant N-AChRs.
Fig. 7.
Concentration-response curves of acetylcholine and nicotine derivatives on the C. elegans (A) and P. equorum (B) N-AChRs expressed in Xenopus oocytes. The N-AChRs were challenged with acetylcholine (ACh), nicotine (Nic), anabasine (Ana) and nornicotine (Nor). All responses are normalized to 1 mM Ach. Results are shown as the mean ± se.
Table 1.
Summary of the EC50 and Hill coefficient values for acetylcholine and nicotine derivatives on the C. elegans and P. equorum N-AChRs expressed in Xenopus oocytes. Results are shown as the mean ± sd. The number of eggs recorded is indicated (n).
| Cel N-AChR | Peq N-AChR | ||
|---|---|---|---|
| Acetylcholine | EC50 (μM) | 22.0 ± 1.2 | 6.4 ± 0.6 |
| Hill slope | 2.1 ± 0.4 | 2.0 ± 0.2 | |
| n | 12 | 6 | |
| Nicotine | EC50 (μM) | 15.1 ± 1.3 | 2.9 ± 0.5 |
| Hill slope | 2.2 ± 0.2 | 1.7 ± 0.2 | |
| n | 11 | 8 | |
| Nornicotine | EC50 (μM) | 67.7 ± 6.6 | 34.9 ± 7.2 |
| Hill slope | 2.7 ± 0.4 | 1.9 ± 0.3 | |
| n | 7 | 9 | |
| Anabasine | EC50 (μM) | 27.5 ± 0.9 | 1.7 ± 0.1 |
| Hill slope | 2.4 ± 0.4 | 1.8 ± 0.2 | |
| n | 14 | 6 | |
5. Discussion
In the present work, using the ALMA assay we first confirmed the multiresistance status of the H. contortus Kokstad isolate and demonstrate that nicotine and some nicotinic derivatives can efficiently paralyze the Lev/Pyr/Ivm-resistant worms. The ALMA technology has been originally designed to quantify subtle motility modification of H. contortus L2 larvae associated with gene silencing (Blanchard et al., 2018). Here we show that ALMA is also suitable to monitor H. contortus L3 motility allowing the determination of IC50 values for cholinergic agonists but also macrocyclic lactones. This result open the way for the systematic determination of resistance status in other H. contortus isolates and lays the basis for a novel drug screening approach for the identification of resistance breaking drugs.
Because different cholinergic agonists are selective for different nematode AChR subtypes, the cholinergic receptor diversity could be potentially exploited for the development of novel anthelminthic able to control resistant parasites (Martin et al., 2012; Beech and Neveu, 2015; Wolstenholme and Neveu, 2017). In strongylid nematodes, Lev resistance has been shown to be associated with changes in binding characteristics or in the number of L-AChRs expressed in muscle cells (Sangster et al., 1988, 1998). In accordance with these observations, molecular investigations performed on Lev-resistant isolates from H. contortus identified truncated isoforms of two L-AChR subunits (i.e. ACR-8 and or UNC-63) associated with resistance (Neveu et al., 2010; Fauvin et al., 2010). In the pig parasitic nematode Oesophogostomum dentatum resistance to Lev has been characterized as the loss of a Lev receptor while nicotine-sensitive receptors were unaffected (Robertson et al., 1999). In accordance with this result, electrophysiological studies performed in C. elegans showed that genetic ablation of L-AChR resulting in Lev/Pyr resistance did not impact the functionality of ACR-16-containing receptors. In addition, in the present work, we showed that C. elegans lev-8 null mutants are sensitive to nicotine, nornicotine and anabasine, whereas these worms are resistant to both Lev and Pyr (Blanchard et al., 2018). Taken together these results support the hypothesis that drugs targeting the nicotinic receptors including the ACR-16 subunit might be efficient at controlling Lev/Pyr-resistant parasites.
In C. elegans, the anthelminthic activity of nicotine at the neuro-muscular junction is mainly mediated by the N-AChR which is, a homomeric receptor subtype made of the ACR-16 subunit (Richmond and Jorgensen, 1999; Touroutine et al., 2005). In accordance, in the present work we report that the nicotinic derivatives such as Nor or Ana induce a paralysis on wild-type and Lev-8 null mutant worms whereas acr-16 null mutants are resistant to these drugs highlighting the N-AChR as a major contributor of nicotinic derivative sensitivity in C. elegans. In addition, these results further support the use of drug targeting the N-AChR as a way to control Lev/Pyr-resistant nematodes. Interestingly, in parasitic species such as H. contortus and O. dentatum, a recombinant L-AChR subtype made of UNC-63, UNC-38 and UNC-29 (i.e. Hco-L-AChR-2 and Ode 29-38-63 respectively) subunits was found to be responsive to Nic (Boulin et al., 2011; Buxton et al., 2014). Here we reported that in contrast with Nic, Hco-L-AChR-2 is readily insensitive to both Nor and Ana. Even though the contribution of Hco-L-AChR-2 to Nic sensitivity in vivo remains to be elucidated, it is tempting to speculate that the reduced sensitivity to Nic observed in Kok (in comparison with Wey) could be associated with a putative impairment of this Hco-L-AChR-2 subtype. Nonetheless, such a reduced sensitivity to nicotine had no impact on Nor and Ana response of Kok L3 supporting the hypothesis that a putative N-AChR subtype including the ACR-16 subunit might be a preferential target for nicotine and nicotinic derivative in H. contortus.
In C. elegans, electrophysiological studies and expression in Xenopus oocytes strongly suggested that the ACR-16 subunit can associate to form a homopentameric channel both in vivo and in vitro (Ballivet et al., 1996; Touroutine et al., 2005). In the distantly related pig parasite A. suum, the ACR-16 subunit was also able to form a functional homopentameric channel when co-expressed in Xenopus oocytes with the RIC-3 ancillary protein. These results suggested that homomeric recombinant N-AChR made of ACR-16 should be obtained for other parasitic species such as H. contortus and the horse parasite P. equorum for which Pyr resistance is an increasing concern (Kaplan, 2002; Matthews, 2014; Lassen and Peltola, 2015). In accordance with this assumption, in the present study we report that the co-expression of the ACR-16 subunit from P. equorum with the RIC-3 from C. elegans led to the robust expression of a functional AChR. In comparison with the prototypical C. elegans N-AChR, the P. equorum N-AChR was found to be more responsive to Nic, Nor and Ana. Such differences could lay the basis for directed mutagenesis experiments in both C. elegans and P. equorum respective ACR-16 subunit that will provide critical information about the binding site of their respective homomeric N-AChR.
Interestingly, if Ana has been identified as the most potent agonist on the recombinant Peq-N-AChR, this nicotine alkaloid was also the most efficient on H. contortus Wey and Kok L3 as revealed during ALMA assays. However, because of its potential toxicity for the host (Lee et al., 2006), Ana is unlikely to be used as resistance breaking drugs for livestock. In that respect, the pharmacomodulation of Ana could represent an attractive approach to improve its efficacy as a potential anthelminthic. Recently, Zheng et al. (Zheng et al., 2017) showed that (S)-5-ethynyl-anabasine has higher agonist potency than other nicotine alkaloids on the recombinant A. suum N-AChR. If such studies open the way for the discovery of novel compounds targeting the N-AChR, there is now an urgent need to evaluate their efficacy on the parasites and also evaluate their potential toxicity for the host.
In contrast with P. equorum, into our hands the ACR-16 subunit from H. contortus failed to form a functional channel when co-expressed with RIC-3 from C. elegans but also using the RIC-3.1 and/or RIC-3.2 from H. contortus (data not shown). Here, we hypothesize that additional subunits or ancillary proteins are required to obtain a functional recombinant H. contortus N-AChR in Xenopus oocytes. Clearly, additional investigations are now required to further investigate ACR-16 containing receptors in H. contortus. In that respect recent progress concerning the efficient silencing of AChR subunit genes in H. contortus using RNAi will provide a valuable approach to decipher its role in nicotinic compound sensitivity in vivo (Blanchard et al., 2018).
In conclusion, we provide a proof of concept that drug targeting the nematode N-AChR can efficiently control Lev/Pyr-resistant parasites.
Research effort should now focus on the identification of a wider range of N-AChR from parasitic species laying the basis for the identification of novel compounds targeting these attractive targets.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
This research program was supported by INRA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. C. elegans strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). The vectors containing the cDNAs of Cel-acr-16 and Cel-ric-3 were kindly provided by Dr. Thomas Boulin (Institut NeuroMyogène, Lyon, France). We thank Noémie Descloux and Hadile Gabaj for valuable technical help.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2018.11.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
S1Fig. tif.
H. contortus L3 larvae migration assay using auto-fluorescence quantification. Correlation between the fluorescence counting (counts/sec) and the number of L3 larvae that migrated to the recording chamber during 5min. Migration assays were performed using 1000, 2000, 3500, 5000 and 7500 L3 larvae respectively. Each data point represents mean ± SE of three independent runs
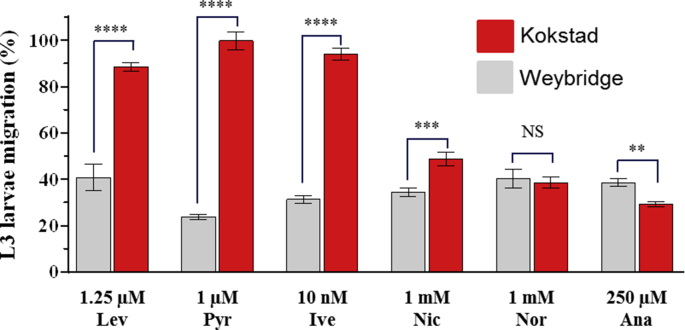
S2Fig. tif.
Comparison of the motility reduction of H. contortus L3 from the Weybridge vs Kokstad isolates exposed to Levamisole (Lev), Pyrantel (Pyr), Ivermectin (Ivm), Nicotine (Nic), Nornicotine (Nor), Anabasine (Ana) as determined by ALMA assays. Mean data of the twenty last fluorescence measures ± SEM. Welsh two sample t-test, Wey vs Kok. ****p < 0.001, ***p < 0.001, **p < 0.01
S3Fig. tif.
1. Maximum likelihood tree showing relationships of ACR-16 acetylcholine receptor (AChR) subunits from C.elegans, H. contortus and P. equorum with other C. elegans AChR subunits. Tree was built upon an alignment of AChR subunit sequences excluding the predicted signal peptide and the variable region between TM3 and TM4. The tree was rooted with DEG-3 group subunit sequences. Scale bar represents the number of substitution per site. Boostrap values > 80% are indicated on branches. Accession numbers for sequences used in the phylogenetic analysis are provided in Material and Methods section. C. elegans AChR subunit groups are named as proposed by Mongan et al. (Mongan et al., 2002). Cel, Hco and Peq refer to Caenorhabitis elegans, Haemonchus contortus and Parascaris equorum respectively
S4Fig. tif.
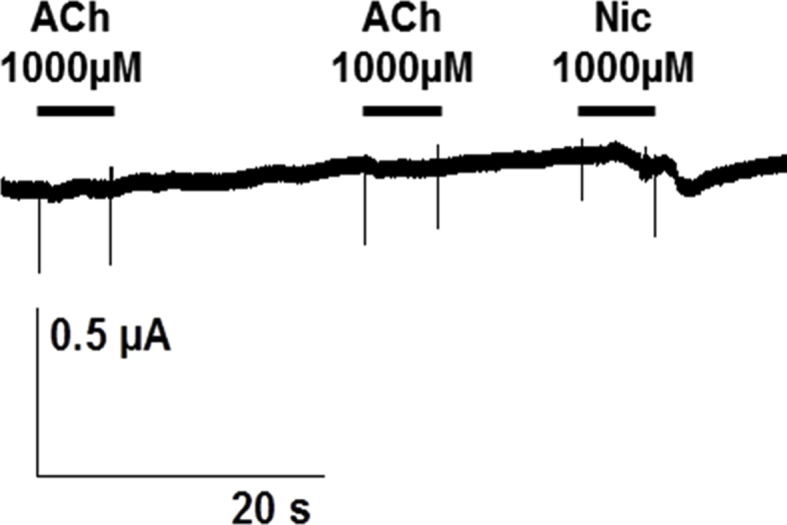
Tentative expression of Hco-ACR-16 subunit in Xenopus oocytes. Representative recording traces from a single oocyte challenged with 1 mM ACh and 1 mM Nic. Experiment repeated in three independent batches of oocytes.
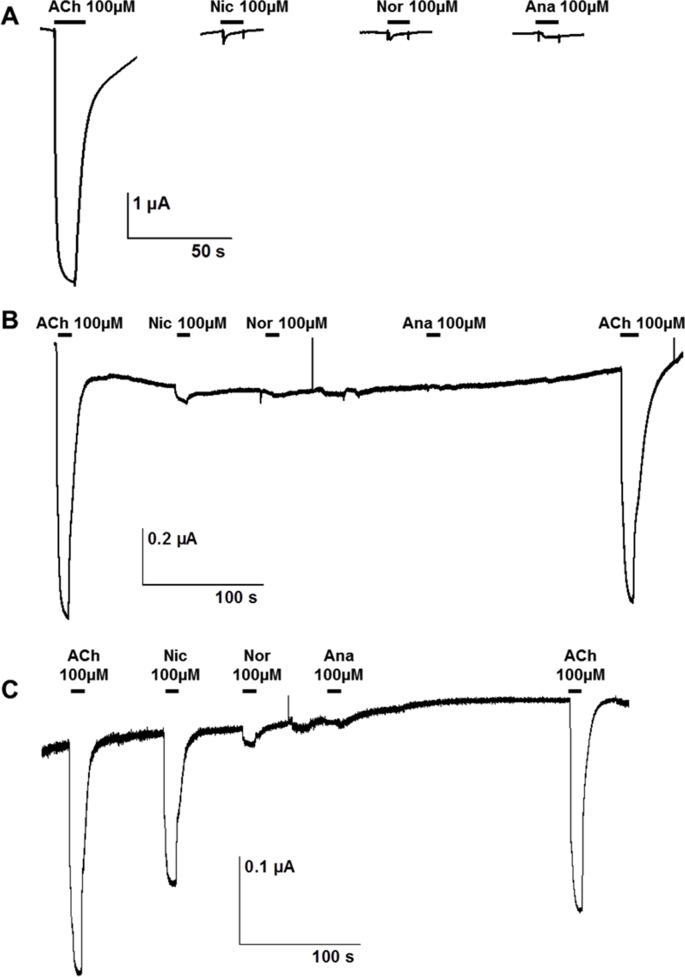
S5Fig. tif.
Representative recording traces showing the effect of 100 μM acetylcholine (ACh), nicotine (Nic), anabasine (Ana) and nornicotine (Nor) on Xenopus oocytes expressing the C. elegans L-AChR (A), the H. contortus L-AChR-1 (B) and the the H. contortus L-AChR-2 (C)
References
- Abongwa M., Buxton S.K., Courtot E., Charvet C., Neveu C., McCoy C.J., Verma S., Robertson A.P., Martin R.J. Pharmacological profile of Asu-acr-16, a new homomeric nAChR widely distributed in Ascaris tissues. Br. J. Pharmacol. 2016;173(16):2463–2477. doi: 10.1111/bph.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceves J., Erlij D., Martinez-Maranon R. The mechanism of the paralysing action of tetramisole on Ascaris somatic muscle. Br. J. Pharmacol. 1970;38:602–607. doi: 10.1111/j.1476-5381.1970.tb10601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25 doi: 10.1093/nar/25.17.3389. 3389e3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry M.L., Cowell P., Davey M.J., Shevde S. Aspects of the pharmacology of a new anthelmintic: pyrantel. Br. J. Pharmacol. 1970;38:332–344. doi: 10.1111/j.1476-5381.1970.tb08521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballivet M., Alliod C., Bertrand S., Bertrand D. Nicotinic acetylcholine re-ceptors in the nematode Caenorhabditis elegans. J. Mol. Biol. 1996;258 doi: 10.1006/jmbi.1996.0248. 261e269. [DOI] [PubMed] [Google Scholar]
- Beech R.N., Wolstenholme A.J., Neveu C., Dent J.A. Nematode parasite genes: what's in a name? Trends Parasitol. 2010;26(7):334–340. doi: 10.1016/j.pt.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Beech R.N., Neveu C. The evolution of pentameric ligand-gated ion-channels and the changing family of anthelmintic drug targets. Parasitology. 2015;142(2):303–317. doi: 10.1017/S003118201400170X. [DOI] [PubMed] [Google Scholar]
- Bendtsen J.D., Nielsen H., von Heijne G., Brunak S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004;340(4):783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Blanchard A., Guégnard F., Charvet C.L., Crisford A., Courtot E., Sauvé C., Harmache A., Duguet T., O'Connor V., Castagnone-Sereno P., Reaves B., Wolstenholme A.J., Beech R.N., Holden-Dye L., Neveu C. Deciphering the molecular determinants of cholinergic anthelmintic sensitivity in nematodes: when novel functional validation approaches highlight major differences between the model Caenorhabditis elegans and parasitic species. PLoS Pathog. 2018;14(5) doi: 10.1371/journal.ppat.1006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulin T., Gielen M., Richmond J.E., Williams D.C., Paoletti P., Bessereau J.L. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc. Natl. Acad. Sci. Unit. States Am. 2008;105:18590–18595. doi: 10.1073/pnas.0806933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulin T., Fauvin A., Charvet C.L., Cortet J., Cabaret J., Bessereau J.L., Neveu C. Functional reconstitution of Haemonchus contortus acetylcholine receptors in Xenopus oocytes provides mechanistic insights into levamisole resistance. Br. J. Pharmacol. 2011;164:1421–1432. doi: 10.1111/j.1476-5381.2011.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton S.K., Charvet C.L., Neveu C., Cabaret J., Cortet J., Peineau N., Abongwa M., Courtot E., Robertson A.P., Martin R.J. Investigation of acetylcholine receptor diversity in a nematode parasite leads to characterization of tribendimidine and derquantel-sensitive nAChRs. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles G.C., Bauer C., Borgsteede F.H.M., Geerts S., Klei T.R., Taylor M.A., Waller P.J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.). Methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- Courtot E., Charvet C.L., Beech R.N., Harmache A., Wolstenholme A.J., Holden-Dye L., O'Connor V., Peineau N., Woods D.J., Neveu C. Functional characterization of a novel class of morantel-sensitive acetylcholine receptors in nematodes. PLoS Pathog. 2015;11(12) doi: 10.1371/journal.ppat.1005267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culetto E., Baylis H.A., Richmond J.E., Jones A.K., Fleming J.T., Squire M.D., Lewis J.A., Sattelle D.B. The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor alpha subunit. J. Biol. Chem. 2004;279(41):42476–42483. doi: 10.1074/jbc.M404370200. [DOI] [PubMed] [Google Scholar]
- De Lourdes Mottier M., Prichard R.K. Genetic analysis of a relationship between macrocyclic lactone and benzimidazole anthelmintic selection on Haemonchus contortus. Pharmacogenetics Genom. 2008;18:129–140. doi: 10.1097/FPC.0b013e3282f4711d. [DOI] [PubMed] [Google Scholar]
- Delannoy-Normand A., Cortet J., Cabaret J., Neveu C. A suite of genes expressed during transition to parasitic lifestyle in the trichostrongylid nematode Haemonchus contortus encode potentially secreted proteins conserved in Teladorsagia circumcincta. Vet. Parasitol. 2010;174(1–2):106–114. doi: 10.1016/j.vetpar.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvin A., Charvet C.L., Issouf M., Cortet J., Cabaret J., Neveu C. cDNA-AFLP analysis in levamisole-resistant Haemonchus contortus reveals alternative splicing in a nicotinic acetylcholine receptor subunit. Mol. Biochem. Parasitol. 2010;170(2):105–107. doi: 10.1016/j.molbiopara.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Fleming J.T., Squire M.D., Barnes T.M., Tornoe C., Matsuda K., Ahnn J. Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J. Neurosci. 1997;17(15):5843–5857. doi: 10.1523/JNEUROSCI.17-15-05843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk A., Almedom R.B., Schedletzky T., Anderson S.D., Yates J.R., Schafer W.R. Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans. EMBO J. 2005;24(14):2566–2578. doi: 10.1038/sj.emboj.7600741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevi S., Yassin L., Eshel M., Sala F., Sala S., Criado M., Treinin M. Con-servation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J. Biol. Chem. 2003;278 doi: 10.1074/jbc.M300170200. [DOI] [PubMed] [Google Scholar]
- Harrow I.D., Gration K.A.F. Mode of action of the anthelmintics morantel, pyrantel and levamisole on muscle cell membrane of the nematode Ascaris suum. Pestic. Sci. 1985;16(6):662–672. [Google Scholar]
- Hernando G., Berge I., Rayes D., Bouzat C. Contribution of subunits to Caenorhabditis elegans levamisole-sensitive nicotinic receptor function. Mol. Pharmacol. 2012;82:550e560. doi: 10.1124/mol.112.079962. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Anthelmintic resistance in nematodes of horses. Vet. Res. 2002;33(5):491–507. doi: 10.1051/vetres:2002035. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance. A status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kelly J.D., Hall C.A. Resistance of animal helminths to anthelmintics. Adv. Pharmacol. Chemother. 1979;16:89–128. doi: 10.1016/s1054-3589(08)60243-4. [DOI] [PubMed] [Google Scholar]
- Lassen B., Peltola S.M. Anthelmintic resistance of intestinal nematodes to ivermectin and pyrantel in Estonian horses. J. Helminthol. 2015;89(6):760–763. doi: 10.1017/S0022149X14000510. [DOI] [PubMed] [Google Scholar]
- Lee S.T., Wildeboer K., Panter K.E., Kem W.R., Gardner D.R., Molyneux R.J., Chang C.W., Soti F., Pfister J.A. Relative toxicities and neuromuscular nicotinic receptor agonistic potencies of anabasine enantiomers and anabaseine. Neurotoxicol. Teratol. 2006;28(2):220–228. doi: 10.1016/j.ntt.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Lewis J.A., Wu C.H., Berg H., Levine J.H. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics. 1980;95(4):905–928. doi: 10.1093/genetics/95.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J.A., Elmer J.S., Skimming J., McLafferty S., Fleming J., McGee T. Cholinergic receptor mutants of the nematode Caenorhabditis elegans. J. Neurosci. 1987;7(10):3059–3071. doi: 10.1523/JNEUROSCI.07-10-03059.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.J., Robertson A.P., Buxton S.K., Beech R.N., Charvet C.L., Neveu C. Levamisole receptors: a second awakening. Trends Parasitol. 2012;28(7):289–296. doi: 10.1016/j.pt.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J.B. Anthelmintic resistance in equine nematodes. Int J Parasitol Drugs Drug Resist. 2014;4(3):310–315. doi: 10.1016/j.ijpddr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar Q.A., Jackson F. Veterinary anthelmintics: old and new. Trends Parasitol. 2004;20(10):456–461. doi: 10.1016/j.pt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Ménez C., Alberich M., Kansoh D., Blanchard A., Lespine A. Acquired tolerance to ivermectin and moxidectin after drug selection pressure in the nematode Caenorhabditis elegans. Antimicrob. Agents Chemother. 2016;60(8):4809–4819. doi: 10.1128/AAC.00713-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongan N.P., Jones A.K., Smith G.R., Sansom M.S.P., Sattelle D.B. Novel alpha 7-like nicotinic acetylcholine receptor subunits in the nematode Caenorhabditis elegans. Protein Sci. 2002;11(5):1162–1171. doi: 10.1110/ps.3040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen L.L., Williamson L.H., Terrill T.H., Kircher R.A., Larsen M., Kaplan R.M. Evaluation of prevalence and clinical implications of anthelmintic resistance in gastrointestinal nematodes of goats. J. Am. Vet. Med. Assoc. 2003;223:495–500. doi: 10.2460/javma.2003.223.495. [DOI] [PubMed] [Google Scholar]
- Neveu C., Charvet C., Fauvin A., Cortet J., Castagnone-Sereno P., Cabaret J. Identification of levamisole resistance markers in the parasitic nematode Haemonchus contortus using a cDNA-AFLP approach. Parasitology. 2007;134:1105–1110. doi: 10.1017/S0031182007000030. [DOI] [PubMed] [Google Scholar]
- Neveu C., Charvet C., Fauvin A., Cortet J., Beech R.N., Cabaret J. Genetic diversity of levamisole receptor subunits in parasitic nematodes and abbreviated transcripts associated with resistance. Pharmacogenetics Genom. 2010;20(7):414–425. doi: 10.1097/FPC.0b013e328338ac8c. [DOI] [PubMed] [Google Scholar]
- Peter J.W., Chandrawathani P. Haemonchus contortus: parasite problem No. 1 from tropics - polar Circle. Problems and prospects for control based on epidemiology. Trop. Biomed. 2005;22:131–137. [PubMed] [Google Scholar]
- Richmond J.E., Jorgensen E.M. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat. Neurosci. 1999;2(9):791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A.P., Bjorn H.E., Martin R.J. Resistance to levamisole resolved at the single-channel level. Faseb. J. 1999;13:749–760. doi: 10.1096/fasebj.13.6.749. [DOI] [PubMed] [Google Scholar]
- Roos M.H., Boersema H.J., Borgsteede F.H.M., Cornelissen J., Taylor M., Ruitenberg E.J. Molecular analysis of selection for benzimidazole resistance in the sheep parasite Haemonchus contortus. Mol. Biochem. Parasitol. 1990;43(1):77–88. doi: 10.1016/0166-6851(90)90132-6. [DOI] [PubMed] [Google Scholar]
- Sangster N.C., Riley F.L., Collins G.H. Investigation of the mechanism of levamisole resistance trichostrongylid nematodes of sheep. Int. J. Parasitol. 1988;18:813–818. doi: 10.1016/0020-7519(88)90123-3. [DOI] [PubMed] [Google Scholar]
- Sangster N.C., Riley F.L., Wiley L.J. Binding of [3H]m-aminolevamisole to receptors in levamisole-susceptible and -resistant Haemonchus contortus. Int. J. Parasitol. 1998;28:707–717. doi: 10.1016/s0020-7519(98)00033-2. [DOI] [PubMed] [Google Scholar]
- Schultz J., Milpetz F., Bork P., Ponting C.P. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. U. S. A. 1998;95(11):5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutine D., Fox R.M., Von Stetina S.E., Burdina A., Miller D.M., Richmond J.E. acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J. Biol. Chem. 2005;280(29):27013–27021. doi: 10.1074/jbc.M502818200. [DOI] [PubMed] [Google Scholar]
- Towers P.R., Edwards B., Richmond J.E., Sattelle D.B. The Caenorhabditis elegans lev-8 gene encodes a novel type of nicotinic acetylcholine receptor alpha subunit. J. Neurochem. 2005;93(1):1–9. doi: 10.1111/j.1471-4159.2004.02951.x. [DOI] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Van Wyk J.A., Stenson M.O., Van der Merwe J.S., Vorste r R.J., Viljoen P.G. Anthelmintic resistance in South-Africa: surveys indicate an extremely serious situation in sheep and goat farming. Onderspoort J Vet Res. 1999;66:273–284. [PubMed] [Google Scholar]
- Waller P.J., Bernes G., Thamsborg S.M., Sukura A., Richter S.H., Ingebrigtsen K., Höglund J. Plants as de-worming Agents of livestock in the nordic countries: historical perspective, popular beliefs and prospects for the future. Acta Vet. Scand. 2001;42(1):31–44. doi: 10.1186/1751-0147-42-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A.J., Neveu C. The interactions of anthelmintic drugs with nicotinic receptors in parasitic nematodes. Emerging Topics in Life Sciences. 2017;1(6):667–673. doi: 10.1042/ETLS20170096. [DOI] [PubMed] [Google Scholar]
- Zheng F., Du X., Chou T.H., Robertson A.P., Yu E.W., VanVeller B., Martin R.J. S)-5-ethynyl-anabasine, a novel compound, is a more potent agonist than other nicotine alkaloids on the nematode Asu-ACR-16 receptor. Int J Parasitol Drugs Drug Resist. 2017;7(1):12–22. doi: 10.1016/j.ijpddr.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.