Abstract
Ion channels are membrane protein complexes that underlie electrical excitability in cells, allowing ions to diffuse through cell membranes in a regulated fashion. They are essential for normal functioning of the neuromusculature and other tissues. Ion channels are also validated targets for many current anthelmintics, yet the properties of only a small subset of ion channels in parasitic helminths have been explored in any detail. Transient receptor potential (TRP) channels comprise a widely diverse superfamily of ion channels with important roles in sensory signaling, regulation of ion homeostasis, organellar trafficking, and other functions. There are several subtypes of TRP channels, including TRPA1 and TRPV1 channels, both of which are involved in, among other functions, sensory, nociceptive, and inflammatory signaling in mammals. Several lines of evidence indicate that TRPA1-like channels in schistosomes exhibit pharmacological sensitivities that differ from their mammalian counterparts and that may signify unique physiological properties as well. Thus, in addition to responding to TRPA1 modulators, schistosome TRPA1-like channels also respond to compounds that in other organisms modulate TRPV1 channels. Notably, TRPV channel genes are not found in schistosome genomes. Here, we review the evidence leading to these conclusions and examine potential implications. We also discuss recent results showing that praziquantel, the current drug of choice against schistosomiasis, selectively targets host TRP channels in addition to its likely primary targets in the parasite. The results we discuss add weight to the notion that schistosome TRP channels are worthy of investigation as candidate therapeutic targets.
Keywords: Schistosoma, Schistosomiasis, Ion channels, TRP channels, Capsaicin, TRPA1, TRPV1, Praziquantel
1. Introduction
Blood flukes of the genus Schistosoma cause schistosomiasis, a neglected tropical disease that affects hundreds of millions globally, with nearly one billion people at risk (Colley et al., 2014; King and Dangerfield-Cha, 2008). Adult worms reside within the blood vessels of the definitive host, depositing large numbers of eggs, many of which are excreted by the host, continuing the parasite life cycle and disease transmission. A large number of eggs also remain within the host however, evoking an immunopathological response with associated morbidity that can result in compromised childhood development, increased susceptibility to other infectious agents such as HIV, and, in some cases, death (Brodish and Singh, 2016; Colley et al., 2014; Hotez and Fenwick, 2009; King, 2010; King and Dangerfield-Cha, 2008; Ndeffo Mbah et al., 2013; van der Werf et al., 2003).
In the absence of a vaccine, chemotherapy remains the main strategy for managing the disease and controlling the spread of infection. Praziquantel (PZQ), the current drug of choice, is effectively the only antischistosomal treatment currently available (Bergquist et al., 2017; Danso-Appiah et al., 2013; Greenberg and Doenhoff, 2017; Kramer et al., 2013). Reports of PZQ-resistance (Day and Botros, 2006; Doenhoff and Pica-Mattoccia, 2006; Greenberg, 2013; Wang et al., 2012), along with known shortcomings of PZQ (Cioli et al., 2014; Greenberg and Doenhoff, 2017) lend added urgency to development of new or repurposed antischistosomals.
Ion channels underlie electrical excitability in cells, forming gated pores in cell membranes, selectively allowing ions to diffuse through the membrane, down their electrochemical gradients. They are essential to normal functioning of the nervous system and musculature as well as other cells and tissues. Helminth ion channels are outstanding targets for anthelmintic drugs. Anthelmintics known to act at least in part on parasite ion channels include ivermectin and other macrocyclic lactones, pyrantel, levamisole, monepantel, emodepside, piperazine, derquantel, tribendimidine, and likely PZQ (Epe and Kaminsky, 2013; Greenberg, 2014; Wolstenholme, 2011). No other class of parasite molecules is targeted nearly as extensively by current anthelmintics.
One set of helminth ion channels that has recently come under scrutiny, particularly in the platyhelminths, is the transient receptor potential (TRP) channel superfamily. TRP channels are highly diverse and involved in a wide variety of critical functions. They play key physiological roles and have been touted for their potential as candidate targets for therapeutics (Nilius and Szallasi, 2014), including antiparasitics (Bais and Greenberg, 2016; Wolstenholme et al., 2011). Here, building upon and summarizing progress since a prior review (Bais and Greenberg, 2016), we consider recent results from our lab and others to provide an assessment of current knowledge about TRP channels in schistosomes and related organisms. We also examine surprising and provocative recent reports showing that PZQ selectively activates TRP channels of the mammalian host, interactions that could possibly play a role in the mode of action of the drug.
2. TRP channels
TRP channels comprise a superfamily of largely non-selective cation channels that function as sensors for an extraordinary range of physical and chemical signals, including cellular messengers such as Ca2+, cyclic nucleotides, membrane lipids, osmotic pressure, and phosphorylation, as well as environmental stimuli such as thermal, light, mechanical, and chemical signals, including triggers of nociceptive and inflammatory pathways (Gees et al., 2010; Hoffstaetter et al., 2018; Venkatachalam and Montell, 2007; Zheng, 2013). TRP channels are also often polymodal; a single TRP channel can be activated through different, seemingly unrelated, stimuli. Thus, TRPV1, a single mammalian TRP channel subtype, is activated by noxious heat, endogenous lipid-derived molecules, protons, toxins, food ingredients (eg, capsaicin), and a host of nociceptive and inflammatory signals (Bevan et al., 2014; Jordt et al., 2003; Julius, 2013).
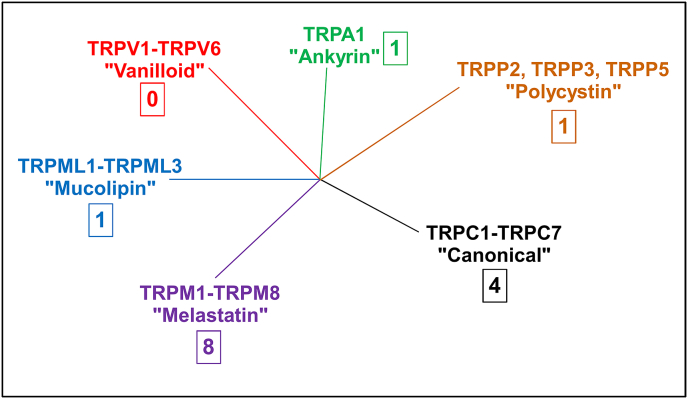
Originally discovered and characterized in Drosophila, with later identification in other organisms, metazoan TRP channels fall into 7–8 subfamilies based on structural homology (Nilius and Owsianik, 2011; Peng et al., 2015; Venkatachalam and Montell, 2007). Within those subfamilies are different subtypes with their own unique properties and functions. For example, mammals have 25–30 isoforms that fall within 6 subfamilies (Fig. 1).
Fig. 1.
Families of TRP channels. Shown are the 6 mammalian subfamilies of TRP channels. The number of S. mansoni genes for members of each subfamily is indicated in the box. TRPC (Canonical) channels are most closely related to the original Drosophila founding member of the TRP channel superfamily. They are activated by the phospholipase C cascade, among other signals. They may also sense mechanical stretch, and possibly Ca2+ store depletion. TRPV (Vanilloid) channels transduce thermal, nociceptive, and inflammatory signals. TRPV1 is the receptor for capsaicin, the primary active ingredient in hot peppers. There are no predicted TRPV channels in schistosomes. TRPA (Ankyrin) channels contain multiple N-terminal ankyrin domains. They are gated by temperature and noxious mechanical stimuli, and like TRPV channels, by nociceptive and inflammatory signals. They are also activated by pungent electrophilic compounds such as allyl isothiocyanate (AITC; found in mustard oil). As in schistosomes, TRPA1 is the sole mammalian TRPA channel. TRPM (Melastatin) channels transduce a variety of sensory signals, including taste and temperature (cold perception). They also respond to osmotic swelling and many chemical compounds. There are several subtypes in mammals, and they represent the most diverse subfamily in schistosomes. TRPP (Polycystin) channels appear to be mechano- or proton sensors. A mutation in one human subtype underlies autosomal dominant polycystic kidney disease (ADPKD), a disorder in which both kidneys show age-dependent massive enlargement. TRPML (Mucolipin) channels are intracellular channels found in endolysosomal vesicles. They play roles in organellar ion homeostasis, autophagy, and nutrient (amino acid) utilization. Mutations in human TRPML1 are the genetic cause of the lysosomal storage disease, mucolipidosis type IV (ML IV), a childhood neurodegenerative disorder. There is one predicted TRPML channel in schistosomes. TRPN and TRPVL subfamilies, absent in both mammals and schistosomes, are not shown (TRPVL is found only in cnidarians and polychaete worms). More details and references can be found in various reviews (Bais and Greenberg, 2016; Nilius and Szallasi, 2014; Peng et al., 2015; Rosasco and Gordon, 2017; Venkatachalam and Montell, 2007).
The functional repertoire of TRP channels remains incomplete, but a common theme is the key role these channels play in transduction of sensory signals, mediated primarily by modulation of intracellular Ca2+ concentrations (Gees et al., 2010). TRP channels also function in many additional physiological processes and pathologies, including: cellular volume and osmotic regulation (Passante-Morales, 2016); cell death and apoptosis (Fliniaux et al., 2018); organellar trafficking and autophagy (Venkatachalam et al., 2015); nociceptive and inflammatory signaling (Bautista et al., 2013; Julius, 2013; Parenti et al., 2016); detection of redox status (Ogawa et al., 2016); oncogenesis, anti-oncogenesis, and tumor progression (Fliniaux et al., 2018; Liberati et al., 2013; Santoni et al., 2011; Shapovalov et al., 2016); metabolic, respiratory, and cardiovascular physiology and disorders (Grace et al., 2014; Yue et al., 2014; Zhu et al., 2011); and others (Kaneko and Szallasi, 2014; Moran, 2018; Nilius and Szallasi, 2014). Notably, the same TRP channel orthologue from different species can have widely varying - and sometimes opposite - responses to sensory input or pharmacological agents. Thus, TRPA1 is a heat-sensor in Drosophila, but may be a cold-sensor in mammals (Viswanth et al., 2003), and TRPV3 is activated by heat in mice, but by cold in frogs (Saito et al., 2011). Similarly, caffeine activates mouse TRPA1, while suppressing human TRPA1 (Nagatomo et al., 2010).
Despite the limited amount of information about TRP channels in parasitic helminths, these channels have been characterized extensively in the free-living nematode Caenorhabditis elegans. As expected, TRP channels in C. elegans mediate sensory signaling, but they are also involved in several other functions, including fertilization, apoptosis, nicotine dependence, lifespan regulation, organelle biogenesis and trafficking, and regulation of gene expression, among others (Venkatachalam et al., 2014; Xiao and Xu, 2011). Recently, intriguing properties of TRP channels, particularly TRPA1, have been identified in free-living platyhelminths, and will be discussed in section 3.1, below.
3. TRP channels in schistosomes and other platyhelminths
3.1. Likely roles for TRP channels in schistosomes and other platyhelminths
As discussed in previous reviews (Bais and Greenberg, 2016; Wolstenholme et al., 2011), TRP channels are undoubtedly critical for realization of parasite life cycles. Dysfunctional TRP channels in mammals and other organisms that are produced genetically or by pharmacological manipulation have notable phenotypes, including sensory and other defects (Venkatachalam et al., 2014; Wu et al., 2010). Similar effects in schistosomes would likely disrupt essential sensory signaling required for fulfillment of the parasite life cycle.
In particular, TRP channels may play major roles in transduction of the types of photic, thermal, and chemical cues used by free-swimming miracidia and cercariae to orient and find appropriate (snail/mammalian) hosts (Haas, 2003; McKerrow and Salter, 2002; Tucker et al., 2013; Wang et al., 2016). This notion is supported by recent studies on free-living platyhelminths. Behavioral and RNA interference (RNAi) experiments implicate a TRPM-like channel in thermo-signaling in the planarian Dugesia japonica (Inoue et al., 2014). Similarly, knocking down expression of a TRPA1-like channel by RNAi in the planarian Schmidtea mediterranea disrupts extraocular avoidance responses to near-ultraviolet light, perhaps reflective of channel interaction with reactive oxygen species (ROS) that are byproducts of UV-light exposure (Birkholz and Beane, 2017). TRPA1 is also required in S. mediterranea for avoidance behaviors in response to noxious heat or irritant chemicals such as allyl isothiocyanate (AITC), a TRPA1 activator (Arenas et al., 2017). Notably, the S. mediterranea TRPA1 channel can restore avoidance of noxious heat in TRPA1-deficient Drosophila yet is itself not activated directly by heat when expressed in a heterologous system (Drosophila S2 cells). Instead, it appears that activation of S. mediterranea TRPA1 by thermal signals is, similar to the response to UV light, mediated by and ROS and H2O2 produced from heat-generated tissue damage, and has been hypothesized to represent an early nociceptive system triggered by ROS activation of TRPA1 (Arenas et al., 2017).
Upon entering their hosts and shortly thereafter, schistosomes also likely rely on TRP channels to interpret and respond to an array of thermal, chemical, osmotic, and other signals required for successful and rapid adaptation to the drastically dissimilar environments of a free-living vs. parasitic existence. Thus, the free-swimming cercaria must acclimatize almost instantaneously from living in fresh water at ambient environmental temperatures to residing in a saline-like milieu at 37 °C, surrounded by host cells and tissues as well as signaling compounds and the mammalian immune system. Within the mammalian host, schistosomes undergo extensive migration to reach their predilection sites (mesenteries, venous plexus of bladder), likely responding to host sensory and immunological cues to guide migration and development, and exhibiting extensive, organized neuromuscular activity to feed, generate movement, and maintain position. As adults, flukes also need to regulate sensory and neuromuscular activity to maintain position, move, feed, mate, lay eggs, and respond quickly to rapid changes within the host's circulatory system; TRP channels are likely candidates for mediating these (and other) functions. Additionally, as in other organisms, endolysosomal TRPML-like channels in schistosomes are likely critical for organellar trafficking, autophagy, nutrient acquisition, and perhaps neuronal development, and may function in iron transport and utilization in mammalian-stage blood-feeding schistosomes, as they do in trypanosomes (Taylor et al., 2013).
An earlier review (Bais and Greenberg, 2016) listed several agents that act on mammalian TRP channels, including endogenous, host-derived compounds, that also have effects on schistosomes or other platyhelminths (killing, paralysis, hyperactivity, attraction, repulsion). In the short period since that review, there have been several reports assessing the activity of physiological and pharmacological signals on TRP channels from schistosomes and other platyhelminths (summarized in Table 1). In addition, investigations have begun into the activity of PZQ on host TRP channels. Of particular note are four findings: 1) as predicted (Bais and Greenberg, 2016), schistosome TRPA1-like channels do indeed appear to exhibit mixed TRPV1/TRPA1-like pharmacology; 2) the host-derived inflammatory compound 4-hydoxynonenal (4-HNE) activates S. mansoni and S. haematobium TRPA1-like channels (and increases S. mansoni locomotory activity), suggesting a route by which mammalian signals might influence schistosomes within the host (Bais et al., 2018); 3) there are differences in the pharmacology of S. mansoni and S. haematobium TRPA1-like channels; 4) PZQ activates mammalian host TRP channels, most notably TRPM8 (Babes et al., 2017; Gunaratne et al., 2018), suggesting, along with evidence of PZQ activity against other mammalian receptors (Chan et al., 2017), that the efficacy of PZQ is not solely dependent on its activity against the parasite, and that host targets may also play a role.
Table 1.
Sensory signals and pharmacological agents that affect platyhelminths and act on platyhelminth TRP channels.
| Compound | Effects on schistosomes and other platyhelminths | Primary mammalian TRP channel activity | Schistosome/platyhelminth TRP channel activity |
|---|---|---|---|
| Capsaicin |
S. mansoni adults and schistosomula: increased locomotor activity (≥10 μM)1 S. mansoni cercariae: inhibition of swimming (60 μM)1 |
↑ TRPV1 (EC50: 10 nM - 1 μM)2 | ↑ SmTRPA, ↑ ShTRPA (≥5 μM)3 |
| AITC | S. mansoni adults: increased locomotor activity (10–20 μM)1; S. haematobium adults: no effect (60 μM)3 | ↑ TRPA1 (EC50: ∼20 μM)4 | ↑ SmTRPA, ↔ ShTRPA (20 μM)3; ↑ Smed-TRPA1 (500 μM)5 |
| Olvanil | S. mansoni adults: increased locomotor activity (10 μM)3 | ↑ TRPV1 (EC50: 0.7 nM)2 | ↑ SmTRPA, ↑ ShTRPA (10 μM)3 |
| 4-hydroxynonenal (4-HNE) | S. mansoni adults: increased locomotor activity (10 μM)3 | ↑ TRPA1 (EC50: ∼50 μM)6 | ↑ SmTRPA, ↑ ShTRPA (50 μM)3 |
| H2O2; ROS (generated by heat, near-UV light) | Schmidtea mediterranea: avoidance behaviors5,7 | ↑ TRPA1 (EC50: 220 μM)8 | ↑ Smed-TRPA1 (≥1 mM) |
| Heat | Dugesia japonica: thermotaxis (towards lower temperatures)9 | ↑ several TRP channels10 | ↑ DjTRPMa?9 |
| Praziquantel | Paralyzes, kills several platyhelminths, including schistosomes; causes "hepatic shift" of schistosomes in vivo | racemic: ↑ hTRPM8, hTRPV111; R-, S-enantiomers: ↑ hTRPA1, hTRPC3, hTRPC712; S-enantiomer: ↑ hTRPM812 |
ND |
↑, activation or sensitization; ↔, no effect;?, uncertain; ND, not determined.
SmTRPA = S. mansoni TRPA1 channel; ShTRPA = S. haematobium TRPA1 channel; Smed-TRPA1 = S. mediterranea TRPA1 channel; DjTRPMa = D. japonica TRPMa channel; hTRPA1, hTRPC3, hTRPC7, hTRPM8 = human TRP channel.
3.2. Schistosomes and other parasitic platyhelminths lack TRPV channels
Examination of the S. mansoni genome predicts 15 TRP channel genes, falling into 5 subfamilies (Bais and Greenberg, 2016; Prole and Taylor, 2011; Wolstenholme et al., 2011). TRPM channels are the most heterogeneous subfamily, with 8 predicted subtypes, followed by TRPC channels (4 subtypes), while TRPA, TRPP, and TRPML subfamilies each appear to have a single representative (Fig. 1). Genomes of other schistosomes and other platyhelminths also exhibit a diverse collection of TRP channel genes (Bais and Greenberg, 2016).
TRPV channels are found in most (though not all) metazoans, ranging from cnidarians to mammals (Peng et al., 2015). However, despite a diverse repertoire of 5 TRP channel subfamilies, it is striking that there are no predicted TRPV channels in any schistosome genomes, nor in those of any parasitic platyhelminth genomes described to date (Bais and Greenberg, 2016). Thus, sensory and other functions that act through TRPV channels in other organisms may in parasitic platyhelminths instead be mediated by TRP channels from other subfamilies. This apparent absence of TRPV channels in the parasitic platyhelminths becomes even more intriguing given that TRPV channel genes are found in the genomes of non-parasitic, free-living platyhelminths that have been examined (Brandl et al., 2015), perhaps indicating that parasitic platyhelminths have lost the TRPV sub-family as an adaptation to parasitism. Interestingly, parasitic nematodes do have TRPV-like channel genes (Wolstenholme et al., 2011).
3.3. Schistosome TRPA1-like channels exhibit mixed TRPA1/TRPV1-like pharmacology and respond to 4-HNE, a host-derived inflammatory compound
The initial evidence suggesting that schistosome TRPA1-like channels might have unusual pharmacology arose from two related observations: 1) capsaicin, the active ingredient in hot peppers and a highly-selective TRPV1 activator (Caterina et al., 1997), has dramatic stimulatory effects on S. mansoni motor activity and disrupts worm pairing despite the absence of any genes encoding TRPV channels in this organism; and 2) these effects are dependent upon expression of SmTRPA, the lone TRPA1 homolog in S. mansoni (Bais et al., 2015). Thus, capsaicin evokes hyperactivity in adult worms in a dose-dependent fashion but has no measurable effects on motor activity following knockdown by RNAi of SmTRPA. A selective TRPV1 inhibitor also eliminates the response of worms to capsaicin, indicating that the hyperactivity evoked by capsaicin displays a pharmacological profile reminiscent of TRPV1channels. SmTRPA is also required for a similar response of worms to AITC, a selective TRPA1 activator, suggesting that SmTRPA also has pharmacological sensitivities comparable to TRPA1 channels from other organisms.
Although persuasive, this evidence for unique, mixed TRPA1/TRPV1-like pharmacological sensitivity of SmTRPA was not conclusive. However, subsequent Ca2+ imaging studies on heterologously-expressed TRPA1-like channels from both S. mansoni and S. haematobium have provided more compelling evidence that these schistosome TRPA1-like channels are atypically activated by capsaicin (Bais et al., 2018). Thus, CHO cells expressing either SmTRPA or ShTRPA (the S. haematobium TRPA1-like channel) in combination with the genetically encoded Ca2+ indicator GCaMP6f (Chen et al., 2013), exhibit a robust, dose-dependent increase in intracellular Ca2+ signals in response to ≥5 μM capsaicin. The response is dependent on the presence of extracellular Ca2+. In contrast, the rat TRPA1 channel expressed under the same conditions shows no measurable Ca2+ increase in response to similar concentrations of capsaicin (Bais et al., 2018).
Surprisingly, although AITC, a classic activator of TRPA1 channels, produces a rise in intracellular Ca2+ in cells expressing SmTRPA (or, as expected, rat TRPA1), cells expressing ShTRPA do not respond to AITC. Furthermore, unlike S. mansoni adult worms, S. haematobium adult worms do not respond to AITC with hyperactivity (Bais et al., 2018). The structural basis for this differential effect of AITC is under investigation, but it is tempting to speculate, as we have, that the difference in AITC responsiveness could possibly mirror divergent TRPA1-mediated responses to host cues in these two schistosome species (which migrate to and occupy distinct host predilection sites).
Schistosome TRPA1-like channels also respond to at least one host-derived compound. Mammalian TRPA1 (and TRPV) channels transduce endogenous mammalian nociceptive and inflammatory signals (Bautista et al., 2013; Dai, 2015; Viana, 2016). For example, 4-HNE (4-hydroxy-2-nonenal), an α,β-unsaturated aldehyde produced in response to tissue injury, inflammation, or oxidative stress, activates mammalian TRPA1 on nociceptive neurons to promote acute pain, neuropeptide release, and neurogenic inflammation (Trevisani et al., 2007). 4-HNE also triggers a significant rise in intracellular Ca2+ in cells expressing SmTRPA or ShTRPA and evokes hyperactivity in adult S. mansoni (Bais et al., 2018). Schistosome TRPA-like channels (and perhaps other TRP channels) may therefore be receptors for host signaling compounds against which the parasites can mount a response or perhaps use to their own benefit. Schistosomes are thought to exploit and regulate host inflammatory responses required for their development (Riner et al., 2013); if schistosome TRPA channels play a role in this process, they may provide a novel mechanism by which parasites interact with these types of host cues and a potential point for therapeutic attack.
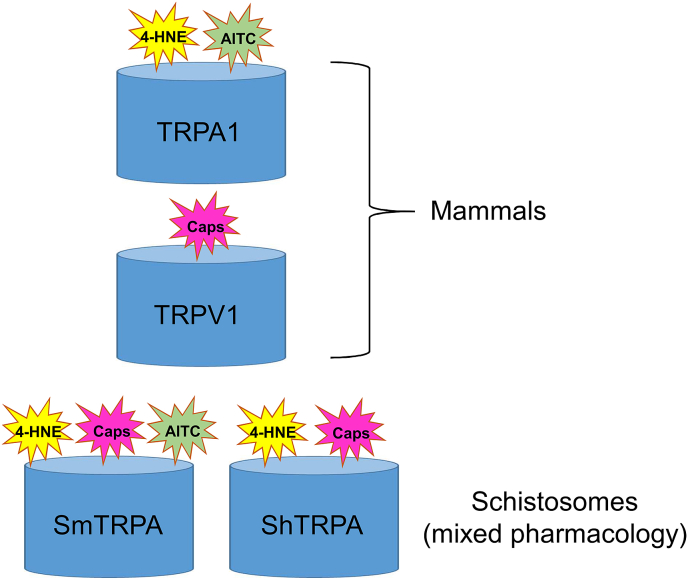
A graphic representation of some of the unique pharmacological properties of schistosome TRPA1-like channels compared with mammalian counterparts is presented in Fig. 2.
Fig. 2.
Summary of mammalian vs. schistosome TRPA1 pharmacology. Shown is a cartoon representation of the effects of TRPA1 (AITC, 4-HNE) and TRPV1 (capsaicin) activators on mammalian TRPA1 channels (top) and schistosome SmTRPA and ShTRPA channels (bottom), as assayed by heterologous expression and Ca2+ imaging (Bais et al., 2018). Capsaicin (Caps, purple) activates mammalian TRPV1 channels but not TRPA1 channels. In contrast, capsaicin activates the schistosome TRPA1-like channels SmTRPA and ShTRPA; there are no TRPV channels in schistosomes (Bais and Greenberg, 2016; Wolstenholme et al., 2011). AITC is a classic TRPA activator (green) that selectively activates mammalian TRPA1 channels. Surprisingly, AITC appears to activate only SmTRPA, and not ShTRPA, a species difference also reflected in dissimilar responses of adult worms to the compound; S. mansoni adults exhibit AITC-induced hyperactivity while S. haematobium adults do not (Bais et al., 2018). In contrast, both SmTRPA and ShTRPA respond to 4-HNE (yellow), a host-derived inflammatory compound that activates TRPA1 channels in other organisms and is associated with nociceptive and inflammatory pathways (Trevisani et al., 2007). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. PZQ interacts with host TRP channels
The mode of action of PZQ, the drug of choice against schistosomiasis, remains unresolved. However, recent evidence has suggested that in addition to its activity on schistosomes and other platyhelminths, part of the drug's efficacy can be attributed to interactions with host receptors, including mammalian TRP channels. Previous work had shown that the active, (R)-enantiomer of PZQ acts as a partial agonist of the G protein-coupled mammalian serotonergic 5-HT2B receptor to effect a constriction of basal tone in mesenteric vessels (Chan et al., 2017), a predilection site for adult S. mansoni and S. japonicum. Thus, the efficacy of PZQ may depend in part on a combination of effects on the host that promote parasite clearance along with deleterious effects on the parasite (paralysis, tegumental disruption). Full effectiveness of the drug may ironically be due to its lack of “clean” selectivity for parasite receptors (Chan et al., 2017).
More recently, this notion has been extended to host TRP channels, most notably a TRPM8 channel (Babes et al., 2017; Gunaratne et al., 2018). Thus, PZQ has been shown by two independent groups to act as a partial agonist of the human TRPM8 channel over a micromolar range (Babes et al., 2017; Gunaratne et al., 2018). Surprisingly, this effect on TRPM8 was shown to be due to the (S)-enantiomer of PZQ (Gunaratne et al., 2018), the enantiomer with less or no activity on schistosomes themselves (reviewed in Greenberg and Doenhoff, 2017). The (S)-enantiomer also evokes a marked relaxation of precontracted mesenteric arteries, similar to effects of known TRPM8 agonists. Interestingly, (S)-PZQ also produces a transient “hepatic shift” of S. mansoni from the mesenteries to the liver in vivo (Meister et al., 2014); it also reduces S. haematobium worm burden in vivo, despite showing 500-fold lower activity against these worms in vitro (Kovac et al., 2017). However, the effects of (S)-PZQ on vessel strips are not eliminated in TRPM8-knockout mice (Gunaratne et al., 2018), though the number of dorsal root ganglion cells responding to racemic PZQ with an increase in intracellular Ca2+ is significantly reduced (though not eliminated) in TRPM8-knockout mice (Babes et al., 2017). Thus, the physiological significance of the interaction of (S)-PZQ with host TRPM8 remains unclear. Notably, there appear to be no TRPM8-like channel genes in schistosomes (Bais and Greenberg, 2016).
Interestingly, although TRPM8 was the only human TRP channel to show stereoselective activation by PZQ, other human TRP channels were also activated by PZQ. Depending on the study, these included TRPV1 (but not TRPA1) at high concentrations of racemic PZQ (Babes et al., 2017), or TRPA1 (but not TRPV1), TRPC3. and TRPC7, all non-stereoselectively (Gunaratne et al., 2018). The role, if any, of these interactions of PZQ and host TRP channels remains unclear.
5. Conclusions and future questions
It is striking that even with investigation of schistosome TRP channels having begun only very recently and having covered only a few subtypes, it is already clear that they exhibit unique and potentially exploitable characteristics. Nonetheless, there remain huge gaps in our knowledge of the physiological roles, signaling pathways, and pharmacological sensitivities of TRP channels in schistosomes and other parasitic helminths.
A number of very fundamental questions about schistosome TRP channels remain unanswered. Some of these are listed below, along with possible strategies for addressing them:
-
1What are the functions of schistosome TRP channels? TRP channels are best known for the critical roles they play in transducing sensory stimuli, including photic, thermal, chemical, and mechanical signals. As noted above, these types of sensory signals are likely to be important for realization of the parasite life cycle and perhaps long-term survival within the mammalian host. However, we have shown that SmTRPA dysfunction can impact locomotor activity and male-female pairing, suggesting important roles in these functions. Furthermore, TRP channels in other organisms are involved in a wide range of physiological activities, and it will be interesting to determine if the same is true for schistosome TRP channels. For example, intracellular mammalian TRPML channels are critical for endolysosomal function and trafficking.
-
-develop new assays and adapt existing ones to assess whether TRP channel modulators and RNAi knockdown of specific TRP channel sequences can alter sensory and other responses of worms (caveat: with the exception of those targeting a few subtypes such as TRPV1 and TRPA1, there are not many TRP channel modulators with high selectivity).
-
-
-
2Do schistosome TRP channels have pharmacological properties that differ from mammalian TRP channels? As noted in this review, SmTRPA and ShTRPA have pharmacological properties that differ significantly from mammalian TRPA1 channels (as well as each other). Do other schistosome TRP channels also exhibit atypical sensitivities? If so, such TRP channels may prove useful as candidate targets for new therapeutics, particularly if inappropriate activation or inhibition of these channels leads to parasite dysfunction. Pharmacological differences may also indicate divergent physiological roles for particular channels.
-
-pharmacologically and genetically dissect responses of schistosomes to various TRP channel modulators
-
-test effects of candidate compounds on schistosome TRP channels expressed in a heterologous system
-
-screen for and test candidate compounds for their effects on worm and egg burdens in schistosome infections in model organisms (eg, mice)
-
-
-
3When and where are different TRP channels expressed in the parasite? Information on expression levels of TRP channels at different schistosome stages (cercariae, schistosomula, adults) is readily available from the schistosome genome database websites. For example, SmTRPA is most highly expressed in cercariae and early schistosomula and exhibits relatively low expression in adults. In contrast, it is not known where the different channel subtypes are being expressed within the parasite. Such patterns of expression will be important for inferring physiological roles and perhaps functional interactions. For example, TRP channels expressed at parasite-host interfaces (eg, tegument, gut) could suggest a role for these channels in parasite-host interactions at intramammalian stages. Furthermore, since TRP channels are tetramers of individual channel subunits, channel subunits that are coexpressed may form heterotetrameric channels, adding to the diversity of channel functions.
-
-localize TRP channel proteins with antibodies, in situ hybridization, fluorescent ligands, etc.
-
-test hypotheses about function that are generated from localization data with knockdown and pharmacological studies in cells and intact worms
-
-test coexpressed channel subtypes in a heterologous system to determine whether they form functional heteromeric channels
-
-
-
4Do schistosome TRP channels transduce host signals that affect parasite growth, differentiation, pathology, and host immune evasion and tolerance? As noted, mammalian TRP channels (particularly TRPV and TRPA1 channels) respond to endogenous signaling molecules, and we have shown that schistosome TRPA1-like channels respond to 4-HNE, a host-derived inflammatory compound. Do SmTRPA and ShTRPA respond to other host-derived signals? Do other schistosome TRP channels also respond to mammalian signals? These types of studies could lead to previously unrecognized, novel pathways for schistosomes and other parasites to integrate and respond to host signals, and perhaps new strategies for therapeutic targeting.
-
-test effects of host-derived signaling molecules on intact larval and adult worms in culture
-
-determine whether pharmacological agents that modulate particular TRP channels or whether knockdown of particular TRP channel sequences affects those responses
-
-test effects of host-derived compounds on schistosome TRP channels expressed in a heterologous system
-
-examine effects on survival, migration, egg production, etc. of knockdown of schistosome TRP channel sequences on schistosomes residing within the host
-
-
-
5Does PZQ interact with schistosome TRP channels, as it does with host TRP channels? PZQ induces a rapid influx of Ca2+ into the worm and a Ca2+-dependent contraction and paralysis. Previous reports have suggested a variety of candidate schistosome targets, including voltage-gated Ca2+ channels (Jeziorski and Greenberg, 2006), but a potential role for parasite TRP channels is certainly worth exploring, especially in light of the interaction of PZQ with host TRP channels.
-
-test PZQ against heterologously-expressed schistosome TRP channels
-
-test effects of PZQ in worms in which expression of specific TRP channels has been altered (eg, by RNA interference)
-
-
The answers to these and other questions have the potential to provide critical information about schistosome and platyhelminth TRP channels that could lead to new candidate therapeutic targets. Additionally, since TRP channels play critical roles in cellular and organismal physiology, understanding their properties and functions will provide a better understanding of parasite biology, including parasite-host interactions. Finally, although not directly related to the worms themselves, information gleaned about these parasite ion channels will almost certainly provide new insights into the evolution of TRP channels and the structure-function relationships that underlie divergent pharmacological and physiological properties of these channels.
Acknowledgments
The authors were supported in part by National Institutes of Health grants R01AI123173 and R21AI132912 to RMG. The sponsors played no role in the preparation or writing of this report, nor in the decision to submit the article for publication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijpddr.2018.08.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Arenas O.M., Zaharleva E.E., Para A., Vasquez-Doorman C., Petersen C.P., Gallio M. Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nature Neuorscience. 2017;20:1686–1693. doi: 10.1038/s41593-017-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babes R.M., Selescu T., Domocos D., Babes A. The anthelminthic drug praziquantel is a selective agonist of the sensory transient receptor potential melastatin type 8 channel. Toxicol. Appl. Pharmacol. 2017;336:55–65. doi: 10.1016/j.taap.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Bais S., Berry C.T., Liu X., Ruthel G., Freedman B.D., Greenberg R.M. Atypical pharmacology of schistosome TRPA1-like ion channels. PLoS Neglected Trop. Dis. 2018;12:e0006495. doi: 10.1371/journal.pntd.0006495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais S., Churgin M.A., Fang-Yen C., Greenberg R.M. Evidence for novel pharmacological sensitivities of transient receptor potential (TRP) channels in Schistosoma mansoni. PLoS Neglected Trop. Dis. 2015;9:e0004295. doi: 10.1371/journal.pntd.0004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais S., Greenberg R.M. TRP channels in schistosomes. Int. J. Parasitol.: Drugs and Drug Resist. 2016;6:335–342. doi: 10.1016/j.ijpddr.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M., Story G.M., Hwang S.W., Viswanath V., Eid S.R., Petrus M.J., Earley T.J., Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista D.M., Pellegrino M., Tsunozaki M. TRPA1: a gatekeeper for inflammation. Annu. Rev. Physiol. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist R., Utzinger J., Keiser J. Controlling schistosomiasis with praziquantel: how much longer without a viable alternative? Infect. Dis. Poverty. 2017;6:74. doi: 10.1186/s40249-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Quallo T., Andersson D.A. TRPV1. Handb. Exp. Pharmacol. 2014;222:207–245. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- Birkholz T.R., Beane W.S. The planarian TRPA1 homolog mediates extraocular behavioral responses to near-ultraviolet light. J. Exp. Biol. 2017;220:2616–2625. doi: 10.1242/jeb.152298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl H., Moon H., Vila-Farré M., Liu S.-Y., Henry I., Rink J.C. PlanMine - a mineable resource of planarian biology and biodiversity. Nucleic Acids Res. 2015;44(D1):764–773. doi: 10.1093/nar/gkv1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodish P.H., Singh K. Association between Schistosoma haematobium exposure and Human Immunodeficiency Virus infection among females in Mozambique. Am. J. Trop. Med. Hyg. 2016;94:1040–1044. doi: 10.4269/ajtmh.15-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chan J.D., Cupit P.M., Gunaratne G.S., McCorvy J.D., Yang Y., Stoltz K., Webb T.R., Dosa P.I., Roth B.L., Abagyan R., Cunningham C., Marchant J.S. The anthelmintic praziquantel is a human serotoninergic G-protein-coupled receptor ligand. Nat. Commun. 2017;8:1910. doi: 10.1038/s41467-017-02084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A., Schreiter E.R., Kerr R.A., Orger M.B., Jayaraman V., Looger L.L., Svoboda K., Kim D.S. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioli D., Pica-Mattoccia L., Basso A., Guidi A. Schistosomiasis control: praziquantel forever? Mol. Biochem. Parasitol. 2014;195:23–29. doi: 10.1016/j.molbiopara.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y. TRPs and pain. Semin. Immunopathol. 2015;38:277–291. doi: 10.1007/s00281-015-0526-0. [DOI] [PubMed] [Google Scholar]
- Danso-Appiah A., Olliaro P.L., Donegan S., Sinclair D., Utzinger J. Drugs for treating Schistosoma mansoni infection. Cochrane Db Syst. Rev. 2013;2013:CD000528. doi: 10.1002/14651858.CD000528.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T.A., Botros S. Drug resistance in schistosomes. In: Maule A., Marks N.J., editors. Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology. CAB International; Oxfordshire, UK: 2006. pp. 256–268. [Google Scholar]
- Doenhoff M.J., Pica-Mattoccia L. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Rev. Anti-infect. Ther. 2006;4:199–210. doi: 10.1586/14787210.4.2.199. [DOI] [PubMed] [Google Scholar]
- Epe C., Kaminsky R. New advancement in anthelmintic drugs in veterinary medicine. Trends Parasitol. 2013;29:129–134. doi: 10.1016/j.pt.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Fliniaux I., Germain E., Farafariello V., Prevarskaya N. TRPs and Ca2+ in cell death and survival. Cell Calcium. 2018;69:4–18. doi: 10.1016/j.ceca.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Gees M., Colsoul B., Nilius B. Vol. 2. 2010. The role of transient receptor potential cation channels in Ca2+ signaling; p. a003962. (Cold Spring Harbor Perspectives in Biology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace M.S., Baxter M., Dubuis E., Birrell M.A., Belvisi M.G. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br. J. Pharmacol. 2014;171:2593–2607. doi: 10.1111/bph.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R.M. New approaches for understanding mechanisms of drug resistance in schistosomes. Parasitology. 2013;140:1534–1546. doi: 10.1017/S0031182013000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R.M. Vol. 1. 2014. Ion channels and drug transporters as targets for anthelmintics; pp. 51–60. (Current Clinical Microbiology Reports). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R.M., Doenhoff M. Chemotherapy and drug resistance in schistosomiasis and other trematode and cestode infections. In: Mayers D.L., Ouellette M., Marchaim D., Sobel J.D., Kaye K.S., editors. Antimicrobial Drug Resistance, Vol. 1: Mechanisms of Drug Resistance, 2nd Edition. second ed. Springer Nature; Cham, Switzerland: 2017. pp. 705–734. [Google Scholar]
- Gunaratne G.S., Yahya N., Dosa P.I., Marchant J.S. Activation of host transient receptor potential (TRP) channels by praziquantel stereoisomers. PLoS Neglected Trop. Dis. 2018;12:e0006420. doi: 10.1371/journal.pntd.0006420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas W. Parasitic worms: strategies of host finding, recognition and invasion. Zoology. 2003;106:349–364. doi: 10.1078/0944-2006-00125. [DOI] [PubMed] [Google Scholar]
- Hill K., Schaefer M. Ultraviolet light and photosensitising agents activate TRPA1 via generation of oxidative stress. Cell Calcium. 2009;45:155–164. doi: 10.1016/j.ceca.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Hoffstaetter L.J., Bagriantsev S.N., Gracheva E.O. TRPs et al.: a molecular toolkit for thermosensory adaptations. Pflügers Archiv. 2018;470:745–759. doi: 10.1007/s00424-018-2120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Neglected Trop. Dis. 2009;3:e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Yamashita T., Agata K. Thermosensory signaling by TRPM is processed by brain serotonergic neurons to produce planarian thermotaxis. J. Neurosci. 2014;34:15701–15714. doi: 10.1523/JNEUROSCI.5379-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeziorski M.C., Greenberg R.M. Voltage-gated calcium channel subunits from platyhelminths: potential role in praziquantel action. Int. J. Parasitol. 2006;36:625–632. doi: 10.1016/j.ijpara.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt S.E., McKemy D.D., Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr. Opin. Neurobiol. 2003;13:487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Julius D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br. J. Pharmacol. 2014;171:2474–2507. doi: 10.1111/bph.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.H. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.H., Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chron. Illness. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- Kovac J., Vargas M., Keiser J. In vitro and in vivo activity of R- and S- praziquantel enantiomers and the main human metabolite trans-4-hydroxy-praziquantel against Schistosoma haematobium. Parasites Vectors. 2017;10:365. doi: 10.1186/s13071-017-2293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer C.V., Zhang F., Sinclair D., Olliaro P.L. Drugs for treating urinary schistosomiasis. Cochrane Db Syst. Rev. 2013;2013:CD000053. doi: 10.1002/14651858.CD000053.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati S., Morelli M.B., Nabissi M., Santoni M., Santoni G. Oncogenic and anti-oncogenic effects of transient receptor potential channels. Curr. Top. Med. Chem. 2013;13:344–366. doi: 10.2174/1568026611313030011. [DOI] [PubMed] [Google Scholar]
- McKerrow J.H., Salter J. Invasion of skin by Schistosoma cercariae. Trends Parasitol. 2002;18:193–195. doi: 10.1016/s1471-4922(02)02309-7. [DOI] [PubMed] [Google Scholar]
- Meister I., Ingram-Sieber K., Cowan N., Todd M., Robertson M.N., Meli C., Patra M., Gasser G., Keiser J. Activity of praziquantel enantiomers and main metabolites against Schistosoma mansoni. Antimicrob. Agents Chemother. 2014;58:5466–5472. doi: 10.1128/AAC.02741-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M.M. TRP channels as potential drug targets. Annu. Rev. Pharmacol. Toxicol. 2018;58:309–330. doi: 10.1146/annurev-pharmtox-010617-052832. [DOI] [PubMed] [Google Scholar]
- Nagatomo K., Ishii H., Yamamoto T., Nakajo K., Kubo Y. The Met268Pro mutation of mouse TRPA1 changes the effect of caffeine from activation to suppression. Biophys. J. 2010;99:3609–3618. doi: 10.1016/j.bpj.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndeffo Mbah M.L., Poolman E.M., Atkins K.E., Orenstein E.W., Meyers L.A., Townsend J.P., Galvani A.P. Potential cost-effectiveness of schistosomiasis treatment for reducing HIV transmission in Africa – the case of Zimbabwean women. PLoS Neglected Trop. Dis. 2013;7:e2346. doi: 10.1371/journal.pntd.0002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol. Rev. 2014;66:676–814. doi: 10.1124/pr.113.008268. [DOI] [PubMed] [Google Scholar]
- Ogawa N., Kurokawa T., Mori Y. Sensing of redox status by TRP channels. Cell Calcium. 2016;60:115–122. doi: 10.1016/j.ceca.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Parenti A., De Logu F., Geppetti P., Benemei S. What is the evidence for the role of TRP channels in inflammatory and immune cells? Br. J. Pharmacol. 2016;173:953–969. doi: 10.1111/bph.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passante-Morales H. Channels and volume changes in the life and death of the cell. Mol. Pharmacol. 2016;90:358–370. doi: 10.1124/mol.116.104158. [DOI] [PubMed] [Google Scholar]
- Peng G., Shi X., Kadowaki T. Evolution of TRP channels inferred by their classification in diverse animal species. Mol. Phylogenet. Evol. 2015;84:145–157. doi: 10.1016/j.ympev.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Prole D.L., Taylor C.W. Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riner D.K., Ferragine C.E., Maynard S.K., Davies S.J. Regulation of innate responses during pre-patent schistosome infection provides an immune environment permissive for parasite development. PLoS Pathog. 2013;9:e1003708. doi: 10.1371/journal.ppat.1003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosasco M.G., Gordon S.E. TRP channels: what do they look like? In: Emir T.L.R., editor. Neurobiology of TRP Channels. second ed. CRC Press/Taylor & Francis; Boca Raton, FL: 2017. [PubMed] [Google Scholar]
- Saito S., Fukuta N., Shingai R., Tominaga M. Evolution of vertebrate transient receptor potential vanilloid 3 channels: opposite temperature sensitivity between mammals and western clawed frogs. PLoS Genet. 2011;7:e1002041. doi: 10.1371/journal.pgen.1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni G., Farfariello V., Amantini C. TRPV channels in tumor growth and progression. Adv. Exp. Med. Biol. 2011;704:947–967. doi: 10.1007/978-94-007-0265-3_49. [DOI] [PubMed] [Google Scholar]
- Shapovalov G., Ritaine A., Skryma R., Prevarskaya N. Role of TRP ion channels in cancer and tumorigenesis. Semin. Immunopathol. 2016;38:357–369. doi: 10.1007/s00281-015-0525-1. [DOI] [PubMed] [Google Scholar]
- Taylor M.C., McLatchie A.P., Kelly J.M. Evidence that transport of iron from the lysosome to the cytosol in African trypanosomes is mediated by a mucolipin orthologue. Mol. Microbiol. 2013;89:420–432. doi: 10.1111/mmi.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M., Siemens J., Materazzi S., Bautista D.M., Nassini R., Campi B., Imamachi N., Andre E., Patacchini R., Cottrell G.S., Gatti R., Basbaum A.I., Bunnett N.W., Julius D., Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. Unit. States Am. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M.S., Karunaratne L.B., Lewis F.A., Freitas T.C., Liang Y.S. Schistosomiasis. Curr. Protoc. Im. 2013;103:19.11.11–19.11.58. doi: 10.1002/0471142735.im1901s103. [DOI] [PubMed] [Google Scholar]
- van der Werf M.J., de Vlas S.J., Brooker S., Looman C.W., Nagelkerke N.J., Habbema J.D., Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K., Luo J., Montell C. Evolutionarily conserved, multitasking TRP channels: lessons from worms and flies. Handb. Exp. Pharmacol. 2014;223:937–962. doi: 10.1007/978-3-319-05161-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K., Montell M. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K., Wong C.O., Zhu M.X. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium. 2015;58:48–56. doi: 10.1016/j.ceca.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F. TRPA1 channels: molecular sentinels of cellular stress and tissue damage. J. Physiol. 2016;594:4151–4169. doi: 10.1113/JP270935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanth V., Story G.M., Peier A.M., Petrus M.J., Lee V.M., Hwang S.W., Patapoutian A., Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- Voets T. TRP channels and thermosensation. Handb. Exp. Pharmacol. 2014;223:729–741. doi: 10.1007/978-3-319-05161-1_1. [DOI] [PubMed] [Google Scholar]
- Vriens J., Appendino G., Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Molecular Phamacology. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- Wang T., Zhao M., Rotgans B.A., Strong A., Liang D., Ni G., Limpanont Y., Ramasoota P., McManus D.P., Cummins S.F. Proteomic analysis of the Schistosoma mansoni miracidium. PLoS One. 2016;11:e0147247. doi: 10.1371/journal.pone.0147247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang L., Liang Y.S. Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol. Res. 2012;111:1871–1877. doi: 10.1007/s00436-012-3151-z. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A. Ion channels and receptor as targets for the control of parasitic nematodes. Int. J. Parasitol.: Drugs and Drug Resist. 2011;1:2–13. doi: 10.1016/j.ijpddr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A.J., Williamson S.M., Reaves B.J. TRP channels in parasites. Adv. Exp. Med. Biol. 2011;704:358–371. doi: 10.1007/978-94-007-0265-3_20. [DOI] [PubMed] [Google Scholar]
- Wu L.J., Sweet T.B., Clapham D.E. International Union of Basic and Clinical Pharmacology LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R., Xu X.Z. C. elegans TRP channels. Adv. Exp. Med. Biol. 2011;704:323–339. doi: 10.1007/978-94-007-0265-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z., Xie J., Yu A.S., Stock J., Du J., Yue L. Role of TRP channels in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2014;308:H157–H182. doi: 10.1152/ajpheart.00457.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. Molecular mechanism of TRP channels. Comprehensive Physiology. 2013;3:221–242. doi: 10.1002/cphy.c120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Luo Z., Ma S., Liu D. TRP channels and their implications in metabolic diseases. Pflügers Archiv. 2011;46:211–223. doi: 10.1007/s00424-010-0902-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.