Abstract
Background
Secreted frizzled-related protein 5 (SFRP5) plays a key role in the development and progression of multiple tumors. However, the role and underlying mechanisms of SFRP5 in melanoma cells remain unknown.
Materials and methods
We used immunohistochemistry and Western blot analysis to detect the expression of SFRP5 in melanoma tissues and melanoma cells, respectively. Furthermore, both in vitro and in vivo assays were used to determine the effect of SFRP5 on malignant behavior in melanoma cells.
Results
We found that SFRP5 was markedly downregulated in melanoma tissues and cell lines. The SFRP5 overexpression exhibited no effect on the proliferation and apoptosis of melanoma cells but markedly suppressed the migration and invasion of melanoma cells in vitro. Regarding mechanisms, the SFRP5 overexpression inhibited the migration and invasion of melanoma cells by suppressing the epithelial–mesenchymal transition process and decreasing the matrix metalloproteinase-2/9 expression through the Wnt signaling pathway. Finally, in a xenograft animal model, we illustrated that the SFRP5 overexpression suppressed the tumor growth by decreasing angiogenesis and declined lung metastasis.
Conclusion
This study suggests that SFRP5 expression could be potentially useful in the invasion and metastasis of melanoma and serve as a putative promising target for human melanoma therapy.
Keywords: SFRP5, Wnt signaling, melanoma, migration, invasion
Introduction
Melanoma is the most severe form of skin cancer that is highly invasive and metastatic.1 It is the fifth and sixth leading cancer among males and females, respectively.2 In 2017, over 94,000 individuals were diagnosed with melanoma in the US.3 Despite remarkable advancements in the treatment options, the prognosis of patients with malignant melanoma remains poor, thereby necessitating the investigation of additional therapeutic targets for managing this disease.
Wnt signaling is vital for the development of melanocytes in the neural crest and is associated with the transformed phenotype of melanoma cells.4 Wnt signaling comprises canonical and noncanonical pathways. In the canonical pathway, signal transduction activates disheveled, which releases the adenomatous polyposis coli-glycogen synthase kinase 3 (GSK3) complex, resulting in the stabilization and accumulation of β-catenin in the cytoplasm.5 Although it is well established that β-catenin is critical in the early stages of the melanocyte transformation and melanomagenesis,6 contradictory studies have been published on the role of β-catenin in melanoma metastasis.7 The noncanonical signaling pathway, however, does not depend on β-catenin. Prior research has illustrated two noncanonical pathways, including the Wnt/Ca2+ pathway and the Wnt polarity (PCP) pathway.8 In the Wnt/Ca2+ pathway, the protein kinase C (PKC) is activated by calcium, and the PCP pathway is relying on the JNK activation.9 Reportedly, the noncanonical signaling pathway correlates with the increased metastatic potential of melanoma cells and tumor grade.10,11
Secreted frizzled-related proteins (SFRPs), an evolutionary conserved family of secreted proteins, have been recognized as extracellular regulators of the Wnt signaling pathway.12 To date, five human SFRPs have been identified, which, based on the sequence homology, are categorized into two subfamilies as follows: (a) SFRP1, 2, and 5 and (b) SFRP3 and 4.13 Reportedly, SFRP1 and SFRP2 are tumor suppressors or indicators of the poor prognosis in various human malignancies, including breast cancer, gastric cancer, colorectal cancer, hepatocellular carcinoma, and squamous cell carcinoma.14–17 Likewise, SFRP5, as one of the members of the SFRP family, has been reported to play a potential role in cancer. For example, in human breast cancer, the epigenetic inactivation of the SFRP5 gene correlates with the unfavorable prognosis.18 In addition, in human gastric cancer, SFRP5 inhibits the gastric epithelial cell migration induced by macrophage-derived Wnt5a.19 However, the biological function and underlying mechanism of SFRP5 in melanoma remain unclear.
Our early research has revealed that the SFRP5 expression was reduced in melanoma tissues. We hypothesized that SFRP5 plays a vital role in the melanoma progression. Hence, this study aims to investigate the effect of SFRP5 overexpression on the proliferation, apoptosis, migration, and invasion of human melanoma cells and to identify the underlying mechanism in vitro and in vivo.
Materials and methods
Human tissue samples
Twenty normal skin and twenty-five melanoma tissues were tested from the First Affiliated Hospital of Chongqing Medical University (Chongqing, China). None of the patients had received radiotherapy or chemotherapy before surgery. Written informed consent was obtained from each patient at the time of surgery. All collections were approved by the Clinical Ethics Committee of the hospital and were subject to the Declaration of Helsinki.
Immunohistochemistry
Melanoma tissues, normal skin tissues, and nude mouse xenograft melanoma tumors were embedded with paraffin and sectioned to 4 µm. Then, the sections were incubated with primary antibody at 4°C overnight after the antigen retrieval. Then, all sections were subjected to immunohistochemical (IHC) analysis using immunohistochemistry SP-9000 Kit (Beijing Zhongshan Golden Bridge Biotechnology, Co., Ltd., Beijing, China).
Cell culture
The melanoma cell lines Mel-888, Mel-624, A375, B16 were kindly donated by Dr Tongchuan He (University of Chicago Medical Center, Chicago, IL, USA). The human melanoma GLL-19 cells were kindly donated by Dr Le Qu (China Medical University, China). The human epidermal melanocytes PIG1 were kindly donated by Dr Chunying Li (Xijing Hospital, Xian, China). The use of all cell lines was approved by the Clinical Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. The melanoma cell lines were cultured in the Dulbecco’s Modified Eagle’s Medium (DMEM; HyClone Laboratories, Inc., Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA), 1% penicillin, and 1% streptomycin. PIG1 cells were cultured in Medium 254 (Cascade Biologics®; Thermo Fisher Scientific) supplemented with human melanocyte growth supplement (Cascade Biologics/Thermo Fisher Scientific), 5% FBS (Thermo Fisher Scientific), 1% penicillin, and 1% streptomycin. All cells were cultured at 37°C in a 5% CO2 incubator.
Adenoviral transfections
Recombinant adenoviruses SFRP5 (AdSFRP5) and recombinant adenoviruses red fluorescent protein (AdRFP, as control) were kindly donated by Dr Tongchuan He. AdSFRP5 or AdRFP was transfected into the A375 and GLL-19 cells with polybrene (Sigma-Aldrich Co., St Louis, MO, USA). After 8 hours of cultivation, the medium was replaced with a fresh medium. Then, the fluorescence was observed after 36 hours.
Western blot analysis
The protein was extracted from cells after 3-day culture and lysed in radio immunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Shanghai, China). After being separated by 10% SDS-polyacrylamide gel electrophoresis and membrane transfer, the polyvinylidene fluoride membranes were blocked with 5% bovine serum albumin (Beijing Solarbio Science and Technology, Co., Ltd., Beijing, China) in TBST at 37°C for 2 hours. Then, the membranes were incubated with primary antibodies against SFRP5 (1:1,000; GeneTex, Irvine, CA, USA), E-cadherin (1:5,000; Abcam, Cambridge, MA, USA), N-cadherin (1:1,000; Abcam), matrix metalloproteinase-2 (MMP-2) (1:1,000; Abcam), MMP-9 (1:1,000; Cell Signaling Technology, Danvers, MA, USA), vimentin (1:1,000; Cell Signaling Technology), β-catenin (1:1,000; Cell Signaling Technology), GSK3β (1:1,000; Cell Signaling Technology), p-GSK3β (1:1,000; Cell Signaling Technology), LRP6 (1:1,000; Cell Signaling Technology), c-Myc (1:1,000; Cell Signaling Technology), cyclin D1 (1:1,000; Cell Signaling Technology), JNK (1:1,000; Cell Signaling Technology), p-PKC-α/βII (1:1,000; Cell Signaling Technology), VEGFA (1:1,000; Cell Signaling Technology), VEGFR2 (1:1,000; Cell Signaling Technology), and β-actin (1:1,000; Cell Signaling Technology). Next, the membranes were washed with TBST three times and incubated with an appropriate secondary antibody (1:5,000; Beijing Zhongshan Golden Bridge Biotechnology, Co., Ltd.) for 1 hour at 37°C. Finally, the protein bands were visualized with SuperSignal West Pico Chemiluminescent Substrate Kit (EMD Millipore, Billerica, MA, USA).
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Cells were treated with AdSFRP5 in FBS-free DMEM for 36 hours. The total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific) in accordance with the RNA extraction protocol. Then, the total RNA (1.5 µg) was used for cDNA synthesis by reverse transcriptase polymerase chain reaction (PCR). The cDNA was amplified by a real-time PCR (qPCR) system (Bio-Rad Laboratories Inc., Hercules, CA, USA) using SYBR-Green PCR Master Mix. β-actin was used as the endogenous control. The primers were designed and synthesized by Takara (Table 1).
Table 1.
Primers sequences
| Targets | Sequence |
|---|---|
|
| |
| β-Actin | |
| Forward | 5′-CACCACACCTTCTACAATGAGC-3′ |
| Reverse | 5′-GTGATCTCCTTCTGCATCCTGT-3′ |
| SFRP5 | |
| Forward | 5′-TGCCCTTGCCCACAGTTAGA-3′ |
| Reverse | 5′-GAGGGAACAGGGATAGGAGAACA-3′ |
| E-cadherin | |
| Forward | 5′-TGAGGAGATCGGCACATTCAT-3′ |
| Reverse | 5′-ATAGTCTTGGTCGTTTCCTGAGC-3′ |
| N-cadherin | |
| Forward | 5′-CCATCAAGCCTGTGGGAATC-3′ |
| Reverse | 5′-GCCGCTTTAAGGCCCTCAT-3′ |
| Vimentin | |
| Forward | 5′-TCTGGATTCACTCCCTCTGGTT-3′ |
| Reverse | 5′-ATCGTGATGCTGAGAAGTTTCGT-3′ |
| MMP-9 | |
| Forward | 5′-CCCTTGTGCTCTTCCCTGGA-3′ |
| Reverse | 5′-TCTGCCACCCGAGTGTAACC-3′ |
| MMP-2 | |
| Forward | 5′-ACATCAAGGGCATTCAGGAGC-3′ |
| Reverse | 5′-CACAGTCCGCCAAATGAACC-3′ |
| Snail | |
| Forward | 5′-TCCAGCAGCCCTACGACCAG-3′ |
| Reverse | 5′-AGGCCGAGGTGGACGAGAA-3′ |
| ZEB1 | |
| Forward | 5′-AGCAGTGAAAGAGAAGGGAATGC-3′ |
| Reverse | 5′-GGTCCTCCTCAGGTGCCTCAG-3′ |
| VEGFA | |
| Forward | 5′-GAGGGCAGAATCATCACGAAGT-3′ |
| Reverse | 5′-GCACACAGGATGGCTTGAAGA-3′ |
| VEGFR2 | |
| Forward | 5′-ATTGTATTGAAGGATGGGAACCG-3′ |
| Reverse | 5′-AATCACCGCCGTGCCTACTAG-3′ |
| bFGF | |
| Forward | 5′-GGAGAAGAGCGACCCTCACAT-3′ |
| Reverse | 5′-TTCCTTCATAGCCAGGTAACGG-3′ |
| PDGFA | |
| Forward | 5′-ACCAGGACGGTCATTTACGAGA-3′ |
| Reverse | 5′-GAGGGCTGGCACTTGACACTG-3′ |
Abbreviations: bFGF, basic fibroblast growth factor; MMP-2, matrix metalloproteinase-2; MMP-9, matrix metallo proteinase-9; PDGFA, platelet-derived growth factor alpha; SFRP5, secreted frizzled-related protein 5; VEGFA, vascular endothelial growth factor A; VEGFR2, vascular endothelial growth factor receptor 2; ZEB1, zincfinger ebox binding homeobox 1.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cell viability was detected by MTT assay. Then, 3,000 cells were seeded into each well of 96-well plates in quintuplicate and cultured for 1, 2, 3, and 4 days. At the indicated time, 10 µL of MTT (5 mg/mL; Sigma-Aldrich Co.) was added into each well and then incubated for 4 hours at 37°C. Next, 150 µL of dimethyl sulfoxide was added to the 96-well plates to dissolve the formazan product. Finally, the absorbance was measured at 492 nm with the use of a microplate reader.
Colony-forming test
Log-phase cells were collected and 1,000 cells were plated into each well of a six-well plate. Then, the cells were cultured in 5% FBS medium after transfection. When clones were observed, the cells were fixed with 4% paraformaldehyde, washed two times with PBS, and stained with 0.1% crystal violet. The visible colonies were counted.
Cellular apoptosis analysis
Cells were harvested and washed three times with cold PBS and resuspended in the binding buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, and 2.5 mM CaCl2) at a concentration of 1×106 cells/mL. Then, we stained cells with Annexin V-FITC and propidium iodide for 15 minutes in the dark before flow cytometry (Becton-Dickinson, San Jose, CA, USA).
Wound-healing test
The cells were seeded into a six-well plate and it grew by 90% after transfection. A wound was made with a 200-µL pipette tip and the cells were incubated for 24 hours. The specific wound area was photographed at time points of 0 and 24 hours. The migration of cells was evaluated as the migration rate: (original scratch width − new scratch width)/original scratch width × 100%.
Migration and invasion assays
Transwell chambers (24-well Transwell chambers, 8-µm pore size; Corning Incorporated, Corning, NY, USA) were utilized for migration and invasion assays. For the migration assay, 2×104/400 µL cells in serum-free media were seeded into the upper chambers after 48-hour infection. The lower chamber contained medium with 20% FBS. The cells were cultured for 24 hours and fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The Transwell invasion assay course was similar to the migration assay except that the Transwell membrane was coated with 1:5 diluted Matrigel beforehand. Each experiment was repeated three times.
Xenograft mouse experiment
The in vivo experiments were approved by the Animal Experiment Administration Committee of Chongqing Medical University Laboratory Animal Research. Briefly, approximately 5×106 AdRFP- or AdSFRP5-infected A375 cells were suspended in 100 µL of serum-free media and injected subcutaneously into nude mice. Tumor dimensions were recorded every week with vernier calipers, and the volumes were calculated using the following formula: length × width2 × 0.5. The mice were sacrificed by cervical vertebra dislocation after 4 weeks, and tumor tissues and lung tissues were collected, and embedded in paraffin for IHC and hematoxylin and eosin (H&E) analysis, respectively. All procedures related to animal handling, care, and treatment were performed in strict accordance with the recommendations of regulations on the management of experimental animals. Additionally, the protocol was approved by the Animal Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Statistical analysis
Data were presented as mean ± SD and GraphPad Prism (Prism 5.0; GraphPad Software, Inc., La Jolla, CA, USA) was used to analyze all values. The Student’s t-test was used to evaluate the differences between the two groups. P<0.05 was considered statistically significant.
Results
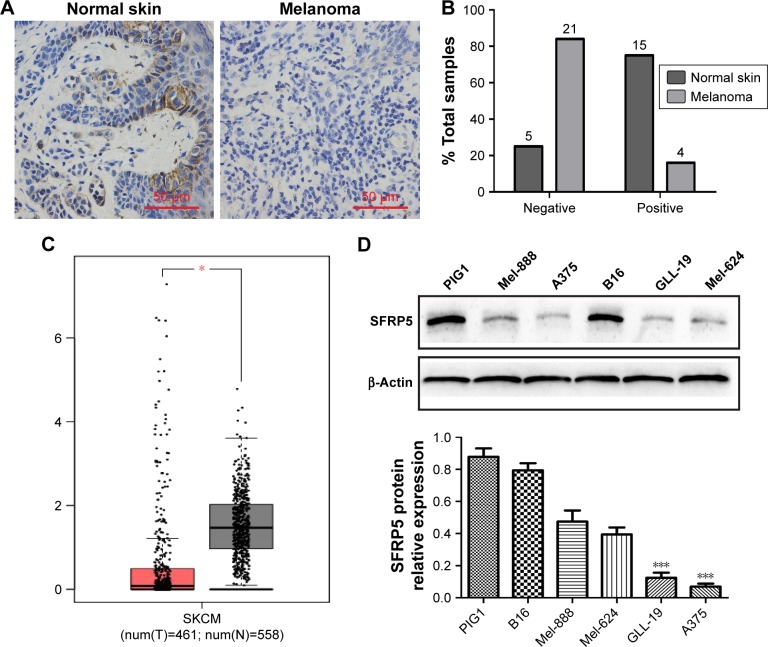
SFRP5 is downregulated in human melanoma tissues and melanoma cells
We detected the endogenous SFRP5 expression in human normal skin and human melanoma tissues by IHC to investigate the role of SFRP5 in human melanoma. The findings revealed that the protein expression of SFRP5 was decreased in melanoma tissues compared with the normal skin (Figure 1A). Samples were scored on the basis of immunoreactivity scores, negative (1–4) and positive (5–12).20 The positive SFRP5 expression rates were 75% (15/20) in the normal skin and 16% (4/25) in melanoma (Figure 1B). In addition, the mRNA level of SFRP5 was markedly down-regulated in melanoma clinical samples compared with that in the normal tissues, which was analyzed by the Gene Expression Profiling Interactive Analysis (GEPIA) online database (http://gepia.cancer-pku.cn/), a web server providing customizable functions (Figure 1C).21 The samples of the GEPIA were derived from the Cancer Genome Atlas (TCGA) and the Genotype-Tissue Expression (GTEx) projects. For further validating the role of SFRP5 in melanoma, we detected SFRP5 expression in human epidermal melanocytes PIG1 and five melanoma cell lines (Mel-888, A375, B16, GLL-19, and Mel-624) by Western blot analysis. PIG1 exhibited higher SFRP5 expression; however, melanoma cell lines exhibited lower expression, especially A375 and GLL-19 cells (Figure 1D). Hence, we used A375 and GLL-19 cells as models to assess the biological effects of SFRP5 on melanoma.
Figure 1.
SFRP5 expression is downregulated in melanoma tissues and melanoma cell lines.
Notes: (A) Representative immunohistochemical staining of SFRP5 in normal skin (n=20) and melanoma tissue (n=25) paraffin sections. (B) The percentage of SFRP5-negative and -positive staining scores in normal skin and melanoma tissues. (C) SFRP5 expression in normal skin (n=558) and skin cutaneous melanoma (n=461) in the GEPIA database. *P<0.05. (D) SFRP5 expression in human epidermal melanocytes PIG1 cell line and melanoma cell lines was measured by Western blot analysis. Data are shown as mean ± SD. ***P<0.001.
Abbreviations: GEPIA, Gene Expression Profiling Interactive Analysis; SFRP5, secreted frizzled-related protein 5.
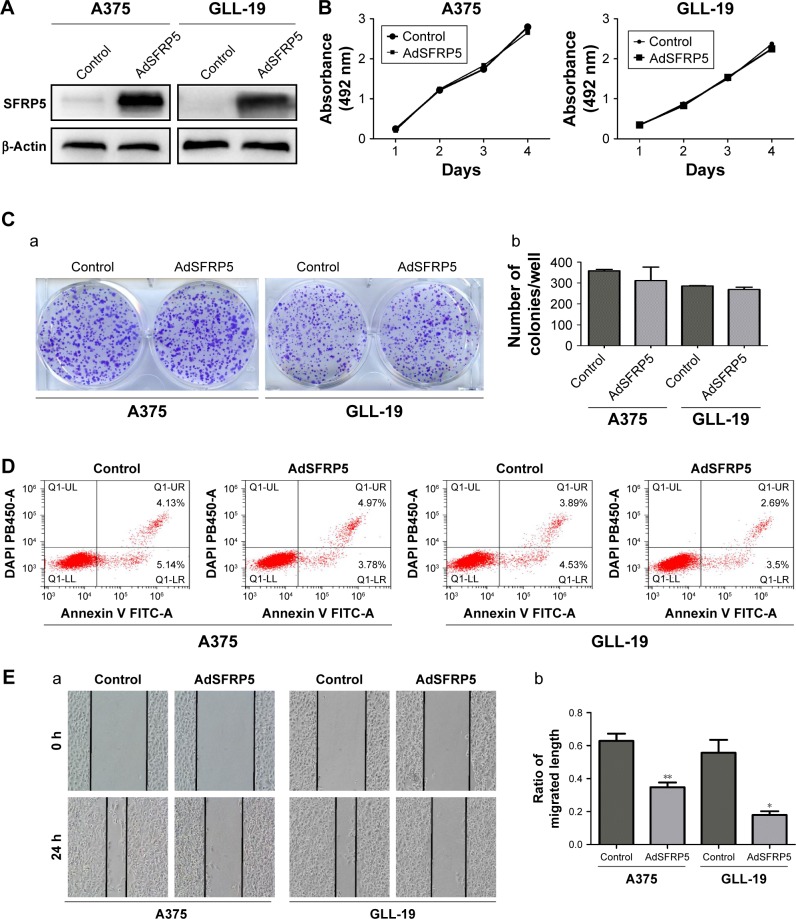
SFRP5 overexpression does not alter cell proliferation or survival but inhibits the invasion and migration of melanoma cells
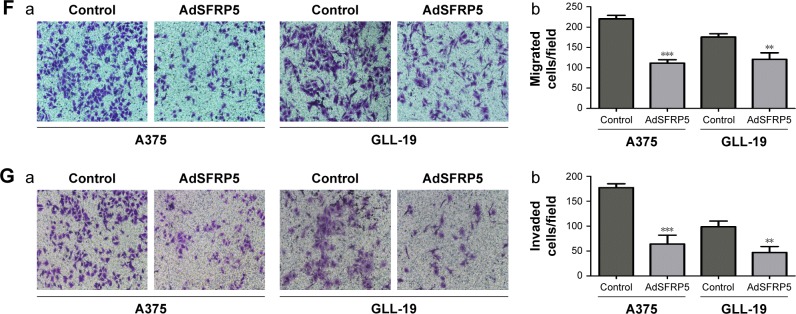
We transfected A375 and GLL-19 cells with AdSFRP5 and AdRFP (as control). The Western blot analysis revealed that AdSFRP5 was successfully transfected into A375 and GLL-19 cells (Figure 2A). The MTT assay revealed that the cell proliferation was not markedly different between AdSFRP5-infected and control cells (Figure 2B). In addition, the colony formation after 8 days in culture was not altered by SFRP5 overexpression (Figure 2C). Moreover, flow cytometry revealed that apoptosis was not altered by SFRP5 overexpression (Figure 2D). Subsequently, we measured cell migration by the wound-healing test and the Transwell migration assay. Cell invasion was tested by the Transwell invasion assay. Notably, AdSFRP5-infected cells displayed a statistically significant lower rate of migration and invasion than controls for both A375 and GLL-19 cells (Figure 2E and G).
Figure 2.
SFRP5 overexpression does not alter cell proliferation or survival but inhibits the invasion and migration of melanoma cells.
Notes: (A) Western blot analysis of the expression levels of SFRP5 in AdSFRP5-infected A375 and GLL-19 cells. (B) Cell viability was measured by MTT assay in A375 and GLL-19 cells. (C) Images of colony formation (a) and the colony formation rates (b) are exhibited. (D) Apoptosis of A375 and GLL-19 cells was tested by flow cytometry. (E) The representative images (a, magnifcation ×200) and percentages of cell migration (b). (F) The representative images (a, magnifcation ×200) and number of migrated cells (b). (G) The representative images (a, magnifcation ×200) and number of invaded cells (b). Data are shown as mean ± SD. *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: AdSFRP5, recombinant adenoviruses secreted frizzled-related protein 5; MTT, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide); SFRP5, secreted frizzled-related protein 5.
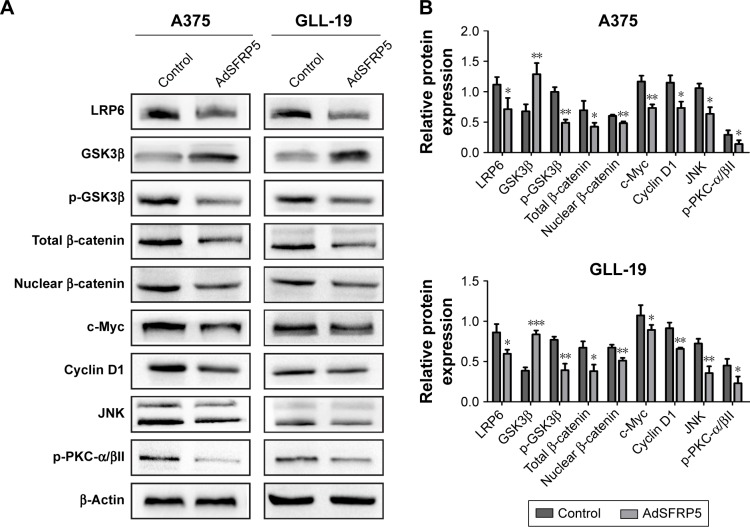
Tumor-suppressing effects of SFRP5 overexpression through both canonical and noncanonical Wnt signaling
We measured Wnt-related proteins by Western blot analysis to detect the regulation of SFRP5 on the Wnt signaling pathway in melanoma cells. In the canonical Wnt signaling pathway, SFRP5 overexpression substantially decreased the expression of LRP6, p-GSK3β, total β-catenin, nuclear β-catenin, c-Myc, and cyclin D1, whereas it increased the GSK3β expression in A375 and GLL-19 cells compared with control groups (Figure 3A and B). Then, we investigated the effect of SFRP5 on the noncanonical Wnt signaling pathway. In addition, the JNK levels were downregulated in the PCP pathway, and the p-PKC-α/βII expression was decreased in the Wnt/Ca2+ pathway (Figure 3A and B). The findings indicated that the SFRP5 function could be a negative regulator of the Wnt signaling pathway in melanoma cells.
Figure 3.
The expression levels of Wnt-related proteins.
Notes: (A) Western blot analysis of the protein expression levels of LRP6, GSK3β, p-GSK3β, total β-catenin, nuclear β-catenin, c-Myc, and cyclin D1, JNK, and p-PKC-α/βII. (B) Relative expression of each protein to β-actin in A375 and GLL-19 cells. Compared with the control group, LRP6, p-GSK3β, total β-catenin, nuclear β-catenin, c-Myc, and cyclin D1, JNK, and p-PKC-α/βII were downregulated, whereas GSK3β was upregulated. Data are shown as mean ± SD. *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: AdSFRP5, recombinant adenoviruses secreted frizzled-related protein 5; GSK3β, glycogen synthase kinase 3 beta; JNK, c-Jun N-terminal kinase; LRP6, low-density lipoprotein receptor-related protein 6; p-PKC/α/βII, phosphor-protein kinase C-alpha/beta II.
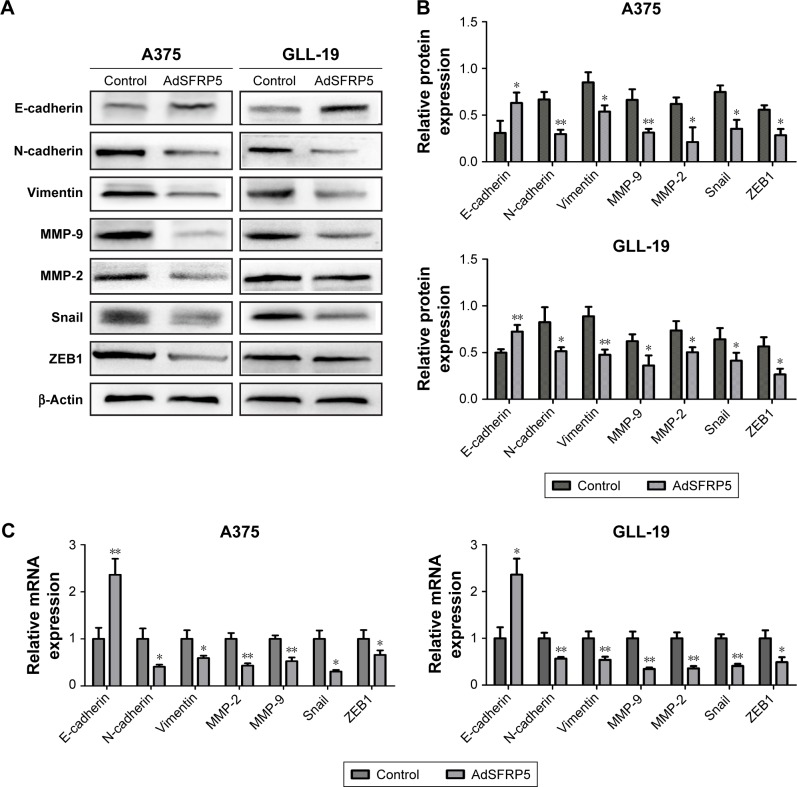
SFRP5 overexpression inhibits epithelial– mesenchymal transition (EMT) and reduces the expression levels of MMP-2/9
The EMT is a vital step in the early stage of metastasis. Therefore, we detected EMT markers and related transcription factors by qRT-PCR and Western blot analysis to determine whether SFRP5 overexpression inhibited the invasion and migration of A375 and GLL-19 cells through the EMT. The findings revealed that the protein expression of E-cadherin was markedly increased, whereas that of N-cadherin, vimentin, Snail, and ZEB1 were remarkably decreased (Figure 4A and B). In addition, the RNA expression exhibited the same results (Figure 4C). Furthermore, we determined the invasion-related indicators MMP-2 and MMP-9 post-transfection. The SFRP5 overexpression markedly decreased the MMP-2 and MMP-9 expression at both protein levels and mRNA (Figure 4A and C).
Figure 4.
The expression of EMT markers and MMP-2/9 in A375 and GLL-19 cells.
Notes: (A) Western blot analysis of protein expression levels of E-cadherin, N-cadherin, vimentin, MMP-9, MMP-2, Snail, and ZEB1. (B) Relative expression of each protein to β-actin in A375 and GLL-19 cells. (C) qRT-PCR analysis of the mRNA expression levels of E-cadherin, N-cadherin, vimentin, MMP-9, MMP-2, Snail, and ZEB1. Data are shown as mean ± SD. *P<0.05, **P<0.01.
Abbreviations: AdSFRP5, recombinant adenoviruses secreted frizzled-related protein 5; EMT, epithelial–mesenchymal transition; MMP-9, matrix metalloproteinase-9; MMP-2, matrix metalloproteinase-2; qRT-PCR, quantitative real-time reverse transcription polymerase chain reaction; ZEB1, zinc finger ebox binding homeobox 1.
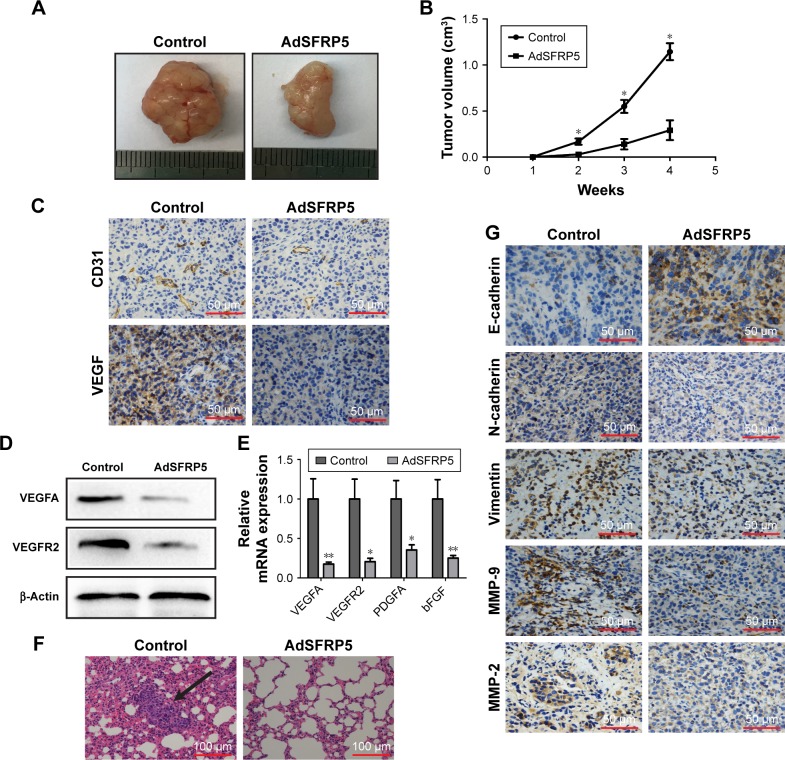
SFRP5 suppressed tumor growth and lung metastases of A375 cells in vivo
We established a xenograft model in nude mice to investigate the effect of SFRP5 on A375 cells in vivo. The tumor volume was measured once a week. Surprisingly, the tumor volume was markedly decreased in the AdSFRP5 group compared with that in the control group (Figure 5A and B). Considering angiogenesis is essential for tumor growth, we assessed angiogenesis-related markers. Immunohistochemistry showed that the CD31 and VEGF expression were decreased in the AdSFRP5 group (Figure 5C). In addition, the Western blot analysis revealed that the VEGFA and VEGFR2 expressions in the AdSFRP5 group were less than that in the control group (Figure 5D). Furthermore, the mRNA expression levels of VEGF, VEGFR2, PDGFA, and basic fibroblast growth factor were declined in the AdSFRP5 group (Figure 5E). The data revealed that SFRP5 might inhibit tumor growth by decreasing angiogenesis. Our previously mentioned results revealed that SFRP5 overexpression inhibited the invasion and migration of melanoma cells in vitro. To validate these results in vivo, we next collected the lung tissues for H&E staining. The findings revealed that the AdSFRP5 group exhibited fewer compact tumor cell islands in the alveoli tissues compared with the control group (Figure 5F). Corroborating prior observations, immunohistochemistry showed that the staining of vimentin, N-cadherin, and MMP-2/9 was decreased, whereas that of E-cadherin was enhanced (Figure 5G).
Figure 5.
SFRP5 overexpression suppressed tumor growth and lung metastases of A375 cells in vivo.
Notes: (A) Representative images of primary implanted tumors and tumor mass sacrificed at 4 weeks. (B) Tumor growth curve. (C) Immunohistochemical staining of CD31 and VEGF. (D) Western blot analysis of the expression levels of VEGFA and VEGFR2. (E) qRT-PCR analysis of the expression levels of VEGFA, VEGFR2, PDGFA, and bFGF. (F) Representative hematoxylin and eosin images of lungs. Black arrow indicates metastatic lung nodule. (G) Immunohistochemical staining of E-cadherin, N-cadherin, vimentin, MMP-9, and MMP-2. Data are shown as mean ± SD. *P<0.05, **P<0.01.
Abbreviations: AdSFRP5, recombinant adenoviruses secreted frizzled-related protein 5; bFGF, basic fibroblast growth factor; MMP, matrix metalloproteinase; PDGFA, platelet-derived growth factor alpha; qRT-PCR, quantitative real-time reverse transcription polymerase chain reaction; VEGF, vascular endothelial growth factor; VEGFA, vascular endothelial growth factor A; VEGFR2, vascular endothelial growth factor receptor 2.
Discussion
Globally, the incidence and mortality rates of melanoma are escalating,22 accounting for a majority of skin cancer-related deaths.23 Wnt signaling plays a vital role in melanoma initiation and invasion.24 Lately, SFRPs, regulators of Wnt signaling, have garnered considerable attention for their role in tumors. As one of the SFRP family members, SFRP5 has been reported as a suppressor gene in various human cancer types,18,19 indicating that it might be a potential new approach to anticancer treatment.
This study first determined SFRP5 expression by immunohistochemistry in normal skin and melanoma tissues, indicating that SFRP5 was markedly decreased in melanoma tissues. Consistently, the data derived from TCGA and the GTEx projects by the online GEPIA database validated that SFRP5 expression was downregulated in melanoma tissues and might play a crucial role in the melanoma progression. Hence, we enforced SFRP5 expression by recombinant adenovirus and, then, explicitly focused on the direct biological effects of SFRP5 on melanoma cells. The results showed that overexpression of SFRP5 did not alter the proliferation and apoptosis of A375 and GLL-19 cells. However, the wound-healing, Transwell migration, and Matrigel invasion assays revealed that the migration and invasion of A375 and GLL-19 cells were markedly suppressed by SFRP5 overexpression.
Then, we examined the protein expression levels of the Wnt signaling pathway by Western blot analysis in A375 and GLL-19 cells to investigate the anti-invasion and anti-migratory mechanisms of SFRP5. A prior study reported that SFRP5 antagonized both canonical and noncanonical Wnt signaling in the foregut epithelium.25 Corroborating this study, we determined that SFRP5 overexpression downregulated the protein expression of key factors within the Wnt/β-catenin, Wnt/Ca2+, and Wnt PCP pathways, indicating that SFRP5 inhibited canonical Wnt signaling, as well as noncanonical Wnt signaling in melanoma cells. Nevertheless, which Wnt signaling is primarily regulated by SFRP5 and the specific correlation between SFRP5 and Wnt signaling in melanoma warrant further investigation in future studies.
In carcinogenesis, the EMT process transforms melanoma in situ to invasive, motile melanoma.26 The loss of E-cadherin is a critical step in the EMT. Along with this, the concurrent increased expression of mesenchymal proteins promotes invasive character, including the increased expression of N-cadherin, vimentin, fibronectin, and MMPs.27 Reportedly, the Wnt pathway plays a vital role in regulating the tumor cell EMT behavior.28 There is increasing research that Wnt/β-catenin contributes to invasion, EMT, and metastatic phenotypes of melanoma.29,30 In addition, the upregulation of noncanonical Wnt ligand and activation of noncanonical Wnt components, including PKC and JNK, have also been observed during EMT.31–33 Aberrant activation of the Wnt/PCP signaling pathway in human cancer, including melanoma, leads to more malignant phenotypes, such as abnormal tissue polarity, invasion, and metastasis.34 This study established that SFRP5 overexpression induced the downregulation of N-cadherin, vimentin, Snail, ZEB1, and MMP-2/9 but upregulated E-cadherin. These findings suggested that SFRP5 might suppress the EMT processes by attenuating the Wnt signaling pathway, resulting in the inhibition of invasion and migration in A375 and GLL-19 cells.
Finally, this study assessed the efficacy of the SFRP5 overexpression on A375 cells in vivo. Remarkably, the subcutaneously transplanted tumor test revealed that SFRP5 markedly inhibited tumor growth. Considering SFRP5 over-expression did not alter cell proliferation in vitro, we decided to investigate how SFRP5 affects tumor growth in vivo. As it is known, angiogenesis is a key step in tumor growth and metastasis.35 The loss or gain of function of Wnt pathway components results in abnormal vascular development and angiogenesis.36 SFRP2, another modulator of Wnt signaling, has been reported to stimulate the angiogenesis in breast tumors.37 A study reported that DKK1 and DKK2, known as Wnt antagonists, affect B16F10 melanoma growth by modulating tumor angiogenesis with no direct action on tumor cell proliferation.38 This study determined that angiogenic molecules were decreased by SFRP5, indicating that SFRP5 suppressed angiogenesis in vivo. Furthermore, the number of lung metastases decreased in the SFRP5-treated group, and the immunohistochemistry of EMT markers of the xenograft tumor corroborated the previous experiments.
In recent years, tumor immunotherapy has drawn a great attention because of its success in cancer treatment.39 As a strategy among numerous immunotherapies, the delivery of immune checkpoint inhibitors holds great promise in melanoma treatment.40 Despite remarkable progress, not all melanoma patients benefit from immune checkpoint blockade therapy, which is partially due to the activation of the Wnt/β-catenin signaling pathway.41 Aberrant Wnt signaling may subvert cancer immunosurveillance and thereby promote immunoevasion and resistance to immune checkpoint blockade therapy.42 Therefore, a combination of SFRP5 and immunotherapy may provide a method to improve the treatment of melanoma patients. However, more experimental models and further validation in patient samples are required to translate them to clinical application.
Conclusion
This study illustrates that SFRP5 can inhibit the migration and invasion of melanoma cells by suppressing the EMT process and by decreasing the expression of MMP-2/9 through the Wnt signaling pathway in vitro and exhibits antiangiogenesis and antimetastatic activity in vivo, suggesting that SFRP5 could be an effective strategy for melanoma therapy.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (81773307).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Uong A, Zon LI. Melanocytes in development and cancer. J Cell Physiol. 2010;222(1):38–41. doi: 10.1002/jcp.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Xue G, Romano E, Massi D, Mandalà M. Wnt/β-catenin signaling in melanoma: preclinical rationale and novel therapeutic insights. Cancer Treat Rev. 2016;49:1–12. doi: 10.1016/j.ctrv.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 6.Larue L, Beermann F. Cutaneous melanoma in genetically modified animals. Pigment Cell Res. 2007;20(6):485–497. doi: 10.1111/j.1600-0749.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 7.Webster MR, Kugel CH, 3rd, Weeraratna AT. The Wnts of change: how Wnts regulate phenotype switching in melanoma. Biochim Biophys Acta. 2015;1856(2):244–251. doi: 10.1016/j.bbcan.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281(32):22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 9.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5(3):367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 10.Da Forno PD, Pringle JH, Hutchinson P, et al. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14(18):5825–5832. doi: 10.1158/1078-0432.CCR-07-5104. [DOI] [PubMed] [Google Scholar]
- 11.Bittner M, Meltzer P, Chen Y, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406(6795):536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 12.Surana R, Sikka S, Cai W, et al. Secreted frizzled related proteins: implications in cancers. Biochim Biophys Acta. 2014;1845(1):53–65. doi: 10.1016/j.bbcan.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Yan J, Jia H, Ma Z, et al. The evolutionary analysis reveals domain fusion of proteins with Frizzled-like CRD domain. Gene. 2014;533(1):229–239. doi: 10.1016/j.gene.2013.09.083. [DOI] [PubMed] [Google Scholar]
- 14.Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 15.Qu Y, Ray PS, Li J, et al. High levels of secreted frizzled-related protein 1 correlate with poor prognosis and promote tumourigenesis in gastric cancer. Eur J Cancer. 2013;49(17):3718–3728. doi: 10.1016/j.ejca.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Li R, He Y, Huang S. Effects of secreted frizzled-related protein 1 on proliferation, migration, invasion, and apoptosis of colorectal cancer cells. Cancer Cell Int. 2018;18:48. doi: 10.1186/s12935-018-0543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paluszczak J, Hemmerling D, Kostrzewska-Poczekaj M, et al. Frequent hypermethylation of WNT pathway genes in laryngeal squamous cell carcinomas. J Oral Pathol Med. 2014;43(9):652–657. doi: 10.1111/jop.12178. [DOI] [PubMed] [Google Scholar]
- 18.Veeck J, Geisler C, Noetzel E, et al. Epigenetic inactivation of the secreted frizzled-related protein-5 (SFRP5) gene in human breast cancer is associated with unfavorable prognosis. Carcinogenesis. 2008;29(5):991–998. doi: 10.1093/carcin/bgn076. [DOI] [PubMed] [Google Scholar]
- 19.Zhao C, Ma H, Bu X, Wang W, Zhang N. SFRP5 inhibits gastric epithelial cell migration induced by macrophage-derived Wnt5a. Carcinogenesis. 2013;34(1):146–152. doi: 10.1093/carcin/bgs309. [DOI] [PubMed] [Google Scholar]
- 20.Sun R, Jiang B, Qi H, et al. SOX4 contributes to the progression of cervical cancer and the resistance to the chemotherapeutic drug through ABCG2. Cell Death Dis. 2015;6:e1990. doi: 10.1038/cddis.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B. The Role of B-RAF Mutations in Melanoma and the Induction of EMT via Dysregulation of the NF-κB/Snail/RKIP/PTEN Circuit. Genes Cancer. 2010;1(5):409–420. doi: 10.1177/1947601910373795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potrony M, Badenas C, Aguilera P, et al. Update in genetic susceptibility in melanoma. Ann Transl Med. 2015;3(15):210. doi: 10.3978/j.issn.2305-5839.2015.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur A, Webster MR, Weeraratna AT. In the Wnt-er of life: Wnt signalling in melanoma and ageing. Br J Cancer. 2016;115(11):1273–1279. doi: 10.1038/bjc.2016.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22(21):3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 27.Pearlman RL, Montes de Oca MK, Pal HC, Afaq F. Potential therapeutic targets of epithelial-mesenchymal transition in melanoma. Cancer Lett. 2017;391:125–140. doi: 10.1016/j.canlet.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao JH, Luo Y, Jiang YG, He DL, Wu CT. Knockdown of β-Catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF-1α. Cancer Invest. 2011;29(6):377–382. doi: 10.3109/07357907.2010.512595. [DOI] [PubMed] [Google Scholar]
- 29.Sinnberg T, Levesque MP, Krochmann J, et al. Wnt-signaling enhances neural crest migration of melanoma cells and induces an invasive phenotype. Mol Cancer. 2018;17(1):59. doi: 10.1186/s12943-018-0773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster MR, Weeraratna AT. A Wnt-er migration: the confusing role of β-catenin in melanoma metastasis. Sci Signal. 2013;6(268):pe11. doi: 10.1126/scisignal.2004114. [DOI] [PubMed] [Google Scholar]
- 31.Dissanayake SK, Wade M, Johnson CE, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282(23):17259–17271. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan NV, Prat A, Abell AN, et al. SWI/SNF chromatin-remodeling factor Smarcd3/Baf60c controls epithelial-mesenchymal transition by inducing Wnt5a signaling. Mol Cell Biol. 2013;33(15):3011–3025. doi: 10.1128/MCB.01443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheel C, Eaton EN, Li SH, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145(6):926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14(6):1583–1588. [PubMed] [Google Scholar]
- 35.Dai J, Peng L, Fan K, et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene. 2009;28(38):3412–3422. doi: 10.1038/onc.2009.189. [DOI] [PubMed] [Google Scholar]
- 36.Choi HJ, Park H, Lee HW, Kwon YG. The Wnt pathway and the roles for its antagonists, DKKS, in angiogenesis. IUBMB Life. 2012;64(9):724–731. doi: 10.1002/iub.1062. [DOI] [PubMed] [Google Scholar]
- 37.Courtwright A, Siamakpour-Reihani S, Arbiser JL, et al. Secreted frizzle-related protein 2 stimulates angiogenesis via a calcineurin/NFAT signaling pathway. Cancer Res. 2009;69(11):4621–4628. doi: 10.1158/0008-5472.CAN-08-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park H, Jung HY, Choi HJ, et al. Distinct roles of DKK1 and DKK2 in tumor angiogenesis. Angiogenesis. 2014;17(1):221–234. doi: 10.1007/s10456-013-9390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller JF, Sadelain M. The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell. 2015;27(4):439–449. doi: 10.1016/j.ccell.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Wang Ye Y, Zhang CX, et al. A melanin-mediated cancer immunotherapy patch. Sci Immunol. 2017;2(17):eaan5692. doi: 10.1126/sciimmunol.aan5692. [DOI] [PubMed] [Google Scholar]
- 41.Pitt JM, Vétizou M, Daillère R, et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016;44(6):1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Galluzzi L, Spranger S, Fuchs E, López-Soto A. WNT signaling in cancer immunosurveillance. Trends Cell Biol. 2018 Sep 13; doi: 10.1016/j.tcb.2018.08.005. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]