Abstract
The luteinizing hormone (LH) surge is essential for ovulation, but the intrafollicular factors induced by LH that mediate ovulatory processes (e.g., angiogenesis) are poorly understood, especially in women. The role of secretogranin II (SCG2) and its cleaved bioactive peptide, secretoneurin (SN), were investigated as potential mediators of ovulation by testing the hypothesis that SCG2/SN is induced in granulosa cells by human chorionic gonadotropin (hCG), via a downstream LH receptor signaling mechanism, and stimulates ovarian angiogenesis. Humans, nonhuman primates, and rodents were treated with hCG in vivo resulting in a significant increase in the messenger RNA and protein levels of SCG2 in granulosa cells collected early during the periovulatory period and just prior to ovulation (humans: 12 to 34 hours; monkeys: 12 to 36 hours; rodents: 4 to 12 hours post-hCG). This induction by hCG was recapitulated in an in vitro culture system utilizing granulosa-lutein cells from in vitro fertilization patients. Using this system, inhibition of downstream LH receptor signaling pathways revealed that the initial induction of SCG2 is regulated, in part, by epidermal growth factor receptor signaling. Further, human ovarian microvascular endothelial cells were treated with SN (1 to 100 ng/mL) and subjected to angiogenesis assays. SN significantly increased endothelial cell migration and new sprout formation, suggesting induction of ovarian angiogenesis. These results establish that SCG2 is increased in granulosa cells across species during the periovulatory period and that SN may mediate ovulatory angiogenesis in the human ovary. These findings provide insight into the regulation of human ovulation and fertility.
SCG2 levels are increased in human, monkey, and rodent periovulatory granulosa cells via LH-dependent signaling. Secretoneurin induces ovulatory angiogenesis in human ovarian endothelial cells.
Ovulation is a strictly coordinated process that is the cornerstone of female fertility. In the ovarian preovulatory follicle, the midcycle luteinizing hormone (LH) surge initiates a cascade of cellular and molecular events, such as stimulating meiosis resumption in the oocyte, cumulus-oocyte expansion, induction of angiogenesis, follicle wall breakdown, oocyte release, and corpora luteal formation. LH signaling, through its receptor [LH/chorionic gonadotropin receptor (LHCGR)] stimulates the production of local granulosa cell–derived hormones/proteins that serve as mediators of the numerous ovulatory processes outlined above (1). The most well-known downstream mediators of ovulation induced by LH include epidermal growth factor (EGF)–like peptides (2), progesterone (P4) and its receptor (PGR) (3, 4), and prostaglandins (5, 6). These mediators can directly execute the biological processes leading to ovulation, or they can serve as signaling intermediates to induce their own downstream ovulatory mediators.
The majority of our understanding of the ovulatory process stems from experiments conducted in rodents. Specifically, reduced ovulation rates are observed in knockout mice lacking epiregulin (an EGF-like peptide induced by LH), PGR, and prostaglandin endoperoxide synthase 2 (PTGS2; a rate limiting synthase for prostaglandin production) (7–10). Likewise, ovulation is inhibited in the rodent following treatment with EGF receptor (EGFR) antagonists, PGR antagonists, and PTGS2 inhibitors (4, 7, 8, 11). Thus, it is clear that these granulosa cell–derived factors serve as mediators of ovulation downstream of the ovulatory LH stimulus in rodents. However, much less is known about the control of ovulation in women. This is primarily because the access to timed periovulatory ovarian samples is extremely limited. Previous studies have shown that some of the mediators of ovulation that were discovered in the rodent (specifically EGF-like peptides, P4/PGR, and prostaglandin synthases and transporters) are also induced in nonhuman primates and women (12–14); yet the existence of additional mediators of ovulation is largely unknown. Infertility treatment protocols, or conversely contraceptive treatment protocols, would greatly benefit from the identification of new mediators of the ovulatory process in humans.
The current study investigated the expression, regulation, and role of secretogranin II (SCG2) as a new mediator of the ovulatory process during the periovulatory period. To our knowledge, SCG2 has never been studied in the mammalian ovary, and thus has no known profile or role during the critical periovulatory period. SCG2 is a member of the chromogranin family of acidic secretory proteins involved with secretory vesicle formation and the sorting and packaging of peptide hormones into vesicles (15). It is localized to large dense core secretory vesicles of numerous endocrine, neuroendocrine, and neuronal tissues, including the gonadotropes of the anterior pituitary, islet cells of the pancreas, the adrenal medulla, gastrointestinal tract, hypothalamus, hippocampus, and several neurotransmitters within the central and peripheral nervous systems (16).
SCG2 is a 71-kDa propeptide precursor that is also rapidly cleaved to bioactive peptides. In fact, greater than 90% of SCG2 is rapidly cleaved in the tissues where it is produced, which is similar to the majority of costored precursor proteins (such as proopiomelanocortin) (17–21). The three bioactive peptide products of SCG2 cleavage are secretoneurin (SN), EM66, and manserin (16–21). SN has been shown to be involved in LH secretion, neurotransmitter release, leukocyte migration, and angiogenesis (22–24). The roles of the other peptides are much less understood, but EM66 is reported to be involved in the control of food intake, whereas manserin is postulated to be involved with stress responses (25, 26). Important for the current study, angiogenesis is a required biological process for ovulation to occur. Specifically, the previously avascular granulosa cells undergo a massive induction of angiogenesis just prior to ovulation, and inhibition of this angiogenesis results in anovulation (6, 14, 27–31). However, the role of SCG2/SN to promote angiogenesis in the ovary to aid in ovulation has not been investigated. One of the goals of this study was to elucidate the actions of SCG2 during the periovulatory period; specifically, the ability of SN to promote the vascular changes in the ovary that are required for ovulation.
Human, nonhuman primate, and rodent ovarian models were used to test the hypothesis that SCG2/SN is induced in granulosa cells via the LH receptor (LHCGR) through its downstream LHCGR-dependent signaling pathways, and stimulates angiogenesis in the ovary. We characterized the ovarian induction of SCG2 by human chorionic gonadotropin (hCG; potent LH analog) across the tested species and determined how SCG2 expression is regulated in human granulosa cells. We then demonstrated that SN is able to induce angiogenesis in human ovarian endothelial cells.
Materials and Methods
Materials
Unless otherwise noted, all chemicals and reagents were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO) or Invitrogen Life Technologies, Inc. (Waltham, MA). RU486 was purchased from Cayman Chemical (Ann Arbor, MI), NS398 was purchased from EMD Chemicals, Inc. (San Diego, CA), and SN was purchased from PolyPeptide Group (Strasbourg, France). In the nonhuman primate studies, follicle-stimulating hormone (FSH) and Ganirelix were kindly provided by Merck and Co., Inc. (Kenilworth, NJ), and LH, Antide, and hCG were generously provided by Serono Reproductive Biology Institute (Randolph, MA).
Human tissue collection in vivo: periovulatory follicles and granulosa cells
Human ovarian follicles and granulosa cells were collected from women across the periovulatory period at Sahlgrenska University Hospital (Gothenburg, Sweden), as previously described (13, 32, 33). The Human Ethics Committee of the Sahlgrenska Academy at the University of Gothenburg approved the collection protocol, and the patients had given their informed written consent before participating. Patient characteristics of the cohort have been previously published (32). Briefly, samples were collected from 30- to 38-year-old women who were healthy, had proven fertility, had regular menstrual cycles, had not taken hormonal contraceptives for 3 months prior to their enrollment of the study, and were undergoing laparoscopic sterilization via tubal ligation. Prior to the tubal ligation surgery, the patients were monitored by transvaginal ultrasound to establish cycle regularity via normal antral follicle development resulting in ovulation and to monitor the size of the developing antral follicle.
After confirming normal cyclicity, surgery was scheduled at four specific intervals of the periovulatory period to collect ovarian samples prior to the induction of the LH surge, following an ovulatory stimulus of hCG treatment but prior to oocyte release, and following follicle rupture. Once the dominant follicle reached a diameter of 14 to 17.5 mm, serum levels of LH, estradiol, and P4 were measured to confirm that ovulation had not been initiated, and the women were randomly assigned to one of the four experimental groups. Women assigned to the preovulatory stage served as the controls (did not receive hCG treatment) and had surgery and samples collected prior to the endogenous LH surge when the follicle was between 14 and 17.5 mm. The remaining women received an ovulatory dose of hCG to mimic the LH surge via a subcutaneous injection (250 μg recombinant hCG from Ovitrelle; Merck Serono) and were assigned to the other experimental groups. These women underwent surgery and had ovarian samples collected either at the early ovulatory stage (12 to 18 hours post-hCG), late ovulatory stage (18 to 34 hours post-hCG), or postovulatory stage (44 to 70 hours post-hCG). Frequent transvaginal ultrasound of these patients has determined that ovulation occurs ~36 hours after hCG administration (33). Depending on the experimental group, the dominant follicle/periovulatory follicle/corpus luteum (CL) was excised with laparoscopic scissors while diathermy was not performed. The follicle/CL was either fixed and processed for immunostaining or bisected and scraped to collect granulosa/luteal cells for messenger RNA (mRNA) analysis, as described below. We have previously determined that there is no detectable contamination of white blood cells in the isolated granulosa cell samples, which also suggests that there is very minimal contamination of theca cells in the samples (34).
Human tissue collection in vitro: granulosa-lutein cells
We have refined an in vitro culture system using granulosa-lutein cells (GLCs) from patients undergoing in vitro fertilization (IVF) that recapitulates periovulatory outcomes as observed in the human in vivo (13, 32, 35, 36). The Institutional Review Board of the University of Kentucky Office of Research Integrity approved the protocol for GLC collection. Women undergoing IVF at the Bluegrass Fertility Center (Lexington, KY) underwent a standardized ovarian stimulation protocol by administration of recombinant human FSH for 9 to 11 days. Following this FSH treatment regimen, the patients were given 10,000 U of hCG to induce the ovulatory cascade, and follicular aspiration was conducted 36 hours post-hCG treatment to collect the contents of the follicles, which is the time point just prior to ovulation. Oocytes were isolated in the fertility clinic, and the remaining GLCs were subjected to a Percoll gradient to separate the GLCs from red blood cells in the aspirate providing a purified population of cells for culture. The isolated GLCs were cultured in OptiMEM media containing 10% fetal bovine serum and 1% antibiotic-antimycotic (2.5 × 105 cells/mL). The GLCs were cultured for 6 days, and the media were changed every 24 hours. Following this 6-day acclimation period, the GLCs regain responsiveness to hCG and are able to recapitulate periovulatory outcomes. Specifically, these revitalized granulosa cells now possess functional LH/hCG receptors, produce P4 in response to hCG, and have increased mRNA levels of EGF-like peptides, PGR, and prostaglandin synthases/transporters in response to hCG (13, 32). Prior to hCG treatment, the GLCs are cultured in media without serum for 1 hour. Some GLCs are collected following serum removal (0 hours), whereas others are treated with or without hCG (1 IU/mL) and collected at 6, 12, and 24 hours after treatment for mRNA analysis. In other experiments using downstream LH/hCG inhibitors, the GLCs were processed as stated above, but some GLCs were treated with one of the following inhibitors for 1 hour and then treated with or without hCG: 10 μM AG1478 (EGF receptor tyrosine kinase inhibitor), 20 μM RU486 (P4 nuclear receptor antagonist), or 15 μM NS398 (selective PTGS2 inhibitor). The doses of these inhibitors have effectively inhibited EGF-, P4-, and prostaglandin-mediated signaling, respectively, in our GLC in vitro model as well as in other human ovarian models (13, 37–39). The GLCs from these experiments were collected at 0, 6, and 12 hours after treatment and processed for mRNA analysis.
Nonhuman primate tissue collection in vivo: periovulatory granulosa cells
Ovarian granulosa cells were obtained from adult cynomolgus macaques (Macaca fascicularis) at the Eastern Virginia Medical School (Norfolk, VA). All of the animal protocols and collections were approved by the Eastern Virginia Medical School Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Regularly cycling macaques underwent ovarian stimulation, as previously established (40–42). Briefly, the macaques received recombinant human FSH (90 IU) for 6 to 8 days, followed by a combination of FSH and recombinant human LH (60 IU) for 2 to 3 days to stimulate multiple follicles to develop. A gonadotropin-releasing hormone antagonist (0.5 mg/kg Antide or 0.03 mg/kg Ganirelix) was also administered daily to inhibit an endogenous LH surge. Serum estradiol levels were measured, and ovarian follicles were visualized by ultrasonography to monitor follicle development. Some macaques underwent surgery to collect granulosa cells via follicular aspiration prior to treatment (0 hours), whereas the remaining macaques received 1000 IU of recombinant hCG to induce ovulatory events. Granulosa cells from these macaques were collected at 12, 24, and 36 hours post-hCG treatment via follicular aspiration. Ovulation is anticipated 37 to 42 hours after the ovulatory gonadotropin surge (43). Postoperative pain control was provided by buprenorphine and either meloxicam or ketoprofen. Following each collection, the aspirates were subjected to a Percoll gradient to isolate a purified population of granulosa cells (40). The granulosa cells were processed for mRNA analysis, as described below.
Rodent tissue collection in vivo and in vitro: periovulatory granulosa cells
Immature C57BL/6 mice and immature Sprague Dawley rats were obtained from Harlan, Inc. (Indianapolis, IN) to collect periovulatory granulosa cells at the University of Kentucky (Lexington, KY). All animal protocols were approved by the University of Kentucky Animal Care and Use Committee, and the animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animals were housed in the University of Kentucky Division of Laboratory Animal Resources, were maintained on a 12-hour light/12-hour dark cycle, and were provided food and water ad libitum. For the in vivo experiments, the well-characterized pregnant mare serum gonadotropin (PMSG)/hCG model of superovulation was used to collect timed periovulatory granulosa cells, as described previously (44). Briefly, mice at postnatal day (PND) 25 and rats at PND 22 were subcutaneously injected with PMSG (5 and 10 IU, respectively) to stimulate the development of multiple follicles. After 48 hours, some animals were euthanized to serve as the control group (0 hours). The remaining mice and rats were subcutaneously injected with hCG (5 and 10 IU, respectively) to induce the ovulatory cascade. These animals were euthanized at set times across the periovulatory period (4, 8, 12, and 24 hours post-hCG treatment). Both mice and rats ovulate shortly following the 12-hour time point. Some ovaries were removed and processed for immunostaining experiments, and the remaining ovaries were removed to collect granulosa cells via follicular aspiration for mRNA analysis. For the in vitro experiments, immature mice (PND 25) and rats (PND 22) were subcutaneously injected with PMSG (5 and 10 IU, respectively) to stimulate the development of multiple follicles, as described previously (44). After 48 hours, the animals were euthanized, ovaries were isolated, and follicular aspiration was used to collect granulosa cells. Granulosa cells were cultured in OptiMEM supplemented with gentamycin and 1× insulin/transferrin/selenium with or without hCG (1 IU/mL). Granulosa cells were collected at 0, 4, 8, 12, and 24 hours after treatment for mRNA analysis.
Analysis of SCG2 mRNA levels
Total RNA (200 ng) was extracted from the granulosa cell samples using the RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) and was then reverse transcribed to complementary DNA (cDNA) using the SuperScriptIII kit according to the manufacturer’s protocol. SCG2 mRNA expression was analyzed for each tested species via quantitative real-time polymerase chain reaction (qPCR). Human SCG2 mRNA expression was measured using a TaqMan primer (Hs01920882_s1), with GAPDH (Hs99999905_m1) serving as the reference gene. Macaque SCG2 mRNA expression was measured using a TaqMan primer (Rh01026452_m1), with ACTB (Mf04354341_g1) serving as the reference gene. Rodent Scg2 mRNA expression was measured using TaqMan primers (mouse: Mm00843883_s1; rat: Rn02042691_s1), with Rpl32 (mouse: Mm02528467_g1; rat: Rn00820748_g1) serving as the reference gene. For each species, the reference gene did not significantly differ across time point or treatment group. The AriaMX Real-Time PCR System (Agilent Technologies, Santa Clara, CA) quantifies the amount of PCR product generated by measuring the fluorescence from the TaqMan Gene Expression Master Mix. The qPCR program included the following steps: 2 minutes at 50°C to permit AmpErase uracil-N-glycosylase optimal activity, denaturation step for 10 minutes at 95°C, 15 seconds at 95°C, and 1 minute at 60°C for 50 cycles, followed by 1 minute at 95°C, 30 seconds at 58°C, and 30 seconds at 95°C for ramp dissociation. Expression data were generated using the mathematical standard comparative (ΔΔCt) method. The ΔCt was calculated by subtracting the reference gene Ct value from the SCG2 Ct value. The ΔΔCt was calculated as the difference between the ΔCt between the treatment groups and the 0 hour/preovulatory control groups. The relative fold-change of expression was then equaled to 2−ΔΔCT for each sample.
Immunohistochemistry
Timed periovulatory follicles from the human in vivo study were fixed in 4% formaldehyde, whereas timed periovulatory ovaries from the mouse in vivo study were fixed in 4% neutral buffered formalin for immunohistochemical analysis of SCG2. The tissues were then embedded in paraffin, sectioned at 7 μm, and processed for immunostaining using the Starr Trek Universal Horseradish Peroxidase Detection System (Biocare Medical, LLC, Concord, CA), as previously described (32, 36). The sections were incubated with SCG2 primary antibody (1:500; Abcam; catalog no. ab192824) overnight in a humidified chamber at 4°C. Vulcan Fast Red was used to visualize the immunostaining and was applied to each slide for an equal amount of time until optimal color development, and the slides were counterstained with hematoxylin. Thus, positive immunostaining is indicated by the pink/red color, and negative staining is indicated by the blue/purple color. The negative control slides were prepared identically but without primary antibody.
Human ovarian microvascular endothelial cell isolation
Human ovarian microvascular endothelial cells (hOMECs) were used to measure the ability of SN to induce ovarian angiogenesis. The hOMECs were isolated from the follicular aspirates of women undergoing oocyte retrieval for oocyte donation following an ovarian stimulation protocol at the Jones Institute for Reproductive Medicine, Eastern Virginia Medical School, as described previously (45). Because the cells are obtained from discarded contents of the aspirate, their use does not constitute human subjects research as determined by the Eastern Virginia Medical School Institutional Review Board. Aspirated cells were cultured in fibronectin coated flasks containing EGM2 media (Lonza, Walkersville, MD), which is optimized for microvascular endothelial cells. The cells were allowed to reach confluency, and then endothelial cells were isolated using CD31 Dynabeads following the manufacturer’s protocol, as described previously (40, 45). A second Dynabead isolation at the second passage results in a purified population (>99%) of primary proliferating endothelial cells for experimentation, as previously characterized (45–47). Four cell lines were established using this technique, each cell line from a different woman.
hOMEC migration assay
The ability of SN to induce endothelial cell migration in the human ovary was assessed using a previously published migration assay (40, 45). The hOMECs were cultured on 6-well plate inserts with 8-µm pores (BD Biosciences, San Jose, CA) in basal media with or without the addition of SN (1, 10, 100 ng/mL). These doses of SN have previously been shown to induce angiogenesis in several other model systems (24, 48–50). EGM2 media were used as the positive control to ensure successful migration in each assay (45). Following 24 hours of culture, five images of the opposite side of the porous membrane were photographed, and the cells that migrated across the membrane were counted. The percentage of cells migrated in the SN groups relative to the basal control group for each cell line was calculated and used for statistical purposes.
hOMEC sprouting assay
The ability of SN to induce new capillary sprout formation in the human ovary was measured using a previously published endothelial cell sprouting assay (40, 45). Cytodex microcarrier beads (GE Healthcare, Uppsala, Sweden) coated with 500 to 1000 hOMECs were embedded in a fibrin matrix. The hOMECs were then treated with basal media with or without the addition of SN (1 and 100 ng/mL). EGM2 media were used as the positive control to ensure successful sprout formation (45). Following 24 hours of culture, photographs of five representative beads were used to count the number of sprouts/bead and to measure the length of each new capillary sprout.
Statistical analysis
Data analysis was conducted using SPSS statistical software (SPSS, Inc., Chicago, IL). Data were expressed as means ± standard error of the mean (SEM). Multiple comparisons between normally distributed experimental groups were made using one-way analysis of variance followed by Tukey post hoc comparison. Multiple comparisons between nonnormally distributed experimental groups were made using Kruskal-Wallis tests when appropriate. Statistical significance was assigned at P ≤ 0.05.
Results
Effect of hCG stimulation on SCG2 mRNA and protein in human granulosa cells in vivo
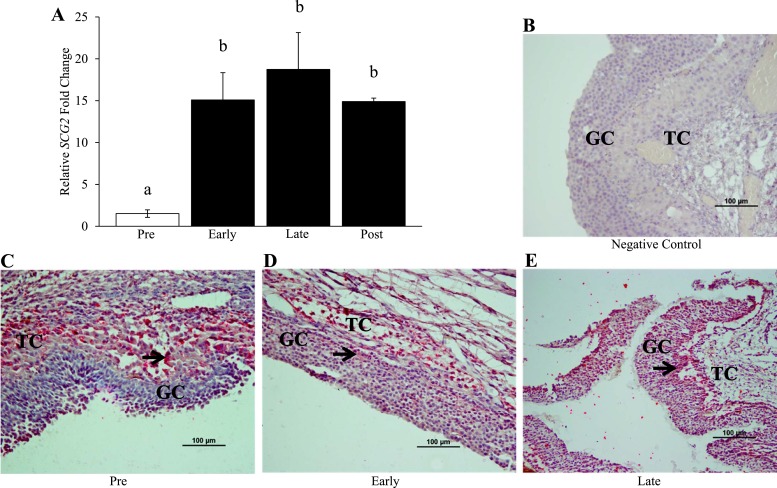
Administration of hCG to fertile, normally cycling women resulted in a significant 15-fold increase in the mRNA levels of SCG2 at the early ovulatory stage when compared with the preovulatory control samples (Fig. 1A). The mRNA levels of SCG2 remained significantly elevated by hCG treatment at the late ovulatory stage (19-fold increase) and the postovulatory stage (15-fold increase) when compared with the preovulatory controls (Fig. 1A). In accordance with the increased mRNA levels, SCG2 protein staining in the human samples obtained in vivo appears to increase in response to hCG treatment. SCG2 staining is prominent in the theca and stroma at all time points tested (Fig. 1C–1E). However, staining intensity in the theca and stroma does not appear to change across time, and staining is minimal in the granulosa cells of the preovulatory control samples (Fig. 1C). In response to hCG, SCG2 staining is moderately increased in the granulosa cells at the early ovulatory stage (Fig. 1D). This staining is then dramatically increased in the granulosa cells at the late ovulatory stage, with an abundant staining pattern in the granulosa cells near the basement membrane (Fig. 1E).
Figure 1.
Effect of hCG stimulation on (A) SCG2 mRNA and (B) protein in human granulosa cells in vivo. Whole follicles and granulosa cells from fertile, naturally cycling women were collected across the periovulatory period as described in “Materials and Methods.” (A) Granulosa cells were processed for mRNA isolation and cDNA synthesis and were subjected to qPCR to measure the mRNA levels of SCG2. All values were normalized to GAPDH. Graph represents mean ± SEM. Bars that do not share a letter designation are significantly different (n = 3 to 6 patients per group; P ≤ 0.05). (B–D) Whole follicles were processed for immunohistochemistry for SCG2 protein expression and localization. SCG2 staining is shown for (B) negative control, (C) preovulatory, (D) early ovulatory, and (E) late ovulatory samples. Pink/red indicates positive SCG2 staining, whereas blue/purple indicates negative staining as the slides were counterstained with hematoxylin. Black arrows indicate a specific cell/area that was stained positively for SCG2. Images are representative images from three to four patients per group. Scale bars, 100 μm. Early, early ovulatory stage; GC, granulosa cell; Late, late ovulatory stage; Post, postovulatory stage; Pre, preovulatory stage; TC, theca cell.
Effect of hCG stimulation on SCG2 mRNA in human granulosa cells in vitro
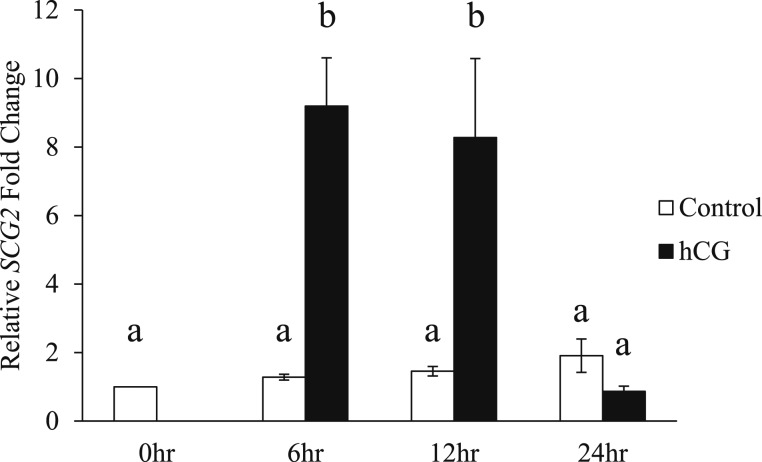
To further characterize SCG2 expression/regulation in human granulosa cells, this study employed an in vitro culture system using GLCs from IVF patients. This model has previously been shown to be hCG responsive and recapitulate periovulatory outcomes observed in vivo (13, 32). Using this model, SCG2 expression was induced by hCG in vitro. As anticipated, SCG2 expression was not induced in the control groups at each time point compared with the 0-hour control group. However, hCG treatment significantly increased the mRNA levels of SCG2 at the 6- and 12-hour time points when compared with the control groups (Fig. 2). The mRNA levels of SCG2 returned to control levels following 24 hours of exposure to hCG (Fig. 2).
Figure 2.
Effect of hCG stimulation on SCG2 mRNA in human granulosa cells in vitro. GLCs were collected at the time of oocyte retrieval from women undergoing IVF. The cells were processed as described in “Materials and Methods” to regain responsiveness to hCG. Following this acclimation period, the cells were treated with or without hCG and collected across the periovulatory period. The cells were processed for mRNA isolation and cDNA synthesis and were subjected to qPCR to measure the mRNA levels of SCG2. All values were normalized to GAPDH. Graph represents mean ± SEM. Bars that do not share a letter designation are significantly different (n = 5 to 12 patients per group, P ≤ 0.05).
Effect of hCG stimulation on SCG2 mRNA in nonhuman primate granulosa cells in vivo
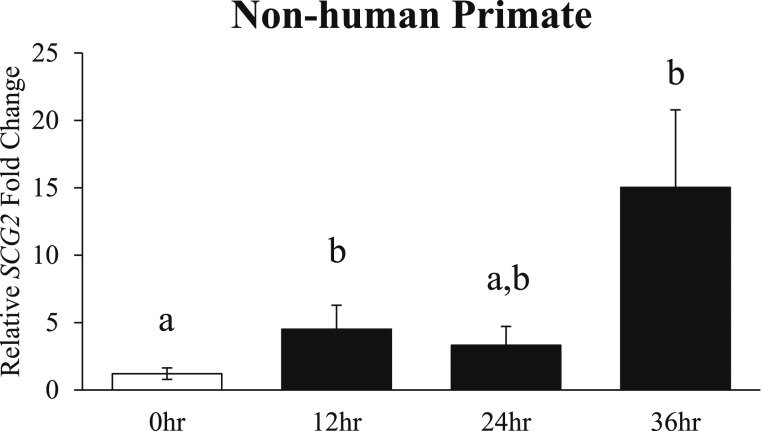
To explore SCG2 expression in the nonhuman primate, the periovulatory induction of SCG2 was characterized in the cynomolgus macaque superovulation model in vivo. In response to hCG treatment, the mRNA levels of SCG2 were significantly increased at the 12-hour time point when compared with the 0-hour control group (Fig. 3). Further, SCG2 expression increased to its highest level at the 36-hour time point (prior to ovulation) when compared with controls (Fig. 3).
Figure 3.
Effect of hCG stimulation on SCG2 mRNA in nonhuman primate granulosa cells in vivo. Granulosa cells from adult cynomolgus macaques were collected across the periovulatory period following ovarian stimulation as described in “Materials and Methods.” Granulosa cells were processed for mRNA isolation and cDNA synthesis and were subjected to qPCR to measure the mRNA levels of SCG2. All values were normalized to ACTB. Graph represents mean ± SEM. White bar indicates control samples, and black bars indicate hCG treatment samples. Bars that do not share a letter designation are significantly different (n = 3 to 6 macaques per group; P ≤ 0.05).
Effect of hCG stimulation on SCG2 mRNA and protein in rodent granulosa cells
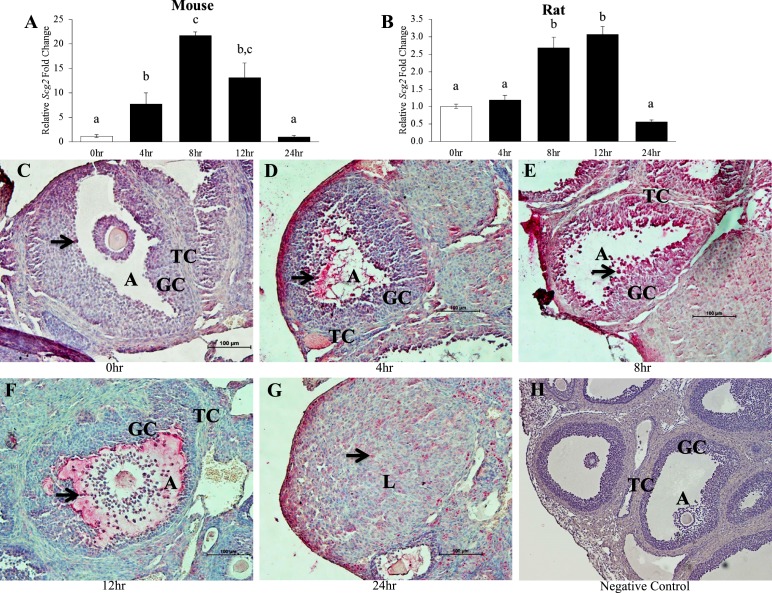
This study also used the immature PMSG/hCG model of superovulation in mice and rats in vivo. Similar to the human and nonhuman primate, hCG treatment induced Scg2 expression in the rodent, albeit the induction was much more pronounced in the mouse compared with the rat (Fig. 4A and 4B). In the mouse, hCG treatment significantly increased the mRNA levels of Scg2 at the 4-, 8- (peak expression levels of a 22-fold increase), and 12-hour time points when compared with the 0-hour control group, and then returned to control levels at the 24-hour time point following ovulation (Fig. 4A). In the rat, hCG treatment significantly increased the mRNA levels of Scg2 only at the 8- and 12-hour (peak expression levels of a threefold increase) time points when compared with the controls before declining to control levels at the 24-hour time point (Fig. 4B).
Figure 4.
Effect of hCG stimulation on (A and B) SCG2 mRNA and (C–G) protein in rodent granulosa cells in vivo. Whole ovaries and granulosa cells from immature mice and rats were collected across the periovulatory period following an ovarian stimulation protocol as described in “Materials and Methods.” (A and B) Granulosa cells were processed for mRNA isolation and cDNA synthesis and were subjected to qPCR to measure the mRNA levels of Scg2. All values were normalized to Rpl32. Graphs represent mean ± SEM. White bars indicate control samples, and black bars indicate hCG treatment samples. Bars that do not share a letter designation are significantly different (n = 5 to 8 rodents per group; P ≤ 0.05). (C–G) Whole ovaries from mice were processed for immunohistochemistry for SCG2 protein expression and localization. SCG2 staining is shown for (C) 0 hours, (D) 4 hours, (E) 8 hours, (F) 12 hours, (G) 24 hours, and (H) negative control samples. Pink/red indicates positive SCG2 staining, whereas blue/purple indicates negative staining as the slides were counterstained with hematoxylin. Black arrows indicate a specific cell/area that was stained positively for SCG2. Images are representative images from three mice per group. Scale bars, 100 μm. A, antrum; GC, granulosa cell; L, corpus luteum; TC, theca cell.
Because Scg2 induction by hCG was more pronounced in the mouse, immunohistochemistry was performed to determine if protein expression mimicked the mRNA expression. SCG2 staining in the mouse ovary was not constitutively present in the theca and stromal cells, as evident in the 0-hour control samples (Fig. 4C). Similar to the human, SCG2 staining was minimal in the granulosa cells of the dominant follicles of the 0-hour control group (Fig. 4C). Much like the increase in the levels of mRNA, hCG treatment increased the protein staining of SCG2 in the granulosa cells of the periovulatory follicles at the 4-hour time point (Fig. 4D). The level of staining in the granulosa cells of the periovulatory follicles was strongest in response to hCG treatment at the 8-hour time point, which correlates to the time point with peak mRNA expression (Fig. 4E). Around the time of ovulation at the 12-hour time point, SCG2 staining in the periovulatory follicles was largely localized to the antrum, granulosa cells that surround the antrum, and cumulus cells surrounding the oocyte (Fig. 4F). Staining in the mural granulosa cells at this time point appeared to decline relative to the 8-hour time point, but it was still increased compared with the 0-hour controls. Following ovulation at the 24-hour time point, SCG2 staining declined in the newly formed corpora lutea, which parallels the decline in mRNA levels at this time point (Fig. 4G).
Interestingly, hCG did not (in the mouse) or only moderately (in the rat) induce the expression of Scg2 in vitro, by using the immature PMSG primed model (Supplemental Fig. 1A and 1B). Specifically, the only time point that hCG significantly increased the mRNA levels of Scg2 was at the 4-hour time point in the rat (Supplemental Fig. 1B), which was not a time point when Scg2 was induced by hCG in the rat in vivo (Fig. 4B).
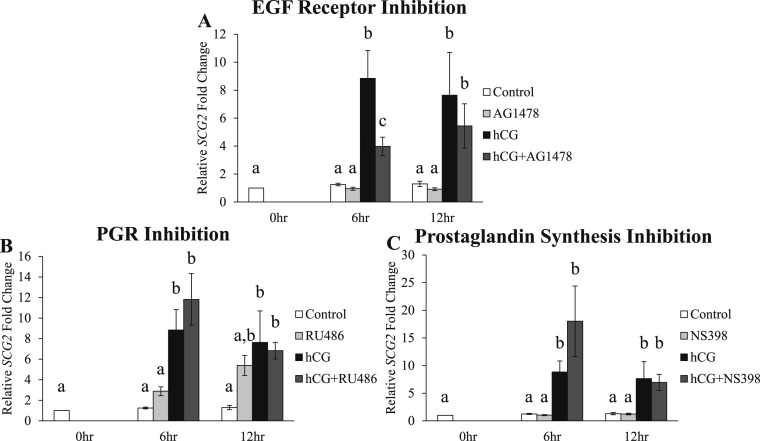
Effect of signaling inhibitors on the hCG-induced increase in SCG2 expression in human granulosa cells in vitro
Because hCG increased the levels of SCG2 in periovulatory granulosa cells, this study next determined how the induction of SCG2 expression is regulated by LH/hCG downstream signaling pathways. Human GLCs were treated with AG1478 (EGF receptor tyrosine kinase inhibitor), RU486 (P4 nuclear receptor antagonist), or NS398 (selective PTGS2 inhibitor) in vitro because these three compounds inhibit essential ovulatory signaling pathways downstream of LH receptor signaling. Treatment with hCG alone significantly increased the mRNA levels of SCG2 at the 6- and 12-hour time points (Fig. 5A–5C), as illustrated previously (Fig. 2). Treatment with the signaling inhibitors alone did not alter the expression of SCG2. However, AG1478 treatment at the 6-hour time point significantly inhibited the hCG-induced increase in the mRNA levels of SCG2 by over 55% when compared with the hCG alone treatment group (Fig. 5A). This suggests that the initial induction of SCG2 is partially regulated by EGF signaling in human granulosa cells. Contrary to AG1478, treatment with RU486 and NS398 did not block the hCG-induced increase in SCG2 levels (Fig. 5B and 5C).
Figure 5.
Effect of signaling inhibitors on the hCG-induced increase in SCG2 expression in human granulosa cells in vitro. GLCs were collected at the time of oocyte retrieval from women undergoing IVF. The cells were processed as described in “Materials and Methods” to regain responsiveness to hCG. Following this acclimation period, the cells were treated with one of the following inhibitors for 1 hour and then treated with or without hCG: (A) AG1478 (10 μM; EGF receptor tyrosine kinase inhibitor), (B) RU486 (20 μM; P4 receptor antagonist), or (C) NS398 (15 μM; selective PTGS2 inhibitor). The cells were processed for mRNA isolation and cDNA synthesis and were subjected to qPCR to measure the mRNA levels of SCG2. All values were normalized to GAPDH. Graphs represent mean ± SEM. Bars that do not share a letter designation are significantly different (n = 5 to 8 patients per group, P ≤ 0.05).
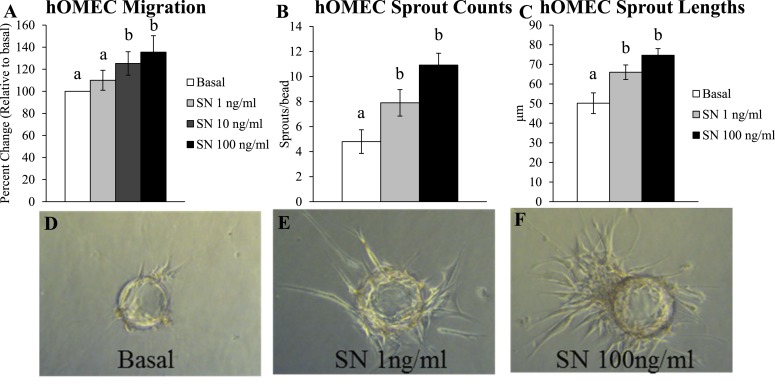
Effect of SN on hOMEC angiogenesis
Given that SCG2 levels are increased in granulosa cells during the periovulatory period, this study next determined how SCG2 may function during the ovulatory process in the human. SCG2 is rapidly cleaved to SN throughout the body (17–21). SN has been shown to induce angiogenesis in several nonovarian systems (24, 48–50), and angiogenesis is a requisite process for ovulation and subsequent corpora luteal formation/functionality (6, 14, 27–31, 51). Thus, this study subjected hOMECs to migratory and endothelial sprouting assays to address the angiogenic properties of SN in the ovary. In the migration assay, SN treatment at the 10 and 100 ng/mL doses significantly increased hOMEC migration by 25% and 35%, respectively, when compared with the basal control group (Fig. 6A). In the endothelial sprouting assay, SN treatment at the 1 and 100 ng/mL doses significantly increased the number of endothelial sprouts (Fig. 6B), and the length of these sprouts (Fig. 6C), when compared with the basal controls.
Figure 6.
Effect of SN on hOMEC angiogenesis. hOMEC lines were processed from oocyte donor patients as described in “Materials and Methods.” (A) Endothelial cell migration assay. hOMECs were cultured for 24 hours in basal media (control) or basal media+SN (1 to 100 ng/mL). Cells migrating to the opposite side of the membrane were counted, and analysis was conducted as the percentage of migrating cells relative to basal control for each hOMEC line. Graph represents mean ± SEM. Bars that do not share a letter designation are significantly different (n = 4 hOMEC lines per group; P ≤ 0.05). (B–F) Endothelial cell sprouting assay. Cytodex microcarrier beads were coated with hOMECs, embedded in a fibrin matrix, and treated with basal media (control) or basal media+SN (1 and 100 ng/mL). Photographs were taken 24 hours later from five representative beads/hOMEC line to (B) quantify sprouts/bead and (C) measure the length of sprouts/bead. (D–F) Assay images are representative images from four hOMEC lines per group. Graphs represent mean ± SEM. Bars that do not share a letter designation are significantly different (n = 4 hOMEC lines per group; P ≤ 0.05).
Discussion
This study characterizes the ovarian induction and function of SCG2 during the periovulatory period in any species. Thus, a major strength is the comparison of SCG2 expression across humans, nonhuman primates, and rodents. Our main findings conclude that SCG2 mRNA and protein levels are increased in granulosa cells of the periovulatory follicle in response to hCG treatment. Further, the initial induction of SCG2 by hCG is partially regulated by EGF signaling in human granulosa cells in vitro, suggesting that SCG2 induction is regulated via this downstream LHCGR-dependent signaling pathway. The increase in SCG2 levels likely leads to an increase in ovarian SN levels, which we have shown is involved in human ovarian angiogenesis. Because ovarian angiogenesis is a required process for ovulation to occur (27–31), these results suggest that SCG2/SN is a periovulatory factor that may stimulate angiogenesis during the ovulatory process in humans.
The majority of knowledge regarding the regulation of ovulation stems from work conducted in the rodent, and in many cases, it is largely unknown if we can extrapolate these findings to human ovulation. A major strength of this study overcomes this issue through the use of timed periovulatory ovarian samples obtained in vivo from fertile, regularly cycling women. We have established that the mRNA and protein levels of SCG2 are increased in granulosa cells following hCG treatment in vivo. The increase in the mRNA levels of SCG2 was also observed in our refined human in vitro model using GLCs from IVF patients. We have previously characterized this model system and have confirmed that following a suitable acclimation period, the GLCs treated with hCG successfully recapitulate periovulatory outcomes that are observed in humans and rodents in vivo, including increases in EGF-like peptides, PGR, and PTGS2 (13, 32, 35, 36). Because SCG2 levels are increased in both human model systems, these findings of an hCG induction of SCG2 have direct relevance to the regulation of human ovulation and fertility.
The characterization experiments established that hCG increases SCG2 mRNA and protein levels relatively early in the periovulatory period, and these levels remain elevated right before ovulation (36 hours in the human and monkey, and 12 hours in the rodents), a time in which the induction of angiogenesis is also increased (27). Interestingly, SCG2 levels decline in the postovulatory period in rodents, but they remain elevated in the postovulatory period in humans. This is perhaps attributed to the timing of collection or differences in the model systems. Rodent samples were collected strictly at 12 hours after ovulation (24-hour collection time point), whereas human samples were collected at a much wider range of 4 to 34 hours after ovulation (40- to 70-hour collection time point). This can also be attributed to species differences, specifically the differences in the lifespan and role of the CL between primates and rodents. Following ovulation, primates form a true, long-lived CL while rodents form a transient, short-lived CL if mating does not occur (52). Regardless of these differences, these data suggest for the first time that SCG2 is an hCG-inducible factor in periovulatory granulosa cells across species.
Contrary to the rodent findings in vivo, the mRNA levels of Scg2 did not increase in response to hCG in rodent granulosa cells in vitro. We hypothesize that the induction of Scg2, the parent precursor protein involved in intracellular/extracellular vesicle communication, requires direct cellular contact with surrounding granulosa and theca cells in the culture. When utilizing the well characterized immature PMSG primed model, follicles are treated to break cell–cell junctions and then punctured to collect granulosa cells, and the cells are immediately treated with hCG before they have time to adhere on the bottom of the culture plate to reestablish cell–cell contacts. This is in contrast to the human in vitro model, where the GLCs acclimate in culture for 6 days prior to hCG treatment where they adhere to the bottom of the culture dish and reestablish cell–cell contacts. Perhaps the granulosa cells need to reestablish connections with each other in order for hCG to induce a gene involved in vesicle communication among cells, such as Scg2. We also do not expect that PMSG alone induces SCG2 expression because the minimal positive immunostaining in the mouse 0-hour control preovulatory follicles (Fig. 4) is similar to all other follicle types and ovarian cells following PMSG administration. Although the in vitro PMSG/hCG model has been instrumental in understanding the regulation of ovulation in the rodent, it is possible that this in vitro model cannot fully recapitulate the vast, dynamic changes that take place in the granulosa cells during the periovulatory period in vivo.
We next demonstrated that SCG2 expression in human periovulatory granulosa cells in vitro is initially mediated, in part, by EGF signaling, which is a downstream LHCGR-dependent signaling mechanism. LH/hCG binding to its G protein-coupled receptor results in the increased production of the EGF-like peptides, amphiregulin and epiregulin (2). These EGF-like peptides bind to EGFR, which results in the induction of additional downstream mediators that ultimately lead to oocyte release and corpora lutea formation (53). AG1478 is a selective EGF receptor tyrosine kinase inhibitor, and when human GLCs were treated with AG1478 in the presence of hCG, there was a significant decrease in the mRNA levels of SCG2 when compared with hCG alone. Thus, the initial upregulation of SCG2 in periovulatory granulosa cells appears to be regulated in part by EGF signaling. The EGFR-mediated increase in SCG2 levels via treatment with EGF has also been observed in SH-SY5Y human neuroblastoma cells (54), GH4C1 rat pituitary tumor cells (55), and GH3B6 prolactin cells (56).
In the human GLC in vitro model, hCG+AG1478 treatment resulted in a 50% reduction of SCG2 levels. Treatment with hCG+AG1478 in this model has been shown to only partially inhibit other ovulatory mediators as well (13, 32). Perhaps additional secondary messengers of LHCGR signaling contribute to the initial induction (6 hours) and maintained elevation (12 hours) of SCG2 mRNA levels, which will be the focus of subsequent studies. These may include cyclic adenosine monophosphate (57), protein kinase B (58), and mitogen-activated protein kinase (59), which are shared secondary messengers of several G protein coupled receptors, like that of LHCGR.
Following the increase in ovarian SCG2 levels, it is likely that it is rapidly cleaved to SN. Consistent with the majority of posttranscriptional proteolytic processing that occurs in large dense core vesicles throughout the body, greater than 90% of SCG2 is rapidly cleaved to SN more so than its other cleaved peptides, EM66 and manserin (17–21). This rapid cleavage of a propeptide precursor to a bioactive peptide is shared among other costored precursor proteins, such as preprohormones, neuropeptide precursors, and the other members of the chromogranin family (60). We exhausted several avenues to quantify SN in the granulosa cells or following secretion in the media; however, we were unsuccessful in each approach. This is in large part to the small molecular weight of SN (3.6 kDa), which resulted in unsuccessful attempts using standard and modified Western blotting protocols for low molecular weight peptides. Further, immunohistochemistry using SN antibodies and enzyme-linked immunosorbent assays cannot definitively distinguish specific immunobinding to cleaved/free SN or the 33-amino acid sequence pertaining to SN on the precursor, uncleaved SCG2 protein. Although we were unable to measure SN in the ovary, it is expected that ~90% of SCG2 is rapidly cleaved to SN, as observed in other systems (17–21). Based on the fact that SN is the predominant bioactive peptide, it was selected for use in the hOMEC angiogenesis assays.
In the current study, SN significantly increased human ovarian endothelial cell migration. These findings are coupled with the increased number and length of newly developed capillary sprouts in the endothelial cell sprouting assay. Together, these findings demonstrate that SN induces angiogenesis in human ovarian endothelial cells. SN has been shown to induce angiogenesis in other model systems including coronary endothelial cells (61), the cornea (24), and the hind limb (49). The mechanism by which SN induces angiogenesis is presently unknown because the receptor of SN has not been fully characterized. The only characterization of the SN receptor has revealed that, on monocytes, it is a novel G-protein coupled receptor (62, 63).
Angiogenesis in periovulatory granulosa cells is vital for fertility because inhibition of ovarian angiogenesis has been shown to cause anovulation (6, 14, 27–31). During follicular development, the granulosa cells are devoid of blood vessels and remain avascular until just around the time of ovulation, which we have shown is the time period in which SCG2 levels are at their highest. At this time, ovarian endothelial cells migrate from the stroma into the granulosa cells, where they establish new capillary networks required for oocyte release and functionality of the CL (27, 40). Thus, the hOMEC cell line and the migration and endothelial cell sprouting assays used in the current study are ideal for studying the capabilities of SN to induce human ovarian angiogenesis. Because SCG2 (and likely SN) increases prior to ovulation and angiogenesis is required for ovulation to occur, these data suggest that SCG2/SN serves a vital functional role in human ovulation.
The full characterization of SCG2 knockout mice has not been conducted, so SCG2’s role in fertility is unknown. Our findings suggest that SCG2 may be a periovulatory factor induced by LH/hCG that may be directly involved in human ovulation. Specifically, this study discovered that SCG2 is increased in granulosa cells around the time of ovulation, and its cleaved bioactive peptide, SN, induces ovarian angiogenesis, which is a requisite ovulatory process. Discovering mediators of the ovulatory process and identifying their roles in ovulation are paramount to obtaining a complete understanding of human ovulation. This understanding will lead to innovative infertility/contraceptive treatment protocols and a decrease in the physical, mental, and socioeconomic agonies associated with infertility, all of which will greatly impact the overall well-being of women. Future projects will investigate the potential of SCG2 to serve as a measureable biomarker of fertility and/or as a targetable protein for infertility treatment or contraceptive purposes.
Supplementary Material
Acknowledgments
The authors thank Drs. Misung Jo, Yohan Choi, Linah Al-Alem; Ms. Carole Bryant; Miss Madison Lane; members of the Bluegrass Fertility Center; and the Jones Institute for Reproductive Medicine. Recombinant human FSH and Ganirelix were generously provided by Merck and Co., Inc. Serono Reproductive Biology Institute kindly provided recombinant human LH and Antide.
Financial Support: This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants HD071875 (to T.E.C., D.M.D., and M.B.) and HD057446 (to T.E.C.), the Lalor Foundation Postdoctoral Fellowship (to P.R.H.), and National Institutes of Health Loan Repayment Program (to P.R.H.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- cDNA
complementary DNA
- CL
corpus luteum
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- FSH
follicle-stimulating hormone
- GLC
granulosa-lutein cell
- hCG
human chorionic gonadotropin
- hOMEC
human ovarian microvascular endothelial cell
- IVF
in vitro fertilization
- LH
luteinizing hormone
- LHCGR
luteinizing hormone/chorionic gonadotropin receptor
- mRNA
messenger RNA
- P4
progesterone
- PGR
progesterone receptor
- PMSG
pregnant mare serum gonadotropin
- PND
postnatal day
- PTGS2
prostaglandin endoperoxide synthase 2
- qPCR
quantitative real-time polymerase chain reaction
- SCG2
secretogranin II
- SEM
standard error of the mean
- SN
secretoneurin
References
- 1. Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120(4):963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. [DOI] [PubMed] [Google Scholar]
- 3. Robker RL, Russell DL, Espey LL, Lydon JP, O’Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA. 2000;97(9):4689–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loutradis D, Bletsa R, Aravantinos L, Kallianidis K, Michalas S, Psychoyos A. Preovulatory effects of the progesterone antagonist mifepristone (RU486) in mice. Hum Reprod. 1991;6(9):1238–1240. [DOI] [PubMed] [Google Scholar]
- 5. Hedin L, Gaddy-Kurten D, Kurten R, DeWitt DL, Smith WL, Richards JS. Prostaglandin endoperoxide synthase in rat ovarian follicles: content, cellular distribution, and evidence for hormonal induction preceding ovulation. Endocrinology. 1987;121(2):722–731. [DOI] [PubMed] [Google Scholar]
- 6. Duffy DM. Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Hum Reprod Update. 2015;21(5):652–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27(5):1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146(1):77–84. [DOI] [PubMed] [Google Scholar]
- 9. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 10. Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91(2):197–208. [DOI] [PubMed] [Google Scholar]
- 11. Mikuni M, Pall M, Peterson CM, Peterson CA, Hellberg P, Brännström M, Richards JS, Hedin L. The selective prostaglandin endoperoxide synthase-2 inhibitor, NS-398, reduces prostaglandin production and ovulation in vivo and in vitro in the rat. Biol Reprod. 1998;59(5):1077–1083. [DOI] [PubMed] [Google Scholar]
- 12. Wissing ML, Kristensen SG, Andersen CY, Mikkelsen AL, Høst T, Borup R, Grøndahl ML. Identification of new ovulation-related genes in humans by comparing the transcriptome of granulosa cells before and after ovulation triggering in the same controlled ovarian stimulation cycle. Hum Reprod. 2014;29(5):997–1010. [DOI] [PubMed] [Google Scholar]
- 13. Choi Y, Wilson K, Hannon PR, Rosewell KL, Brännström M, Akin JW, Curry TE Jr, Jo M. Coordinated regulation among progesterone, prostaglandins, and EGF-like factors in human ovulatory follicles. J Clin Endocrinol Metab. 2017;102(6):1971–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stouffer RL, Xu F, Duffy DM. Molecular control of ovulation and luteinization in the primate follicle. Front Biosci. 2007;12:297–307. [DOI] [PubMed] [Google Scholar]
- 15. Beuret N, Stettler H, Renold A, Rutishauser J, Spiess M. Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J Biol Chem. 2004;279(19):20242–20249. [DOI] [PubMed] [Google Scholar]
- 16. Fischer-Colbrie R, Laslop A, Kirchmair R. Secretogranin II: molecular properties, regulation of biosynthesis and processing to the neuropeptide secretoneurin. Prog Neurobiol. 1995;46(1):49–70. [DOI] [PubMed] [Google Scholar]
- 17. Kirchmair R, Hogue-Angeletti R, Gutierrez J, Fischer-Colbrie R, Winkler H. Secretoneurin–a neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C). Neuroscience. 1993;53(2):359–365. [DOI] [PubMed] [Google Scholar]
- 18. Marksteiner J, Kirchmair R, Mahata SK, Mahata M, Fischer-Colbrie R, Hogue-Angeletti R, Saria A, Winkler H. Distribution of secretoneurin, a peptide derived from secretogranin II, in rat brain: an immunocytochemical and radioimmunological study. Neuroscience. 1993;54(4):923–944. [DOI] [PubMed] [Google Scholar]
- 19. Marksteiner J, Saria A, Kirchmair R, Pycha R, Benesch H, Fischer-Colbrie R, Haring C, Maier H, Ransmayr G. Distribution of secretoneurin-like immunoreactivity in comparison with substance P- and enkephalin-like immunoreactivities in various human forebrain regions. Eur J Neurosci. 1993;5(12):1573–1585. [DOI] [PubMed] [Google Scholar]
- 20. Egger C, Kirchmair R, Hogue-Angeletti R, Fischer-Colbrie R, Winkler H. Different degrees of processing of secretogranin II in large dense core vesicles of bovine adrenal medulla and sympathetic axons correlate with their content of soluble PC1 and PC2. Neurosci Lett. 1993;159(1–2):199–201. [DOI] [PubMed] [Google Scholar]
- 21. Egger C, Kirchmair R, Kapelari S, Fischer-Colbrie R, Hogue-Angeletti R, Winkler H. Bovine posterior pituitary: presence of p65 (synaptotagmin), PC1, PC2 and secretoneurin in large dense core vesicles. Neuroendocrinology. 1994;59(2):169–175. [DOI] [PubMed] [Google Scholar]
- 22. Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SR. The extended granin family: structure, function, and biomedical implications. Endocr Rev. 2011;32(6):755–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montero-Hadjadje M, Vaingankar S, Elias S, Tostivint H, Mahata SK, Anouar Y. Chromogranins A and B and secretogranin II: evolutionary and functional aspects. Acta Physiol (Oxf). 2008;192(2):309–324. [DOI] [PubMed] [Google Scholar]
- 24. Kirchmair R, Gander R, Egger M, Hanley A, Silver M, Ritsch A, Murayama T, Kaneider N, Sturm W, Kearny M, Fischer-Colbrie R, Kircher B, Gaenzer H, Wiedermann CJ, Ropper AH, Losordo DW, Patsch JR, Schratzberger P. The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation. 2004;109(6):777–783. [DOI] [PubMed] [Google Scholar]
- 25. Boutahricht M, Guillemot J, Montero-Hadjadje M, Bellafqih S, El Ouezzani S, Alaoui A, Yon L, Vaudry H, Anouar Y, Magoul R. Biochemical characterisation and immunohistochemical localisation of the secretogranin II-derived peptide EM66 in the hypothalamus of the jerboa (Jaculus orientalis): modulation by food deprivation. J Neuroendocrinol. 2005;17(6):372–378. [DOI] [PubMed] [Google Scholar]
- 26. Kamada N, Tano K, Oyabu A, Imura Y, Narita N, Tashiro Y, Uchida A, Komada Y, Narita M. Immunohistochemical localization of manserin, a novel neuropeptide derived from secretogranin II, in rat adrenal gland, and its upregulation by physical stress. Int J Pept Res Ther. 2010;16(2):55–61. [Google Scholar]
- 27. Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and vascular function in the ovary. Reproduction. 2009;138(6):869–881. [DOI] [PubMed] [Google Scholar]
- 28. Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology. 2002;143(7):2797–2807. [DOI] [PubMed] [Google Scholar]
- 29. Hazzard TM, Rohan RM, Molskness TA, Fanton JW, D’Amato RJ, Stouffer RL. Injection of antiangiogenic agents into the macaque preovulatory follicle: disruption of corpus luteum development and function. Endocrine. 2002;17(3):199–206. [DOI] [PubMed] [Google Scholar]
- 30. Hazzard TM, Xu F, Stouffer RL. Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkeys. Biol Reprod. 2002;67(4):1305–1312. [DOI] [PubMed] [Google Scholar]
- 31. Xu F, Hazzard TM, Evans A, Charnock-Jones S, Smith S, Stouffer RL. Intraovarian actions of anti-angiogenic agents disrupt periovulatory events during the menstrual cycle in monkeys. Contraception. 2005;71(4):239–248. [DOI] [PubMed] [Google Scholar]
- 32. Al-Alem L, Puttabyatappa M, Rosewell K, Brännström M, Akin J, Boldt J, Muse K, Curry TE Jr. Chemokine ligand 20: a signal for leukocyte recruitment during human ovulation? Endocrinology. 2015;156(9):3358–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thoroddsen A, Dahm-Kähler P, Lind AK, Weijdegård B, Lindenthal B, Müller J, Brännström M. The water permeability channels aquaporins 1-4 are differentially expressed in granulosa and theca cells of the preovulatory follicle during precise stages of human ovulation. J Clin Endocrinol Metab. 2011;96(4):1021–1028. [DOI] [PubMed] [Google Scholar]
- 34. Lind AK. Human Ovulation: Studies on Collagens, Gelatinases and Tissue Inhibitors of Metalloproteinases. Göteborg, Sweden: The Sahlgrenska Academy at Göteborg University; 2006. [Google Scholar]
- 35. Rosewell KL, Al-Alem L, Zakerkish F, McCord L, Akin JW, Chaffin CL, Brännström M, Curry TE Jr. Induction of proteinases in the human preovulatory follicle of the menstrual cycle by human chorionic gonadotropin. Fertil Steril. 2015;103(3):826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosewell KL, Li F, Puttabyatappa M, Akin JW, Brännström M, Curry TE Jr. Ovarian expression, localization, and function of tissue inhibitor of metalloproteinase 3 (TIMP3) during the periovulatory period of the human menstrual cycle. Biol Reprod. 2013;89(5):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsai EM, Chan TF, Chen YH, Hsu SC, Chuang CY, Lee JN. Mifepristone attenuates human chorionic gonadotropin-induced extracellular signal-regulated kinase 1/2 phosphorylation, cyclooxygenase-2, and prostaglandin E2 production in human granulosa luteal cells. Fertil Steril. 2008;89(Suppl 5):1522–1529. [DOI] [PubMed] [Google Scholar]
- 38. Yung Y, Maman E, Ophir L, Rubinstein N, Barzilay E, Yerushalmi GM, Hourvitz A. Progesterone antagonist, RU486, represses LHCGR expression and LH/hCG signaling in cultured luteinized human mural granulosa cells. Gynecol Endocrinol. 2014;30(1):42–47. [DOI] [PubMed] [Google Scholar]
- 39. Fang L, Yu Y, Zhang R, He J, Sun YP. Amphiregulin mediates hCG-induced StAR expression and progesterone production in human granulosa cells. Sci Rep. 2016;6(1):24917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trau HA, Davis JS, Duffy DM. Angiogenesis in the primate ovulatory follicle is stimulated by luteinizing hormone via prostaglandin E2. Biol Reprod. 2015;92(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vandevoort CA, Baughman WL, Stouffer RL. Comparison of different regimens of human gonadotropins for superovulation of rhesus monkeys: ovulatory response and subsequent luteal function. J In Vitro Fert Embryo Transf. 1989;6(2):85–91. [DOI] [PubMed] [Google Scholar]
- 42. Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci USA. 1996;93(5):1897–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weick RF, Dierschke DJ, Karsch FJ, Butler WR, Hotchkiss J, Knobil E. Periovulatory time courses of circulating gonadotropic and ovarian hormones in the rhesus monkey. Endocrinology. 1973;93(5):1140–1147. [DOI] [PubMed] [Google Scholar]
- 44. Jo M, Curry TE Jr. Luteinizing hormone-induced RUNX1 regulates the expression of genes in granulosa cells of rat periovulatory follicles. Mol Endocrinol. 2006;20(9):2156–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trau HA, Brännström M, Curry TE Jr, Duffy DM. Prostaglandin E2 and vascular endothelial growth factor A mediate angiogenesis of human ovarian follicular endothelial cells. Hum Reprod. 2016;31(2):436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ratcliffe KE, Anthony FW, Richardson MC, Stones RW. Morphology and functional characteristics of human ovarian microvascular endothelium. Hum Reprod. 1999;14(6):1549–1554. [DOI] [PubMed] [Google Scholar]
- 47. Bohgaki M, Kitaguchi H. Conversion of cultured monocytes/macrophages into endothelial-like cells through direct contact with endothelial cells. Int J Hematol. 2007;86(1):42–48. [DOI] [PubMed] [Google Scholar]
- 48. Kirchmair R, Egger M, Walter DH, Eisterer W, Niederwanger A, Woell E, Nagl M, Pedrini M, Murayama T, Frauscher S, Hanley A, Silver M, Brodmann M, Sturm W, Fischer-Colbrie R, Losordo DW, Patsch JR, Schratzberger P. Secretoneurin, an angiogenic neuropeptide, induces postnatal vasculogenesis. Circulation. 2004;110(9):1121–1127. [DOI] [PubMed] [Google Scholar]
- 49. Albrecht-Schgoer K, Barthelmes J, Schgoer W, Theurl M, Nardin I, Lener D, Gutmann C, Dünnhaupt S, Bernkop-Schnürch A, Kirchmair R. Nanoparticular delivery system for a secretoneurin derivative induces angiogenesis in a hind limb ischemia model. J Control Release. 2017;250:1–8. [DOI] [PubMed] [Google Scholar]
- 50. Albrecht-Schgoer K, Schgoer W, Theurl M, Stanzl U, Lener D, Dejaco D, Zelger B, Franz WM, Kirchmair R. Topical secretoneurin gene therapy accelerates diabetic wound healing by interaction between heparan-sulfate proteoglycans and basic FGF. Angiogenesis. 2014;17(1):27–36. [DOI] [PubMed] [Google Scholar]
- 51. Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod. 2002;17(11):2825–2831. [DOI] [PubMed] [Google Scholar]
- 52. Stouffer RL, Hennebold JD. Structure, Function, and Regulation of the Corpus Luteum. In: Plat T, Zeleznik A, eds. Knobil and Neill’s Physiology of Reproduction: Two-Volume Set. Vol 1. 4th ed San Diego, CA: Academic Press; 2014:1023–1076. [Google Scholar]
- 53. Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA. 2005;102(45):16257–16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weiss C, Winkler H, Laslop A. Regulation of chromogranin biosynthesis by neurotrophic growth factors in neuroblastoma cells. Neurochem Int. 2001;38(1):43–52. [DOI] [PubMed] [Google Scholar]
- 55. Scammell JG, Rosa P, Hille A, Huttner WB. Regulation of chromogranin B/secretogranin I and secretogranin II storage in GH4C1 cells. J Histochem Cytochem. 1990;38(7):949–956. [DOI] [PubMed] [Google Scholar]
- 56. Muller L, Tougard C. Production and secretion of N-terminal secretogranin II derived peptides in GH3B6 prolactin cells. Mol Cell Endocrinol. 1995;112(1):101–112. [DOI] [PubMed] [Google Scholar]
- 57. Marsh JM. The stimulatory effect of luteinizing hormone on adenyl cyclase in the bovine corpus luteum. J Biol Chem. 1970;245(7):1596–1603. [PubMed] [Google Scholar]
- 58. Carvalho CR, Carvalheira JB, Lima MH, Zimmerman SF, Caperuto LC, Amanso A, Gasparetti AL, Meneghetti V, Zimmerman LF, Velloso LA, Saad MJ. Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology. 2003;144(2):638–647. [DOI] [PubMed] [Google Scholar]
- 59. Su YQ, Denegre JM, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev Biol. 2003;263(1):126–138. [DOI] [PubMed] [Google Scholar]
- 60. Kirchmair R, Leitner B, Fischer-Colbrie R, Marksteiner J, Hogue-Angeletti R, Winkler H. Large variations in the proteolytic formation of a chromogranin A-derived peptide (GE-25) in neuroendocrine tissues. Biochem J. 1995;310(Pt 1):331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Albrecht-Schgoer K, Schgoer W, Holfeld J, Theurl M, Wiedemann D, Steger C, Gupta R, Semsroth S, Fischer-Colbrie R, Beer AG, Stanzl U, Huber E, Misener S, Dejaco D, Kishore R, Pachinger O, Grimm M, Bonaros N, Kirchmair R. The angiogenic factor secretoneurin induces coronary angiogenesis in a model of myocardial infarction by stimulation of vascular endothelial growth factor signaling in endothelial cells. Circulation. 2012;126(21):2491–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kong C, Gill BM, Rahimpour R, Xu L, Feldman RD, Xiao Q, McDonald TJ, Taupenot L, Mahata SK, Singh B, O’Connor DT, Kelvin DJ. Secretoneurin and chemoattractant receptor interactions. J Neuroimmunol. 1998;88(1-2):91–98. [DOI] [PubMed] [Google Scholar]
- 63. Schneitler C, Kähler C, Wiedermann CJ, Hogue-Angeletti R, Fischer-Colbrie R. Specific binding of a 125I-secretoneurin analogue to a human monocytic cell line. J Neuroimmunol. 1998;86(1):87–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.