Abstract
Pharmacologic expansion of endogenous β cells is a promising therapeutic strategy for diabetes. To elucidate the molecular pathways that control β-cell growth we screened ∼2400 bioactive compounds for rat β-cell replication-modulating activity. Numerous hit compounds impaired or promoted rat β-cell replication, including CC-401, an advanced clinical candidate previously characterized as a c-Jun N-terminal kinase inhibitor. Surprisingly, CC-401 induced rodent (in vitro and in vivo) and human (in vitro) β-cell replication via dual-specificity tyrosine phosphorylation–regulated kinase (DYRK) 1A and 1B inhibition. In contrast to rat β cells, which were broadly growth responsive to compound treatment, human β-cell replication was only consistently induced by DYRK1A/B inhibitors. This effect was enhanced by simultaneous glycogen synthase kinase–3β (GSK-3β) or activin A receptor type II–like kinase/transforming growth factor-β (ALK5/TGF-β) inhibition. Prior work emphasized DYRK1A/B inhibition–dependent activation of nuclear factor of activated T cells (NFAT) as the primary mechanism of human β-cell–replication induction. However, inhibition of NFAT activity had limited effect on CC-401–induced β-cell replication. Consequently, we investigated additional effects of CC-401–dependent DYRK1A/B inhibition. Indeed, CC-401 inhibited DYRK1A-dependent phosphorylation/stabilization of the β-cell–replication inhibitor p27Kip1. Additionally, CC-401 increased expression of numerous replication-promoting genes normally suppressed by the dimerization partner, RB-like, E2F and multivulval class B (DREAM) complex, which depends upon DYRK1A/B activity for integrity, including MYBL2 and FOXM1. In summary, we present a compendium of compounds as a valuable resource for manipulating the signaling pathways that control β-cell replication and leverage a DYRK1A/B inhibitor (CC-401) to expand our understanding of the molecular pathways that control β-cell growth.
Chemical screening identifies novel regulators of β-cell replication, including CC-401, which acts via DYRK1A/B inhibition, NFAT activation, p27 destabilization, and DREAM complex derepression.
Diabetes is a disorder of glucose homeostasis that negatively affects the health of nearly 1 in 12 individuals worldwide. Loss of insulin-producing β cells is a fundamental feature of diabetes, and compensatory β-cell mass expansion may be an important mechanism for avoiding hyperglycemia (1–3). The causal and protective associations of genetic variants in growth-associated loci with type 2 diabetes (e.g., CDKN2A/B and CCND2) support this supposition (4, 5). Interestingly, the cellular origin of new β cells in adult animals remains somewhat controversial, with potential sources including partially defined facultative progenitor cell populations, cells with the capacity to transdifferentiate (α cells), and, most convincingly, previously existing β cells (6–9). Presently, a variety of strategies for replacing (transplantation based) or expanding (regeneration based) an individual’s β-cell mass are being pursued. Among these, expansion of endogenous insulin-producing pancreatic islet β cells remains paramount.
A major challenge to expanding β-cell mass is the limited replication capacity of mature β cells. Consequently, developing a precise understanding of the molecular pathways that restrain β-cell growth and identifying drugs capable of surmounting these restraints are critical efforts. Recently, several small molecules [namely, harmine, 5-iodotubercidin (5-IT) and aminopyrazines] were shown to stimulate human β-cell replication, in part by inhibiting the dual-specificity tyrosine phosphorylation–regulated kinases 1A/B (DYRK1A/B) and activating nuclear factor of activated T cells (NFAT) signaling (10–14). However, the contribution of other signaling pathways to DYRK1A/B inhibition–dependent induction of β-cell replication has not been thoroughly investigated. Additionally, the potential benefit of simultaneously inhibiting multiple replication-associated pathways to maximize human β-cell–replication induction has not been explored.
To expand our understanding of the molecular pathways that restrain β-cell growth, potentially contributing to diabetes susceptibility, and identify compounds that stimulate β-cell replication, we developed a high-content screening platform (14–16). Herein, we report the successful use of this platform to screen ∼2400 bioactive compounds and identify novel replication-modulating compounds. Importantly, negative regulators of β-cell replication include commonly prescribed medications that could be linked to diabetes risk. We found compound combinations that synergistically promote β-cell replication, highlighting the potential benefit of tailored ligand targeting for improved human β-cell–replication induction. Additionally, we characterized CC-401 as a DYRK1A/B inhibitor that promotes β-cell replication in vitro and in vivo, and developed a CC-401 derivative (STF-200785) with improved replication-promoting activity. Finally, we highlight additional β-cell replication pathways, including p27Kip1 phosphorylation/stabilization and dimerization partner, RB-like, E2F and multivulval class B (DREAM) complex–dependent gene repression, controlled by CC-401–dependent DYRK1A/B inhibition. These studies provide important insights for understanding and manipulating β-cell replication.
Materials and Methods
β-cell replication in vitro
All animal work was approved and carried out in accordance with our institutional animal care and use committee. Islets were isolated from male Sprague Dawley rats [250- to 300-g animals; Research Resource Identifier (RRID): RGD_1566457] as previously described (17). Screened compounds were derived from a variety of collections, including the following: LOPAC (Sigma-Aldrich, St. Louis, MO), Spectrum Microsource Collection (Discovery Systems, Gaylordsville, CT), National Institutes of Health Clinical Collection, and KINASet (ChemBridge, San Diego, CA). Replication was assessed via automated image acquisition and analysis using a Cellomics ArrayScanVTI. The acquisition thresholds and parameters were established such that the computer-based calls of replication events were consistent with human-based calls. The number of β cells per well was determined using the Cellomics ArrayScan VTI to image the entire well and count the number of DAPI+PDX1+− or DAPI+insulin+ cells (n = 4 to 8 per condition). Human islets (from 10 donors) of high purity (90% to 95%) and viability (>87%) from nondiabetic donors (aged 25 to 62 years; median age, 50 years) were obtained through the National Disease Research Interchange and Integrated Islet Distribution Program. Donors were of mixed race (n = 5 white, n = 1 Hispanic, and n = 4 black) and sex (n = 5 male, n = 5 female); donors were generally obese (average body mass index ± standard deviation, 30.1 ± 5 kg/m2).
β-cell replication in vivo
For in vivoβ-cell replication assessment, 8-week-old female C57BL/6J (JAX 0664; RRID: IMSR) mice received twice-daily intraperitoneal (IP) injections with vehicle or CC-401 [25 mg/kg (10 µL/g)] for 7 days. This dose of CC-401 did not have an observable effect on animal behavior or acutely affect blood glucose levels; however, higher CC-401 doses led to weight loss. After the first IP dose, animals were provided bromodeoxyuridine (BrdU)-containing water (0.8 mg/mL) in opaque bottles that were changed every 2 days. Mice were killed and the pancreata were harvested on day 8. Paraffin sections were prepared and immunofluorescently stained with insulin, BrdU, and 4′,6-diamidino-2-phenylindole (DAPI).
Manual imaging of pancreatic sections and quantitation of the percentage of β cells that coexpressed BrdU were performed by investigators blinded to the treatment cohort. A minimum of 2000 β cells from nonconsecutive sections (>50 µm apart) were used to determine the β-cell replication rate for each animal. The treatment group comprised five animals. Exocrine cell replication was approximated by counting all nuclei outside the islet structure but within the pancreatic parenchyma.
Kinase assays
Recombinant DYRK1B (2.5 ng per reaction; catalog no. D09-10BG-10; SignalChem, Richmond, BC, Canada) phosphorylation of DyrkTide (40 µM;, RRRFRPASPLRGPPK; catalog no. D96-58; SignalChem) was measured in a reaction buffer containing 10 mM MgCl2, 20 mM Tris pH 7.5, and 10 μM adenosine triphosphate. Drug candidates (CC-401 and STF-785) were assayed at fourfold dilutions starting at 20 μM; final 2% dimethyl sulfoxide (DMSO). Reactions were terminated after 90 minutes at 30°C and kinase activity was measured using ADP-Glo Kinase Assay (V6930; Promega, Madison, WI). An analogous experiment was conducted with p27 (25 ng per reaction; catalog no. 56279; Abcam) as the reaction substrate instead of DyrkTide, with other reaction components unchanged. Reactions were conducted in the presence and absence of 1 μM CC-401 or 1% DMSO. Control reactions lacking DYRK1A (2.5 ng per reaction, SignalChem D09-10G-10) or adenosine triphosphate were also conducted. After 60 minutes at 30°C, reactions were quenched and run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions. Total p27 and phospho-p27 were quantified by western blot. Kinome screening and kinase inhibition assays were performed by DiscoveRx (San Diego, CA) (18).
Antibodies and compounds
The following antibodies were used for immunostaining: pancreatic and duodenal homeobox 1 (PDX1) (1:300; catalog no. AF2419; RRID: AB_355257; R&D Systems, Minneapolis, MN); Ki67 (1:300; catalog no. 556003; RRID: AB_396287; BD Bioscience, San Jose, CA); insulin (1:300; catalog no. A0564; RRID: AB_10013624; Dako, Santa Clara, CA); glucagon (1:500; catalog no. A0565; RRID: AB_10013726; Dako); somatostatin (1:300; catalog no. A0566; RRID: AB_2688022; Dako); vimentin (1:200; catalog no. ab24525; RRID: AB_778824; Abcam, Cambridge, MA); proliferating cell nuclear antigen (PCNA) (1:50; catalog no. sc-7907; RRID: AB_2160375; Santa Cruz Biotechnology, Dallas, TX); BrdU (1:50; M074401; RRID: AB_2721915; Dako); phosphor-RB Ser608 (1:50; catalog no. 2181; RRID: AB_331517; Cell Signaling Technology, Danvers, MA); p27 (1 µg/mL; catalog no. ab7961; RRID: AB_2244722; Abcam), phospho-p27 (1 µg/mL; catalog no. ab62364; RRID: AB_944575; Abcam); β-actin (1:15,000; catalog no. A5316; RRID: AB_476743; Sigma-Aldrich); and histone H3 (1:1000; catalog no. ab1791; RRID: AB_302613; Abcam). Antigen retrieval was performed for staining of pancreatic sections by heating slides to 90°C for 10 minutes in 10 mM sodium citrate (pH 6.0) solution. All compounds used are available from multiple commercial vendors except the CC-401 derivatives, which were synthesized according to published techniques as outlined in Supplemental Material.
Determination of the rat β-cell transcriptome and quantitative polymerase chain reaction
Rat islets were isolated as described previously in Materials and Methods from 16 rats, rested overnight, trypsinized, and plated into four 35-mm wells. The next day, islet cultures were infected with the HIP-LA lentivirus, which expresses green fluorescent protein (GFP) in insulin+ cells; lentivirus was packaged as described previously (19). The next day (48 hours postplating), the medium was changed. At 72 hours postplating, duplicate cultures were treated with vehicle or CC-401. At 120 hours postplating, cultures were harvested, and GFP+ cells were fluorescence-activated cell sorted for plating or for RNA isolation (∼550,000 cells per condition; RNAqueous-Micro; catalog no. AM1931; Ambion).
Expression library construction was performed with the Encore Complete RNA-Seq DR Multiplex System 1–8 (part no. 0333; NuGen) with an input of 100 ng RNA. Sequencing was performed using Illumina HiSeq 2000 with paired 100 base-pair reads (50 million to 75 million paired-end reads per sample). Analysis was carried out using TopHat (RRID: SCR_013035) and HTseq/DEseq2 (RRID: SCR_005514/RRID:SCR_015687) to assess levels of gene expression and differential expression between treated and untreated β cells. Gene ontology and transcription factor network analysis were performed using Bioconductor goseq (https://bioconductor.org/packages/release/bioc/html/goseq.html) and MetaCore (RRID: SCR_008125; Thomson Reuters, Toronto, ON, Canada) (20).
For quantitative polymerase chain reaction (qPCR) experiments, total RNA was isolated using Zymo Research (Irvine, CA) Quick-RNA MicroPrep kit, with DNAse digestion. RNA was obtained from growth-arrested R7T1 cells (maintained for 7 days in doxycycline-free low glucose Dulbecco’s modified Eagle medium with 10% serum, glutamine, and penicillin/streptomycin; RRID: AB_302613) and primary human islets after 48-hour treatment with DMSO or CC-401 in the aforementioned medium (21). Isolated RNA was reverse transcribed by qScript cDNA Synthesis Kit (Quantabio, Beverly, MA). Real-time qPCR was performed with Power SYBR master mix (Applied Biosystems, Foster City, CA) on the QuantStudio 6 Flex Real-Time PCR System with fast 96-well block (Applied Biosystems). Relative quantification of gene expression was analyzed by the comparative threshold cycle method with GAPDH gene as the endogenous reference (22). The primers used in qPCR are listed in Supplemental Material.
Expression constructs and luciferase assay
Constructs encoding human NFATc1 (NM_001278669) and human DYRK1A (NM_001396) were generated and sequence confirmed. The interleukin 2–based pGL3-NFAT luciferase reporter construct was obtained from Addgene (catalog no. 17870; Cambridge, MA). Luciferase assays were performed by transfecting (0.625 µg polyethylenimine/1 µg of DNA) 10-cm tissue culture plates of 90% confluent HEK 293T (RRID: CVCL_0063) cells with DYRK1A (7.5 µg), Renilla plasmid (10.5 µg; Promega), PGL3-NFAT luciferase (4.5 µg), and NFATc1 (1.5 µg). The next day, cells were trypsinized and transferred to 96-well plates (50 µL/well, 1/300th of total cell volume). After 6 hours, wells were treated with vehicle or compound as indicated (n = 4 per treatment condition) for 24 hours before being lysed (catalog no. E1500; Promega) for luciferase measurement (Modulus Microplate; Turner Biosystems/Promega).
Statistical analysis
Statistically significant differences between treatment conditions were determined using the Student two-tailed t test; P ≤ 0.5 was taken to be significant. Experimental results were confirmed in independent experimentation in all cases except for the primary β-cell replication screening and RNA sequencing experiments. Data are reported with standard deviations.
Results
Chemical screening identified β-cell replication–modulating compounds
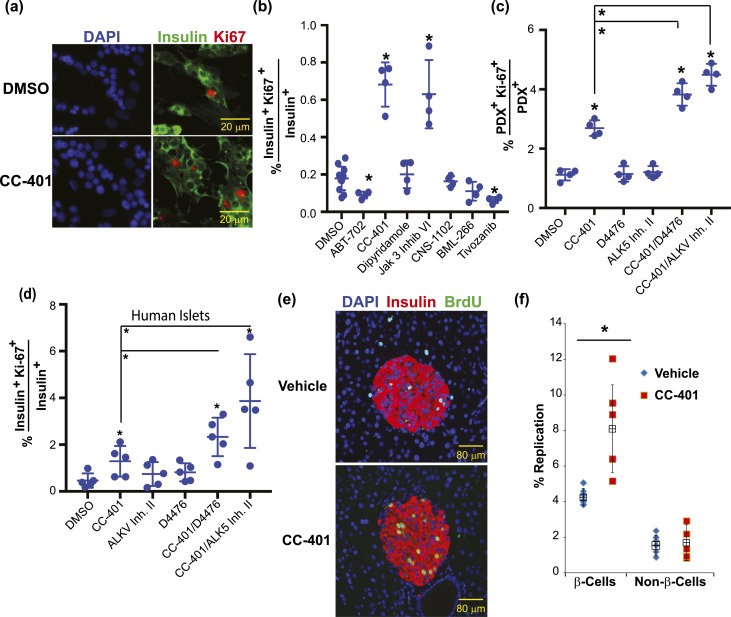
We screened ∼2400 small molecules for the ability to modulate β-cell replication activity [Fig. 1(a); Supplemental Table 1]. Compound screening was conducted at 10 μM (n = 1 to 3) using dispersed primary rat islets (14). β cells were identified by PDX1 expression, also expressed by less prevalent δ cells, and replication events by Ki67 expression (23). Hit compounds were defined by a twofold increase in β-cell replication compared with vehicle-treated (DMSO) wells. Using these criteria, we identified 254 replication-inducing compounds (10.3%). Subsequently, 157 of these compounds were retested and 62 were confirmed by a twofold induction of β-cell replication at any concentration or >1.4-fold induction at more than one concentration [Fig. 1(a); Supplemental Table 2].
Figure 1.
Identification of small-molecule inducers of rat β-cell replication and their combinatorial use. (a) Summary of rat β-cell replication screening results. Compounds are clustered according to their annotated bioactivity. Supplemental Tables 1 and 2 list primary screening and confirmatory testing β-cell–replication results. (b) The β-cell replication–promoting profile of prioritized compounds. Data are presented as mean ± standard deviation (SD; n = 3 to 5 wells per treatment condition; results confirmed in n ≥ 3 independent experiments). (c) Representative images of rat islet cultures treated with vehicle (DMSO) or CC-401. (d) Representative images of compound-treated rat islet cultures. The percentage of replicating PDX1+ cells and compound treatments are indicated. (e) Rat islet β-cell replication indices Ki67 (blue) and PCNA (red). Compound concentrations were as indicated in (b) and was 1 μM for CHIR99021. Individual data points represent 2000 to 3000 β cells (n = 4 to 8 replicates per condition; mean ± SD shown). All compounds increased β-cell replication above DMSO (P < 0.01). All compound combinations increased β-cell replication above the relevant individual compound-treatment conditions (P < 0.01). (f) Rat islet β-cell replication index after compound treatment as indicated. Single compound treatment, CNS-1102 combinations, and BML-266 combinations are shown. All individual compound treatments increased replication (vs DMSO; P < 0.01); select combinations increased β-cell replication vs relevant individual compound-treated conditions. *P < 0.01. Individual data points represent 2000 to 3000 β cells (n = 4 to 8 replicates per condition; mean ± SD shown). Error bars represent the standard deviation of an experimental condition (n ≥ 3). cAMP, cyclic adenosine monophosphate; CAS#, Chemical Abstracts Service number; PDE, phosphodiesterase.
The purported activities of confirmed replication-inducing compounds clustered into several functional categories. Among the hit compounds were established β-cell replication–promoting compound classes including the adenosine kinase inhibitors 5-iodotubercidin and ABT-702, the insulin-secretion modulator glibenclamide, the phosphodiesterase inhibitor dipyridamole, and the glycogen synthase kinase-3β (GSK-3β) inhibitor CHIR99021 (14, 15, 24, 25). Reidentification of established β-cell replication–promoting compounds confirmed prior findings and demonstrated high-quality screen performance. In addition, numerous β-cell replication stimulators were identified [Fig. 1(b); Supplemental Table 2]. Notably, the replication-promoting activity of hit compounds was frequently not shared by similarly acting molecules. For example, two chemical vascular endothelial growth factor (VEGF) receptor antagonists [tivozanib, 5.1-fold replication (5 µM); and XL880, 1.6-fold replication (10 µM); Supplemental Table 2] promoted β-cell replication, whereas recombinant soluble VEGF receptor, a highly specific VEGF inhibitor, was inactive [Supplemental Fig. 1(a)]. Similarly, the replication-promoting activity of Janus kinase 3 (JAK3) inhibitor VI (Supplemental Tables 1 and 2) was not shared by the JAK3 inhibitors tofacitnib and ruxolitinib [Supplemental Fig. 1(a)]. This discordance indicated that off-target effects were the likely basis of compound activity.
We selected compounds for follow-up studies primarily based on robustness of activity, greater than twofold induction of rat β-cell replication, and confirmation with distinct cell-identity and replication markers [Fig. 1(b) and 1(c)]. Prioritized hits included previously identified compounds namely, ABT-702 and dipyridamole) and newly recognized compounds (namely, CC-401, Jak3 inhibitor VI, CNS-1102, BML-266, and tivozanib).
In addition to uncovering compounds that promoted β-cell replication, we identified 153 compounds (2.15%) that reduced β-cell replication by ≥50% (Supplemental Table 1). Several of these compounds have shared annotated activities: seven calcium channel blockers, two adenosine receptor antagonists, 11 adrenergic agonists, 10 cyclooxygenase/prostaglandin modulators, 27 modulators of neurotransmission (e.g., dopamine, acetylcholine, serotonin, and histamine), eight histone deacetylase inhibitors, and two melatonin signaling modulators. Although these molecules highlight pathways potentially relevant to β-cell dysfunction and diabetes predisposition, subsequent work herein focused on β-cell replication–inducing compounds.
Compound combinations cooperatively promoted rat β-cell replication
Given the limited replication capacity of β cells, we tested whether combinations of hit compounds could be used to cooperatively enhance β-cell replication. Indeed, on rat islet cultures, CC-401 and Jak3 inhibitor VI positively interacted with most other replication-inducing compounds [Fig. 1(d)–1(f)]. The most efficacious drug combination was CC-401 and BML-266 (31.3% ±3.0%]). Notably, some drug combinations did not show cooperativity. For instance, combined treatment with ABT-702 and BML-266 yielded a replication index of 7.5% ± 0.8%, which was not statistically different from cells treated with BML-266 alone (7.1% ± 1.7%). These studies further validated the replication-promoting activity of CC-401 and demonstrated the extraordinary power of combinatorial treatment of promoting considerable levels (>25-fold above baseline) of rat β-cell replication.
CC-401 induced human β-cell replication
Next, we evaluated the ability of select compounds to promote human β-cell replication and the feasibility of synergistic human β-cell–replication induction with combinatorial compound treatment. Whereas CC-401 and Jak 3 inhibitor VI consistently increased the percentage of insulin+ Ki67+ double-positive cells (approximately 2- to 10-fold), other hit compounds did not [Fig. 2(a) and 2(b)]. The GSK-3β inhibitor CHIR99021 demonstrated inconsistent human β-cell replication–promoting activity. For further experimentation, we focused on CC-401, an advanced lead compound that was developed as a chemotherapeutic c-Jun N-terminal kinase (JNK) inhibitor (Celgene, Summit, NJ) and has been used in a phase 1 human study (26). Interestingly, with the exception of the GSK-3β inhibitor, CHIR99021, compound combinations that synergistically promoted rat β-cell replication [Fig. 1(d)–1(f)] did not cooperatively increase human β-cell replication in either the absence or presence of exenatide [Supplemental Fig. 1(b)], highlighting differences between rat and human β-cell replication control.
Figure 2.
CC-401 stimulates human β-cell replication in vitro and mouse β-cell replication in vivo. (a) Representative immunofluorescence images of vehicle- (DMSO) and CC-401–treated human islet cultures [DAPI (blue); insulin (green)]. (b) The β-cell–replication index of dispersed human islet cultures after compound treatment (48 hours). Independent treatments along with mean ± standard deviation (SD) are shown. *P < 0.05. Similar data were obtained from at least five independent islet procurements. (c) Rat β-cell–replication index of compound-treated (CC-401, 10 µM; D4476, 5 µM; and ALK5 inhibitor II, 2 µM) primary islet cultures. Independent replicates (n = 4) are shown with mean ± SD. *P < 0.01; >1000 β cells counted per data point. (d) Human β-cell–replication index of compound-treated islet cultures. Independent replicates (n = 5) are shown with mean ± SD. *P < 0.05; >1000 β cells counted per data point. (e) Representative images of pancreatic sections from 8-week female vehicle- and CC-401–treated mice stained for insulin (red), BrdU (green), and nuclei (blue). See Supplemental Fig. 2 for determination of CC-401’s in vivo half-life and in vitro potency. (f) The BrdU incorporation index (percentage of replication) of β cells (insulin+) and non–β cells (insulin−) after treatment with vehicle or CC-401 (25 mg/kg) for 1 week. Data from individual mice (n = 5) and mean ± SD shown. *P < 0.05. Error bars represent the standard deviation of an experimental condition (n ≥ 3). Two independent experiments were performed with similar results. See Supplemental Fig. 2 for in vitro replication effects on α cells, δ cells, and dermal fibroblasts. ALKV Inh. II, activin A receptor type II–like kinase inhibitor II.
In contrast to prior work demonstrating a β-cell replication–promoting activity for transforming growth factor-β (TGF-β) inhibitors, these compounds were not identified in primary screening: activin A receptor type II–like kinase (ALK5) inhibitor II, 1.79-fold; Alk5 inhibitor VI, 1.40-fold; Alk5 inhibitor V, 1.29-fold; and LY 364947, 1.29-fold (27). To explain this discrepancy, we hypothesized that the growth-promoting activity of TGF-β inhibition is context dependent. Therefore, we assessed the rat and human β-cell replication–promoting activity of TGF-β inhibitors (5 µM D4476 and 2 µM Alk5 inhibitor II) in isolation and in combination with CC-401 [Fig. 2(c) and 2(d)]. Consistent with the primary screening results, these compounds had no replication promoting activity when used in isolation. However, combining TGF-β receptor inhibitors with CC-401 increased both rat and human β-cell replication, indicating a context-specific replication-promoting activity of TGF-β receptor inhibitors.
CC-401 stimulated mouse β-cell replication in vivo
Having demonstrated CC-401 promoted both rat and human β-cell replication in vitro, we assessed whether CC-401 also stimulated mouse β-cell replication in vivo. To develop a dosing strategy, we measured the half-life of CC-401 delivered by IP injection. At a dose of 25 mg/kg, CC-401 achieved a maximum plasma concentration of ∼5 µM with a half-life of ∼53 minutes [Supplemental Fig. 2(a)]. Based on the in vitro proliferation data, CC-401 had a half maximal effective concentration (EC50) of 5.2 ± 0.8 µM and a minimum effective concentration of ∼1.0 µM [Supplemental Fig. 2(b)]. At the examined dose, plasma levels of CC-401 remained above the minimum effective concentration for ∼2 hours. Although 25 mg/kg achieved limited drug exposure, increased dosing led to weight loss. Consequently, the effect of 25 mg/kg/d IP for 7 days on pancreatic-cell replication (BrdU incorporation) was evaluated. CC-401 increased β-cell replication in treated rats compared with vehicle-treated animals [8.1% ±3% vs 4.3% ±0.5%; P = 0.02; Fig. 2(e) and 2(f)]. No replication effect was observed on the extraislet non–β-cell population (CC-401, 1.7% ± 1% vs vehicle, 1.5% ± 0.6%; P = 0.78). However, CC-401 did promote islet α-cell and δ-cell but not primary dermal fibroblast replication in vitro [Supplemental Fig. 2(c) and 2(d)]. Therefore, CC-401 increased mouse β-cell replication in vivo and demonstrated limited lineage-restricted growth-promoting activity.
CC-401 promoted β-cell replication via DYRK1A/B inhibition
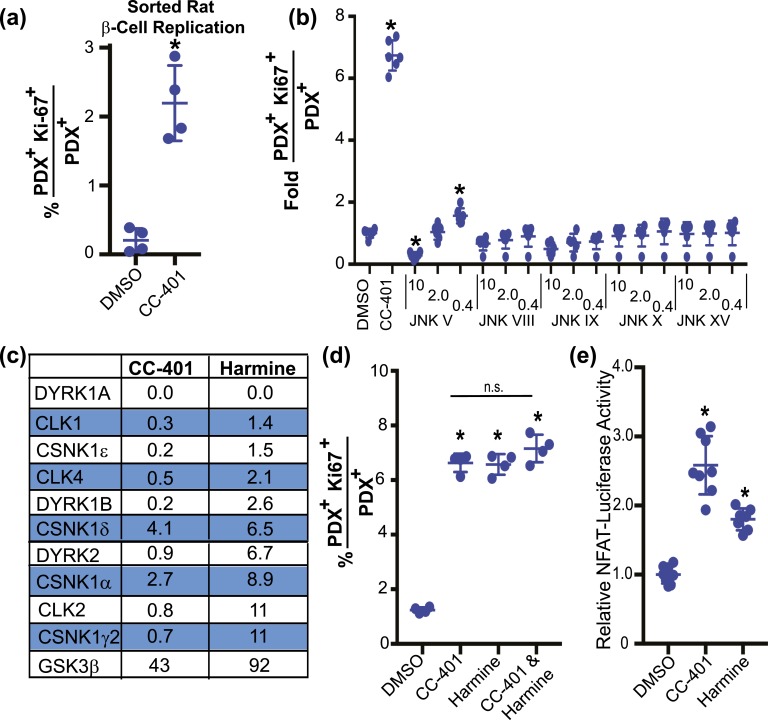
To explore CC-401’s mechanism of action and minimize potential influences of other cell types, we assessed the replication response of purified β cells (∼95% purity) to CC-401 treatment [Fig. 3(a); Supplemental Fig. 3]. β cells were purified by fluorescence-activated cell sorting using a lentivirus-based insulin-GFP reporter system (19). CC-401 treatment increased purified rat β-cell replication (P < 0.01) from 0.2% ± 0.2% to 2.6% ± 0.3%, indicating CC-401 likely acted directly on β cells. Because CC-401 was developed as a JNK inhibitor, we hypothesized that CC-401 promoted β-cell replication via JNK inhibition. However, no other tested JNK inhibitor recapitulated the robust replication-promoting activity of CC-401 [Fig. 3(b)], indicating an alternative kinase was likely to be the relevant target.
Figure 3.
CC-401 promotes β-cell replication via DYRK1A/B inhibition. (a) Replication index of vehicle- and CC-401–treated fluorescence-activated cell sorting–purified rat β cells (see also Supplemental Fig. 3). Individual data points and mean ± standard deviation (SD) are shown (n = 4 wells per treatment condition with >1000 β cells per well). *P < 0.05. (b) Rat β-cell–proliferation index of JNK inhibitor–treated islet cultures. Individual wells and mean ± SD shown (n = 6 wells per treatment condition). *P < 0.05 vs DMSO treatment. (c) Percent residual kinase activity after incubation with the specified compound (10 µM). See Supplemental Table 4 for complete kinome datasets and Supplemental Fig. 4(a)–4(c) for the replication-promoting activity of additional DYRK1A/B inhibitors. (d) In vitro rat β-cell–replication response to the indicated compound treatment(s) (48 hours). Data from individual wells with mean ± SD are shown (n = 4 wells per treatment condition; >1000 β cells counted per well). *P < 0.05. (e) Fold-induction of NFAT-firefly luciferase reporter activity from cells cotransfected with Renilla (used for normalization across transfections), NFATc1, and DYRK1A expression plasmids, and treated with DMSO, CC-401, or harmine. Data from individual wells (n = 8) are presented with mean ± SD. *P < 0.05. Error bars represent the standard deviation of an experimental condition (n ≥ 3).
To explore potential kinase targets, we performed kinome screening with 10 µM CC-401 using 453 human kinases (DiscoveRx, San Diego, CA; Fig. 3(c); Supplemental Table 3). CC-401 displayed only modest in vitro selectivity at high concentration (10 µM) (18). Among the most inhibited kinases were the DYRK1A and DYRK1B previously implicated in harmine-induced β-cell replication (10). Notably, Jak3 inhibitor VI, the only other human β-cell replication–inducing compound we identified, is also a high-affinity DYRK1A-binding molecule [Supplemental Fig. 4(a)]. Hence, DYRK1A/B inhibition was the most likely mechanism of CC-401–dependent β-cell–replication induction.
Harmine is a β-cell replication–promoting compound used to establish the role of DYRK1A/B in controlling β-cell replication (10). Given our suspicion that CC-401 promoted β-cell replication via DYRK1A/B inhibition, we assessed whether CC-401 and harmine had a shared mechanism of action. As a first step, we measured β-cell replication in response to treatment with these compounds independently and in combination [Fig. 3(d)]. We hypothesized that the combination of these compounds would not substantially increase β-cell replication above independent compound treatment if they used a common growth-promoting pathway.
Indeed, CC-401 and harmine independently induced β-cell replication (5.4 ± 0.3-fold and 5.3 ± 0.3-fold, respectively), whereas the combination provided an insignificant increase in β-cell replication (5.8 ± 0.4-fold), consistent with the hypothesis that these molecules have a shared mechanism of action. In addition, activation of NFAT-dependent transcription is the proposed mechanism by which DYRK inhibitors, including harmine, promote β-cell replication (10, 11, 13). CC-401 and harmine demonstrated similar ability to activate an NFAT-luciferase reporter system [Fig. 3(e)]. These data supported the hypothesis that CC-401 promoted β-cell replication via DYRK1A/B inhibition.
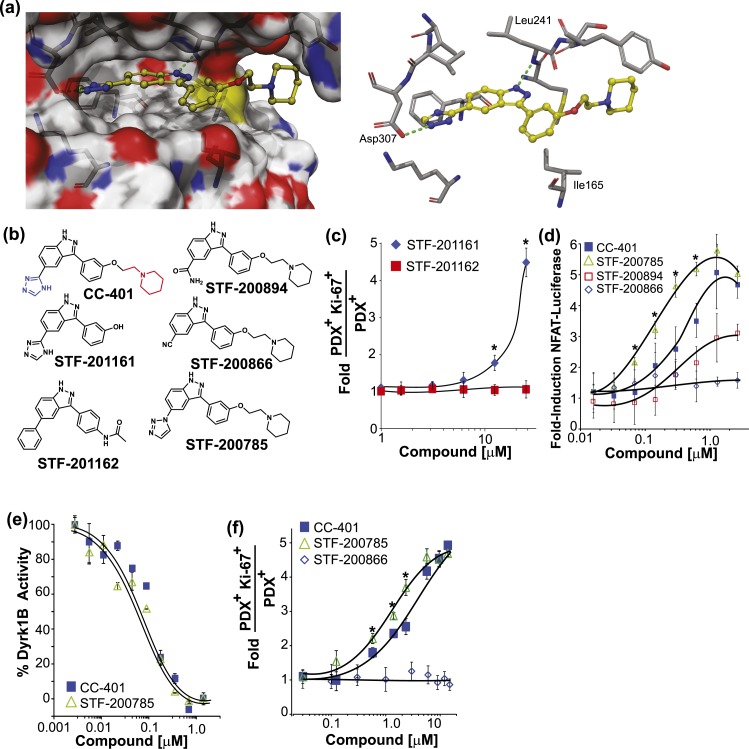
To establish DYRK1A/B more firmly as the relevant β-cell replication target, we used structure-based design to guide synthesis of molecules that interrogate key molecular interactions between CC-401 and DYRK1A. In silico docking of CC-401 into the DYRK1A active site predicted key interactions for ligand binding including a hydrogen bonds between a triazole ring nitrogen with the carboxyl group of Asp307 and an indazole nitrogen with the amide hydrogen of Leu241 [Fig. 4(a)]. In addition, several van der Waals contacts between CC-401 and DYRK1A were predicted, most notably between the phenyl ring and Ile165, and the indazole ring with the side chain of Leu241. Consistent with this model, STF-201161, which lacks the solvent-exposed piperidine group, retained β-cell replication–promoting activity, whereas STF-201162, which lacks the Asp307-binding triazole group, was no longer active [Fig. 4(b) and 4(c)]. Furthermore, substitution of the triazole with a nitrile group (STF-200866) disrupted compound activity [Fig. 4(b) and 4(d)], whereas inclusion of a basic amide group (STF-200894), predicted to retain the ability to hydrogen bond with Asp307, retained cellular DYRK1A-inhibition activity, albeit with reduced potency [CC-401 (EC50, 0.37 ± 0.07 µM) vs STF-200894 (EC50, 1.59 ± 0.7 µM). Surprisingly, STF-200785, which replaced the 1,2,4-triazole of CC-401 with a 1,2,3-triazole, demonstrated a ∼2.5-fold improvement in potency (STF-200785 (EC50, 0.15 ± 0.03 µM) with respect to NFAT-luciferase induction in the cellular luciferase assay [Fig. 4(d)]. However, consistent with our model, CC-401 and STF-200785 demonstrated equivalent in vitro DYRK1B kinase inhibition activity [CC-401, half maximal inhibitory concentration, 0.08 ± 0.05 µM vs STF-200785, half maximal inhibitory concentration, 0.09 ± 0.03 µM; Fig. 4(e)], indicating enhanced NFAT induction was independent of intrinsic kinase-inhibition activity. Indeed, STF-200785 displayed substantially enhanced diffusion in the Caco-2 cell assay (STF-200785 PAPP A to B = 5.75 ± 1.9 × 10−6 cm/s vs CC-401 PAPP A to B = 0.50 ± 0.45 × 10−6 cm/s), consistent with improved cellular permeability. Accordingly, STF-200785 displayed significantly improved potency with respect to β-cell–replication induction [STF-200785, EC50, 1.0 ± 0.4 µM vs CC-401, EC50, 4.8 ± 0.6 µM; Fig. 4(f)]. Therefore, CC-401 induces β-cell replication via DYRK1A/B inhibition and STF-200785 is a CC-401 derivative with modestly improved β-cell replication–promoting activity.
Figure 4.
Rationally designed CC-401 derivatives confirm DYRK1A/B as the relevant β-cell–replication target. (a) Space-filling (left) and stick (right) models of CC-401 binding to DYRK1A [4MQ2; Protein Data Bank (28)] generated with Schrodinger Glide software. Key hydrogen bond interactions are highlighted (green dashed line). (b) Chemical structures of CC-401 and synthesized derivatives. The piperidine group (red) and the triazole group (blue) are highlighted on CC-401. (c) Rat β-cell–proliferation response to STF-201161 and STF-201162. Data are presented as mean ± standard deviation (SD; n = 4 wells per treatment condition). *P < 0.05. (d) Fold-induction of NFAT-luciferase reporter activity in cells cotransfected with NFATc1, DYRK1A, and Renilla luciferase. Cells were compound treated as indicated. Data are presented as mean ± SD (n = 4 wells per experimental point). *P < 0.01 shown for STF-785 vs CC-401. A significant (P < 0.05) luciferase induction above DMSO occurred in response to CC-401 at a concentration >0.1 µM, STF-200785 (>0.05 µM), and STF-200894 (>2.0 µM). No luciferase induction occurred in response to STF-200866. (e) Recombinant DYRK1B activity measured in the presence of CC-401 or STF-200785 (n = 2 wells per experimental point). *P < 0.01. Half maximal inhibitory concentrations were not statistically different. (f) Fold increase in rat β-cell replication in response to the indicated compound treatment (n = 4 wells per treatment condition presented as mean ± SD). *P < 0.05 shown for STF-785 vs CC-401. No replication response occurred in response to STF-200866. Error bars represent the standard deviation of an experimental condition (n ≥ 3).
CC-401 promoted β-cell replication via p27 destabilization and derepression of the DREAM complex
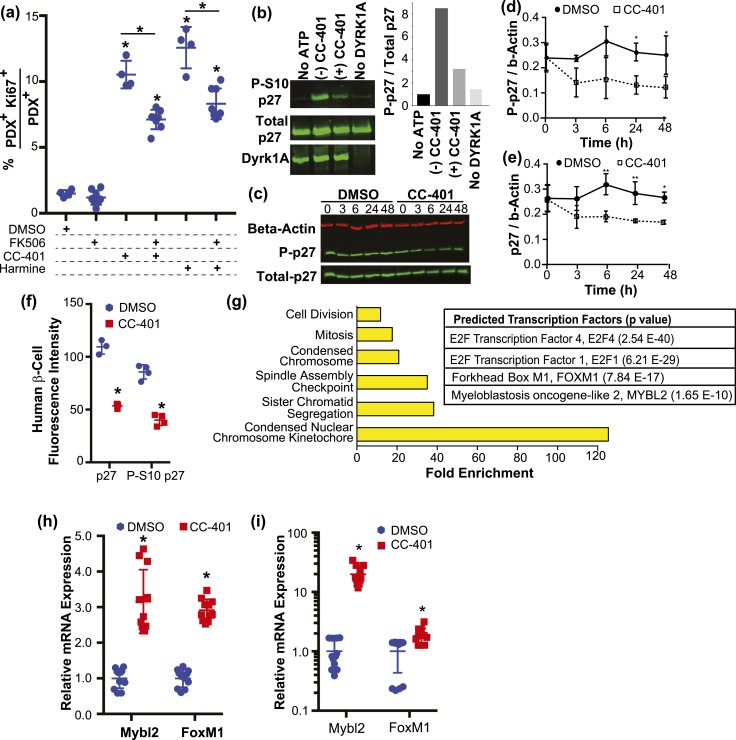
Prior work identified NFAT activation as the primary mechanism of DYRK1A inhibition–dependent induction of β-cell replication (10, 11, 13). However, FK506, a potent inhibitor of NFAT activity, only partially inhibited CC-401– or harmine-dependent induction of β-cell replication [Fig. 5(a)], suggesting NFAT-independent pathways contribute to CC-401–induced β-cell replication. Interestingly, the cyclin-dependent kinase inhibitor p27kip1 is a primary controller of β-cell transition from quiescence to proliferation and is stabilized by phosphorylation at serine 10 (S10), a potential phosphorylation target of DYRK1A/B (29–31). Accordingly, we assessed whether recombinant DYRK1A increased phosphorylation of p27kip1 at S10 in vitro.
Figure 5.
Inhibition of DYRK1A/B promotes β-cell replication via NFAT activation, p27kip1 destabilization, and DREAM-complex target gene derepression. (a) In vitro rat β-cell–replication indices after compound treatment as indicated. The results from individual wells (n = 4 to 7) are shown with mean ± standard deviation (SD). *P < 0.05 vs the DMSO-treated condition or as indicated. (b) Western blot analysis of phospho-S10p27, p27, and DYRK1A after in vitro incubation of recombinant p27 and DYRK1A with and without adenosine triphosphate, CC-401, or DYRK1A. Quantitation of S10 phosphorylated p27 intensity normalized to total p27 are shown in the right-side panel. (c) Representative western blot of R7T1 β-cell lysates probed for β-actin, p27, and phosphoS10-p27 after treatment of cultures with DMSO or CC-401 for the indicated time (hours). Quantitation of the relative levels of (d) phosphoS10-p27 and (e) total p27 obtained from western blots (n = 3) performed as in (c). Protein levels were normalized to β-actin levels. *P < 0.05 vs DMSO condition at the relevant time point. (f) The average fluorescence intensity of p27 and phospho-S10p27 measured in β cells (PDX1+) from human islet cultures treated with DMSO or CC-401. Individual wells (n = 4; >1500 β cells per well) are shown with mean ± SD. *P < 0.01. (g) Enriched gene ontology categories (left) and transcription factor networks (right) identified by MetaCore analysis of differentially expressed genes from RNA sequencing performed on fluorescence-activated cell–sorted β cells after vehicle- and CC-401–treatment (48 hours); see Supplemental Table 4 for complete results. (h) Relative gene expression levels determined by qPCR (normalized to Gapdh) after treatment of growth-arrested R7T1 β-cell cultures with DMSO or CC-401 for 48 hours. (i) Relative gene expression levels determined by qPCR (normalized to GAPDH) after treatment of human islet cultures with DMSO or CC-401 for 48 hours. Error bars represent the standard deviation of an experimental condition (n ≥ 3). ATP, adenosine triphosphate.
We observed this activity [Fig. 5(b)]. In addition, incubation with CC-401 reduced DYRK1A phosphorylation of p27kip1 at S10 [Fig. 5(b)], confirming CC-401 as a DYRK1A inhibitor and S10p27kip1 as a DYRK1A phosphorylation target. Furthermore, CC-401 treatment of R7T1 β cells led to a reduction in total and P-S10 p27kip1 levels [Fig. 5(c)–5(e)] (21). CC-401 treatment of human islet cultures for 24 hours also reduced β-cell p27kip1 and P-S10 p27kip1 levels [Fig. 5(f)]. These findings indicate that inhibition of DYRK1A/B promotes destabilization of p27kip1, a known mechanism for β-cell proliferation.
To further understand how DYRK1A/B inhibition promotes β-cell replication, we determined the transcriptional program induced by CC-401 treatment. RNA sequencing was performed on sorted vehicle- and CC-401–treated rat β cells (48 hours; n = 2 for each condition) and compared. Eighty-two (adjusted P < 0.05) or 583 (unadjusted P < 0.05) differentially expressed genes were identified (Supplemental Table 3). As anticipated, gene ontology analysis demonstrated differential expression of cell division–associated transcripts, consistent with CC-401’s replication-promoting activity [Fig. 5(g)]. To better understand how CC-401 induced a replication-associated gene expression program, transcription factor network analysis (MetaCore) was performed on differentially expressed genes. Interestingly, E2F transcription factor 1, an established regulator of β-cell replication (32), as well as the multiprotein DREAM (comprising DP, RBL1, RBL2, E2F4, and the MuvB core) and MMB (Myb-MuvB, including MYBL2, FOXM1, and the MuvB core) complexes were strongly implicated [Fig. 5(g)].
The function of the DREAM complex is to maintain cellular quiescence through repression of cell-cycle-promoting genes, and the functional integrity of this complex depends on DYRK1A/B activity (33, 34). Notably, seven of the top 10 CC-401–induced β-cell genes (Mcm5, Asf1b, Aurkb, Arhgap11a, Kifc1, Rrm2, and Ccna2; Supplemental Table 4) are bound by the DREAM complex in G0 (repressed) and have established roles in promoting cellular division (35). In contrast to the DREAM complex, the function of the MMB complex is to promote cell-cycle progression (36, 37). Indeed, inhibition of DYRK1A/B with CC-401 also induced rat β-cell expression of the cell-cycle–associated MMB complex genes, Mybl2 (1.6-fold) and Foxm1 (2.0-fold; Supplemental Table 4). Furthermore, CC-401–dependent induction of Mybl2 and Foxm1 expression was confirmed by qPCR in growth-arrested R7T1 mouse β cells and primary human islets [Fig. 5(h) and 5(i)]. Although human islets demonstrated the highest fold-induction of MYBL2 gene, baseline Mybl2 expression levels in growth-arrested R7T1 cells was ∼50 times more than that of human islets. This observation is consistent with the very low basal replication and limited compound-induced replication response of human β cells. Hence, CC-401–dependent DYRK1A/B inhibition promotes β-cell replication in part via destabilization of the DREAM complex, as evidenced by derepression of numerous DREAM complex–regulated cell-cycle–promoting genes including the MMB complex components, MYBL2 and FOXM1.
Discussion
We have used primary β-cell replication screening to characterize the replication-modulating activity of ∼2400 bioactive compounds on primary rat β cells. Our investigation focused on characterizing CC-401, an advanced lead molecule that stimulated rodent and human β-cell replication. Several key findings and concepts with broad implications emerged from our studies. First, the plethora of identified replication-modulating compounds provide a new resource for probing the molecular pathways that control β-cell replication and function. Second, DYRK1A/B-inhibition appears sufficient to induce β-cell replication; although CC-401 is a promiscuous kinase inhibitor, structurally diverse DYRK1A/B inhibitors with distinct target profiles all promoted β-cell replication. Third, combinatorial treatment with replication-promoting compounds or compounds engineered toward multiple therapeutic targets (e.g., TGF-β receptor and DYRK1A/B) may achieve an enhanced human β-cell replication response. Notably, replication induction of human β cells generally requires DYRK1A/B inhibition, though we observed a variable replication response to isolated GSK-3β inhibition. Fourth, DYRK1A/B inhibition drives β-cell replication via simultaneous modulation of multiple growth regulatory pathways including NFAT signaling activation, p27kip1 destabilization, and derepression of DREAM-complex target genes. Finally, as shown for CC-401, improved β-cell replication–promoting compounds (e.g., STF-200785) may be derived from hit molecules identified in this screen.
Multiple lines of evidence support the conclusion that DYRK1A and DYRK1B are the relevant targets of CC-401–induced β-cell replication. First, numerous structurally diverse DYRK1A/B inhibitors, including CC-401, Jak 3 inhibitor VI, harmine, leucettine, AZ-191, TG-003, and 5-IT, demonstrate β-cell replication promoting activity. Notably, kinome inhibition analysis of 466 kinases with mL-401, harmine, and AZ191 at high concentration (10 µM) reveal only eight shared targets (namely, DYRK1A/B, CLK1/4, HASPIN, CSNK1D/A, and DYRK2). Suggesting that one of these kinases normally represses β-cell replication. The established role of DYRK1A/B in controlling cell-cycle entry makes these kinases the most likely relevant targets. Second, assessment of selective CSNK1A/D inhibitors (i.e., CKI 7, LH 846, PF 4800567, and PF 670462) did not promote β-cell replication (data not shown). Third, structure-based design and synthesis of CC-401 derivatives predicted not to inhibit DYRK1A/B failed to activate the DYRK1A-dependent NFAT reporter system or to induce β-cell replication. Finally, in prior work, knockdown of DYRK1A promoted β-cell replication (11, 38). These data strongly suggest that DYRK1A/B are the relevant β-cell replication targets for CC-401 but do not entirely exclude a contribution from the other potential mechanisms.
Although our investigation focused on β-cell replication–inducing compounds, we also uncovered numerous β-cell replication inhibitors. Interestingly, human variants in at least two recurrently identified β-cell replication–inhibiting compound targets, the adrenergic receptor ADRA2A and the melatonin receptor MTNR1B, are associated with increased risk for diabetes (39, 40). Not surprisingly, the mTORC1 inhibitor rapamycin, which increases diabetes risk, also inhibited β-cell replication (41). Additionally, several antidepressant (namely, fluoxetine, imipramine, despiramine) and antipsychotic (namely, reserpine, quetiapine) medications linked to an increased risk of developing diabetes were among the most effective inhibitors of β-cell replication we identified (42, 43). Consequently, the collection of β-cell–replication inhibitors provided herein is valuable resource for uncovering pathways that impair β-cell replication, compromise β-cell function, and potentially contribute to an increased diabetes risk.
The different replication capacities of rodent and human β cells are well established (44). Accordingly, diverse chemical entities induced rodent β-cell replication, whereas only DYRK1A/B inhibitors consistently induced human β-cell replication. Whereas prior work emphasized induction of NFAT signaling, a well-characterized β-cell replication pathway (10–12), as the central mechanism of human β-cell–replication induction by DYRK1A/B inhibitors, we propose that the coordinated activation of multiple growth-promoting pathways is a more likely basis for the unique activity of DYRK1A/B inhibitors. Indeed, multiple compounds (e.g., glucose and sulfonylureas) that promote NFAT activation and rodent β-cell replication via β-cell depolarization and calcium flux do not induce human β-cell replication (45). Consequently, additional DYRK1A/B-regulated pathways significantly contribute to human β-cell–replication induction. In neuronal precursor cells, overexpression of DYRK1A induces cell-cycle arrest via phosphorylation/stabilization of the cell-cycle inhibitor p27kip1 (30, 46). Accordingly, we found that DYRK1A/B inhibition with CC-401 led to destabilization of p27kip1, an important negative regulator of β-cell replication (47). Additionally, DYRK1A/B act as potent negative regulators of cellular replication by stabilizing the DREAM complex, which maintains cells in quiescence via repression of cell cycle–associated gene expression (34).
Although the function of the DREAM complex has been explored in a variety of cell types, its role in maintaining β-cell quiescence has not been considered, to our knowledge. Herein, we establish DREAM-complex target genes as the most highly regulated factors in response to DYRK1A/B inhibition and β-cell–replication induction. In addition, CC-401–dependent inhibition of DYRK1A/B led to derepression of cell cycle entry or progression–associated MMB complex genes (i.e., Mybl2 and Foxm1) in rodent and human β cells. Notably, Foxm1 expression is critical for postnatal β-cell expansion, but overexpression of an activated form of FOXM1 is insufficient to promote β-cell replication (48, 49). This may reflect the dependence of Foxm1 and Mybl2 for recruitment to and activation of cell-cycle genes (50), highlighting, why inhibition of DYRK1A/B, the master regulator of DREAM-complex stability, might be uniquely capable of coordinating the multifaceted expression program necessary to drive human β cells into the cell cycle. Importantly, the induction of Foxm1 and Mybl2 correlated with the induction of β-cell replication but was not shown to be necessary or sufficient.
Given the relatively limited replication response of human β cells to DYRK1A/B inhibition (typically <1% Ki67 positivity), we explored whether combinations of replication-inducing compounds acted cooperatively. Although such an effect was observed in rat β cells, human β-cell replication was only further increased, above treatment with CC-401 alone, when GSK-3β (as previously recognized) (11) or ALK5/TGF-β inhibitors were added. Notably, isolated treatment with ALK5/TGF-β inhibitors failed to induce rat or human β-cell replication in our in vitro system, indicating the potential of identifying human β-cell replication–promoting compounds that increase replication competence without directly promoting β-cell replication. Importantly, to our knowledge, a systematic combinatorial treatment strategy has not been applied to the challenge of understanding and uncovering the interactions among the disparate pathways that modulate human β-cell replication.
An important limitation of the current study is the failure to demonstrate that in vivo treatment with CC-401 increased β-cell mass or rescued the diabetic condition. Unfortunately, extending the compound treatment duration led to weight loss, confounding the interpretation of CC-401’s effects on glucose homeostasis and β-cell replication/expansion (data not shown). Additional exploration of CC-401’s therapeutic potential will require development of optimized derivatives. Notably, prior efforts to demonstrate therapeutic β-cell mass expansion in vivo are inconclusive. For example, harmine treatment of diabetic mice that had received a subtherapeutic human islet transplant demonstrated improved glucose homeostasis within days of initiating harmine treatment (10). This rapid therapeutic response is more consistent with harmine-induced improvements in β-cell function or peripheral insulin sensitivity, possibly via PPARγ induction, rather than β-cell expansion (51). Similarly, long-term in vivo treatment with the aminopyrazines also demonstrated glucose homeostasis improvements in diabetic mice (11). However, these effects were at least partially related to reduced insulin resistance, given that compound-treated animals demonstrated dramatically increased insulin-dependent glucose reduction (insulin tolerance test) when calculated by percent reduction rather than absolute glucose reduction. Finally, mice treated with the β-cell replication–inducing compound 5-IT displayed treatment-related improvement in glucose tolerance after biweekly dosing (0.25 mg/kg IP, six total doses) (13). Pharmacodynamic data substantiating 5-IT–dependent β-cell expansion as the cause of restored glucose homeostasis was not provided. Hence, in vivo pharmacologic expansion of β-cell mass remains an unproven strategy for resolving diabetes.
The observation that pharmacologic DYRK1A/B inhibition promotes mature β-cell replication must be reconciled with conflicting in vivo data. Surprisingly, DYRK1A-haploinsufficient mice are diabetic with reduced β-cell mass and proliferation (52). In addition, treatment of E11.5 explants with harmine reduced insulin+ cell generation, raising the possibility that DYRK inhibition has discrepant effects on immature or progenitor cells and mature β cells. Consistent with DYRK1A activity potentiating β-cell growth in vivo, BAC transgenic overexpression of DYRK1A increases β-cell mass and protects against a diabetogenic diet (53). The relevance of the DYRK1A-haploinsufficient mice to the pharmacologic effects of CC-401 and harmine is complicated by the residual activity of DYRK1B, which may have an opposing function. In humans, a low-frequency DYRK1B variant (L28P) that is predicted to impair kinase function is protective against diabetes, whereas activating DYRK1B mutations R102C and H90P are causally associated with developing metabolic syndrome features, including diabetes (54, 55). Work will need to address the discrepant effects of pharmacologic and genetic inhibition of DYRK1A/B activity on β-cell replication.
In conclusion, we have identified CC-401 as a human β-cell replication–promoting small molecule that acts via DYRK1A/B inhibition. We leveraged CC-401 to demonstrate that DYRK1A/B inhibition–dependent induction of β-cell replication is multifactorial, including activation of NFAT signaling, destabilization of p27kip1, and disruption of DREAM complex–dependent repression of cell-cycle genes, including MYBL2 and FOXM1. Additionally, we demonstrated the potential of combining small molecule inhibitors to augment the limited replication response of human β cells. Key issues to address in the future are the development of strategies to target regenerative compounds selectively to the β cell, because DYRK1A/B inhibition has broad replication-promoting activity, and to assess whether forced expansion of β cells leads to compromised function (i.e., reduced glucose-stimulated insulin secretion or cellular viability).
Supplementary Material
Acknowledgments
We thank Sara Sun (Stanford University, Stanford, CA) for technical support. We thank Dr. Donald Kohn for providing the β-cell reporter virus (University of California, Los Angeles, CA).
Financial Support: This research was supported by the Friedenrich BII Diabetes Fund and National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK101530 and P30DK116074 (to J.P.A.); the SPARK and CHRI programs at Stanford University School of Medicine (NIH, National Center for Advancing Translational Sciences Clinical and Translation Science Awards Grant UL1TR001085 to Y.A.); Endocrinology Training Grant T32DK007217 (to P.A.); Molecular Pharmacology Training Grant 5T32GM113854 (to H.P.M.); and Stanford Chemistry, Engineering and Medicine for Human Health Chemistry/Biology Interface Predoctoral Training Program and Bio-X and Stanford Interdisciplinary Graduate Fellowship (to T.M.H.).
Author Contributions: J.P.A. conceived the study and provided resources. Y.A., S.L., P.A., T.M.H., R.J.N., D.M., and A.S. determined the study methodology. Y.A., Z.Z., H.X., P.A., T.M.H., B.Y., R.J.N., D.M., A.S., M.S., and J.P.A. conducted the investigations. Y.A., S.L., P.A., N.A.A. and J.P.A. wrote the original manuscript. Y.A., Z.Z., S.L., P.A., T.M.H., B.Y., H.P.M., M.S., N.A.A., and J.P.A. reviewed and edited the manuscript. Y.A., S.L., T.M.H., and J.P.A. acquired funding for the study. M.S. and J.P.A. supervised the study.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 5-IT
5-iodotubercidin
- ALK5
activin A receptor type II–like kinase
- BrdU
bromodeoxyuridine
- DAPI
4′,6-diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- DREAM
dimerization partner, RB-like, E2F and multivulval class B
- DYRK
dual-specificity tyrosine phosphorylation–regulated kinase
- DYRK1A/B
dual-specificity tyrosine phosphorylation–regulated kinases 1A/B
- EC50
half maximal effective concentration
- GFP
green fluorescent protein
- GSK-3β
glycogen synthase kinase–3β
- IP
intraperitoneal
- JAK3
Janus kinase 3
- JNK
c-Jun N-terminal kinase
- MMB
MYBL2, FOXM1, and the MuvB core
- NFAT
nuclear factor of activated T cells
- PCNA
proliferating cell nuclear antigen
- PDX1
pancreatic and duodenal homeobox 1
- qPCR
quantitative polymerase chain reaction
- RRID
Research Resource Identifier
- S10
serine 10
- TGF-β
transforming growth factor-β
- VEGF
vascular endothelial growth factor
References
- 1. Nichols RJ, New C, Annes JP. Adult tissue sources for new β cells. Transl Res. 2014;163(4):418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Assche FA, Aerts L, De Prins F. A morphological study of the endocrine pancreas in human pregnancy. Br J Obstet Gynaecol. 1978;85(11):818–820. [DOI] [PubMed] [Google Scholar]
- 3. Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care. 2006;29(3):717–718. [DOI] [PubMed] [Google Scholar]
- 4. Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A, Prokopenko I, Kang HM, Dina C, Esko T, Fraser RM, Kanoni S, Kumar A, Lagou V, Langenberg C, Luan J, Lindgren CM, Müller-Nurasyid M, Pechlivanis S, Rayner NW, Scott LJ, Wiltshire S, Yengo L, Kinnunen L, Rossin EJ, Raychaudhuri S, Johnson AD, Dimas AS, Loos RJ, Vedantam S, Chen H, Florez JC, Fox C, Liu CT, Rybin D, Couper DJ, Kao WH, Li M, Cornelis MC, Kraft P, Sun Q, van Dam RM, Stringham HM, Chines PS, Fischer K, Fontanillas P, Holmen OL, Hunt SE, Jackson AU, Kong A, Lawrence R, Meyer J, Perry JR, Platou CG, Potter S, Rehnberg E, Robertson N, Sivapalaratnam S, Stančáková A, Stirrups K, Thorleifsson G, Tikkanen E, Wood AR, Almgren P, Atalay M, Benediktsson R, Bonnycastle LL, Burtt N, Carey J, Charpentier G, Crenshaw AT, Doney AS, Dorkhan M, Edkins S, Emilsson V, Eury E, Forsen T, Gertow K, Gigante B, Grant GB, Groves CJ, Guiducci C, Herder C, Hreidarsson AB, Hui J, James A, Jonsson A, Rathmann W, Klopp N, Kravic J, Krjutškov K, Langford C, Leander K, Lindholm E, Lobbens S, Männistö S, Mirza G, Mühleisen TW, Musk B, Parkin M, Rallidis L, Saramies J, Sennblad B, Shah S, Sigurðsson G, Silveira A, Steinbach G, Thorand B, Trakalo J, Veglia F, Wennauer R, Winckler W, Zabaneh D, Campbell H, van Duijn C, Uitterlinden AG, Hofman A, Sijbrands E, Abecasis GR, Owen KR, Zeggini E, Trip MD, Forouhi NG, Syvänen AC, Eriksson JG, Peltonen L, Nöthen MM, Balkau B, Palmer CN, Lyssenko V, Tuomi T, Isomaa B, Hunter DJ, Qi L, Shuldiner AR, Roden M, Barroso I, Wilsgaard T, Beilby J, Hovingh K, Price JF, Wilson JF, Rauramaa R, Lakka TA, Lind L, Dedoussis G, Njølstad I, Pedersen NL, Khaw KT, Wareham NJ, Keinanen-Kiukaanniemi SM, Saaristo TE, Korpi-Hyövälti E, Saltevo J, Laakso M, Kuusisto J, Metspalu A, Collins FS, Mohlke KL, Bergman RN, Tuomilehto J, Boehm BO, Gieger C, Hveem K, Cauchi S, Froguel P, Baldassarre D, Tremoli E, Humphries SE, Saleheen D, Danesh J, Ingelsson E, Ripatti S, Salomaa V, Erbel R, Jöckel KH, Moebus S, Peters A, Illig T, de Faire U, Hamsten A, Morris AD, Donnelly PJ, Frayling TM, Hattersley AT, Boerwinkle E, Melander O, Kathiresan S, Nilsson PM, Deloukas P, Thorsteinsdottir U, Groop LC, Stefansson K, Hu F, Pankow JS, Dupuis J, Meigs JB, Altshuler D, Boehnke M, McCarthy MI;Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steinthorsdottir V, Thorleifsson G, Sulem P, Helgason H, Grarup N, Sigurdsson A, Helgadottir HT, Johannsdottir H, Magnusson OT, Gudjonsson SA, Justesen JM, Harder MN, Jørgensen ME, Christensen C, Brandslund I, Sandbæk A, Lauritzen T, Vestergaard H, Linneberg A, Jørgensen T, Hansen T, Daneshpour MS, Fallah MS, Hreidarsson AB, Sigurdsson G, Azizi F, Benediktsson R, Masson G, Helgason A, Kong A, Gudbjartsson DF, Pedersen O, Thorsteinsdottir U, Stefansson K. Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat Genet. 2014;46(3):294–298. [DOI] [PubMed] [Google Scholar]
- 6. Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. [DOI] [PubMed] [Google Scholar]
- 7. Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57(6):1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36(1):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, Herrera PL. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature. 2014;514(7523):503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang P, Alvarez-Perez JC, Felsenfeld DP, Liu H, Sivendran S, Bender A, Kumar A, Sanchez R, Scott DK, Garcia-Ocaña A, Stewart AF. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med. 2015;21(4):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen W, Taylor B, Jin Q, Nguyen-Tran V, Meeusen S, Zhang YQ, Kamireddy A, Swafford A, Powers AF, Walker J, Lamb J, Bursalaya B, DiDonato M, Harb G, Qiu M, Filippi CM, Deaton L, Turk CN, Suarez-Pinzon WL, Liu Y, Hao X, Mo T, Yan S, Li J, Herman AE, Hering BJ, Wu T, Martin Seidel H, McNamara P, Glynne R, Laffitte B. Inhibition of DYRK1A and GSK3B induces human β-cell proliferation. Nat Commun. 2015;6(1):8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443(7109):345–349. [DOI] [PubMed] [Google Scholar]
- 13. Dirice E, Walpita D, Vetere A, Meier BC, Kahraman S, Hu J, Dančík V, Burns SM, Gilbert TJ, Olson DE, Clemons PA, Kulkarni RN, Wagner BK. Inhibition of DYRK1A stimulates human β-cell proliferation. Diabetes. 2016;65(6):1660–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Annes JP, Ryu JH, Lam K, Carolan PJ, Utz K, Hollister-Lock J, Arvanites AC, Rubin LL, Weir G, Melton DA. Adenosine kinase inhibition selectively promotes rodent and porcine islet β-cell replication. Proc Natl Acad Sci USA. 2012;109(10):3915–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao Z, Low YS, Armstrong NA, et al. Repurposing cAMP-modulating medications to promote beta-cell replication. Mol Endocrinol. 2014;28(10):1682–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tuomi T, Nagorny CLF, Singh P, Bennet H, Yu Q, Alenkvist I, Isomaa B, Östman B, Söderström J, Pesonen AK, Martikainen S, Räikkönen K, Forsén T, Hakaste L, Almgren P, Storm P, Asplund O, Shcherbina L, Fex M, Fadista J, Tengholm A, Wierup N, Groop L, Mulder H. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 2016;23(6):1067–1077. [DOI] [PubMed] [Google Scholar]
- 17. Gotoh M, Maki T, Satomi S, Porter J, Bonner-Weir S, O’Hara CJ, Monaco AP. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987;43(5):725–730. [DOI] [PubMed] [Google Scholar]
- 18. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29(11):1046–1051. [DOI] [PubMed] [Google Scholar]
- 19. Shaw KL, Pais E, Ge S, Hardee C, Skelton D, Hollis RP, Crooks GM, Kohn DB. Lentiviral vectors with amplified beta cell-specific gene expression. Gene Ther. 2009;16(8):998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11(2):R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Efrat S, Fusco-DeMane D, Lemberg H, al Emran O, Wang X. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc Natl Acad Sci USA. 1995;92(8):3576–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 23. Serup P, Petersen HV, Pedersen EE, Edlund H, Leonard J, Petersen JS, Larsson LI, Madsen OD. The homeodomain protein IPF-1/STF-1 is expressed in a subset of islet cells and promotes rat insulin 1 gene expression dependent on an intact E1 helix-loop-helix factor binding site. Biochem J. 1995;310(Pt 3):997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, Dadon D, Granot Z, Ben-Hur V, White P, Girard CA, Karni R, Kaestner KH, Ashcroft FM, Magnuson MA, Saada A, Grimsby J, Glaser B, Dor Y. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab. 2011;13(4):440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang W, Walker JR, Wang X, Tremblay MS, Lee JW, Wu X, Schultz PG. Identification of small-molecule inducers of pancreatic beta-cell expansion [published correction appears in Proc Natl Acad Sci USA. 2009;106(17):7264]. Proc Natl Acad Sci USA. 2009;106(5):1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uehara T, Xi Peng X, Bennett B, Satoh Y, Friedman G, Currin R, Brenner DA, Lemasters J. c-Jun N-terminal kinase mediates hepatic injury after rat liver transplantation. Transplantation. 2004;78(3):324–332. [DOI] [PubMed] [Google Scholar]
- 27. Dhawan S, Dirice E, Kulkarni RN, Bhushan A. Inhibition of TGF-β signaling promotes human pancreatic β-cell replication. Diabetes. 2016;65(5):1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Protein Data Bank. 4MQ2. https://www.rcsb.org/structure/4mq2. Accessed 6 March 2018.
- 29. Georgia S, Bhushan A. p27 Regulates the transition of beta-cells from quiescence to proliferation. Diabetes. 2006;55(11):2950–2956. [DOI] [PubMed] [Google Scholar]
- 30. Soppa U, Schumacher J, Florencio Ortiz V, Pasqualon T, Tejedor FJ, Becker W. The Down syndrome-related protein kinase DYRK1A phosphorylates p27(Kip1) and Cyclin D1 and induces cell cycle exit and neuronal differentiation. Cell Cycle. 2014;13(13):2084–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deng X, Mercer SE, Shah S, Ewton DZ, Friedman E. The cyclin-dependent kinase inhibitor p27Kip1 is stabilized in G(0) by Mirk/dyrk1B kinase. J Biol Chem. 2004;279(21):22498–22504. [DOI] [PubMed] [Google Scholar]
- 32. Harb G, Vasavada RC, Cobrinik D, Stewart AF. The retinoblastoma protein and its homolog p130 regulate the G1/S transition in pancreatic beta-cells [published correction appears in Diabetes 2009;58(10):2425.]Diabetes. 2009;58(8):1852–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sadasivam S, DeCaprio JA. The DREAM complex: master coordinator of cell cycle-dependent gene expression [published correction appears in Nat Rev Cancer. 2013;13:752]. Nat Rev Cancer. 2013;13(8):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Litovchick L, Florens LA, Swanson SK, Washburn MP, DeCaprio JA. DYRK1A protein kinase promotes quiescence and senescence through DREAM complex assembly. Genes Dev. 2011;25(8):801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Litovchick L, Sadasivam S, Florens L, Zhu X, Swanson SK, Velmurugan S, Chen R, Washburn MP, Liu XS, DeCaprio JA. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell. 2007;26(4):539–551. [DOI] [PubMed] [Google Scholar]
- 36. Lorvellec M, Dumon S, Maya-Mendoza A, Jackson D, Frampton J, García P. B-Myb is critical for proper DNA duplication during an unperturbed S phase in mouse embryonic stem cells. Stem Cells. 2010;28(10):1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sala A, Saitta B, De Luca P, Cervellera MN, Casella I, Lewis RE, Watson R, Peschle C. B-MYB transactivates its own promoter through SP1-binding sites. Oncogene. 1999;18(6):1333–1339. [DOI] [PubMed] [Google Scholar]
- 38. Song WJ, Song EA, Jung MS, Choi SH, Baik HH, Jin BK, Kim JH, Chung SH. Phosphorylation and inactivation of glycogen synthase kinase 3β (GSK3β) by dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A). J Biol Chem. 2015;290(4):2321–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosengren AH, Jokubka R, Tojjar D, et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327(5962):217–220. [DOI] [PubMed] [Google Scholar]
- 40. Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu H, Remedi MS, Pappan KL, Kwon G, Rohatgi N, Marshall CA, McDaniel ML. Glycogen synthase kinase-3 and mammalian target of rapamycin pathways contribute to DNA synthesis, cell cycle progression, and proliferation in human islets. Diabetes. 2009;58(3):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andersohn F, Schade R, Suissa S, Garbe E. Long-term use of antidepressants for depressive disorders and the risk of diabetes mellitus. Am J Psychiatry. 2009;166(5):591–598. [DOI] [PubMed] [Google Scholar]
- 43. Nanasawa H, Sako A, Mitsutsuka T, Nonogaki K, Kondo T, Mishima S, Uju Y, Ito T, Enomoto T, Hayakawa T, Yanai H. Development of diabetes mellitus associated with quetiapine: a case series. Medicine (Baltimore). 2017;96(3):e5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61(9):2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lawrence MC, McGlynn K, Park BH, Cobb MH. ERK1/2-dependent activation of transcription factors required for acute and chronic effects of glucose on the insulin gene promoter. J Biol Chem. 2005;280(29):26751–26759. [DOI] [PubMed] [Google Scholar]
- 46. Latreille M, Abu-Thuraia A, Oliva R, Zuo D, Larose L.. Casein kinase igamma2 impairs fibroblasts actin stress fibers formation and delays cell cycle progression in g1. Int J Cell Biol. 2012;2012:684684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhong L, Georgia S, Tschen SI, Nakayama K, Nakayama K, Bhushan A. Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. J Clin Invest. 2007;117(10):2869–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang H, Ackermann AM, Gusarova GA, et al. The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Mol Endocrinol. 2006;20(8):1853–1866. [DOI] [PubMed] [Google Scholar]
- 49. Golson ML, Maulis MF, Dunn JC, et al. Activated FoxM1 attenuates streptozotocin-mediated beta-cell death. Mol Endocrinol. 2014;28(9):1435–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sadasivam S, Duan S, DeCaprio JA. The MuvB complex sequentially recruits B-Myb and FoxM1 to promote mitotic gene expression. Genes Dev. 2012;26(5):474–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Waki H, Park KW, Mitro N, Pei L, Damoiseaux R, Wilpitz DC, Reue K, Saez E, Tontonoz P. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARgamma expression. Cell Metab. 2007;5(5):357–370. [DOI] [PubMed] [Google Scholar]
- 52. Rachdi L, Kariyawasam D, Guez F, Aïello V, Arbonés ML, Janel N, Delabar JM, Polak M, Scharfmann R. Dyrk1a haploinsufficiency induces diabetes in mice through decreased pancreatic beta cell mass. Diabetologia. 2014;57(5):960–969. [DOI] [PubMed] [Google Scholar]
- 53. Rachdi L, Kariyawasam D, Aiello V, et al. Dyrk1A induces pancreatic beta cell mass expansion and improves glucose tolerance. Cell Cycle. 2014;13(14):2221–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mirshahi T, Murray MF, Carey DJ. The metabolic syndrome and DYRK1B. N Engl J Med. 2014;371(8):784–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Keramati AR, Fathzadeh M, Go GW, Singh R, Choi M, Faramarzi S, Mane S, Kasaei M, Sarajzadeh-Fard K, Hwa J, Kidd KK, Babaee Bigi MA, Malekzadeh R, Hosseinian A, Babaei M, Lifton RP, Mani A. A form of the metabolic syndrome associated with mutations in DYRK1B. N Engl J Med. 2014;370(20):1909–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.