Abstract
Alzheimer’s disease (AD) is characterized by two primary pathologies: tau-related neurofibrillary tangles and the extracellular accumulation of amyloid-β (Aβ). The development of these pathologies is topologically distinct early in the disease, with Aβ beginning to accumulate as a diffuse, neocortical pathology, while tau-related pathology begins to form in mesial temporal regions. This study investigated the hypothesis that, by virtue of this distinction, there exist preferential associations between the primary pathologies and aspects of the cognitive phenotype. We investigated the relationship between cerebrospinal fluid (CSF) biomarkers for tau and Aβ pathologies with neurocognitive measures in 191 patients with mild cognitive impairment (MCI). Participants completed cognitive tests of new learning, information processing speed, and working memory. Separate regression models were computed and then followed up with mediation analyses to examine the predictive status of CSF biomarkers. The effect of Aβ on learning was mediated by phospho-tau (p = 0.008). In contrast, Aβ had a direct effect on information processing speed that was not mediated by phospho-tau (p = 0.59). No predictors were significant for working memory. This study provided evidence for a differential relationship of Aβ and phospho-tau pathologies on the neurocognitive phenotype of MCI. This supports the proposition that these primary AD pathologies maximally affect different aspects of cognition, and has potential implications for cognitive assessments and the use of biomarkers in disease-modifying therapeutic trials.
Keywords: Amyloid-β, cerebrospinal fluid, cognition, mild cognitive impairment, tau
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative condition that results in a progressive clinical syndrome of deteriorating neurocognitive function of insidious onset [1]. In typical AD, the neurocognitive phenotype follows a well-documented progression, beginning with impairments in new learning before evolving to affect semantic memory, praxis, and visuospatial function [2]. The evolution of neurocognitive dysfunction corresponds to the severity and pathological staging of tau-related neurofibrillary tangles (NFTs) [3–5]. The accumulation of NFTs follow a predictable course, beginning in transentorhinal cortex, and then spreading to nearby limbic regions (including the hippocampus) before infiltrating broad regions of isocotex [6]. The fact that that fundamental memory impairment is an early neurocognitive marker of the disease is not surprising given the early involvement of the mesial temporal regions [7]. The spread of this pathology to lateral temporal neocortex also accords with the evolution of the clinical syndrome in the form of deficits in semantic function. Similarly, the later development of impairments in praxis and spatial function corresponds to pathology in the temporo-parietal neo-cortical regions [8].
Unlike the evolution of tau-related pathology, which corresponds to the development of the neurocognitive phenotype, the nexus between amyloid-β (Aβ) and cognition is not well established. When measures of Aβ-related pathology are compared directly to tau-related pathology with regard to their respective contributions to global cognitive dysfunction, Aβ consistently accounts for less variance [9, 10]. In contrast to the tau-based NFTs, which begin to accumulate in the mesial temporal region, Aβ deposition begins in basal isocortex before spreading inwards to mesial temporal regions and finally involving more diffuse isocortical regions [11]. A number of in vivo imaging studies of Aβ have revealed deposition to be a diffuse neocortical pathology, with greatest binding in anterior neocortex and relatively little uptake in mesial temporal structures [12–15].
Given the neocortical predominance of Aβ deposition, particularly in anterior regions, it is of note that clinical syndrome is not dominated by neurocognitive signs considered typical of these regions (e.g., primary executive impairments). The question then becomes, how does Aβ pathology contribute to the neurocognitive phenotype? There are two possible answers to this suggested by the amyloid cascade hypotheses [16]. The first is that the effect of Aβ is entirely indirect, and influences cognitive function by causing the accumulation of tau-related pathology. In this way, the effect of Aβ on the neurocognitive phenotype is mediated by the effect of tau-related pathology. This possibility is supported not only by the nexus between tau-related pathology and cognition, but also by evidence that the relationship between amyloid load and cognition is attenuated when the effect of tau-related pathology is taken into account [3, 17]. A recent study also reported that the relationship between Aβ load and memory function is mediated by hippocampal volume, which further supports this view [18]. This indirect effect may also explain why the correlation between cognition and Aβ is weaker than that of tau.
The second possibility suggested by the amyloid cascade hypothesis is that Aβ may also have a direct and tau-independent effect on cognition. There is evidence to suggest that Aβ oligomers, an intermediate species in the amyloid-aggregation process, can directly cause subtle synaptic dysfunction [19–23]. Unlike tau-related pathology, which likely exerts focal and specific neurocognitive effects (especially early in the disease), Aβ is likely to exert general neurocognitive dysfunction as a result of its diffuse cortical distribution. In support of this view, a recent study reported that successful clearance of Aβ pathology in patients with AD resulted in improvements on tasks of executive function, but not of memory[24].
The possibility that Aβ pathology may result in neurocognitive dysfunction via two pathways suggests it may be possible to differentiate the effects of Aβ and phospho-tau. To our knowledge no previous study has attempted to dissociate the pathology-cognition relationship in this way. The purpose of this study was to examine this using data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). The analysis focused on patients with MCI, in order to increase the likelihood of demonstrating differential relationships between the two pathological hallmarks and cognition. Specifically, as tau-related pathology begins in mesial temporal regions and spreads out, and Aβ deposition begins in neocortex and spreads inwards, it stands to reason that in the early the stage of the disease there will be less overlap between the two pathologies. Patients with MCI are more likely than elderly controls to carry AD pathology and as such, without accurate in vivo diagnostics, MCI presents the best clinical model of early AD [25].
CSF biomarkers of phospho-tau and Aβ were utilized, which remain the only currently accessible methods for measuring multiple pathological hallmarks in AD using a common modality. CSF levels of Aβ and phospho-tau are abnormal in AD, with decreased levels of Aβ, and increased phospho-tau compared to cognitively normal controls [26–28]. In MCI, lower baseline Aβ and higher phospho-tau is associated with more rapid cognitive decline, greater cortical thinning, and increased likelihood of transition to dementia of the Alzheimer’s type (DAT) [29–33]. Critically for the present study, CSF levels of Aβ and phospho-tau from lumbar puncture are significantly correlated with cortical brain biopsy histology, suggesting they are adequate surrogates for ex vivo histopathological measurements [34, 35].
Several studies have investigated the relationship between CSF biomarkers and different aspects of the cognitive phenotype; however, a consensus is yet to emerge [36, 37]. One possible reason for this is that these studies used CSF total tau as a marker of tau-pathology. CSF total-tau is obtained from assays that detect all available isoforms or tau, while assays for CSF p-tau are specific to phosphorylated tau protein [38, 39]. Given NFTs are comprised of hyper-phosphorylated tau, this renders p-tau a conceptually more specific marker of tau-related pathology in AD. Further, CSF t-tau likely reflects non-specific neuronal damage, including from trauma, stroke, and Creutzfeldt-Jakob disease. Critically for the present study, although t-tau and p-tau CSF levels are both elevated in AD, this correlation is not seen in stroke or Creutzfeldt-Jakob Disease where t-tau levels are elevated but p-tau levels are not [40–46]. Taken together, this suggests that CSF p-tau levels might be more specific to NFT-related pathology, while t-tau may be a more general marker of neuronal damage [47, 48]. As such, CSF p-tau will be used as the primary marker for tau-related pathology in the present enquiry.
The hypothesis proposed here is not one of absolute dissociation. Patients at the MCI stage of the disease are likely to have some NFT formation in neocortical regions. Rather, it is expected that there will be preferential association between CSF biomarkers and aspects of the cognitive syndrome. It is predicted that phospho-tau will be most strongly associated with memory functions, while Aβ will be most strongly associated non-memory functions (such as executive functions).
MATERIALS AND METHODS
ADNI
The data used in this study were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. The ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies and non-profit organizations, as a $60 million, 5-year public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Determination of sensitive and specific markers of early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, with subjects recruited from over 50 sites across the U.S. and Canada. The initial target for ADNI was to recruit 800 adults, ages 55 to 90, to participate in the research, approximately 200 cognitively normal older individuals to be followed for 3 years, 400 people with MCI to be followed for 3 years and 200 people with early AD to be followed for 2 years (for more information, see http://www.adni-info.org).
Participants
The ADNI study includes healthy controls, participants with MCI, and those with DAT. The characteristics of the clinical cohort, including inclusion and exclusion criteria, are described elsewhere [49]. For the present study, the ADNI database was queried for participants who, at baseline, met the criteria for MCI. Participants were classified as satisfying MCI criteria if, in addition to the general study criteria referred to above, they: (a) had a subjective memory complaint verified by an informant; (b) demonstrated objective memory dysfunction on the Logical Memory II subtest of the Wechsler Memory Scale; (c) had a Mini-Mental Status Examination (MMSE)scoreofbetween24and30(inclusive);(d)had a Clinical Dementia Rating (CDR) score of 0.5 with a Memory Box Score of at least 0.5; and (e) did not meet criteria for DAT [50].
Of MCI participants in ADNI cohort, 200 underwent CSF collection. Nine subjects were excluded for use in the current study: one participant failed screening and was excluded from further participation in the ADNI study, one participant had missing biomarker data, and seven participants had missing neuropsychological data. The remaining 191 participants were included for analysis. Due to deliberate oversampling of male participants in order to guard against differential life expectancy, the sample included 126 males (66%) and 65 females (34%). Participants were administered a range of screening instruments, including the modified Hachinski Ischemic Index [51], Geriatric Depression Scale (GDS), Functional Assessment Questionnaire (FAQ), Alzheimer’s Disease Assessment Scale-Cognitive (ADAS-Cog), and MMSE.
Pre-morbid verbal intelligence was estimated using the American version of the National Adult Reading Test, a test of single word reading (ANART) [52]. The number of errors (mispronounced words) on the ANART was used in combination with years of education to estimate each participant’s pre-morbid verbal intelligence quotient (VIQ) using an approach described elsewhere [53].
Cerebrospinal fluid biomarkers
CSF was sampled from all participants via lumbar puncture. Samples were subsequently analyzed by the ADNI Core Biomarkers Team for levels of (Aβ1–42) and tau hyperphosphorylated at the threonine 181 (p-tau181P). The detailed protocol for this analysis, including quality control procedures, is described elsewhere [54]. The mean delay between lumbar puncture and neuropsychological assessment was 0.52 days (SE = 0.26).
Cognitive measures
Memory
The Rey Auditory Verbal Learning Test (RAVLT) [55] is a test of verbal supra-span memory widely used in research and clinical practice [56]. For this study, four indices derived from the RAVLT were analyzed. The first index, total learning, is the number of words correctly recalled, summed over trials the five learning trials. The second index, post interference recall included the number of words correctly recalled following the interference trial. Third, delayed recall, is the number of words correctly recalled from list A following a 30-min delay. Finally, recognition was calculated as the number of false positives subtracted from the number of correctly recognized words on the delayed recognition trial.
Non-memory cognitive tests
A number of non-memory cognitive measures were used in the present study. Digit Span Forwards and Digit Span Backwards from the WAIS-R [57] were included as measures of the ability to register, update, manipulate, and report information in working memory. Digit Symbol Substitution from the WAIS-R [58] was included as a test of attention and speed of information processing. Part A of the Trail Making Test was included as a further measure of information processing speed. Part B was included as a measure of working memory and attentional switching. To correct for the effect of information processing speed, the score for Part B was divided by Part A [59].
Statistical analyses
The individual cognitive variables were reduced using principal component analysis (PCA) primarily for the purposes of assisting in data interpretation, but also to reduce the problem of multiple comparisons. Unlike the construction of scales by adding conceptually related items together, PCA allows for the item loadings for each scale to be determined empirically, thus ensuring the resulting component scores explain the maximum possible variance in the original items, while still fulfilling the practical requirement of data reduction [60].
Initial analysis entailed the estimation of separate multiple regression models with biomarkers, age, gender, and VIQ regressed onto each of the component scores. The pattern of significant regression coefficients was interpreted to evaluate the unique contribution of each biomarker variable to the model. These regression analyses were followed up with mediation analyses.
Mediation analyses were conducted in SPSS 22 (IBM Corporation) using the PROCESS package [61]. For each cognitive composite variable, a simple mediation model was computed to examine whether the relationship between Aβ1–42 and cognition was either direct, or mediated by p-tau181P. age, gender, and VIQ were added to all models as covariates. The significance of the mediation effect was determined using Sobel’s test [62]. Standardized regression coefficients were used to interpret the predictive relationship between variables. Given the conceptual relationship between VIQ and other cognitive measures, these regression models were repeated with VIQ removed to confirm the pattern of findings.
RESULTS
Biomarkers and control variables
Participant demographics are included in Table 1. Mean CSF Aβ1–42 was 163.31 pg/ml (SE = 54.74) and mean CSF p-tau181P levels were 35.98 pg/ml (SE = 18.12). Mean CSF t-tau levels were 104.86 pg/ml (SE = 4.48). Aβ1–42 and p-tau181P were moderately negatively correlated, r = −0.53, p < 0.001. P-tau181P was not significantly correlated with age, r = 0.02, p = 0.76, nor VIQ, r = 0.06, p = 0.40. Similarly, A−β1–42 was not correlated with age, r = 0.02, p = 0.80, nor VIQ, r = −0.04, p = 0.61. No gender differences were observed for either Aβ1–42, t(189) = 1.56, p = 0.11, or p-tau181P, t(189) = −1.54, p = 0.16.
Table 1.
Participant characteristics
| Variable | Range | M | SE | |
|---|---|---|---|---|
| Min | Max | |||
| Age (years) | 55 | 89 | 74.72 | 0.54 |
| Education (years) | 3 | 20 | 15.81 | 0.22 |
| Hachinski Score | 0 | 4 | 0.60 | 0.06 |
| GDS | 0 | 5 | 1.67 | 0.10 |
| FAQ | 0 | 21 | 3.71 | 0.32 |
| ADAS-Cog | 2.00 | 26.67 | 11.54 | 0.33 |
| MMSE | 24 | 30 | 26.95 | 0.13 |
| VIQ | 83.94 | 131.00 | 115.3 | 0.70 |
GDS, Geriatric Depression Scale; FAQ, Functional Assessment Questionnaire; ADAS-Cog; MMSE, Mini-Mental State Examination; VIQ, verbal intelligence quotient.
Cognitive component scores
Descriptive statistics for all cognitive measures are shown in Table 2. The PCA solution revealed three components, all with eigenvalues greater than one, which together explained 68% of variance in the original items. Following the application of a promax rotation (κ= 2), these components were labeled learning (containing the four items from the RAVLT), working memory (digits forwards, digits backwards, and corrected trail making test part B), and processing speed (trail making test part A and digit symbol coding). The pattern matrix is show in Table 3. Component scores were generated for each participant using the regression method.
Table 2.
Cognitive measures
| Variable | Range | M | SE | |
|---|---|---|---|---|
| Min | Max | |||
| Delayed Recall | 0 | 13 | 2.59 | 0.22 |
| Post-interference Recall | 0 | 14 | 3.52 | 0.22 |
| Total Learning | 11 | 55 | 30.33 | 0.63 |
| Recognition | 0 | 15 | 8.10 | 0.26 |
| Digit Span Forwards | 4 | 12 | 8.34 | 0.14 |
| Digit Span Backwards | 2 | 12 | 6.16 | 0.16 |
| Trail Making – B | 1.15 | 8.88 | 3.08 | 5.30 |
| Trail Making – A | 17 | 150 | 45.24 | 1.70 |
| Digit Symbol Coding | 9 | 64 | 37.16 | 0.82 |
Table 3.
Component loading for principal components analysis
| Component | |||
|---|---|---|---|
| L | WM | PS | |
| Delayed Recall | 0.903 | ||
| Post-interference Recall | 0.896 | ||
| Total Learning | 0.798 | ||
| Recognition | 0.744 | ||
| Digit Span Forwards | 0.832 | ||
| Digit Span Backwards | 0.774 | ||
| Trail Making – B | –0.496 | ||
| Trail Making – A | –0.932 | ||
| Digital Symbol Coding | 0.793 | ||
L, new learning; WM, working memory; PS, processing speed.
Regression analyses
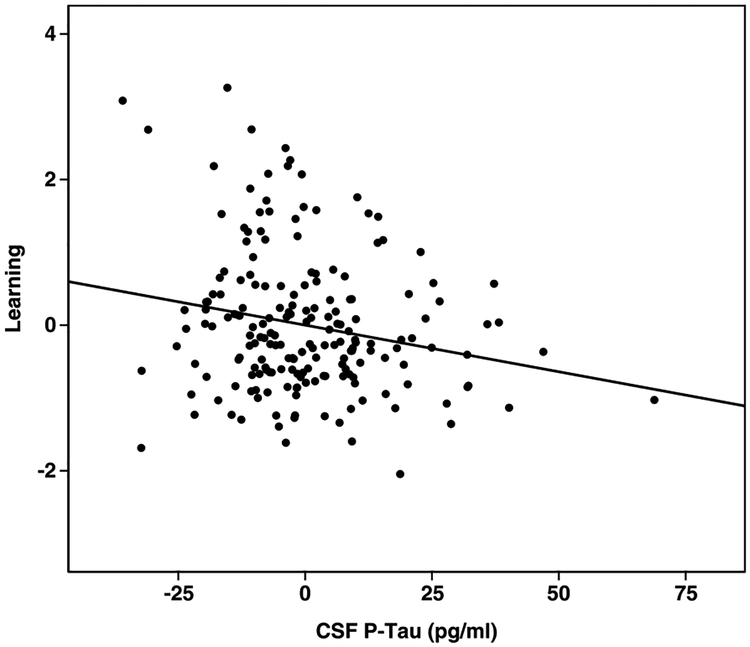
Results for the regression analyses are shown in Table 4. The overall regression model for learning was significant, R = 0.31, F(5,190) = 4.00, p = 0.002, with p-tau181P a significant predictor. Aβ1–42 was a non-significant predictor, as were gender (p = 0.05), VIQ (p = 0.18), and age (p = 0.66). The relationship between p-tau181P and new learning persisted after re-running the regression model with VIQ omitted (p = 0.006), and with VIQ replaced with MMSE (p = 0.005). The relationship between learning and p-tau is shown in Fig. 1.
Table 4.
Regression models and CSF biomarker coefficients
| Model | β | t | sr2 | p |
|---|---|---|---|---|
| Learning | ||||
| P-tau181P | –0.23 | –2.82 | 0.03 | 0.005** |
| Aβ1–42 | 0.07 | 0.84 | <0.01 | 0.402 |
| Working Memory | ||||
| P-tau181P | 0.06 | 0.77 | <0.01 | 0.445 |
| Aβ1–42 | 0.12 | 1.41 | <0.01 | 0.161 |
| Processing Speed | ||||
| P-tau181P | 0.05 | 0.55 | <0.01 | 0.583 |
| Aβ1–42 | 0.20 | 2.38 | 0.03 | 0.018* |
p < 0.05,
p < 0.01,
df = 190, β = standardized regression coefficients, sr2 = semi-partial correlation coefficient squared.
Fig. 1.
Partial regression plot for learning and CSF-p-tau.
While the overall model for working memory was significant, R = 0.31, F(5,190) = 4.04, p = 0.002, neither p-tau181P nor Aβ1–42, were significant predictors. VIQ was a statistically significant covariate (p < 0.001), while gender (p = 0.93) and age were not (p = 0.77).
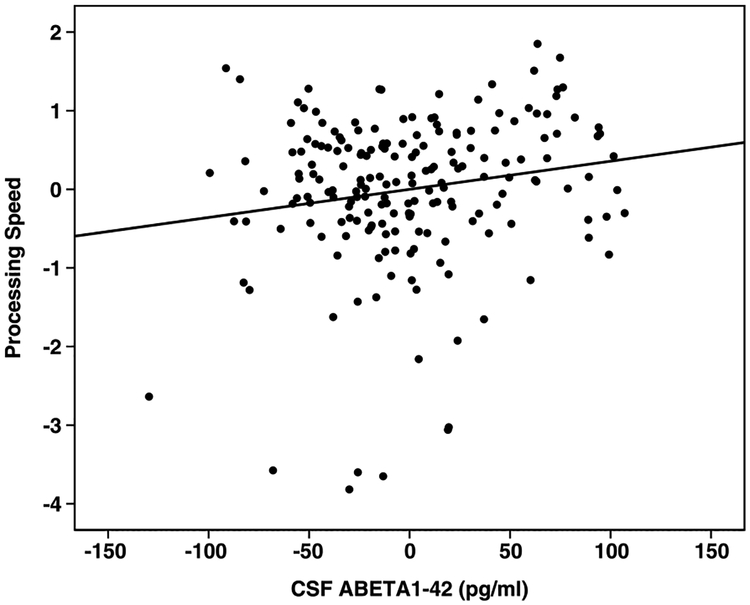
The overall model for processing speed was signifi-cant, R = 0.31, F(5,190) = 3.92, p = 0.002, with Aβ1–42 a significant predictor. P-tau181P was non significant, as were gender (p = 0.054) and age (p = 0.341). VIQ was a significant covariate (p = 0.007). The relationship between Aβ1–42 and processing speed persisted after the regression analysis was re-run with VIQ omitted (p = 0.02). After including MMSE in the regression instead of VIQ, Aβ1–42 became non-significant (p = 0.07). The relationship between Aβ1–42 and processing speed is shown in Fig. 2.
Fig. 2.
Partial regression plot for processing speed and CSF Aβ.
Mediation analyses
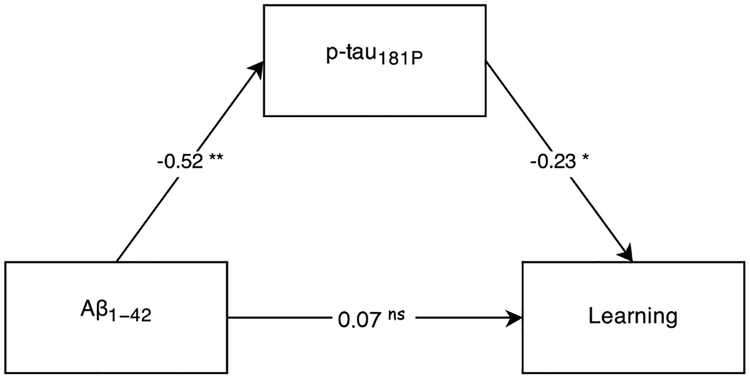
As biomarkers were only significant predictors in these learning and processing speed models, mediation analyses were limited to these cognitive variables. For learning, the direct effect of Aβ1–42 was completely mediated by p-tau181P, Sobel’s Z = 2.65, p = 0.008. As shown in Fig. 3, the effect was indirect, with Aβ1–42 significantly predicting p-tau181P and p-tau181P subsequently predicting learning. The same mediation effect was not observed when the analysis was repeated with t-tau instead of p-tau181P, Sobel’s Z = 1.92, p = 0.06.
Fig. 3.
Learning Mediation Model. Numbers along paths are standardized regression coefficients. Statistical significance: *p < 0.05, **p < 0.01, nsp > 0.05.
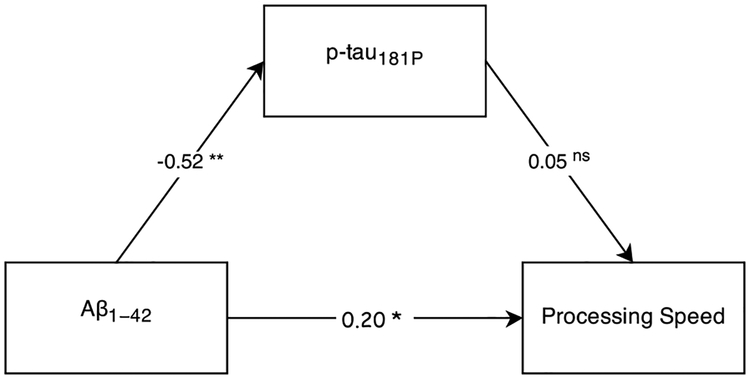
In contrast, as shown in Fig. 4, the direct effect of Aβ1–42 on processing speed was not mediated by p-tau181P, Sobel’s Z = −0.55, p = 0.59, and remained significant with p-tau181P included in the model. Further, the indirect effect was also non-significant with p-tau181P failing to predict processing speed. The indirect effect remained non-significant when t-tau was entered as the mediator, Sobel’s Z = −0.80, p = 0.42.
Fig. 4.
Processing Speed Mediation Model. Numbers along paths are standardized regression coefficients. Statistical significance: *p < 0.05, **p<0.01, nsp > 0.05.
DISCUSSION
The results of this study support the hypothesis of preferential association between AD biomarkers of tau and Aβ related pathology and cognitive dysfunction. We found that the degree of memory impairment was maximally associated with tau-related pathology, but not Aβ. The effect of Aβ was indirect, that is, while Aβ levels predicted phospho-tau, only phospho-tau was statistically associated with memory function. This same effect was not observed for total tau. This finding is consistent with the early accumulation of tau-related pathology in the mesial temporal regions in AD compared to the relatively diffuse deposition of Aβ [7]. In a previous study, Mormino and colleagues reported that the relationship between cortical Aβ deposition and memory function was mediated by hippocampal volume [18]. Although a direct measure of tau-related pathology was not included in their study, the authors speculated that hippocampal volume might have acted as an indirect measure of NFT load. This explanation is supported by our finding that the effect of Aβ on memory function was mediated by phospho-tau. The mediating effect of tau has been documented in previous work by Bennett and colleagues [17].
Previous work from Hedden and colleagues [63] reported that Aβ binding was correlated with the degree of memory impairment healthy older adults. The results presented here to not contradict such findings. Our finding of an indirect relationship between Aβ and memory (mediated by tau) suggests that a correlation would be observed between Aβ and memory if tau had not been included in mediation analysis. As the study by Hedden and colleagues did not investigate cerebral phospho-tau accumulation, it is not possible to speculate whether the relationship between memory and Aβ would persist once levels of tau-pathology had been considered. A similar finding was reported by Lim and coworkers [64] who showed that MCI patients with high Aβ were characterized by memory impairment, while low-Aβ MCI patients presented with impairments in multiple domains, including executive functions. Without considering the burden of tau-related pathology, it is difficult to reconcile these findings with the present study. This underscores the need to replicate our findings, as well as the need to consider markers of both primary pathologies in studies of cognitive impairment in AD.
We also demonstrated a direct, tau-independent association between Aβ and impairments in processing speed. This association was small, but statistically significant and remained after controlling for phospho-tau. It also remained after controlling for total tau. This finding is consistent with proposition that Aβ deposition, as a diffuse neocortical pathology, has a direct and independent effect on the neurocognitive phenotype. It is likely processing speed behaved as a marker of diffuse cerebral involvement, as fundamental processing speed impairment characterizes a number of diffuse pathologies [65–69]. This association did not persist when global cognitive status was taken into account statistically, further supporting the global effects of amyloid as a diffuse pathology.
These findings are also consistent with the amyloid cascade hypothesis. Specifically, the finding that the effect of Aβ on memory function was indirect is consistent with the view that the accumulation of tau-related pathology is secondary to the accumulation of Aβ [16, 70–74]. A direct effect of Aβ on cognition is also consistent with the amyloid cascade hypothesis, and accords with evidence that Aβ oligomers directly disrupt synaptic function [19–23]. This is also congruent with the recent finding that treatment with an anti-Aβ therapy resulted in improvements in executive functions (including very fundamental aspects such as processing speed), but not memory [24].
The absence of a detectable association between any of the pathological biomarkers and working memory was unexpected. One possibility is that any relationship between working memory and Aβ is overshadowed by the variance shared with processing speed such that any residual relationship between working memory and Aβ could not be statistically detected. An association between working memory and phospho-tau was also not observed. The further investigation of the relationship between working memory and disease biomarkers is a clear direction for future research. Estimated verbal intelligence was the only variable to significantly predict working memory. This might be explained by the fact performances on the working memory tasks (digit span and the trail making test) are mediated by verbal abilities. While the effect of tau on memory persisted when a measure of global cognitive status was included, the effect of Aβ on information processing speed did not. This supports the global and diffuse nature of amyloid-pathology, but also confirms the importance of more sensitive cognitive assessment in future studies to further differentiate the preferential effects of amyloid.
Given the overlap of tau- and amyloid-pathology, even in patients with MCI, it will be necessary to confirm these findings in a longitudinal context with patients in the very early stages of the disease (e.g., healthy controls who later convert to DAT). This would help address issues related to inter-individual variation in the development of AD-related pathology. It would also help address the uncertainty of using MCI to investigate underlying AD pathology. A further factor to take into account involves white-matter integrity. Previous work has shown a relationship between executive function impairments and quantification of white matter hyperintensities [63]. The participants in this study were at relatively low risk for cerebrovascular disease, based on screening using the Hachniski Ischemia score. Nevertheless, quantification of cerebrovascular status would be appropriate for inclusion in future work.
This study found evidence for preferential association between biomarkers of tau and Aβ AD pathology in their relationship with different aspects of neurocognitive function in participants with MCI. The data demonstrated a direct association between phospho-tau and memory function, and a tau independent association between Aβ and processing speed. On this basis we proposed a model for AD where phospho-tau related pathology is maximally related to fundamental memory function by virtue of its early accumulation in mesial temporal regions, and Aβ pathology is maximally related to processing speed by virtue of its diffuse neocortical distribution. The data presented here provide tentative support for this model in patients with MCI. The effects reported here are small, but statistically significant, underscoring the need for replication of these findings.
A particular focus of future work would involve the differential effects of phospho-tau and total-tau. While both isoforms are present in AD, levels of phospho-tau are more conceptually aligned with NFTs [47, 48]. Nevertheless, the neurocognitive effects of total-tau are likely to be significant, especially in cases of comorbid pathology, such as cerebrovascular disease [40–46]. A task for future work is to confirm the special effects of phospho-tau, compared to total tau.
These findings have potential implications for disease-modifying therapeutic trials in AD. Specifically, it is possible that therapeutic interventions targeting one of either Aβ or phospho-tau may be optimally assessed using different cognitive paradigms. While the measurement of fundamental memory function may be most appropriate for a potential anti-tau therapy, measures of more diffuse neocortical functions may be more appropriate for anti-Aβ therapies. This point is supported by the findings of Faux and colleagues [24] referred to previously.
ACKNOWLEDGMENTS
Charles Malpas is the recipient of an Alzheimer’s Australia Dementia Research Foundation Viertel postgraduate research scholarship.
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative(ADNI)(NationalInstitutesofHealthGrantU01 AG024904) and DOD ADNI (Department of Defense awardnumberW81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation;AraclonBiotech;BioClinica,Inc.;Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; Euro Immun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Health-care; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research and Development, LLC.; Johnson and Johnson Pharmaceutical Research and Development LLC.; Medpace, Inc.; Merck and Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Authors’ disclosures available online (http://jalz.com/manuscript-disclosures/14-2643r2).
Footnotes
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
REFERENCES
- [1].Hodges JR (2006) Alzheimer’s centennial legacy: Origins, landmarks and the current status of knowledge concerning cognitive aspects. Brain 129, 2811–2822. [DOI] [PubMed] [Google Scholar]
- [2].Galton CJ, Patterson K, Xuereb JH, Hodges JR (2000) Atypical and typical presentations of Alzheimer’s disease: A clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 123, 484–498. [DOI] [PubMed] [Google Scholar]
- [3].Giannakopoulos P, Herrmann FR, Bussière T, Bouras C, Kövari E, Perl DP, Morrison JH, Gold G, Hof PR (2003) Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 60, 1495–1500. [DOI] [PubMed] [Google Scholar]
- [4].Nagy Z, Hindley NJ, Braak H, Braak E, Yilmazer-Hanke DM, Schultz C, Barnetson L, King EM-F, Jobst KA, Smith AD (1999) The progression of Alzheimer’s disease from limbic regions to the neocortex: Clinical, radiological and pathological relationships. Dement Geriatr Cogn Dis 10, 115–120. [DOI] [PubMed] [Google Scholar]
- [5].Nelson PT, Braak H, Markesbery WR (2009) Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J Neuropathol Exp Neurol 68, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- [7].Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, Lipton RB (1999) Memory and mental status correlates of modified Braak staging. Neurobiol Aging 20, 573–579. [DOI] [PubMed] [Google Scholar]
- [8].Apostolova LG, Lu P, Rogers S, Dutton RA, Hayashi KM, Toga AW, Cummings JL, Thompson PM (2008) 3D mapping of language networks in clinical and pre-clinical Alzheimer’s disease. Brain Lang 104, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gold G, Kövari E, Corte G, Herrmann FR, Canuto A, Bussière T, Hof PR, Bouras C, Giannakopoulos P (2001) Clinical validity of Aβ-protein deposition staging in brain aging and Alzheimer disease. J Neuropathol Exp Neurol 60, 946–952. [DOI] [PubMed] [Google Scholar]
- [10].Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J Neuropathol Exp Neurol 71, 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800. [DOI] [PubMed] [Google Scholar]
- [12].Fripp J, Bourgeat P, Acosta O, Raniga P, Modat M, Pike KE, Jones G, O’Keefe G, Masters CL, Ames D, Ellis KA, Maruff P, Currie J, Villemagne VL, Rowe CC, Salvado O, Ourselin S (2008) Appearance modeling of 11C PiB PET images: Characterizing amyloid deposition in Alzheimer’s disease, mild cognitive impairment and healthy aging. Neuroimage 43, 430–439. [DOI] [PubMed] [Google Scholar]
- [13].Kemppainen NM, Aalto S, Wilson IA, Någren K, Helin S, Brück A, Oikonen V, Kailajärvi M, Scheinin M, Viitanen M, Parkkola R, Rinne JO (2007) PET amyloid ligand [11C] PIB uptake is increased in mild cognitive impairment. Neurology 68, 1603–1606. [DOI] [PubMed] [Google Scholar]
- [14].Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Guo-feng H, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 55, 306–319. [DOI] [PubMed] [Google Scholar]
- [15].Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL (2007) Imaging B-amyloid burden in aging and dementia. Neurology 68, 1718–1725. [DOI] [PubMed] [Google Scholar]
- [16].Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297, 353–356. [DOI] [PubMed] [Google Scholar]
- [17].Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE (2004) Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol 61, 378–384. [DOI] [PubMed] [Google Scholar]
- [18].Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ, Alzheimer’s Disease Neuroimaging Initiative (2009) Episodic memory loss is related to hippocampal-mediated β-amyloid deposition in elderly subjects. Brain 132, 1310–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Benilova I, Karran E, De Strooper B (2012) The toxic Aβ oligomer and Alzheimer’s disease: An emperor in need of clothes. Nat Neurosci 15, 349–357. [DOI] [PubMed] [Google Scholar]
- [20].Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol 8, 101–112. [DOI] [PubMed] [Google Scholar]
- [21].Hsia AY, Masliah E, McConlogue L, Yu G-Q, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L (1999) Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci U S A 96, 3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL (1998) Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A 95, 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepard-son NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ (2008) Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14, 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Faux NG, Ritchie CW, Gunn A, Rembach A, Tsatsanis A, Bedo J, Harrison J, Lannfelt L, Blennow K, Zetterberg H, Ingelsson M, Masters CL, Tanzi RE, Cummings JL, Herd CM, Bush AI (2010) PBT2 rapidly improves cognition in Alzheimer’s disease: Additional phase II analyses. J Alzheimers Dis 20, 509–516. [DOI] [PubMed] [Google Scholar]
- [25].Markesbery WR (2010) Neuropathologic alterations in mild cognitive impairment: A review. J Alzheimers Dis 19, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rosenmann H (2012) CSF biomarkers for amyloid and tau pathology in Alzheimer’s disease. J Mol Neurosci 47, 1–14. [DOI] [PubMed] [Google Scholar]
- [27].Wallin AK, Blennow K, Andreasen N, Minthon L (2006) CSF biomarkers for Alzheimer’s disease: Levels of β-amyloid, tau, phosphorylated tau relate to clinical symptoms and survival. Dement Geriatr Cogn Dis 21, 131–138. [DOI] [PubMed] [Google Scholar]
- [28].Andreasen N, Sjögren M, Blennow K (2003) CSF markers for Alzheimer’s disease: Total tau, phospho-tau and Aβ42. World J Biol Psychiatry 4, 147–155. [DOI] [PubMed] [Google Scholar]
- [29].Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L (2006) Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol 5, 228–234. [DOI] [PubMed] [Google Scholar]
- [30].Palmqvist S, Hertze J, Minthon L, Wattmo C, Zetterberg H, Blennow K, Londos E, Hansson O (2012) Comparison of brief cognitive tests and CSF biomarkers in predicting Alzheimer’s disease in mild cognitive impairment: Six-year follow-up study. PloS One 7, e38639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Seppälä TT, Koivisto AM, Hartikainen P, Helisalmi S, Soininen H, Herukka S-K (2011) Longitudinal changes of CSF biomarkers in Alzheimer’s disease. J Alzheimers Dis 25, 583–594. [DOI] [PubMed] [Google Scholar]
- [32].Tosun D, Schuff N, Truran-Sacrey D, Shaw LM, Trojanowski JQ, Aisen P, Peterson R, Weiner MW, Alzheimer’s Disease Neuroimaging Initiative (2010) Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: A longitudinal MRI study. Neurobiol Aging 31, 1340–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund L-O, Freund-Levi Y, Tsolaki M, Minthon L, Wallin AK, Hampel H, Bürger K, Pirttila T, Soininen H, Rikkert MO, Verbeek MM, Spiru L, Blennow K (2009) Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: A prospective cohort study. Lancet Neurol 8, 619–627. [DOI] [PubMed] [Google Scholar]
- [34].Buerger K, Ewers M, Pirttilä Tuula, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, Hampel H (2006) CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 129, 3035–3041. [DOI] [PubMed] [Google Scholar]
- [35].Seppälä TT, Nerg O, Koivisto A, Rummukainen J, Puli L, Zetterberg H, Pyykkö OT, Helisalmi S, Alafuzoff I, Hiltunen M, Jääskeläinen JE, Rinne J, Soininen H, Leinonen V, Herukka SK (2012) CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology 78, 1568–1575. [DOI] [PubMed] [Google Scholar]
- [36].Nordlund A, Rolstad S, Klang O, Lind K, Pedersen M, Blennow K, Edman Aring, Hansen S, Wallin A (2008) Episodic memory and speed/attention deficits are associated with Alzheimer-typical CSF abnormalities in MCI. J Int Neuropsychol Soc 14, 582–590. [DOI] [PubMed] [Google Scholar]
- [37].Rolstad S, Berg AI, Bjerke M, Blennow K, Johansson B, Zetterberg H, Wallin A (2011) Amyloid-β42 is associated with cognitive impairment in healthy elderly and subjective cognitive impairment. J Alzheimers Dis 26, 135–142. [DOI] [PubMed] [Google Scholar]
- [38].Kohnken R, Buerger K, Zinkowski R, Miller C, Kerkman D, DeBernardis J, Shen J, Möller H-J, Davies P, Hampel H (2000) Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer’s disease patients. Neurosci Lett 287, 187–190. [DOI] [PubMed] [Google Scholar]
- [39].Vanmechelen E, Vanderstichele H, Davidsson P, Van Kerschaver E, Van Der Perre B, Sjögren M, Andreasen N, Blennow K (2000) Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: A sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett 285, 49–52. [DOI] [PubMed] [Google Scholar]
- [40].Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E, Blennow K (2001) Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett 297, 187–190. [DOI] [PubMed] [Google Scholar]
- [41].Öst M, Nylén K, Csajbok L, Öhrfelt AO, Tullberg M, Wikkelsö C, Nellgård C, Rosengren P, Blennow L, Nellgård KB (2006) Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology 67, 1600–1604. [DOI] [PubMed] [Google Scholar]
- [42].Zetterberg H, Hietala MA, Jonsson M, Andreasen N, Styrud E, Karlsson I, Edman Aring, Popa C, Rasulzada A, Wahlund L-O (2006) Neurochemical aftermath of amateur boxing. Arch Neurol 63, 1277–1280. [DOI] [PubMed] [Google Scholar]
- [43].Magnoni S, Esparza TJ, Conte V, Carbonara M, Carrabba G, Holtzman DM, Zipfel GJ, Stocchetti N, Brody DL (2012) Tau elevations in the brain extracellular space correlate with reduced amyloid-β levels and predict adverse clinical outcomes after severe traumatic brain injury. Brain 135, 1268–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Franz G, Beer R, Kampfl A, Engelhardt K, Schmutzhard E, Ulmer H, Deisenhammer F (2003) Amyloid beta 1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology 60, 1457–1461. [DOI] [PubMed] [Google Scholar]
- [45].Neselius S, Brisby H, Theodorsson A, Blennow K, Zetterberg H, Marcusson J (2012) CSF-biomarkers in Olympic boxing: Diagnosis and effects of repetitive head trauma. PloS One 7, e33606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Riemenschneider M, Wagenpfeil S, Vanderstichele H, Otto M, Wiltfang J, Kretzschmar H, Vanmechelen E, Förstl H, Kurz A (2003) Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt–Jakob disease from other dementias. Mol Psychiatry 8, 343–347. [DOI] [PubMed] [Google Scholar]
- [47].Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, Sjoegren M, DeBernardis J, Kerkman D, Ishiguro K (2004) Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: A comparative cerebrospinal fluid study. Arch Gen Psychiatry 61, 95–102. [DOI] [PubMed] [Google Scholar]
- [48].Koopman K, Le Bastard N, Martin J-J, Nagels G, De Deyn PP, Engelborghs S (2009) Improved discrimination of autopsy-confirmed Alzheimer’s disease (AD) from non-AD dementias using CSF P-tau 181P. Neurochem Int 55, 214–218. [DOI] [PubMed] [Google Scholar]
- [49].Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR, Jagust WJ, Shaw LM, Toga AW, Trojanowski JQ, Weiner MW (2010) Alzheimer’s Disease Neuroimaging Initiative (ADNI) clinical characterization. Neurology 74, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease. Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–939. [DOI] [PubMed] [Google Scholar]
- [51].Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, Russell RWR, Symon L (1975) Cerebral blood flow in dementia. Arch Neurol 32, 632–637. [DOI] [PubMed] [Google Scholar]
- [52].Nelson H (1982) National Adult Reading Test Manual, The National Hospital for Nervous Disease, London. [Google Scholar]
- [53].Grober E, Sliwinsk M (1991) Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol 13, 933–949. [DOI] [PubMed] [Google Scholar]
- [54].Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ, Alzheimer’s Disease Neuroimaging Initiative (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rey A (1958) L’examen Clinique en Psychologie, Presses Universitaries de France, Paris. [Google Scholar]
- [56].Lezak MD (2004) Neuropsychological Assessment, Oxford University Press, Oxford. [Google Scholar]
- [57].Wechsler D (1987) Wechsler Memory Scale-Revised, Psychological Corporation, Texas. [Google Scholar]
- [58].Wechsler D (1981) Wechsler Adult Intelligence Scale-Revised, The Psychological Corporation, Texas. [Google Scholar]
- [59].Lamberty GJ, Putnam SH, Chatel DM, Bieliauskas LA, Adams KM (1994) Derived Trail Making Test indices: A preliminary report. Neuropsychiatry Neuropsychol Behav Neurol 7, 230–234. [Google Scholar]
- [60].Jolliffe I (2002) Principal Components Analysis, Springer, New York. [Google Scholar]
- [61].Hayes AF (2013) Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, Guilford Press, New York. [Google Scholar]
- [62].Sobel ME (1986) Some new results on indirect effects and their standard errors in covariance structure models. Sociol Methodol 16, 159–186. [Google Scholar]
- [63].Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Buckner RL, Johnson KA, Sperling RA, Rentz DM (2012) Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J Neurosci 32, 16233–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lim YY, Ellis KA, Harrington K, Kamer A, Pietrzak RH, Bush AI, Darby D, Martins RN, Masters CL, Rowe CC (2013) Cognitive consequences of high Aβ amyloid in mild cognitive impairment and healthy older adults: Implications for early detection of Alzheimer’s disease. Neuropsychology 27, 322. [DOI] [PubMed] [Google Scholar]
- [65].Battistone M, Woltz D, Clark E (2008) Processing speed deficits associated with traumatic brain injury: Processing inefficiency or cautiousness? Appl Neuropsychol 15, 69–78. [DOI] [PubMed] [Google Scholar]
- [66].Maddocks D, Saling M (1996) Neuropsychological deficits following concussion. Brain Inj 10, 99–104. [DOI] [PubMed] [Google Scholar]
- [67].Ponsford J, Kinsella G (1992) Attentional deficits following closed-head injury. J Clin Exp Neuropsychol 14, 822–838. [DOI] [PubMed] [Google Scholar]
- [68].Schmitter-Edgecombe M, Marks W, Fahy JF (1993) Semantic priming after severe closed head trauma: Automatic and attentional processes. Neuropsychology 7, 136–148. [Google Scholar]
- [69].Spikman JM, Zomeren AHv, Deelman BG (1996) Deficits of attention after closed-head injury: Slowness only? J Clin Exp Neuropsychol 18, 755–767. [DOI] [PubMed] [Google Scholar]
- [70].Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lublin AL, Gandy S (2010) Amyloid-β oligomers: Possible roles as key neurotoxins in Alzheimer’s disease. Mt Sinai J Med 77, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ (2010) Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science 330, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Perl DP (2010) Neuropathology of Alzheimer’s disease. Mt Sinai J Med 77, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Van Broeck B, Van Broeckhoven C, Kumar-Singh S (2007) Current insights into molecular mechanisms of Alzheimer disease and their implications for therapeutic approaches. Neurodegener Dis 4, 349–365. [DOI] [PubMed] [Google Scholar]