Abstract
Chronic kidney disease (CKD) is becoming a worldwide epidemic, driven largely by the dramatic rise in the prevalence of diabetes and obesity. Novel targets and treatments for CKD are, therefore, desperately needed—to both mitigate the burden of this disease in the general population and reduce the necessity for renal replacement therapy in individual patients. This Review highlights new insights into the mechanisms that contribute to CKD, and approaches that might facilitate the development of disease-arresting therapies for CKD. Particular focus is given to therapeutic approaches using antifibrotic agents that target the transforming growth factor β superfamily. In addition, we discuss new insights regarding the roles of vascular calcification, the NADPH oxidase family, and inflammation in the pathogenesis of CKD. We also highlight a new understanding regarding kidney energy sensing pathways (AMPK, sirtuins, and mTOR) in a variety of kidney diseases and how they are linked to inflammation and fibrosis. Finally, exciting new insights have been made into the role of mitochondrial function and mitochondrial biogenesis in relation to progressive kidney disease. Prospective therapeutics based on these findings will hopefully renew hope for clinicians and patients in the near future.
Introduction
Chronic kidney disease (CKD) has become a major burden on the economies of many countries and severely impairs the quality of life of affected patients. The prevalence of CKD is estimated to be 8–16% worldwide.1 The number of patients requiring renal replacement therapies has also increased tremendously over the past decade, with currently >500,000 patients on dialysis in the USA alone. Kidney disease was ranked the eighteenth most common cause of mortality in 2010, and CKD was ranked third highest for years of life lost due to premature mortality (82%), behind only AIDS and diabetes mellitus.1 The market for dialysis is worth over US$60 billion worldwide and expected to grow considerably in the next 5 years.2 With the ongoing rise in the prevalence of diabetes, which is expected to continue, and the lag time between onset of diabetes and late-stage complications, the prevalence of CKD will likely increase even more dramatically in the future.
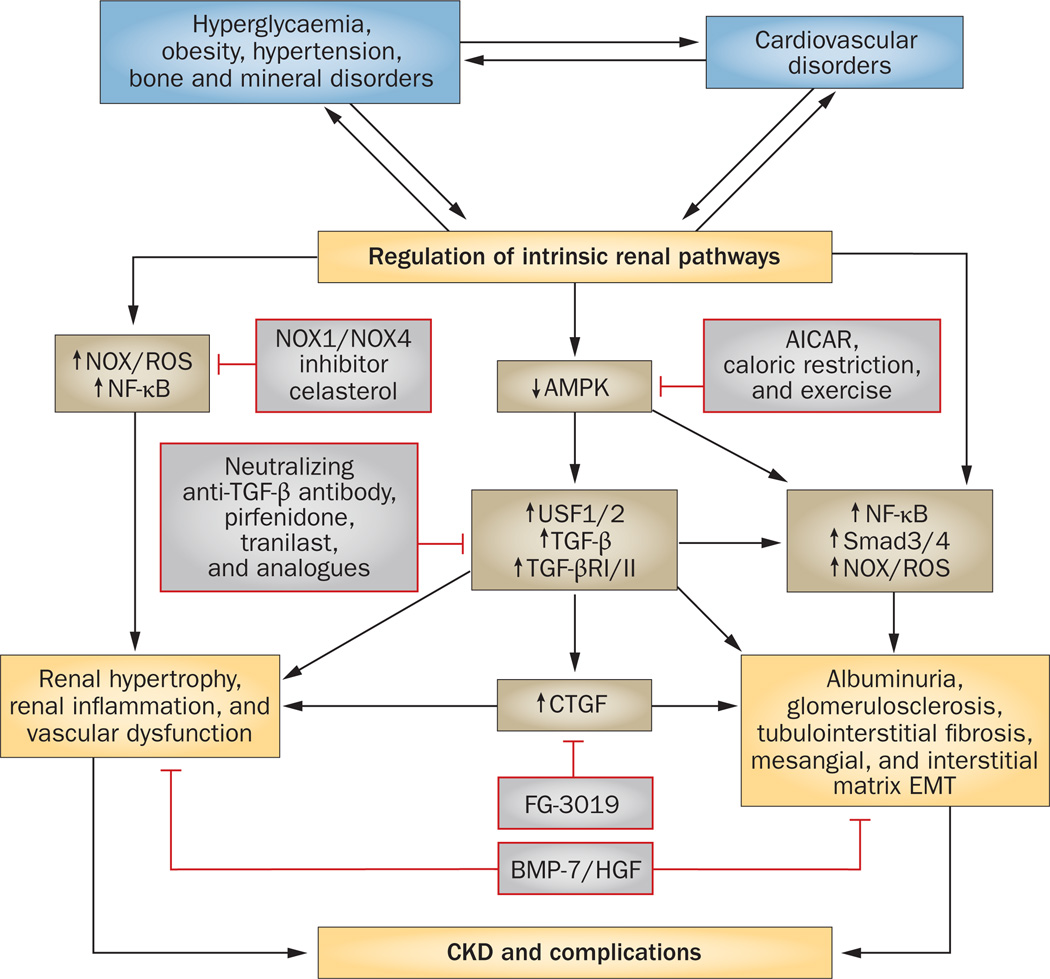
The current therapeutic strategy for treating diabetic and nondiabetic CKD largely involves the control of hyperglycaemia, dyslipidaemia, and systemic blood pressure (Figure 1). Although activation of the renin– angiotensin–aldosterone system (RAAS) has a central role in hypertension, and RAAS blockade has beneficial effects in terms of reducing albuminuria and slowing the progression of CKD, an urgent demand clearly exists for novel disease-modifying therapies that can arrest the progression of CKD.
Figure 1.
Specific targets and potential therapeutic strategies to inhibit or slow the progression of CKD. There is a complex feed-forward relationship between the initiating factors (hyperglycaemia, obesity, hypertension, bone and mineral disorders) and cardiovascular disorders that stimulate and regulate a variety of major pathways leading to CKD and its complications. Inhibiting intrinsic renal pathways linked to inflammation (NADPH oxidase) and fibrosis (Smads, TGF-β, and CTGF) might prove beneficial. A possible central pathway would be the activation of AMPK that can reduce both inflammatory and profibrotic pathways. Abbreviations: AICAR, AMP analogue; AMPK, 5’-AMP-activated protein kinase; BMP-7, bone morphogenetic protein 7; CKD, chronic kidney disease; CTGF, connective tissue growth factor; EMT, epithelial–mesenchymal transition; HGF, hepatocyte growth factor; NF, nuclear factor; NOX, NADPH oxidase; R, receptor; ROS, reactive oxygen species; Smad, mothers against decapentaplegic homologue; TGF-β, transforming growth factor β; USF, upstream stimulatory factor.
The role of TGF-β in renal fibrosis
Robust evidence suggests that essentially all progressive forms of CKD are characterized by marked accumulation of extracellular matrix proteins in the glomerulus and tubulointerstitium. As progressive fibrosis might be a driving force for the disruption of glomerular and tubular architecture, inhibition of the major mediators responsible for matrix accumulation might slow or arrest the progression of CKD. Support for this concept has been provided by the results of a number of studies in animal models of CKD, in which inhibiting factors that promote fibrosis, such as transforming growth factor β (TGF-β), connective tissue growth factor (CTGF), and myofibroblast activation,3–7 or enhancing factors that attenuate fibrosis, such as bone morphogenetic protein 7 (BMP-7) and hepatocyte growth factor (HGF),8,9 improved renal architecture and/or function (Table 1). Renal fibrosis is the final common manifestation defining CKD and is characterized by progressive tissue scarring that leads to glomerulosclerosis and tubulo interstitial fibrosis.10 Although the precise sequence of molecular events that result in renal fibrosis has not been completely elucidated, present data indicate that TGF-β is the master regulator of this process. TGF-β is the major driver of matrix synthesis, inhibition of matrix degradation, and myofibroblast activation.
Table 1.
Antifibrotic strategies for treating CKD
| Agent | Setting | Primary outcome and/or results |
|---|---|---|
| Preclinical studies | ||
| Anti-TGF-β antibody | Leprdb/db diabetic mice3,4 | Reduction in plasma TGF-β1 levels, prevention of increases in plasma creatinine levels and glomerular mesangial matrix expansion, associated with decreased renal mRNAs encoding collagen IV and fbronectin |
| Anti-TGF-β antibody | Rats with chronic allograft rejection nephropathy5 | Reduction of proteinuria, attenuation of mononuclear cell infltration and interstitial fbrosis along with downregulation of mRNA levels of TGF-β1, TGF-β2, and proinfammatory cytokines, or with upregulation of mRNA levels of HGF, BMP-5, and BMP-7 |
| Anti-TGF-β antibody | Mice with STZ-induced diabetes12 | Prevention of glomerular hypertrophy, attenuated gain in kidney weight, and attenuation of increased mRNA levels of TGF-β1, type II TGF-β receptor, collagen I V, and fbronectin |
| Anti-TGF-β antibody | Rats with puromycin aminonucleoside nephropathy13 | Reduction of TGF-β isoform mRNA expression and collagen III production, and amelioration of histological sclerosis with low dose of antibody |
| Soluble β-glycan | Leprdb/db diabetic mice14 | Reduction in serum creatinine, albuminuria, and structural renal damage |
| siRNA for TGF-β type II receptor | Mice with UUO15 | Decreased TGF-β receptor and α-SMA overexpression at the mRNA level, collagen deposition, and fbrotic area |
| Tranilast | Rats with remnant kidney,28 rats with chronic cyclosporin-induced nephrotoxicity,29 hypertensive Ren-2 rats with STZ-induced diabetes,30 and rats with UUO31 | Inhibition of TGF-β-induced glomerulosclerosis and tubulointerstitial fbrosis, reduction in albuminuria and plasma creatinine, attenuation of macrophage accumulation, and decreased oxidative stress |
| Analogue of tranilast (FT061) | Rats with progressive diabetic nephropathy6 | Reduction of albuminuria |
| Analogue of tranilast (FT011) | 5/6 nephrectomized rats and rats with STZ-induced diabetic nephropathy35 | Reduction in proteinuria, infammation, and glomerulosclerosis |
| Pirfenidone | Leprdb/db diabetic mice25 and 5/6 nephrectomized rats26 | Reduction in ECM accumulation and infammatory cell infltration |
| CTGF antisense oligonucleotide | Mice with STZ-induced diabetes19 and Leprdb/db diabetic mice19 | Reduction in CTGF mRNA expression, decreased proteinuria and albuminuria, attenuation in mRNA expression of fbronectin, and collagens I and IV |
| CTGF siRNA | Rats with chronic allograft rejection nephropathy20 | Reduction in mRNA CTGF expression, amelioration of serum creatinine level, reduction in mRNA expression of α-SMA and collagens I and IV |
| miR-21 | Leprdb/db diabetic mice39 | Amelioration of albuminuria, renal fbrosis, and infammation |
| Recombinant human BMP-7 | Mouse model of CKD44 | Decreased damage to renal tubular epithelial cells, in association with reversal of chronic renal injury |
| Exogenous HGF | Rats with remnant kidney,46,48,50 5/6 nephrectomized rats,52 and mice with STZ-induced diabetic nephropathy53 | Amelioration of renal fbrosis and tubulointerstitial collagen deposition; decreased renal infammation, glomerular hypertrophy, and sclerosis |
| Hgf gene transfection | Mouse model of chronic graft-versus-host disease49 | Prevention of proteinuria and histopathological changes associated with glomerulonephritis |
| Genetic deletion of CDA1 | Diabetic mice with CDA1 knockout55 | Reduction in ECM accumulation, decreased mRNA levels of TGF-β, TGF-β receptor, and Smad3 |
| Clinical studies | ||
| Anti-TGF-β antibody (fresolimumab) | Phase I Patients with primary steroid-resistant FSGS17 | Single dose was well tolerated |
| Anti-TGF-β antibody (LY2382770) | Phase II (ongoing) Patients with type 1 or type 2 diabetes mellitus and diabetic nephropathy16 | Change in serum creatinine levels from baseline to 12 months, results not yet reported |
| Tranilast | Phase I Patients with diabetic nephropathy32 | Amelioration of urinary excretion of type IV collagen and albumin |
| Analogue of tranilast (FT061) | Phase I Patients with type 2 diabetes mellitus and diabetic nephropathy36 | Reduction in proteinuria, infammation, and glomerulosclerosis |
| Pirfenidone | Phase I Patients with diabetic nephropathy27 | Encouraging results for increased mean eGFR but without improvement in proteinuria |
| Anti-CTGF monoclonal antibody (FG-3019) | Phase I Patients with diabetic nephropathy21 | FG-3019 was well tolerated and associated with decreased albuminuria |
| Anti-CTGF monoclonal antibody (FG-3019) and ACE inhibitors or ARBs | Phase I Patients with type 1 or type 2 diabetes mellitus and diabetic nephropathy22 | Safety and tolerability of two doses of FG-3019 administered for 12 weeks; results not yet reported |
| Anti-CTGF monoclonal antibody (FG-3019) | Phase II Patients with type 2 diabetes mellitus and diabetic nephropathy23 | To assess the effect of FG-3019 on proteinuria as assessed by urinary ACR, trial terminated |
| Anti-CTGF monoclonal antibody (FG-3019) | Phase I Patients with steroid-resistant FSGS24 | To assess safety and tolerability of FG3019; trial terminated |
Abbreviations: α-SMA, α smooth muscle actin; ACE, angiotensin-converting enzyme; ACR, albumin:creatinine ratio; ARB, angiotensin receptor blocker; BMP, bone morphogenetic protein; CDA1, cell division autoantigen 1; CKD, chronic kidney disease; CTGF, connective tissue growth factor; ECM, extracellular matrix; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; HGF, hepatocyte growth factor; siRNA, small interfering RNA; Smad3, mothers against decapentaplegic homologue 3; STZ, streptozotocin; TGF-β, transforming growth factor β; UUO, unilateral ureteral obstruction.
Therapeutic agents that inhibit TGF-β have been shown to reduce matrix accumulation in animal models of diabetes, puromycin nephropathy, unilateral ureteral obstruction (UUO), diseases involving antibodies to glomerular basement membrane components, and hypertensive renal disease.3,11–13 Many potential therapeutic approaches based on inhibition of TGF-β have been tested in experimental models of CKD, such as the administration of neutralizing anti-TGF-β anti-bodies,3–5,12 soluble TGF-β receptor14 or small interfering RNAs that target the TGF-β type II receptor (Table 1).15 These therapies reduced structural renal injury and decreased renal fibrosis. Interestingly, although anti-TGF-β antibodies reduced matrix accumulation in glomerular and tubulointer stitial disease, reductions in albuminuria were not consistently observed, demonstrating that the two processes might operate through separate pathways. In 2011, the results of a phase I clinical trial of fresolimumab, an anti-TGF-β antibody, showed that this agent was well tolerated in patients with primary resistant focal segmental glomerulo sclerosis (FSGS).16 Phase II studies of another anti-TGF-β antibody, LY2382770, are ongoing in patients with either refractory FSGS or diabetic nephropathy.17
Although TGF-β has a central and dominant role in renal fibrosis, inhibition of TGF-β might promote inflammation and epithelial cell proliferation as well as inhibit fibrosis. CTGF, which is also closely associated with the progression of renal fibrosis but is thought to act downstream of TGF-β,18–20 might, therefore, be a more fibrosis-specific target than TGF-β itself. The results of a phase I trial of FG-3019, an anti-CTGF antibody, demonstrated that this agent reduced albuminuria in patients with diabetic nephropathy.21 Other trials of FG-3019 in combination with angiotensin-converting-enzyme inhibitors and/ or angiotensin-receptor blockers have been conducted in patients with diabetic nephropathy.22,23 A further trial of FG-3019 was conducted in patients with steroid-resistant FSGS.24 The principal objective of these trials was to assess the safety and tolerability of FG-3019 as well as to determine its effect on proteinuria. However, the phase II trial in patients with diabetic kidney disease was stopped early; the FSGC trial has been completed, but the results are not yet publicly available.22–24
Several oral agents have been shown to delay the progression of renal fibrosis in animal models of CKD. Pirfenidone is an orally bioavailable compound that inhibits TGF-β as well as platelet-derived growth factor and tumour necrosis factor (TNF) via unknown mechanisms. Several studies in animal models of CKD have demonstrated beneficial effects of pirfenidone in reducing extracellular matrix accumulation and inflammatory cell infiltration.25,26 An exploratory clinical trial of pirfenidone in 77 patients with diabetic nephropathy has been completed27 in which an encouraging beneficial effect on the mean estimated glomerular filtration rate (eGFR), but not on proteinuria, was observed at a dose of 1,200 mg daily.27 Baseline levels of plasma biomarkers of inflammation and fibrosis—such as TNF, soluble TNF receptor, and fibroblast growth factor 23 (FGF-23)— correlated with baseline eGFR in these patients, but did not predict a response to pirfenidone treatment.27 Although these results are promising, the lack of effect on albuminuria and on the measured biomarkers makes pirfenidone dosing and monitoring its effect challenging.
Tranilast, another oral antifibrotic agent, reduced glomerulosclerosis, tubulointerstitial fibrosis, and renal inflammation in animal models of CKD.28–31 Two small-scale clinical studies, conducted in nine and 20 patients with diabetic nephropathy, respectively, demonstrated that tranilast treatment showed promise for slowing the progression of renal disease.32,33 An analogue of tranilast, FT061, has demonstrated encouraging beneficial effects in in vitro and in vivo models by reducing collagen production through inhibition of TGF-β in renal mesangial cells, as well as preventing worsening of albuminuria in a rat model of diabetic nephropathy.6 Another oral analogue of tranilast, FT011, has been tested for antifibrotic effects in experimental models of kidney and heart fibrosis.34 In two different rat models of CKD (5/6 nephrectomized rats and hypertensive Ren-2 transgenic rats with streptozotocin-induced diabetes), FT011 reduced protein uria, inflammation, and glomerulosclerosis.35 FT011 also had a cardioprotective role in the diabetic Ren-2 rat model, attenuating cardiomyocyte hypertrophy as well as macrophage infiltration and interstitial fibrosis of heart tissue. A phase I clinical trial demonstrated safety and tolerability of FT011 in healthy volunteers and patients with type 2 diabetes mellitus who had diabetic nephropathy.36 A secondary outcome of this clinical study is also ongoing to evaluate the effect of FT011 on kidney function.36 The results of this trial could provide a new perspective in preventing the progression of renal fibrosis in patients with CKD.
Various studies have focused on potential interventions downstream of TGF-β signalling. Although testing of TGF-β receptor kinase inhibitors has not advanced to clinical studies, several approaches involve interruption of mothers against decapentaplegic homologue (Smad)—a mediator of TGF-β activity— post-receptor signalling. For example, the effects of FT011 might be mediated via inhibition of pathways involving Smad2 and mitogen-activated protein kinases 1 and 3.34 Moreover, Smad3 has emerged as potentially the most important receptor-regulated phosphopeptide in the Smad family in relation to matrix accumulation: knock out of Smad3 protects against diabetic nephropathy, hypertensive kidney disease, and obstructive nephropathy. 37 Importantly, Smad3 is also phosphorylated via stimulation of angiotensin II, independent of TGF-β.36 Smad4 is a cofactor involved in all Smad-mediated signalling that has emerged as essential for transcriptional initiation of Smad3 target genes. Deletion of Smad4 in tubular epithelial cells, tubulointerstitial fibroblasts, and mesangial cells protects against TGF-β-induced matrix accumulation.37,38 TGF-β-induced stimulation of a variety of microRNAs (miR-21, miR-29, and miR-192) is mediated via Smad3.39 Blocking miR-2139 and miR-19240 seems to be a promising approach to inhibit TGF-β-induced fibrosis, and this strategy is being considered for clinical studies.
BMP-7 is another member of the TGF-β superfamily. Signalling via BMP-7 receptors results in phosphorylation of Smad1, Smad5, and Smad8. As Bmp7–/– mice die of renal failure41 and BMP-7 levels are reduced in patients with CKD,42,43 this protein has been recognized as a potential therapeutic agent in this setting. BMP-7 administration attenuates renal fibrosis and, probably via Smad7, inhibits TGF-β signalling.44 Although administration of BMP-7 itself might be of limited use in clinical practice, owing to its propensity to induce soft tissue calcification, treatments based on BMP-7 receptor agonists or modulating antagonists of BMP-7 seem to show promise.45
HGF is well known to promote tissue repair in many diseases, and is also considered to function as an anti-fibrotic regulator in CKD. Its biological actions in the kidney depend on the cell type. The administration of anti-HGF antibodies to rats or mice with CKD worsened progression of tubulointerstitial fibrosis, suggesting a role for HGF in suppressing fibrosis.46,47 In addition, treatment with exogenous HGF was effective in terms of ameliorating declining renal function and decreasing fibrosis in various experimental models of CKD (including 5/6 nephrectomized rats, renal allograft recipients, animals with diabetic nephropathy, and aristolochic acid-induced nephrotoxicity).46,48–53
Testis-specific Y-encoded-like protein 2 (TSPY-like protein 2, also known as cell division autoantigen 1 or CDA1) has been highlighted as a potential target to reduce renal TGF-β signalling.54,55 CDA1 was first considered for its antiproliferative effects;56 however, the critical role of CDA1 in TGF-β signalling was demonstrated in models of diabetic nephropathy. CDA1 was upregulated in tubular cells and podocytes in rodent and human diabetic kidneys.54 In the same study, knockdown of CDA1 in cultured cells reduced production of extra- cellular matrix proteins and TGF-β-stimulated expression of genes encoding collagen I and collagen III. In a separate study, the role of TSPY-like protein 2 in regulating TGF-β signalling was demonstrated.55 In diabetic Tspyl2–/– mice there was reduced renal accumulation of extra cellular matrix proteins and decreased glomerular and tubulointerstitial injury were observed, along with decreased gene expression of TGF-β and TGF-β type I receptor, and decreased levels of phosphorylated Smad3.55 Interestingly, targeted deletion of Tspyl2 did not affect other features, such as hyperglycaemia, renal hypertrophy, or hyperfiltration, in these diabetic mice.55 Although additional studies are required to evaluate the potential effect of CDA1 inhibition on sustained protein-uria and progressive fibrosis, its actions in reducing TGF-β-mediated matrix accumulation provide support for CDA1 as a potential therapeutic target to slow the progression of CKD.
Vascular calcification factors
Vascular calcification and associated hyperphosphat-aemia is a common feature in CKD and both type 1 and type 2 diabetes mellitus. Studies of the effects of FGF-23 and Klotho have revealed many new insights regarding the pathogenesis of vascular calcification. Although FGF-23 is produced by bone, the kidney is its principal target organ—FGF-23 (along with Klotho) increases urinary phosphate excretion and suppresses production of 1,25- dihydroxyvitamin D3. In addition, FGF-23 acts on the parathyroid gland to decrease the secretion of para thyroid hormone, and has been identified as a potential mediator of cardiac hypertrophy in patients with CKD (through a Klotho-independent pathway).57
The actions of FGF-23 occur through FGF receptors and Klotho.58 Klotho is produced in the tubular epithelium of the kidney and exists in membrane-bound as well as soluble forms.59 The membrane-bound form of Klotho acts as a co-receptor for FGF-23 on renal tubular cells, where it augments the phosphaturic activity of FGF-23.59 Circulating levels of FGF-23 in humans increase with decreasing renal function, reaching very high concentrations in patients with end-stage renal disease.60,61 This observation is likely to represent a compensatory response to maintain the phosphate balance. However, since the progressive decline of renal function and the loss of functional nephrons in individuals with CKD lead to decreased Klotho expression in the kidney, the ability of FGF-23 to increase urinary phosphate excretion is limited. A potential therapeutic approach to targeting FGF-23 could involve administration of neutralizing anti-FGF-23 antibodies; however, inhibition of FGF-23 might not be appropriate in the early stages of renal disease owing to the resulting inhibition of phosphaturia.
In view of the aforementioned results, the role of Klotho in vascular calcification is attracting considerable interest. New insight into the roles of Klotho has been gained by studying Klotho-knockout (Kl/–/–) mice, which have severe vascular calcification and CKD.62 Klotho has a beneficial role in preserving phosphaturia and the glomerular filtration rate, but also acts on vascular smooth muscle to suppress dedifferentiation of vascular smooth muscle cells (Table 2). Of note, loss of Klotho contributes to renal fibrosis. Klotho-mediated regulation of aldosterone might have a role in vascular calcification, as Kl–/– mice seem to have hyperaldosteronism;63 treatment of these mice with spironolactone reduces vascular calcification and moderately increases survival.64 Key regulators of vascular calcification (such as sodium- dependent phosphate transporter 1, TNF, homeobox protein MSX2, and others) are upregulated in the aorta of Kl–/– mice, although expression levels could be reduced with spinorolactone treatment.64 Moreover, vascular smooth muscle cells express mineralocorticoid receptors, and activation of these receptors leads to differentiation and mineralization of human vascular smooth muscle cells.64–66 The emerging recognition of this connection between Klotho–aldosterone signalling and vascular calcification requires further validation in additional animal models and in humans; nevertheless, many options exist for targeting this pathway, thereby improving kidney and cardiovascular disease (Table 2).
Table 2.
Non-antifibrotic strategies for treating CKD
| Agent | Setting | Primary outcome and/or results |
|---|---|---|
| Vascular calcifcation | ||
| Klotho overexpression | Mice with CKD62 | Enhanced phosphaturia, improved renal function, and decreased calcifcation |
| Exogenous Klotho | Mice with UUO67 and mice with adriamycin-induced CKD67 | Inhibition of renal β-catenin activation, suppression of myofbroblast activation, reduction in extracellular matrix protein expression, and amelioration of renal fbrosis |
| Oxidative stress and NADPH oxidases | ||
| NOX-2 defciency | Mice with STZ-induced diabetic nephropathy74 | No benefcial effect on renal function, and marked increase in NOX-4 at the protein level in the kidney |
| GKT136901* | Leprdb/db diabetic mice80,81 | Reduction in albuminuria and oxidative stress |
| GKT137831* | Diabetic Apoe–/– mice83 | Reduction in ROS production, infammation, vascular complications, and profbrotic markers, such as CTGF, collagen I V, and fbronectin |
| GKT137831* | Ongoing phase I/II study in patients with type 2 diabetes mellitus and diabetic nephropathy84 | GKT137831 was well tolerated |
| NOX-4 defciency | Mice with STZ-induced diabetes,76 mice with UUO,76 and 5/6 nephrectomized mice76 | No benefcial effect on renal function |
| NOX-4 defciency | Mice with UUO85 | Increased tubulointerstitial fbrosis, increased oxidative stress, and decreased antioxidant markers, such as NRf2, at the protein level as well as glutathione S-transferase α at the mRNA level |
| NF-κB | ||
| PDTC | Rats with gentamicin-induced nephropathy,86 5/6 nephrectomized rats,87 and rats with aldosterone and salt-induced CKD88 | Attenuation of renal infammation and renal injury |
| Celastrol | Leprdb/db diabetic mice89 | Reduction in albuminuria and glomerular mesangial matrix along with reduced TGF-β expression at the protein level and collagen IV deposition |
| Energy sensing | ||
| AICAR | High-fat-diet-induced kidney disease in mice102 | Reduction in albuminuria, glomerular mesangial matrix, renal infammation, and lipid accumulation |
| AICAR | OVE26 mice104 | Reduction in albuminuria and renal hypertrophy along with decreased NOX-4 and tumour-suppressor p53 expression at the protein level |
Dual NOX-1 and NOX-4 inhibitor.
Abbreviations: AICAR, AMP analogue; ApoE, apolipoprotein E; CKD, chronic kidney disease; CTGF, connective tissue growth factor; NF, nuclear factor; NOX, NADPH oxidase; NRf2, nuclear factor erythroid 2-related factor 2; PDTC, pyrrolidine dithiocarbamate; ROS, reactive oxygen species; STZ, streptozotocin; TGF-β, transforming growth factor β; UUO, unilateral ureteral obstruction.
Administration of exogenous Klotho has a potent antifibrotic effect, as Klotho seems to be an endogenous antagonist of Wnt–β-catenin activity, which promotes fibrogenesis.67 In addition, Klotho seems to be inhibited by TGF-β, and increased secretion of Klotho has a role in suppressing myofibroblast activation.67 Loss of Klotho in renal tissue might, therefore, lead to progression of renal fibrosis. Future studies exploring the antifibrotic potential of Klotho might reveal therapeutic potential.67
Oxidative stress and NADPH oxidases
The critical role of oxidative stress as a mechanism underlying all diabetic complications has been emphasized.68 According to this view, the initial increase in mitochondrial superoxide (driven by exposure to high glucose levels) resulting from increased electron transport chain activity contributes to alterations in downstream pathways, such as stimulation of protein kinase C signalling, increased intracellular formation of advanced glycation end-products, and upregulation of the sorbitol pathway.68–71 Although clear evidence from numerous groups attests to increased production of specific reactive oxygen species (ROS),72,73 demonstration that mitochondrial superoxide production is increased, and is the initial catalyst of downstream deleterious pathways, remains to be established. In this context, the NADPH oxidases might act downstream of mitochondrial superoxide production driven by the electron transport chain. These enzymes are widely expressed in the kidney and well-known to be a major source of ROS.
Several publications have focused on isoforms of these enzymes as potential major sources of oxidant production in CKD.74,75 Of the major isoforms that have been identified, NOX-1, NOX-2 (also known as cytochrome b-245 heavy chain), and NOX-4 are expressed in both rodent76 and human77 kidneys and might have a role in mediating oxidative stress in CKD by promoting vascular dysfunction, inflammation, and fibrosis.74,78 By contrast, little is known about the role of NOX-5 in kidney disease, as it is only expressed in human kidneys. Transgenic mice with podocyte- specific expression of human NOX-5 (Nox5pod+) develop albuminuria, podocyte foot process effacement, and elevated blood pressure.79 These changes were all shown to be exacerbated in animals with streptozotocin- induced diabetes.79 Moreover, NOX-5 expression was increased in kidney biopsy specimens from patients with diabetic nephropathy.79 The results of this study represent a first step towards an improved understanding of the role of NOX-5 in the development of kidney diseases.
Although the roles of NOX-1, NOX-2, and NOX-4 in kidney disease have not been fully elucidated, several studies have provided new insights. Our group found that NOX-2-knockout mice (Nox2–/–) have the same degree of hyperglycaemia and weight loss after induction of diabetes using streptozotocin as is observed in wild-type mice with streptozotocin-induced diabetes; furthermore, the degree of diabetic nephropathy was unchanged in the Nox2–/– mice with streptozotocin- induced diabetes.74 The degree of albuminuria, glomerular matrix expansion, and urinary levels of hydrogen peroxide were all essentially the same in both groups (wild-type and Nox2–/–) of diabetic mice. As expression of NOX-4 was markedly increased in the kidneys of diabetic Nox2–/– mice, it is possible that upregulation of NOX-4 compensates for NOX-2 deficiency, and might be sufficient to promote diabetic nephropathy. By contrast, the utility of simultaneously inhibiting NOX-1 and NOX-4 was demonstrated in studies of GKT136901, a dual inhibitor of NOX-1 and NOX-4.80,81 Markers of oxidative stress and profibrotic signalling were reduced in mouse proximal tubule cells exposed to high glucose levels, and GKT13691 reduced albuminuria and oxidative stress in diabetic Leprdb/db mice.80,81 In other studies, another dual inhibitor of NOX-1 and NOX-4, GKT137831, protected against experimentally induced liver fibrosis,73 and also reduced oxidative stress in human aortic endothelial cells.82,83 The role of NOX-1 in diabetes-associated vascular complications has also been evaluated in mice.83 Deletion of Nox1 in atherosclerosis-prone, apolipo-protein E-deficient (Apoe–/–) mice with streptozotocin-induced diabetes prevented the development of atherosclerotic plaques in the aorta, and similar results were reported for treatment with GKT137831 in diabetic Apoe–/– mice. By contrast, knockout of Nox4 in diabetic Apoe–/– mice did not prevent plaque formation in the aorta, suggesting that NOX-1 has a crucial role in diabetes-induced vascular complications. In addition, administration of GKT137831 to diabetic Apoe–/– mice reduced ROS production, inflammation, and expression of vascular adhesion molecules and profibrotic markers.83 A phase I clinical trial using a single dose of GKT137831 demonstrated that it was safe and well tolerated.84 A double-blind, randomized, placebo- controlled phase II study is underway to evaluate the effect of GKT137831 on albuminuria in patients with type 2 diabetes mellitus.84
Many studies support the concept that overproduction of NADPH oxidase isoforms is implicated in several pathological processes relevant to CKD; however, some NADPH oxidase isoforms might also elicit protective functions. In particular, the protective role of NOX-4 has been investigated. The pathogenic role of NOX-4 was questioned based on a study that used three different models of CKD (streptozotocin-induced diabetes, UUO, and 5/6 nephrectomized mice) to define the role of NOX-4 in renal fibrosis.76 NOX-4 knockout did not reduce progression of the disease in these three models. In the streptozotocin-induced diabetes model, NOX-4 deficiency led to increased albuminuria but to a similar degree of matrix deposition in wild-type and knockout mice. In the UUO mice, renal expression of ICAM-1, CTGF, and fibronectin were higher in knockout than wild-type mice, and in the 5/6 nephrectomy model, the development of hypertension was not prevented in the knockout mice. In addition, a separate study revealed a protective role of NOX-4 in the UUO model of CKD.85 Nox4-deficient mice exhibited extensive tubulo interstitial fibrosis, tubular apoptosis, and oxidative stress and reduced expression of antioxidant markers, such as hypoxia-inducible factor 1α and nuclear factor erythroid 2-related factor 2; renal expression of the other NADPH oxidase isoforms remained unchanged.85 The function of NOX-4 might, therefore, differ across the whole body, individual organs, or types of cells. Moreover, its effect might also be time-dependent, changing over the course of the disease. Although complete deletion of Nox4 might be detrimental, the body of evidence collected to date suggests that reducing NOX-4 overactivity is a worthwhile strategy in treating renal disease progression in diabetic nephropathy.
Inflammation and NF-κB signalling
As nuclear factor κB (NF-κB) activates a large number of proinflammatory genes, it is an attractive therapeutic target for the regulation of the inflammatory processes involved in CKD. New insights into NF-κB signalling and its crucial role in renal inflammation have been gained with the use of pyrrolidine dithiocarbamate (PDTC), which inhibits the NF-κB pathway to block to chronic inflammation in gentamicin-treated rats and in the 5/6 nephrectomy rat model.86,87 In both models, PDTC treatment attenuated renal injury and renal inflammation.86,87 In a rat model of renal disease induced by aldosterone and salt administration,88 PDTC treatment reduced renal injury by inhibiting the expression of genes encoding TGF-β, intercellular adhesion molecule 1 (ICAM-1), collagen IV, and CTGF, as well as expression of CTGF and ICAM-1 proteins. Although the exact mode of action of PDTC is unclear, these data reinforce the concept that NF-κB has a pivotal role in the progression of chronic renal inflammation. However, these studies only showed a partial renoprotective effect of PDTC.
The effect of celastrol, another inhibitor of NF-κB, on insulin resistance and renal function has been tested in Leprdb/db diabetic mice.89 Celastrol is derived from a plant (Tripterygium wilfordi) used in traditional Chinese medicine that has anti-inflammatory, antioxidant, and anticancer activities. Celastrol treatment improved insulin resistance, reduced albuminuria, glomerular matrix expansion, TGF-β expression, and deposition of collagen IV in these mice.89 Urinary levels of several proinflammatory cytokines were also significantly decreased, as was the accumulation of renal lipids.89 Celastrol prevents acute kidney injury (caused by ischaemia–reperfusion injury) in rats by reducing renal inflammation and tubular injury90 In these studies, only partial inhibition of NF-κB was observed, suggesting that the protective effects of celastrol might be mediated via combined inhibition of other inflammatory proteins such as mitogen-activated protein kinases and transcription factor AP-1.91 Currently, no clinical trials have assessed the effects of NF-κB inhibitors in patients with CKD; however, these new data might facilitate such an approach.
Energy-sensing molecules
The kidney is prone to injury associated with states of excess caloric intake, such as diabetes and obesity, with similar renal manifestations. Structural alter-ations include mesangial matrix expansion, glomerulomegaly glomerulosclerosis, thickening of the glomerular basement membrane, progressive detachment of podocytes, renal haemodynamic alterations, inflammation, and the progressive development of interstitial fibrosis.92–94 Functional impairment is usually characterized by increased proteinuria, as well as glomerular hyperfiltration.94–96
For a long time, adipose tissue was considered to act as a passive fat storage depot; now, it is recognized to be an active endocrine organ that produces a number of adipo-kines (such as leptin and adiponectin) and TNF that are involved not only in physiological functions, such as energy and cell homeostasis and metabolism, but also in pathophysiological processes, such as inflammation and insulin resistance.97 Numerous adipocyte-derived molecules are involved in the pathological changes leading to RAAS activation, hyperinsulinaemia, inflammation, and hormonal disturbances.90,98,99
Among the adipokines, adiponectin might provide renal protection via its anti-inflammatory properties. Studies of adiponectin and its downstream signalling pathway, 5’-AMP-activated protein kinase (AMPK), have established an important causal link between metabolic disease and the development of CKD.75 The role of AMPK in the kidney has highlighted a key pathway that links energy sensing with the development of renal inflammation and fibrosis.
AMPK in CKD and inflammation
AMPK is activated in response to depletion of ATP or an increased intracellular ratio of AMP to ATP, which preserves cell survival under low-caloric conditions.100 The central role of AMPK in mediating the effects of adiponectin was established by studies in adiponectin receptor 1 (AdipoR1)-knockout mice.101 AMPK has a role in early renal inflammation and sustained fibrosis in mice with diet-induced obesity and renal injury.92,102 Potent inhibition of CCL2 (also known as MCP-1) by AMPK was observed in vivo and in mesangial cells; furthermore, activation of AMPK completely blocked lipid vacuolization in proximal tubule cells.92 Part of the basis for this latter finding might be reduced activity of HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase and reduced cholesterol production, which occurs in response to AMPK activation.102 AMPK also seems to have a prominent role in regulating macrophage infiltration and activation. Infiltrated macrophages in the kidneys of mice fed a high-fat diet were completely reduced by AMPK activation.92 Furthermore, AMPK activation lowered the ratio of CD11c to CD11b, indicating a reduction specifically in M1 macrophages. These observations highlight a role for AMPK in the regulation of macrophage activation,100 which will probably become an active area of future research.
AMPK also seems to have a key role in regulating NADPH oxidase isoforms. NOX-4 is prominently expressed in podocytes, and the upregulation of NOX-4 induced by exposure to high glucose levels can be blocked by treatment with adiponectin or by activation of AMPK.75 In other studies, activation of AMPK inhibited expression of NOX-2 subunits p67 and p47, via upregulation of IκB-α, which blocks NF-κB from stimulating expression of the genes encoding these subunits in endothelial cells.103 A role for AMPK regulation of NOX-4 was demonstrated in diabetic nephropathy by several groups, leading to a growing consensus that NOX-4 might be the most critical NAPDH oxidase isoform linked to progression of diabetic nephropathy.80,104
AMPK activation and fibrosis
In addition to inflammation, AMPK has also been closely linked to fibrosis-promoting pathways. In mice fed a high-fat diet, chronic activation of AMPK with the AMP analogue, AICAR (5-aminoimidazole-4-carboxamide ribonucleotide) reduced both mesangial matrix expansion and urinary levels of TGF-β1.102 Our group reported that AMPK activation also markedly reduced glomerular accumulation of TGF-β, collagen and fibronectin in several mouse models of diabetic nephropathy.105 Similar findings were also observed in the OVE26 mouse model of diabetic nephropathy.104
The mechanistic basis of how AMPK activation inhibits TGF-β is unclear at present. Adiponectin and AMPK reduce TGF-β-induced secretion of the extracellular matrix proteins collagen types I and IV and fibronectin, and also inhibits myofibroblast transdifferentiation;106 however, phosphorylation of Smad2 and Smad3 was unaffected.106 The key transcription factor, upstream stimulatory factor 1 (USF1), is translocated to the nucleus in response to high glucose levels, and this effect is completely blocked by AMPK activation.107 As USF1 mediates glucose-induced stimulation of TGF-β1 gene transcription, AMPK might have an important role in regulating USF1-induced TGF-β1 synthesis.108
AMPK activation, sirtuins, and mTOR
A key downstream event in AMPK activation involves stimulation of members of the sirtuin family of class III protein deacetylases, of which seven different forms have been identified (Sirt1–7). Sirt1 and Sirt3 are induced by calorie restriction, and the catalytic α-subunit of AMPK regulates sirtuin synthesis in macrophages.109 Interestingly, Sirt3 is regulated by angiotensin receptors and might have a role in cell senescence.110
The serine– threonine protein kinase mammalian target of rapamycin (mTOR) is activated in patients with diabetic nephropathy,111 and inhibition of mTOR signalling prevented glomerulosclerosis and ameliorated the progression of glomerular disease in mice.111 However, mTOR knockout in podocytes also contributes to renal disease,111,112 and treatment with rapamycin worsens proteinuria in some patients,113 limiting its potential as a therapeutic agent for diabetic nephropathy. In another study, mTOR inhibition also led to reduced levels of NOX-4 in podocytes, suggesting that mTOR might directly regulate NOX-4 independently of AMPK.114
Mitochondrial biogenesis and kidney disease
A key pathway by which AMPK activation protects cells in a calorie-deprived state involves stimulating the master regulator of mitochondrial biogenesis, peroxi-some proliferator-activated receptor γ co-activator 1α (PGC-1α, encoded by PPARGC1A). This transcriptional co- activator is a potent stimulator of many mitochondrial proteins, and its activation increases the mitochondrial content of the cell.115 In states of reduced AMPK activation, PGC-1α activity would also be expected to be reduced. Indeed, PGC-1α levels are markedly reduced in muscle tissue of patients with diabetes,116 which might result in part from epigenetic modification of the PPARGC1A promoter. PGC-1α levels are reduced in diabetic kidneys, in association with reduced AMPK, reduced mitochondrial content, and reduced mitochondrial complex activity.105 This observation has led researchers to question whether mitochondrial complex activity in the electron transport chain is also reduced, and whether a concomitant change might occur in mitochondrial superoxide production. Indeed, when our group conducted real-time imaging of diabetic kidneys, we showed that superoxide production was reduced; this finding was further verified in ex vivo studies using electron paramagnetic resonance measurements.105 These observations indicate that the diabetic kidney is actually in a state of reduced mitochondrial activity with reduced mitochondrial super-oxide production, in direct contrast to the prevailing notion that diabetic complications result from an excess of mitochondrial superoxide. Clearly, the question of mitochondrial superoxide production will need to be further addressed in CKD using tools that can accurately measure superoxide production in vivo and in specific subcellular compartments.
In other work, our research group obtained an index of mitochondrial activity in patients with established diabetic nephropathy by quantitative measurements of a variety of metabolites linked to various relevant biochemical pathways.117 The predominant signature identified was a reduction in metabolites produced by mitochondrial enzymes. Semiquantitative analysis revealed a reduction in mitochondrial complex IV in kidney biopsy samples from patients with diabetic nephropathy. Furthermore, PPARGC1A expression was shown to be reduced in kidney tissues from patients with diabetic nephropathy, but not in those from patients with minimal change disease.117 These studies have established a new paradigm for understanding diabetic nephropathy. An early and progressive reduction in mitochondrial content, potentially driven by reduced activity of AMPK and PGC-1α, seems to be linked to early renal inflammation and profibrotic pathways. Results from animal studies suggest that reversal of these features is beneficial, and similar approaches might be useful in treating patients with diabetes as well. Of note, nonpharmacological means of increasing AMPK activity (such as exercise and caloric restriction) reduce diabetic nephropathy independent of weight loss and glucose lowering.118
Novel activators of AMPK are under investigation. For example, ZNL024 (a small-molecule allosteric activator of AMPK) has beneficial effects in mice.119 In this study, Lepr db/db diabetic mice treated with ZNL024 demonstrated improved glucose tolerance, reduced liver weight, and decreased liver content of total cholesterol and triacylglycerol.119 Valproic acid, a potent antiepileptic agent, is effective in activating AMPK in vitro and in vivo.120 Treatment of Lepob/ob hyperphagic obese mice with valproic acid significantly reduced plasma glucose levels and decreased both liver mass and fat content, owing to AMPK activation.120 Further studies will need to be conducted to define the role of these agents in activating AMPK in different stages of CKD.
Conclusions
The exciting new research linking energy-sensing pathways, kidney inflammation, and fibrosis suggest novel paradigms in the approach to treating CKD. Activation of the AMPK–sirtuin–PGC-1α pathways and inhibition of mTOR will probably find a place in the treatment of metabolic disorders as well as various types of progressive kidney disease. Anti-inflammatory approaches to mitigate NF-κB activation and NOX activity using oral inhibitors are promising. Oral agents that inhibit fibrosis and vascular calcification via multiple mechanisms of action might prove valuable adjuncts to currently available therapies in patients with moderate to advanced stages of CKD. In addition, molecules identified by microRNA and metabolomic analysis might serve as useful targets for new therapies, as well as markers for monitoring disease progression. Coupled with the advances in biomarker research and the close collaboration of academia, industry, and government agencies, the coming years could herald a new era of drug discovery in the treatment of CKD.
Key points.
-
▪
The incidence of chronic kidney disease (CKD) has increased dramatically over the past 10 years, and new strategies for slowing its progression are urgently needed
-
▪
Many novel approaches to control renal fibrosis in CKD are currently in clinical development
-
▪
NADPH oxidase isoforms have emerged as important mediators of vascular dysfunction, inflammation, and fibrosis in CKD
-
▪
New insights into the role of vascular calcification in the pathogenesis of CKD have led to novel therapeutic approaches, which are under preclinical and clinical development
-
▪
Further animal studies and clinical trials are urgently needed to determine the potential beneficial effects of activating energy-sensing molecules in slowing the progression of CKD
-
▪
Mitochondrial function and biogenesis are now considered as active players in the development of CKD
Acknowledgements
K.S. is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DP3DK094352), the Veterans Administration Merit Grant (5101BX000277), and the Juvenile Diabetes Research Foundation.
K.S. has received research funding from AbbVie, has consulted for Boerhinger-Ingelheim, Genkyotex, and Sanofi, and holds equity in Clinical Metabolomics.
Footnotes
Competing interests
A.-E.D. declares no competing interests.
Author contributions
A.-E.D. and K.S. contributed equally to researching the data for the article, discussions of its content, writing, reviewing and editing of the manuscript before submission.
References
- 1.Jha V, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.ReporterLinker.. Dialysis Market [(Hemodialysis —Machine, Dialyzer, Bloodlines, Concentrates, Services), (Peritoneal Dialysis—Cycler, Catheter, Dialysate, CCPD, CAPD, IPD), (End Users—Hospital, Independent Dialysis Center, Home Dialysis)]—Global Forecast to 2018. 2013 [online] http://www.reportlinker.com/p01887614/Dialysis-Market-Hemodialysis-Machine-Dialyzer-Bloodlines-Concentrates-Services-Peritoneal-Dialysis-Cycler-Catheter-Dialysate-CCPD-CAPD-I PD-End-Users-Hospital-Independent-Dialysis-Center-Home-Dialysis-Global-Forecast-to.html.
- 3.Ziyadeh FN, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-β antibody in db/db diabetic mice. Proc. Natl Acad. Sci. USA. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, et al. Reversibility of established diabetic glomerulopathy by anti-TGF-β antibodies in db/db mice. Biochem. Biophys. Res. Commun. 2003;300:16–22. doi: 10.1016/s0006-291x(02)02708-0. [DOI] [PubMed] [Google Scholar]
- 5.Guan Q, et al. Reduction of chronic rejection of renal allografts by anti-transforming growth factor-β antibody therapy in a rat model. Am. J. Physiol. Renal Physiol. 2013;305:F199–F207. doi: 10.1152/ajprenal.00665.2012. [DOI] [PubMed] [Google Scholar]
- 6.Williams SJ, et al. 3’,4’-Bis-difluoromethoxycinnamoylanthranilate (FT061): an orally-active antifibrotic agent that reduces albuminuria in a rat model of progressive diabetic nephropathy. Bioorg. Med. Chem. Lett. 2013;23:6868–6873. doi: 10.1016/j.bmcl.2013.09.100. [DOI] [PubMed] [Google Scholar]
- 7.Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am. J. Physiol. Renal Physiol. 2003;284:F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- 8.Negri AL. Prevention of progressive fibrosis in chronic renal diseases: antifibrotic agents. J. Nephrol. 2004;17:496–503. [PubMed] [Google Scholar]
- 9.Zeisberg M, Kalluri R. Reversal of experimental renal fibrosis by BMP7 provides insights into novel therapeutic strategies for chronic kidney disease. Pediatr. Nephrol. 2008;23:1395–1398. doi: 10.1007/s00467-008-0818-x. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 11.Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N. Engl. J. Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 12.Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-β by anti-TGF-β antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes. 1996;45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 13.Ma LJ, et al. Divergent effects of low versus high dose anti-TGF-β antibody in puromycin aminonucleoside nephropathy in rats. Kidney Int. 2004;65:106–115. doi: 10.1111/j.1523-1755.2004.00381.x. [DOI] [PubMed] [Google Scholar]
- 14.Juarez P, et al. Soluble β glycan reduces renal damage progression in db/db mice. Am. J. Physiol. Renal Physiol. 2007;292:F321–F329. doi: 10.1152/ajprenal.00264.2006. [DOI] [PubMed] [Google Scholar]
- 15.Kushibiki T, Nagata-Nakajima N, Sugai M, Shimizu A, Tabata Y. Delivery of plasmid DNA expressing small interference RNA for TGF-β type II receptor by cationized gelatin to prevent interstitial renal fibrosis. J. Control. Release. 2005;105:318–331. doi: 10.1016/j.jconrel.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Trachtman H, et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79:1236–1243. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US National Library of Medicine. ClinicalTrials.gov. 2013 doi: 10.1080/15360280801989377. [online], http://clinicaltrials.gov/ct2/show/NCT01113801. [DOI] [PubMed]
- 18.Boor P, Floege J. Chronic kidney disease growth factors in renal fibrosis. Clin. Exp. Pharmacol. Physiol. 2011;38:441–450. doi: 10.1111/j.1440-1681.2011.05487.x. [DOI] [PubMed] [Google Scholar]
- 19.Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007;21:3355–3368. doi: 10.1096/fj.06-6713com. [DOI] [PubMed] [Google Scholar]
- 20.Luo GH, et al. Inhibition of connective tissue growth factor by small interfering RNA prevents renal fibrosis in rats undergoing chronic allograft nephropathy. Transplant. Proc. 2008;40:2365–2369. doi: 10.1016/j.transproceed.2008.07.100. [DOI] [PubMed] [Google Scholar]
- 21.Adler SG, et al. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin. J. Am. Soc. Nephrol. 2010;5:1420–1428. doi: 10.2215/CJN.09321209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US National Library of Medicine. ClinicalTrials.gov. 2011 doi: 10.1080/15360280801989377. [online] http://clinicaltrials.gov/ct2/show/NCT00754143. [DOI] [PubMed]
- 23.US National Library of Medicine. ClinicalTrials.gov. 2012 doi: 10.1080/15360280801989377. [online] http://clinicaltrials.gov/ct2/show/NCT00913393. [DOI] [PubMed]
- 24.US National Library of Medicine. ClinicalTrials.gov. 2009 doi: 10.1080/15360280801989377. [online] http://clinicaltrials.gov/ct2/show/NCT00782561. [DOI] [PubMed]
- 25.Ramachandrarao SP, et al. Pirfenidone is renoprotective in diabetic kidney disease. J. Am. Soc. Nephrol. 2009;20:1765–1775. doi: 10.1681/ASN.2008090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JF, et al. Pirfenidone inhibits macrophage infiltration in 5/6 nephrectomized rats. Am. J. Physiol. Renal Physiol. 2013;304:F676–F685. doi: 10.1152/ajprenal.00507.2012. [DOI] [PubMed] [Google Scholar]
- 27.Sharma K, et al. Pirfenidone for diabetic nephropathy. J. Am. Soc. Nephrol. 2011;22:1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly DJ, Zhang Y, Gow R, Gilbert RE. Tranilast attenuates structural and functional aspects of renal injury in the remnant kidney model. J. Am. Soc. Nephrol. 2004;15:2619–2629. doi: 10.1097/01.ASN.0000139066.77892.04. [DOI] [PubMed] [Google Scholar]
- 29.Tao Y, et al. Tranilast attenuates chronic cyclosporine nephrotoxicity in rats. Transplant. Proc. 2009;41:4373–4375. doi: 10.1016/j.transproceed.2009.09.073. [DOI] [PubMed] [Google Scholar]
- 30.Tan SM, Zhang Y, Cox AJ, Kelly DJ, Qi W. Tranilast attenuates the up-regulation of thioredoxin-interacting protein and oxidative stress in an experimental model of diabetic nephropathy. Nephrol. Dial. Transplant. 2011;26:100–110. doi: 10.1093/ndt/gfq355. [DOI] [PubMed] [Google Scholar]
- 31.Kaneyama T, Kobayashi S, Aoyagi D, Ehara T. Tranilast modulates fibrosis, epithelial-mesenchymal transition and peritubular capillary injury in unilateral ureteral obstruction rats. Pathology. 2010;42:564–573. doi: 10.3109/00313025.2010.508784. [DOI] [PubMed] [Google Scholar]
- 32.Soma J, Sato K, Saito H, Tsuchiya Y. Effect of tranilast in early-stage diabetic nephropathy. Nephrol. Dial. Transplant. 2006;21:2795–2799. doi: 10.1093/ndt/gfl325. [DOI] [PubMed] [Google Scholar]
- 33.Soma J, Sugawara T, Huang YD, Nakajima J, Kawamura M. Tranilast slows the progression of advanced diabetic nephropathy. Nephron. 2002;92:693–698. doi: 10.1159/000064071. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, et al. FT011, a new anti-fibrotic drug, attenuates fibrosis and chronic heart failure in experimental diabetic cardiomyopathy. Eur. J. Heart Fail. 2012;14:549–562. doi: 10.1093/eurjhf/hfs011. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert RE, et al. A purpose-synthesised anti-fibrotic agent attenuates experimental kidney diseases in the rat. PLoS ONE. 2012;7:e47160. doi: 10.1371/journal.pone.0047160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Australian New Zealand Clinical Trials Registry. Anzctr.org. 2013 [online] https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000386730.
- 37.Meng XM, Chung AC, Lan HY. Role of the TGF-β /BMP-7/Smad pathways in renal diseases. Clin. Sci. (Lond.) 2013;124:243–254. doi: 10.1042/CS20120252. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchida K, Zhu Y, Siva S, Dunn SR, Sharma K. Role of Smad4 on TGF-β -induced extracellular matrix stimulation in mesangial cells. Kidney Int. 2003;63:2000–2009. doi: 10.1046/j.1523-1755.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhong X, et al. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56:663–674. doi: 10.1007/s00125-012-2804-x. [DOI] [PubMed] [Google Scholar]
- 40.Putta S, et al. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J.Am.Soc. Nephrol. 2012;23:458–469. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vukicevic S, et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J. Clin. Invest. 1998;102:202–214. doi: 10.1172/JCI2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spanjol J, et al. Bone morphogenetic protein-7 expression in human pyelonephritis. Coll. Antropol. 2010;34(Suppl. 2):61–64. [PubMed] [Google Scholar]
- 43.Bramlage CP, et al. Bone morphogenetic protein (BMP)-7 expression is decreased in human hypertensive nephrosclerosis. BMC Nephrol. 2010;11:31. doi: 10.1186/1471-2369-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeisberg M, et al. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nature Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka M, et al. Expression of BMP-7 and USAG-1 (a BMP antagonist) in kidney development and injury. Kidney Int. 2008;73:181–191. doi: 10.1038/sj.ki.5002626. [DOI] [PubMed] [Google Scholar]
- 46.Gong R, Rifai A, Tolbert EM, Centracchio JN, Dworkin LD. Hepatocyte growth factor modulates matrix metalloproteinases and plasminogen activator/ plasmin proteolytic pathways in progressive renal interstitial fibrosis. J. Am. Soc. Nephrol. 2003;14:3047–3060. doi: 10.1097/01.asn.0000098686.72971.db. [DOI] [PubMed] [Google Scholar]
- 47.Mizuno S, Matsumoto K, Kurosawa T, Mizuno-Horikawa Y, Nakamura T. Reciprocal balance of hepatocyte growth factor and transforming growth factor-β1 in renal fibrosis in mice. Kidney Int. 2000;57:937–948. doi: 10.1038/sj.ki.4491416. [DOI] [PubMed] [Google Scholar]
- 48.Dworkin LD, et al. Hepatocyte growth factor ameliorates progression of interstitial fibrosis in rats with established renal injury. Kidney Int. 2004;65:409–419. doi: 10.1111/j.1523-1755.2004.00417.x. [DOI] [PubMed] [Google Scholar]
- 49.Kuroiwa T, et al. Hepatocyte growth factor prevents lupus nephritis in a murine lupus model of chronic graft-versus-host disease. Arthritis Res. Ther. 2006;8:R123. doi: 10.1186/ar2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang HY, et al. Hepatocyte growth factor-induced amelioration in chronic renal failure is associated with reduced expression of α-smooth muscle actin. Ren. Fail. 2012;34:862–870. doi: 10.3109/0886022X.2012.687344. [DOI] [PubMed] [Google Scholar]
- 51.Okada H, et al. Transgene-derived hepatocyte growth factor attenuates reactive renal fibrosis in aristolochic acid nephrotoxicity. Nephrol. Dial. Transplant. 2003;18:2515–2523. doi: 10.1093/ndt/gfg440. [DOI] [PubMed] [Google Scholar]
- 52.Gong R, et al. Hepatocyte growth factor ameliorates renal interstitial inflammation in rat remnant kidney by modulating tubular expression of macrophage chemoattractant protein-1 and RANTES. J. Am. Soc. Nephrol. 2004;15:2868–2881. doi: 10.1097/01.ASN.0000141962.44300.3A. [DOI] [PubMed] [Google Scholar]
- 53.Mizuno S, Nakamura T. Suppressions of chronic glomerular injuries and TGF-β 1 production by HGF in attenuation of murine diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2004;286:F134–F143. doi: 10.1152/ajprenal.00199.2003. [DOI] [PubMed] [Google Scholar]
- 54.Tu Y, et al. Cell division autoantigen 1 enhances signaling and the profibrotic effects of transforming growth factor-β in diabetic nephropathy. Kidney Int. 2011;79:199–209. doi: 10.1038/ki.2010.374. [DOI] [PubMed] [Google Scholar]
- 55.Chai Z, et al. Genetic deletion of cell division autoantigen 1 retards diabetes-associated renal njury. J. Am. Soc. Nephrol. 2013;24:1782–1792. doi: 10.1681/ASN.2013010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chai Z, Sarcevic B, Mawson A, Toh BH. SET-related cell division autoantigen-1 (CDA1) arrests cell growth. J. Biol. Chem. 2001;276:33665–33674. doi: 10.1074/jbc.M007681200. [DOI] [PubMed] [Google Scholar]
- 57.Faul C, et al. FGF23 induces left ventricular hypertrophy. J. Clin. Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Komaba H, Fukagawa M. The role of FGF23 in CKD—with or without Klotho. Nat Rev. Nephrol. 2012;8:484–490. doi: 10.1038/nrneph.2012.116. [DOI] [PubMed] [Google Scholar]
- 59.Hu MC, Kuro-o M, Moe OW. Klotho and kidney disease. J. Nephrol. 2010;23(Suppl. 16):S136–S144. [PMC free article] [PubMed] [Google Scholar]
- 60.Gutierrez O, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 61.Shigematsu T, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am. J. Kidney Dis. 2004;44:250–256. doi: 10.1053/j.ajkd.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 62.Hu MC, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischer SS, et al. Hyperaldosteronism in Klotho-deficient mice. Am. J. Physiol. Renal Physiol. 2010;299:F1171–F1177. doi: 10.1152/ajprenal.00233.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voelkl J, et al. Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice. J. Clin. Invest. 2013;123:812–822. doi: 10.1172/JCI64093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler. Thromb. Vasc. Biol. 2007;27:799–805. doi: 10.1161/01.ATV.0000258414.59393.89. [DOI] [PubMed] [Google Scholar]
- 66.Sanz-Rosa D, et al. Participation of aldosterone in the vascular inflammatory response of spontaneously hypertensive rats: role of the NFκB/IκB system. J. Hypertens. 2005;23:1167–1172. doi: 10.1097/01.hjh.0000170379.08214.5a. [DOI] [PubMed] [Google Scholar]
- 67.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J. Am. Soc. Nephrol. 2013;24:771–785. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosca MG, et al. Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. Am. J. Physiol. Renal Physiol. 2005;289:F420–F430. doi: 10.1152/ajprenal.00415.2004. [DOI] [PubMed] [Google Scholar]
- 69.Brouwers O, et al. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J. Biol. Chem. 2011;286:1374–1380. doi: 10.1074/jbc.M110.144097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coughlan MT, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 2009;20:742–752. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 72.Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann. Med. 2012;44(Suppl. 1):S2–S16. doi: 10.3109/07853890.2011.653393. [DOI] [PubMed] [Google Scholar]
- 73.Popolo A, Autore G, Pinto A, Marzocco S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic. Res. 2013;47:346–356. doi: 10.3109/10715762.2013.779373. [DOI] [PubMed] [Google Scholar]
- 74.You YH, et al. Role of Nox2 in diabetic kidney disease. Am. J. Physiol. Renal Physiol. 2013;304:F840–F848. doi: 10.1152/ajprenal.00511.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma K, et al. Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Babelova A, et al. Role of Nox4 in murine models of kidney disease. Free Radic. Biol. Med. 2012;53:842–853. doi: 10.1016/j.freeradbiomed.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 77.Gorin Y, Block K. Nox as a target for diabetic complications. Clin. Sci. (Lond.) 2013;125:361–382. doi: 10.1042/CS20130065. [DOI] [PubMed] [Google Scholar]
- 78.Sedeek M, Nasrallah R, Touyz RM, Hebert RL. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J. Am. Soc. Nephrol. 2013;24:1512–1518. doi: 10.1681/ASN.2012111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holterman CE, et al. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J. Am. Soc. Nephrol. doi: 10.1681/ASN.2013040371. http://dx.doi.org/10.1681/ASN.2013040371 [DOI] [PMC free article] [PubMed]
- 80.Sedeek M, et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2010;299:F1348–F1358. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- 81.Sedeek M, et al. Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of type 2 diabetes. Clin. Sci. (Lond.) 2013;124:191–202. doi: 10.1042/CS20120330. [DOI] [PubMed] [Google Scholar]
- 82.Aoyama T, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gray SP, et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127:1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 84.US National Library of Medicine. ClinicalTrials.gov. 2014 doi: 10.1080/15360280801989377. [online]. http://clinicaltrials.gov/ct2/show/NCT02010242. [DOI] [PubMed]
- 85.Nlandu Khodo S, et al. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J. Am. Soc. Nephrol. 2012;23:1967–1976. doi: 10.1681/ASN.2012040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Volpini RA, Costa RS, da Silva CG, Coimbra TM. Inhibition of nuclear factor-κB activation attenuates tubulointerstitial nephritis induced by gentamicin. Nephron Physiol. 2004;98:97–106. doi: 10.1159/000081558. [DOI] [PubMed] [Google Scholar]
- 87.Fujihara CK, et al. Chronic inhibition of nuclear factor-κB attenuates renal injury in the 5/6 renal ablation model. Am. J. Physiol. Renal Physiol. 2007;292:F92–F99. doi: 10.1152/ajprenal.00184.2006. [DOI] [PubMed] [Google Scholar]
- 88.Ding W, Yang L, Zhang M, Gu Y. Chronic inhibition of nuclear factor κB attenuates aldosterone/salt-induced renal injury. Life Sci. 2012;90:600–606. doi: 10.1016/j.lfs.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 89.Kim JE, et al. Celastrol, an NF-κB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice. PLoS ONE. 2013;8:e62068. doi: 10.1371/journal.pone.0062068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Briffa JF, McAinch AJ, Poronnik P, Hryciw DH. Adipokines as a link between obesity and chronic kidney disease. Am. J. Physiol. Renal Physiol. 2013;305:F1629–F1636. doi: 10.1152/ajprenal.00263.2013. [DOI] [PubMed] [Google Scholar]
- 91.Li Y, et al. Protective effect of celastrol in rat cerebral ischemia model: down-regulating p-JNK, p-c-Jun and NF-κB. Brain Res. 2012;1464:8–13. doi: 10.1016/j.brainres.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 92.Decleves AE, Mathew AV, Cunard R, Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J. Am. Soc. Nephrol. 2011;22:1846–1855. doi: 10.1681/ASN.2011010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deji N, et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am. J. Physiol. Renal Physiol. 2009;296:F118–F126. doi: 10.1152/ajprenal.00110.2008. [DOI] [PubMed] [Google Scholar]
- 94.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 95.Goumenos DS, et al. Early histological changes in the kidney of people with morbid obesity. Nephrol. Dial. Transplant. 2009;24:3732–3738. doi: 10.1093/ndt/gfp329. [DOI] [PubMed] [Google Scholar]
- 96.Chagnac A, et al. Glomerular hemodynamics in severe obesity. Am. J. Physiol. Renal Physiol. 2000;278:F817–F822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]
- 97.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 98.Ruster C, Wolf G. The role of the renin-angiotensin-aldosterone system in obesity-related renal diseases. Semin. Nephrol. 2013;33:44–53. doi: 10.1016/j.semnephrol.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 99.Ruster C, Wolf G. Adipokines promote chronic kidney disease. Nephrol. Dial. Transplant. 2013;28(Suppl. 4):v8–iv14. doi: 10.1093/ndt/gft191. [DOI] [PubMed] [Google Scholar]
- 100.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 101.Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74–80. doi: 10.1016/j.febslet.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 102.Decleves AE, et al. Regulation of lipid accumulation by AMK-activated kinase in high fat diet-induced kidney injury. Kidney Int. doi: 10.1038/ki.2013.462. http://dx.doi.org/10.1038/ki.2013.462 [DOI] [PMC free article] [PubMed]
- 103.Wang S, et al. AMPKα2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ. Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eid AA, et al. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J. Biol. Chem. 2010;285:37503–37512. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dugan LL, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J. Clin. Invest. 2013;123:4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mishra R, et al. AMP-activated protein kinase inhibits transforming growth factor-β-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J. Biol. Chem. 2008;283:10461–10469. doi: 10.1074/jbc.M800902200. [DOI] [PubMed] [Google Scholar]
- 107.Sanchez AP, et al. Role of the USF1 transcription factor in diabetic kidney disease. Am. J. Physiol. Renal Physiol. 2011;301:F271–F279. doi: 10.1152/ajprenal.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu Y, Casado M, Vaulont S, Sharma K. Role of upstream stimulatory factors in regulation of renal transforming growth factor-β1. Diabetes. 2005;54:1976–1984. doi: 10.2337/diabetes.54.7.1976. [DOI] [PubMed] [Google Scholar]
- 109.Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage α1 AMP-activated protein kinase (α1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benigni A, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J. Clin. Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Godel M, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest. 2011;121:2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cina DP, et al. Inhibition of MTOR disrupts autophagic flux in podocytes. J. Am. Soc. Nephrol. 2012;23:412–420. doi: 10.1681/ASN.2011070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ponticelli C, Graziani G. Proteinuria after kidney transplantation. Transpl. Int. 2012;25:909–917. doi: 10.1111/j.1432-2277.2012.01500.x. [DOI] [PubMed] [Google Scholar]
- 114.Eid AA, et al. Mammalian target of rapamycin regulates Nox4-mediated podocyte depletion in diabetic renal injury. Diabetes. 2013;62:2935–2947. doi: 10.2337/db12-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-α. Int. J. Obes. (Lond.) 2005;29(Suppl. 1):S5–S9. doi: 10.1038/sj.ijo.0802905. [DOI] [PubMed] [Google Scholar]
- 116.Barres R, et al. Non-CpG methylation of the PGC-1α promoter through DNMT3B controls mitochondrial density. Cell. Metab. 2009;10:189–198. doi: 10.1016/j.cmet.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 117.Sharma K, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J. Am. Soc. Nephrol. 2013;24:1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghosh S, et al. Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice. Am. J. Physiol. Renal Physiol. 2009;296:F700–F708. doi: 10.1152/ajprenal.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang LN, et al. Novel small-molecule AMP-activated protein kinase allosteric activator with beneficial effects in db/db mice. PLoS ONE. 2013;8:e72092. doi: 10.1371/journal.pone.0072092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Avery LB, Bumpus NN. Valproic acid is a novel activator of AMP-activated protein kinase and decreases liver mass, hepatic fat accumulation, and serum glucose in obese mice. Mol. Pharmacol. 2014;85:1–10. doi: 10.1124/mol.113.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]