Abstract
Literature reports suggest that ataxia telangiectasia mutated (ATM) can activate the AMP-activated protein kinase (AMPK), a protein that can stimulate glucose transport in skeletal muscle. We hypothesized that 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), an AMPK activator, would increase glucose transport in mouse extensor digitorum longus (EDL) muscles in an ATM-dependent manner. AICAR-stimulated glucose transport was prevented by the ATM inhibitor KU-55933 despite normal stimulation of AMPK phosphorylation. Consistent with this, AICAR caused AMPK phosphorylation but not an increase of glucose transport in ATM-deficient (ATM−/−) muscles. S231 of TBC1D1 matches the sequence motif of ATM substrates, and phosphorylation of this site is known to inhibit TBC1D1 and lead to increased glucose transport. Accordingly, we assessed TBC1D1 phosphorylation and found that AICAR-stimulated phosphorylation of TBC1D1 at S231 did not occur in ATM−/− muscles. However, activation of ATM without activation of AMPK was insufficient to increase TBC1D1 phosphorylation. The data suggest that ATM plays a role in AICAR-stimulated glucose transport downstream of AMPK.
Keywords: AMP-activated protein kinase, ataxia telangiectasia mutated, TBC1D1, AICAR, glucose transport, skeletal muscle
INTRODUCTION
The serine-threonine kinase ataxia telangiectasia mutated (ATM) appears to play a role in glucose homeostasis. For example, recent genome-wide association studies have found that genetic variations near the ATM gene are related to glycemic responses to metformin [1, 2], a commonly-prescribed drug for blood glucose control. While the mechanism for metformin’s effect on blood glucose levels is under debate [3–6], it is known that metformin acutely stimulates glucose transport into skeletal muscle concomitant with activation of the AMP-activated protein kinase (AMPK) [7].
Activation of AMPK is sufficient to stimulate insulin-independent glucose transport into skeletal muscle [8, 9]. Intriguingly, ATM dependence has been reported for activation of AMPK in response to DNA damage or insulin-like growth factor 1 in HeLa cells and fibroblasts, exposure of lung cancer cells to ionizing radiation, exposure of lymphoblasts to H2O2, or treatment of HeLa cells and mouse embryonic fibroblasts with the adenosine analog AICAR [10–14]. Despite these suggestive data on the role of ATM upstream of AMPK, the potential role of ATM in AMPK-dependent stimulation of glucose transport has not previously been investigated in skeletal muscle, the predominant whole-body storage depot for glucose.
Accordingly, the purpose of this study was to test the hypothesis that glucose uptake stimulated by the AMPK activator AICAR would be dependent on ATM in skeletal muscle.
MATERIALS AND METHODS
Materials
Antibodies against TBC1D1, AMPK, phosphorylated AMPKα T172 (P-AMPK), and phosphorylated ATM S1981 (P-ATM) were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against phosphorylated TBC1D1 (P-TBC1D1) S237 (S231 in mouse) were purchased from EMD Millipore Corporation (Billerica, MA, USA). Antibodies against tubulin and ATM were obtained from Sigma-Aldrich Corporation (St. Louis, MO, USA). Horseradish peroxidase-conjugated secondary antibodies were obtained from Pierce Biotechnology (Rockford, IL, USA). The ATM inhibitor KU-55933 was a generous gift from Dr. Graeme Smith (KuDOS Phramaceuticals, Cambridge, UK). The AMPK inhibitor Compound C was provided by Merck & Co., Inc. (Rahway, NJ, USA). Doxorubicin was purchased from Sigma-Aldrich Corporation. Radiolabeled 2-deoxyglucose and mannitol were purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO, USA).
Collection and processing of animal muscle
All procedures using live animals were approved by the Saint Louis University Institutional Animal Care and Use Committee. Transgenic mice expressing a truncation mutation of ATM [15] were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Mice that were heterozygous for the transgene were used to breed wild-type (ATM+/+) and ATM-deficient (ATM−/−) mice. After weaning, each mouse was anesthetized with ketamine/xylazine (55 mg ketamine and 5.5 mg xylazine per kg), and a tail sample was obtained for genotyping as previously described [15, 16].
Mice were anesthetized with sodium pentobarbital (50 mg/kg), and extensor digitorum longus (EDL) muscles were removed and incubated in vitro as described previously [16, 17]. The incubation media for the muscle consisted of Krebs Henseleit bicarbonate buffer (KHB) containing 8 mM glucose and 32 mM mannitol. Vials containing EDL muscles were gassed with 95% O2: 5% CO2 and kept gently shaking at 35 °C.
Muscles were incubated for one hour to allow recovery from dissection. Muscles were then transferred into KHB containing 32 mM mannitol and 8 mM glucose in the presence of 0.1% dimethyl sulfoxide vehicle (DMSO) or 1 μM KU-55933, a concentration sufficient to inhibit ATM [18, 19] but low enough to avoid inhibition of phosphatidylinositol 3-kinase [19]. After 30 minutes, muscles were incubated in KHB with 8 mM glucose in the absence or presence of 2 mM AICAR for one hour with the continued presence of DMSO or KU and 32 mM or 30 mM mannitol to keep osmolarity constant across media. At this point, some muscles were blotted and clamp-frozen with aluminum tongs cooled in liquid nitrogen and stored at −80 °C for later Western blot analysis. Other muscles were subjected to 2-deoxyglucose (2DG) uptake assays as described below.
In parallel procedures, EDL muscles from wild-type or ATM-deficient animals were allowed to recover in vitro for one hour, incubated in KHB containing mannitol as described above in the absence or presence of 2 mM AICAR for one hour and then either clamp-frozen or subjected to 2DG uptake assays as previously reported [16, 17] and briefly described below.
2DG uptake
Muscles were washed at 30 °C in glucose-free KHB containing 40 mM mannitol in the absence or presence of KU-55933 (DMSO vehicle) or, for procedures with the ATM−/− mice, in medium containing neither KU nor DMSO. Muscles were then incubated in KHB containing 4 mM 2DG, 2 μCi/ml 3H-2DG, 36 mM mannitol, 0.3 μCi/ml 14C-mannitol, and 0.1% DMSO or 1 μM KU-55933 if they had been present in earlier incubations. Muscles were clamp-frozen and stored at −80 °C. Muscles were then homogenized in Kontes ground glass tubes in ice-cold buffer containing protease and phosphatase inhibitors (50 mM HEPES, pH 7.4, 2 mM Na3VO4, 150 mM NaF,10 μg/ml leupeptin, 10 μg/ml aprotinin, 0.5 μg/mL pepstatin and 1 mM phenylmethylsulfonyl fluoride). Homogenates were centrifuged at 4 °C for 10 minutes at 14,000xg, and supernatant protein concentration was analyzed by the bicinchoninic acid (BCA) method (Pierce Protein Technologies, Rockland, IL, USA). Supernatant aliquots and aliquots of the incubation media were mixed with Ultima Gold XR scintillation fluid (Perkin Elmer, Boston, MA, USA), and samples were assessed by scintillation counting (TriCarb 3110TR, Perkin Elmer, Boston, MA, USA). The disintegrations per minute (DPM) of 14C-mannitol were used to measure the extracellular volume, and intracellular 2DG was calculated from 3H DPM after accounting for 3H DPM in the extracellular space. 2DG transport was expressed as nmol 2DG/mg protein/10 minutes.
Western blotting
Samples were homogenized, centrifuged, assayed for protein content as described above, diluted in Laemmli sample buffer containing dithiothreitol, and boiled for 5 minutes. Samples were then analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis as described previously [20]. Samples were run on 4–20% Tris-HEPES gels (Pierce) and then transferred onto nitrocellulose membranes. After transfer, membranes were blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween. Proteins on the nitrocellulose membranes were probed with primary and secondary antibodies described in the ‘Materials’ section and then visualized using enhanced chemiluminescence (Western Lightning; PerkinElmer, Waltham, MA, USA). Western blots were quantified using TotalLab software purchased from TotalLab Nonlinear Dynamics (Newcastle on Tyre, UK). For probing ATM and P-ATM, samples were run on 3–8% Tris-Acetate gels (Invitrogen, Carlsbad, CA, USA) alongside HiMark (Invitrogen) molecular weight markers.
Statistics
Data were analyzed by analysis of variance with post hoc least significant difference comparisons. A level of P < 0.05 was regarded as significant.
RESULTS
AICAR-stimulated glucose transport
ATM’s role in AICAR-stimulated glucose transport was assessed in isolated EDL muscles by using either ATM-deficient mice or by using the specific ATM inhibitor, KU-55933. As shown in Figure 1A, ATM protein was present in only background levels in EDL from ATM−/− mice. As shown in Figure 1B, AICAR increased glucose transport in muscles from wild-type mice (P < 0.05 vs. transport in the absence of AICAR). However, AICAR did not stimulate a statistically significant increase in glucose transport in muscles from ATM−/− mice, and glucose transport in muscles exposed to AICAR was only about half as much in ATM-deficient muscles compared to muscles from wild-type mice (P < 0.05). As shown in Figure 1C, AICAR increased glucose transport in wild-type muscles in the presence of the vehicle (P < 0.05). This increase was abolished when muscles were pretreated with the ATM inhibitor KU-55933.
Figure 1. ATM-dependence of AICAR-stimulated glucose uptake.
(A) EDL muscles from wild-type (ATM+/+) and ATM-deficient mice (ATM−/−) were analyzed by Western blot for ATM and tubulin. (B) EDL muscles from ATM+/+ and ATM−/− mice were incubated in vitro with or without 2 mM AICAR for one hour and then assayed for 2-deoxyglucose (2DG) uptake (n = 5–6/group). (C) EDL muscles from wild-type mice were pre-treated with 1 μM KU-55933 (KU) or vehicle (0.1% DMSO) for thirty minutes. They were then incubated with or without 2 mM AICAR for one hour and then assayed for 2DG uptake (n = 5–6/group). *: greater than corresponding control without AICAR, P < 0.05. †: lower than corresponding control with AICAR. P < 0.05. Data represent means ± SE.
AICAR-stimulated phosphorylation of AMPK
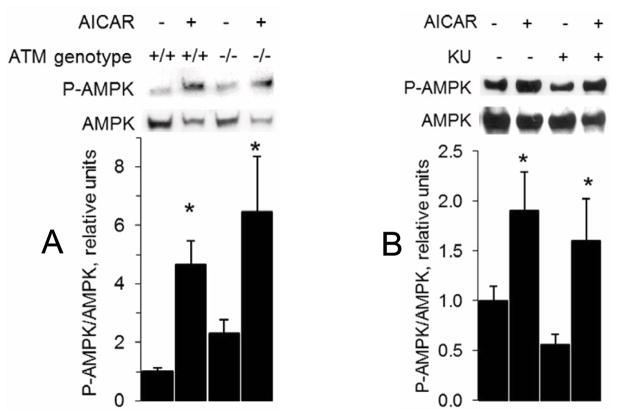
It has previously been reported that ATM plays a role in AICAR-stimulated AMPK phosphorylation in HeLa cells and mouse embryonic fibroblasts [12]. Thus, we assessed phosphorylation of AMPK to determine whether ATM’s role in AICAR-stimulated glucose transport was through an influence on AMPK phosphorylation. As shown in Figure 2A, AICAR-stimulated AMPK phosphorylation was normal in muscles from ATM−/− mice. Likewise, AICAR-stimulated AMPK phosphorylation was unaffected by the ATM inhibitor KU-55933 (Figure 2B).
Figure 2. No influence of ATM on AICAR-stimulated AMPK phosphorylation.
Muscle homogenates were analyzed by Western blot for P-AMPK and AMPK for (A) ATM+/+ and ATM−/− EDL muscles that were incubated with or without 2 mM AICAR for one hour (n = 6/group) and (B) wild-type EDL muscles that were pretreated with either 1 μM KU or DMSO (vehicle) for 30 min and then exposed to 2 mM AICAR for one hour (n = 6/group). *: P < 0.05 for an AICAR effect Data represent means ± SE.
Phosphorylation of TBC1D1
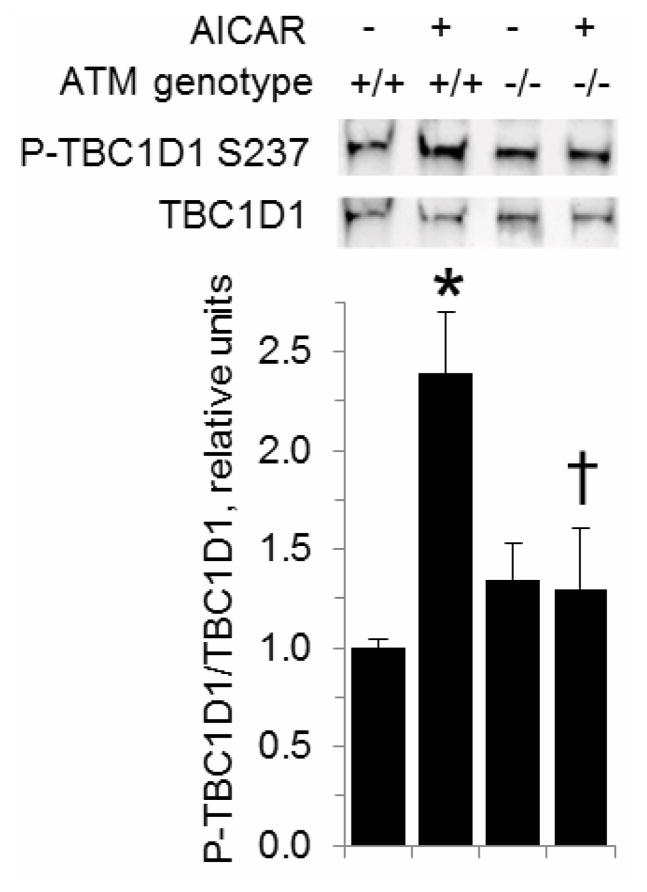
The Rab GTPase activating protein (GAP) TBC1D1 is required for stimulation of glucose transport by AICAR [21]. Furthermore, phosphorylation of mouse TBC1D1 at S231 (corresponding to S237 of human TBC1D1) in response to AICAR occurs concomitant with an increase in glucose transport [22–24], and S231 phosphorylation appears to be necessary to convey insulin-responsiveness to TBC1D1 [25]. Intriguingly, S231 and the surrounding amino acids (F-S-Q) match the consensus hydrophobic-serine/threonine-glutamine (Φ-S/T-Q) motif of ATM targets [26, 27]. Even though this site is both an in vitro target of AMPK and phosphorylated in response to the AMPK activators AICAR and phenformin [28], this does not rule out the possibility that another kinase could also act on S231. Thus, we hypothesized that S231 phosphorylation in response to AICAR would be dependent on ATM. As shown in Figure 3, AICAR increased phosphorylation of TBC1D1 S231 in EDL from wild-type mice (P < 0.05). However, there was no AICAR-dependent increase in phosphorylation of TBC1D1 S231 in EDL from ATM-deficient mice. Given this result, we examined whether activation of ATM would be sufficient to increase TBC1D1 S231 phosphorylation. As shown in Figure 4A, 1 μM doxorubicin (DXR), which activates ATM in muscle cells [29], caused phosphorylation of ATM at S1981, the autophosphorylation site of ATM. However, phosphorylation of AMPK was unaffected by DXR As shown in Figure 4B, DXR did not increase TBC1D1 S231 phosphorylation, while the AMPK inhibitor Compound C (20 μM with DMSO vehicle) tended to suppress S231 phosphorylation. Thus, the data suggest that activation of ATM is not in itself sufficient to cause phosphorylation of TBC1D1 S231.
Figure 3. Decreased AICAR-stimulated phosphorylation of TBC1D1 S237 in muscles from ATM-deficient mice.
ATM+/+ and ATM−/− EDL muscles were incubated in the absence or presence of 2 mM AICAR. Muscle lysates were analyzed by Western blot with antibodies against phosphorylated TBC1D1 S231 and TBC1D1 (n = 3/group). *: P < 0.05, greater than wild-type muscle in the absence of AICAR. †: lower than wild-type muscle exposed to AICAR, P < 0.05. Data represent means ± SE.
Figure 4. The ATM activator doxorubicin does not affect TBC1D1 phosphorylation.

EDL muscles from wild-type mice were incubated in the absence or presence of the AMPK inhibitor Compound C (Comp C, 20 μM) and then in the absence or presence of 1 μM doxorubicin (DXR) before Western blots for A) phosphorylation of the ATM autophosphorylation site, S1981, and B) phosphorylation of AMPK. Data are means with standard errors, n = 3/group.
DISCUSSION
The new information provided by this study is that AICAR-stimulated glucose uptake in skeletal muscle is dependent on ATM. Additionally, this role for ATM in AICAR-stimulated glucose uptake does not involve an effect at the level of AMPK phosphorylation but instead is associated with altered phosphorylation of TBC1D1.
Based on data that the ATM inhibitor KU-55933 blunted activation of AMPK by metformin in a hepatoma cell line, Zhou et al. proposed that ATM acts upstream of AMPK [2]. However, two independent groups have shown that KU-55933 prevents AMPK activation by metformin through inhibition of the cation transporter responsible for metformin uptake rather than through inhibition of ATM [3, 4]. In hepatocytes, ultraviolet light irradiation stimulated phosphorylation of the ATM target H2AX, but had no effect on AMPK activity [4]. Additionally, caffeine, which inhibits ATM, suppressed phosphorylation of H2AX but not activation of AMPK by metformin [4]. Finally, while hydrogen peroxide activated both AMPK and ATM in HEK293 cells, KU-55933 prevented ATM autophosphorylation but did not interfere with AMPK activity [4]. Together, these data [4] suggest that ATM does not act upstream of AMPK, at least in hepatocytes or HEK293 cells.
While it has been reported that ATM acts upstream of AMPK in HeLa cells, lung cancer cells, fibroblasts, lymphoblasts, and embryonic fibroblasts [10–14], it seems unlikely that tissues corresponding to these cell lines would play a meaningful role in glucose homeostasis. Intriguingly, however, the increase in insulin sensitivity and a concomitant increase in autophosphorylated ATM in L6 myotubes in response to serum starvation was found to be dependent on AMPK, while inhibition of ATM prevented increased insulin action but not an increase in AMPK phosphorylation in serum starved myotubes [18]. Together, the data from serum-starved myotubes [18] suggest that ATM could act downstream of AMPK in the regulation of glucose transport. The current data showing blunted glucose transport despite normal phosphorylation of AMPK in response to AICAR in ATM-deficient skeletal muscles or muscles exposed to KU-55933 are consistent with the idea of ATM acting downstream of AMPK.
AMPK is a heterotrimer of α, β, and γ subunits, each with multiple isoforms [30]. The two main activating upstream kinases for AMPK are liver kinase B1 (LKB1) and calcium/calmodulin-dependent kinase kinase β [31], though there are some reports that ATM-dependent phosphorylation of AMPK does not require LKB1 [11, 32] and could indeed be through direct phosphorylation of AMPK by ATM [11]. Intriguingly, LKB1 is an in vitro substrate for ATM [33], suggesting a potential mechanism for the ATM-dependent phosphorylation of AMPK [14]. However, phosphorylation of LKB1 by ATM does not affect LKB1 activity in vitro or LKB1 localization in vivo [33]; hence the precise role of LKB1 phosphorylation in the activation of AMPK remains uncertain. Clearly, there are cell-type differences in the role of ATM upstream of AMPK, and perhaps these are influenced by factors including the expression profile of AMPK subunit isoforms or the subcellular localizations of ATM, AMPK, and LKB1.
The current study, as the first to demonstrate a role of ATM in insulin-independent glucose transport, adds to the growing body of literature suggesting a role for ATM in glucoregulation. For example, young mice that lack functional ATM are hyperglycemic compared to wild-type animals during oral glucose tolerance tests [34]. Likewise, for mice with an ApoE−/− background, animals that have only one allele of ATM that codes for functional protein are hyperglycemic during intraperitoneal glucose tolerance tests and insulin tolerance tests compared to mice with two wild-type ATM alleles [35]. Finally, skeletal muscles from mice lacking ATM and L6 cells expressing kinase-dead ATM have blunted insulin-stimulated glucose uptake [16, 32]. Quite interestingly, while ATM plays a role upstream of Akt in response to insulin in some cell lines and in glycolytic skeletal muscle [16, 20, 36], the point of influence of ATM in insulin signaling leading to glucose transport in oxidative muscle is downstream of Akt at the Rab GAP AS160/TBC1D4 [16, 20] which, like TBC1D1, acts on Rabs 2A, 8A, 8B, 10, and 14 [37]. Thus, ATM influences both insulin-stimulated phosphorylation of AS160 [16, 20] and AICAR-stimulated phosphorylation of TBC1D1 in skeletal muscle.
CONCLUSION
In summary, this study provides the first evidence for a role of ATM in AICAR-stimulated glucose uptake by skeletal muscle. Thus, ATM plays key roles in both insulin-dependent [16] and insulin-independent stimulation of glucose uptake in skeletal muscle, suggesting a basis for the association of ATM variants with glycemic profiles recently reported [2, 7].
Acknowledgments
The project was supported by Grant Numbers R15DK080437 and R15DK080437-01S3 (American Recovery and Reinvestment Act) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to Jonathan Fisher. Stanley Andrisse received support from the William Townsend Porter Pre-doctoral Fellowship from the American Physiological Society (APS). The manuscript content is solely the responsibility of the authors and does not necessarily represent the official views of NIDDK, the National Institutes of Health, or the APS. The funding agencies played no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
References
- 1.van Leeuwen N, Nijpels G, Becker ML, Deshmukh H, Zhou K, Stricker BH, Uitterlinden AG, Hofman A, van ‘t Riet E, Palmer CN, Guigas B, Slagboom PE, Durrington P, Calle RA, Neil A, Hitman G, Livingstone SJ, Colhoun H, Holman RR, McCarthy MI, Dekker JM, t Hart LM, Pearson ER. Diabetologia. 2012;55:1971. doi: 10.1007/s00125-012-2537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou K, Bellenguez C, Spencer CC, Bennett AJ, Coleman RL, Tavendale R, Hawley SA, Donnelly LA, Schofield C, Groves CJ, Burch L, Carr F, Strange A, Freeman C, Blackwell JM, Bramon E, Brown MA, Casas JP, Corvin A, Craddock N, Deloukas P, Dronov S, Duncanson A, Edkins S, Gray E, Hunt S, Jankowski J, Langford C, Markus HS, Mathew CG, Plomin R, Rautanen A, Sawcer SJ, Samani NJ, Trembath R, Viswanathan AC, Wood NW, Harries LW, Hattersley AT, Doney AS, Colhoun H, Morris AD, Sutherland C, Hardie DG, Peltonen L, McCarthy MI, Holman RR, Palmer CN, Donnelly P, Pearson ER. Nat Genet. 2011;43:117. [Google Scholar]
- 3.Yee SW, Chen L, Giacomini KM. Nat Genet. 2012;44:359. doi: 10.1038/ng.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods A, Leiper JM, Carling D. Nat Genet. 2012;44:360. doi: 10.1038/ng.2235. [DOI] [PubMed] [Google Scholar]
- 5.Zhou K, Bellenguez C, Sutherland C, Hardie G, Palmer C, Donnelly P, Pearson E. Nat Genet. 2012;44:361. doi: 10.1038/ng.2234. [DOI] [PubMed] [Google Scholar]
- 6.Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O’Neill HM, Ford RJ, Palanivel R, O’Brien M, Hardie DG, Macaulay SL, Schertzer JD, Dyck JR, van Denderen BJ, Kemp BE, Steinberg GR. Nat Med. 2013;19:1649. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. J Clin Invest. 2001;108:1167. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Diabetes. 1998;47:1369. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 9.Merrill GF, Kurth EJ, Hardie DG, Winder WW. Am J Physiol. 1997;273:E1107. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 10.Fu X, Wan S, Lyu YL, Liu LF, Qi H. PLoS ONE. 2008;3:e2009. doi: 10.1371/journal.pone.0002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki A, Kusakai G, Kishimoto A, Shimojo Y, Ogura T, Lavin MF, Esumi H. Biochem Biophys Res Commun. 2004;324:986. doi: 10.1016/j.bbrc.2004.09.145. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y, Connors KE, Yang DQ. Mol Cell Biochem. 2007;306:239. doi: 10.1007/s11010-007-9575-6. [DOI] [PubMed] [Google Scholar]
- 13.Sanli T, Rashid A, Liu C, Harding S, Bristow RG, Cutz JC, Singh G, Wright J, Tsakiridis T. Int J Radiat Oncol Biol Phys. 2010;78:221. doi: 10.1016/j.ijrobp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, Inoki K, Guan KL, Shen J, Person MD, Kusewitt D, Mills GB, Kastan MB, Walker CL. Proc Natl Acad Sci USA. 2010;107:4153. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Cell. 1996;86:159. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 16.Ching JK, Spears LD, Armon JL, Renth AL, Andrisse S, Collins RL, 4th, Fisher JS. Appl Physiol Nutr Metab. 2013;38:589. doi: 10.1139/apnm-2012-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Am J Physiol Endocrinol Metab. 2002;282:E18. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- 18.Ching JK, Rajguru P, Marupudi N, Banerjee S, Fisher JS. Am J Physiol Cell Physiol. 2010;299:C1171. doi: 10.1152/ajpcell.00514.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ, Smith GC. Cancer Res. 2004;64:9152. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 20.Jeong I, Patel AY, Zhang Z, Patil PB, Nadella ST, Nair S, Ralston L, Hoormann JK, Fisher JS. Acta Physiol (Oxf) 2010;198:465. doi: 10.1111/j.1748-1716.2009.02069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szekeres F, Chadt A, Tom RZ, Deshmukh AS, Chibalin AV, Bjornholm M, Al-Hasani H, Zierath JR. Am J Physiol Endocrinol Metab. 2012;303:E524. doi: 10.1152/ajpendo.00605.2011. [DOI] [PubMed] [Google Scholar]
- 22.An D, Toyoda T, Taylor EB, Yu H, Fujii N, Hirshman MF, Goodyear LJ. Diabetes. 2010;59:1358. doi: 10.2337/db09-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. J Biol Chem. 2008;283:9787. doi: 10.1074/jbc.M708839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vichaiwong K, Purohit S, An D, Toyoda T, Jessen N, Hirshman MF, Goodyear LJ. Biochem J. 2010;431:311. doi: 10.1042/BJ20101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatakeyama H, Kanzaki M. Mol Biol Cell. 2013;24:809. doi: 10.1091/mbc.E12-10-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Neill T, Dwyer AJ, Ziv Y, Chan DW, Lees-Miller SP, Abraham RH, Lai JH, Hill D, Shiloh Y, Cantley LC, Rathbun GA. J Biol Chem. 2000;275:22719. doi: 10.1074/jbc.M001002200. [DOI] [PubMed] [Google Scholar]
- 27.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. Science. 2007;316:1160. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Biochem J. 2008;409:449. doi: 10.1042/BJ20071114. [DOI] [PubMed] [Google Scholar]
- 29.Andrisse S, Patel GD, Chen JE, Webber AM, Spears LD, Koehler RM, Robinson-Hill RM, Ching JK, Jeong I, Fisher JS. PLoS ONE. 2013;8:e66027. doi: 10.1371/journal.pone.0066027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moffat C, Ellen Harper M. IUBMB Life. 2010;62:739. doi: 10.1002/iub.387. [DOI] [PubMed] [Google Scholar]
- 31.Hardie DG, Ross FA, Hawley SA. Nat Rev Mol Cell Biol. 2012;13:251. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halaby MJ, Hibma JC, He J, Yang DQ. Cell Signal. 2008;20:1555. doi: 10.1016/j.cellsig.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Sapkota GP, Deak M, Kieloch A, Morrice N, Goodarzi AA, Smythe C, Shiloh Y, Lees-Miller SP, Alessi DR. Biochem J. 2002;368:507. doi: 10.1042/BJ20021284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miles PD, Treuner K, Latronica M, Olefsky JM, Barlow C. Am J Physiol Endocrinol Metab. 2007;293:E70. doi: 10.1152/ajpendo.00259.2006. [DOI] [PubMed] [Google Scholar]
- 35.Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, Bernal-Mizrachi C, Muslin AJ, Kastan MB, Semenkovich CF. Cell Metab. 2006;4:377. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Viniegra JG, Martinez N, Modirassari P, Hernandez Losa J, Parada Cobo C, Sanchez-Arevalo Lobo VJ, Aceves Luquero CI, Alvarez-Vallina L, Ramon y Cajal S, Rojas JM, Sanchez-Prieto R. J Biol Chem. 2005;280:4029. doi: 10.1074/jbc.M410344200. [DOI] [PubMed] [Google Scholar]
- 37.Roach WG, Chavez JA, Miinea CP, Lienhard GE. Biochem J. 2007;403:353. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]