Abstract
The carcinogenic potential of erionite has sparked concern about human exposure in areas where it is present in regional bedrock. The Arikaree Formation in western North Dakota contains altered tuffaceous units with authigenic zeolites. We sampled stratigraphic profiles in the Killdeer Mountains, Dunn County, North Dakota, to determine the distribution and chemical composition of zeolites. Powder X-ray diffraction, SEM/EDS and electron microprobe analyses were carried out on sample concentrates. Only samples stratigraphically in or below the distinctive burrowed marker unit were found to contain zeolites. Erionite and offretite were the most common zeolites identified, with offretite being more abundant based on frequency of measured Mg/(Ca+Na) ratios. Intermediate chemical compositions could be natural or due to intimate intergrowths of the two minerals. A better understanding is needed of the potential toxicity across the range of erionite and offretite compositions.
Keywords: Erionite, offretite, zeolite, Killdeer Mountains, North Dakota, Arikaree
Introduction
During the late 1970s, an epidemic of mesothelioma was discovered in three villages in the Cappadocian region of central Turkey (Baris et al. 1978; Artvinli and Baris 1979). Subsequent studies investigated the link between the high incidence of deaths within the group caused by malignant pleural mesothelioma (MPM) and the occurrence of erionite in the region’s bedrock (Baris et al. 1987). Emigrants from the region were found to have increased risk of MPM and 49% or more of the deaths in the Cappadocian region of Turkey due to MPM had a potential link to erionite exposure (Metintas et al. 1999). It was reported that 78% of the deaths that had occurred in the study group were due to malignant mesothelioma, and it is estimated that 50% of the total deaths in the area can be attributed to mesothelioma (Metintas et al. 1999; Emri et al. 2002).
Experimental studies show erionite has up to 300–800 times more carcinogenic potency and may be 20–40 times more active than some asbestos forms (U.S. EPA 2010). It has been classified as a Group I carcinogen by the International Agency for Research on Cancer (IARC 1987). Physical and chemical differences between the minerals could explain these differences (Emri et al. 2002). Supporting studies on rats have shown that inhaled erionite fibers resulted in increased incidence of mesothelioma in those animals (Wagner et al. 1985). A North American case of mesothelioma attributed to erionite exposure was reported by Kliment et al. (2009), however the mineral identification did not include a crystallographic tool such as XRD or TEM. Increasing interest in the subject prompted many more studies on the health effects of erionite, as well as new investigations into its carcinogenic potential, mechanisms of carcinogenesis, and potential genetic predispositions (Carbone and Yang 2012), its identification and classification (Dogan and Dogan 2008), erionite mineral structure, and the similarities between the mineral erionite and other closely related zeolites. A summary is provided by Carbone et al. (2007).
The concern with the carcinogenic potential of erionite has sparked an interest within North Dakota and other areas containing erionite in regional bedrock or sediments. These areas include other high butte formations scattered across western North Dakota as well as the badland formations of North Dakota, South Dakota, and Montana (Goodman and Pierson 2010). There is concern with exposure and transmission of airborne dusts and particulates possibly containing erionite fibers from gravel pits, roads, parking lots, playgrounds, feed lots, building and construction, mining operations, oil extraction, and farming/ranching operations (Carbone et al. 2011; Maher 2010). The study reported here was undertaken to characterize the distribution and chemical composition of erionite and related zeolites in rocks exposed in the Killdeer Mountains of Dunn County, North Dakota.
Geologic setting and previous work
Bluemle (2000), Murphy (2001), Murphy et al. (1993), and Hoganson et al. (1998) provide descriptions of the general geology, the geologic time setting, and the past geologic processes that resulted in the formations and stratigraphy found in the study area.
The majority of the bedrock in the area surrounding the Killdeer Mountains consists of the sandstones, siltstones, claystones, and lignites of the Paleocene Fort Union Group. During Eocene time, rivers and streams cut into the Fort Union sediments, ultimately depositing coarse gravel and sand beds, which would become a part of the Chalky Buttes Member of the Chadron Formation of the White River Group. Presently, river and stream erosion along with mass wasting is still the primary form of erosion affecting the southwestern North Dakota landscape (Bluemle 2000). These processes contribute to redistribution of any zeolite bearing sediments that are present.
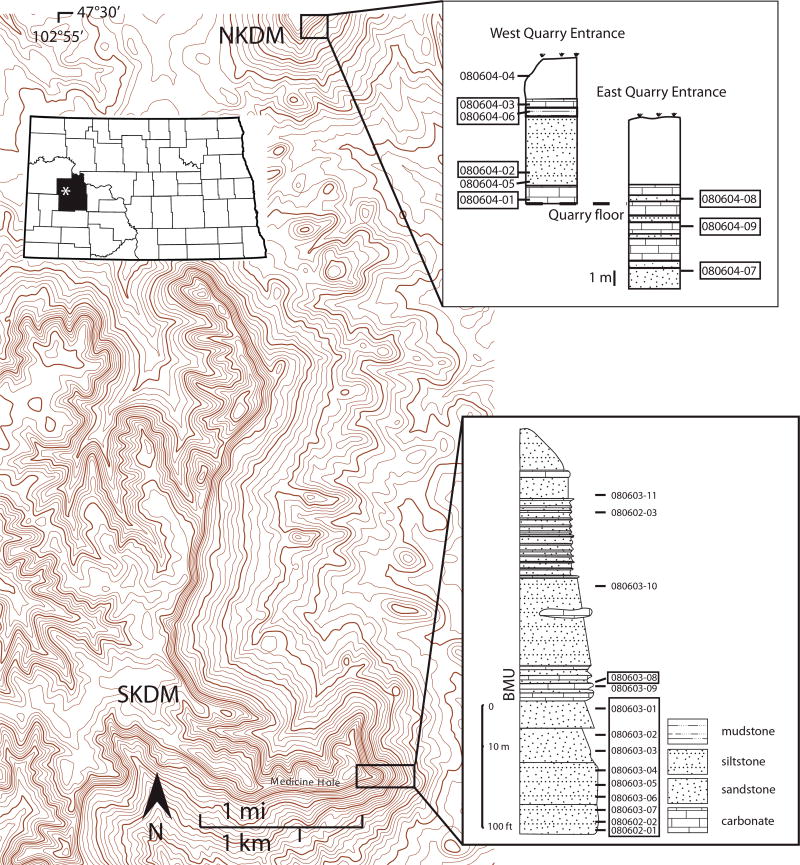
The Killdeer Mountains consist of two predominant mesas located in northern Dunn County of western North Dakota (Fig. 1). The two mesas rise about 200 m above the surrounding landscape and cover an area of approximately 2000 hectares. They are composed of, from top to bottom, rock units from the Arikaree Formation, Chadron Formation (Chalky Buttes Member), and the Golden Valley Formation (Bear Den Member and Camel Buttes Member). No Brule Formation appears to be present in this location (Denson and Gill 1965; Murphy et al. 1993).
Figure 1.
Topography, stratigraphic profiles, and sample identifications in the study area. SKDM: South Killdeer Mountain. NKDM: North Killdeer Mountain. BMU: “burrowed marker unit.” Inset shows general location of study area. SKDM stratigraphy from Murphy et al. (1993). Base map from U.S. Geological Survey. (Online color.)
The mesas are located in an area once covered by a large lake or a series of many smaller lakes during Miocene time. The Arikaree Formation, which constitutes the caprock of the Killdeer Mountain complex, is the most well-recognized erionite-bearing unit. It consists of approximately 100 m of tuffaceous siltstones, sandstones, and carbonates, with the sandstones and siltstones being calcareous (Denson and Gill 1965). Most units contain some volcanic glass (Delimata 1975; Forsman 1986), which characterizes them as slightly to highly tuffaceous. Volcanic ash in these units is believed to have originated from volcanic eruptions westward of the Killdeer Mountains (Delimata 1975). The ash would have been deposited across western North Dakota by eolian processes and then transported to the lake systems by fluvial processes, eventually accumulating to approximately 30 m thickness in locations. The ash-rich, tuffaceous sediment eventually lithified into tuffaceous limestone beds. Since the Pliocene, the erosional cycle in the area removed large amounts of surrounding sediment. The geologic setting of the area has been described as an inverted lake basin (Delimata 1975). Diagenetic processes resulted in glass shards being altered to a clay and zeolite assemblage (Delimata 1975; Forsman 1986).
A predominant cliff-forming unit of the Killdeer Mountain caprock contains interbedded tuffaceous sandstone and siltstone layers with carbonate lenses (Fig. 2). This unit, dated using fission track analysis to 25.1 ± 2.2 Ma, was termed the “burrowed marker unit” (BMU) by Forsman (1986) for the presence of an abundance of what has been classified as fossilized burrows of unknown origins (Murphy et al. 1993).
Figure 2.
Photo of “burrowed marker unit” (BMU) of Forsman (1986) on South Killdeer Mountain. Sample 080603-08 is from the more weathered, friable material between harder calcareous layers; sample 080603-09 is from the more resistant calcareous material. (Online color.)
Delimata (1975) carried out XRD analysis on samples from the Killdeer Mountains and reported the occurrence of clinoptilolite, offretite [which he considered identical to erionite following Hey and Fejer (1962)], and chabazite. He described the habits as radiating acicular and columnar void fillings, along with fibrous habits found in matrix pores. Forsman (1986) reported on zeolites within the pores and vugs of tuffaceous ash units. Based on standard powder X-ray diffraction (XRD) and electron microprobe (EMP) analysis on single mineral crystals, he concluded that erionite composed the majority of the zeolite content in the samples collected. Due to the possible health risks associated with erionite, hazard mapping (Forsman 2006) was undertaken by the North Dakota Department of Health, in cooperation with the North Dakota Geological Survey (NDGS) and the Environmental Protection Agency (EPA). These investigations led to gravel quarry restrictions, gravel use restrictions, dust control measures, and guidance plans to control and reduce the overall exposure by businesses and private landowners working in close proximity to the bedrock formations and/or gravel quarries, which potentially contain erionite (North Dakota Department of Health 2005).
Lowers and Meeker (2007) conducted a study by the USGS on 20 soil and roadbed samples collected from western North Dakota for zeolite identification. The SEM/EDS analysis determined the zeolite composition as intermediate between erionite and offretite as determined by Passaglia et al. (1998), and as similar to zeolites associated with high incidences of malignant diseases in Turkey (Dogan et al. 2006). Their EMP data plot within the offretite field on a Mg–Ca+Na–K diagram, and agree with the EMP data collected by Forsman (1986). XRD data supported the presence of erionite, but because both minerals have similar diffraction patterns, the presence of offretite could not be ruled out (Lowers and Meeker 2007). Eylands et al. (2009) studied sandstones and siltstones from buttes in Dunn, Stark, and Slope Counties of North Dakota and identified erionite using XRD and SEM. Lowers et al. (2010) and Carbone et al. (2011) found erionite from the Killdeer Mountains with that from villages in Turkey to have similar physical and chemical characteristics. However, the EMP data from Lowers et al. (2010) plot in the offretite field. Steele (2011) carried out single-crystal studies on zeolite from North Dakota and Turkey and confirmed the presence of erionite in both areas.
The U.S. EPA carried out chest X-rays and sensitive high-resolution computed tomography (HRCT) scans to detect pleural and interstitial changes associated with fiber exposure in current or past residents of western North Dakota with exposure to road gravels and erionite containing rock units (U.S. EPA, October, 2010; Ryan et al. 2011). Chest X-ray results did not indicate a significant increase in interstitial or localized pleural changes. The HRCT scans did indicate an increase in interstitial changes. Results of that study suggested that exposure to erionite containing rock units and road gravels could increase the risk of pleural and interstitial changes in humans that are commonly associated with asbestos exposure.
Sampling and analysis
Fieldwork and sampling for this study was carried out during 2008 at North and South Killdeer Mountains (Fig. 1). At South Killdeer Mountain (SKDM), samples were taken along the southeast edge of the mesa in the region of Medicine Hole Plateau (approximately 47°26˝38´ N; 102°53˝40´ W). The measured geologic section of Murphy et al. (1993) is used here as a basis for locating samples (Fig. 1). At North Killdeer Mountain (NKDM), samples were taken on both sides of the entry to a former quarry at the top of the mesa (47°29˝53´ N, 102°53˝36´ W). Sampling was also conducted at West and East Rainy Buttes and at White Butte (Chalky Butte complex) in southwest North Dakota; these results and analyses of Killdeer Mountain samples provided by Forsman from his 1986 collection are presented in Triplett (2012). Sample locations and descriptions are provided in the supplemental materials file1.
Small portions of samples were disaggregated into a coarse powder to liberate any zeolite minerals. Some samples were well enough cemented that an agate mortar and pestle was used, but the majority were friable enough to disaggregate easily without grinding. This disaggregated material was considered as “unprocessed” and used for SEM/EDS analysis, before further processing for powder XRD and EMP analysis.
Initial sample preparation for SEM/EDS was removal of small portions of the rock by pressing carbon tape onto the sample surface. Visual inspection and qualitative SEM/EDS analyses were carried out on unprocessed samples to identify any zeolite minerals.
Samples that showed apparent zeolite minerals in initial screening were subjected to a simple floatation process. Disaggregated material was placed into distilled water in a 1000 mL graduated cylinder and allowed to settle. A ball pipette was used to transfer all of the suspension, the water and fine suspended particles, into a vacuum filter system. Filtered material was used for powder XRD, SEM/EDS, and EMP analysis.
Filtered material was pulverized for XRD using an agate mortar and pestle. Powder XRD mounts were prepared using ethanol on glass slides and were analyzed at the NDSU Department of Chemistry and Biochemistry on a Phillips X’Pert MPD X-ray powder diffractometer. Search match was carried out using Jade+ and X’Pert Highscore software.
Samples were prepared for SEM/EDS and EMP analysis by placing concentrated sample material onto carbon tape and then carbon coated. SEM/EDS analysis was carried out at the North Dakota State University Electron Microscopy Center on a JEOL JSM-7600F with a field-emission source. EMP analysis was conducted at the University of Minnesota Electron Microprobe Lab, Department of Earth Sciences using a JXA-8900 SuperProbe. Microprobe analysis was carried out using the standards: Na, Amelia albite; Ba, barite; K, asbestos microcline; Mg, Si, Al, Ca, and Fe, Kakanui hornblende. H2O was calculated by difference in ZAF correction. Analytical conditions were 15 kV with a beam current of 10 nA; the beam diameter was nominally 5 µm, although it was modified for particular grains from 2 to 10 µm. Counting times were 10 s on-peak and 10 s off-peak. Experimental error was assessed by analyzing 8 spots on Kakanui hornblende. The measured average and standard deviations are: 40.92 (0.31) wt% SiO2, 15.14 (0.21) wt% Al2O3, 11.08 (0.20) wt% FeO, 12.99 (0.13) wt% MgO, 10.33 (0.18) wt% CaO, 2.84 (0.08) wt% Na2O, and 2.22 (0.05) wt% K2O. Depending on the zeolite concentration and the accessibility of the mineral grains within the surrounding matrix, 1 to 6 grains were analyzed by EMP from each of the nine samples. We analyzed 27 grains on 77 different points.
Results and discussion
Powder XRD analysis identified some type of zeolite in 10 of the 14 SKDM samples and 7 of the 9 NKDM samples (Table 1, Fig. 1). The processed material often contained calcite, quartz, or other minerals, and these are noted. Erionite and offretite were the most common zeolites, but chabazite, heulandite, and clinoptilolite were also identified. XRD identification of zeolites in one of the samples, 080603-05 is questionable.
Table 1.
Powder XRD identification of minerals in processed samples, Killdeer Mountains, North Dakota
| Sample | Minerals |
|---|---|
| 080602-01* | Eri, Qz |
| 080602-02* | Off, Eri, Cal |
| 080602-03 | Cal, Ank |
| 080603-01 | Eri, Cal |
| 080603-02 | Eri, Off |
| 080603-03 | Off, Eri, Cal |
| 080603-04 | Eri, Cal |
| 080603-05 | Eri?, Off? |
| 080603-06 | Eri, Cal, Qz |
| 080603-07* | Off, Cal |
| 080603-08 | Eri, Qz |
| 080603-09 | Cal, Qz |
| 080603-10 | Dol, Qz |
| 080603-11 | Cal, Qz |
| 080604-01 | Off, Cal |
| 080604-02 | Cal, Qz |
| 080604-03 | Eri, Cbz, Cal, Qz |
| 080604-04 | Eri, Hul, Cal, Qz |
| 080604-05 | Cal, Qz |
| 080604-06 | Cpt, Eri, Off, Cal, Qz |
| 080604-07 | Off |
| 080604-08 | Eri, Off, Cal |
| 080604-09 | Off, Cal, Qz |
Notes: Eri = erionite, Off = offretite, Cbz = chabazite, Hul = heulandite, Cpt = clinoptilolite, Cal = calcite, Dol = dolomite, Ank = ankerite, Qz = quartz. ? = tentative identification.
Sample location possibly slumped.
SEM/EDS and EMP analyses were carried out on samples with detectable zeolite based on the XRD analyses. Additional SEM/EDS analyses were carried out on samples provided by Forsman from his 1986 study. Some grains measured using EMP were the identical ones measured by SEM/EDS. EMP analyses are presented in Table 2 and Figure 3 is a plot of the compositions as measured by both EMP and SEM/EDS.
Table 2.
Chemical compositions of erionite and offretite from the Killdeer Mountains
| Sample no./ | 080603 | 080603 | 080603 | 080603 | 080603 | 080603 | 080603 | 080604 | 080604 | 080604 | 080603 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grain no. | −08/1 | −08/2 | −08/3 | −07/1 | −07/2 | −07/3 | −07/4 | −09/3 | −08/1 | −07/2 | −01/1 |
| SiO2 | 44.86 | 53.54 | 43.49 | 61.82 | 48.06 | 54.70 | 48.72 | 64.87 | 67.52 | 62.45 | 58.89 |
| Al2O3 | 12.03 | 13.90 | 12.03 | 15.74 | 12.03 | 14.31 | 12.97 | 16.36 | 17.27 | 15.14 | 12.90 |
| Fe2O3 | 1.99 | 0.28 | 0.05 | 0.66 | 0.17 | 6.53 | 0.31 | 0.12 | 0.32 | 0.57 | 0.08 |
| MgO | 1.78 | 1.04 | 0.74 | 1.62 | 1.35 | 4.73 | 1.08 | 0.37 | 0.77 | 0.69 | 1.29 |
| CaO | 2.77 | 3.85 | 2.85 | 3.59 | 2.63 | 1.68 | 3.66 | 6.00 | 5.95 | 4.57 | 3.14 |
| Na2O | 0.16 | 0.22 | 0.23 | 0.16 | 0.09 | 0.14 | 0.17 | 0.03 | 0.03 | 0.05 | 0.11 |
| K2O | 2.41 | 3.22 | 3.65 | 3.02 | 2.13 | 2.51 | 2.71 | 2.35 | 2.33 | 3.20 | 2.62 |
| H2O | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Si | 26.79 | 27.54 | 26.60 | 27.73 | 27.94 | 25.80 | 27.35 | 27.92 | 27.78 | 28.05 | 28.70 |
| Al | 8.47 | 8.43 | 9.37 | 8.32 | 8.23 | 7.96 | 8.61 | 8.30 | 8.38 | 8.01 | 7.39 |
| Fe3+ | 0.90 | 0.11 | 0.03 | 0.22 | 0.07 | 2.32 | 0.13 | 0.04 | 0.10 | 0.19 | 0.03 |
| Mg | 1.58 | 0.80 | 0.73 | 1.08 | 1.17 | 3.33 | 0.90 | 0.24 | 0.47 | 0.46 | 0.92 |
| Ca | 1.78 | 2.13 | 2.14 | 1.72 | 1.64 | 0.85 | 2.21 | 2.77 | 2.62 | 2.20 | 1.65 |
| Na | 0.18 | 0.22 | 0.35 | 0.14 | 0.11 | 0.13 | 0.18 | 0.02 | 0.03 | 0.04 | 0.11 |
| K | 1.84 | 2.12 | 3.21 | 1.73 | 1.58 | 1.51 | 1.94 | 1.29 | 1.22 | 1.84 | 1.69 |
| Number of points | 1 | 3 | 2 | 2 | 2 | 1 | 3 | 1 | 1 | 1 | 3 |
| Alth | 8.73 | 8.19 | 9.30 | 7.48 | 7.29 | 9.99 | 8.35 | 7.33 | 7.44 | 7.20 | 6.94 |
| E% | 7.19 | 4.25 | 1.06 | 14.03 | 13.89 | 2.89 | 4.64 | 13.82 | 13.95 | 13.95 | 6.91 |
| Si+Al | 35.26 | 35.97 | 35.97 | 36.04 | 36.17 | 33.76 | 35.97 | 36.22 | 36.15 | 36.06 | 36.08 |
| Mg/(Ca+Na) | 0.81 | 0.34 | 0.31 | 0.58 | 0.68 | 3.40 | 0.38 | 0.09 | 0.18 | 0.21 | 0.55 |
| 080603 | 080602 | 080602 | 080604 | 080604 |
|---|---|---|---|---|
| −01/2 | −03/1 | −03/2 | −01/1 | −01/3 |
| 48.20 | 61.46 | 59.53 | 45.10 | 64.51 |
| 10.98 | 14.93 | 14.33 | 14.44 | 16.24 |
| 0.21 | 0.24 | 0.29 | 0.17 | 0.03 |
| 2.35 | 2.52 | 2.61 | 1.95 | 1.99 |
| 2.73 | 3.08 | 3.35 | 2.92 | 3.42 |
| 0.29 | 0.02 | 0.05 | 0.08 | 0.05 |
| 1.28 | 1.57 | 1.30 | 2.35 | 2.79 |
| n.a. | n.a. | n.a. | n.a. | n.a. |
| 28.08 | 28.06 | 27.99 | 26.29 | 27.94 |
| 7.54 | 8.04 | 7.95 | 9.92 | 8.29 |
| 0.09 | 0.08 | 0.10 | 0.07 | 0.01 |
| 2.04 | 1.72 | 1.83 | 1.70 | 1.28 |
| 1.70 | 1.51 | 1.69 | 1.82 | 1.59 |
| 0.32 | 0.02 | 0.05 | 0.09 | 0.04 |
| 0.95 | 0.92 | 0.78 | 1.75 | 1.54 |
| 1 | 1 | 2 | 1 | 1 |
| 8.76 | 7.39 | 7.86 | 8.88 | 7.32 |
| –12.91 | 9.89 | 2.35 | 12.46 | 13.39 |
| 35.62 | 36.10 | 35.94 | 36.20 | 36.23 |
| 1.01 | 1.12 | 1.06 | 0.89 | 0.79 |
Note: Atomic ratios based on 72 O. The balance error E% = [(Al + Fe3+) − Alth]/Alth × 100 where Alth = Na + K + 2(Ca + Mg + Sr + Ba) (Passaglia 1970).
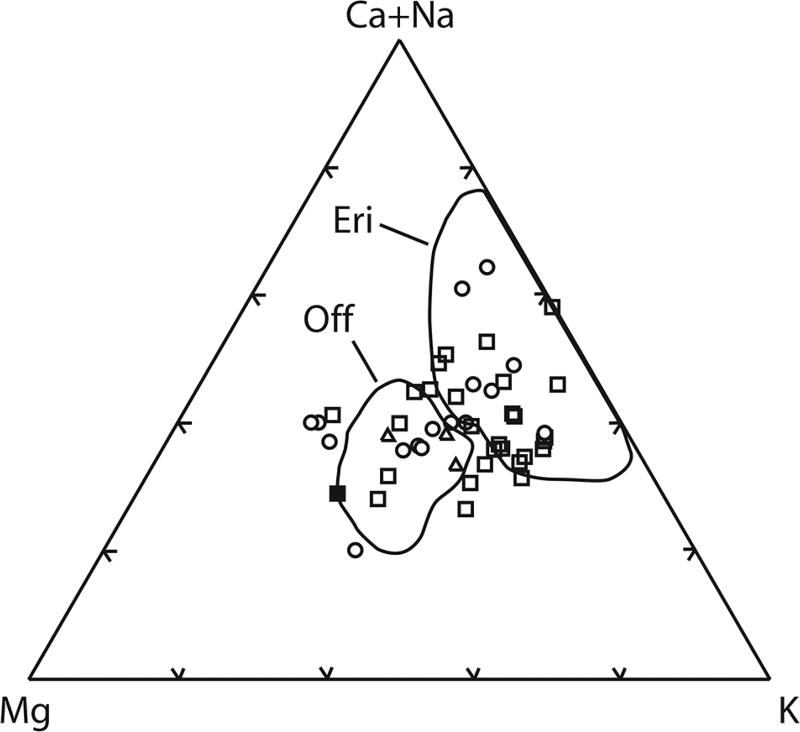
Figure 3.
Ternary compositional plot of zeolite minerals. This study squares = SEM/EDS (filled square is mineral pictured in Fig. 5); circles = EMPA. Triangles = Forsman (1986). Eri (erionite) and Off (offretite) fields after Gualtieri et al. (1998).
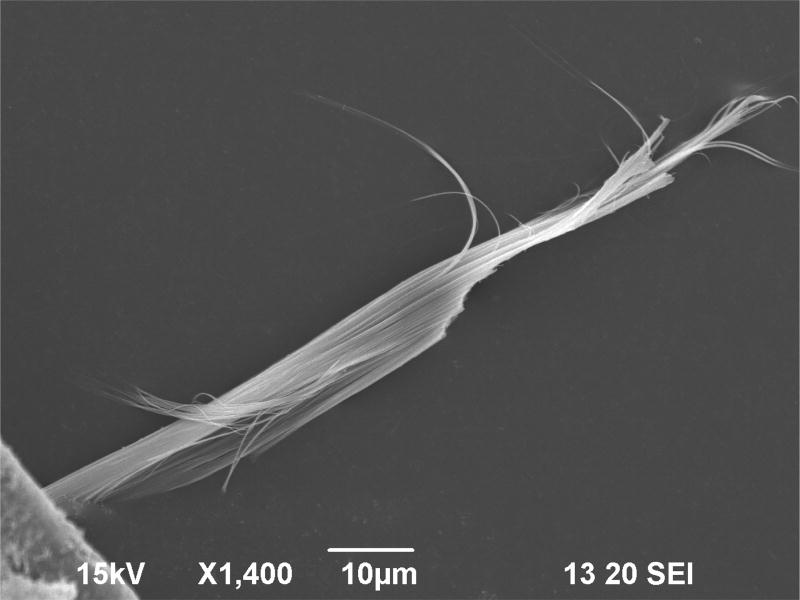
Figures 4 and 5 are micrographs of representative grains. As discussed in Gunter et al. (2007) relating to amphiboles, terms such as “fibers” and “fibrous” are applied differently by different groups. Gunter et al. (2007) also discuss use of the terms “particle,” “cleavage-fragment,” and “fragment.” To mineralogists, the morphological term “fiber” is a textural description for flexible thin partings. The grain shown in Figure 5 exhibits such fibrous morphology. The grains in Figure 4 could be single crystals, or based on aspect-ratio criteria, could be considered as fibers by the regulatory community. In zeolite nomenclature, however, the term “fibrous zeolite” is used as part of a crystal-chemical classification scheme referring to zeolites containing T5O10 chains of tetrahedra (Gottardi and Galli 1985; Armbruster and Gunter 2001), with no implication on a particular mineral fragment’s flexibility.
Figure 4.
Electron micrographs of zeolite minerals. (a) 080603-06 grain 1, (b) 080603-06 grain 2, (c) 080603-07, (d) 080603-08. Scale bar 10 µm.
Figure 5.
Electron micrograph of a zeolite mineral separated from a North Killdeer Mountain sample provided by N. Forsman. Scale bar 10 µm.
Identification of and distinction between erionite and offretite can be difficult because of their structural and chemical similarities (Passaglia et al. 1998), and because of the possibility of intergrowth of the two species within each crystal (Tschernich 1992; Coombs et al. 1997). The erionite general formula is (K2 Na2 Ca3)[Al10 Si26 O72]·30H2O (Passaglia et al. 1998) with a hexagonal space group symmetry P63/mmc and unit-cell parameters a ≈ 13.15 and c ≈ 15.05 Å (Passaglia et al. 1998). Three erionite species have been identified, erionite-Ca, -Na, and -K (Coombs et al. 1997). The offretite general formula is (Ca K Mg) [Al5 Si13 O36]·16H2O (Passaglia et al. 1998) with a hexagonal space group symmetry P6m2 and unit-cell parameters a ≈ 13.30 and c ≈ 7.60 Å (Gualtieri et al. 1998). In erionite, Si + Al [+Fe3+] should be equal to 36 atoms based on 72 oxygen atoms, while in offretite, Si + Al [+Fe3+] should be equal to 18 atoms based on 36 oxygen atoms. Here, all data are calculated on the basis of 72 oxygen atoms.
The reliability of a chemical analysis used to determine the zeolite species (or any framework silicate) can be evaluated by using a balance error formula (Passaglia 1970):
where Alth = Na + K + 2(Ca + Mg + Sr + Ba).
An extended balance formula is presented in Coombs et al. (1997). Chemical analyses for zeolites are considered to be reliable if the balance error (E%) is equal to or less than ±10% (Passaglia et al. 1998). If the E% falls within the set conditions, then the mineral may be erionite or may be another closely related zeolite with similar chemical composition. While some EMP analyses in Table 2 fall outside the ±10% range, all are presented here for completeness.
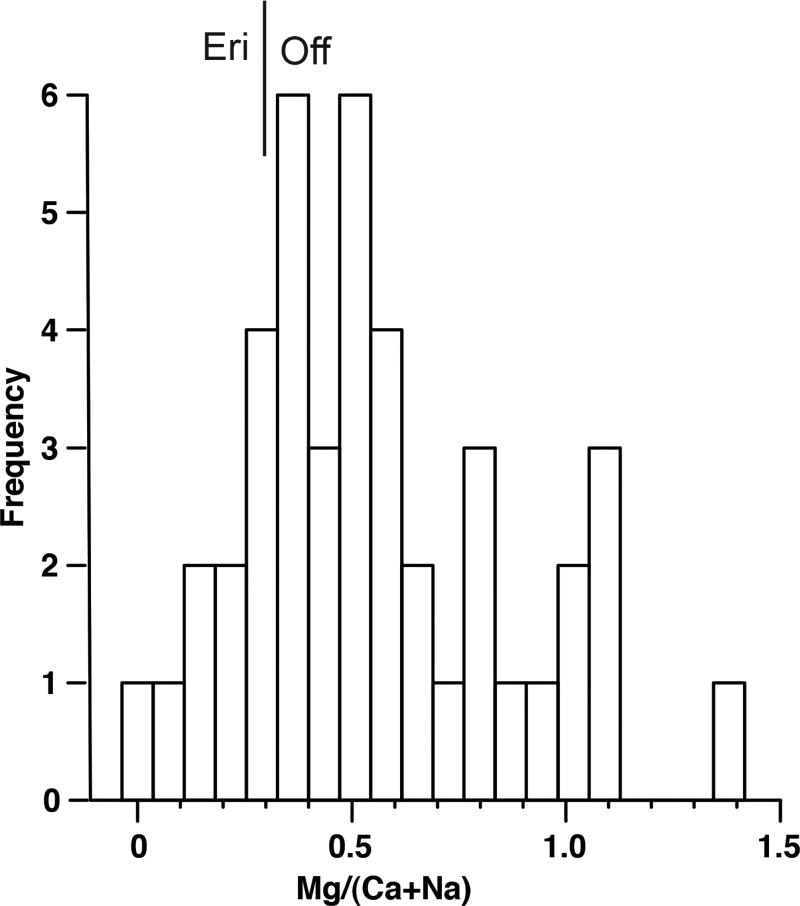
A chemical attribute relevant to distinguishing erionite from offretite is the ratio of Mg to (Ca+Na). Passaglia et al. (1998) defined the ratio Mg/(Ca+Na) = 0.30 as the boundary between the two minerals. As seen on the fields depicted on Figure 3 and discussed in Gualtieri et al. (1998), erionite is generally magnesium poor due to crystal structural limitations, whereas offretite is more magnesium rich with a Ca/Mg ratio close to 1.0. However, Rinaldi (1976) as cited in Tschernich (1992) reported a magnesium rich erionite from Sasbach, Germany. It should be noted, that the structural and chemical conclusions of Gualtieri et al. (1998) and Passaglia et al. (1998) were based on zeolites that were not collected from tuffs such as in Turkey or North Dakota and so may not be directly applicable to zeolites formed in other geologic environments (Steele, pers. comm., 2013). Figure 6 is a histogram of Mg/(Ca+Na) ratio for zeolite grains analyzed in this study. The data set includes SEM/EDS and EMP analyses of samples collected for this study, and of samples provided to us by Forsman from his 1986 study. The relative frequency of Mg/(Ca+Na) > 0.3 is approximately 80%. Following Passaglia et al. (1998), these high ratios indicate compositions consistent with offretite occur more frequently than those consistent with erionite. The apparent lack of a compositional gap could be the result of analytical error, grain scale intergrowth of erionite with offretite, or real compositional variation.
Figure 6.
Histogram of Mg/(Ca+Na) ratios for zeolite fibers analyzed in this study. Note: One measured value of 3.40 not included.
The study by Lowers and Meeker (2007) of zeolite grains from 20 soil and roadbed samples from the Killdeer Mountain region showed comparable results. SEM/EDS analyses overlap the erionite and offretite fields of Passaglia et al. (1998), and EMP analyses indicate the presence of offretite. XRD analysis showed the presence of erionite but offretite could not be ruled out. For the South Killdeer Mountain profile studied here, all samples except those stratigraphically above the BMU contained erionite or offretite, while six of the nine samples collected from NKDM contained erionite or offretite. At SKDM, erionite-or offretite-containing rock units were identified down to the base unit of the Arikaree Formation, which at that location is described as a 7.6 m (25 ft) thick moderately cemented siltstone with sand lenses and concretions approximately 94 m (308 ft) from the top of the mesa (Murphy et al. 1993). Zeolite was not found in samples above the BMU: from the entrance to Medicine Hole, from the massive sandstone unit in the middle of the Arikaree Formation (unit 10 of Murphy et al. 1993), from the calcareous portion of the burrowed marker unit, nor from the sandstone near the top of the mesa (unit 13 of Murphy et al. 1993). Because this was the extent of the sampling for this study, it is possible that the zeolite bearing rock units extend below the Arikaree Formation into the Chadron and Golden Valley Formations.
At North Killdeer Mountain, erionite or offretite were identified in six of nine samples taken (Fig. 1; Tables 1 and 2). Zeolites were present in rock units from just below the uppermost weathered horizon of NKDM down to the massive sandstone unit in the middle of the Arikaree Formation, and additionally from the base of the massive unit down to the stratigraphically lowest exposed outcrop of the NKDM east quarry wall. That unit is interpreted to be the bottom of the burrowed marker unit, the stratigraphically lowest sampling for this project at North Killdeer Mountain. One of the samples without zeolite (080604-04) was from weathered surficial material, and another (080604-05) was a lithic fragment. Because this was the extent of sampling at NKDM, it is possible that stratigraphically lower rock units may contain zeolite minerals.
In this study, we have documented the extent and composition of erionite and offretite in sampled profiles of exposed Killdeer Mountain rock units. It is unclear whether the mineralogic distinction between erionite and offretite has any health implications. However, as has been seen for the case of asbestos minerals (Gunter et al. 2007; Berndt and Brice 2008; Thompson et al. 2011), codification of nomenclature such as specific mineral names or habits into laws or regulations may have consequences in the application of health and legal policy. An area of research to be explored in environmental health may be to better understand any differences in potential toxicity between erionite and offretite including the varieties and intergrowths of these minerals.
Supplementary Material
Acknowledgments
Nels Forsman, Edward C. Murphy, and John Hoganson were helpful in providing direction and preliminary information on zeolites in western North Dakota, and Edward is acknowledged for reviewing the geology section. Angel Ugrinov, Scott Payne, Jayma Moore, and Ellery Frahm provided analytical support. Dillon Dolezal and Sharon (Brozo) Feit assisted with field and analytical work. Landowners Craig Dvirnak, Brian Benz, Sheila Murphy, Kenny Urlacher, and Marry Dennis are thanked for allowing access for sample collection, and Wendell and Linda Vigen provided welcome hospitality. We especially thank Ian Steele, an anonymous reviewer, and the editorship of Mickey Gunter for their meticulous reviews and suggestions on improving the manuscript. However, interpretations presented are strictly those of the authors. Funding from National Institutes of Health grant number P20 RR016471 from the INBRE program of the National Center for Research Resources, and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM12345 are gratefully acknowledged.
Footnotes
Special Collection: Minerals in the Human Body. Special associate editors are Mickey Gunter and Gregory Meeker. Special collection papers can be found on GSW at http://ammin.geoscienceworld.org/site/misc/virtual_special_list.xhtml. Additionally the GSW site has a classification tag found on the right-hand side of the article that is a link to all the collection papers. The MSA website (http://www.minsocam.org/MSA/AmMin/AmMineral.html) has complete information.
Deposit item AM-14-110, Supplemental Data. Deposit items are stored on the MSA web site and available via the American Mineralogist Table of Contents. Find the article in the table of contents at GSW (ammin.geoscienceworld.org) or MSA (www.minsocam.org), and then click on the deposit link.
References cited
- Armbruster T, Gunter ME. Crystal structures of natural zeolites. In: Bish DL, Ming DW, editors. Natural Zeolites; occurrence, properties, applications. Vol. 45. Reviews in Mineralogy and Geochemistry, Mineralogical Society of America; Chantilly, Virginia: 2001. pp. 1–67. [Google Scholar]
- Artvinli M, Baris YI. Malignant mesothelioma in a small village in the Anatolian region of Turkey: An epidemiologic study. Journal of the National Cancer Institute. 1979;63:17–22. [PubMed] [Google Scholar]
- Baris YI, Sahin AA, Ozesmi M, Kerse I, Ozen E, Kolacan B, Altinörs M, Göktepeli A. An outbreak of pleural mesothelioma and chronic fibrosing pleurisy in the village of Karain/Ürgüp in Anatolia. Thorax. 1978;33:181–192. doi: 10.1136/thx.33.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baris I, Artvinli M, Saracci R, Simonato L, Pooley F, Skidmore J, Wagner C. Epidemiological and environmental evidence of the health effects of exposure to erionite fibres: A four-year study in the Cappadocian region of Turkey. International Journal of Cancer. 1987;39:10–17. doi: 10.1002/ijc.2910390104. [DOI] [PubMed] [Google Scholar]
- Berndt ME, Brice WC. The origins of public concern with taconite and human health: Reserve Mining and the asbestos case. Regulatory Toxicology and Pharmacology. 2008;52:S31–S39. doi: 10.1016/j.yrtph.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Bluemle JP. The Face of North Dakota (3rd ed.) North Dakota Geological Survey Educational Series. 2000;26:206. [Google Scholar]
- Carbone M, Yang H. Molecular pathways: Targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clinical Cancer Research. 2012;18:598–604. doi: 10.1158/1078-0432.CCR-11-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M, Emri S, Dogan AE, Steele I, Tuncer M, Pass HI, Baris YI. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nature Reviews Cancer. 2007;7:147–154. doi: 10.1038/nrc2068. [DOI] [PubMed] [Google Scholar]
- Carbone M, Baris YI, Bertino P, Brass B, Comertpay S, Dogan AU, Gaudino G, Jube S, Kanodia S, Partridge CR, et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proceedings of the National Academy of Sciences. 2011;108:13618–13623. doi: 10.1073/pnas.1105887108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs DS, Alberti A, Armbruster T, Artioli G, Colella C, Galli E, Grice JD, Liebau F, Mandarino JA, Minato H, et al. Recommended nomenclature for zeolite minerals: Report of the Subcommittee on Zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Canadian Mineralogist. 1997;35:1571–1606. [Google Scholar]
- Delimata JJ. Ph.D. thesis. University of North Dakota; Grand Forks: 1975. Petrology and geochemistry of the Killdeer carbonates; p. 256. [Google Scholar]
- Denson NM, Gill JR. Uranium-Bearing Lignite and Carbonaceous Shale in the Southwestern Part of the Williston Basin-A Regional Study. U.S. Geological Survey Professional Paper. 1965;463:75. [Google Scholar]
- Dogan AU, Dogan M. Re-evaluation and re-classification of erionite series minerals. Environmental Geochemistry and Health. 2008;30:355–366. doi: 10.1007/s10653-008-9163-z. [DOI] [PubMed] [Google Scholar]
- Dogan AU, Baris YI, Dogan M, Emri S, Steele I, Elmishad AG, Carbone M. Genetic predisposition to fiber carcinogenesis causes a mesothelioma epidemic in Turkey. Cancer Research. 2006;66:5063–5068. doi: 10.1158/0008-5472.CAN-05-4642. [DOI] [PubMed] [Google Scholar]
- Emri S, Demir A, Dogan M, Akay H, Bozkurt B, Carbone M, Baris I. Lung diseases due to environmental exposures to erionite and asbestos in Turkey. Toxicology Letters. 2002;127:251–257. doi: 10.1016/s0378-4274(01)00507-0. [DOI] [PubMed] [Google Scholar]
- Eylands KE, Azenkeng A, Mibeck BA, Raymond LJ. Subtask 1.1 – Characterization of Erionite. Report 2009-EERC-12-06. North Dakota University, Energy & Environmental Research Center; 2009. [Google Scholar]
- Forsman NF. Documentation and diagenesis of tuffs in the Killdeer Mountains, Dunn County, North Dakota. Report of Investigation No. 87, North Dakota Geological Survey. 1986:13. [Google Scholar]
- Forsman NF. Erionite in tuffs of North Dakota: the need for erionite hazard maps. Geological Society of America Abstracts with Programs. 2006;38:366. [Google Scholar]
- Gottardi G, Galli E. Natural Zeolites. Springer-Verlag; Berlin: 1985. p. 409. [Google Scholar]
- Goodman BS, Pierson MP. Erionite, a naturally occurring fibrous mineral hazard in the tri-state area of North Dakota, South Dakota, and Montana. Geological Society of America Abstracts with Programs. 2010;42(3):5. [Google Scholar]
- Gualtieri A, Artioli G, Passaglia E, Bigi S, Viani A, Hanson JC. Crystal structure-crystal chemistry relationships in the zeolites erionite and offretite. American Mineralogist. 1998;83:590–606. [Google Scholar]
- Gunter ME, Belluso E, Mottana A. Amphiboles: Environmental and health concerns. In: Hawthorne FC, Oberti R, Della Verntura G, Mottana A, editors. Amphiboles: Crystal Chemistry, Occurrence, and Health Issues. Vol. 67. Reviews in Mineralogy and Geochemistry, Mineralogical Society of America; Chantilly, Virginia: 2007. pp. 453–516. [Google Scholar]
- Hey MH, Fejer EE. The identity of erionite and offretite. Mineralogical Magazine. 1962;33:66–67. [Google Scholar]
- Hoganson JW, Murphy EC, Forsman NF. Lithostratigraphy, paleontology, and biochronology of the Chadron, Brule, and Arikaree Formations in North Dakota. In: Terry DO Jr, LaGarry HE, Hunt RM, editors. Geological Society of America Special Paper. Vol. 325. 1998. pp. 185–196. [Google Scholar]
- IARC. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volume 42. IARC Monographs on the evaluation of carcinogenic risks to humans, Supplement. 1987;7:203. [PubMed] [Google Scholar]
- Kliment CR, Clemens K, Oury TD. North American erionite-associated mesothelioma with pleural plaques and pulmonary fibrosis: A case report. International Journal of Clinical and Experimental Pathology. 2009;2:407–410. [PMC free article] [PubMed] [Google Scholar]
- Lowers HA, Meeker GP. Denver microbeam laboratory administrative report 14012007: U.S. Geological Survey Administrative Report. 2007:11. [Google Scholar]
- Lowers HA, Adams DT, Meeker GP, Nutt CJ. Chemical and morphological comparison of erionite from Oregon, North Dakota, and Turkey. U.S. Geological Survey Open-File Report 2010-1286. 2010:13. [Google Scholar]
- Maher B. Epidemiology: Fear in the dust. Nature. 2010;468:884–885. doi: 10.1038/468884a. [DOI] [PubMed] [Google Scholar]
- Metintas M, Hillerdal G, Metintas S. Malignant mesothelioma due to environmental exposure to erionite: follow-up of a Turkish emigrant cohort. European Respiratory Journal. 1999;13:523–526. doi: 10.1183/09031936.99.13352399. [DOI] [PubMed] [Google Scholar]
- Murphy EC. Geology of Dunn County. North Dakota Geological Survey Bulletin 68 Part 1, Bismarck, North Dakota. 2001:36. [Google Scholar]
- Murphy EC, Hoganson JW, Forsman NF. The Chadron, Brule, and Arikaree Formations in North Dakota. North Dakota Geological Survey Report of Investigation No. 96, Bismarck, North Dakota. 1993:144. [Google Scholar]
- North Dakota Department of Health. [Accessed Aug. 17, 2009];Erionite. 2005 www.health.state.nd.us/EHS/Erionite/
- Passaglia E. The crystal chemistry of chabazites. American Mineralogist. 1970;55:1278–1301. [Google Scholar]
- Passaglia E, Artioli G, Gualtieri A. Crystal chemistry of the zeolites erionite and offretite. American Mineralogist. 1998;83:577–589. [Google Scholar]
- Ryan PH, Dihle M, Griffin S, Partridge C, Hillbert TJ, Taylor R, Adjei S, Lockey JE. Erionite in road gravel associated with interstitial and pleural changes—an occupational hazard in western United States. Journal of Occupational Environmental Medicine. 2011;53:892–898. doi: 10.1097/JOM.0b013e318223d44c. [DOI] [PubMed] [Google Scholar]
- Steele IM. Comparison of erionite from N. Dakota and central Turkey. Geological Society of America Abstracts with Programs. 2011;43:138. [Google Scholar]
- Thompson BD, Gunter ME, Wilson MA. Amphibole asbestos soil contamination in the U.S.A.: A matter of definition. American Mineralogist. 2011;96:690–693. [Google Scholar]
- Tschernich RW. Zeolites of the World. Geoscience Press; Pheonix, Arizona: 1992. p. 563. [Google Scholar]
- Triplett JW. M.S. thesis. North Dakota State University; Fargo: 2012. Identification and characterization of zeolites in western North Dakota; p. 112. [Google Scholar]
- U.S. EPA. Radiographic changes associated with exposure to erionite in road gravel in North Dakota. Report EP-R8-06-02/TO no.0804. 2010:90. [Google Scholar]
- Wagner JC, Skidmore JW, Hill RJ, Griffiths DM. Erionite exposure and mesotheliomas in rats. British Journal of Cancer. 1985;51:727–730. doi: 10.1038/bjc.1985.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.