Abstract
Secreted phosphoprotein one (SPP1, osteopontin) may regulate conceptus implantation and placentation. We investigated effects of progesterone (P4) and the conceptus on expression and localization of SPP1 in the ovine uterus. Steady-state levels of SPP1 mRNA in the endometrium of unilaterally pregnant ewes did not differ significantly between nongravid and gravid horns within their respective days of pregnancy; however, levels did increase as pregnancy progressed. SPP1 mRNA was detectable in the glandular epithelium (GE) of both nongravid and gravid horns via in situ hybridization. SPP1 protein was localized to the apical surface of the luminal epithelium of both nongravid and gravid uterine horns. Gravid horns exhibited extensive stromal SPP1 on Days 40 through 120, whereas SPP1 was markedly lower in the stroma of nongravid uterine horns through Day 80 of pregnancy. By Day 120, stromal expression of SPP1 between nongravid and gravid horns was similar. Long-term P4 treatment of ovariectomized ewes induced SPP1 in the uterine stroma and GE. A bioactive 45-kDa SPP1 fragment was purified from uterine secretions and promoted ovine trophectoderm cell attachment in vitro. Interestingly, increased stromal cell expression of SPP1 was positively associated with vascularization as assessed by von Willebrand factor staining. Finally, ovine uterine artery endothelial cells produced SPP1 during outgrowth into three-dimensional collagen matrices in an in vitro model system that recapitulates angiogenesis. Collectively, P4 induces and the conceptus further stimulates SPP1 in uterine GE and stroma, where SPP1 likely influences histotrophic and hematotrophic support of conceptus development..
Keywords: hematotroph, histotroph, osteopontin, ovine, placenta, pregnancy, progesterone, SPP1, uterus
Introduction
The ovine uterus undergoes considerable morphologic and functional changes to facilitate placentation and development of the fetus [1]. During the peri-implantation period, the uterine glandular epithelial (GE) and luminal epithelial (LE) cells begin to synthesize and secrete histotroph, an incompletely defined mixture of nutrients, adhesion molecules, enzymes, growth factors, cytokines, and other proteins that support development of the free-floating conceptus (embryo/fetus and associated placental membranes) and continue to be taken up by the areolae of the placenta throughout pregnancy [2–5]. Secreted phosphoprotein one (SPP1), also known as osteopontin, is a candidate factor associated with histotrophic support of the developing conceptus. This integrin-binding extracellular matrix protein, with properties of a cytokine, may regulate conceptus implantation, placentation, and development [6]. Previous research has demonstrated that uterine SPP1 is regulated by steroid hormones in mammals [7–10]. In ewes, SPP1 is induced in uterine GE in response to progesterone (P4) during the peri-implantation period, and it increases coordinately with overall production of histotroph [8, 9, 11]. Although, studies using ovariectomized ewes suggest that P4 regulates SPP1 in concert with interferon tau and chorionic somatomammotropin one [6, 12–14], postimplantation regulation of SPP1 expression in GE has not been well studied.
Complementary to the uptake of histotroph derived from uterine epithelia, uterine and placental blood vessels grow and/or dilate to facilitate maximal transfer of nutrients from maternal to placental vasculatures for hematotrophic support of conceptus development [15, 16]. SPP1 is a candidate for promoting angiogenesis to provide hematotrophic support of the developing conceptus, because SPP1 has increasingly been linked to angiogenesis in multiple tissues. SPP1 induces tumor cell angiogenesis in mice [17, 18], and it enhances vascularization in ectopic bone [19]. Physiologically, SPP1 is upregulated during cardiovascular development and myocardial remodeling [20, 21]. In ewes, increases in SPP1 expression in interplacentomal stroma between Days 20 and 25, and later in the uterine stroma of developing placentomes, are temporally and spatially associated with increasing angiogenesis within the pregnant uterus [9]. However, endocrine regulation of expression of SPP1 in stromal cells remains unknown.
Therefore, the objectives of this study were to identify the influence of P4 and/or conceptus-derived signals on SPP1 expression in uterine GE and stroma throughout pregnancy, and to investigate the potential functions of SPP1 in histotrophic and hematotrophic support of conceptus development during pregnancy.
Materials and Methods
Animals and Experimental Design
Experimental and surgical procedures involving animals in the present studies were approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Unilateral Pregnant Ewes
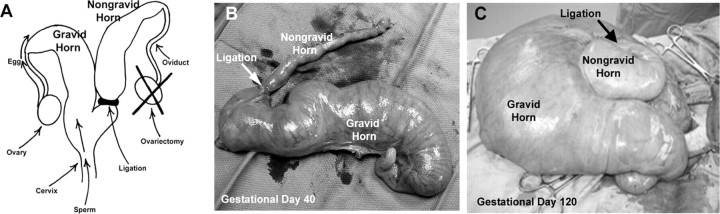
Ewes were checked daily for estrous behavior using vasectomized rams. Following at least one estrous cycle of normal duration, the ovary ipsilateral to the right uterine horn was removed and a double ligature was placed at the base of the right uterine horn at the bifurcation. At the following estrus (Day 0), ewes were mated to intact rams to generate a nongravid and a gravid uterine horn in each bred ewe (Fig. 1A) [2]. All ewes were hysterectomized on Gestational Days 40, 80, and 120 (n = 4 ewes per day; Fig. 1, B and C). Several sections (thickness ∼1–1.5 cm) of placentomal (or caruncular) and interplacentomal (or intercaruncular) tissues from each uterine horn (nongravid and gravid, respectively) were placed in fresh 4% paraformaldehyde fixative for 24 h and then embedded in Paraplast Plus (Oxford Labware, St. Louis, MO). Several similar sections were frozen in Tissue-Tek Optimal Cutting Temperature compound (Miles, Oneonta, NY). The remainder of the endometrium was dissected from the myometrium, frozen in liquid nitrogen, and stored at −80°C for RNA extraction. Uterine luminal as well as placental allantoic and amniotic fluids were collected from the uterine horns at Gestational Days 40, 80, and 120 (Fig. 1C) [22].
Fig. 1.
Unilaterally pregnant ewe model (A) in which the ovary ipsilateral to the right uterine horn was removed and a double ligature was placed at the base of the right uterine horn at the bifurcation. At the following estrus (Day 0), ewes were mated to intact rams to generate a nongravid and a gravid uterine horn in each pregnant ewe. Unilaterally pregnant uteri collected on gestational Days 40 (B) and 120 (C) are shown. Width of B is 12 inches. Width of C is 24 inches.
Slot Blot Hybridization Analysis
Steady-state levels of SPP1 mRNA were assessed by slot blot hybridization as described previously [8]. Denatured total endometrial RNA (10 μg) from each uterine horn (n = 4 samples per) was analyzed using a radiolabeled antisense cRNA probe for ovine SPP1 [8]. To correct for variation in total RNA loading, a duplicate RNA slot blot membrane was hybridized with radiolabeled antisense 18S rRNA and cRNA (pT718S; Ambion, Austin, TX). Following washing, nonspecific hybridization was removed by RNase A digestion. The radioactivity associated with each slot was quantified by electronic autoradiography using a Typhoon Scanner (Amersham Pharmacia Biotech, Piscataway, NJ) and expressed as relative units (RUs). Data from slot blot hybridization analyses were subjected to least-squares analysis of variance (LS-ANOVA) by the general linear models procedures of Statistical Analysis System version 8.1 for Windows (SAS Institute, Cary, NC) using the 18S rRNA data as a covariate. All tests of significance were performed using the appropriate error terms according to the expectation of the mean squares for error. Data are presented as LS mean RUs with SEM.
In Situ Hybridization Analysis
SPP1 mRNA was localized in paraffin-embedded uterine tissue using methods previously described [8]. Briefly, deparaffinized, rehydrated, and deproteinated uterine cross sections (∼5 μm; n = 4 samples per) were hybridized with radiolabeled antisense or sense ovine SPP1 RNA probes [8] synthesized by in vitro transcription with [α-35S]uridine 5-triphosphate (Perkin-Elmer Life Sciences, Wellesley, MA). After hybridization, washes, and RNase A digestion, autoradiography was performed using NTB-2 liquid photographic emulsion (Eastman Kodak, Rochester, NY). Slides were exposed at 4°C for 5 days, developed in Kodak D-19 developer, counterstained with Harris modified hematoxylin (Fisher Scientific, Fairlawn, NJ), dehydrated, and protected with cover slips.
Immunofluorescence Analyses
SPP1 and von Willebrand Factor (vWF) proteins were localized in frozen uterine cross sections (∼8–10 μm; n = 3 samples per) by immunofluorescence staining as previously described [22]. Briefly, tissues were fixed in −20°C methanol, washed in PBS containing 0.3% vol/vol Tween-20 in PBS, blocked in 10% vol/vol normal goat serum, and incubated overnight at 4°C with 2 μg/ml of a cocktail of rabbit anti-human recombinant SPP1 (LF-123 and LF-124, kindly provided by Dr. Larry Fisher, National Institutes of Health [23], rabbit anti-vWF, or rabbit immunoglobulin G [IgG; negative control; Molecular Probes, Eugene, OR]). Tissue-bound primary antibody was then detected with fluorescein-conjugated goat anti-rabbit IgG (2 μg/ml; Molecular Probes). Slides were overlaid with a Prolong antifade mounting reagent containing 4′,6′-diamidino-2-phenylindole (DAPI; Molecular Probes) and a cover glass.
Histology and Morphometry
Embedded tissues were sectioned (5 μm), deparaffinized, and stained with Mayer hematoxylin and eosin for general histomorphological evaluation as described previously [24]. A uterine gland cross section with an open lumen was counted as a gland. Uterine gland numbers were determined for at least six nonsequential sections from each uterine horn of each ewe (n = 4 ewes per). Endometrial gland density was defined by counting the number of glands in a 940-μm field of view of the interplacentomal endometrium and subjected LS-ANOVA by the general linear models procedures of Statistical Analysis System version 8.1 for Windows. All tests of significance were performed using the appropriate error terms according to the expectation of the mean squares for error.
P4-Treated Ewes
During seasonal anestrus, adult virgin control ewes (n = 4) were hemihysterectomized and bilaterally ovariectomized (Day 0), then received daily i.m. injections of 100 mg P4 in corn oil vehicle for 60 days. On Day 60, the remaining uterine horn was surgically recovered. Several sections (thickness ∼1–1.5 cm) from the middle of each uterine horn (P4 treatment) were placed in fresh 4% paraformaldehyde fixative for 24 h and then embedded in Paraplast Plus (Oxford Labware, St. Louis, MO).
Western Blot Analysis
Proteins from uterine luminal fluids (n = 4) and allantoic and amniotic fluids (n = 2 per) were denatured in Laemmli buffer, separated on 10% SDS–PAGE gels, and transferred to nitrocellulose. Proteins from ovine uterine artery endothelial cells (oUAECs) invading in 3D collagen matrices (300 μl) were concentrated 10-fold using trichloracetic acid precipitation prior to analysis using SPP1-specific antisera. Samples were separated on 12% SDS–PAGE gels and transferred to polyvinylidene fluoride (PVDF) membranes. Blots were blocked in 5% nonfat milk/TBST (Tris-buffered saline, 0.1% Tween 20) at room temperature for 1 h, incubated with a cocktail of rabbit anti-human recombinant SPP1 (LF-123 and LF-124; 5 μg/ml) [23] in 2% nonfat milk/TBST overnight at 4°C, rinsed three times for 10 min each with TBST at room temperature, incubated with goat anti-rabbit IgG horseradish peroxidase conjugate (1:20 000 dilution of 1 mg/ml stock; KPL, Bethesda, MD), then rinsed three times for 10 min each with TBST. Immunoreactive proteins were detected by enhanced chemiluminescence (SuperSignal West Pico Luminol System; Pierce Chemical Co., Rockford, IL) according to the manufacturer's recommendations using a FluorChem IS-8800 (Alpha Innotech, San Leandro, CA).
Purification of SPP1 from Ovine Uterine Secretions
SPP1 was isolated from uterine secretions using previously established methods [25]. Uterine secretions were collected from the nongravid horn of three unilaterally pregnant sheep on Day 120 of gestation and combined (n = 3). The secretions (400 ml) were mixed overnight with 40 ml DEAE-Sephacel beads (Pharmacia) at 4°C. To remove unbound protein, the mixture was added to a 5 × 20-cm column and washed with 400 ml of 0.2 M NaCl in 10 mM NaH2PO4, pH 7.4, and 250 ml of 0.25 M NaCl in 10 mM NaH2PO4, pH 7.4. Bound protein was eluted with 250 ml of 0.3 M NaCl in 10 mM NaH2PO4, pH 7.4, and analyzed by SDS-PAGE. Fractions containing the protein of interest (Mr = 45 000 or 70 000) were combined and increased to 4 M using NaCl and loaded onto a 20-ml phenyl-Sepharose (Sigma) column (2.5 × 10 cm). Proteins were eluted with 200 ml of 2 M NaCl in 10 mM NaH2PO4, pH 7.4, and fractions of interest were again determined, pooled, and increased to 5 M with NaCl. This suspension was loaded onto a 5-ml phenyl-Sepharose column (1 × 10 cm), and proteins were eluted with 30 ml of 2 M NaCl in 10 mM NaH2PO4, pH 7.4. Purity of the isolated protein was assessed by analyzing 10 μg protein by SDS-PAGE and Coomassie blue R-250 staining.
Cell Attachment Assay
Polystyrene microwells (Corning-Costar, Cambridge, MA) were coated overnight at 4°C with 5 μg/ml of the following proteins (50 μl) in phosphate-buffered saline (PBS): ovine uterine SPP1 (SPP1) purified from ovine uterine secretions, recombinant rat SPP1 with an intact Arg-Gly-Asp (RGD) integrin-binding sequence (rSPP1 [RGD]), or mutant rSPP1 with Arg-Ala-Asp (RAD) in place of the RGD sequence (rSPP1 [RAD]). An ovine trophectoderm cell line (oTr1) was isolated from Day 15 conceptuses and cultured as previously described [22, 26]. After blocking each well in 10 mg/ml BSA in PBS, 50 000 oTr1 cells were added per well and allowed to attach for 1 h (37°C, 5% CO2). Nonadherent cells were removed by washing in isotonic saline, and wells were fixed in 10% formalin. Plates were stained with 0.1% Amido black for 15 min, rinsed, and solubilized with 2 N NaOH to obtain an absorbance reading at 595 nm, which directly corresponds to the number of cells stained in each well [27, 28].
Ovine UAEC Invasion in 3D Collagen Matrices
Primary oUAECs from three individual pregnant ewes (n = 3; passages 4–5) [29] were seeded on the upper surface of 3D collagen matrices (2.5 mg/ml) containing 1 μM sphingosine-1-phosphate (S1P) to promote invasion as previously described [30]. Cell monolayers were allowed to attach for 30 min prior to addition of MEM (Invitrogen) containing a reduced serum II supplement, vascular endothelial growth factor (40 ng/ml), basic fibroblast growth factor (40 ng/ml), and ascorbic acid (50 μg/ml). Cultures (n = 4 per time point) were harvested for analysis 6, 12, 18, or 36 h after addition of growth factors to the medium and were fixed in 3% glutaraldehyde and stained with toluidine blue. Additional collagen matrices (n = 5 per time point) were harvested for analysis at 6, 12, 18, or 24 h after of addition of growth factors to the medium and placed directly into preheated Laemmli buffer (95°C) for subsequent Western blot analysis.
Photomicrography
Digital photomicrographs of in situ hybridization (bright-field and dark-field images) and immunofluorescence staining were evaluated using an Axioplan 2 microscope (Carl Zeiss, Thornwood, NY) interfaced with an Axiocam HR digital camera and Axiovision 4.1 software (Carl Zeiss). Digital photographs of oUAEC invasion assays were captured using an Olympus CKX41 microscope (Leeds Instruments Inc., Irving, TX). Photographic plates were assembled using Adobe Photoshop (version 6.0; Adobe Systems Inc., San Jose, CA).
Results
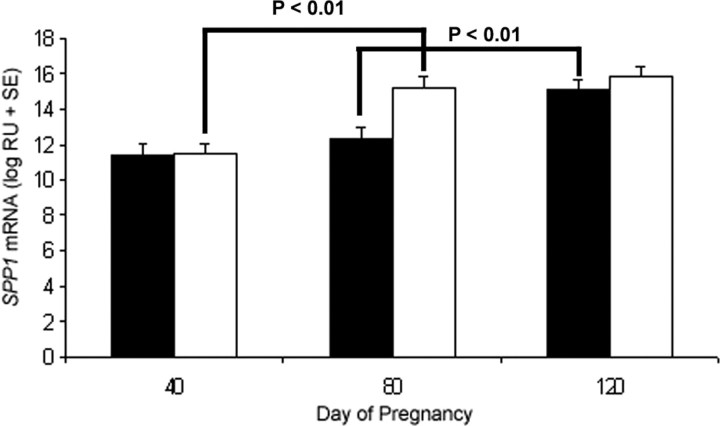
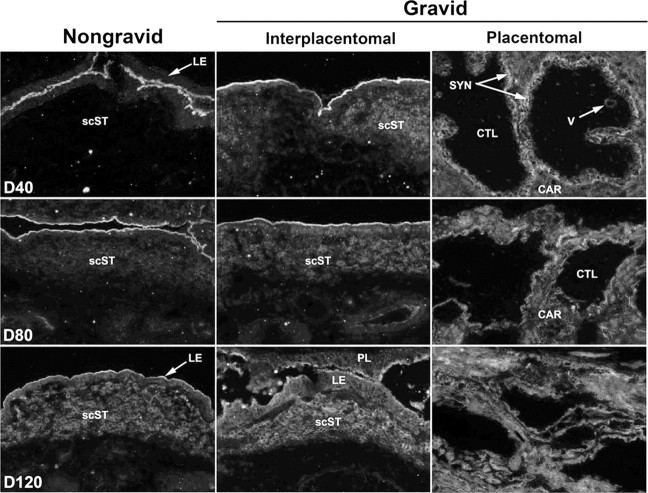
Steady-state levels of SPP1 mRNA were not different between nongravid and gravid uterine horns within the respective days of pregnancy (P > 0.10; Fig. 2). However, SPP1 mRNA levels significantly increased between Gestational Days 40 and 80 in gravid horns (P < 0.01) and between Days 80 and 120 (P < 0.01) in nongravid horns (Fig. 2). SPP1 mRNA was detectable via in situ hybridization in the GE of both nongravid and gravid uterine horns (Fig. 3A). SPP1 mRNA (Fig. 3B) and protein (Fig. 4) were more abundant in the stratum compactum stroma of gravid than nongravid horns on Days 40 and 80 of unilateral pregnancy as detected by in situ hybridization and immunofluorescence microscopy. SPP1 protein was also present in caruncular stroma of placentomes. By Day 120, immunoreactive SPP1 protein was not different between gravid and nongravid horns (Fig. 4).
Fig. 2.
Steady-state levels of SPP1 mRNA in endometria determined by slot blot hybridization analysis. SPP1 expression was not different between nongravid (black bars) and gravid (white bars) uterine horns within the respective days of pregnancy (P > 0.10). However, SPP1 mRNA levels significantly increased between gestational Days 40 and 80 in the gravid horn (P < 0.01) as well as between Days 80 and 120 (P < 0.01) in the nongravid horn.
Fig. 3.
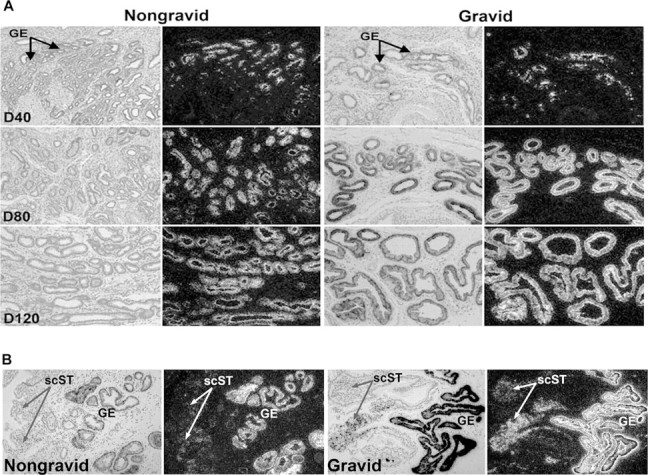
In situ hybridization analysis of SPP1 mRNA in uteri collected from unilaterally pregnant ewes. A) SPP1 mRNA in glandular epithelium (GE) on Gestational Days (D) 40, 80, and 120. B) SPP1 mRNA in stratum compactum stroma (scST) on Day 80. Corresponding brightfield and darkfield images of representative endometrial cross sections are shown. A section hybridized with radiolabeled sense cRNA probe shown in Figure 6 serves as a negative control. Width of each field is 940 μm.
Fig. 4.
Immunofluorescence localization of SPP1 (polyclonal rabbit anti-human SPP1 IgG [LF-123 + LF-124]) in frozen sections of uteri from nongravid and gravid horns collected from unilaterally pregnant ewes on Gestational Days (D) 40, 80, and 120. Rabbit IgG (IgG) serves as a negative control and is shown in Figure 8. scST, Stratum compactum stroma; LE, luminal epithelium; PL, placental membranes; CTL, cotyledon; CAR, caruncle; SYN, syncytia; V, blood vessel. Width of each field is 940 μm.
Histological evaluation of the uterine cross sections of gravid and nongravid uterine horns revealed differences in gland development (Fig. 5). As pregnancy progressed, the number of glands visible in a single field of view decreased (P < 0.05) in both gravid and nongravid horns (Fig. 5B). However, fewer glands were visible per field in gravid than nongravid horns (P < 0.05), indicating that the presence of a conceptus within the horn advanced GE hypertrophy (Fig. 5B).
Fig. 5.
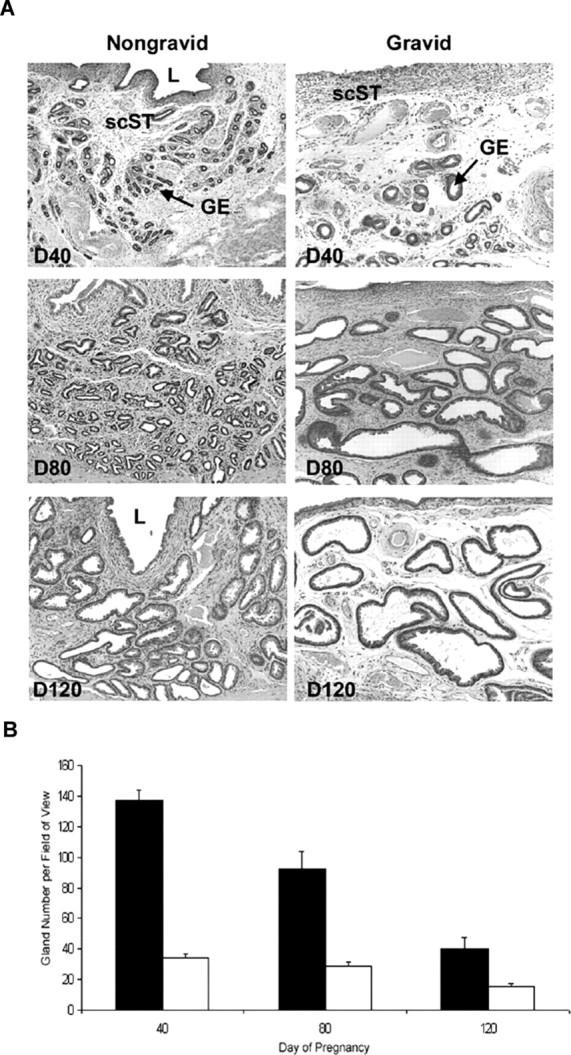
A) Representative photomicrographs of hematoxylin and eosin-stained uterine cross sections of nongravid and gravid uterine horns from unilaterally pregnant ewes collected on Gestational Days (D) 40, 80, and 120 are shown. L, Lumen; GE, glandular epithelium; scST, stratum compactum stroma. Width of each field is 940 μm. B) Quantification of uterine gland number per 940-μm field of view. Fewer glands were visible per field in gravid (white bars) than nongravid (black bars) uterine horns (P < 0.001) throughout gestation.
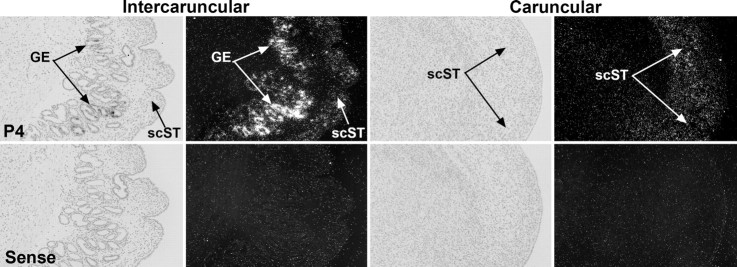
SPP1 mRNA was present in the intercaruncular and caruncular stroma, as well as the GE of endometrium from ovariectomized ewes that received P4 treatment for 60 days (Fig. 6), suggesting that P4 alone is sufficient to induce stromal and GE expression of SPP1.
Fig. 6.
In situ hybridization analysis of SPP1 mRNA expression in the intercaruncular and caruncular endometria of ovariectomized ewes after 60 days of P4 treatment (P4). Corresponding brightfield and darkfield images are shown. A section from an ewe treated with P4 hybridized with a radiolabeled sense RNA probe serves as a negative control (Sense). scST, Stratum compactum stroma; GE, glandular epithelium. Width of each field is 940 μm.
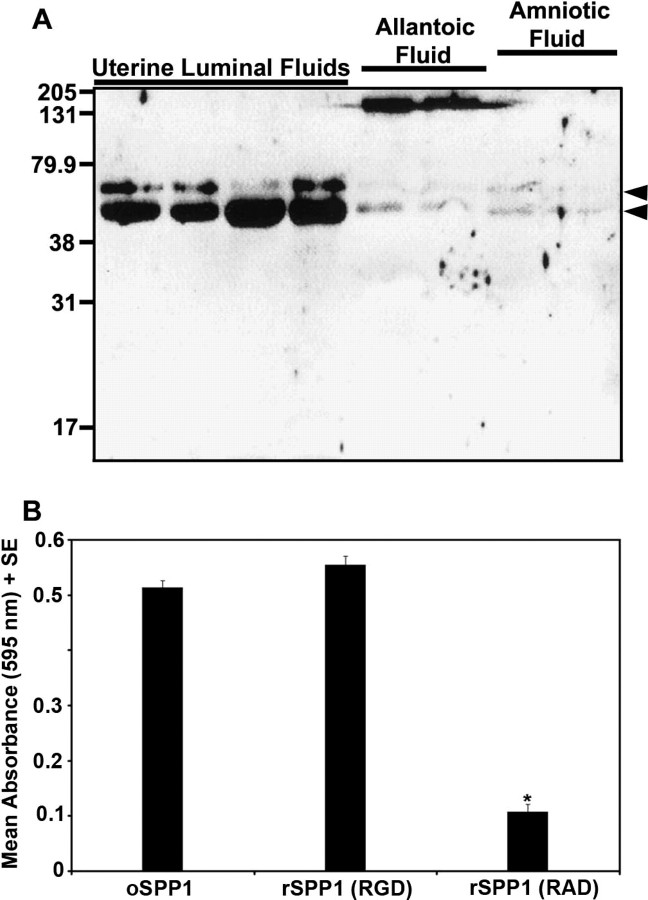
Evaluation of uterine luminal fluids from nongravid horns of Day 120 unilaterally pregnant ewes via Western blot analysis revealed two forms of SPP1 that migrated at ∼70 and ∼45 kDa, with the smaller form being more abundant (Fig. 7A). These proteins were also detectable, although less abundant, in allantoic and amniotic fluids of gravid horns (Fig. 7A). A prominent immunoreactive band of ∼140 kDa was also prominent in allantoic fluid (Fig. 7A), and likely represents SPP1 dimers that remain, despite utilization of reducing conditions, due to the ability of SPP1 to cross-link to itself or other proteins via two conserved glutamine residues that have transglutaminase activity [8, 31]. Uterine secretions from the nongravid uterine horn were collected and SPP1 purified (oSPP1) using previously established methods [25]. The oTr1 cell line was used to determine whether SPP1 purified from ovine uterine secretions can promote trophectoderm cell adhesion. Adhesion of oTR1 cells was supported by oSPP1 at similar levels to intact recombinant rat SPP1 (rSPP1 [RGD]), but not by rSPP1 containing a mutated RGD integrin-binding sequence (rSPP1 [RAD]). These results indicate that SPP1 purified from uterine secretions is bioactive and supports adhesion of oTr1 cells to SPP1 in an integrin-dependent manner.
Fig. 7.
A) Western blot analysis of SPP1 in uterine luminal secretions recovered from nongravid uterine horns and from allantoic and amniotic fluids of conceptuses in the gravid uterine horn of unilaterally pregnant ewes on Gestational Day 120. Each lane represents a sample from a different ewe. Immunoreactive SPP1 was detected using a cocktail of polyclonal rabbit anti-hSPP1 IgG (LF-123 + LF-124). Positions of prestained molecular weight standards (kDa) are indicated. Arrowheads denote SPP1 fragments. B) SPP1 promotes attachment of ovine trophectoderm cells. Adhesion assays were conducted with rSPP1 (RGD), rSPP1 (RAD), or ovine uterine luminal SPP1 (oSPP1). Data represent absorbance values (595 nm) + SEM (n = 3 wells per data point). Attachment did not differ between oSPP1 and rSPP1 (RGD) (P > 0.10), but was significantly less using rSPP1 (RAD) than oSPP1 or rSPP1 (RGD) (*P < 0.01).
Development of the vasculature in the uterine stroma was assessed using immunofluorescence staining for the endothelial cell marker vWF. In parallel with SPP1 expression, increased levels of vascular development and organization were detected in the gravid over nongravid horns (Figs. 4 and 8A). Staining for vWF increased steadily in placentomes throughout pregnancy. In addition, immunostaining was greater in gravid than in nongravid interplacentomal tissues at Days 40 and 80 of pregnancy, where endothelial staining was most prominent just beneath the uterine LE, as well as throughout the stratum compactum stroma (Fig. 8A). In nongravid tissue, no differences in caruncular and intercaruncular immunostaining were observed (data not shown). The coordinate temporal and spatial increases of SPP1 expression and vascular development suggest a role for SPP1 in angiogenesis to enhance hematotrophic nutrition for the developing conceptus.
Fig. 8.
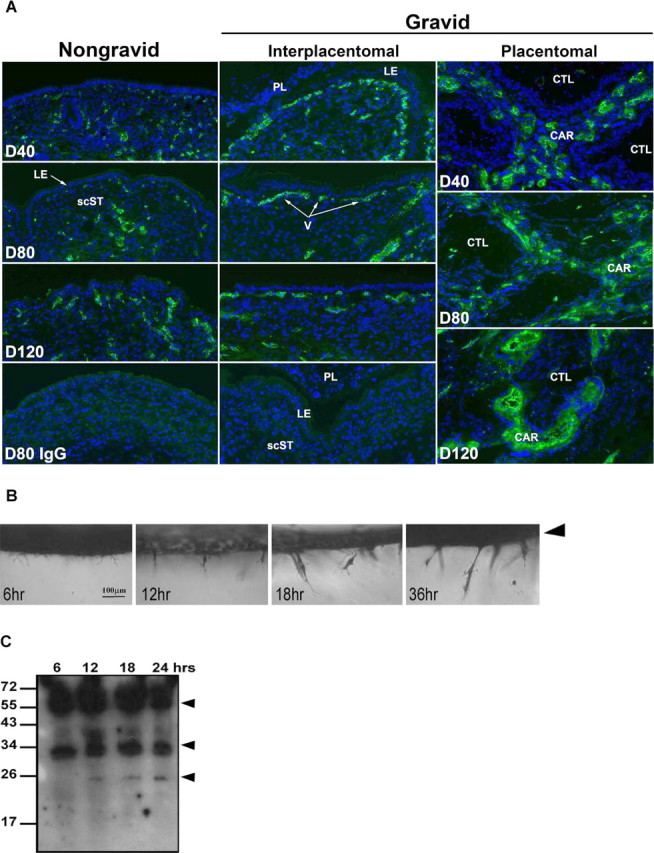
A) Immunofluorescence localization of vWF (polyclonal rabbit anti-human vWF) in frozen sections of uteri from nongravid and gravid horns collected from unilaterally pregnant ewes on Gestational Days (D) 40, 80, and 120. Rabbit IgG (IgG) serves as a negative control. Nuclei are also stained with DAPI. scST, Stratum compactum stroma; LE, luminal epithelium; PL, placental membranes; CTL, cotyledon; CAR, caruncle; V, blood vessel. Width of each field is 940 μm. B) Photographs of fixed cultures illustrating oUAEC outgrowth in three-dimensional collagen matrices over time. Cultures were fixed at 6, 12, 18, and 36 h and stained with toluidine blue prior to imaging. Arrowhead indicates the surface of collagen gel where cells were originally seeded. A side view of cultures is shown. C) Western blot analysis of SPP1 in oUAECs grown on 3D collagen matrices for 6, 12, 18, or 24 h. Immunoreactive SPP1 was detected using a cocktail of polyclonal rabbit anti-hSPP1 IgG (LF-123 + LF-124). Positions of prestained molecular weight standards (kDa) are indicated. Arrowheads denote SPP1 fragments.
To determine whether SPP1 production is associated with new blood vessel growth, as suggested by in vivo results, we used an in vitro model of endothelial cell invasion that mimics angiogenesis. In these studies, primary oUAECs were allowed to invade into 3D collagen matrices. Photographs of invasion illustrate that invading structures increase in size and number from 0 to 36 h (Fig. 8B). Western blot analysis of SPP1 expression in protein extracts revealed that invading cells produce proteins of ∼70 to ∼50 kDa, similar to those observed in vivo, as well as two smaller cleavage fragments (Fig. 8C). Variation in size and number of SPP1 fragments in vitro as opposed to in vivo is not unexpected, as SPP1 is a complex molecule, possessing multiple cleavage sites, acidic amino acids, serine phosphorylations, and glycosylation [31–33]. These results suggest a possible role for SPP1 in vasculature development in the pregnant ovine uterus.
Discussion
The present studies assessed the influence of P4 as well as physical and/or paracrine factors, including the fetus and associated placental membranes, on ovine uterine SPP1 expression to better understand how these signals direct SPP1 production, which is hypothesized to provide histotrophic and hematotrophic support of the conceptus. Results suggest that SPP1 is a major component of histotroph secreted in response to P4 by GE as it undergoes hypertrophy and hyperplasia. In addition, P4 induces, and unidentified conceptus factors further stimulate, expression of SPP1 in uterine stroma that is coordinate with development of the vasculature to provide hematotrophic nutrition to the conceptus.
Morphometric analysis of endometrial tissue within the gravid and nongravid uterine horns of unilaterally pregnant ewes was used to assess changes in the number and area of uterine glands in response to the presence of placental membranes and day of pregnancy. During pregnancy there is an increase in gland number (hyperplasia), followed by an increase in area (hypertrophy) [12]. In the present study, a decrease in gland number per endometrial cross section corresponded with an increase in individual gland cross sectional area per endometrial cross section in both the nongravid and gravid uterine horns, indicating gland hypertrophy. An influence of placental membranes on the architecture of endometrial GE was suggested by the presence of larger glands in the gravid compared with the nongravid uterine horns. Previous research has shown that members of the lactogenic and somatogenic family of hormones are key regulators of endometrial glandular development and function [4, 34, 35]. Specifically, the servomechanism proposed by Spencer et al. [34] stated that sequential exposure of the pregnant ovine endometrium to P4, interferon tau, CSH1, and placental growth hormone is necessary for the regulation of uterine gland function, including secretion of SPP1 and uterine milk protein or SERPIN [34]. Consistent with that hypothesis, previous research found that SPP1 mRNA increases in response to P4 in the ovine uterine GE during the peri-implantation period and is further increased in response to CSH1 but not growth hormone [6, 14]. The use of the long-term P4 treatment of ewes in the present study provides firm evidence that P4 alone can not only stimulate, but also maintain SPP1 expression in the ovine GE. In addition, use of the unilaterally pregnant ewe model demonstrated that increased SPP1 levels in the GE are likely the result of increased GE number and size in response to hormones from placental membranes, such as placental lactogen. Collectively, the present results strongly suggest that SPP1 is induced and maintained in ovine GE by P4 and that placental factors, including placental lactogen, increase GE hyperplasia, leading to increased SPP1 gene expression and secretion.
It is known that SPP1 produced by ovine GE is secreted into the uterine lumen during the attachment phase of implantation as a 45-kDa fragment of the full-length 70-kDa protein [8]. This fragment then localizes along the entire conceptus-maternal interface throughout gestation. The present data provide the first definitive evidence that SPP1 continues to be secreted as a major component of histotroph throughout ovine pregnancy. Large amounts of native 70-kDa SPP1 and the 45-kDa fragment were detected in ovine uterine secretions (uterine milk), and the 45-kDa SPP1 was subsequently purified using an established protocol [25]. Our demonstration that ovine oTr1 cells adhere to immobilized ovine uterine SPP1 at levels similar to rSPP1 (RGD) but not rSPP1 (RAD) suggests that integrins on trophectoderm cells bind directly to SPP1, and this interaction may support adhesion of these cells to the uterus to support implantation and placentation. The 45-kDa SPP1 fragment contains the integrin-binding RGD sequence and has enhanced cell adhesion and spreading properties over full-length SPP1 [34, 36]. Therefore, it is reasonable to propose that interaction between conceptus integrins and the 45-kDa form of uterine SPP1 contributes to stable adhesion of the conceptus to the uterine LE, and therefore facilitates intimate communication, including nutrient exchange between the uterus and developing conceptus.
Fetal development during gestation is supported by epithelial secretions, as well as vascular transfer of nutrients, or hematotrophic support. In ruminants, placentomes (discrete regions of the maternal-fetal interface formed from the interdigitation of the aglandular uterine caruncles with fetal cotyledons) are separated by extensive regions (interplacentomal) of true epitheliochorial placentation. Primarily placentomal, but also interplacentomal regions of the placenta contribute to hematotrophic support for the developing conceptus.
The presence of SPP1 in the stroma of placentomal and interplacentomal ovine endometrium after Day 25 of gestation [37] led to the hypothesis that its expression was regulated by either the physical presence of the placenta and/or paracrine factors from the placenta. Results from the present study demonstrate that stromal SPP1 is induced and maintained by P4. In addition, it appears that a placental factor(s) further stimulates P4-induced expression of SPP1 in uterine stroma because gravid uterine horns exhibited extensive stromal expression of SPP1 on all days examined (i.e., Days 40 to 120), whereas levels of SPP1 in the nongravid uterine horns were not equivalent to those in the gravid uterine horn until Day 120. However, it should be noted that in our studies, P4 treatment for 60 days resulted in hydration of the endometrium and the accumulation of uterine secretions. Therefore, it is plausible that SPP1 in interplacentomal stroma may also be stimulated by the stretch generated from accumulation of these fluids. Further, SPP1 protein was increased in the caruncular stroma of placentomes throughout pregnancy (Fig. 4), suggesting a physiological role for SPP1 in these regions of extensive interdigitation between cotyledenary and caruncular tissues.
These increases in SPP1 expression were temporally and spatially associated with increases in angiogenesis within the endometrium based on the presence of vWF as an endothelial cell marker. Consistent with the delay in increased SPP1 mRNA expression observed in the nongravid horn (Day 80) as opposed to the gravid horn (Day 40), the increase in vascular development in the subepithelial compartment of the nongravid horn was not detected until gestational Day 80, whereas the stroma of the gravid uterine horn displayed a marked increase in organization of endothelial cell networks in the subepithelial compartment by Day 40 of gestation. In addition, SPP1 protein also increased in the caruncular component of placentomes, and it was associated temporally and spatially with increased vWF staining in these tissues. Therefore, SPP1 is positioned to facilitate angiogenesis essential to providing hematotrophic nutrition to the conceptus.
The process of angiogenesis can be characterized by four fundamental stages in which an endothelial cell will sprout from the vessel of origin and give rise to a new capillary [38]. This process was largely recapitulated in vitro by placing oUAECs in 3D collagen matrices [30]. A potential role for SPP1 in regulating endothelial cell invasion is suggested, as there was substantial SPP1 production by the oUAECs as they invaded into the matrices (Fig. 8C). Indeed, a recent microarray evaluating gene expression changes in human umbilical vein endothelial cells (HUVECs) as they invade into 3D collagen matrices showed that SPP1 is the most highly upregulated extracellular matrix protein (Bayless, unpublished results). Previous research conducted using SPP1 knockout mice further supports a role for SPP1 in development and repair of the vasculature associated with the skeletal and cardiovascular systems [19, 20]. Additionally, SPP1 has been shown to induce angiogenesis in murine tumor cells [17, 18]. SPP1 is an important regulator of endothelial cell invasion and subsequent angiogenesis in multiple species and tissues. Indeed, recent studies in the pig have identified considerable levels of SPP1 mRNA associated with the endothelium of endometrial vasculature (Johnson, unpublished results). Collectively, results of these in vitro and in vivo studies suggest that stromal SPP1 is an excellent candidate for influencing endometrial angiogenesis and subsequent hematotrophic support of the conceptus in sheep.
In summary, levels of P4 in pregnancy are sufficient to induce SPP1 expression by endometrial GE and stroma, but paracrine and/or physical effects of the conceptus enhance expression of SPP1 in each of these cell types. However, SPP1 likely exerts distinct effects in compartment-specific regions of the ovine uterus to increase both histotrophic (GE) and hematotrophic (stromal) support of implantation, placentation, fetal growth, and development. A biologically active fragment of SPP1 is secreted by GE throughout pregnancy, and it functions as an adhesive extracellular matrix glycoprotein, capable of binding mononuclear trophoblasts of the placenta to LE of the uterus via integrins. In the stromal compartment of the endometrium (placentomal and interplacentomal), SPP1 increases in unison with growth of blood vessels and may play a role in endothelial cell invasion and sprouting that is necessary for provision of hematotrophic nutrition to the conceptus. Clearly, the extensive temporal and spatial pattern of SPP1 demonstrated both in vivo and in vitro implies physiological relevance to several mammalian species that show similar patterns of SPP1 expression, including goats, pigs, mice, and primates [10, 39–42].
References
- 1. Spencer TE, Johnson GA, Bazer FW, Burghardt RC. Fetal-maternal interactions during the establishment of pregnancy in ruminants. Soc Reprod Fertil Suppl 2007; 64:379–396. [DOI] [PubMed] [Google Scholar]
- 2. Bazer FW. Uterine protein secretions: relationship to development of the conceptus. J Anim Sci 1975; 41:1376–1382. [DOI] [PubMed] [Google Scholar]
- 3. Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod 2001; 65:1311–1323. [DOI] [PubMed] [Google Scholar]
- 4. Gray CA, Burghardt RC, Johnson GA, Bazer FW, Spencer TE. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002; 124:289–300. [PubMed] [Google Scholar]
- 5. Roberts RM, Bazer FW. The functions of uterine secretions. J Reprod Fertil 1988; 82:875–892. [DOI] [PubMed] [Google Scholar]
- 6. Johnson GA, Burghardt RC, Bazer FW, Spencer TE. Osteopontin: roles in implantation and placentation. Biol Reprod 2003; 69:1458–1471. [DOI] [PubMed] [Google Scholar]
- 7. Apparao KB, Murray MJ, Fritz MA, Meyer WR, Chambers AF, Truong PR, Lessey BA. Osteopontin and its receptor alphavbeta(3) integrin are coexpressed in the human endometrium during the menstrual cycle but regulated differentially. J Clin Endocrinol Metab 2001; 86:4991–5000. [DOI] [PubMed] [Google Scholar]
- 8. Johnson GA, Spencer TE, Burghardt RC, Bazer FW. Ovine osteopontin: I. Cloning and expression of messenger ribonucleic acid in the uterus during the periimplantation period. Biol Reprod 1999; 61:884–891. [DOI] [PubMed] [Google Scholar]
- 9. Johnson GA, Spencer TE, Burghardt RC, Taylor KM, Gray CA, Bazer FW. Progesterone modulation of osteopontin gene expression in the ovine uterus. Biol Reprod 2000; 62:1315–1321. [DOI] [PubMed] [Google Scholar]
- 10. White FJ, Burghardt RC, Hu J, Joyce MM, Spencer TE, Johnson GA. Secreted phosphoprotein 1 (osteopontin) is expressed by stromal macrophages in cyclic and pregnant endometrium of mice, but is induced by estrogen in luminal epithelium during conceptus attachment for implantation. Reproduction 2006; 132:919–929. [DOI] [PubMed] [Google Scholar]
- 11. Johnson GA, Burghardt RC, Spencer TE, Newton GR, Ott TL, Bazer FW. Ovine osteopontin: II. Osteopontin and alpha(v)beta(3) integrin expression in the uterus and conceptus during the periimplantation period. Biol Reprod 1999; 61:892–899. [DOI] [PubMed] [Google Scholar]
- 12. Spencer TE, Johnson GA, Bazer FW, Burghardt RC. Implantation mechanisms: insights from the sheep. Reproduction 2004; 128:657–668. [DOI] [PubMed] [Google Scholar]
- 13. Spencer TE, Johnson GA, Burghardt RC, Bazer FW. Progesterone and placental hormone actions on the uterus: insights from domestic animals. Biol Reprod 2004; 71:2–10. [DOI] [PubMed] [Google Scholar]
- 14. Johnson GA, Burghardt RC, Joyce MM, Spencer TE, Bazer FW, Gray CA, Pfarrer C. Osteopontin is synthesized by uterine glands and a 45-kDa cleavage fragment is localized at the uterine-placental interface throughout ovine pregnancy. Biol Reprod 2003; 69:92–98. [DOI] [PubMed] [Google Scholar]
- 15. Reynolds LP, Killilea SD, Redmer DA. Angiogenesis in the female reproductive system. FASEB J 1992; 6:886–892. [PubMed] [Google Scholar]
- 16. Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci 1995; 73:1839–1851. [DOI] [PubMed] [Google Scholar]
- 17. Takahashi F, Akutagawa S, Fukumoto H, Tsukiyama S, Ohe Y, Takahashi K, Fukuchi Y, Saijo N, Nishio K. Osteopontin induces angiogenesis of murine neuroblastoma cells in mice. Int J Cancer 2002; 98:707–712. [DOI] [PubMed] [Google Scholar]
- 18. Hirama M, Takahashi F, Takahashi K, Akutagawa S, Shimizu K, Soma S, Shimanuki Y, Nishio K, Fukuchi Y. Osteopontin overproduced by tumor cells acts as a potent angiogenic factor contributing to tumor growth. Cancer Lett 2003; 198:107–117. [DOI] [PubMed] [Google Scholar]
- 19. Asou Y, Rittling SR, Yoshitake H, Tsuji K, Shinomiya K, Nifuji A, Denhardt DT, Noda M. Osteopontin facilitates angiogenesis, accumulation of osteoclasts, and resorption in ectopic bone. Endocrinology 2001; 142:1325–1332. [DOI] [PubMed] [Google Scholar]
- 20. Zhao X, Johnson JN, Singh K, Singh M. Impairment of myocardial angiogenic response in the absence of osteopontin. Microcirculation 2007; 14:233–240. [DOI] [PubMed] [Google Scholar]
- 21. Leali D, Dell'Era P, Stabile H, Sennino B, Chambers AF, Naldini A, Sozzani S, Nico B, Ribatti D, Presta M. Osteopontin (Eta-1) and fibroblast growth factor-2 cross-talk in angiogenesis. J Immunol 2003; 171:1085–1093. [DOI] [PubMed] [Google Scholar]
- 22. Johnson GA, Bazer FW, Jaeger LA, Ka H, Garlow JE, Pfarrer C, Spencer TE, Burghardt RC. Muc-1, integrin, and osteopontin expression during the implantation cascade in sheep. Biol Reprod 2001; 65:820–828. [DOI] [PubMed] [Google Scholar]
- 23. Fisher LW, Stubbs JT III, Young MF. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand Suppl 1995; 266:61–65. [PubMed] [Google Scholar]
- 24. Spencer TE, Stagg AG, Joyce MM, Jenster G, Wood CG, Bazer FW, Wiley AA, Bartol FF. Discovery and characterization of endometrial epithelial messenger ribonucleic acids using the ovine uterine gland knockout model. Endocrinology 1999; 140:4070–4080. [DOI] [PubMed] [Google Scholar]
- 25. Bayless KJ, Davis GE, Meininger GA. Isolation and biological properties of osteopontin from bovine milk. Protein Expr Purif 1997; 9:309–314. [DOI] [PubMed] [Google Scholar]
- 26. Dunlap KA, Palmarini M, Varela M, Burghardt RC, Hayashi K, Farmer JL, Spencer TE. Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc Natl Acad Sci U S A 2006; 103:14390–14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bayless KJ, Meininger GA, Scholtz JM, Davis GE. Osteopontin is a ligand for the alpha4beta1 integrin. J Cell Sci 1998; 111(pt 9):1165–1174. [DOI] [PubMed] [Google Scholar]
- 28. Davis GE, Camarillo CW. Regulation of integrin-mediated myeloid cell adhesion to fibronectin: influence of disulfide reducing agents, divalent cations and phorbol ester. J Immunol 1993; 151:7138–7150. [PubMed] [Google Scholar]
- 29. Bird IM, Sullivan JA, Di T, Cale JM, Zhang L, Zheng J, Magness RR. Pregnancy-dependent changes in cell signaling underlie changes in differential control of vasodilator production in uterine artery endothelial cells. Endocrinology 2000; 141:1107–1117. [DOI] [PubMed] [Google Scholar]
- 30. Bayless KJ, Davis GE. Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. Biochem Biophys Res Commun 2003; 312:903–913. [DOI] [PubMed] [Google Scholar]
- 31. Sorensen ES, Rasmussen LK, Moller L, Jensen PH, Hojrup P, Petersen TE. Localization of transglutaminase reactive glutamine residues in bovine osteopontin. Biochem J 1994; 304:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Christensen B, Kazanecki CC, Petersen TE, Rittling SR, Denhardt DT, Sorensen ES. Cell type-specific post-translational modifications of mouse ostepontin are associated with different adhesive properties. J Biol Chem 2007; 282:19463–19472. [DOI] [PubMed] [Google Scholar]
- 33. Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem 2007; 102:912–924. [DOI] [PubMed] [Google Scholar]
- 34. Spencer TE, Gray A, Johnson GA, Taylor KM, Gertler A, Gootwine E, Ott TL, Bazer FW. Effects of recombinant ovine interferon tau, placental lactogen, and growth hormone on the ovine uterus. Biol Reprod 1999; 61:1409–1418. [DOI] [PubMed] [Google Scholar]
- 35. Noel S, Herman A, Johnson GA, Gray CA, Stewart MD, Bazer FW, Gertler A, Spencer TE. Ovine placental lactogen specifically binds to endometrial glands of the ovine uterus. Biol Reprod 2003; 68:772–780. [DOI] [PubMed] [Google Scholar]
- 36. Senger DR, Perruzzi CA. Cell migration promoted by a potent GRGDS-containing thrombin-cleavage fragment of osteopontin. Biochim Biophys Acta 1996; 1314:13–24. [DOI] [PubMed] [Google Scholar]
- 37. Johnson GA, Burghardt RC, Joyce MM, Spencer TE, Bazer FW, Pfarrer C, Gray CA. Osteopontin expression in uterine stroma indicates a decidualization-like differentiation during ovine pregnancy. Biol Reprod 2003; 68:1951–1958. [DOI] [PubMed] [Google Scholar]
- 38. Risau W. Mechanisms of angiogenesis. Nature 1997; 386:671–674. [DOI] [PubMed] [Google Scholar]
- 39. White FJ, Ross JW, Joyce MM, Geisert RD, Burghardt RC, Johnson GA. Steroid regulation of cell specific secreted phosphoprotein 1 (osteopontin) expression in the pregnant porcine uterus. Biol Reprod 2005; 73:1294–1301. [DOI] [PubMed] [Google Scholar]
- 40. Joyce MM, Gonzalez JF, Lewis S, Woldesenbet S, Burghardt RC, Newton GR, Johnson GA. Caprine uterine and placental osteopontin expression is distinct among epitheliochorial implanting species. Placenta 2005; 26:160–170. [DOI] [PubMed] [Google Scholar]
- 41. Garlow JE, Ka H, Johnson GA, Burghardt RC, Jaeger LA, Bazer FW. Analysis of osteopontin at the maternal-placental interface in pigs. Biol Reprod 2002; 66:718–725. [DOI] [PubMed] [Google Scholar]
- 42. von Wolff M, Bohlmann MK, Fiedler C, Ursel S, Strowitzki T. Osteopontin is up-regulated in human decidual stromal cells. Fertil Steril 2004; 81(suppl 1):741–748. [DOI] [PubMed] [Google Scholar]