Abstract
This pilot study evaluated the use of smartphone ecological momentary assessments (EMA) for self-monitoring to optimize treatment outcomes among gay and bisexual men enrolled in an outpatient methamphetamine abuse treatment service program. Participants (N = 34) received EMA prompts five times daily to self-monitor their methamphetamine use, cravings, sexual risk behaviors, and associated triggers and affect throughout the 8-week treatment program. Participants were randomized into either a self-directed condition with access to a web-based EMA response visualization dashboard (“EMA+Dashboard”; n = 16); or, a counselor-supported condition incorporating weekly, 30-minute, one-on-one counseling sessions to review and discuss the participant’s self-monitoring data on the dashboard (“EMA+Dashboard+Counselor”; n = 18). Pilot participants were compared with historical controls (n = 102) as the reference group in multiple regression analyses to assess the impact of the two study conditions on the treatment service program outcomes. Study participants with weekly counseling (EMA+Dashboard+Counselor) exhibited significantly greater reductions in the number of condomless anal intercourse episodes than historical controls (IRR = 0.02, 95% CI [0.00, 0.30]), whereas the reduction was of similar magnitude as controls in the EMA+Dashboard self-directed condition (IRR = 0.23, 95% CI [0.02, 3.56]). Treatment effects were not significant for comparisons between the two study conditions and historical controls for self-reported methamphetamine use (EMA+Dashboard: IRR = 1.06, 95% CI [0.32, 3.49]; EMA+Dashboard+Counselor: IRR = 0.46, 95% CI [0.14, 1.49]), number of male partners (EMA+Dashboard: IRR = 1.02, 95% CI [0.39, 2.61]; EMA+Dashboard+Counselor: IRR = 0.54, 95% CI [0.20, 1.45]), and the likelihood of providing a urine sample that tested positive for methamphetamine metabolites (EMA+Dashboard : OR = 1.00, 95% CI [0.79, 1.25]; EMA+Dashboard+Counselor: OR = 0.93, 95% CI [0.74, 1.16]). The pilot study provides preliminary evidence that the treatment outcome for condomless anal intercourse can be improved through a combination of smartphone- and counselor-assisted self-monitoring.
Keywords: methamphetamine, MSM, sexual risk behavior, intervention, ecological momentary assessment, self-monitoring
1. Introduction
1.1. Methamphetamine Use and HIV among Gay and Bisexual Men in the U.S.
In the United States (U.S.), methamphetamine use is more prevalent among gay and bisexual men (GBM) than among heterosexual males. According to the 2015 National Survey on Drug Use and Health, 3.4% of GBM aged 18 or older used methamphetamine in the past year, compared to 0.9% of adult heterosexual men (Medley et al., 2016). Comprehensive epidemiological data on geographic patterns in methamphetamine use of GBM is lacking, but smaller-scale behavioral studies suggest that methamphetamine use is particularly elevated among GBM in major urban centers such as Los Angeles or New York City (Grov, Bimbi, Nanin, & Parsons, 2006; Halkitis, Levy, Moreira, & Ferrusi, 2014; Reback, Fletcher, Shoptaw, & Grella, 2013; Solomon, Halkitis, Moeller, & Pappas, 2012).
The adverse effects of chronic methamphetamine use are wide-ranging, including neurocognitive impairments (Curtin et al., 2015; Dean, Groman, Morales, & London, 2013), psychiatric illness (Salo et al., 2011), and increased risk of cardiovascular disease and stroke (Huang et al., 2016). Among GBM and other men who have sex with men (MSM), methamphetamine use has also been shown to increase engagement in unsafe sexual behavior and risk for infection with HIV (Grov et al., 2014a; Hirshfield, Remien, Walavalkar, & Chiasson, 2004; Nakamura, Mausbach, Ulibarri, Semple, & Patterson, 2011; Shoptaw & Reback, 2006). This deleterious combination of both direct (e.g., neurological, dental, cardiovascular) and secondary (i.e., increased risk for infection with HIV) consequences of methamphetamine use for GBM in the U.S. has created a pressing need for efficacious and evidence-based interventions specifically tailored to methamphetamine-using GBM engaged in HIV sexual risk-taking.
1.2. Gay-specific Cognitive Behavioral Therapy (GCBT) for Methamphetamine-using GBM
Shoptaw and Reback (Reback & Shoptaw, 2014; Shoptaw et al., 2005, 2008) developed and manualized a gay-specific, cognitive behavioral therapy (GCBT) small-group intervention for GBM that supplements standard cognitive-behavioral techniques (Rawson et al., 1995) with gay male cultural references and targets both methamphetamine use and HIV-related sexual risk behaviors. Two randomized controlled trials demonstrated efficacy of the GCBT intervention, showing significant and sustained reductions in methamphetamine use, number of male sex partners, and unprotected anal sex (Shoptaw et al., 2005, 2008). A modified version of the GCBT intervention, named Getting Off: A Behavioral Treatment Intervention for Gay and Bisexual Male Methamphetamine Users, was designed to increase cost and time effectiveness (Reback & Shoptaw, 2014) for application in community settings. The Getting Off outpatient treatment program comprises 24 group sessions over an 8-week time period. An ancillary low-cost contingency management intervention provides incentives to participants who submit methamphetamine-metabolite-free urine samples. Urine drug screenings are administered thrice weekly for the duration of the program. The Getting Off intervention was shown to be similarly efficacious in reducing methamphetamine use and sexual risk behaviors as the original GCBT intervention (Reback & Shoptaw, 2014).
1.3. Augmenting Getting Off through Ecological Momentary Assessment Self-Monitoring
Evidence-based treatment interventions, such as the Getting Off intervention for GBM methamphetamine users, are well-poised to take advantage of recent advancements in mobile, technology-based health interventions. Self-monitoring, a core element of evidence-based interventions (Chorpita, Daleiden, & Weisz, 2005; Michie et al., 2013) and theories of behavior change and self-regulation (Bandura, 1991; Carver, 1979; Kanfer, 1970), has been made more feasible and accessible through the widespread use of mobile phones and may enhance intervention effects. There is modest meta-analytic evidence that self-monitoring supports self-management of diabetes (Warsi, Wang, LaValley, Avorn, & Solomon, 2004) and obesity (Burke, Wang, & Sevick, 2011). Substance abuse intervention research suggests that repeated assessments may increase self-monitoring and thereby improve targeted outcomes (Jenkins, McAlaney, & McCambridge, 2009; McCambridge, 2009). A similar effect may also underlie sexual risk reduction on the order of 15% to up to 30% that was observed in control groups in some HIV prevention trials (Healthy Living Project Team, 2007; Kamb et al., 1998; NIMH Multisite HIV Prevention Trials Group, 1998).
Ecological Momentary Assessment (EMA) is an intensive self-report methodology involving multiple time- and/or event-based prompts for reporting experiences and behaviors throughout a day in natural settings, and has been used extensively in basic behavioral research on substance use (Shiffman, Stone, & Hufford, 2008; Shiffman, 2009). EMA researchers have noted methodological challenges of potential reactivity (i.e., changes in awareness and behaviors or “assessment effects”) in response to intensive self-assessments, particularly when a sample population is motivated to change (Heron & Smyth, 2010; Shiffman et al., 2008).
EMA methods have been used extensively in tobacco and alcohol research, including with in-treatment and in-recovery populations, but relatively few studies have examined other substances such as heroin and cocaine (Serre, Fatseas, Swendsen, & Auriacombe, 2015). For example, EMA was instrumental in assessing the impact of cognitive, affective, and motivational factors (Huhn et al., 2016; Marhe, Waters, van de Wetering, & Franken, 2013; Waters, Marhe, & Franken, 2012) as well as stress (Preston et al., 2017) on drug craving and use among heroin- and/or cocaine-dependent patients. One study examined the feasibility and acceptability of EMA in a sample of four male and two female adult methamphetamine-dependent users (Galloway, Didier, Garrison, & Mendelson, 2008). Approximately 30 EMA studies targeted sexual behavior, and a subsample of these included GBM. Wray, Kahler, and Monti (2016) found EMA to be feasible and acceptable in a sample of twelve high-risk MSM who reported on sexual behaviors and substance use. To date, no EMA study has worked with GBM methamphetamine users at high-risk for HIV acquisition and transmission.
EMA and reliable self-monitoring have historically been both costly and labor intensive, relying on paper-based methods, instructions and alarms (i.e., watches or pagers), or, more recently, early technological portals through personal digital assistants, interactive voice response calls or websites. The rapid development and proliferation of smartphone technology over the past decade has enabled EMA and self-monitoring methods to become affordable, portable, and scalable. Acceptability of technology-based interventions is high among GBM who, as early as the 1990s, became vanguard users of emerging digital technologies by adopting the Internet for sexual purposes (e.g., finding sex partners, seeking sexual health information, pornography Grov, Breslow, Newcomb, Rosenberger, & Bauermeister, 2014b). Recent data indicate more widespread use of mobile technologies by GBM than in other adult populations (Grov et al., 2014b).
Prior research has demonstrated promising results in the adoption of technology-based self-monitoring among MSM. In a 6-month prospective study with young adult MSM, participants who completed web-based diaries about their sexual behaviors reported fewer unprotected anal sex acts in retrospective surveys and had fewer new HIV/STI diagnoses than participants without diaries (Glick, Winer, & Golden, 2013; Horvath, Beadnell, & Bowen, 2007). A mixed methods study of self-monitoring and web-dashboards for substance use, sexual risks, medication adherence, and quality of life among people living with HIV suggest that multiple theory-linked mechanisms are at play in supporting behavior change and self-management, including increased awareness of behaviors and triggers, comparison to a personal standard or social norm, reminders, goal progress tracking and accountability, self-rewards, and reinforcement (Swendeman et al., 2015). In summary, theory and emerging empirical research suggest that technology-based self-monitoring using EMA methods may serve to enhance impacts of traditional interventions.
The aim of the present study was to evaluate pilot data on the use of EMA for optimizing treatment outcomes among GBM enrolled in the Getting Off outpatient methamphetamine abuse treatment service program in Los Angeles. The pilot study examined the feasibility, acceptability, and potential utility of EMA using smartphones and an open-source mobile health application platform, accompanied by a web-based visualization dashboard, with and without counseling. It was hypothesized that EMA self-monitoring would optimize methamphetamine outpatient treatment outcomes by prompting participants to self-monitor their methamphetamine use, cravings, HIV sexual risk behaviors, and associated triggers and affect throughout the intervention period. The postulated effects of EMA were evaluated by comparing study participants’ treatment outcomes with the outcomes of historical controls who participated in the same Getting Off outpatient program. To our knowledge, this is the first study to adopt EMA as a self-monitoring intervention support strategy with methamphetamine users and, specifically, the high-risk population of GBM methamphetamine users.
2. Materials and Methods
2.1. Participants
Pilot participants (N = 34) were enrolled from December 2013 through July 2014. Eligibility criterion was within the first week of enrollment in the Getting Off methamphetamine abuse outpatient treatment program. The eligibility criteria for the Getting Off program was broad, given that Getting Off was a community-based service program and not a research study: 1) identified as a gay or bisexual male; 2) used methamphetamine in the previous 12 months; and, 3) seeking treatment for their methamphetamine use.
2.2. Procedures
2.2.1. Recruitment and enrollment
Participants were recruited from the Getting Off methamphetamine abuse treatment program (Reback & Shoptaw, 2014) in Los Angeles, California. Getting Off participants were invited to participate in the EMA pilot study at the time that they enrolled, and could enroll in the study at any time during their first week of participation in Getting Off. Getting Off participants were informed that participation in the EMA study was voluntary and that participation in the Getting Off treatment program was not dependent upon participation in the EMA study. Interested participants met with a research assistant (RA) in a private room who answered all questions and provided written informed consent.
Participants were assisted by the RA on how to install the EMA app, set up a personalized time frame for prompts (alarms) to complete the EMAs, and how to access and utilize the password protected study visualization dashboard website. Each participant received a unique study login ID and password to securely access the app and dashboard website. Participants used their personal cell phones. If a participant did not own a cell phone that was compatible with the EMA mobile app, either an Android or iPhone, and did not have a minimum of 8 weeks left on their contract with an unlimited data plan, he was lent an Android study cell phone plus two chargers, for the duration of his participation in the study (n = 17). A study cell phone had a data plan but did not have a calling plan.
2.2.2. Intervention phase
The web-based dashboard displayed all EMA survey responses by time and by location. The goal of the time and location feature was to enable participants to look for time and location trends and patterns of association between different EMA survey responses and methamphetamine use and/or sexual risk behaviors. The RA monitored EMA data on a daily basis (during weekdays) and contacted, via a phone call or text message, any participant who did not complete an EMA survey for three consecutive days. The RA also provided trouble-shooting support for any technical problems that a participant experienced in using the app or dashboard.
2.2.3. Incentives and compensation
Participants were compensated $25 for completing the 4-week follow-up evaluation, $50 for completing the 8-week follow-up evaluation, and $40 for completing the 12-week follow-up evaluation. Additionally, there was an increasingly valuable incentive schedule for EMA surveys completed. Participants earned up to $20 every two weeks during the 8-week study intervention, based on how many EMA surveys were completed. There were 5 surveys each day for a total of 70 surveys every two weeks. Table 1 illustrates the incentive schedule for survey completion. Participants were compensated in gift cards, no cash was given. Study procedures were approved by the Friends Research Institute’s and University of California, Los Angeles Institutional Review Boards.
Table 1.
Incentive Schedule for EMA Survey Completion
| Participants could earn: | If participant completed: | # of surveys the participant must complete every two weeks: |
|---|---|---|
| $20.00 | 90% of the surveys or greater | 63 to 70 surveys |
| $17.50 | Between 80% and 89% of the surveys | 56 to 62 surveys |
| $15.00 | Between 70% and 79% of the surveys | 49 to 55 surveys |
| $12.50 | Between 60% and 69% of the surveys | 42 to 48 surveys |
| $10.00 | Between 50% and 59% of the surveys | 35 to 41 surveys |
| $7.50 | Between 40% and 49% of the surveys | 28 to 34 surveys |
| $5.00 | Between 25% and 39% of the surveys | 17 to 27 surveys |
| $2.50 | Between 10% and 24% of the surveys | 7 to 16 surveys |
| $1.00 | Between 1% and 9% of the surveys | 1 to 6 surveys |
| -0- | No surveys | No surveys |
2.3. Interventions
Participants were randomized either to: 1) cell phone EMA self-monitoring plus a web-based visualization dashboard (“EMA+Dashboard”; n = 16); or, 2) the same intervention as above plus a 30-minute, once a week, one-on-one counseling session to review and discuss the participant’s self-monitoring data on the web dashboard (“EMA+Dashboard+Counselor”; n = 18). The EMA self-monitoring and counseling activities extended over the 8-week Getting Off intensive outpatient treatment program.
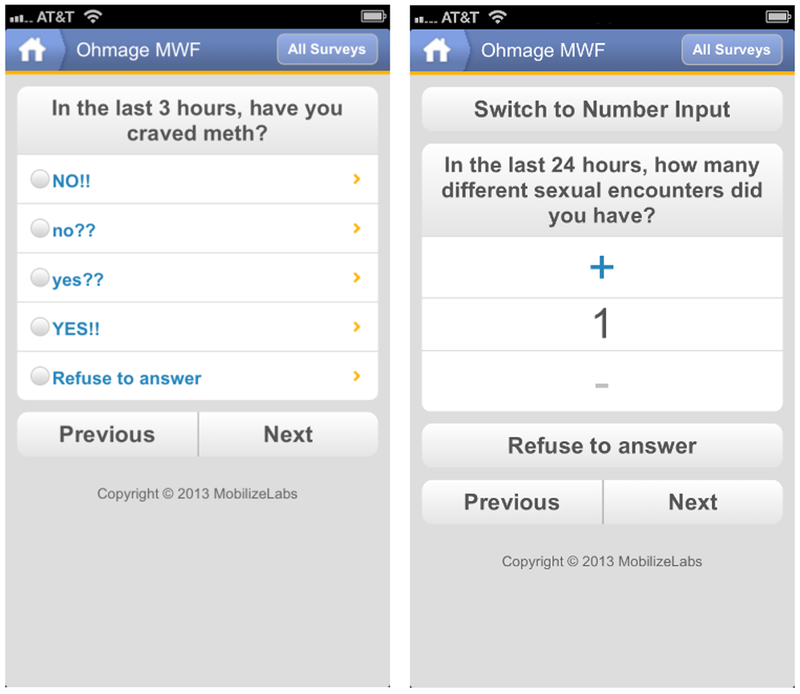
All participants were prompted to complete EMA surveys five times each day between the hours of 9:00 AM and 12:00 AM, approximately every three hours, unless the participant preferred to schedule alerts for a different 15-hour period (e.g., between 12:00 noon and 3:00 AM). Each EMA survey covered the prior 3 hours and assessed the following domains with 6 to 23 questions (depending on responses): 1) external triggers, 2) internal triggers/affect, 3) substance use cravings and substance use, and 4) sexual encounters (Figure 1). One of the five EMA surveys, administered between the hours of 3:00 PM and 6:00 PM, included additional questions on sexual encounters in the last 24 hours (reported on collectively in 1 to 8 questions, depending on responses) and HIV and/or psychotropic medications adherence (if appropriate; 1 question for each type of medication). EMA surveys took less than three minutes to complete and the sexual encounter questions took less than two minutes to complete for each sexual encounter reported. Each EMA entry was automatically time-stamped and location-stamped using the cell phone’s GPS, which enabled the dashboard to display data by time and by location. Additionally, participants could complete an EMA whenever they chose, for example, if a participant wanted to report a trigger, craving, substance use, or sexual encounter just after it occurred. Participants could also turn off the EMA application’s survey prompt notifications (reminder alarms) if they did not want to be disturbed.
Figure 1.
Examples of EMA survey questions administered through the mobile app about craving for methamphetamine (left) and number of sexual encounters (right).
2.3.1. EMA+Dashboard
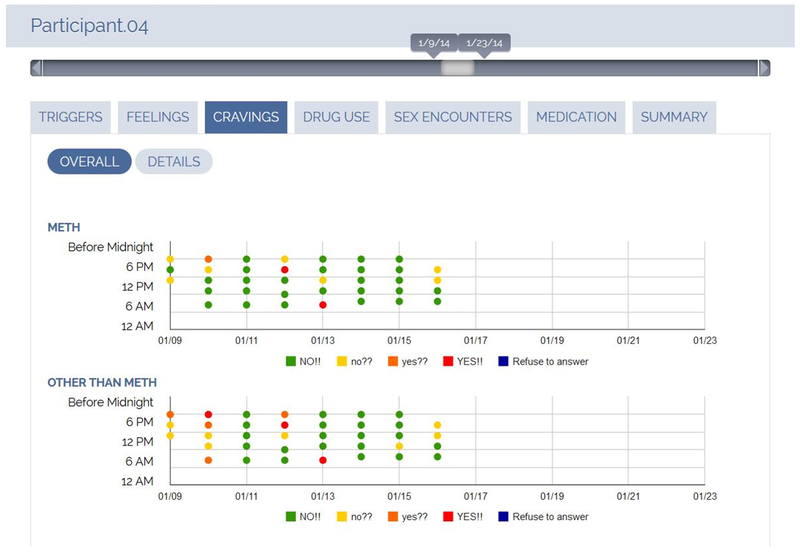
Participants randomly assigned to the “EMA+Dashboard” condition were asked to complete EMA surveys when alerted by the app five times a day during the 8-week Getting Off outpatient treatment program. Participants were also encouraged to complete EMA surveys whenever an event occurred that was covered in the survey questions such as experiencing a substance use craving, a substance use episode, a sexual encounter. The web-based visualization dashboard provided a detailed chronological review of a participant’s momentary assessments, structured by domain (Figure 2). Participants were able to review their personal dashboard at any time during the 8-week intervention.
Figure 2.
Example of the visual dashboard displaying a participant’s responses to EMA survey questions about craving for methamphetamine and other substances over a two-week period.
2.3.2. EMA+Dashboard+Counselor
Participants randomly assigned to the “EMA+Dashboard+Counselor” condition participated in the same activities and dashboard access as the “EMA+Dashboard” condition. Additionally, participants in the “EMA+Dashboard+Counselor” condition met with the RA (cross-trained as a certified substance abuse counselor) for an on-site, one-on-one counseling session once a week for up to 30 minutes over the 8-week EMA study and Getting Off program. During the counseling sessions, the RA facilitated a discussion on the participant’s progress by reviewing the web-based dashboard and EMA survey question responses and urinalysis results. The counseling sessions focused on identifying trends and patterns of association between different EMA surveys and urinalysis results through visual inspection of the dashboard, highlighting successes and challenges, and problem solving triggers, cravings, methamphetamine use, and high-risk sex.
2.4. Matched Historical Control Group
A historical matched control group was selected from a pool of 407 participants who were enrolled in the Getting Off program between January 1, 2011 and June 30, 2014. Procedurally, there was no difference between historical controls and study participants, except for the addition of EMA for study participants. Historical controls were selected with a nearest-neighbor propensity score algorithm (Ho, Imai, King, & Stuart; 2007, 2011) that determined the three best control matches for each of the 34 EMA pilot participants, resulting in a sample of 102 historical control participants. A logistic regression model was used to estimate the propensity score, defined as the probability of being in the EMA groups, conditional on the covariates age, race/ethnicity (Caucasian/White, African American/Black, Hispanic/Latino, multiethnic/other), HIV serostatus (positive, negative), and self-reported methamphetamine use level (addict, abuser, problem user, social user). In addition, we imposed exact-matching restrictions on all covariates except age.
2.5. Measures
2.5.1. Admission Form
Originally developed for substance abuse treatment (Rawson et al., 1995) and modified, in consultation with the developers, for use among methamphetamine-using MSM (Shoptaw et al., 2005), the Admission Form was administered at baseline only and collected sociodemographic data (e.g., age, race/ethnicity, educational attainment, income, HIV status, and methamphetamine use [month and year of first use, number of days used in past 30 days, method of use]).
2.5.2. Behavioral Questionnaire – Amphetamine (BQA)
The BQA gathers information on HIV-related drug and sexual risk behaviors (Chesney, Chambers, & Kahn, 1997; Twitchell, Huber, Reback, & Shoptaw, 2002). The BQA was administered at baseline, 4-, 8-, and 12-week follow-ups to obtain information on sexual risk behaviors, including the number of episodes (0-999) of condomless anal intercourse with non-primary partner(s) and number of sexual partners (0-999) in the past 30 days. Participants provided a self-descriptive report on their level of methamphetamine use (i.e., addict, abuser, problem user, social user, non-user) by choosing between five single-sentence statements (e.g., “A crystal methamphetamine addict or recovering crystal methamphetamine addict,” “A crystal methamphetamine abuser”).
2.5.3. Substance Use Form
The Substance Use Form was administered at baseline, 4-, 8-, and 12-week follow-ups to obtain information on substance use, including injection, in the past 30 days.
2.5.4. Urine drug screening
Urine samples were collected three times per week during the 8-week treatment period and once at 12-week follow-up and analyzed for metabolites of methamphetamine. Sample results were coded qualitatively as positive or negative using a cutoff of 1000 ng/ml (W254 Multi-Drug Test 5 in 1, W.H.P.M., Inc., Irwindale, CA, USA).
2.5.5. Retention
Treatment retention was assessed as the last week (0 through 8) of clinic attendance (operationalized as either an attended Getting Off group session or a submitted urine sample).
2.6. Data Analysis
Participants in the EMA+Dashboard condition, the EMA+Dashboard+Counselor condition, and the matched historical control group were compared at baseline (week 1) and at the last follow-up (week 12) time point, which was four weeks following Getting Off treatment completion, on behavioral measures of methamphetamine use and sexual risk behaviors. Analyses for treatment outcome measures based on the results of the urine drug screenings for methamphetamine metabolites were conducted for the intervention period (weeks 1-8) and at follow-up. Specifically, the Joint Probability Index (Ling et al., 1997) is a group-level index that was determined separately for each condition at weeks 8 and 12, respectively. In each instance, it was calculated by dividing the sum of methamphetamine metabolite-free samples across participants by the product of the number of scheduled samples per participant and the number of participants enrolled in the respective condition. The Treatment Effectiveness Score (Ling et al., 1997) equaled the total number of methamphetamine metabolite-free samples per participant. With three scheduled urine samples per week during the 8-week intervention period, a participant’s Treatment Effectiveness Score could range from 0 to 24. Additional outcome measures included the percentage of methamphetamine metabolite-free samples per participant during the 8-week intervention period, the number of methamphetamine metabolite-free samples at follow-up, and the longest consecutive sequence of methamphetamine metabolite-free samples per participant during the intervention period, ranging from 0 to 24. All analyses were based on the intention-to-treat sample, that is, all participants who were enrolled in the study were included in the analysis. In case of missing data, listwise deletion was used in each analysis. Univariate comparisons were carried out using t-test for continuous variables, Kruskal-Wallis (KW) test for count data, and chi-square test for categorical variables or Fisher’s exact test when at least one cell of the contingency table contained fewer than five observations.
Primary study hypotheses concerning the effect of treatment condition on methamphetamine use and sexual risk behaviors were tested in mixed-effects negative binomial regression models with treatment condition (EMA+Dashboard; EMA+Dashboard+Counselor; matched historical control group) and time (week 1, week 12) as predictors. The likelihood of providing a urine sample tested positive for methamphetamine metabolites was investigated in a mixed-effects logistic regression analyses with treatment condition and time (weeks 1-8) as predictors. The significance level for all statistical tests was set to α = .05. All analyses were carried out using the R language and environment for statistical computing, version 3.3.3.
3. Results
3.1. Baseline Characteristics
Table 2 provides a descriptive summary of baseline sociodemographic and methamphetamine use characteristics of the participants enrolled in the EMA pilot (full sample) and the matched historical control group. As a consequence of the matching procedure, both samples were identical in the distribution of race/ethnicity (predominantly Caucasian/White and Hispanic/Latino), had the same rate of HIV-seropositivity (47.1%), and contained the same relative number of methamphetamine-use levels (predominantly self-reported addicts and abusers). There were also no significant group differences in average age, education, lifetime methamphetamine use, income distribution, or in the relative frequency of injection methamphetamine use.
Table 2.
Participant Characteristics by Sociodemographics, HIV Status, and Methamphetamine Use
| Variable | Combined EMA N = 34 |
Historical Matches N = 102 |
Test statistic |
|---|---|---|---|
| Age (years), M (SD)* | 40.6 (9.3) | 40.4 (8.6) | t(53) = −0.08 |
| Race/ethnicity, N (%)* | |||
| White | 12 (35.3%) | 36 (35.3%) | — |
| Hispanic/Latino | 11 (32.4%) | 33 (32.4%) | |
| Black/African American | 7 (20.6%) | 21 (20.6%) | |
| Multi/other | 4 (11.8%) | 12 (11.8%) | |
| HIV seropositivity, N (%)* | 16 (47.1%) | 48 (47.1%) | — |
| Methamphetamine-use level, N (%)* | |||
| Addict | 21 (61.8%) | 63 (61.8%) | — |
| Abuser | 8 (23.5%) | 24 (23.5%) | |
| Problem user | 3 (8.8%) | 9 (8.8%) | |
| Social user | 2 (5.9%) | 6 (5.9%) | |
| Education (years), M (SD) | 13.8 (2.4) | 13.8 (2.6) | t(60) = 0.02 |
| Income (annual), N (%)a | |||
| < $15,001 | 28 (82.4%) | 71 (69.6%) | FET p = .459 |
| $15,001-$30,000 | 3 (8.8%) | 16 (15.7%) | |
| $30,001-$60,000 | 1 (2.9%) | 5 (4.9%) | |
| > $60,000 | 1 (2.9%) | 10 (9.8%) | |
| Reported methamphetamine use | |||
| Lifetime methamphetamine use (in years), M (SD) | 13.6 (8.0) | 14.0 (7.6) | t(54) = 0.27 |
| Any injection methamphetamine use, N (%) | 22 (64.7%) | 57 (55.9%) | χ2(1) = 0.82 |
Note. FET = Fisher’s exact test.
Historical matches were selected based on the variables age, race/ethnicity, HIV seropositivity, and self-described methamphetamine-use level. No statistical tests were conducted for race/ethnicity, HIV seropositivity, and methamphetamine-use level because the matching algorithm was specified to create identical distributions in these variables. There were no significant differences between the two EMA treatment conditions in any of the matching covariates (all ps > .3).
n(EMA) = 33.
3.2. Self-Reported Methamphetamine Use
Univariate analyses showed no significant differences between treatment conditions for the reported number of days of methamphetamine use at baseline (week 1) or at follow-up (week 12; Table 3). The potential differences in the treatment effect were evaluated, i.e., differences in the observed change between baseline and follow-up, in a mixed-effects negative binomial regression (Table 4, Model I). While the main effect of time was significant (IRR = 0.54, 95% CI [0.34, 0.87]), indicating a decrease in the number of days of methamphetamine use from baseline to follow-up, the lack of significant interaction effects showed that this decrease was of similar magnitude in each of the two EMA conditions when compared to the decrease in the control condition, respectively.
Table 3.
Methamphetamine Use, Sexual Risk Behaviors, and Retention
| EMA+Dashboard N = 16 |
EMA+Dashboard+Counselor N = 18 |
Historical Matches N = 102 |
Comparison of the three treatment conditions Test statistic |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | Week 1 (n = 16) |
Week 12 (n = 13) |
Week 1 (n = 18) |
Week 12 (n = 12) |
Week 1 (n = 101) |
Week 12 (n = 76) |
Week 1 | Week 12 |
| Methamphetamine use and sexual risk behaviors, Median (IQR) | ||||||||
| Methamphetamine use (days in previous 30) | 5.0 (0.0-10.5) | 1.0 (0.0-6.0) | 11.5 (2.5-19.5) | 0.0 (0.0-2.0) | 5.5 (1.3-15.0) | 1.0 (0.0-6.3)a | KW χ2(2) = 2.0 | KW χ2(2) = 1.6 |
| Male sexual partners (# in previous 30 days) | 1.5 (0.0-4.3) | 0.5 (0.0-2.8) | 1.5 (1.0-5.5) | 0.0 (0.0-1.0) | 2.0 (1.0-6.0) | 1.0 (0.0-2.0) | KW χ2(2) = 1.6 | KW χ2(2) = 2.8 |
| Unprotected anal sex with non-primary partner (times in previous 30 days) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 2.0 (0.0-7.0) | 0.0 (0.0-0.0) | 1.0 (0.0-5.0) | 0.0 (0.0-1.0) | KW χ2(2) = 7.2 * | KW χ2(2) = 6.1 * |
| Urine drug screening for methamphetamine | ||||||||
| Joint probability index | ||||||||
| 8 weeks | 0.54 | 0.44 | 0.38 | |||||
| 12 weeks | 0.69 | 0.56 | 0.52 | |||||
| Treatment effectiveness score (Weeks 1-8), Median (IQR) | 17.0 (9.0-21.3) | 11.5 (8.0-19.5) | 11.0 (2.3-18.0) | KW χ2(2) = 2.8 | ||||
| Percentage of samples negative (Weeks 1-8), Median (IQR)b | 100.0 (80.9-100.0) | 90.5 (81.7-100.0) | 100.0 (78.3-100.0) | KW χ2(2) = 0.8 | ||||
| Longest consecutive negative samples (Weeks 1-8), Median (IQR)b | 8.5 (4.5-15.5) | 6.0 (4.0-10.0) | 5.0 (1.3-10.8) | KW χ2(2) = 3.8 | ||||
| Samples negative (Week 12), N (%)b | 11 (68.8) | 10 (55.6) | 53 (52.0) | χ2(2) = 1.6 | ||||
| Retention (final week), Median (IQR) | 5.5 (5.0-6.0) | 6.0 (5.0-6.0) | 6.0 (5.0-6.0) | KW χ2(2) = 0.1 | ||||
Note. IQR = interquartile range. KW = Kruskal-Wallis.
n = 64.
Missing urine samples were treated as samples tested positive for methamphetamine metabolites in the analysis.
p ≤ .05.
Table 4:
Mixed-effect Negative Binomial Regressions (I, II, II) and Logistic Regression (IV) of Methamphetamine Use and Sexual Risk Behaviors on Treatment Condition.
| Model I | Model II | Model III | Model IV | |
|---|---|---|---|---|
| Methamphetamine use (past 30 days) | No. of male partners (past 30 days) | Episodes of CAI (past 30 days) | Positive urine samples (Weeks 1-8) | |
| Parameter | Coef. [95% CI] | Coef. [95% CI] | Coef. [95% CI] | Coef. [95% CI] |
| Condition | ||||
| Historical Matches | Ref. Cat. | Ref. Cat. | Ref. Cat. | Ref. Cat. |
| EMA+Dashboard | −0.04 [−0.82, 0.74] | −0.33 [−1.12, 0.47] | −1.11 [−2.79, 0.57] | 0.38 [−2.06, 2.83] |
| EMA+Dashboard+Counselor | 0.36 [−0.38, 1.09] | −0.12 [−0.86, 0.63] | 0.69 [−0.64, 2.02] | 2.09 [−0.25, 4.42] † |
| Time | −0.61 [−1.08, −0.14] * | −0.71 [−1.06, −0.36] *** | −1.00 [−1.79, −0.20] * | −0.17 [−0.30, −0.03] * |
| Condition ✕ Time | ||||
| Historical Matches ✕ Time | Ref. Cat. | Ref. Cat. | Ref. Cat. | Ref. Cat. |
| EMA+Dashboard ✕ Time | 0.06 [−1.13, 1.25] | 0.02 [−0.93, 0.96] | −1.45 [−4.16, 1.27] | 0.00 [−0.23, 0.22] |
| EMA+Dashboard+Counselor ✕ Time | −0.77 [−1.95, 0.40] | −0.62 [−1.60, 0.37] | −3.97 [−6.76, −1.19] ** | −0.07 [−0.30, 0.15] |
| Intercept | 2.12 [1.84, 2.41] *** | 1.22 [0.92, 1.52] *** | 1.13 [0.55, 1.71] *** | −3.67 [−5.20, −2.14] *** |
Note. EMA = ecological momentary assessment. CAI = condomless anal intercourse. CI = confidence interval.
Coefficients and 95% CIs are reported in log-odds units in Table 4 and in exponentiated form as incident rate ratios in Sections 3.2–3.3 and as odds ratios in Section 3.4.
p ≤ .1.
p ≤ .05.
p ≤ .01.
p ≤ .001.
3.3. Sexual Risk Behaviors
The number of male sexual partners in the past 30 days was comparable across treatment conditions at baseline and at follow-up, given the lack of significant univariate effects at each time point (Table 3). Modeling results showed a significant reduction in the number of male sexual partners over time (IRR = 0.49, 95% CI [0.35, 0.70]), but this treatment effect did not differ significantly between conditions (Table 4, Model II).
For number of episodes of condomless anal intercourse (receptive or insertive) with a non-primary partner in the past 30 days, univariate analyses revealed significant differences between treatment conditions at baseline (KW χ2(2) = 7.2) and at follow-up (KW χ2(2) = 6.1; Table 2). In a negative binomial regression analysis (Table 4, Model III), a significant effect of time (IRR = 0.37, 95% CI [0.17, 0.81]) indicated a reduction in the number of episodes from baseline to follow-up. Of note, this treatment effect was stronger in the EMA+Dashboard+Counselor condition than in the matched historical control condition, as evidenced by a significant interaction between EMA+Dashboard+Counselor and time (IRR = 0.02, 95% CI [0.00, 0.30]).
3.4. Urine Drug Screening Outcomes
The aggregate drug screening indices summarized in Table 3 suggests that the EMA+Dashboard condition exhibited consistently superior performance over the EMA+Dashboard+Counselor condition and the matched historical control condition. However, univariate analyses did not reveal any significant differences between the three treatment conditions. A longitudinal logistic regression analysis (Table 4, Model IV) showed that the likelihood of providing a urine sample tested positive for methamphetamine metabolites decreased across the treatment period (OR = 0.85, 95% CI [0.74, 0.97]), but there were no significant observed interaction effects, suggesting that the size of the treatment effect was comparable between conditions.
3.5. Retention
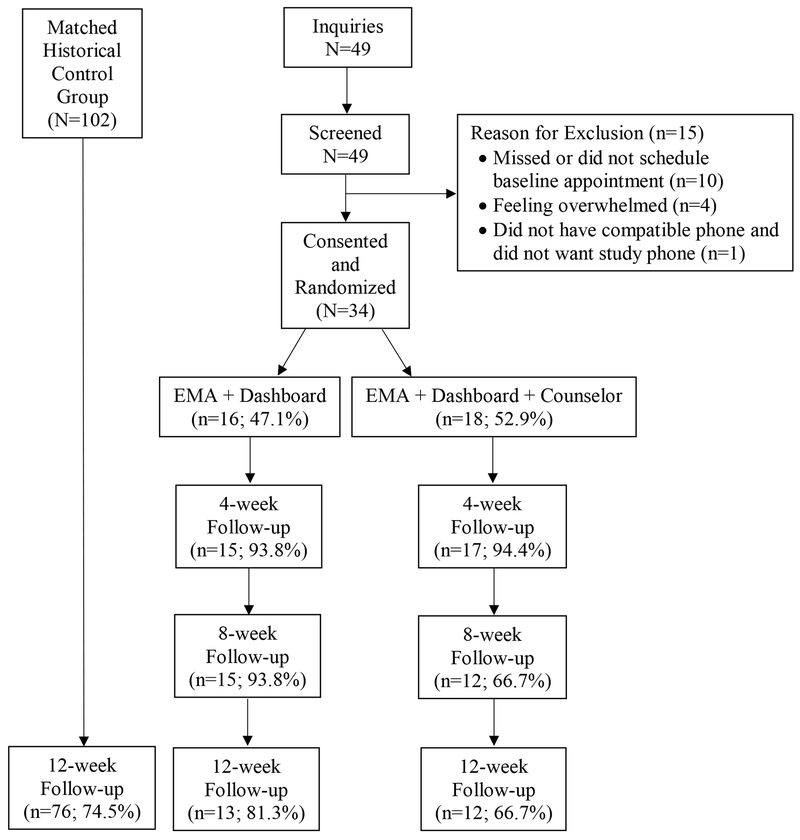
Figure 3 illustrates the progress and retention from initial screening through 12-week follow-up evaluations. There was no statistically significant difference with respect to the last week of clinic attendance between the three treatment conditions (Table 3).
Figure 3.
CONSORT diagram of study progression and retention
4. Discussion
This pilot study sought to evaluate whether treatment outcomes from the intervention, Getting Off: A Behavioral Treatment Intervention for Gay and Bisexual Male Methamphetamine Users (Reback & Shoptaw, 2014; Shoptaw et al., 2005, 2008), could be optimized through the addition of EMA self-monitoring during the 8-week treatment period. It was hypothesized that the additional self-monitoring introduced by EMA would promote behavior change through increased awareness of their own risky behavior and its correlates, as well as reminders from EMA prompts and goal tracking and accountability, thereby positively impacting treatment outcomes. Findings reported here provide partial support for the hypothesis. Most importantly, the results found a significantly greater reduction in the frequency of engagement in condomless anal intercourse from baseline to 12-week follow-up in the EMA+Dashboard+Counselor condition (but not in the EMA+Dashboard condition) compared with matched historical controls. The finding suggests EMA in conjunction with guided self-monitoring optimized sexual risk outcomes of the Getting Off intervention, and that participants did not benefit solely from the EMA visualization dashboard alone. A qualitative analysis by Swendeman and Reback (2015) indicated that participants either did not view their visualization dashboard, or were not as able to derive helpful insights from the survey results summarized in their visualization dashboard. By contrast, when participants discussed their EMA survey reports with a counselor, identifying behavioral patterns and enhancing accountability to behavior change goals, EMA became an effective tool for reducing the frequency of sexual risk behaviors. This finding dovetails with the results of Helzer et al. (2008) who found that feedback-enhanced self-monitoring of alcohol consumption after a brief alcohol intervention had a therapeutic advantage over self-monitoring without feedback.
Reductions in the number of male sexual partners, in the frequency of methamphetamine use, and in the likelihood of submitting a urine sample that was positive for methamphetamine metabolites were observed in both study conditions and did not differ statistically with reductions observed in the matched historical control condition. Comparable rates of relapse to substance use over a four-month period in a group of EMA participants and a non-EMA control group were also reported by Moore et al. (2014). It is worth noting, however, that in the present study, regression coefficient estimates suggested a greater effectiveness of the EMA+Dashboard+Counselor condition for self-reported methamphetamine use and number of male sexual partners, a finding that may emerge as statistically significant in an adequately powered replication study.
An obvious limitation of this pilot study is the small sample size that limited the ability to detect EMA effects. The finding that EMA was only effective in conjunction with a counselor raises the issue of whether there are other variables that moderate the effects of EMA that were not taken into account in this study. Though random condition assignment is meant to control for the influence of such moderators, the small sample size of this pilot study allows for the possibility that such exogenous effects remain. Newcomb and Mustanski (2013) reported that behavior change effects of MSM’s weekly online sexual diaries depended on the moderating effects of social-cognitive variables, specifically, only participants with sufficient risk reduction motivation exhibited a decrease in the likelihood of condomless anal intercourse over time. Thus, much remains to be understood about the conditions under which self-monitoring interventions, either in the form of stand-alone treatments or treatment adjuncts as in the current study, can effectively reduce sexual risk and drug use behaviors in MSM. Swendeman and colleagues (2015) proposed a theoretical framework of self-monitoring that posits different self-monitoring functions (e.g., reflection, reinforcement, cues to action) and mediators (e.g., cognitions, motivations, support) that intervene between self-monitoring and behavioral outcomes. Such a framework would provide helpful guidance for future research on the utility of EMA and self-monitoring in the treatment of methamphetamine-using MSM. Qualitative results from this study suggest that multiple causal mechanisms are at play in self-monitoring, feedback and counseling activities, in conjunction with Getting Off’s small-group and cognitive-behavioral intervention activities (Swendeman & Reback, 2015).
5. Conclusion
Methamphetamine-using GBM who participated in the Getting Off outpatient program were previously shown to reduce both methamphetamine use and sexual risk behavior (Reback & Shoptaw, 2014; Shoptaw et al., 2005, 2008). The results of this pilot study suggest that Getting Off sexual risk outcomes can be optimized through a combination of technology- and counselor-assisted self-monitoring.
Acknowledgments:
Dr. Rünger conducted statistical analyses and wrote the first draft of the manuscript. Dr. Fletcher conducted statistical analyses and provided extensive revisions to the manuscript drafts. Drs. Reback and Swendeman designed the study, developed the protocol, and provided revisions and feedback to the manuscript during multiple iterations. All authors approve the final version of the manuscript for submission.
Funding: Funding for this study was provided by the National Institute of Mental Health, supplement to grant #P30MH58107 (Rotheram-Borus, PI), with the study designed and conducted by Drs. Reback and Swendeman. Funding for the parent service program was provided by the Los Angeles County, Department of Public Health, Division of HIV and STD programs contract #PH-001039 and the City of West Hollywood, Division of Social Services. Dr. Swendeman was also supported by a career development grant from the William T. Grant Foundation. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Declarations of Interest: None.
References
- Bandura A (1991). Social cognitive theory of self-regulation. Organizational Behavior & Human Decision Processes, 50(2), 248–287. https://doi.org/10.1016/0749-5978(91)90022-L [Google Scholar]
- Burke LE, Wang J, & Sevick MA (2011). Self-monitoring in weight loss: a systematic review of the literature. Journal of the American Dietetic Association, 111(1), 92–102. https://doi.org/10.1016/j.jada.2010.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS (1979). A cybernetic model of self-attention processes. Journal of Personality and Social Psychology, 37(8), 1251–1281. https://doi.org/10.1037/0022-3514.37.8.1251. [Google Scholar]
- Chesney MA, Chambers DB, & Kahn JO (1997). Risk behavior for HIV infection in participants in preventive HIV vaccine trials: a cautionary note. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 16(4), 266–271. https://doi.org/10.1097/00042560-199712010-00007 [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Daleiden EL, & Weisz JR (2005). Identifying and selecting the common elements of evidence based interventions: A distillation and matching model. Mental Health Services Research, 7(1), 5–20. https://doi.org/10.1007/s11020-005-1962-6 [DOI] [PubMed] [Google Scholar]
- Curtin K, Fleckenstein AE, Robison RJ, Crookston MJ, Smith KR, & Hanson GR (2015). Methamphetamine/amphetamine abuse and risk of Parkinson’s disease in Utah: a population-based assessment. Drug and Alcohol Dependence, 146, 30–38. https://doi.org/10.1016/j.drugalcdep.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Groman SM, Morales AM, & London ED (2013). An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology, 38(2), 259–274. https://doi.org/10.1038/npp.2012.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway GP, Didier R, Garrison K, & Mendelson J (2008). Feasibility of ecological momentary assessment using cellular telephones in methamphetamine dependent subjects. Substance Abuse: Research and Treatment, 1, 9–14. Retrieved at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2789561/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SN, Winer RL, & Golden MR (2013). Web-based sex diaries and young adult men who have sex with men: assessing feasibility, reactivity, and data agreement. Archives of Sexual Behavior, 42(7), 1327–1335. https://doi.org/10.1007/s10508-012-9984-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grov C, Bimbi DS, Nanin JE, & Parsons JT (2006). Exploring racial and ethnic differences in recreational drug use among gay and bisexual men in New York City and Los Angeles. Journal of Drug Education, 36(2), 105–123. https://doi.org/10.2190/1g84-ena1-uad5-u8vj [DOI] [PubMed] [Google Scholar]
- Grov C, Rendina HJ, Breslow AS, Ventuneac A, Adelson S, & Parsons JT (2014a). Characteristics of men who have sex with men (MSM) who attend sex parties: results from a national online sample in the USA. Sexually Transmitted Infections, 90(1), 26–32. https://doi.org/10.1136/sextrans-2013-051094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grov C, Breslow AS, Newcomb ME, Rosenberger JG, & Bauermeister JA (2014b). Gay and bisexual men’s use of the Internet: research from the 1990s through 2013. The Journal of Sex Research, 51(4), 390–409. https://doi.org/10.1080/00224499.2013.871626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkitis PN, Levy MD, Moreira AD, & Ferrusi CN (2014). Crystal methamphetamine use and HIV transmission among gay and bisexual men. Current Addiction Reports, 1(3), 206–213. https://doi.org/10.1007/s40429-014-0023-x [Google Scholar]
- Healthy Living Project Team. (2007). Effects of a behavioral intervention to reduce risk of transmission among people living with HIV: the healthy living project randomized controlled study. Journal of Acquired Immune Deficiency Syndromes, 44(2), 213–221. https://doi.org/10.1097/qai.0b013e31802c0cae [DOI] [PubMed] [Google Scholar]
- Helzer JE, Rose GL, Badger GJ, Searles JS, Thomas CS, Lindberg SA, & Guth S (2008). Using interactive voice response to enhance brief alcohol intervention in primary care settings. Journal of Studies on Alcohol and Drugs, 69(2), 251–258. https://doi.org/10.15288/jsad.2008.69.251 [DOI] [PubMed] [Google Scholar]
- Heron KE, & Smyth JM (2010). Ecological momentary interventions: incorporating mobile technology into psychosocial and health behaviour treatments. British Journal of Health Psychology, 15(1), 1–39. https://doi.org/10.1348/135910709X466063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield S, Remien RH, Walavalkar I, & Chiasson MA (2004). Crystal methamphetamine use predicts incident STD infection among men who have sex with men recruited online: a nested case-control study. Journal of Medical Internet Research, 6(4), e41 https://doi.org/10.2196/jmir.6.4.e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath KJ, Beadnell B, & Bowen AM (2007). A daily web diary of the sexual experiences of men who have sex with men: comparisons with a retrospective recall survey. AIDS and Behavior, 11(4), 537–548. https://doi.org/10.1007/s10461-007-9206-y [DOI] [PubMed] [Google Scholar]
- Huang MC, Yang SY, Lin SK, Chen KY, Chen YY, Kuo CJ, & Hung YN (2016). Risk of cardiovascular diseases and stroke events in methamphetamine users. The Journal of Clinical Psychiatry, 77(10), 1396–1403. https://doi.org/10.4088/JCP.15m09872 [DOI] [PubMed] [Google Scholar]
- Huhn AS, Harris J, Cleveland HH, Lydon DM, Stankoski D, Cleveland MJ, Bunce SC. (2016). Ecological momentary assessment of affect and craving in patients in treatment for prescription opioid dependence. Brain Research Bulletin, 123(4), 94–101. https://doi.org/10.1016/j.brainresbull.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins RJ, McAlaney J, & McCambridge J (2009). Change over time in alcohol consumption in control groups in brief intervention studies: systematic review and meta-regression study. Drug and Alcohol Dependence, 100(1), 107–114. https://doi.org/10.1016/j.drugalcdep.2008.09.016 [DOI] [PubMed] [Google Scholar]
- Kamb ML, Fishbein M, Douglas JM Jr, Rhodes F, Rogers J, Bolan G, & Kent, C. (1998). Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA, 280(13), 1161–1167. https://doi.org/10.1001/jama.280.13.1161 [DOI] [PubMed] [Google Scholar]
- Kanfer FH (1970). Self-monitoring: Methodological limitations and clinical applications. Journal of Consulting and Clinical Psychology, 35(2), 148–152. https://doi.org/10.1037/h0029874 [Google Scholar]
- Ling W, Shoptaw S, Wesson D, Rawson RA, Compton M, & Klett CJ (1997). Treatment effectiveness score as an outcome measure in clinical trials. NIDA Research Monograph, 175, 208–220. https://doi.org/10.1037/e495552006-011 [PubMed] [Google Scholar]
- Marhe R, Waters AJ, van de Wetering BJM, & Franken IHA (2013). Implicit and explicit drug-related cognitions during detoxification treatment are associated with drug relapse: An ecological momentary assessment study. Journal of Consulting and Clinical Psychology, 81(1), 1–12. https://doi.org/10.1037/a0030754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J (2009). [Commentary] Research assessments: instruments of bias and brief interventions of the future? Addiction, 104(8), 1311–1312. https://doi.org/10.1111/j.1360-0443.2009.02684.x [DOI] [PubMed] [Google Scholar]
- Medley G, Lipari RN, Bose J, Cribb DS, Kroutil LA, & McHenry G (2016). Sexual orientation and estimates of adult substance use and mental health: results from the 2015 National Survey on Drug Use and Health. NSDUH Data Review. Retrieved at http://www.samhsa.gov/data/
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, & Wood CE. (2013). The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine, 46(1), 81–95. https://doi.org/10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- Moore TM, Seavey A, Ritter K, McNulty JK, Gordon KC, & Stuart GL (2014). Ecological momentary assessment of the effects of craving and affect on risk for relapse during substance abuse treatment. Psychology of Addictive Behaviors, 28(2), 619–624. https://doi.org/10.1037/a0034127 [DOI] [PubMed] [Google Scholar]
- Nakamura N, Mausbach BT, Ulibarri MD, Semple SJ, & Patterson TL (2011). Methamphetamine use, attitudes about condoms, and sexual risk behavior among HIV-positive men who have sex with men. Archives of Sexual Behavior, 40(2), 267–272. https://doi.org/10.1007/s10508-009-9566-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb ME, & Mustanski B (2013). Diaries for observation or intervention of health behaviors: factors that predict reactivity in a sexual diary study of men who have sex with men. Annals of Behavioral Medicine, 47(3), 325–334. https://doi.org/10.1007/s12160-013-9549-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMH Multisite HIV Prevention Trail: Reducing HIV sexual risk behavior. Science, 280(5371), 1889–1894. https://doi.org/10.1126/science.280.5371.1889 [DOI] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Epstein DH (2017). Context and craving during stressful events in the daily lives of drug-dependent patients. Psychopharmacology, 234(17), 2631–2642. https://doi.org/10.1007/s00213-017-4663-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Shoptaw SJ, Obert JL, McCann MJ, Hasson AL, Marinelli-Casey PJ, …& Ling W. (1995). An intensive outpatient approach for cocaine abuse treatment: the matrix model. Journal of Substance Abuse Treatment, 12(2), 117–127. https://doi.org/10.1016/0740-5472(94)00080-B [DOI] [PubMed] [Google Scholar]
- Reback CJ, Fletcher JB, Shoptaw S, & Grella CE (2013). Methamphetamine and other substance use trends among street-recruited men who have sex with men, from 2008 to 2011. Drug and Alcohol Dependence, 133(1), 262–265. https://doi.org/10.1016/j.drugalcdep.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reback CJ, & Shoptaw S (2014). Development of an evidence-based, gay-specific cognitive behavioral therapy intervention for methamphetamine-abusing gay and bisexual men. Addictive Behaviors, 39(8), 1286–1291. https://doi.org/10.1016/j.addbeh.2011.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Flower K, Kielstein A, Leamon MH, Nordahl TE, & Galloway GP (2011). Psychiatric comorbidity in methamphetamine dependence. Psychiatry Research, 186(2), 356–361. https://doi.org/10.1016/j.psychres.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre F, Fatseas M, Swendsen J, & Auriacombe M (2015). Ecological momentary assessment in the investigation of craving and substance use in daily life: A systematic review. Drug and Alcohol Dependence, 148, 1–20. https://doi.org/10.1016/j.drugalcdep.2014.12.024 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, & Hufford MR (2008). Ecological momentary assessment. Annual Review of Clinical Psychology, 4, 1–32. https://doi.org/10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- Shiffman S (2009). Ecological Momentary Assessment (EMA) in studies of substance use. Psychological Assessment, 21(4), 486–497. https://doi.org/10.1037/a0017074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, & Hucks-Ortiz C (2005). Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug and Alcohol Dependence, 78(2), 125–134. https://doi.org/10.1016/j.drugalcdep.2004.10.004 [DOI] [PubMed] [Google Scholar]
- Shoptaw S, & Reback CJ (2006). Associations between methamphetamine use and HIV among men who have sex with men: a model for guiding public policy. Journal of Urban Health, 83(6), 1151–1157. https://doi.org/10.1007/s11524-006-9119-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Larkins S, Wang PC, Rotheram-Fuller E, Dang J, & Yang X (2008). Outcomes using two tailored behavioral treatments for substance abuse in urban gay and bisexual men. Journal of Substance Abuse Treatment, 35(3), 285–293. https://doi.org/10.1016/j.jsat.2007.11.004 [DOI] [PubMed] [Google Scholar]
- Solomon TM, Halkitis PN, Moeller RW, & Pappas MK (2012). Levels of methamphetamine use and addiction among gay, bisexual, and other men who have sex with men. Addiction Research & Theory, 20(1), 21–29. https://doi.org/10.3109/16066359.2011.552816 [Google Scholar]
- Swendeman D, Ramanathan N, Baetscher L, Medich M, Scheffler A, Comulada WS, & Estrin D (2015). Smartphone self-monitoring to support self-management among people living with HIV: perceived benefits and theory of change from a mixed-methods randomized pilot study. Journal of Acquired Immune Deficiency Syndromes, 69, S80–91. https://doi.org/10.1097/QAI.0000000000000570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendeman D, & Reback CJ (2015). Self-monitoring by smartphone ecological momentary assessment (EMA) to support outpatient methamphetamine treatment: preliminary efficacy and theory of action from a mixed-methods randomized pilot study Oral presentation at Addiction Health Service Research, Los Angeles, California, October 2015. [Google Scholar]
- Twitchell GR, Huber A, Reback CJ, & Shoptaw S (2002). Comparison of general and detailed HIV risk assessments among methamphetamine abusers. AIDS and Behavior, 6(2), 153–162. https://doi.org/10.1023/A:1015449231848 [Google Scholar]
- Warsi A, Wang PS, LaValley MP, Avorn J & Solomon DH (2004). Self-management education programs in chronic disease. Archives of Internal Medicine, 164(15), 1641–1649. https://doi.org/10.1001/archinte.164.15.1641 [DOI] [PubMed] [Google Scholar]
- Waters AJ, Marhe R, & Franken IHA (2012). Attentional bias to drug cues is elevated before and during temptations to use heroin and cocaine. Psychopharmacology, 219(3), 909–921. https://doi.org/10.1007/s00213-011-2424-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray TB, Kahler CW, & Monti PM (2016). Using ecological momentary assessment (EMA) to study sex events among very high-risk men who have sex with men (MSM). AIDS and Behavior, 20(10), 2231–2242. https://doi.org/10.1007/s10461-015-1272-y [DOI] [PMC free article] [PubMed] [Google Scholar]