Abstract
Ovarian cancer is the most fatal gynecological cancer in the USA and the fifth most common cancer-related cause of death in women. Inflammation has been shown to play many roles in ovarian cancer tumor growth, with the proinflammatory cytokine interleukin-6 (IL-6) having been established as a key immunoregulatory cytokine. Ovarian cancer cells continuously secrete cytokines that promote tumorigenicity in both autocrine and paracrine fashions while also receiving signals from the tumor microenvironment (TME). The TME contains many cells including leukocytes and fibroblasts, which respond to proinflammatory cytokines and secrete their own cytokines, which can produce many effects including promotion of chemoresistance, resistance to apoptosis, invasion, angiogenesis by way of overexpression of vascular endothelial growth factor, and promotion of metastatic growth at distant sites. IL-6 and its proinflammatory family members, including oncostatin M, have been found to directly stimulate enhanced invasion of cancer cells through basement membrane degradation caused by the overexpression of matrix metalloproteinases, stimulate promotion of cell cycle, enhance resistance to chemotherapy, and cause epithelial-to-mesenchymal transition (EMT). IL-6 has been shown to activate signaling pathways that lead to tumor proliferation, the most studied of which being the Janus kinase (JAK) and STAT3 pathway. IL-6-induced JAK/STAT activation leads to constitutive activation of STAT3, which has been correlated with enhanced tumor cell growth and resistance to chemotherapy. IL-6 has also been shown to act as a trigger of the EMT, the hypothesized first step in the metastatic cascade. Understanding the important role of IL-6 and its family members’ effects on the pathogenesis of ovarian cancer tumor growth and metastasis may lead to more novel treatments, detection methods, and improvement of overall clinical outcomes.
Keywords: interleukin-6, IL-6, OSM, inflammatory cytokines, ovarian cancer, metastasis
Inflammation and cancer
In 1863, Rudolf Virchow first described the possible role of inflammation in the progression of cancer through observing the presence of lymphocytes present within a “lymphoreticular infiltrate” that surrounded many cancerous lesions. Virchow hypothesized that this “lymphoreticular infiltrate” served to be the fuel for the uncontrolled growth of tumor.1 If genetic damage was the spark that started the fire, then the chemical signaling pathways involved in inflammation and wound healing could be the fuel that malignant cells needed to proliferate, invade local tissues, and metastasize.1 Since then, the role of inflammation in cancer progression has been widely accepted, with proinflammatory cytokines being shown to play key roles at many stages of tumorigenesis.2,3 The interactions between the proinflammatory TME and a tumor have been shown to be an essential component of tumor development, again supporting Virchow’s initial hypothesis.4,5 Inflammation has many important functions in the various stages of tumor growth that include initiation, promotion, progression, invasion, and metastasis.4,5 Numerous inflammatory mediators have been implicated in cancer metastasis such as interleukin-6 (IL-6), IL-10, and tumor necrosis factor-alpha (TNF-α). However, research so far has established IL-6 as one of the key immunoregulatory cytokines present in the ovarian cancer TME that initiates many different signaling pathways that can lead to a variety of outcomes including tumor proliferation, angiogenesis, and chemoresistance.6–8 In this review, we will focus on the role of IL-6 in the metastasis of ovarian cancer.
Metastatic ovarian cancer
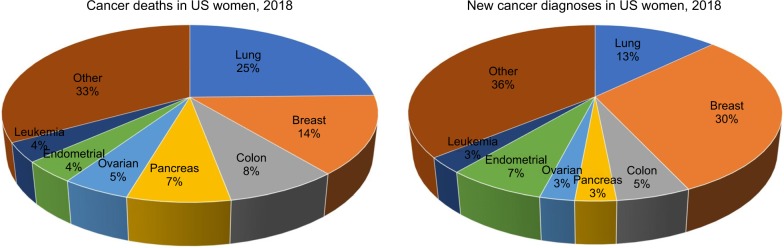
Ovarian cancer is the most lethal gynecological cancer in the USA and the fifth most common cause of death from cancer in women9–14 (Figure 1). The estimated number of new ovarian cancer cases in the USA in 2018 is 22,240 patients or 3% of all cancer diagnoses (Figure 1), and approximately 14,070 deaths are expected in the USA in 2018 alone.15,16 Anatomically, the ovaries are two walnut-shaped organs that are located bilaterally to the uterus on the left and right. They secrete reproductive egg cells through fallopian tubes that carry these cells from either ovary to the uterus. At the junction between the fallopian tube and the ovary, each fallopian tube tapers into fimbriae. Research suggests a dual type I and type II classification system of epithelial ovarian cancers based on the cancer cell phenotype and origin with three distinct subtypes within type I ovarian carcinomas.17,18 Type I ovarian carcinomas generally arise from nonmalignant extraovarian lesions that are able to undergo malignant transformation when implanted onto the ovary. These tumors have excellent prognosis when confined to the ovary and make up only 10% of death due to ovarian cancer. Type I tumors are further categorized into three groups to include endometriosis-related tumors (endometrioid, seromucinous carcinomas, and clear cell type), low-grade serous carcinomas, mucinous carcinomas, and malignant Brenner tumors.19 Type II generally comprises of high-grade serous carcinomas, carcinosarcoma, and undifferentiated carcinoma. These highly aggressive tumors are present in advanced stage in more than 75% of cases and make up 90% of deaths from ovarian cancer.19 A majority of patients with a new diagnosis of ovarian cancer present with widespread and distant metastasis; metastasis plays a major role in ovarian cancer prognosis and accounts for 80%–90% of all ovarian cancer deaths.9,10,12,14,20,21 Therefore, understanding the pathogenesis of ovarian cancer metastasis is an important area of research that may assist to improve clinical outcomes.
Figure 1.
New cancer deaths and diagnoses in US women in 2018; data from Siegel et al.16
Metastasis is a complex process that involves the detachment of cancer cells from the primary tumor site, the spread of cancer cells to different tissues in the body through body systems such as vasculature or lymphatics, the attachment of a cancer cell to tissue, and then the continued uncontrolled growth of that cell in the new tissue. It involves cell motility induction, extracellular matrix (ECM) degradation, angiogenesis, intravasation, circulation, extravasation, and survival in a new environment.22 The local tumor environment, also known as the microenvironment, contains the supporting cells that promote and enhance cancer progression. The tumor microenvironment (TME) is composed of cancer cells, matrix proteins, inflammatory cells, and stromal cells (including macrophages, pericytes, endothelial cells, regulatory T cells, myeloid-derived suppressor cells, fibroblasts, and platelets). The communication among cells of the TME induces apoptosis, invasion, angiogenesis, and growth at distant sites.23,24 Ovarian cancer is known to metastasize early, present aggressively, and spread quickly. It primarily disseminates through peritoneal ascites, spreading throughout the peritoneal cavity, which differs from most cancers that metastasize through vasculature or lymphatics. This means that ovarian cancer can superficially invade peritoneal organs including the small and large intestines and bladder.21 When cancer cells implant in the greater omentum, they often cause ascites, which can be especially pronounced in high-grade serous carcinomas.21,25 Ovarian cancer cells can float freely in malignant ascitic effusions. They are able to proliferate, survive, and spread without a local solid scaffold and vascular structures seen in many other types of cancer metastasis.21,26 Additionally, ovarian cancer cells and peritoneal mesothelial cells are continuously secreting cytokines that promote tumorigenicity in an autocrine and paracrine fashion resulting in ovarian cancer’s preferential metastasis to the greater omentum.25,26
IL-6 function and dysregulation
Cytokines are highly localized soluble signaling proteins that are produced by almost all types of cells of the immune system such as neutrophils, monocytes, macrophages, B cells, and T cells.1,27 They are proteins that can stimulate or inhibit cell growth, regulate cell differentiation, begin cell chemotaxis, and influence the other cytokine expression either directly or indirectly. Cytokines can function as growth factors to increase metastasis by promoting cell adhesiveness and/or tumor angiogenesis.1,24,27 Although cytokines can regulate an antitumor response depending on the TME, they can also contribute to cell transformation and malignancy during chronic inflammation. This process is dependent on the balance between the amounts of proinflammatory and anti-inflammatory cytokines and also which cytokine receptors are expressed.1–3
IL-6 is a proinflammatory pleiotropic cytokine that is present in the TME. It is a 26 kDa low molecular weight protein with 185 amino acids that is produced by many cell types including neutrophils, macrophages, monocytes, fibroblasts, endothelial cells, lymphocytes, and tumor cells.1–3,28,29 IL-6 is produced in response to local proinflammatory cytokines such as TNF-α, which is one of the main cytokines constitutively expressed in most malignant ovarian carcinomas and signals a vast network of other cytokines, chemokines, angiogenic factors, and transcription factors that promote metastatic spread and growth of tumor deposits through an autocrine and paracrine manner. TNF-α influences metastasis via CXCR4, survival of tumor cells via CXCR4/CXCL12, and angiogenesis through vascular endothelial growth factor (VEGF) expression and CXCL12 induction.30 In physiological conditions, IL-6 has many functions such as recruiting neutrophils; promoting the migration, growth, activation, and differentiation of T lymphocytes; and promoting the differentiation of B lymphocytes to plasma cells in order to produce immunoglobulins.28 IL-6 has been found to have direct stimulatory effects on many cancer cells through its actions on several cell signaling pathways that promote the cell cycle and growth. Additionally, at high concentrations IL-6 has been shown to have inhibitory effects on immune cells by inhibiting the expression of IL-2, decreasing activation of T cells, and promoting apoptosis of lymphocytes, which can prevent immune surveillance of cancer cells.31
Overexpression and dysregulation of IL-6 measured by elevated serum levels of IL-6 and IL-6 family members have been associated with ovarian cancer along with other cancers including multiple myeloma and breast cancer.32 Other cytokines in the IL-6 family include oncostatin M (OSM), IL-11, IL-27, IL-31, leukemia inhibitory factor (LIF), ciliary neurotrophic factor, cardiotrophin-1, and cardiotrophin-like cytokine.33 Several of these family members have also been shown to act directly on ovarian carcinoma cells to enhance proliferation, migration, invasion, survival, and chemoresistance.10–12,14 Of that family of cytokines, OSM, a 26 kDa molecular weight secreted cytokine in the glycoprotein 130 (gp130) (IL-6/LIF) family of cytokines, is primarily secreted from neutrophils and macrophages. However, it is also localized to and secreted from tumor cells and lymphocytes and exhibits many pleiotropic effects through inhibition of proliferation of some cancers while inducing proliferation in others.51,70–77 A potential area for future research would be to understand further how OSM functions and how to utilize it to prevent ovarian cancer metastasis.
When IL-6 binds to its IL-6 receptor (IL-6R) or common signal transducer subunit gp130, it can activate multiple signaling pathways.34 These pathways include the Janus tyrosine kinase (JAK), STAT3 pathway,10–12,14,33–35 the mitogen-activated protein kinase pathway,34 and the phosphoinositide 3-kinase/AKT pathway.10–12,14,25,34 Of these pathways, IL-6-induced activation of the JAK/STAT3 pathway is one of the most studied pathways involving IL-6R dimerization, leading to JAK2 recruitment, STAT3 phosphorylation and dimerization, then translocation of the STAT3 dimer from the cytoplasm to the nucleus. In the nucleus, STAT3 alters the transcription of many genes, including genes that play a role in proliferation, migration, differentiation, angiogenesis, survival, and resistance to apoptosis induced by chemotherapy.10–12,14,36 In normal cells, STAT3 activation is highly regulated and transient; however, in cancer cells, there is often a continuous activation of the STAT3 pathway by IL-6 that is correlated with aggressive behavior of high-grade ovarian cancer and poor prognosis.37–41 One study by Silver et al demonstrated that activated STAT3 was found more often in high-grade epithelial ovarian cancer that was diagnosed at a later stage rather than in low-grade cancer. This study also suggests that activation of JAK/STAT signaling pathway is involved in ovarian cancer motility, cell survival, and proliferation and that STAT3 is required for ovarian cancer cell migration.40 Saini et al showed that activation of STAT3, particularly STAT3 phosphorylation at Tyr705, is needed for ovarian tumor metastasis and is greatly expressed by metastatic cells that are present in ascites; ascites is known to be directly related to the peritoneal spread of ovarian cancer.9,42
IL-6 may be either constitutively secreted directly by ovarian carcinoma cells or through secondary inflammatory and tumor-infiltrating cells, including fibroblasts, tumor-associated macrophages (TAMs), T cells, and natural killer cells, and works to promote tumor cell detachment and migration. In peritoneal fluid with metastatic ovarian carcinoma, a study by Isobe et al found that M2-polarized TAMs were the primary IL-6-secreting cells. These types of macrophages have been shown to display mostly tumor-promoting effects and increased number of TAMs within a tumor has been associated with worse prognosis.43 They demonstrated that increased levels of IL-6R are independently prognostic for worse progression-free survival.6 Additionally, they showed that exogenous treatment of IL-6 induced proliferation, invasion, and VEGF expression in all experimental ovarian cancer cell lines. This effect was IL-6 dose-dependent and was attenuated with the preadministration of anti-IL-6R antibodies. Higher levels of serum and peritoneum fluid IL-6 were more commonly associated with aggressive metastatic ovarian carcinomas.6,38,39
IL-6 activation of STAT3 results in the expression of cell cycle-promoting proteins such as c-MYC and cyclins D1, D2, and B1, while also downregulating the cyclin-dependent kinase inhibitor, p21, promoting entry into the cell cycle. Additionally, STAT3 has been shown to alter the resistance of stem cell-like cells to chemotherapy.36,44 STAT3 increases the expression of survival proteins such as BCL-2, BCL-xL, survivin, and MCL-1, which contribute to cancer phenotypes of chemoresistance and survival.10–12 In ovarian cancer, it has been shown that the IL-6-induced JAK/STAT signaling pathway enhances tumor cell growth and resistance to chemotherapy.45
IL-6 also facilitates the invasion of other tissues and blood vessels by cancer cells through its prominent role as an important trigger of epithelial-to-mesenchymal transition (EMT).4,5,46,47 EMT is a transcriptionally regulated natural process where epithelial cells express certain proteins involved in important processes such as embryogenesis, gonadal development in the ovary, and wound healing.48,49 In EMT, cell polarity and expression of anchoring proteins are lost in stationary epithelial cells and replaced with mesenchymal proteins such as vimentin and/or N-cadherin expression, which leads to invasive, migratory, and stem cell properties.50 IL-6-induced EMT activates STAT3, which promotes transcription of ZEB1, a transcription factor that promotes the production of mesenchymal-like proteins and a mesenchymal phenotype.51 This process, however, can be utilized by carcinoma cells to promote invasion, metastasis, and reoccurrence and is hypothesized to be the first step in the metastatic cascade of cancer cells.32,45–47,50,52,53
Additionally, EMT leads to altered E-cadherin expression, which plays a crucial step in cancer progression. E-cadherin is a calcium-dependent cell adhesion glycoprotein expressed by surface epithelial cells that functions as an inhibitory factor for malignant transformation, invasion, and metastasis. Therefore, loss of E-cadherin is a rate-limiting step of invasion and loss of differentiation of cells and is a marker for EMT.54–56 In ovarian cancer, there are lower levels of tumor cell E-cadherin expression in ascites and metastatic site compared with the primary ovarian tumor, which contributes to the tumor’s increased invasiveness.21 EMT-associated transcription factors that suppress E-cadherin expression include TWIST, SNAIL, and ZEB1.45–47,50,57,58 Furthermore, because ovarian cancer metastasizes through peritoneal cavity shedding, the re-expression of E-cadherin during a process called mesenchymal-to-epithelial transition, in which metastatic cells re-express epithelial tight junction proteins and anchor proteins, helps to stabilize metastatic cells through adhesion to the peritoneal cavity further contributing to tumor formation.54,56
In addition to local invasion, another characteristic gained by aggressive metastatic potential of ovarian cancer is angiogenesis or new blood vessel formation from preexisting vessels. Angiogenesis is an adaptive method for cancer cells by which they increase much needed supplies of oxygen and nutrients while growing, creating new conduits for their spread to distant areas.59 Increased IL-6/IL-6R expression has shown to lead to increased expression of VEGF, a potent growth factor, that promotes angiogenesis.10–12,14
Another invasive characteristic in ovarian cancer is the secretion of proteases needed for ECM degradation. IL-6 can also contribute to the proliferation and invasion of cancerous cell lines by increasing their ability to secrete matrix metalloproteinase 9 (MMP-9).60,61 MMP-9 (gelatinase B, 92 kDa type IV collagenase) is a zinc-dependent metalloproteinase that functions as an ECM-degrading protease. MMP-9 is involved in the degradation of type IV collagen (an important barrier to tumor cell invasion as it is a major structure in the basement membrane), gelation, and other ECM macromolecules and plays a role in tumor invasion and metastasis.60,62–64 A meta-analysis of 30 studies that examined the link between increased MMP-9 expression and survival of ovarian cancer showed that MMP-9 expression substantially predicted poor prognosis in ovarian cancer patients.63 Hu et al utilized RNAi to explore the function of MMP-9 in ovarian cancer cell invasion and adhesion and determined that downregulation of MMP-9 expression leads to decreased invasion and adhesion of ovarian cancer cells.64 Furthermore, MMP-9 is also found to be statistically higher in patients with malignant and recurrent ovarian cancer.64 Rabinovich et al studied the mechanisms of how autocrine IL-6 affects ovarian cancer cell line (SKOV-3) through upregulation of MMP-2 and MMP-9 protein production and secretion. Their study determined that IL-6 regulates these secretions in different autocrine manners but suggests that IL-6-increased secretion of MMP-9 plays a role in the tumorigenic potential of ovarian cancer cells.65 Thus, IL-6 appears to play a significant role in contributing to the degradation of ECM, promoting cancer invasion and metastasis.
Anti-IL-6/IL-6R therapy
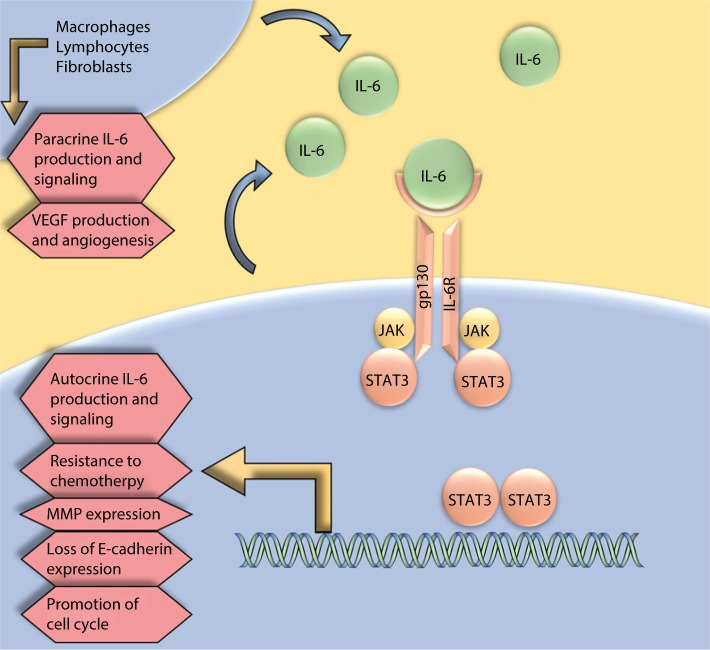
The effects of increased angiogenesis, invasion, and metastasis of IL-6 are often mediated through activation of the IL-6-induced gp130/JAK/STAT signaling pathway (Figure 2), making this pathway a potential target for the development of therapeutics that suppress its constitutive and ligand-induced activation that has been shown to be involved in ovarian cancer motility, cell survival, proliferation, and resistance to chemotherapy.36,40 The utility of STAT3 in monotherapy and combination therapy as a possible target for ovarian cancer treatment has been explored recently. As a novel ovarian cancer treatment, Ma et al created cationic solid lipid nanoparticle (SLN) system that uses STAT3 decoy oligodeoxynucleotides (ODN), 15-mer double-stranded oli-gonucleotides to prevent the decoy degradation and increase in vitro efficiency of cellular uptake.66 This study demonstrated that SLN-STAT3 decoy ODN complexes inhibit cell invasion through the upregulation of E-cadherin expression and downregulation of the SNAI1 transcription factor and MMP-9 expression. The study also showed that SLN-STAT3 decoy ODN carries both suppress growth and cause cell death through apoptosis and autophagy.66 This shows a potential role of SLN-STAT3 decoy ODN in targeted gene delivery in ovarian cancer that can be applied in future clinical trials. In an in vitro study by Tang et al, WP1066, a small molecule capable of selectively blocking STAT3 phosphorylation at tyrosine 705 (Tyr-705) was investigated to determine the antitumor effect of WP1066 in ovarian cancer cells. Their results show that WP1066 is able to suppress proliferation, migration, and invasion while inducing apoptosis in ovarian cancer cells.67 This also demonstrates a role of WP1066 to be researched further in clinical trials.
Figure 2.
IL-6 is expressed in an autocrine and paracrine manner from both ovarian cancer cells and cells in the surrounding tumor microenvironment.
Note: IL-6 expression plays a role in the promotion of epithelial-to-mesenchymal transition, chemotherapy resistance, and metastasis.
Abbreviations: MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor.
Due to the evidence that IL-6 is present in high amounts in malignant ovarian cells and functions to enhance ovarian tumor cell survival, angiogenesis, metastasis, and resistance to chemotherapy, anti-IL-6 therapy has been investigated as a potential therapeutic option to treat ovarian cancer and prevent metastasis. Anti-IL-6 antibodies and anti-IL-6R antibodies have become a popular area of research. Tocilizumab (Genetech, South San Francisco, CA, USA), a humanized antihuman IL-6R antibody that binds to the IL-6-binding site of human IL-6R, has been shown to improve clinical outcomes in Castleman’s disease and rheumatoid arthritis.6 In a Phase II single-arm clinical trial, siltuximab (CNTO 328; Centocor, Inc., Horsham, PA, USA), the monoclonal antibody with high binding affinity for IL-6, was assessed in patients with platinum-resistant ovarian cancer, irrespective of the IL-6 levels present.8 Their study showed that given alone, siltuximab was well tolerated and had therapeutic activity in all 18 women with platinum-resistant ovarian cancer that participated in the study, with stable disease being achieved in eight patients with four of those patients being progression-free for >6 months. However, there were no complete or partial responses, which demonstrates that siltuximab fails to function independently in treating human ovarian carcinoma and does not offer better outcomes than existing chemotherapy regimens.8 In another Phase I/II clinical trial of siltuximab, Angevin et al investigated the safety, efficacy, and pharmacokinetics of anti-IL-6 therapy at increasing doses in 84 patients with a history of previously treated malignant solid tumors including ovarian carcinoma. The results showed little efficacy of siltuximab as a monotherapy in the treatment of platinum-resistant ovarian cancer.68 The lack of objective response seen with the single-drug therapy siltuximab treatment in this study, and in other studies investigating the effects of anti-IL-6 therapy in platinum-resistant ovarian cancer, suggests that IL-6 inhibition may have a limited benefit in advanced-stage ovarian cancer as a single-drug therapy. Additionally, it may mean that these late-stage cancers are IL-6 independent of STAT3 signaling and utilize additional pathways for carcinogenesis.6,8,68
Future of anti-IL-6 therapy in a multitarget approach to treatment
Related to the constitutively activated JAK/STAT3 in ovarian cancer, the EGFR prosurvival signaling pathway is also frequently activated and overexpressed in 70% of ovarian cancers and linked to a poor prognosis. Wen et al explored the clinical role of targeting EGFR in ovarian cancer treatment by studying gefitinib (Iressa, AstraZeneca plc, London, UK), an EGFR inhibitor.41 The study demonstrated that EGFR inhibition enhances phosphorylation of STAT3 significantly in ovarian cancer cells. It also demonstrated that STAT3 activation blockade resulted in increased antitumor activity of gefitinib in vitro and in vivo when inhibiting both EGRF and STAT3 pathways together rather than alone.41 Additionally, epithelial ovarian cancer cells have specific steroid receptors expressed such as estrogen receptor alpha, estrogen receptor beta, and progesterone receptor.69 These receptors have also been researched as potential targets for therapy in epithelial ovarian carcinoma and are already being used as significant therapies in many diseases such as prostate and breast cancer. These studies suggest that blockade of many survival pathways may be necessary to attain maximum antitumor activity.69
The data presented in this review suggest that IL-6 plays a key role in ovarian cancer metastasis, which includes ovarian tumor cell migration, proliferation, invasion, survival, and chemoresistance through induction of pathways such as JAK/STAT3 and processes such as EMT. Rather than being the most potent inflammatory cytokine involved in ovarian cancer metastasis, it is clear that IL-6 is a part of a greater group of cytokines and other inflammatory and noninflammatory growth factors that play an integral role in ovarian cancer metastasis. Future research should investigate each IL-6 family member cytokine and their unique contribution to cancer metastasis. As more is learned about cytokine signaling redundancy and cross talk between receptors, there is promise to reveal the most potent cytokines involved in the promotion of EMT and metastasis, which could help in the discovery of future therapies to control or even stop this process. In addition to studying a multipronged approach to inhibiting ovarian cancer metastasis, another area of future research includes the role of OSM and how to utilize it to prevent the spread of ovarian cancer metastasis. Through more research and understanding of the pathogenesis of ovarian cancer metastasis, more novel treatment regimens and detection methods can be developed to help reduce the morbidity and mortality of women affected by this devastating carcinoma.
Acknowledgments
This study was partially funded by the following grants: NIH grants P20GM103408, P20GM109095, R25GM123927, the METAvivor Quinn Davis Northwest Arkansas METSquerade Fund, the Smylie Family Cancer Fund, and the Boise State University Biomolecular Research Center.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee M, Stühmer T, Herrmann P, Bommert K, Dörken B, Bargou RC. Combined disruption of both the MEK/ERK and the IL-6R/STAT3 pathways is required to induce apoptosis of multiple myeloma cells in the presence of bone marrow stromal cells. Blood. 2004;104(12):3712–3721. doi: 10.1182/blood-2004-04-1670. [DOI] [PubMed] [Google Scholar]
- 3.Gasche JA, Hoffmann J, Boland CR, Goel A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int J Cancer. 2011;129(5):1053–1063. doi: 10.1002/ijc.25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker C, Fantini MC, Wirtz S, et al. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4(2):217–220. [PubMed] [Google Scholar]
- 6.Isobe A, Sawada K, Kinose Y, et al. Interleukin 6 receptor is an independent prognostic factor and a potential therapeutic target of ovarian cancer. PLoS One. 2015;10(2):e0118080. doi: 10.1371/journal.pone.0118080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopinathan G, Milagre C, Pearce OM, et al. Interleukin-6 stimulates defective angiogenesis. Cancer Res. 2015;75(15):3098–3107. doi: 10.1158/0008-5472.CAN-15-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coward J, Kulbe H, Chakravarty P, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17(18):6083–6096. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saini U, Naidu S, ElNaggar AC, et al. Elevated STAT3 expression in ovarian cancer ascites promotes invasion and metastasis: a potential therapeutic target. Oncogene. 2017;36(2):168–181. doi: 10.1038/onc.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colomiere M, Ward AC, Riley C, et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer. 2009;100(1):134–144. doi: 10.1038/sj.bjc.6604794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obata NH, Tamakoshi K, Shibata K, Kikkawa F, Tomoda Y. Effects of interleukin-6 on in vitro cell attachment, migration and invasion of human ovarian carcinoma. Anticancer Res. 1997;17(1A):337–342. [PubMed] [Google Scholar]
- 12.Rath KS, Funk HM, Bowling MC, Richards WE, Drew AF. Expression of soluble interleukin-6 receptor in malignant ovarian tissue. Am J Obstet Gynecol. 2010;203(3):230, e1–e8. doi: 10.1016/j.ajog.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Li L, Guo X, et al. Interleukin-6 signaling regulates anchorage-independent growth, proliferation, adhesion and invasion in human ovarian cancer cells. Cytokine. 2012;59(2):228–236. doi: 10.1016/j.cyto.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 15.American Cancer Society . Cancer Facts & Figures 2016. American Cancer Society; Atlanta, GA, USA: 2016. [Accessed November 28, 2018]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2016/cancer-facts-and-figures-2016.pdf. [Google Scholar]
- 16.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 17.Nezhat FR, Apostol R, Nezhat C, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213(3):262–267. doi: 10.1016/j.ajog.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 18.McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. 2011;43(5):420–432. doi: 10.1097/PAT.0b013e328348a6e7. [DOI] [PubMed] [Google Scholar]
- 19.Kurman RJ, Shih I eM The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186(4):733–747. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pradeep S, Kim SW, Wu SY, et al. Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell. 2014;26(1):77–91. doi: 10.1016/j.ccr.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177(3):1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imanishi Y, Hu B, Jarzynka MJ, et al. Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res. 2007;67(9):4254–4263. doi: 10.1158/0008-5472.CAN-06-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang E, Ngalame Y, Panelli MC, et al. Peritoneal and subperitoneal stroma may facilitate regional spread of ovarian cancer. Clin Cancer Res. 2005;11(1):113–122. [PubMed] [Google Scholar]
- 24.Zhang Y, Tang H, Cai J, et al. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303(1):47–55. doi: 10.1016/j.canlet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Giridhar PV, Funk HM, Gallo CA, et al. Interleukin-6 receptor enhances early colonization of the murine omentum by upregulation of a mannose family receptor, LY75, in ovarian tumor cells. Clin Exp Metastasis. 2011;28(8):887–897. doi: 10.1007/s10585-011-9420-x. [DOI] [PubMed] [Google Scholar]
- 26.Said N, Socha MJ, Olearczyk JJ, Elmarakby AA, Imig JD, Motamed K. Normalization of the ovarian cancer microenvironment by SPARC. Mol Cancer Res. 2007;5(10):1015–1030. doi: 10.1158/1541-7786.MCR-07-0001. [DOI] [PubMed] [Google Scholar]
- 27.Savant SS, Sriramkumar S, O’Hagan HM. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers (Basel) 2018;10(8):E251. doi: 10.3390/cancers10080251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65(23):10794–10800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20(42):5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 30.Kulbe H, Thompson R, Wilson JL, et al. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67(2):585–592. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macciò A, Madeddu C. Inflammation and ovarian cancer. Cytokine. 2012;58(2):133–147. doi: 10.1016/j.cyto.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Wu D, Cheng J, Sun G, et al. p70S6K promotes IL-6-induced epithelial-mesenchymal transition and metastasis of head and neck squamous cell carcinoma. Oncotarget. 2016;7(24):36539–36550. doi: 10.18632/oncotarget.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22(5):347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Wu P, Wu D, et al. Prognostic and clinicopathological significance of serum interleukin-6 expression in colorectal cancer: a systematic review and meta-analysis. Onco Targets Ther. 2015;8:3793–3801. doi: 10.2147/OTT.S93297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abubaker K, Luwor RB, Escalona R, et al. Targeted disruption of the JAK2/STAT3 pathway in combination with systemic administration of paclitaxel inhibits the priming of ovarian cancer stem cells leading to a reduced tumor burden. Front Oncol. 2014;4:75. doi: 10.3389/fonc.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan Z, Foster R, Bell DA, et al. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin Cancer Res. 2006;12(17):5055–5063. doi: 10.1158/1078-0432.CCR-06-0861. [DOI] [PubMed] [Google Scholar]
- 38.Kim G, Davidson B, Henning R, et al. Adhesion molecule protein signature in ovarian cancer effusions is prognostic of patient outcome. Cancer. 2012;118(6):1543–1553. doi: 10.1002/cncr.26449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho CM, Cheng WF, Lin MC, et al. Prognostic and predictive values of E-cadherin for patients of ovarian clear cell adenocarcinoma. Int J Gynecol Cancer. 2010;20(9):1490–1497. doi: 10.1111/IGC.0b013e3181e68a4d. [DOI] [PubMed] [Google Scholar]
- 40.Silver DL, Naora H, Liu J, Cheng W, Montell DJ. Activated signal transducer and activator of transcription (STAT) 3: localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res. 2004;64(10):3550–3558. doi: 10.1158/0008-5472.CAN-03-3959. [DOI] [PubMed] [Google Scholar]
- 41.Wen W, Wu J, Liu L, et al. Synergistic anti-tumor effect of combined inhibition of EGFR and JAK/STAT3 pathways in human ovarian cancer. Mol Cancer. 2015;14:100. doi: 10.1186/s12943-015-0366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mo L, Bachelder RE, Kennedy M, et al. Syngeneic murine ovarian cancer model reveals that ascites enriches for ovarian cancer stem-like cells expressing membrane GRP78. Mol Cancer Ther. 2015;14(3):747–756. doi: 10.1158/1535-7163.MCT-14-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erroi A, Sironi M, Chiaffarino F, Chen ZG, Mengozzi M, Mantovani A. IL-1 and IL-6 release by tumor-associated macrophages from human ovarian carcinoma. Int J Cancer. 1989;44(5):795–801. doi: 10.1002/ijc.2910440508. [DOI] [PubMed] [Google Scholar]
- 44.Chaluvally-Raghavan P, Jeong KJ, Pradeep S, et al. Direct upregulation of STAT3 by microRNA-551b-3p deregulates growth and metastasis of ovarian cancer. Cell Rep. 2016;15(7):1493–1504. doi: 10.1016/j.celrep.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.So KA, Min KJ, Hong JH, Lee JK. Interleukin-6 expression by interactions between gynecologic cancer cells and human mesenchymal stem cells promotes epithelial-mesenchymal transition. Int J Oncol. 2015;47(4):1451–1459. doi: 10.3892/ijo.2015.3122. [DOI] [PubMed] [Google Scholar]
- 46.Ansieau S, Bastid J, Doreau A, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14(1):79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Morel AP, Hinkal GW, Thomas C, et al. EMT inducers catalyze malignant transformation of mammary epithelial cells and drive tumorigenesis towards claudin-low tumors in transgenic mice. PLoS Genet. 2012;8(5):e1002723. doi: 10.1371/journal.pgen.1002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vergara D, Merlot B, Lucot JP, et al. Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett. 2010;291(1):59–66. doi: 10.1016/j.canlet.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 49.Stefania D, Vergara D. The many-faced program of epithelial-Mesenchymal transition: a system biology-based view. Front Oncol. 2017;7:274. doi: 10.3389/fonc.2017.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wellner U, Schubert J, Burk UC, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11(12):1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 51.Guo L, Chen C, Shi M, et al. Stat3-coordinated Lin-28-let-7-HMGA2 and miR-200-ZEB1 circuits initiate and maintain oncostatin M-driven epithelial-mesenchymal transition. Oncogene. 2013;32(45):5272–5282. doi: 10.1038/onc.2012.573. [DOI] [PubMed] [Google Scholar]
- 52.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 53.Cooke VG, LeBleu VS, Keskin D, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21(1):66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Auersperg N, Pan J, Grove BD, et al. E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc Natl Acad Sci U S A. 1999;96(11):6249–6254. doi: 10.1073/pnas.96.11.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Fan N, Yang J. Expression and clinical significance of hypoxia-inducible factor 1α, Snail and E-cadherin in human ovarian cancer cell lines. Mol Med Rep. 2015;12(3):3393–3399. doi: 10.3892/mmr.2015.3786. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Yoshida T, Ozawa Y, Kimura T, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer. 2014;110(6):1497–1505. doi: 10.1038/bjc.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeasmin S, Nakayama K, Rahman MT, et al. Loss of MKK4 expression in ovarian cancer: a potential role for the epithelial to mesenchymal transition. Int J Cancer. 2011;128(1):94–104. doi: 10.1002/ijc.25332. [DOI] [PubMed] [Google Scholar]
- 58.Terauchi M, Kajiyama H, Yamashita M, et al. Possible involvement of TWIST in enhanced peritoneal metastasis of epithelial ovarian carcinoma. Clin Exp Metastasis. 2007;24(5):329–339. doi: 10.1007/s10585-007-9070-1. [DOI] [PubMed] [Google Scholar]
- 59.Keskin D, Kim J, Cooke VG, et al. Targeting vascular pericytes in hypoxic tumors increases lung metastasis via angiopoietin-2. Cell Rep. 2015;10(7):1066–1081. doi: 10.1016/j.celrep.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naylor MS, Stamp GW, Davies BD, Balkwill FR. Expression and activity of MMPS and their regulators in ovarian cancer. Int J Cancer. 1994;58(1):50–56. doi: 10.1002/ijc.2910580110. [DOI] [PubMed] [Google Scholar]
- 61.Ko HS, Park BJ, Choi SK, et al. STAT3 and ERK signaling pathways are implicated in the invasion activity by oncostatin m through induction of matrix metalloproteinases 2 and 9. Yonsei Med J. 2016;57(3):761–768. doi: 10.3349/ymj.2016.57.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piura B, Medina L, Rabinovich A, Dyomin V, Huleihel M. Thalidomide distinctly affected TNF-α, IL-6 and MMP secretion by an ovarian cancer cell line (SKOV-3) and primary ovarian cancer cells. Eur Cytokine Netw. 2013;24(3):122–129. doi: 10.1684/ecn.2013.0342. [DOI] [PubMed] [Google Scholar]
- 63.Li LN, Zhou X, Gu Y, Yan J. Prognostic value of MMP-9 in ovarian cancer: a meta-analysis. Asian Pac J Cancer Prev. 2013;14(7):4107–4113. doi: 10.7314/apjcp.2013.14.7.4107. [DOI] [PubMed] [Google Scholar]
- 64.Hu X, Li D, Zhang W, Zhou J, Tang B, Li L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Arch Gynecol Obstet. 2012;286(6):1537–1543. doi: 10.1007/s00404-012-2456-6. [DOI] [PubMed] [Google Scholar]
- 65.Rabinovich A, Medina L, Piura B, Segal S, Huleihel M. Regulation of ovarian carcinoma SKOV-3 cell proliferation and secretion of MMPs by autocrine IL-6. Anticancer Res. 2007;27(1A):267–272. [PubMed] [Google Scholar]
- 66.Ma Y, Zhang X, Xu X, et al. STAT3 decoy oligodeoxynucleotides-loaded solid lipid nanoparticles induce cell death and inhibit invasion in ovarian cancer cells. PLoS One. 2015;10(4):e0124924. doi: 10.1371/journal.pone.0124924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang YJ, Sun ZL, Wu WG, et al. Inhibitor of signal transducer and activator of transcription 3 (STAT3) suppresses ovarian cancer growth, migration and invasion and enhances the effect of cisplatin in vitro. Genet Mol Res. 2015;14(1):2450–2460. doi: 10.4238/2015.March.30.3. [DOI] [PubMed] [Google Scholar]
- 68.Angevin E, Tabernero J, Elez E, et al. A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2014;20(8):2192–2204. doi: 10.1158/1078-0432.CCR-13-2200. [DOI] [PubMed] [Google Scholar]
- 69.van Kruchten M, de Vries EF, Arts HJ, et al. Assessment of estrogen receptor expression in epithelial ovarian cancer patients using 16α-18F-fluoro-17β-estradiol PET/CT. J Nucl Med. 2015;56(1):50–55. doi: 10.2967/jnumed.114.147579. [DOI] [PubMed] [Google Scholar]
- 70.Gearing DP, Comeau MR, Friend DJ, et al. The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992;255(5050):1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- 71.Mosley B, De Imus C, Friend D, et al. Dual oncostatin M (OSM) receptors. Cloning and characterization of an alternative signaling subunit conferring OSM-specific receptor activation. J Biol Chem. 1996;271(51):32635–32643. doi: 10.1074/jbc.271.51.32635. [DOI] [PubMed] [Google Scholar]
- 72.Liu J, Modrell B, Aruffo A, Scharnowske S, Shoyab M. Interactions between oncostatin M and the IL-6 signal transducer, gp130. Cytokine. 1994;6(3):272–278. doi: 10.1016/1043-4666(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 73.Grenier A, Dehoux M, Boutten A, et al. Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood. 1999;93(4):1413–1421. [PubMed] [Google Scholar]
- 74.West NR, Murray JI, Watson PH. Oncostatin-M promotes phenotypic changes associated with mesenchymal and stem cell-like differentiation in breast cancer. Oncogene. 2014;33(12):1485–1494. doi: 10.1038/onc.2013.105. [DOI] [PubMed] [Google Scholar]
- 75.David E, Guihard P, Brounais B, et al. Direct anti-cancer effect of oncostatin M on chondrosarcoma. Int J Cancer. 2011;128(8):1822–1835. doi: 10.1002/ijc.25776. [DOI] [PubMed] [Google Scholar]
- 76.David E, Tirode F, Baud’huin M, et al. Oncostatin M is a growth factor for Ewing sarcoma. Am J Pathol. 2012;181(5):1782–1795. doi: 10.1016/j.ajpath.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 77.Ryan RE, Martin B, Mellor L, et al. Oncostatin M binds to extracellular matrix in a bioactive conformation: implications for inflammation and metastasis. Cytokine. 2015;72(1):71–85. doi: 10.1016/j.cyto.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]