Abstract
Objective
The objective of this study was to investigate the prognostic significance and the efficacy evaluation of total lymphocyte count (TLC), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) in advanced non–small cell lung cancer (NSCLC) patients treated with chemoradiotherapy.
Patients and methods
A total of 389 advanced NSCLC patients who received chemoradiotherapy from 2011 to 2016 were enrolled in this retrospective study. TLC, NLR, and PLR were analyzed with overall survival (OS). Survival data were identified with the Kaplan–Meier method and optimal cutoff values with receiver operating characteristic curves.
Results
The median OS for all patients was 18.37 months. Pretreatment and median baseline TLC was 2.47×103/μL (±0.78); NLR, 3.15 (±3.96); and PLR, 143.82 (±91.77); corresponding cutoffs were 2.4, 3.4, and 136.1. Higher TLC was associated with superior median OS (21.78 vs 15.66 months, P<0.001), and higher NLR and PLR with worse median OS (NLR: 14.13 vs 23.8 months, P<0.001; PLR: 15.49 vs 22.04 months, P<0.001).
Conclusion
The lymphopenia indicators (TLC, NLR, and PLR) were significant prognostic indicators of survival in advanced NSCLC patients treated with chemoradiotherapy.
Keywords: TLC, NLR, PLR, advanced NSCLC, chemoradiotherapy
Introduction
Lung cancer is a malignant tumor with the highest incidence worldwide, and non– small cell lung cancer (NSCLC) accounts for ~85% of all new lung cancer cases.1 Although surgical techniques and medical treatment have been advanced by leaps and bounds, the prognosis for lung cancer remains poor, with a 5-year survival rate <15%.2 For inoperable locally advanced patients, radiotherapy and chemotherapy are now the main treatment methods, but there is a lack of precise biomarkers for efficacy and prognosis. Therefore, sensitive and prognostic factors are needed to guide clinical practice.
An increasing number of studies have been devoted to explore the relationship between the occurrence and development of cancer and inflammation. The malignant transformation of inflammation as a very complex biological process often requires pathogens, cells, genes, and other factors involved jointly. In March 2011, two professors published an updated review which briefly describes the hot spots and progresses in oncology in the last 10 years and adds four new features on the basis of the original six features. “Tumor Promotion Inflammation” as one of the tumor cell features was known to us.3–7 Peripheral blood total lymphocyte count (TLC), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) are signs of systemic inflammatory response, and they are closely related to prognosis of multiple malignant tumors.8 As TLC, NLR, and PLR can be calculated by using only blood routine results, they have the advantages of being cheaper, more stable, and repeatable. At present, some studies have confirmed their correlation with the prognosis of various tumors, such as gastric cancer,9,10 colorectal cancer,11,12 liver cancer, cholangio carcinoma,13 breast cancer,14 and so on. But there are relatively few studies on the correlation of prognosis of patients with advanced NSCLC alone, especially for unresectable patients. So the aims of this study were to identify the prognostic value of TLC, NLR, and PLR in advanced NSCLC patients treated with chemoradiotherapy and whether they could be better prognostic indicators.
Patients and methods
Patient selection
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Committee Board of Shandong Cancer Hospital (Jinan, China). Written informed consent was obtained from all patients. We retrospectively collected patients with advanced NSCLC from 2011 to 2016. The inclusion criteria were: 1) patients were diagnosed as NSCLC by pathologic examination (histology and/or cytology examination); 2) according to the TNM criteria of NSCLC (Union for International Cancer Control, eighth edition), patients were diagnosed with stage IIIA or IIIB; 3) the whole course of radiotherapy was conducted in our hospital; and 4) availability of laboratory data obtained at the same institution before initiation of any curative treatment (radiotherapy, chemotherapy, or both). The exclusion criteria were: 1) had upfront surgery in the past three years; 2) did not complete radiation therapy. All the enrolled patients received standard treatment in our institution, including chemotherapy followed by concurrent chemoradiotherapy, upfront concurrent chemoradiotherapy followed by adjuvant chemotherapy, induction chemotherapy followed by sequential radiotherapy, or concurrent chemoradiotherapy alone. The standard chemotherapies were platinum-based regimens. Finally, 389 advanced NSCLC patients were included in this study. Patients were generally followed up every 3 months for the first 2 years, every 6 months from 3 to 5 years, and every year thereafter. Thoracic computed tomography (CT) and positron emission tomography-CT scans were performed at each follow-up visit, magnetic resonance imaging (MRI) studies or CT scans of the brain were also obtained at every follow-up visit or on the appearance of any neurologic symptoms. The compliance of making CT was satisfactory, but other examinations were not performed in time unless patients have symptoms.
Data collection
Patient characteristics and hematologic data were obtained from electronic medical records from Shandong Cancer Hospital. Targeted variables included age, gender, Karnofsky Performance Status (KPS) score, smoking status, and laboratory values from pretreatment data of complete blood count (CBC). The most recent CBC data before the initial point of definitive treatment (radiotherapy, chemotherapy, or both) were used. Treatment variables included chemotherapy regimen and dose, radiation technique and dose, receipt of prophylactic cranial irradiation, and receipt of induction or concurrent chemotherapy. Tumor-specific variables included T status, N status, and TNM stage. Variables from CBC included TLC, NLR, and PLR. All the CBC variables were obtained within 3 months before the initial time they received definitive treatment.
Statistical analysis
Overall survival (OS) was measured from the date of definitive therapy to the date of death from any cause or the date on which the patients were last known to be alive. In order to determine the independent prognostic factors, a multivariate analysis was performed using Cox proportional hazard model. The optimal cutoff value of TLC, NLR, and PLR were determined by a running log-rank test. The two category comparisons were performed by χ2 test or Fisher’s exact test. The comparison of continuous variables was performed by t test. P-values reported were bidirectional, and the significant level was at <0.05. All analyses were performed using SPSS statistics 19.0.
Ethics approval and consent to participate
Due to the retrospective nature of the study, informed consent was waived. The study was approved by the committee of our hospital.
Results
A total of 389 patients were treated with definitive therapy for unresectable advanced NSCLC in this study; 345 of these patients had neutrophil, lymphocyte, and platelet counts within 1 month before the initiation of definitive therapy, but only 310 had biopsy-proven stage IIIA or IIIB NSCLC. Of these patients, 43 were excluded from this study, 29 for having been initially treated at other hospital or institution, eight for not receiving integrated course of treatment, and six for having had upfront surgery in the last three years. Finally, 267 patients were enrolled in this study; 170 patients had adenocarcinoma, 89 had squamous cell carcinoma, and eight were not specified further than NSCLC. Patient characteristics and hematologic data are shown in Table 1. The median follow-up for all patients was 28 months.
Table 1.
Patient characteristics
| Pretreatment lymphocyte count | ||||
|---|---|---|---|---|
| Value or no. of patients (%) | ||||
| Characteristic | All patients | TLC <2.4×103/μL | TLC ≥2.4×103/μL | P-value |
| (n=267) | (n=132) | (n=135) | ||
| Demographics | ||||
| Gender | ||||
| Male | 146 | 74 | 72 | 0.654 |
| Female | 121 | 58 | 63 | |
| Age | ||||
| Median (IQR), years | 62(58–71) | 63(59–70) | 62(60–71) | 0.851 |
| KPS score | ||||
| 60–80 | 234 | 113 | 121 | 0.283 |
| ≥80 | 33 | 19 | 14 | |
| Smoking status | ||||
| Current | 92 | 43 | 49 | 0.088 |
| Former | 151 | 72 | 79 | |
| Never | 24 | 17 | 7 | |
| Treatment | ||||
| Radiation technique | ||||
| 3D-CRT | 101 | 49 | 52 | 0.814 |
| IMRT | 166 | 83 | 83 | |
| Chemotherapy | ||||
| No induction chemo | 157 | 77 | 80 | 0.879 |
| Induction chemo | 110 | 55 | 55 | |
| No concurrent chemo | 70 | 36 | 34 | 0.698 |
| Concurrent chemo | 197 | 96 | 101 | |
| T status | ||||
| T1 | 31 | 15 | 16 | 0.494 |
| T2 | 72 | 34 | 38 | |
| T3 | 70 | 37 | 33 | |
| T4 | 88 | 45 | 43 | |
| Tx | 9 | 4 | 5 | |
| N status | ||||
| N0 | 50 | 23 | 27 | 0.905 |
| N1 | 38 | 19 | 19 | |
| N2 | 100 | 52 | 48 | |
| N3 | 79 | 38 | 41 | |
| Disease stage | ||||
| IIIA | 58 | 27 | 31 | 0.641 |
| IIIB | 209 | 105 | 104 | |
| Pretreatment laboratory findings | ||||
| Hb, median (IQR), g/dL | 12.9 (12.0–14.4) | 12.9 (12.1–14.4) | 12.9 (12.0–14.3) | 0.047 |
| WBC, median | ||||
| (IQR), ×103/μL | 7.6 (6.3–8.9) | 6.8 (5.5–8.3) | 8.4 (7.5–9.6) | <0.001 |
| Neutrophils, median | ||||
| (IQR), ×103/μL | 4.8 (3.7–5.9) | 4.4 (3.4–5.9) | 5.1 (4.2–6.1) | 0.063 |
| Platelets, median | ||||
| (IQR), ×103/μL | 249 (201–316) | 245 (197–304) | 262 (217–334) | 0.189 |
Abbreviations: 3D-CRT, 3-dimensional conformal radiation therapy; Hb, hemoglobin; IMRT, intensity-modulated radiation therapy; IQR, interquartile range; KPS, Karnofsky Performance Status; TLC, total lymphocyte count; WBC, white blood cells.
The median time to obtain the indicators of hematology before treatment was 8 days (interquartile range [IQR], 1–12 days). Median baseline value of TLC was 2.47×103/μL (±0.78), NLR was 3.15 (±3.96), and PLR was 143.82 (±91.77). According to receiver operating characteristic (ROC) analysis, the cutoff values of TLC was 2.4 (sensitivity 43%, specificity 56%), NLR was 3.4 (sensitivity 54%, specificity 73%), and PLR was 136.1 (sensitivity 54%, specificity 73%). According to these different cutoff values, patients were divided into two groups for OS analysis.
The median OS time for all patients was 18.37 months (IQR 10.3–48.2). In univariate analysis, KPS score ≥70, TNM stage IIIB, higher TLC (≥2.4× 103/μL), lower NLR (<3.4), and lower PLR (<136.1) were all associated with better survival (P<0.05) (Table 2).
Table 2.
Univariate analysis of factors potentially associated with overall survival
| Characteristics | HR | 95% CI | P-value |
|---|---|---|---|
| Age ≥65 years | 1.32 | 0.91–2.46 | 0.085 |
| Male | 0.95 | 0.74–2.60 | 0.228 |
| KPS score ≥70 | 2.32 | 1.51–4.12 | 0.009 |
| TNM stage IIIB | 1.24 | 0.87–1.94 | 0.013 |
| Current smoker | 0.84 | 0.51–1.85 | 0.182 |
| Receipt of induction chemotherapy | 0.73 | 0.32–1.54 | 0.797 |
| Receipt of concurrent chemotherapy | 0.88 | 0.45–1.84 | 0.791 |
| Pretreatment TLC ≥2.4×103/μL | 0.46 | 0.39–0.91 | 0.035 |
| Pretreatment NLR ≥3.4 | 1.87 | 1.19–2.63 | 0.010 |
| Pretreatment PLR ≥136.1 | 1.78 | 1.16–2.56 | 0.025 |
Abbreviations: HR, hazard ratio; KPS, Karnofsky Performance Status; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; TLC, total lymphocyte count.
From the Kaplan–Meier plots of OS, we can see that patients with higher pretreatment TLC (≥2.4×103/μL) had better OS than patients with lower TLC (<2.4×10 /μL) (21.78 vs 15.66 months, P<0.001) (Figure 1). Inversely, patients with higher pretreatment NLR (≥3.4) and PLR (≥136.1) had worse median OS times than patients with lower NLR (<3.4) and PLR (<136.1) (NLR: 14.13 vs 23.8 months, P<0.001; PLR: 15.49 vs 22.04 months, P<0.001) (Figures 2 and 3). Because TLC, NLR, and PLR have strong collinearity, these factors cannot be tested simultaneously, so they will be tested separately in multivariate analysis.
Figure 1.
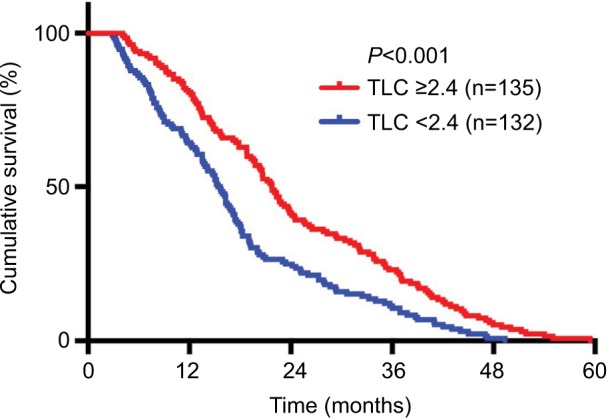
Kaplan–Meier plots of overall survival among patients who received chemoradiotherapy for advanced NSCLC stratified by baseline TLC.
Abbreviations: NSCLC, non–small cell lung cancer; TLC, total lymphocyte count.
Figure 2.
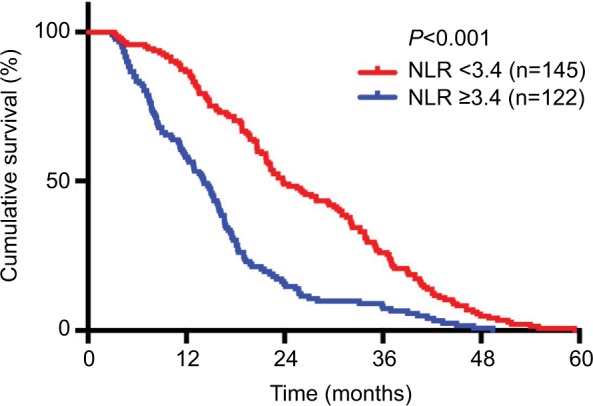
Kaplan–Meier plots of overall survival among patients who received chemoradiotherapy for advanced NSCLC stratified by baseline NLR.
Abbreviations: NSCLC, non–small cell lung cancer; NLR, neutrophil-to-lymphocyte ratio.
Figure 3.
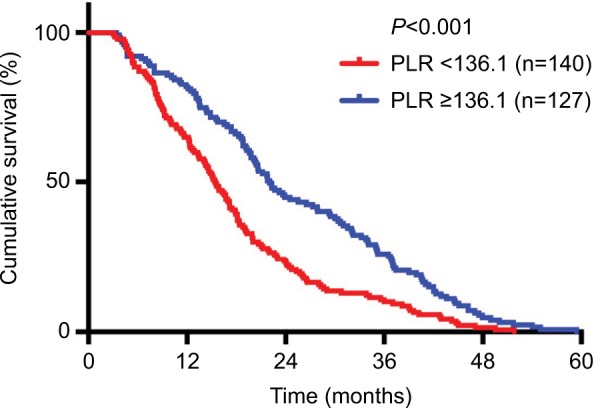
Kaplan–Meier plots of overall survival among patients who received chemoradiotherapy for advanced NSCLC stratified by baseline PLR.
Abbreviations: NSCLC, non–small cell lung cancer; PLR, platelet-to-lymphocyte ratio.
The final multivariate Cox regression analysis was adjusted for age, KPS score, disease stage, and the CBC values (Table 3). Results indicate that along with the positive effect of KPS score on OS, higher TLC (≥2.4×103/μL) was associated with superior OS (HR 0.49, 95% CI 0.33–0.95, P=0.041). Conversely, higher baseline NLR (≥3.4) and higher PLR (≥136.1) were associated with inferior OS (NLR: HR 1.66, 95% CI 1.05–2.79, P=0.041; PLR: HR 1.67, 95% CI 1.09–2.78, P=0.038).
Table 3.
Multivariate analysis of factors potentially associated with overall survival
| Model 1 (TLC included) | Model 2 (NLR included) | Model 3 (PLR included) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Characteristics | |||||||||
| Age ≥65 years | 1.51 | 0.88–2.41 | 0.185 | 1.43 | 0.87–2.35 | 0.176 | 1.43 | 0.89–2.35 | 0.175 |
| KPS score ≥70 | 1.68 | 1.04–3.63 | 0.041 | 1.53 | 1.13–3.24 | 0.032 | 1.52 | 1.1–3.23 | 0.033 |
| TNM stage IIIB | 1.32 | 0.44–2.43 | 0.162 | 1.84 | 0.71–2.82 | 0.148 | 1.82 | 0.74–2.85 | 0.143 |
| Pretreatment TLC ≥2.4×103/μL | 0.49 | 0.33–0.95 | 0.041 | ||||||
| Pretreatment NLR ≥3.4 | 1.66 | 1.05–2.79 | 0.041 | ||||||
| Pretreatment PLR ≥136.1 | 1.67 | 1.09–2.78 | 0.038 | ||||||
Abbreviations: HR, hazard ratio; KPS, Karnofsky Performance Status; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; TLC, total lymphocyte count.
Discussion
In recent years, studies have shown that systemic inflammatory response and the body’s immune system are correlated with tumor progression and prognosis. Inflammatory response may promote tumor metastasis by upregulating cytokines, producing inflammatory mediators, inhibiting cell apoptosis, promoting angiogenesis, and inducing DNA mutations. The invasiveness of cells is not only related to the essential characteristics of tumor cells, but also depends on the microenvironment. Inflammatory cells secrete inflammatory mediators, cytokines, and so on to stimulate the body to produce a series of stress reaction, then excessive inflammatory cells gathered themselves together and ultimately lead to cell oxidative damage and other negative biological effects, which influence the microenvironment of the body. In the end, normal cells are transformed into tumor cells, and the changed microenvironment could accelerate tumor growth, invasion and metastasis process.15 As for the immune system, under normal circumstances, the body relies on a complete immune mechanism to effectively monitor and reject cancerous cells, so the vast majority of individuals do not develop tumors. If cancer cells proliferate to a certain extent because they evade immune surveillance and rejection for some reason, the occurrence of tumors is inevitable.
Lymphocytes are an important part of the immune system and play a key role in immune response to cancer. TLC is closely related to the immune ability of the body and a low lymphocyte count indicates that the body is immunosuppressed.16 Hasenclever and Diehl first detected that lymphocyte reduction was an adverse prognostic factor for advanced Hodgkin’s lymphoma treated with combination chemotherapy, with or without adjuvant radiotherapy. And patients with peripheral blood lymphocyte count of <0.6×109/L had a worse prognosis.17 Ray-Coquard et al reported a prospective multicenter study, peripheral blood lymphocyte count was an independent prognostic factor of OS and progression-free survival (PFS) in patients with metastatic breast adenocarcinoma treated with chemotherapy, and in patients with diffuse large B-cell lymphoma.18
NLR represents the state of balance between neutrophils and lymphocytes. The higher NLR is, the more obvious the imbalance state is, that is, the more severe the inflammatory response and the stronger the immune suppression. The mechanism may be that neutrophils and lymphocytes participate in the escape of the tumor’s own immune system, thus promoting the occurrence and diffusion of the tumor.19 Elevated NLR indicates a decrease in T-lymphocyte-mediated anti-tumor response, and the release of inflammatory cytokines by neutrophils can help stimulate the tumor micro-environment and promote tumor metastasis.20 Ietomi first proposed in 1990 that the progression of malignant tumors was accompanied by increased neutrophils and decreased lymphocytes, and for the first time suggested that NLR may be associated with the prognosis of tumors.21 And recent studies confirmed that PFS and OS of patients with high NLR were significantly inferior to those with low NLR in patients with advanced NSCLC receiving first-line targeted therapy or chemotherapy.22,23
Most studies suggest that PLR has the similar effect with NLR in predicting the prognosis of cancer patients. The mechanism of poor prognosis caused by elevated PLR may be related to tumor metastasis or lymphocyte reduction associated with increased platelet count in cancer patients. During the immune process, platelets are activated and release a certain amount of growth factors, which are involved in the proliferation and adhesion of tumor cells and thereby promote the occurrence and invasion of tumors.24 Qiang et al conducted a meta-analysis on the relationship between PLR and lung cancer prognosis. The research showed that high PLR predicted shorter OS and PFS, which were independent risk factors influencing the prognosis of NSCLC patients.25
In our study we found that in advanced NSCLC patients treated with chemoradiotherapy, higher pretreatment TLC (≥2.4×103/μL) had better OS than patients with lower pretreatment TLC (<2.4×103/μL). Inversely, patients with higher pretreatment NLR (≥3.4) and PLR (≥136.1) had worse median OS times than patients with lower pretreatment NLR (<3.4) and PLR (<136.1). It can be seen that in advanced NSCLC, patients with high TLC and low NLR and PLR can obtain relatively good treatment effect and prognosis, which was consistent with previous research on other tumors by others. Therefore, the detection of TLC, NLR, and PLR before treatment is of certain value in the evaluation of prognosis of patients with advanced NSCLC.
However, there are still some limitations in this study. First, this study is a single-center clinical study with only 267 advanced NSCLC patients included. Therefore, a large sample multicenter clinical study is needed to verify the conclusions of this study. Second, this study did not take into account the mutation of EGFR and ALK in patients with advanced NSCLC, because the prognosis of patients receiving targeted therapy was significantly better than that of patients receiving radiotherapy and chemotherapy, which to some extent affected our evaluation of the value of TLC, NLR, and PLR in the prognosis of advanced NSCLC. In subsequent studies, the genetic status of patients should be further examined to exclude the effect of gene or targeted therapy on this result. Finally, the survival time of patients with advanced NSCLC is affected by a variety of factors, the influence of those factors on the survival time should be excluded as far as possible in subsequent studies.
Conclusion
Advanced NSCLC patients with high TLC and low NLR and PLR can obtain relatively good treatment effect and prognosis. The lymphopenia indicators (TLC, NLR, and PLR) were significant prognostic indicators of survival in advanced NSCLC patients treated with chemoradiotherapy.
Acknowledgments
The authors would like to express their gratitude to the enrolled patients and to the members of the Shandong Cancer Hospital Affiliated to Shandong University.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Li N, Xu M, Cai MY, et al. Elevated serum bilirubin levels are associated with improved survival in patients with curatively resected non-small-cell lung cancer. Cancer Epidemiol. 2015;39(5):763–768. doi: 10.1016/j.canep.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 3.de Martel C, Franceschi S. Infections and cancer: established associations and new hypotheses. Crit Rev Oncol Hematol. 2009;70(3):183–194. doi: 10.1016/j.critrevonc.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17(1):89–97. doi: 10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, Mcmillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. 2018;44(5):607–612. doi: 10.1016/j.ejso.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Kadam PD, Chuan HH. Erratum to: Rectocutaneous fistula with transmigration of the suture: a rare delayed complication of vault fixation with the sacrospinous ligament. Int Urogynecol J. 2016;27(3):505. doi: 10.1007/s00192-016-2952-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Zhang HY, Li J, Shao XY, Zhang CX. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(40):68837–68846. doi: 10.18632/oncotarget.18575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104(8):1288–1295. doi: 10.1038/bjc.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beal EW, Wei L, Ethun CG, et al. Elevated NLR in gallbladder cancer and cholangiocarcinoma–making bad cancers even worse: results from the US Extrahepatic Biliary Malignancy Consortium. HPB. 2016;18(11):950–957. doi: 10.1016/j.hpb.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iimori N, Kashiwagi S, Asano Y, et al. Clinical significance of the neutrophil-to-lymphocyte ratio in endocrine therapy for stage IV breast cancer. In Vivo. 2018;32(3):669–675. doi: 10.21873/invivo.112292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tazzyman S, Barry ST, Ashton S, et al. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. Int J Cancer. 2011;129(4):847–858. doi: 10.1002/ijc.25987. [DOI] [PubMed] [Google Scholar]
- 16.von Bernstorff W, Voss M, Freichel S, et al. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7(3 Suppl):925s–932s. [PubMed] [Google Scholar]
- 17.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339(21):1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 18.Ray-Coquard I, Cropet C, van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58(1):58–64. doi: 10.1016/j.jhep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer. 2009;45(11):1950–1958. doi: 10.1016/j.ejca.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Ietomi K. A study on the role of granulocytes in carcinoma-bearing hosts--G/L ratio as a new host indicator. Nihon Gan Chiryo Gakkai Shi. 1990;25(3):662–671. [PubMed] [Google Scholar]
- 22.Lin GN, Peng JW, Liu PP, Liu DY, Xiao JJ, Chen XQ. Elevated neutrophil-to-lymphocyte ratio predicts poor outcome in patients with advanced non-small-cell lung cancer receiving first-line gefitinib or erlotinib treatment. Asia Pac J Clin Oncol. 2017;13(5):e189–e194. doi: 10.1111/ajco.12273. [DOI] [PubMed] [Google Scholar]
- 23.Berardi R, Rinaldi S, Santoni M. Prognostic models to predict survival in patients with advanced non-small cell lung cancer treated with first-line chemo- or targeted therapy. Oncotarget. 2001;7(18):26916–2624. doi: 10.18632/oncotarget.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majeti BK, Lee JH, Simmons BH, Shojaei F. VEGF is an important mediator of tumor angiogenesis in malignant lesions in a genetically engineered mouse model of lung adenocarcinoma. BMC Cancer. 2013;13:213–216. doi: 10.1186/1471-2407-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiang G, Liang C, Xiao F, et al. Prognostic significance of platelet-to-lymphocyte ratio in non-small-cell lung cancer: a meta-analysis. Onco Targets Ther. 2016;9:869–876. doi: 10.2147/OTT.S96804. [DOI] [PMC free article] [PubMed] [Google Scholar]


